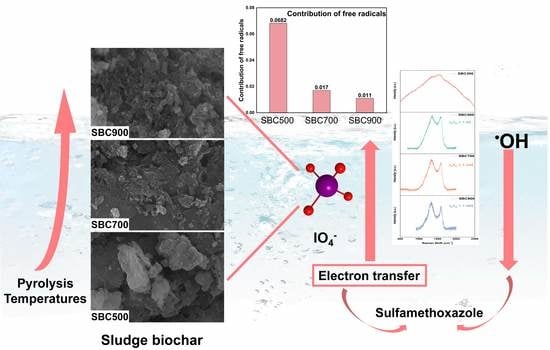
Converting Hybrid Mechanisms to Electron Transfer Mechanism by Increasing Biochar Pyrolysis Temperature for the Degradation of Sulfamethoxazole in a Sludge Biochar/Periodate System
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterizations of SBCs
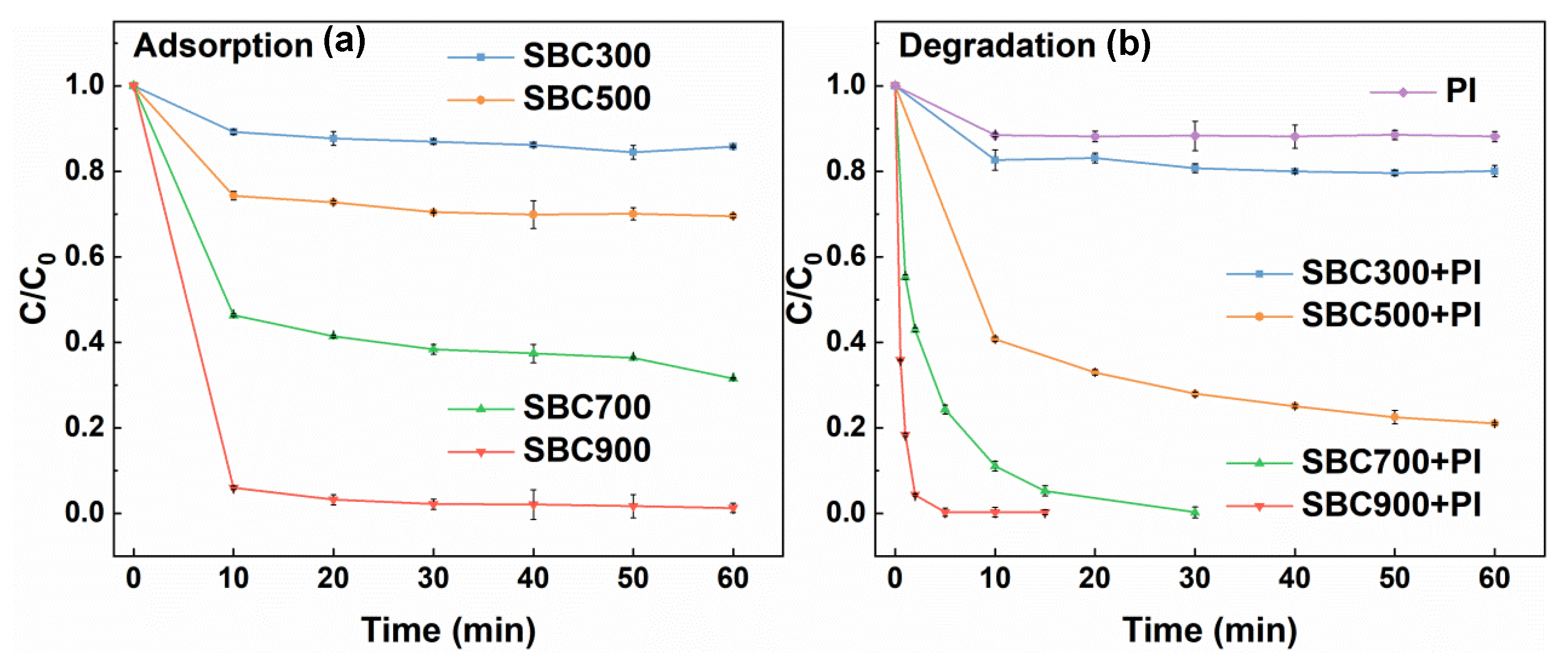
2.2. Activation Performance of SBCs towards PI for SMX Removal
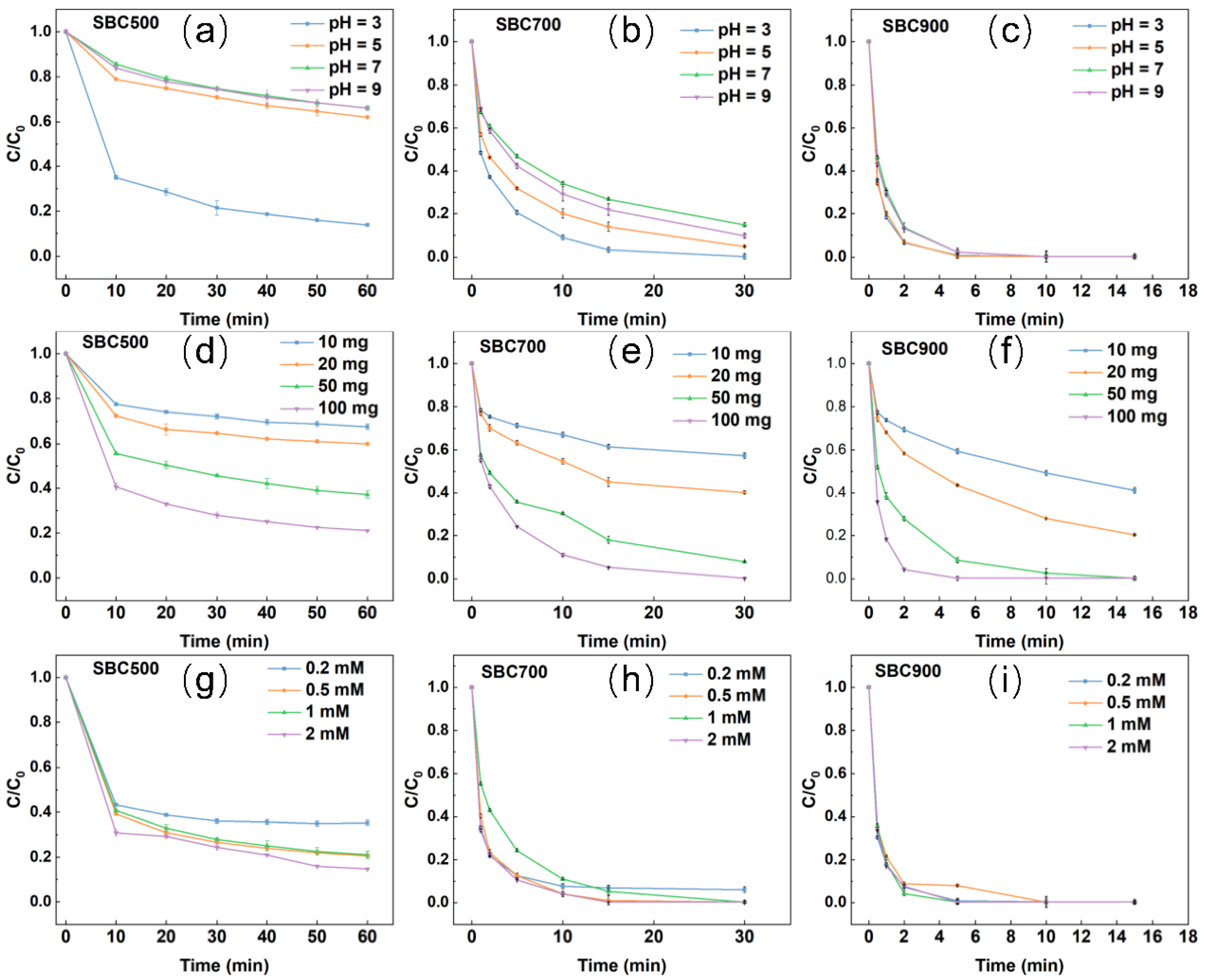
2.3. Influence of Reaction Conditions
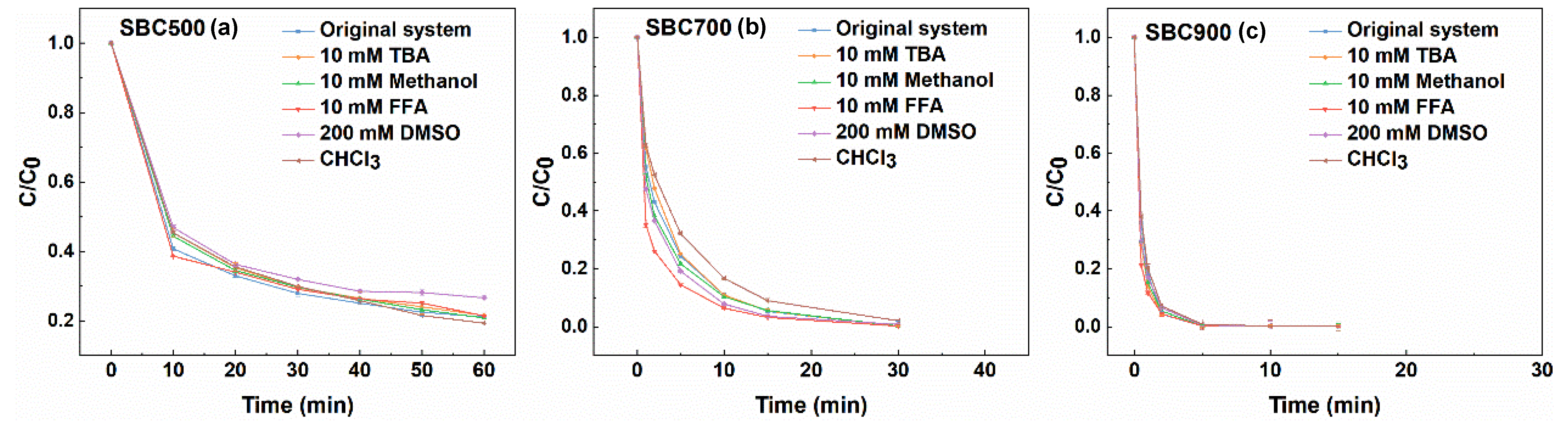
2.4. Influence of Common Matrix Species
2.5. Identification of Major Active Species and Possible Activation Mechanism
2.6. Toxicity Study in SBCs/PI Systems
3. Material and Methods
3.1. Materials
3.2. Preparation and Characterization of SBCs
3.3. Experimental Procedures and Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Syed-Hassan, S.S.A.; Wang, Y.; Hu, S.; Su, S.; Xiang, J. Thermochemical processing of sewage sludge to energy and fuel: Fundamentals, challenges and considerations. Renew. Sustain. Energy Rev. 2017, 80, 888–913. [Google Scholar] [CrossRef]
- Chen, Y.-D.; Wang, R.; Duan, X.; Wang, S.; Ren, N.-Q.; Ho, S.-H. Production, properties, and catalytic applications of sludge derived biochar for environmental remediation. Water Res. 2020, 187, 116390. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Gao, L.; Chen, Z.; Ma, C.; Zhou, Y.; Chen, J.; Ma, S.; Laghari, M.; Xiao, B.; Zhang, B.; et al. Syngas production by catalytic in-situ steam co-gasification of wet sewage sludge and pine sawdust. Energy Convers. Manag. 2016, 111, 409–416. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, J.; Zheng, P.; Qiu, R. Atrazine immobilization on sludge derived biochar and the interactive influence of coexisting Pb(II) or Cr(VI) ions. Chemosphere 2015, 134, 438–445. [Google Scholar] [CrossRef]
- Wu, Q.; Bao, X.; Guo, W.; Wang, B.; Li, Y.; Luo, H.; Wang, H.; Ren, N. Medium chain carboxylic acids production from waste biomass: Current advances and perspectives. Biotechnol. Adv. 2019, 37, 599–615. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Zhou, H.; Wang, J. Pyrolysis of municipal sewage sludges in a slowly heating and gas sweeping fixed-bed reactor. Energy Convers. Manag. 2014, 88, 1151–1158. [Google Scholar] [CrossRef]
- Chen, Y.-d.; Ho, S.-H.; Nagarajan, D.; Ren, N.-q.; Chang, J.-S. Waste biorefineries—Integrating anaerobic digestion and microalgae cultivation for bioenergy production. Curr. Opin. Biotechnol. 2018, 50, 101–110. [Google Scholar] [CrossRef]
- Samolada, M.C.; Zabaniotou, A.A. Comparative assessment of municipal sewage sludge incineration, gasification and pyrolysis for a sustainable sludge-to-energy management in Greece. Waste Manag. 2014, 34, 411–420. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. Peroxymonosulfate activation by Co9S8@ S and N co-doped biochar for sulfamethoxazole degradation. Chem. Eng. J. 2020, 385, 123933. [Google Scholar] [CrossRef]
- Liu, B.; Guo, W.; Wang, H.; Si, Q.; Zhao, Q.; Luo, H.; Ren, N. Activation of peroxymonosulfate by cobalt-impregnated biochar for atrazine degradation: The pivotal roles of persistent free radicals and ecotoxicity assessment. J. Hazard. Mater. 2020, 398, 122768. [Google Scholar] [CrossRef] [PubMed]
- Jellali, S.; Khiari, B.; Usman, M.; Hamdi, H.; Charabi, Y.; Jeguirim, M. Sludge-derived biochars: A review on the influence of synthesis conditions on pollutants removal efficiency from wastewaters. Renew. Sustain. Energy Rev. 2021, 144, 111068. [Google Scholar] [CrossRef]
- Wang, J.; Shen, M.; Gong, Q.; Wang, X.; Cai, J.; Wang, S.; Chen, Z. One-step preparation of ZVI-sludge derived biochar without external source of iron and its application on persulfate activation. Sci. Total Environ. 2020, 714, 136728. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yang, L.; Wu, L.; Li, P.; Qi, X.; He, L.; Cui, S.; Ding, Y.; Zhang, Z. Carbon nanotube supported sludge biochar as an efficient adsorbent for low concentrations of sulfamethoxazole removal. Sci. Total Environ. 2020, 718, 137299. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J. Activation of peroxymonosulfate by sludge-derived biochar for the degradation of triclosan in water and wastewater. Chem. Eng. J. 2019, 356, 350–358. [Google Scholar] [CrossRef]
- Long, Y.; Dai, J.; Zhao, S.; Su, Y.; Wang, Z.; Zhang, Z. Atomically Dispersed Cobalt Sites on Graphene as Efficient Periodate Activators for Selective Organic Pollutant Degradation. Environ. Sci. Technol. 2021, 55, 5357–5370. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, X.; Yu, X.; Kamali, M.; Appels, L.; van der Bruggen, B.; Cabooter, D.; Dewil, R. Efficiency and mechanism of 2,4-dichlorophenol degradation by the UV/IO4− process. Sci. Total Environ. 2021, 782, 146781. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Chen, M.-J.; Huang, C.-P.; Kuo, J.; Lo, S.-L. Efficient sonochemical degradation of perfluorooctanoic acid using periodate. Ultrason. Sonochem. 2016, 31, 499–505. [Google Scholar] [CrossRef]
- Chia, L.H.; Tang, X.M.; Weavers, L.K. Kinetics and mechanism of photoactivated periodate reaction with 4-chlorophenol in acidic solution. Environ. Sci. Technol. 2004, 38, 6875–6880. [Google Scholar] [CrossRef]
- Du, J.; Tang, S.; Faheem; Ling, H.; Zheng, H.; Xiao, G.; Luo, L.; Bao, J.; Faheem; Ling, H. Insights into periodate oxidation of bisphenol A mediated by manganese. Chem. Eng. J. 2019, 369, 1034–1039. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Singlet-Oxygen Generation in Alkaline Periodate Solution. Environ. Sci. Technol. 2015, 49, 14392–14400. [Google Scholar] [CrossRef]
- Choi, Y.; Yoon, H.I.; Lee, C.; Vetrakova, L.; Heger, D.; Kim, K.; Kim, J. Activation of Periodate by Freezing for the Degradation of Aqueous Organic Pollutants. Environ. Sci. Technol. 2018, 52, 5378–5385. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Lv, L.; Pillai, S.C.; Wang, H.; Xue, J.; Ma, Y.; Liu, Y.; Chen, Y.; Wu, L.; Zhang, Z.; et al. Efficient degradation of diclofenac sodium by periodate activation using Fe/Cu bimetallic modified sewage sludge biochar/UV system. Sci. Total Environ. 2021, 783, 146974. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Shi, Y.; Chen, Y.; Shen, S.; Xue, J.; Ma, Y.; Zheng, L.; Wu, L.; Zhang, Z.; Yang, L. Iron-manganese oxide loaded sludge biochar as a novel periodate activator for thiacloprid efficient degradation over a wide pH range. Sep. Purif. Technol. 2022, 288, 120703. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, X.; Mašek, O.; Zimmerman, A. Heterogeneity of biochar properties as a function of feedstock sources and production temperatures. J. Hazard. Mater. 2013, 256–257, 1–9. [Google Scholar] [CrossRef]
- Jin, J.; Li, Y.; Zhang, J.; Wu, S.; Cao, Y.; Liang, P.; Zhang, J.; Wong, M.H.; Wang, M.; Shan, S.; et al. Influence of pyrolysis temperature on properties and environmental safety of heavy metals in biochars derived from municipal sewage sludge. J. Hazard. Mater. 2016, 320, 417–426. [Google Scholar] [CrossRef]
- Zhang, P.; Tan, X.; Liu, S.; Liu, Y.; Zeng, G.; Ye, S.; Yin, Z.; Hu, X.; Liu, N. Catalytic degradation of estrogen by persulfate activated with iron-doped graphitic biochar: Process variables effects and matrix effects. Chem. Eng. J. 2019, 378, 122141. [Google Scholar] [CrossRef]
- Diao, Z.-H.; Dong, F.-X.; Yan, L.; Chen, Z.-L.; Qian, W.; Kong, L.-J.; Zhang, Z.-W.; Zhang, T.; Tao, X.-Q.; Du, J.-J.; et al. Synergistic oxidation of Bisphenol A in a heterogeneous ultrasound-enhanced sludge biochar catalyst/persulfate process: Reactivity and mechanism. J. Hazard. Mater. 2020, 384, 121385. [Google Scholar] [CrossRef]
- Yan, J.; Han, L.; Gao, W.; Xue, S.; Chen, M. Biochar supported nanoscale zerovalent iron composite used as persulfate activator for removing trichloroethylene. Bioresour. Technol. 2015, 175, 269–274. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J. Degradation of sulfamethoxazole by ozonation combined with ionizing radiation. J. Hazard. Mater. 2021, 407, 124377. [Google Scholar] [CrossRef]
- Bu, Q.; Wang, B.; Huang, J.; Deng, S.; Yu, G. Pharmaceuticals and personal care products in the aquatic environment in China: A review. J. Hazard. Mater. 2013, 262, 189–211. [Google Scholar] [CrossRef] [PubMed]
- Choong, Z.-Y.; Gasim, M.F.; Lin, K.-Y.A.; Hamidon, T.S.; Hussin, H.; Oh, W.-D. Unravelling the formation mechanism and performance of nitrogen, sulfur codoped biochar as peroxymonosulfate activator for gatifloxacin removal. Chem. Eng. J. 2023, 451, 138958. [Google Scholar] [CrossRef]
- Yang, Z.; Hou, J.; Wu, J.; Miao, L. The effect of carbonization temperature on the capacity and mechanisms of Pb(II) adsorption by microalgae residue-derived biochar. Ecotoxicol. Environ. Saf. 2021, 225, 112750. [Google Scholar] [CrossRef] [PubMed]
- Ren, N.; Tang, Y.; Li, M. Mineral additive enhanced carbon retention and stabilization in sewage sludge-derived biochar. Process Saf. Environ. Prot. 2018, 115, 70–78. [Google Scholar] [CrossRef]
- Ho, S.-H.; Chen, Y.-D.; Yang, Z.-K.; Nagarajan, D.; Chang, J.-S.; Ren, N.-Q. High-efficiency removal of lead from wastewater by biochar derived from anaerobic digestion sludge. Bioresour. Technol. 2017, 246, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Oh, W.-D.; Hu, Z.-T.; Sun, Y.-M.; Webster, R.D.; Li, S.-Z.; Lim, T.-T. Enhancing sulfacetamide degradation by peroxymonosulfate activation with N-doped graphene produced through delicately-controlled nitrogen functionalization via tweaking thermal annealing processes. Appl. Catal. B Environ. 2018, 225, 243–257. [Google Scholar] [CrossRef]
- Liu, S.; Lai, C.; Zhou, X.; Zhang, C.; Chen, L.; Yan, H.; Qin, L.; Huang, D.; Ye, H.; Chen, W.; et al. Peroxydisulfate activation by sulfur-doped ordered mesoporous carbon: Insight into the intrinsic relationship between defects and 1O2 generation. Water Res. 2022, 221, 118797. [Google Scholar] [CrossRef]
- He, L.; Yang, S.; Shen, S.; Ma, Y.; Chen, Y.; Xue, J.; Wang, J.; Zheng, L.; Wu, L.; Zhang, Z.; et al. Novel insights into the mechanism of periodate activation by heterogeneous ultrasonic-enhanced sludge biochar: Relevance for efficient degradation of levofloxacin. J. Hazard. Mater. 2022, 434, 128860. [Google Scholar] [CrossRef]
- Ji, Y.; Fan, Y.; Liu, K.; Kong, D.; Lu, J. Thermo activated persulfate oxidation of antibiotic sulfamethoxazole and structurally related compounds. Water Res. 2015, 87, 1–9. [Google Scholar] [CrossRef]
- Fan, S.; Wang, Y.; Wang, Z.; Tang, J.; Tang, J.; Li, X. Removal of methylene blue from aqueous solution by sewage sludge-derived biochar: Adsorption kinetics, equilibrium, thermodynamics and mechanism. J. Environ. Chem. Eng. 2017, 5, 601–611. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Qi, C.; Lin, C. Activation of periodate by granular activated carbon for acid orange 7 decolorization. J. Taiwan Inst. Chem. Eng. 2016, 68, 211–217. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Lin, C.; Qi, C.; Zhang, H.; Ma, J. Enhanced activation of periodate by iodine-doped granular activated carbon for organic contaminant degradation. Chemosphere 2017, 181, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.-D.; Lua, S.-K.; Dong, Z.; Lim, T.-T. Performance of magnetic activated carbon composite as peroxymonosulfate activator and regenerable adsorbent via sulfate radical-mediated oxidation processes. J. Hazard. Mater. 2015, 284, 1–9. [Google Scholar] [CrossRef]
- Pan, X.; Chen, J.; Wu, N.; Qi, Y.; Xu, X.; Ge, J.; Wang, X.; Li, C.; Qu, R.; Sharma, V.K.; et al. Degradation of aqueous 2,4,4′-Trihydroxybenzophenone by persulfate activated with nitrogen doped carbonaceous materials and the formation of dimer products. Water Res. 2018, 143, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Yu, Y.; Qu, R.; Chen, J.; Huo, Z.; Zhu, F.; Wang, Z. Fe-Activated Peroxymonosulfate Enhances the Degradation of Dibutyl Phthalate on Ground Quartz Sand. Environ. Sci. Technol. 2020, 54, 9052–9061. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; He, F.; Choi, W. Production of Reactive Oxygen Species by the Reaction of Periodate and Hydroxylamine for Rapid Removal of Organic Pollutants and Waterborne Bacteria. Environ. Sci. Technol. 2020, 54, 6427–6437. [Google Scholar] [CrossRef]
- Zhu, H.; Guo, A.; Wang, S.; Long, Y.; Fan, G.; Yu, X. Efficient tetracycline degradation via peroxymonosulfate activation by magnetic Co/N co-doped biochar: Emphasizing the important role of biochar graphitization. Chem. Eng. J. 2022, 450, 138428. [Google Scholar] [CrossRef]
- Peng, L.; Duan, X.; Shang, Y.; Gao, B.; Xu, X. Engineered carbon supported single iron atom sites and iron clusters from Fe-rich Enteromorpha for Fenton-like reactions via nonradical pathways. Appl. Catal. B Environ. 2021, 287, 119963. [Google Scholar] [CrossRef]
- Li, Y.; Yang, T.; Qiu, S.; Lin, W.; Yan, J.; Fan, S.; Zhou, Q. Uniform N-coordinated single-atomic iron sites dispersed in porous carbon framework to activate PMS for efficient BPA degradation via high-valent iron-oxo species. Chem. Eng. J. 2020, 389, 124382. [Google Scholar] [CrossRef]
- Chen, L.; Duan, J.; Du, P.; Sun, W.; Lai, B.; Liu, W. Accurate identification of radicals by in-situ electron paramagnetic resonance in ultraviolet-based homogenous advanced oxidation processes. Water Res. 2022, 221, 118747. [Google Scholar] [CrossRef]
- Dong, X.; Ren, B.; Zhang, X.; Liu, X.; Sun, Z.; Li, C.; Tan, Y.; Yang, S.; Zheng, S.; Dionysiou, D.D. Diatomite supported hierarchical 2D CoNi3O4 nanoribbons as highly efficient peroxymonosulfate catalyst for atrazine degradation. Appl. Catal. B Environ. 2020, 272, 118971. [Google Scholar] [CrossRef]
- Wang, J.; Shen, M.; Wang, H.; Du, Y.; Zhou, X.; Liao, Z.; Wang, H.; Chen, Z. Red mud modified sludge biochar for the activation of peroxymonosulfate: Singlet oxygen dominated mechanism and toxicity prediction. Sci. Total Environ. 2020, 740, 140388. [Google Scholar] [CrossRef] [PubMed]

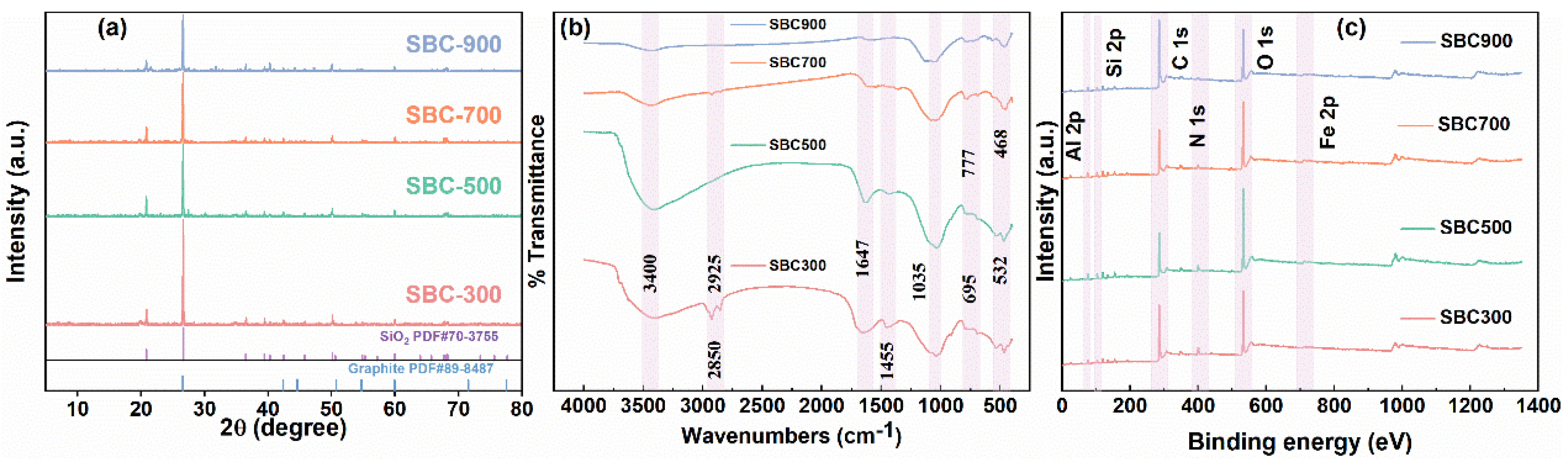



| Catalysts | SBET (m2 g−1) | Vtot (cm3 g−1) | Dp (nm) | O/C Ratio |
|---|---|---|---|---|
| SBC300 | 34.92 | 0.08942 | 10.64 | 1.03 |
| SBC500 | 83.50 | 0.1200 | 8.93 | 1.37 |
| SBC700 | 61.26 | 0.1798 | 12.34 | 1.41 |
| SBC900 | 124.96 | 0.1934 | 6.791 | 1.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, L.; Yang, S.; Yang, L.; Li, Y.; Kong, D.; Wu, L.; Zhang, Z. Converting Hybrid Mechanisms to Electron Transfer Mechanism by Increasing Biochar Pyrolysis Temperature for the Degradation of Sulfamethoxazole in a Sludge Biochar/Periodate System. Catalysts 2022, 12, 1431. https://doi.org/10.3390/catal12111431
He L, Yang S, Yang L, Li Y, Kong D, Wu L, Zhang Z. Converting Hybrid Mechanisms to Electron Transfer Mechanism by Increasing Biochar Pyrolysis Temperature for the Degradation of Sulfamethoxazole in a Sludge Biochar/Periodate System. Catalysts. 2022; 12(11):1431. https://doi.org/10.3390/catal12111431
Chicago/Turabian StyleHe, Liuyang, Shangding Yang, Lie Yang, Yulong Li, Dejin Kong, Li Wu, and Zulin Zhang. 2022. "Converting Hybrid Mechanisms to Electron Transfer Mechanism by Increasing Biochar Pyrolysis Temperature for the Degradation of Sulfamethoxazole in a Sludge Biochar/Periodate System" Catalysts 12, no. 11: 1431. https://doi.org/10.3390/catal12111431
APA StyleHe, L., Yang, S., Yang, L., Li, Y., Kong, D., Wu, L., & Zhang, Z. (2022). Converting Hybrid Mechanisms to Electron Transfer Mechanism by Increasing Biochar Pyrolysis Temperature for the Degradation of Sulfamethoxazole in a Sludge Biochar/Periodate System. Catalysts, 12(11), 1431. https://doi.org/10.3390/catal12111431