Na Promotion of Pt/m-ZrO2 Catalysts for the Steam Reforming of Formaldehyde
Abstract
1. Introduction
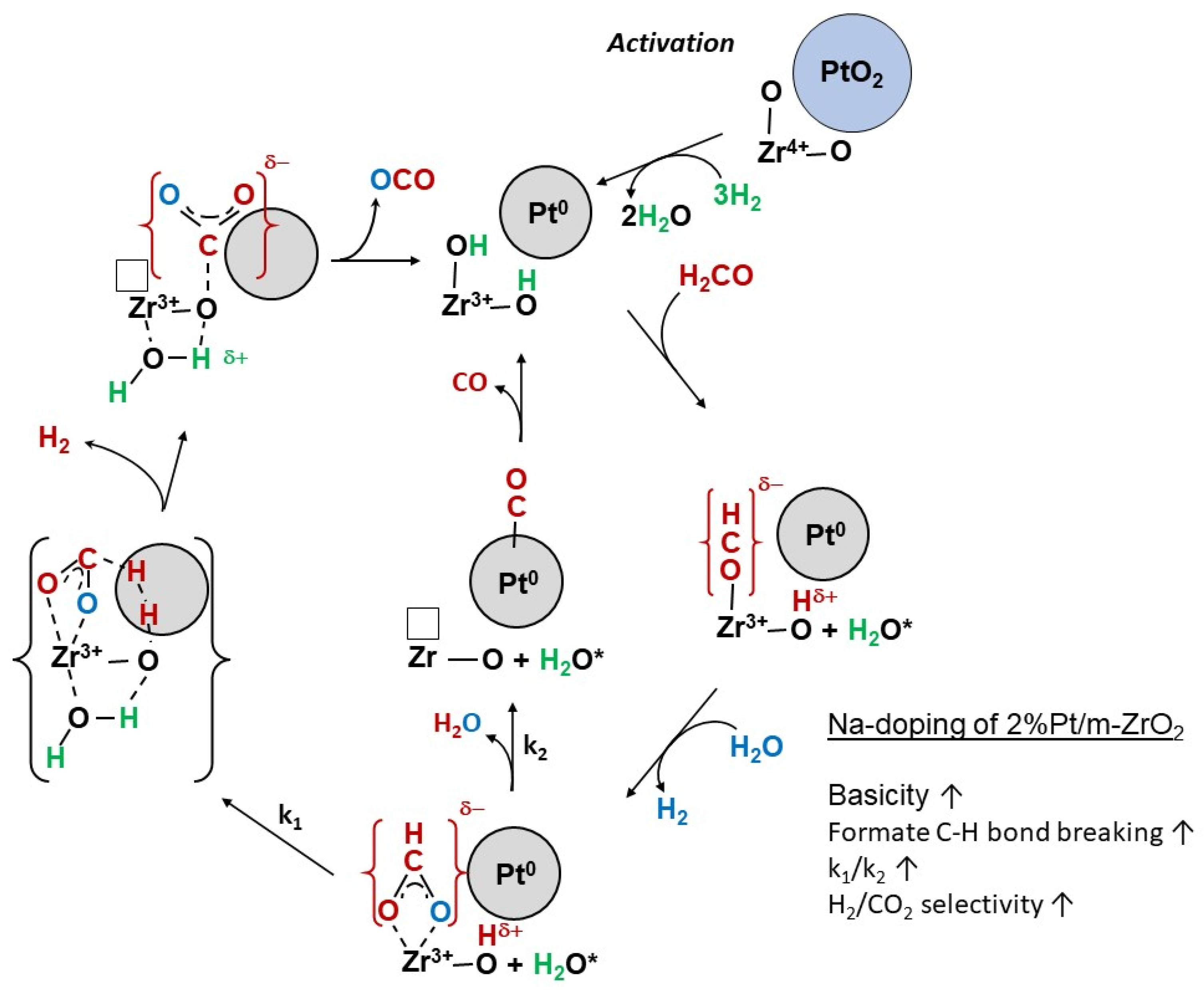
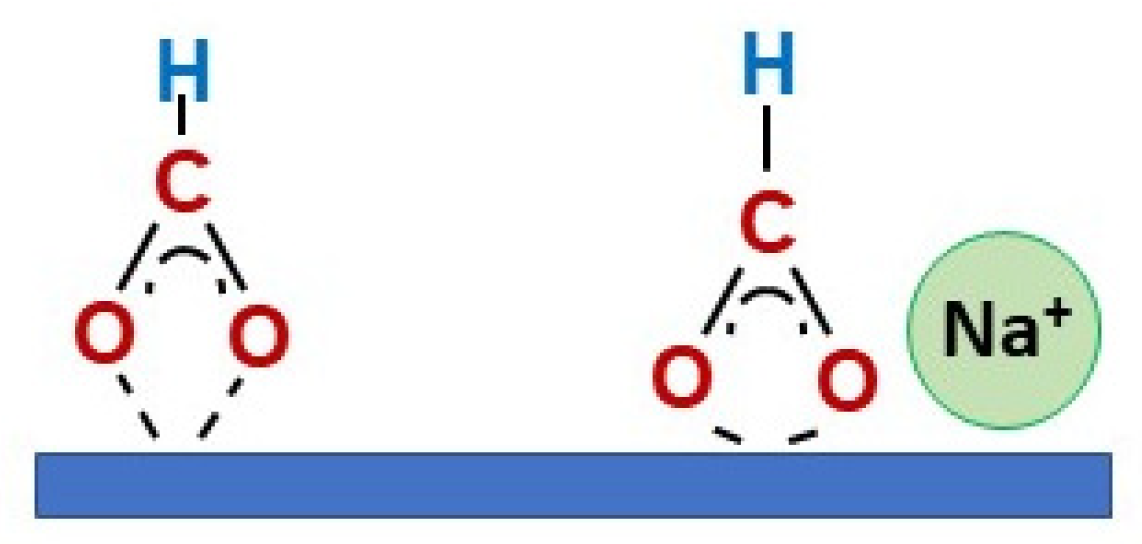
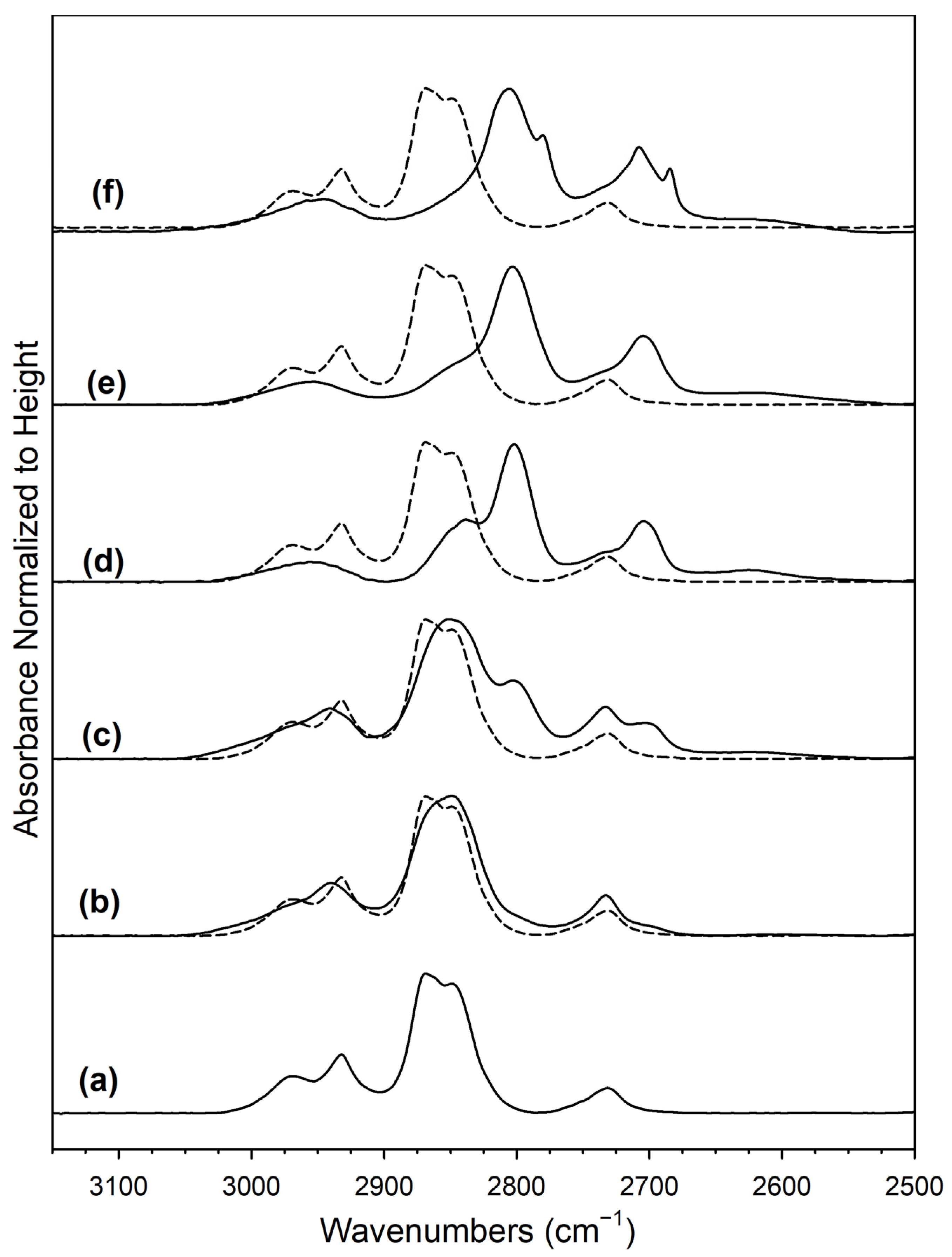
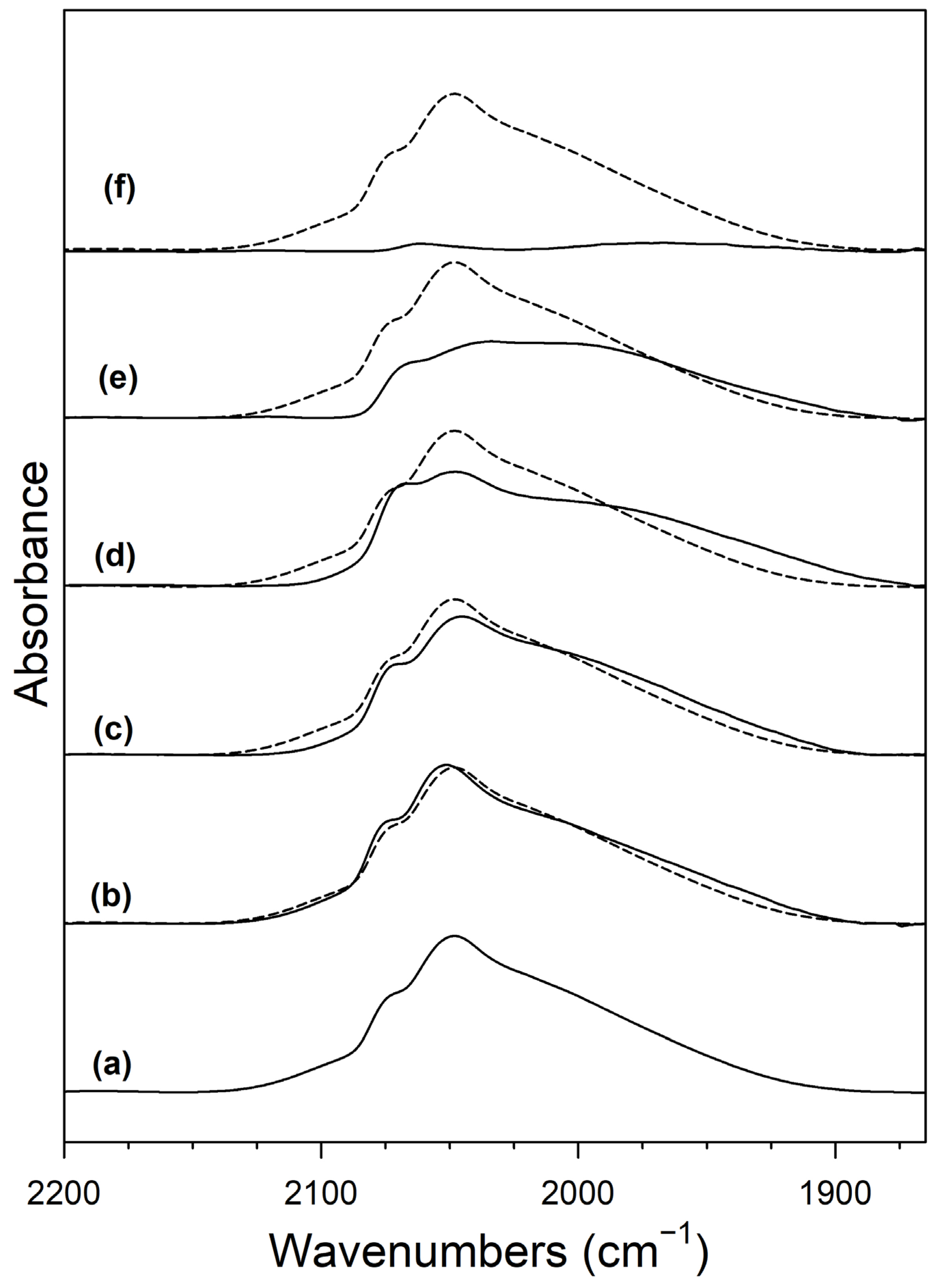
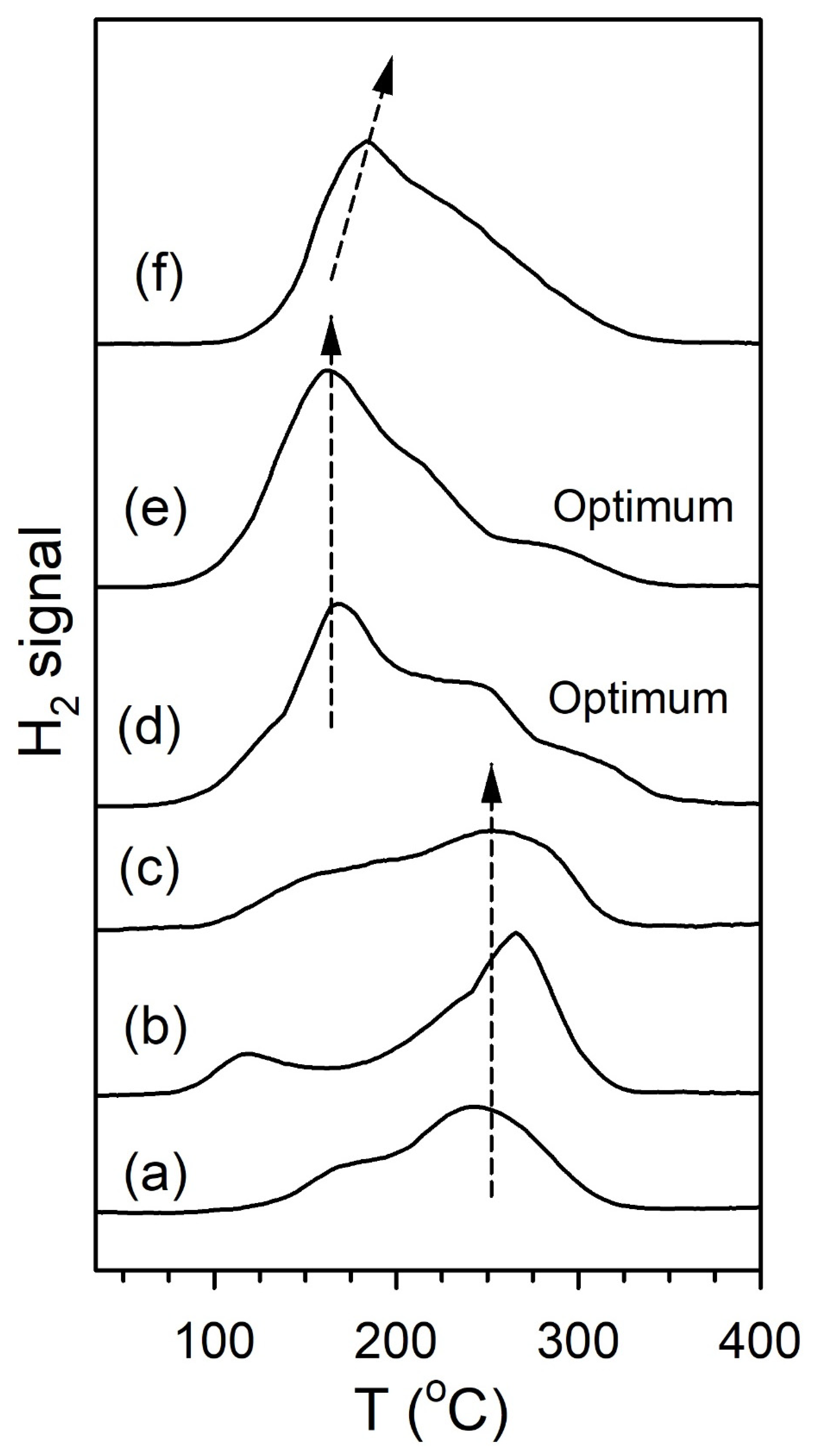
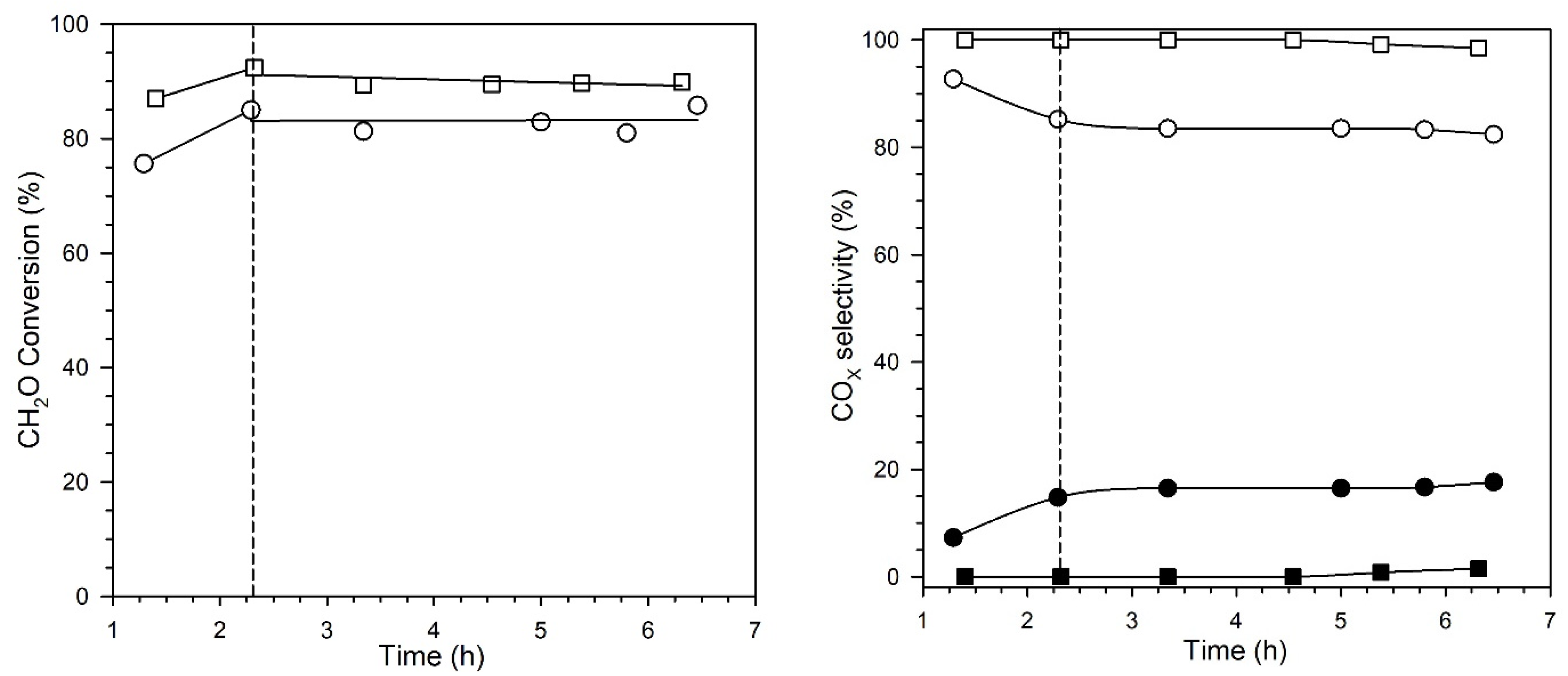
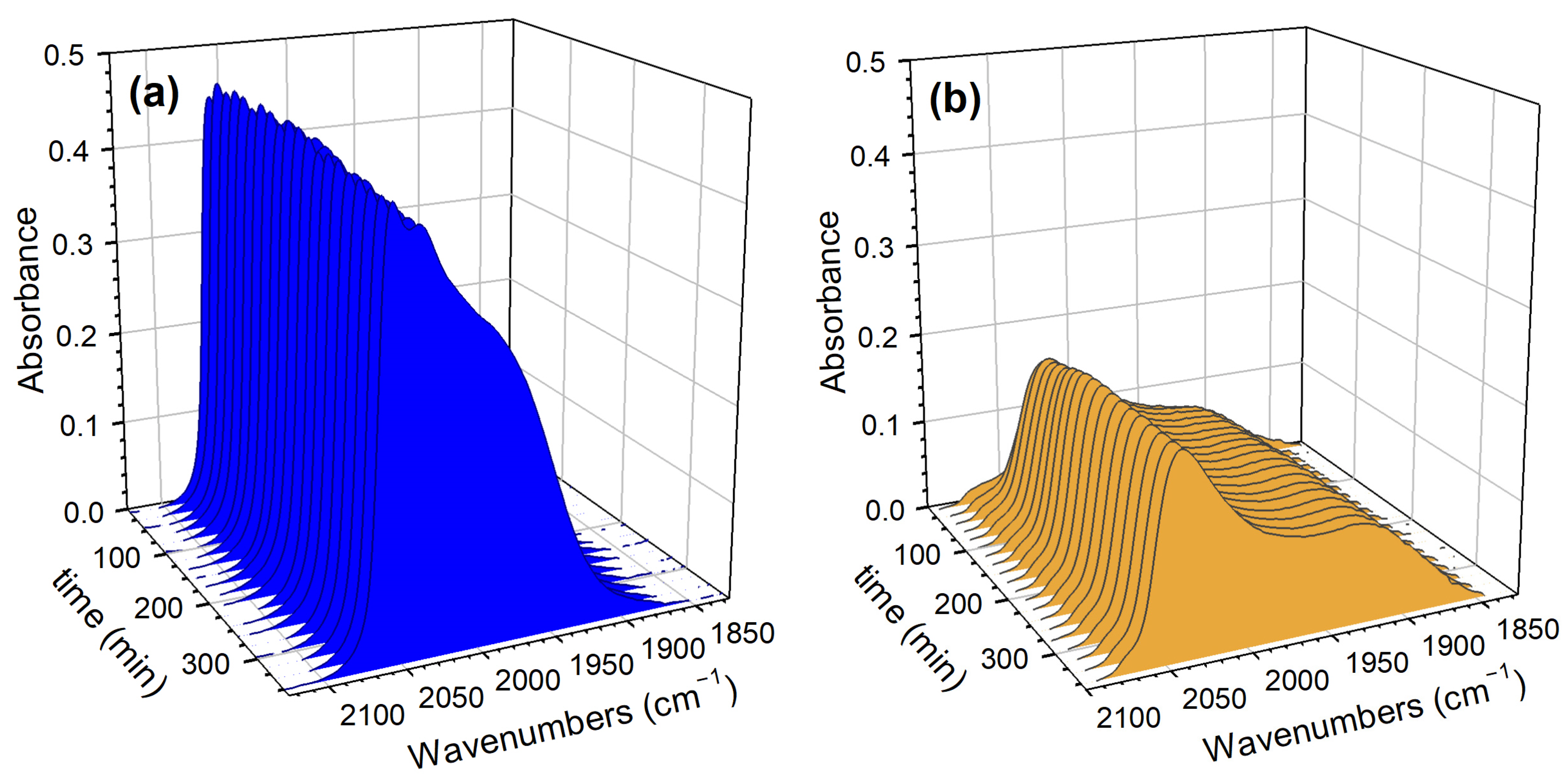
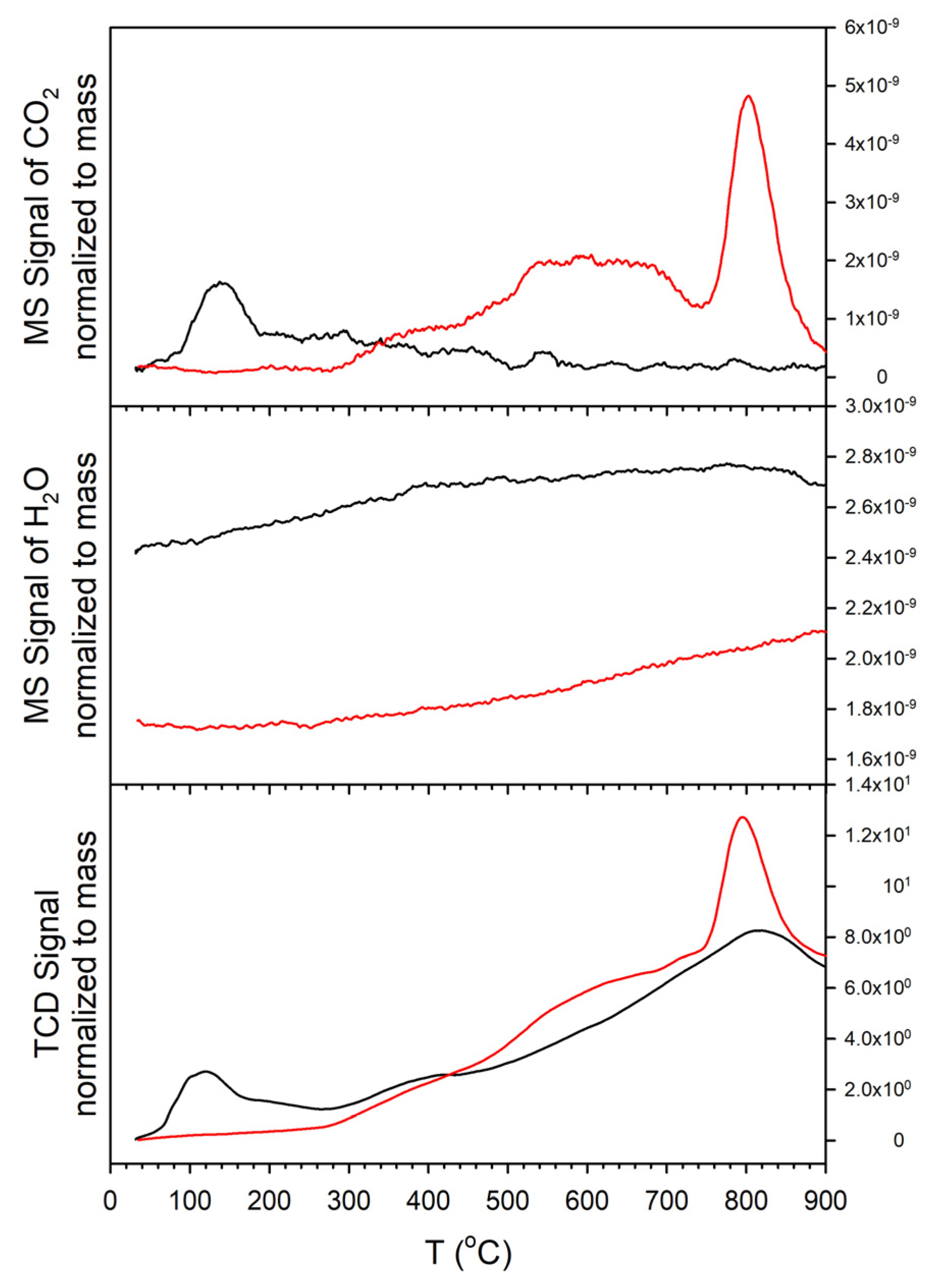
2. Results and Discussion
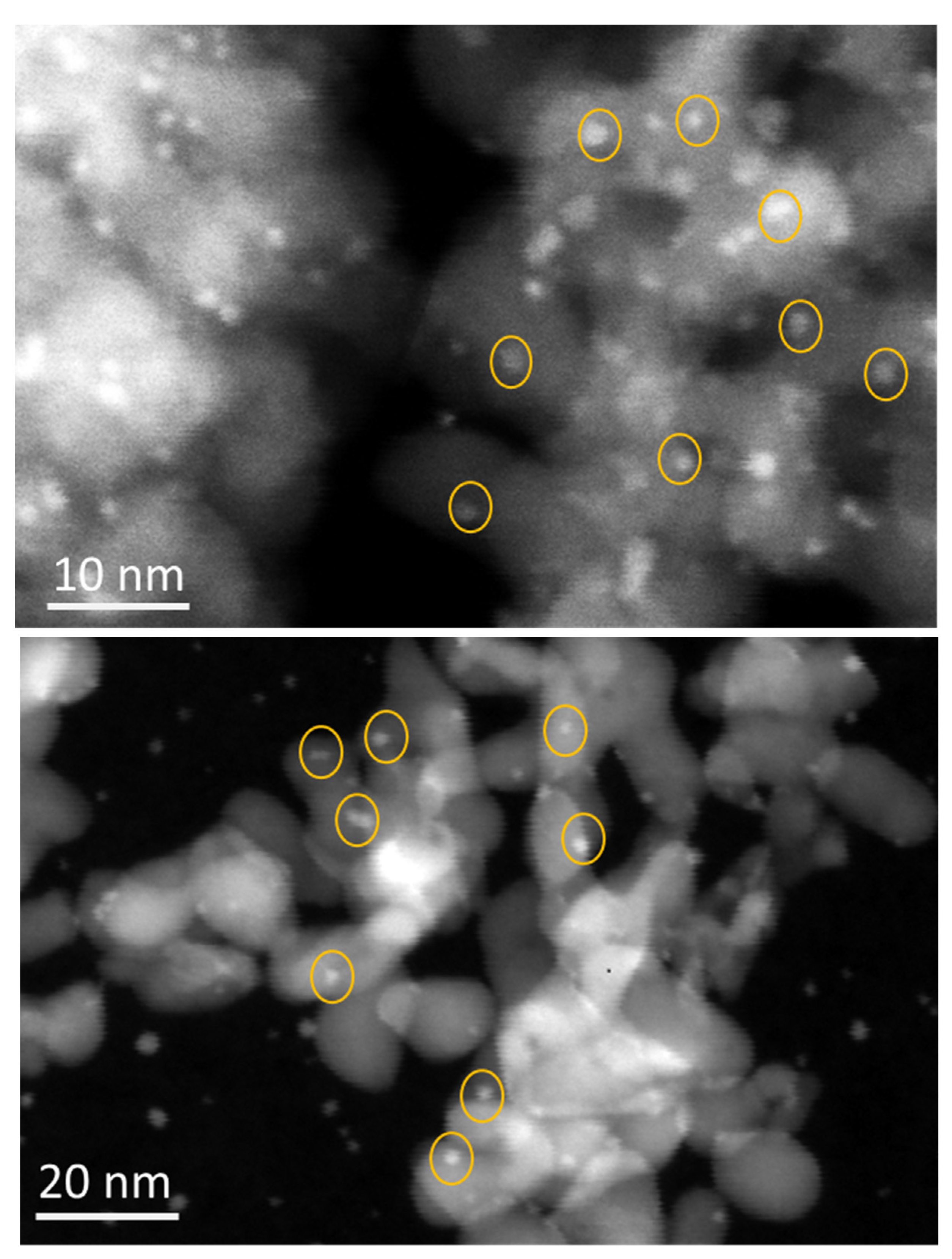
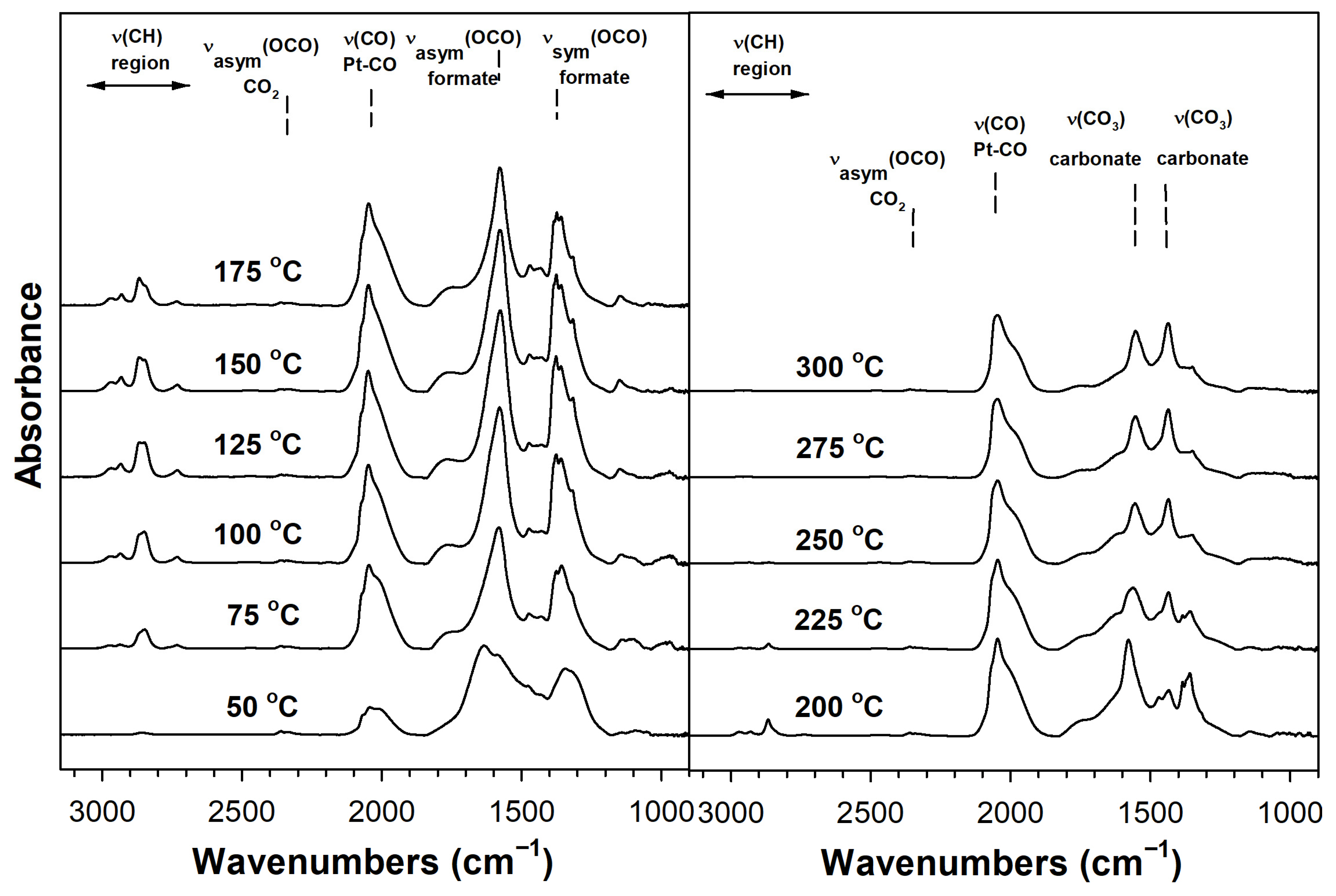
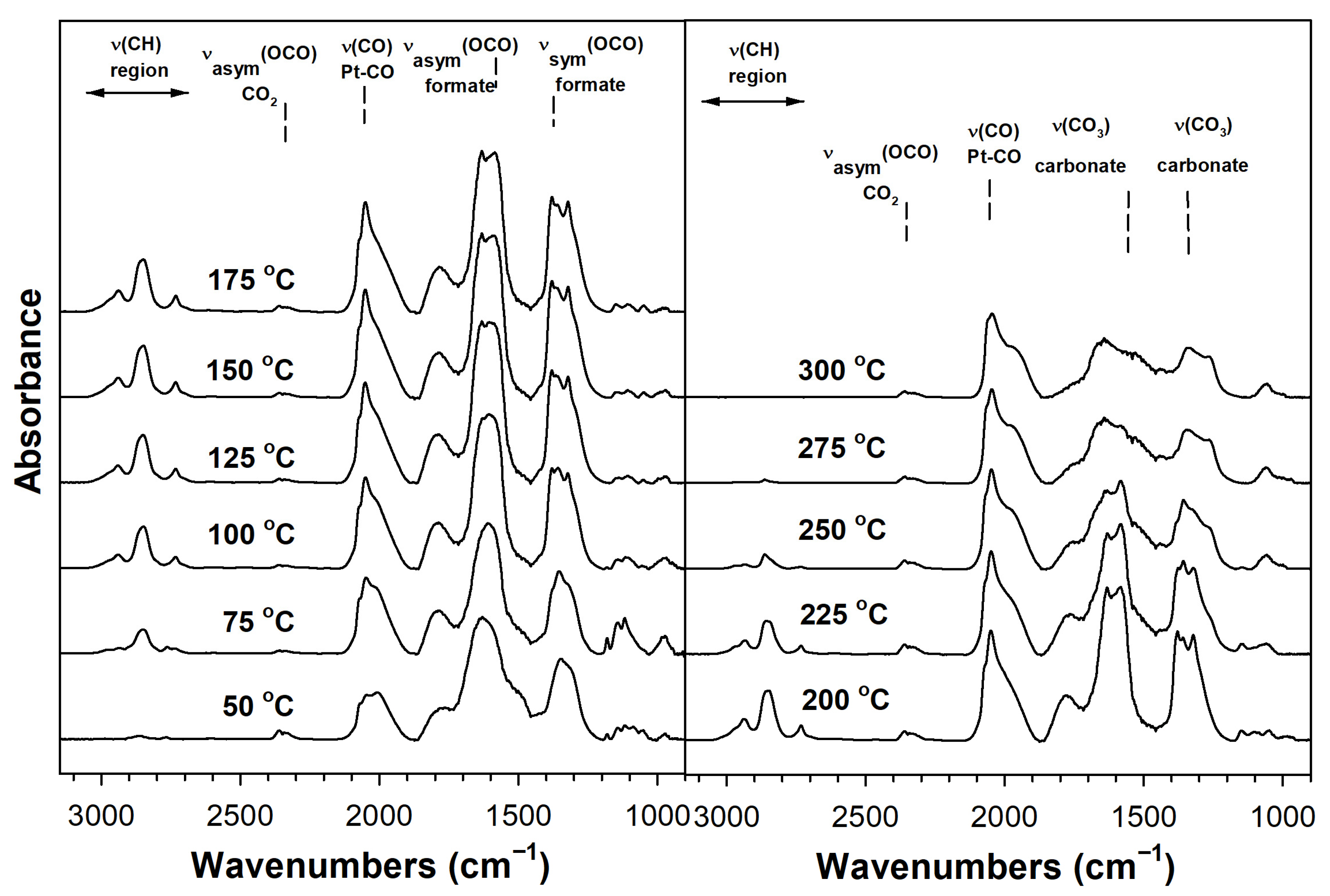
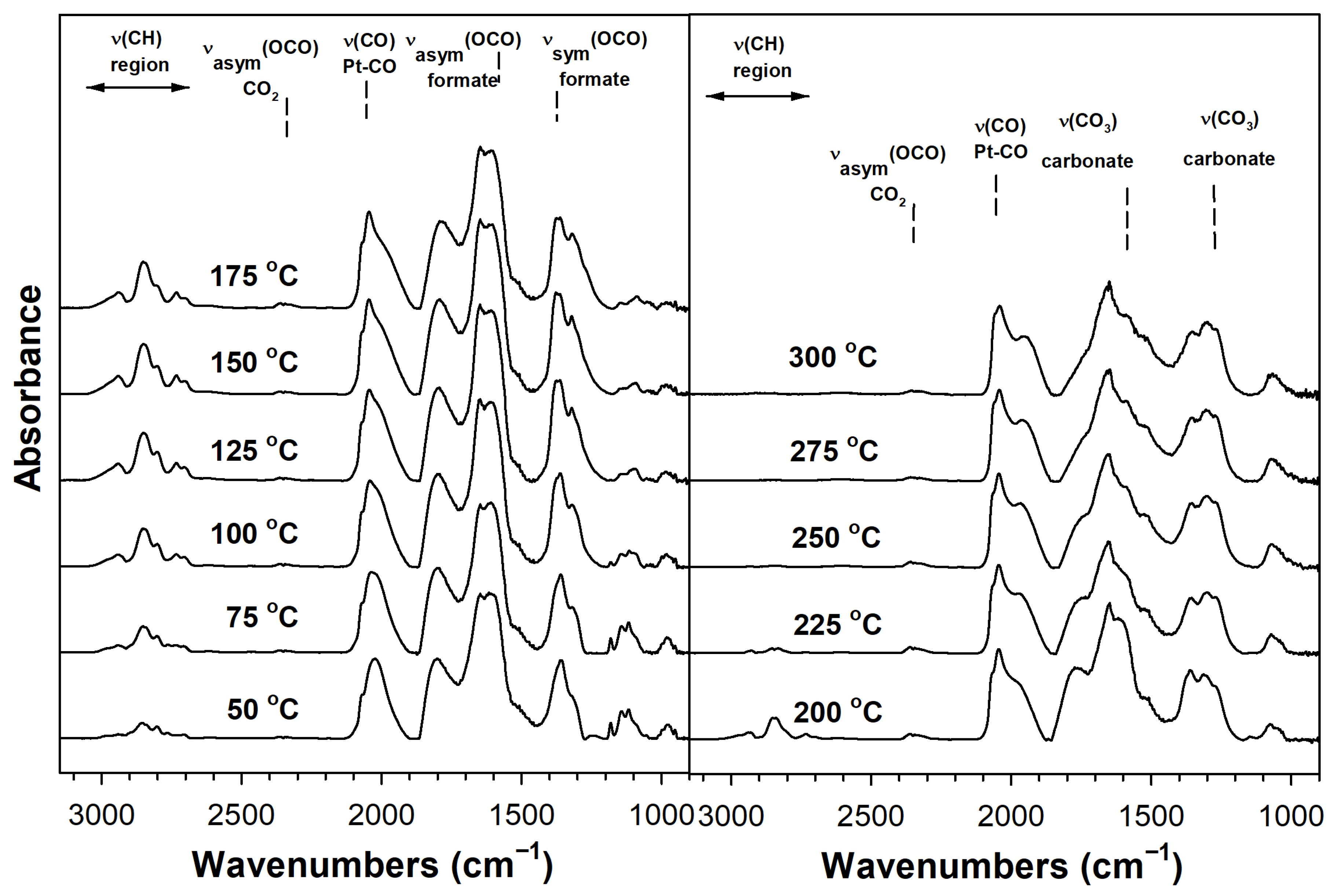
Catalyst Characterization
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Characterization
3.2.1. BET Surface Area
3.2.2. TEM
3.2.3. Temperature Programmed Reduction/Mass Spectrometry
3.2.4. Temperature Programmed Desorption, Reaction, and Oxidation
3.2.5. DRIFTS
3.2.6. Reaction Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anil, S.; Indraja, S.; Singh, R.; Appari, S.; Roy, B. A review on ethanol steam reforming for hydrogen production over Ni/Al2O3 and Ni/CeO2 based catalyst powders. Int. J. Hydrog. Energy 2022, 47, 8177–8213. [Google Scholar] [CrossRef]
- Ogo, S.; Sekine, Y. Recent progress in ethanol steam reforming using non-noble transition metal catalysts: A review. Fuel Proc. Tech. 2020, 199, 106238. [Google Scholar] [CrossRef]
- Bac, S.; Keskin, S.; Avci, A.K. Recent advances in materials for high purity H2 production by ethanol and glycerol steam reforming. Int. J. Hydrog. Energy 2020, 45, 34888–34917. [Google Scholar] [CrossRef]
- Bepari, S.; Kuila, D. Steam reforming of methanol, ethanol and glycerol over nickel-based catalyst: A review. Int. J. Hydrog. Energy 2020, 45, 18090–18113. [Google Scholar] [CrossRef]
- Mattos, L.V.; Jacobs, G.; Davis, B.H.; Noronha, F.B. Production of hydrogen from ethanol: Review of reaction mechanism and catalyst deactivation. Chem. Rev. 2012, 112, 4094–4123. [Google Scholar] [CrossRef] [PubMed]
- Sharma, Y.C.; Kumar, A.; Prasad, R.; Upadhyay, S.N. Ethanol steam reforming for hydrogen production: Latest and effective catalyst modification strategies to minimize carbonaceous deactivation. Renew. Sust. Energy Rev. 2017, 74, 89–103. [Google Scholar] [CrossRef]
- Iulianelli, A.; Ribeirinha, P.; Mendes, A.; Basile, A. Methanol steam reforming for hydrogen generation via conventional and membrane reactors: A review. Renew. Sust. Energy Rev. 2014, 29, 355–368. [Google Scholar] [CrossRef]
- Palo, D.R.; Dagle, R.A.; Holladay, J.D. Methanol steam reforming for hydrogen production. Chem. Rev. 2007, 107, 3992–4021. [Google Scholar] [CrossRef]
- Jacobs, G.; Davis, B.H. In situ DRIFTS investigation of the steam reforming of methanol over Pt/ceria. Appl. Catal. A Gen. 2005, 285, 43–49. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Polychronopoulou, K.; Asif, A.; Goula, M.A. The potential of glycerol and phenol towards H2 production using steam reforming reaction: A review. Surf. Coat. Tech. 2018, 352, 92–111. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Tzounis, L.; Dou, B.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Polychronopoulou, K.; Goula, M.A. Ni/Y2O3–ZrO2 catalyst for hydrogen production through the glycerol steam reforming reaction. Int. J. Hydrogen Energy 2020, 45, 10442–10460. [Google Scholar] [CrossRef]
- Martinelli, M.; Jacobs, G.; Shafer, W.D.; Davis, B.H. Effect of alkali on C-H bond scission over Pt/YSZ catalyst during water-gas shift, steam-assisted formic acid decomposition and methanol steam reforming. Catal. Today 2017, 291, 29–35. [Google Scholar] [CrossRef]
- Chu, L.; Gu, S.; Jin, Q.; Zhu, P.; Shen, Y.; Li, P. Hydrogen production from formaldehyde steam reforming using recyclable NiO/NaF catalyst. Int. J. Hydrog. Energy 2020, 45, 28752–28763. [Google Scholar] [CrossRef]
- Chu, L.; Shen, Y. Hydrogen production from formaldehyde steam reforming using recyclable NiO/NaCl catalyst. Appl. Surf. Sci. 2020, 532, 147376. [Google Scholar] [CrossRef]
- Lorenz, H.; Friedrich, M.; Armbruester, M.; Kloetzer, B.; Penner, S. ZnO is a CO2-selective steam reforming catalyst. J. Catal. 2013, 297, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Bielz, T.; Lorenz, H.; Amann, P.; Klotzer, B.; Penner, S. Water-gas shift and formaldehyde reforming activity determined by defect chemistry of polycrystalline In2O3. J. Phys. Chem. 2011, 115, 6622–6628. [Google Scholar] [CrossRef]
- Jin, Q.; Wang, A.; Lu, B.; Xu, X.; Shen, Y.; Zeng, Y. Steam reforming of formaldehyde for generating hydrogen and coproducing carbon nanotube for enhances photosynthesis. Catal. Sci. Tech. 2020, 10, 4436–4447. [Google Scholar] [CrossRef]
- Jin, Q.; Shen, Y.; Cai, Y.; Chu, L.; Zeng, Y. Resource utilization of waste V2O5-based deNOX catalysts for hydrogen production from formaldehyde and water via steam reforming. J. Hazard. Mat. 2020, 381, 120934. [Google Scholar] [CrossRef]
- Li, X.; Lim, K.H. DFT studies of steam reforming of formaldehyde on Cu, PdZn and Ir. ChemCatChem 2012, 4, 1311–1320. [Google Scholar] [CrossRef]
- Bo, J.-Y.; Zhang, S.; Lim, K.H. Steam reforming of formaldehyde on Cu(100) surface: A density functional study. Catal. Lett. 2009, 129, 444–448. [Google Scholar] [CrossRef]
- Lim, K.H.; Chen, Z.-X.; Neyman, K.M.; Roesch, N. Comparative theoretical study of formaldehyde decomposition on PdZn, Cu, and Pd Surfaces. J. Phys. Chem. B 2006, 110, 14890–14897. [Google Scholar] [CrossRef] [PubMed]
- Chenu, E.; Jacobs, G.; Crawford, A.C.; Keogh, R.A.; Patterson, P.M.; Sparks, D.E.; Davis, B.H. Water-gas Shift: An examination of unpromoted and Pt promoted MgO and tetragonal and monoclinic ZrO2 by in-situ DRIFTS. Appl. Catal. B Environ. 2005, 59, 45–56. [Google Scholar] [CrossRef]
- Azzam, K.G.; Babich, I.V.; Seshan, K.; Lefferts, L. Bifunctional catalysts for single-stage water-gas shift reaction in fuel cell applications. J. Catal. 2007, 251, 153–162. [Google Scholar] [CrossRef]
- Petallidou, K.C.; Kalamaras, C.M.; Efstathiou, A.M. The effect of La3+, Ti4+ and Zr4+ dopants on the mechanism of WGS on ceria-doped supported Pt catalysts. Catal. Today 2014, 228, 183–193. [Google Scholar] [CrossRef]
- Martinelli, M.; Jacobs, G.; Graham, U.M.; Shafer, W.D.; Cronauer, D.C.; Kropf, A.J.; Marshall, C.L.; Khalid, S.; Visconti, C.G.; Lietti, L.; et al. Water-gas shift: Characterization and testing of nanoscale YSZ supported Pt catalysts. Appl. Catal. A Gen. 2015, 497, 184–197. [Google Scholar] [CrossRef]
- Martinelli, M.; Jacobs, G.; Graham, U.M.; Davis, B.H. Methanol steam reforming: Na doping of Pt/YSZ provides fine tuning of selectivity. Catalysts 2017, 7, 148. [Google Scholar] [CrossRef]
- Pigos, J.M.; Brooks, C.J.; Jacobs, G.; Davis, B.H. Low temperature water-gas shift: Assessing formates as potential intermediates over Pt/ZrO2 and Na doped Pt/ZrO2 catalysts employing the SSITKA-DRIFTS technique. In Advances in Fischer-Tropsch Synthesis, Catalysts and Catalysis; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2010; Chapter 19; pp. 365–394. [Google Scholar] [CrossRef]
- Pigos, J.M.; Brooks, C.J.; Jacobs, G.; Davis, B.H. Low temperature water-gas shift: Characterization of Pt-based ZrO2 catalyst promoted with Na discovered by combinatorial methods. Appl. Catal. A General 2007, 319, 47–57. [Google Scholar] [CrossRef]
- Martinelli, M.; Alhraki, N.; Castro, J.D.; Matamoros, M.E.; Jacobs, G. Effect of Na loading on Pt/ZrO2 catalysts for low temperature water-gas shift for the production and purification of hydrogen. In New Dimensions in Production and Utilization of Hydrogen; Nanda, S., Vo, D.-V., Tri, P.N., Eds.; Elsevier Books: Amsterdam, The Netherlands, 2020; Chapter 6; pp. 143–160. ISBN 9780128195536. [Google Scholar]
- Shido, T.; Iwasawa, Y. Reactant-promoted reaction mechanism for water-gas shift reaction on Rh-doped CeO2. J. Catal. 1993, 141, 71–81. [Google Scholar] [CrossRef]
- Martinelli, M.; Castro, J.D.; Alhraki, N.; Matamoros, M.E.; Kropf, A.J.; Cronauer, D.C.; Jacobs, G. Effect of sodium loading on Pt/ZrO2 during ethanol steam reforming. Appl. Catal. A General 2021, 610, 117947. [Google Scholar] [CrossRef]
- Martinelli, M.; Watson, C.D.; Jacobs, G. Sodium doping of Pt/m-ZrO2 promotes C-C scission and decarboxylation during ethanol steam reforming. Internat. J. Hydrogen Energy 2020, 45, 18490–18501. [Google Scholar] [CrossRef]
- Binet, C.; Daturi, M.; Lavalley, J.-C. IR study of polycrystalline ceria properties in oxidised and reduced states. Catal. Today 1999, 50, 207–225. [Google Scholar] [CrossRef]
- Zhu, X.; Xie, Y.; Liu, C.-J.; Zhang, Y.-P. Stability of Pt particles on ZrO2 support during partial oxidation of methane: DRIFT studies of adsorbed CO. J. Molec. Catal. A 2008, 282, 67–73. [Google Scholar] [CrossRef]
- Jongpatiwuta, S.; Trakarnroek, S.; Rirksomboon, T.; Osuwan, S.; Resasco, D.E. n-Octane aromatization on Pt-containing non-acidic large pore zeolite catalysts. Catal. Lett. 2005, 100, 7–15. [Google Scholar] [CrossRef]
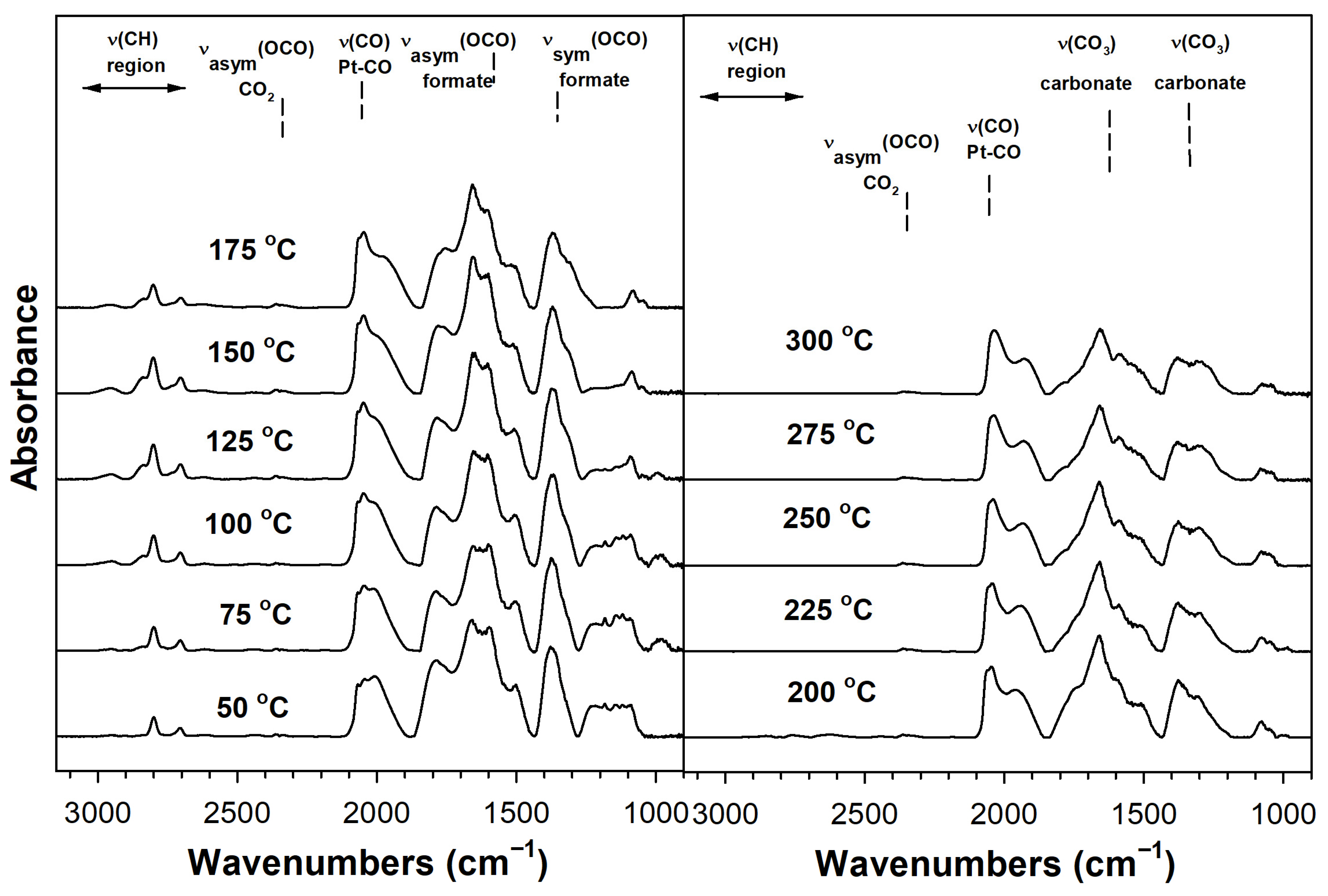
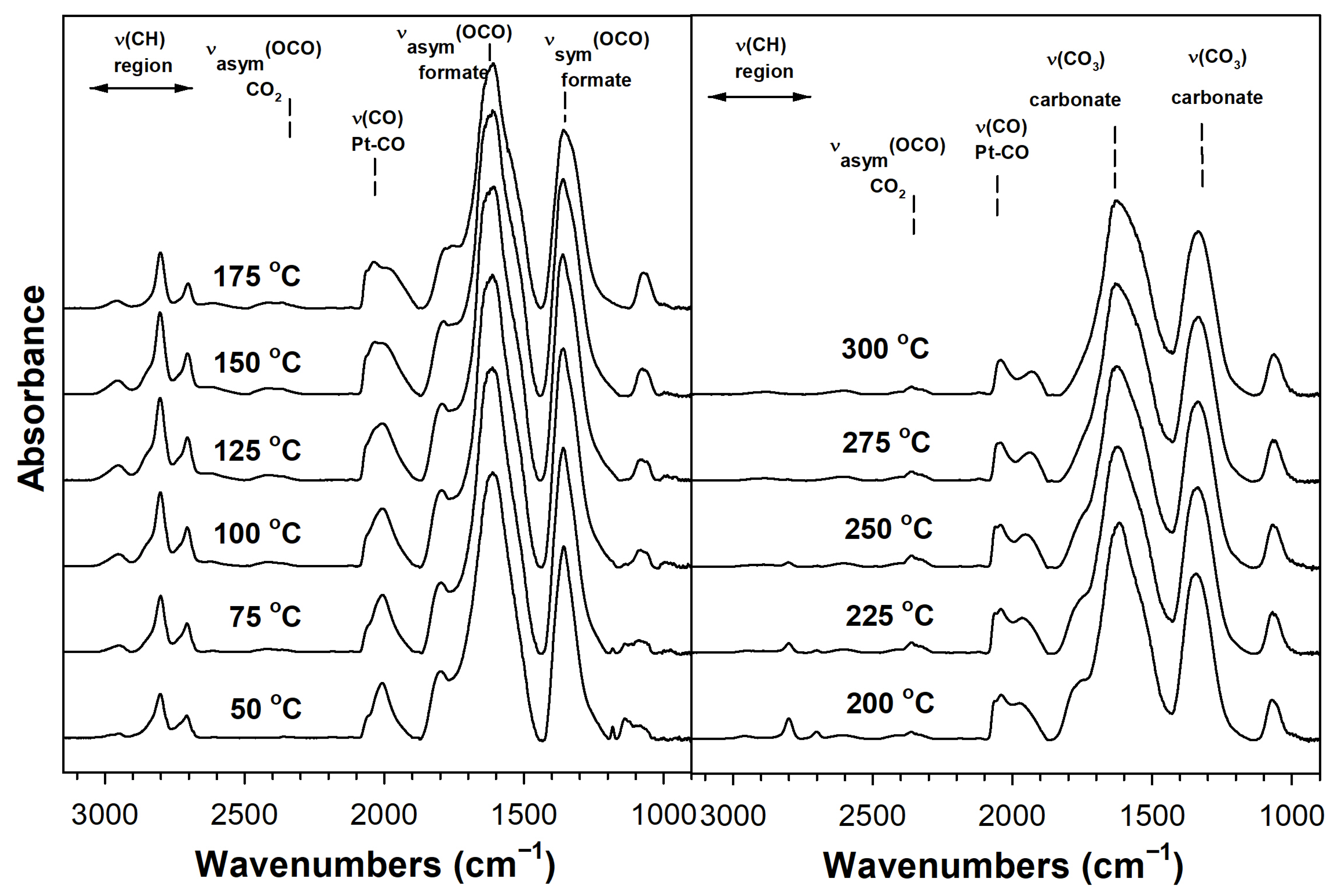
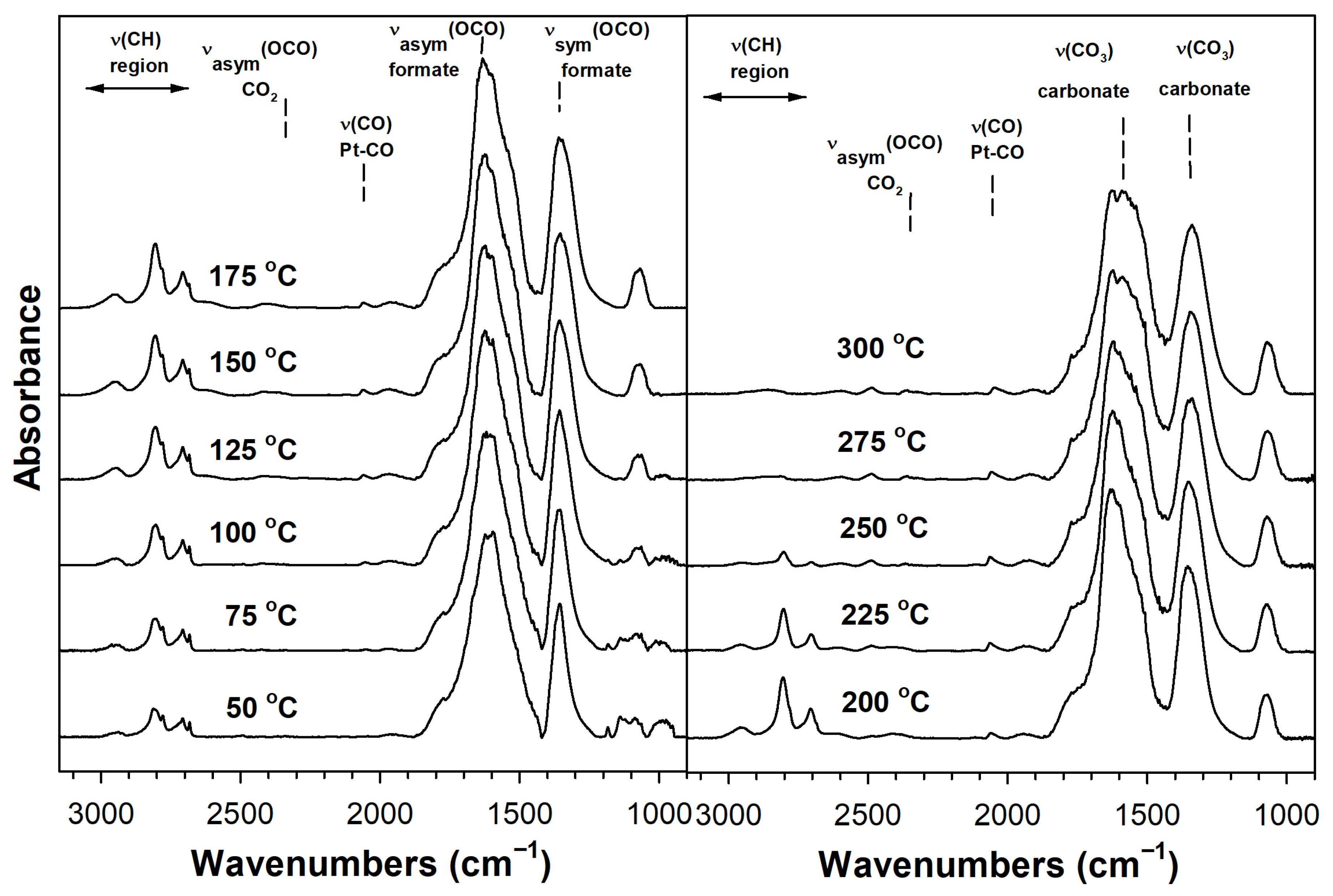

| Sample | Expected As (m2/g) | Actual As (BET) (m2/g) | Vp (BJH Des) (cm3/g) | Dp (BJH Des) (Å) | Pt0 Size from EXAFS (nm) | Expected %Pt Disp. | Formate ν(CH) CO Ads. (cm−1) | Formate Decomp. T (°C) TP-rxn ** | XANES % of Pt L3 Minus L2 Area Rel. to No Na |
|---|---|---|---|---|---|---|---|---|---|
| ZrO2 | 106.6 | 106.6 | 0.324 | 96 | - | - | - | - | - |
| 2%Pt/m-ZrO2 | 104.1 | 95.1 | 0.279 | 93 | 0.84 | 93 | 2868, 2863, 2848 | 304 | 100 |
| Pt/ZrO2 with | |||||||||
| 0.5%Na | 100.8–102.9 | 94.2 | 0.269 | 93 | 0.87 | 92 | (2868, 2856), 2845 | 275 | 108 |
| 1.0%Na | 97.5–101.7 | 94.1 | 0.272 | 94 | 0.92 | 90 | 2869, 2862, 2847 | (wk 190), 287 | 115 |
| 1.8%Na | 92.1–99.7 | 71.8 | 0.232 | 94 | 0.88 * | 91 * | (2850), 2833, 2800 | - | 156 * |
| 2.5%Na | 87.5–98.0 | 66.4 | 0.216 | 97 | 0.91 | 91 | 2802 | 190, 270 | 135 |
| 5.0%Na | 70.9–91.8 | 46.1 | 0.158 | 102 | 0.86 | 86 | 2803 | (wk 212), 280 | 180 |
| Catalyst | Band Position (cm−1) | Δ(OCO) Formate (cm−1) | |||||
|---|---|---|---|---|---|---|---|
| ν(CH) | δ(CH) + νsym(OCO) | 2δ(CH) | ν(OCO) Formate | ν(OCO) Carbonate | % of Formate Band Area at 200 °C Relative to Maximum Area (3050–2500 cm−1) | ||
| 2%Pt/ZrO2 (reference) | 2850, 2868 | 2968, 2934 | (2758), 2731 | sy 1377, 1359, 1317 asy 1576 | 1745, 1553, 1436, 1350, 1138–1025 | 199 | 27 |
| 0.5%Na-2%Pt/ZrO2 | 2849 (2862) | (2971), 2939 | 2733 (2702) | sy, 1379, 1359, 1322 asy 1632, 1586 | (1745), 1644, (1595–1460), 1441, 1341, (1309), 1268, 1060 | 253 | 94 |
| 1%Na-2%Pt/ZrO2 | 2851, 2803 | (2969), 2941 | 2733 (2625) | sy 1377, 1367, 1321 asy 1648, 1605, 1508 | (1745), 1650, (1591, 1531), 1354, 1299, 1272, 1069 | 271 | 33 |
| 1.8%Na-2%Pt/ZrO2 | 2838, 2802 | 2956 | (2735), 2704, 2625 | sy (1430–1382), 1371, (1340–1268) asy 1655, (1634–1550) | (1780, 1745), 1659, 1591, (1555–1460), 1382, 1315, 1294, 1079 | 284 | 15 |
| 2.5%Na-2%Pt/ZrO2 | (2851) 2803 | 2954 | (2735), 2705, (2661–2538) | sy 1361 asy 1609 | (1745) 1625, 1335, 1064 | 248 | 17 |
| 5%Na-2%Pt/ZrO2 | 2805 (2784) | 2955 | (2737) 2708, 2685 | sy (1366–1423), 1360, 1348 asy (1651, 1642), 1632, (1605, 1557–1475) | (1772, 1745), 1625, 1590, 1565, (1540), 1447, 1339, 1070 | 272 | 78 |
| Catalyst | % T < 250 °C | % 250 °C < T < 400 °C | % T > 400 °C |
|---|---|---|---|
| 2%Pt/m-ZrO2 | 54 | 34 | 12 |
| 0.5%Na-2%Pt/m-ZrO2 | 48 | 37 | 16 |
| 1%Na-2%Pt/m-ZrO2 | 51 | 29 | 20 |
| 1.8%Na-2%Pt/m-ZrO2 | 37 | 44 | 19 |
| 2.5%Na-2%Pt/m-ZrO2 | 33 | 37 | 30 |
| 5%Na-2%Pt/m-ZrO2 | 23 | 50 | 26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinelli, M.; Garcia, E.S.; Rajabi, Z.; Watson, C.D.; Kropf, A.J.; Cronauer, D.C.; Jacobs, G. Na Promotion of Pt/m-ZrO2 Catalysts for the Steam Reforming of Formaldehyde. Catalysts 2022, 12, 1294. https://doi.org/10.3390/catal12111294
Martinelli M, Garcia ES, Rajabi Z, Watson CD, Kropf AJ, Cronauer DC, Jacobs G. Na Promotion of Pt/m-ZrO2 Catalysts for the Steam Reforming of Formaldehyde. Catalysts. 2022; 12(11):1294. https://doi.org/10.3390/catal12111294
Chicago/Turabian StyleMartinelli, Michela, Elijah S. Garcia, Zahra Rajabi, Caleb D. Watson, A. Jeremy Kropf, Donald C. Cronauer, and Gary Jacobs. 2022. "Na Promotion of Pt/m-ZrO2 Catalysts for the Steam Reforming of Formaldehyde" Catalysts 12, no. 11: 1294. https://doi.org/10.3390/catal12111294
APA StyleMartinelli, M., Garcia, E. S., Rajabi, Z., Watson, C. D., Kropf, A. J., Cronauer, D. C., & Jacobs, G. (2022). Na Promotion of Pt/m-ZrO2 Catalysts for the Steam Reforming of Formaldehyde. Catalysts, 12(11), 1294. https://doi.org/10.3390/catal12111294










