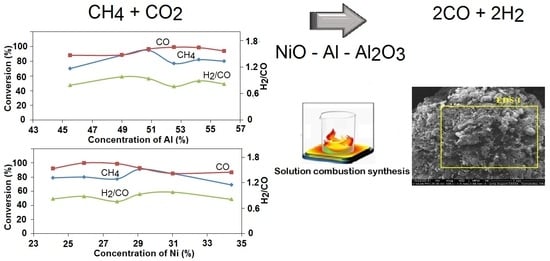
Ni-Al Self-Propagating High-Temperature Synthesis Catalysts in Dry Reforming of Methane to Hydrogen-Enriched Fuel Mixtures
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Catalysts
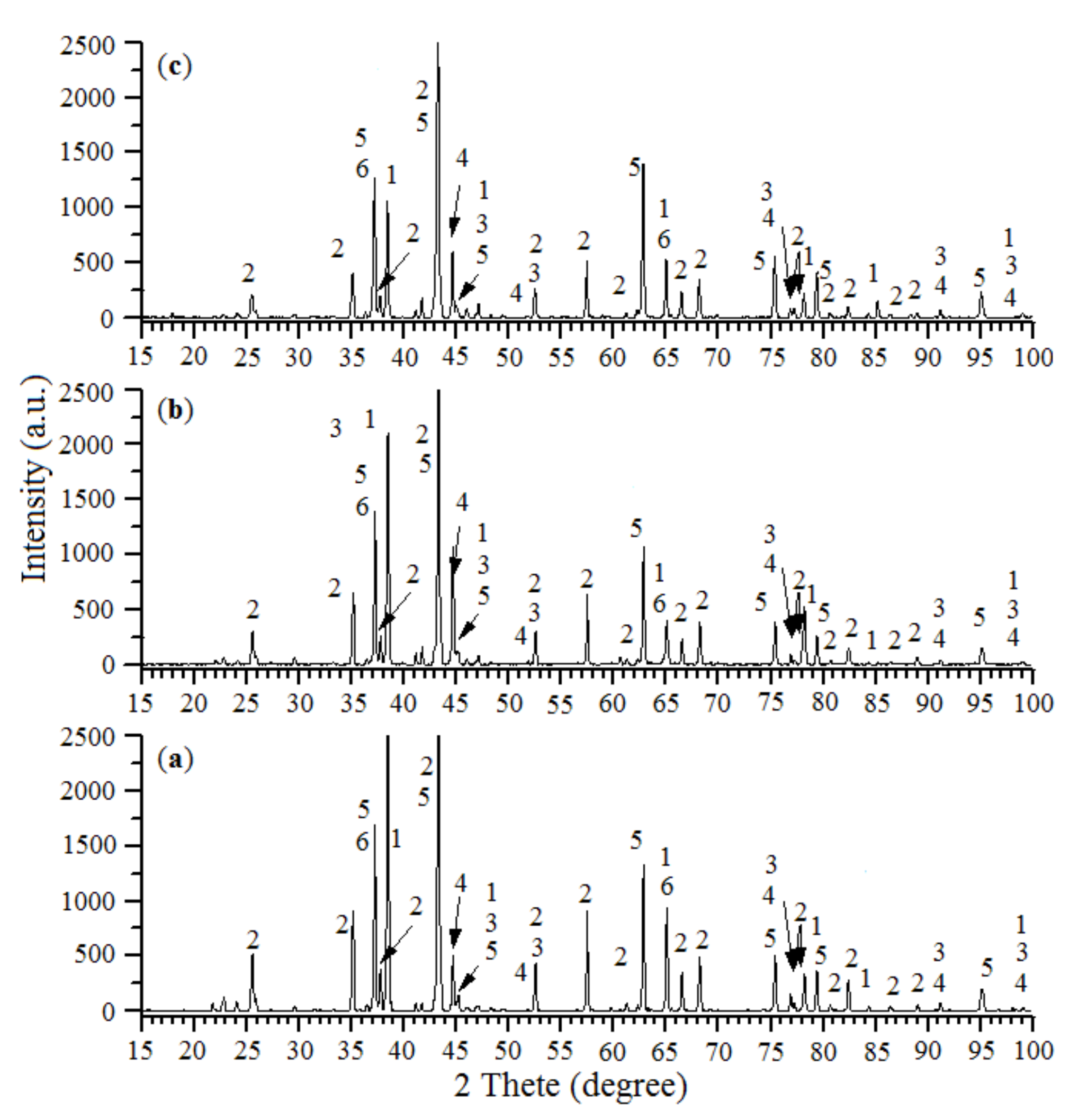
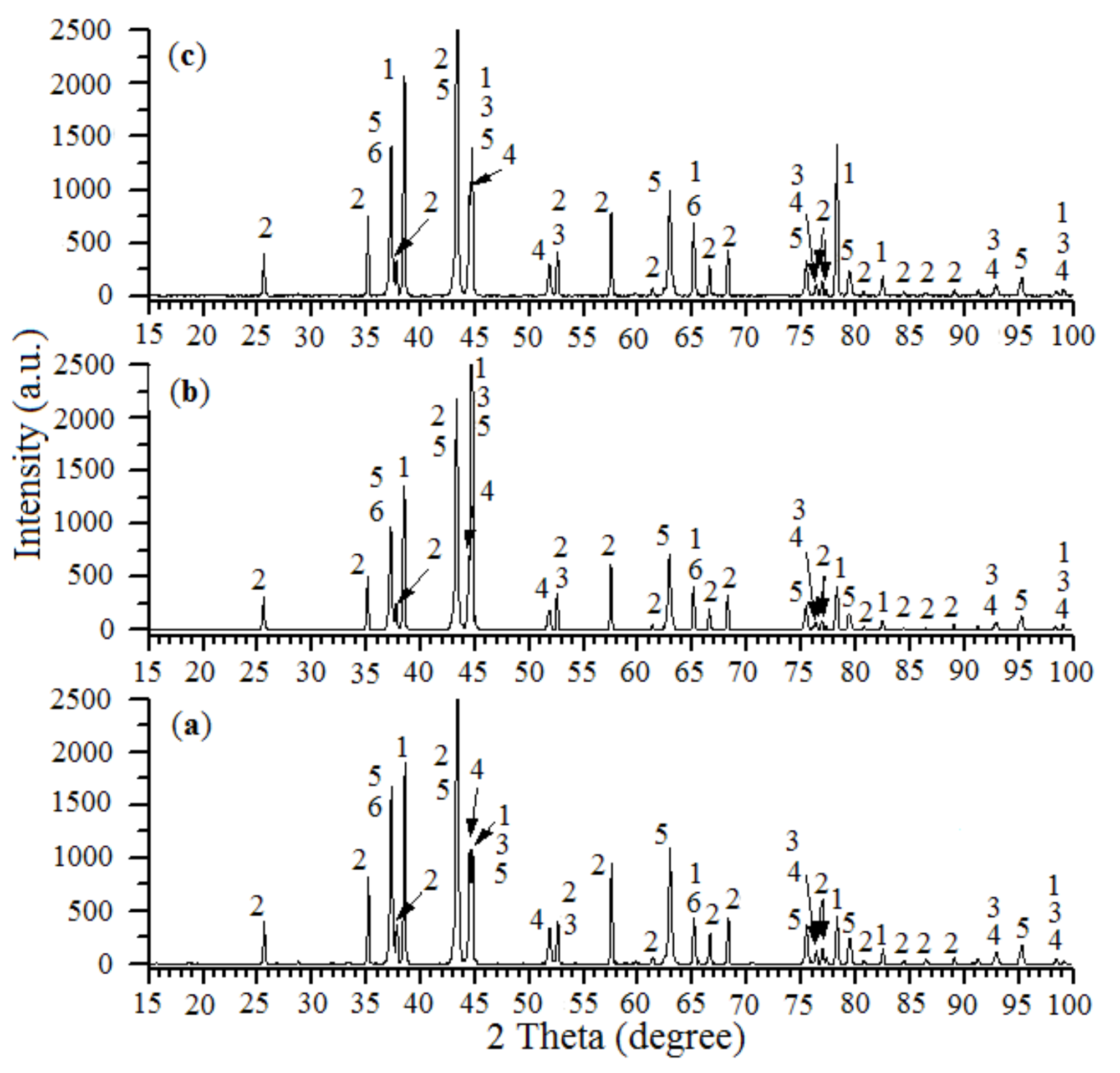
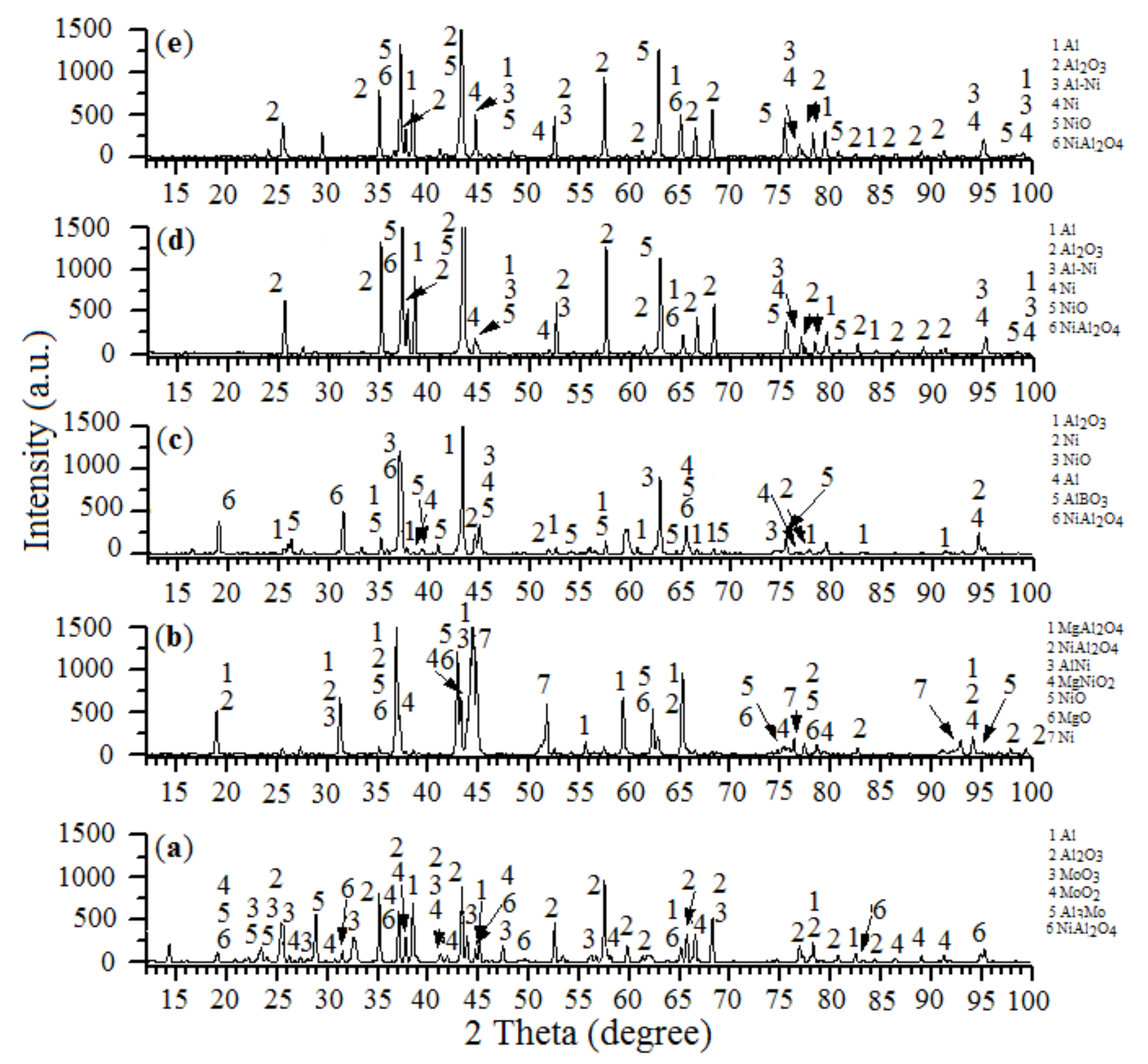
2.1.1. XRD Analysis
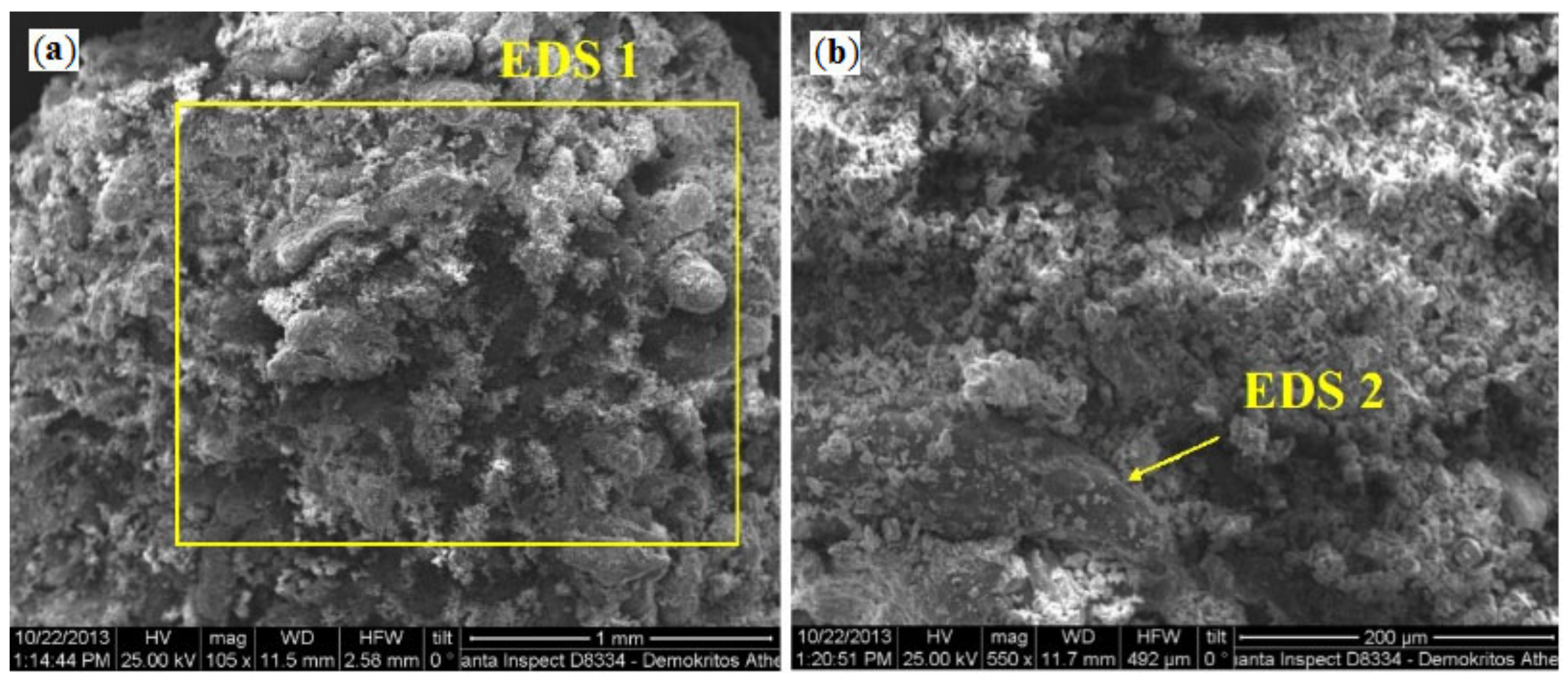
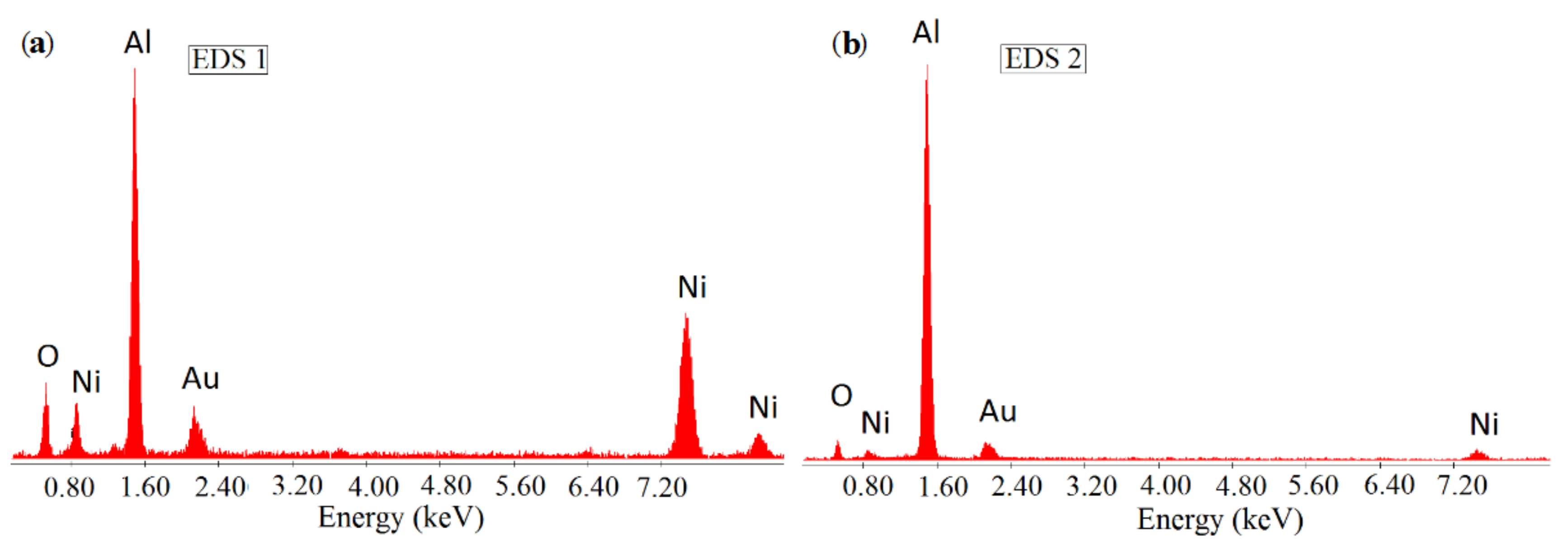
2.1.2. Investigation of the Structure and Composition of SHS Catalysts Using a Scanning Electron Microscope with Chemical Analysis
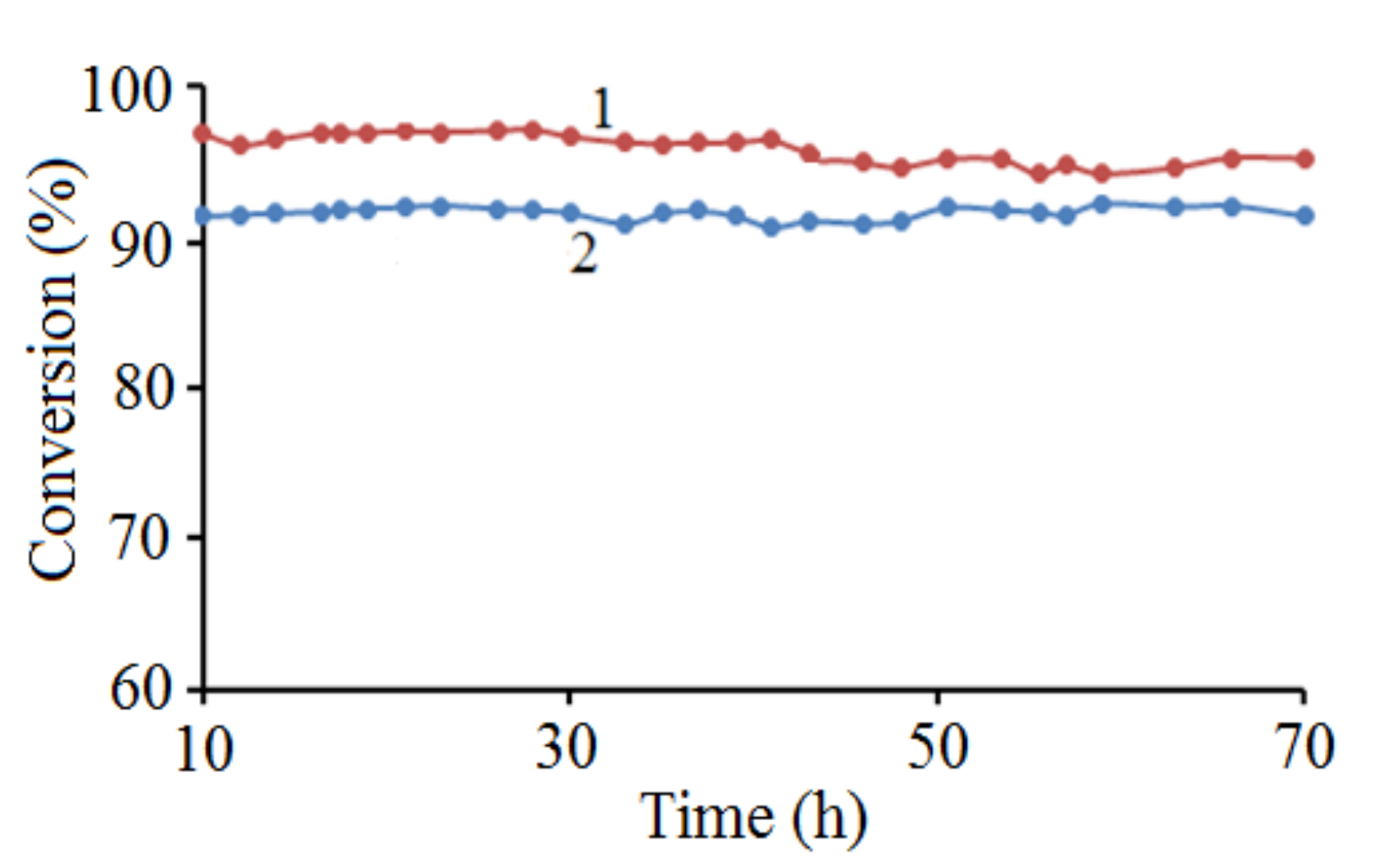
2.2. Catalytic Results
2.2.1. Dry Reforming of Methane
2.2.2. Partial Oxidation of Methane
3. Materials and Methods
3.1. Catalysts Preparation and Characterization
3.2. Catalytic Reaction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fouquet, R. Historical energy transitions: Speed, prices and system transformation. Energy Res. Soc. Sci 2016, 22, 7–12. [Google Scholar] [CrossRef]
- Davidson, D.J. Exnovating for a renewable energy transition. Nat. Energy 2019, 4, 254–256. [Google Scholar] [CrossRef]
- Allen, M.R.; Frame, D.J.; Huntingford, C.; Jones, C.D.; Lowe, J.A.; Meinshausen, M.; Meinshausen, N. Warming caused by cumulative carbon emissions towards the trillionth tonne. Nature 2009, 458, 1163–1166. [Google Scholar] [CrossRef]
- Matthews, H.D.; Gillett, N.P.; Stott, P.A.; Zickfeld, K. The proportionality of global warming to cumulative carbon emissions. Nature 2009, 459, 829–832. [Google Scholar] [CrossRef]
- Hakawati, R.; Smyth, B.M.; McCullough, G.; De Rosa, F.; Rooney, D. What is the most energy efficient route for biogas utilization: Heat, electricity or transport? Appl. Energy 2017, 206, 1076–1087. [Google Scholar] [CrossRef]
- Iyer, M.V.; Norcio, L.P.; Kugler, E.L.; Dadyburjor, D.B. Kinetic modeling for methane reforming with carbon dioxide over a mixed-metal carbide catalyst. Ind. Eng. Chem. Res. 2003, 42, 2712–2721. [Google Scholar] [CrossRef]
- Barrai, F.; Jackson, T.; Whitmore, N.; Castaldi, M. The role of carbon deposition on precious metal catalyst activity during dry reforming of biogas. Catal. Today 2007, 129, 391–396. [Google Scholar] [CrossRef]
- Kohn, M.P.; Castaldi, M.J.; Farrauto, R.J. Auto-thermal and dry reforming of landfill gas over a Rh/γAl2O3 monolith catalyst. Appl. Catal. B 2010, 94, 125–133. [Google Scholar] [CrossRef]
- Lu, S.-M. A global survey of gas hydrate development and reserves: Specifically in the marine field. Renew. Sustain. Energy Rev. 2015, 41, 884–900. [Google Scholar] [CrossRef]
- Statistical Review of World Energy, 69th ed.; BP p.l.c.: London, UK, 2020; p. 65. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2020-full-report.pdf (accessed on 10 September 2022).
- Danghyan, V.; Kumar, A.; Mukasyan, A.; Wolf, E.E. An active and stable NiOMgO solid solution based catalysts prepared by paper assisted combustion synthesis for the dry reforming of methane. Appl. Catal. B 2020, 273, 119056. [Google Scholar] [CrossRef]
- Ghareghashi, A.; Shahraki, F.; Razzaghi, K.; Ghader, S.; Torangi, M.A. Enhancement of gasoline selectivity in combined reactor system consisting of steam reforming of methane and Fischer-Tropsch synthesis. Korean J. Chem. Eng. 2017, 34, 87–99. [Google Scholar] [CrossRef]
- Monteiro, W.F.; Vieira, M.O.; Calgaro, C.O.; Perez-Lopez, O.W.; Ligabue, R.A. Dry reforming of methane using modified sodium and protonated titanate nanotube catalysts. Fuel 2019, 253, 713–721. [Google Scholar] [CrossRef]
- Dedov, A.G.; Loktev, A.S.; Shmigel, A.V.; Tikhonov, P.A.; Lapshin, A.E.; Arsent’ev, M.Y.; Mukhin, I.E.; Ivanov, V.K.; Moiseev, I.I. Selective conversion of methane to synthesis gas: Catalysts based on electrochemically modified nickel foam. Pet. Chem. 2017, 57, 230–235. [Google Scholar] [CrossRef]
- Song, Y.Q.; He, D.H.; Xu, B.Q. Effects of preparation methods of ZrO2 support on catalytic performances of Ni/ZrO2 catalysts in methane partial oxidation to syngas. Appl. Catal. A 2008, 337, 19–28. [Google Scholar] [CrossRef]
- Pengpanich, S.; Meeyoo, V.; Rirksomboon, T. Methane partial oxidation over Ni/CeO2-ZrO2 mixed oxide solid solution catalysts. Catal. Today 2004, 93, 95–105. [Google Scholar] [CrossRef]
- Matsumura, Y.; Nakamori, T. Steam reforming of methane over nickel catalysts at low reaction temperature. Appl. Catal. A 2004, 258, 107–114. [Google Scholar] [CrossRef]
- Das, S.; Ashok, J.; Bian, Z.; Dewangan, N.; Wai, M.H.; Du, Y. Silica-ceria sandwiched Ni core-shell catalyst for low temperature dry reforming of biogas: Coke resistance and mechanistic insights. Appl. Catal. B 2018, 230, 220–236. [Google Scholar] [CrossRef]
- Souza, G.; Marcilio, N.R.; Perez-Lopez, O.W. Dry reforming of methane at moderate temperatures over modified Co-Al Co-precipitated catalysts. Mater. Res. 2014, 17, 1047–1055. [Google Scholar] [CrossRef]
- La Parola, V.; Pantaleo, G.; Venezia, A. Effects of synthesis on the structural properties and methane partial oxidation activity of Ni/CeO2 catalyst. Catalysts 2018, 8, 220. [Google Scholar] [CrossRef]
- Luna, A.E.C.; Iriarte, M.E. Carbon dioxide reforming of methane over a metal modified Ni-Al2O3 catalyst. Appl. Catal. A 2008, 343, 10–15. [Google Scholar] [CrossRef]
- Galanov, S.I.; Kosyreva, K.A.; Litvak, E.A. Partial catalytic oxidation of natural gas to synthesis gas. Bull. Tomsk. State Univ. 2012, 364, 230–233. [Google Scholar]
- Maksimov, Y.M.; Kirdyashkin, A.I.; Arkatova, L.A. Conversion of methane on catalysts obtained via self-propagating high-temperature synthesis. Catal. Ind. 2013, 3, 245–252. [Google Scholar] [CrossRef]
- Fan, X.; Li, L.; Yang, X.; Guo, Z.; Jing, F.; Chu, W. High-performance CoxM3−xAlOy (M = Ni, Mn) catalysts derived from microwave-assisted synthesis of hydrotalcite precursors for methane catalytic combustion. Catal. Today 2018, 347, 23–30. [Google Scholar] [CrossRef]
- Merzhanov, A.G.; Borovinskaja, I.P. Historical retrospective of SHS: An autoreview. Int. J. Self-Propagating High-Temp. Synth. 2008, 17, 242–265. [Google Scholar] [CrossRef]
- Mukasyan, A.S.; White, J.D.E. Combustion joining of refractory materials. Int. J. Self-Propagating High-Temp. Synth. 2007, 16, 154–168. [Google Scholar] [CrossRef]
- Manukyan, K.V.; Cross, A.; Roslyakov, S.; Rouvimov, S.; Rogachev, A.S.; Wolf, E.E.; Mukasyan, A.S. Solution combustion synthesis of nano-crystalline metallic materials: Mechanistic studies. J. Phys. Chem. C 2013, 117, 24417–24427. [Google Scholar] [CrossRef]
- Kingsley, J.J.; Patil, K.C. A novel combustion process for the synthesis of fine particle α-alumina and related oxide materials. Mater. Lett. 1988, 6, 427–432. [Google Scholar] [CrossRef]
- Mimani, T.; Patil, K.C. Solution combustion synthesis of nanoscale oxides and their composites. Mater. Phys. Mech. 2001, 4, 134–137. [Google Scholar]
- Liu, Y.; Parisi, J.; Sun, X.; Lei, Y. Solid-state gas sensors for high temperature applications—A review. J. Mater. Chem. A 2014, 2, 9919–9943. [Google Scholar] [CrossRef]
- Jayathilaka, W.A.D.M.; Qi, K.; Qin, Y.; Chinnappan, A.; Serrano-García, W.; Baskar, C.; Ramakrishna, S. Significance of nanomaterials in wearables: A review on wearable actuators and sensors. Adv. Mater. 2018, 31, 201805921. [Google Scholar] [CrossRef]
- Pokhrel, S.; Mädler, L. Flame made particles for sensors, catalysis and energy storage applications. Energy Fuels 2020, 34, 13209–13224. [Google Scholar] [CrossRef] [PubMed]
- Anthony, L.S.; Perumal, V.; Mohamed, N.M.; Saheed, M.S.M.; Gopinath, S.C.B. Nanomaterials for Healthcare, Energy and Environment; Advanced Structured Materials; Springer: Berlin/Heidelberg, Germany, 2019; Volume 3, pp. 51–69. [Google Scholar]
- Sharma, N.; Ojha, H.; Bharadwaj, A.; Pathak, D.P.; Sharma, R.K. Preparation and catalytic applications of nanomaterials: A review. RSC Adv. 2015, 5, 53381–53403. [Google Scholar] [CrossRef]
- Xin, Y.; Li, S.; Qian, Y.; Zhu, W.; Yuan, H.; Jiang, P.; Wang, L. High-entropy alloys as a platform for catalysis: Progress, challenges, and opportunities. ACS Catal. 2020, 10, 11280–11306. [Google Scholar] [CrossRef]
- Wu, W. Inorganic nanomaterials for printed electronics: A review. Nanoscale 2017, 9, 7342–7372. [Google Scholar] [CrossRef]
- Jin, L.J.; Xie, T.; Ma, B.X.; Li, Y.; Hu, H.Q. Preparation of carbon-Ni/MgO-Al2O3 composite catalysts for CO2 reforming of methane. Int. J. Hydrog. Energy 2017, 42, 5047–5055. [Google Scholar] [CrossRef]
- Nair, M.M.; Kaliaguine, S. Structured catalysts for dry reforming of methane. J. Chem. 2016, 40, 4049–4060. [Google Scholar]
- Pashchenko, D. Experimental investigation of synthesis gas production by methane reforming with flue gas over a NiO-Al2O3 catalyst: Reforming characteristics and pressure drop. Int. J. Hydrog. Energy 2019, 44, 7073–7082. [Google Scholar] [CrossRef]
- González, A.R.; Asencios, Y.J.O.; Assaf, E.M.; Assaf, J.M. Dry reforming of methane on Ni–Mg–Al nano-spheroid oxide catalysts prepared by the sol–gel method from hydrotalcite-like precursors. Appl. Surf. Sci. 2013, 280, 876–887. [Google Scholar] [CrossRef]
- Ali, S.; Khader, M.M.; Almarri, M.J.; Abdelmoneim, A.G. Ni-based Nano-catalysts for the dry reforming of methane. Catal. Today 2019, 343, 26–37. [Google Scholar] [CrossRef]
- Krylov, O.V. Carbon dioxide conversion of methane to synthesis gas. Russ. J. Gen. Chem. 2000, 44, 19–33. [Google Scholar]
- York, A.P.E.; Xiao, T.C.; Green, M.L.H.; Claridge, J.B. Methane oxyforming for synthesis gas production. Catal. Rev. 2007, 49, 511–560. [Google Scholar] [CrossRef]
- Lavoie, J.M. Review on dry reforming of methane, a potentially more environmentally-friendly approach to the increasing natural gas exploitation. Front. Chem. 2014, 2, 81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Vajglova, Z.; Mäki-Arvela, P.; Peurla, M.; Palonen, H.; Murzin, D.Y.; Tungatarova, S.; Baizhumanova, T.; Aubakirov, Y. Mono- and bimetallic Ni-Co catalysts in dry reforming of methane. ChemistrySelect 2021, 6, 3424–3434. [Google Scholar] [CrossRef]
- Xanthopoulou, G.; Varitis, S.; Zhumabek, M.; Karanasios, K.; Vekinis, G.; Tungatarova, S.A.; Baizhumanova, T.S. Direct reduction of greenhouse gases by continuous dry (CO2) reforming of methane over Ni-containing SHS catalysts. Energies 2021, 14, 6078. [Google Scholar] [CrossRef]
- Jiménez, J.D.; Betancourt, L.E.; Danielis, M.; Zhang, H.; Zhang, F.; Orozco, I.; Xu, W.; Llorca, J.; Liu, P.; Trovarelli, A.; et al. Identification of highly selective surface pathways for methane dry reforming using mechanochemical synthesis of Pd–CeO2. ACS Catal. 2022, 12, 12809–12822. [Google Scholar] [CrossRef]
- Shakir, M.; Prasad, M.; Ray, K.; Sengupta, S.; Sinhamahapatra, A.; Liu, S.; Vuthaluru, H.B. NaBH4-assisted synthesis of B–(Ni–Co)/MgAl2O4 nanostructures for the catalytic dry reforming of methane. ACS Appl. Nano Mater. 2022, 5, 10951–10961. [Google Scholar] [CrossRef]
- Taherian, Z.; Gharahshiran, V.S.; Khataee, A.; Orooji, Y. Synergistic effect of freeze-drying and promoters on the catalytic performance of Ni/MgAl layered double hydroxide. Fuel 2022, 311, 122620. [Google Scholar] [CrossRef]
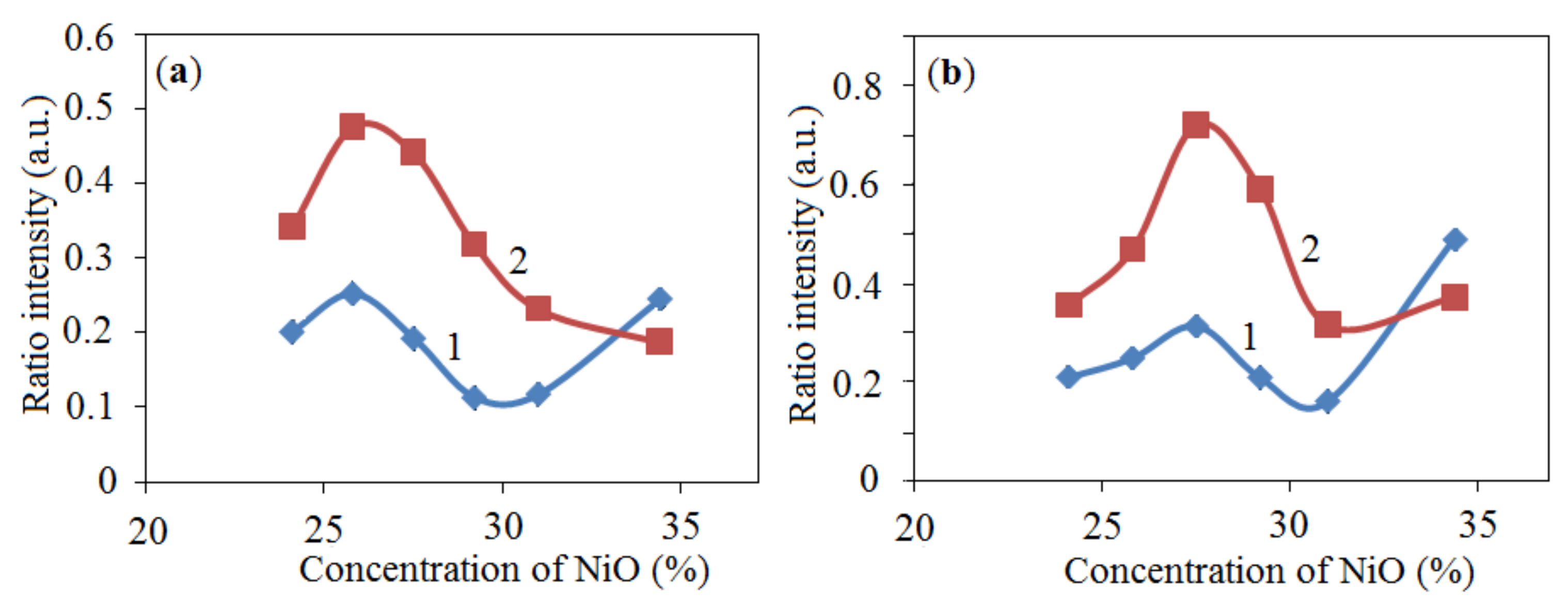
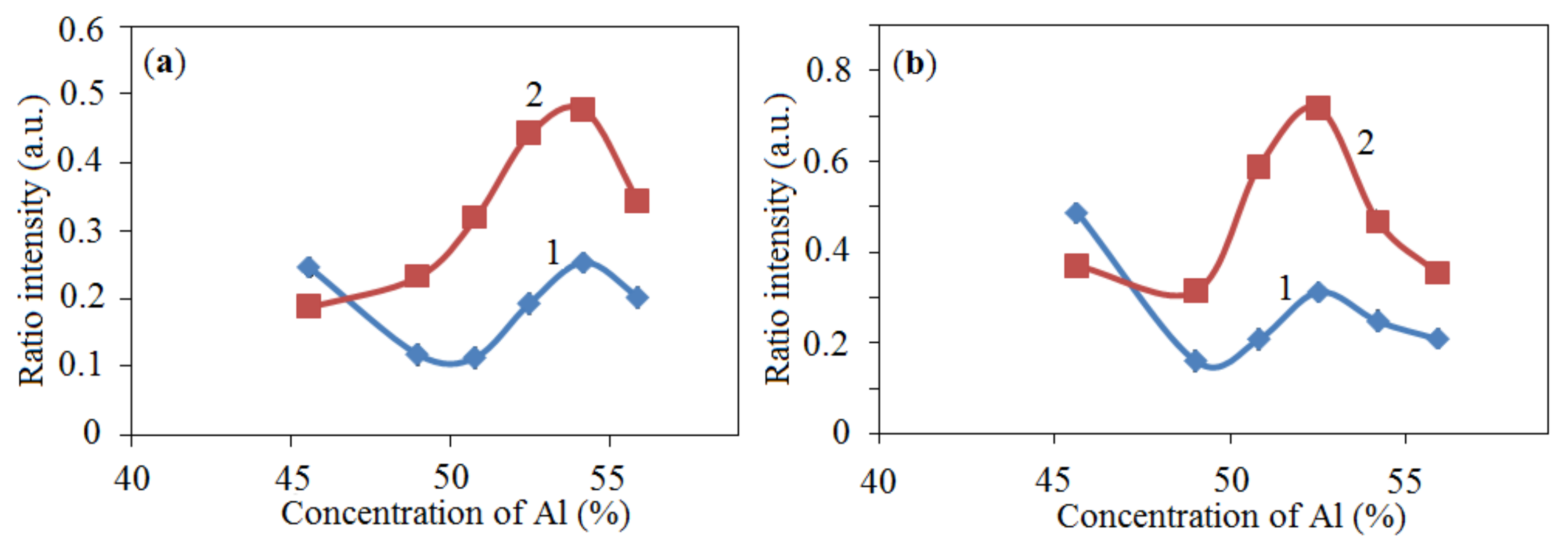



 ), 2—25.8% NiO—54.2% Al + 20% Al2O3 (
), 2—25.8% NiO—54.2% Al + 20% Al2O3 ( ), 3—27.5% NiO—52.5% Al—20% Al2O3 (
), 3—27.5% NiO—52.5% Al—20% Al2O3 ( ), 4—29.2% NiO—50.8% Al—20% Al2O3 (
), 4—29.2% NiO—50.8% Al—20% Al2O3 ( ), 5—31% NiO—49% Al—20% Al2O3 (
), 5—31% NiO—49% Al—20% Al2O3 ( ), 6—34.4% NiO—45.6% Al—20% Al2O3 (
), 6—34.4% NiO—45.6% Al—20% Al2O3 ( ); (a) CH4 conversion, (b) CO2 conversion, (c) H2/CO ratio, (d) H2 yield, (e) CO yield; GHSV—860 h−1.
); (a) CH4 conversion, (b) CO2 conversion, (c) H2/CO ratio, (d) H2 yield, (e) CO yield; GHSV—860 h−1.
 ), 2—25.8% NiO—54.2% Al + 20% Al2O3 (
), 2—25.8% NiO—54.2% Al + 20% Al2O3 ( ), 3—27.5% NiO—52.5% Al—20% Al2O3 (
), 3—27.5% NiO—52.5% Al—20% Al2O3 ( ), 4—29.2% NiO—50.8% Al—20% Al2O3 (
), 4—29.2% NiO—50.8% Al—20% Al2O3 ( ), 5—31% NiO—49% Al—20% Al2O3 (
), 5—31% NiO—49% Al—20% Al2O3 ( ), 6—34.4% NiO—45.6% Al—20% Al2O3 (
), 6—34.4% NiO—45.6% Al—20% Al2O3 ( ); (a) CH4 conversion, (b) CO2 conversion, (c) H2/CO ratio, (d) H2 yield, (e) CO yield; GHSV—860 h−1.
); (a) CH4 conversion, (b) CO2 conversion, (c) H2/CO ratio, (d) H2 yield, (e) CO yield; GHSV—860 h−1.
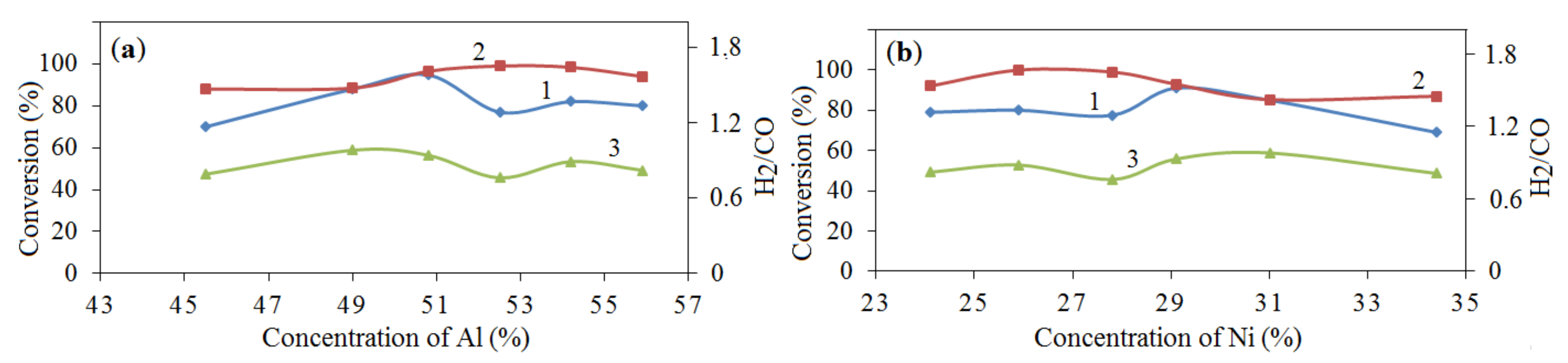
 ), 2—28% NiO—42% Al—30% Al2O3 (
), 2—28% NiO—42% Al—30% Al2O3 ( ), 3—39% NiO—10% Al—30% Al2O3—7% Mg—14% MgO2 (
), 3—39% NiO—10% Al—30% Al2O3—7% Mg—14% MgO2 ( ), 4—7% NiO—12.6% Al—0.4% H3BO3 (
), 4—7% NiO—12.6% Al—0.4% H3BO3 ( ), 5—10% NiO—28% Al—40% Al2O3—22% MoO3 (
), 5—10% NiO—28% Al—40% Al2O3—22% MoO3 ( ); (a)—CH4 conversion, (b)—CO2 conversion, (c)—H2/CO ratio, (d)—H2 yield, (e)—CO yield, GHSV—860 h−1.
); (a)—CH4 conversion, (b)—CO2 conversion, (c)—H2/CO ratio, (d)—H2 yield, (e)—CO yield, GHSV—860 h−1.
 ), 2—28% NiO—42% Al—30% Al2O3 (
), 2—28% NiO—42% Al—30% Al2O3 ( ), 3—39% NiO—10% Al—30% Al2O3—7% Mg—14% MgO2 (
), 3—39% NiO—10% Al—30% Al2O3—7% Mg—14% MgO2 ( ), 4—7% NiO—12.6% Al—0.4% H3BO3 (
), 4—7% NiO—12.6% Al—0.4% H3BO3 ( ), 5—10% NiO—28% Al—40% Al2O3—22% MoO3 (
), 5—10% NiO—28% Al—40% Al2O3—22% MoO3 ( ); (a)—CH4 conversion, (b)—CO2 conversion, (c)—H2/CO ratio, (d)—H2 yield, (e)—CO yield, GHSV—860 h−1.
); (a)—CH4 conversion, (b)—CO2 conversion, (c)—H2/CO ratio, (d)—H2 yield, (e)—CO yield, GHSV—860 h−1.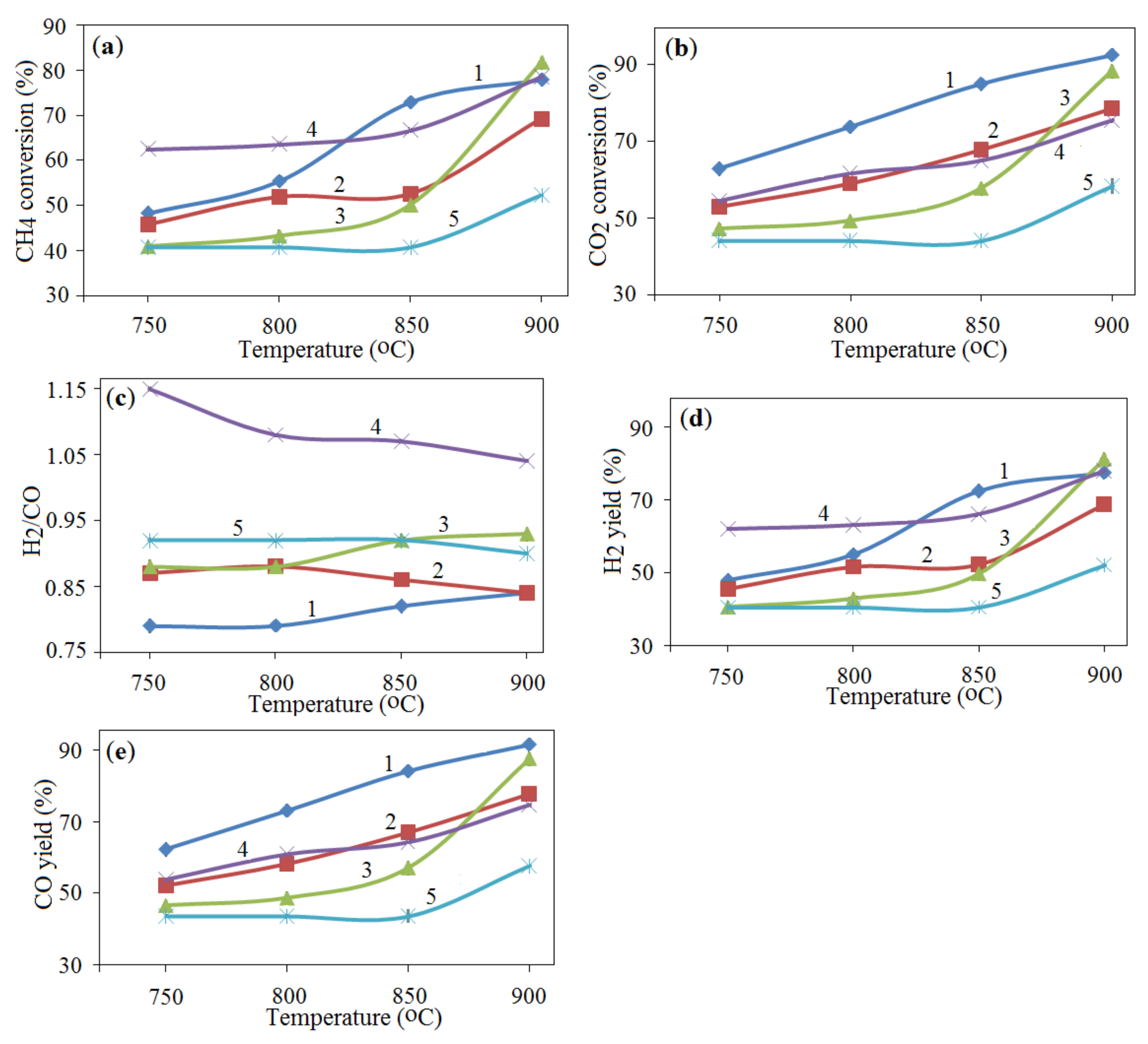
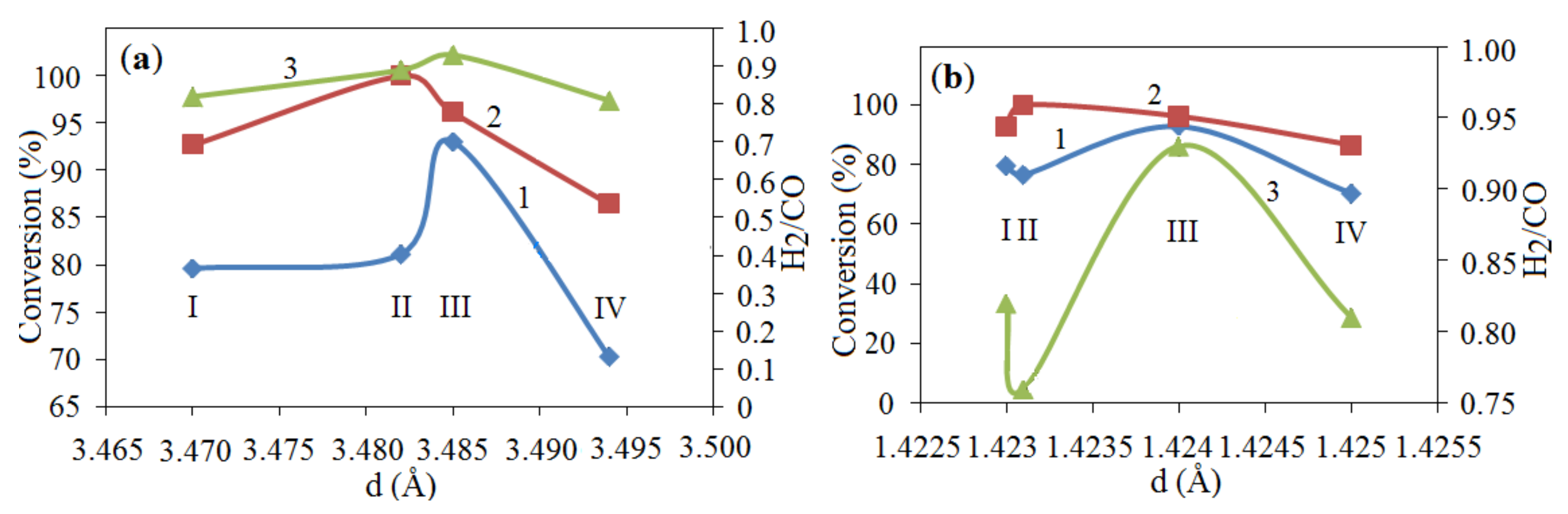

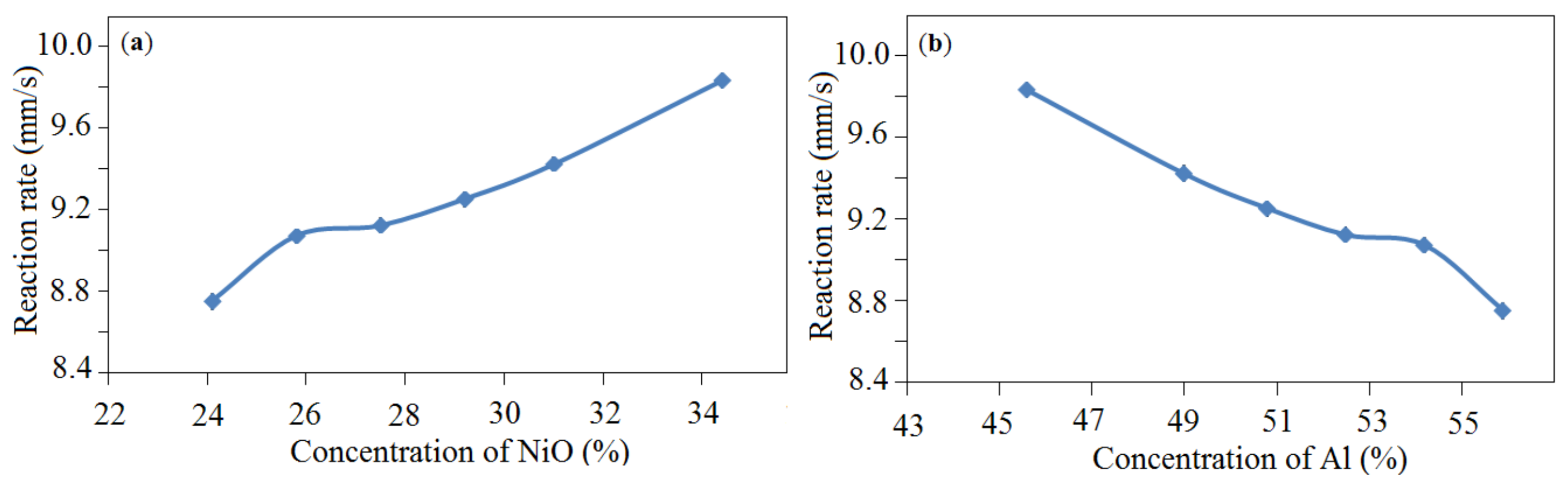
| The Initial Batch Composition | T (°C) | V (mm/s) | Catalysts Composition | S (m2/g) |
|---|---|---|---|---|
| 24.1% NiO—55.9% Al—20.0% Al2O3 | 900 | 8.75 | Al, α-Al2O3, NiAl, Ni, NiO, NiAl2O4 | 0.3 |
| 25.8% NiO—54.2% Al—20.0% Al2O3 | 900 | 9.07 | Al, α-Al2O3, NiAl, Ni, NiO, NiAl2O4 | 0.3 |
| 27.5% NiO—52.5% Al—20.0% Al2O3 | 900 | 9.12 | Al, α-Al2O3, NiAl, Ni, NiO, NiAl2O4 | 0.32 |
| 29.2% NiO—50.8% Al—20.0% Al2O3 | 900 | 9.25 | Al, α-Al2O3, NiAl, Ni, NiO, NiAl2O4 | 0.35 |
| 31.0% NiO—49.0% Al—20.0% Al2O3 | 850 | 9.42 | Al, α-Al2O3, NiAl, Ni, NiO, NiAl2O4 | 0.4 |
| 34.4% NiO—45.6% Al—20.0% Al2O3 | 800 | 9.83 | Al, α-Al2O3, NiAl, Ni, NiO, NiAl2O4 | 0.48 |
| 31.0% NiO—19.0% Al—50.0% Al2O3 | 900 | 9.15 | Al, α-Al2O3, NiAl, Ni, NiO, NiAl2O4 | 0.8 |
| 28.0% NiO—42.0% Al—30.0% Al2O3 | 800 | 9.17 | Al, α-Al2O3, NiAl, Ni, NiO, NiAl2O4 | 0.5 |
| 39.0% NiO—10.0% Al—30.0% Al2O3 | 850 | 7.42 | MgAl2O4, NiAl2O4, NiAl, MgNiO2, | 1.7 |
| 7.0% Mg—14.0% MgO2 | NiO, MgO, Ni | |||
| 87.0% NiO—12.6% Al—0.4% H3BO3 | 650 | 8.17 | Al2O3, Ni, NiO, Al, AlBO3, NiAl2O4 | 2.1 |
| 10.0% NiO—28.0% Al—40.0% Al2O3 | 900 | 12.48 | Al, Al2O3, MoO, MoO2, Al3Mo, | 1.4 |
| 22.0% MoO3 | NiAl2O4 |
| The Initial Batch Composition | CH4 Conversion (%) | H2 Yield (%) | CO Yield (%) | H2/CO | ||||
|---|---|---|---|---|---|---|---|---|
| SHS | IM | SHS | IM | SHS | IM | SHS | IM | |
| 24.1% NiO—55.9% Al—20% Al2O3 | 90 | 78.8 | 61.5 | 54.9 | 21 | 21.9 | 2.9 | 2.5 |
| 25.8% NiO—54.2% Al—20% Al2O3 | 84.2 | 66.9 | 62 | 57.2 | 26.4 | 22.4 | 2.3 | 2.6 |
| 27.5% NiO—52.5% Al—20% Al2O3 | 96 | 69.4 | 67 | 53.9 | 27.1 | 24.2 | 2.5 | 2.2 |
| 29.2% NiO—50.8% Al—20% Al2O3 | 81.7 | 73.7 | 52 | 55.7 | 25.9 | 22.8 | 2 | 2.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tungatarova, S.; Xanthopoulou, G.; Vekinis, G.; Karanasios, K.; Baizhumanova, T.; Zhumabek, M.; Sadenova, M. Ni-Al Self-Propagating High-Temperature Synthesis Catalysts in Dry Reforming of Methane to Hydrogen-Enriched Fuel Mixtures. Catalysts 2022, 12, 1270. https://doi.org/10.3390/catal12101270
Tungatarova S, Xanthopoulou G, Vekinis G, Karanasios K, Baizhumanova T, Zhumabek M, Sadenova M. Ni-Al Self-Propagating High-Temperature Synthesis Catalysts in Dry Reforming of Methane to Hydrogen-Enriched Fuel Mixtures. Catalysts. 2022; 12(10):1270. https://doi.org/10.3390/catal12101270
Chicago/Turabian StyleTungatarova, Svetlana, Galina Xanthopoulou, George Vekinis, Konstantinos Karanasios, Tolkyn Baizhumanova, Manapkhan Zhumabek, and Marzhan Sadenova. 2022. "Ni-Al Self-Propagating High-Temperature Synthesis Catalysts in Dry Reforming of Methane to Hydrogen-Enriched Fuel Mixtures" Catalysts 12, no. 10: 1270. https://doi.org/10.3390/catal12101270
APA StyleTungatarova, S., Xanthopoulou, G., Vekinis, G., Karanasios, K., Baizhumanova, T., Zhumabek, M., & Sadenova, M. (2022). Ni-Al Self-Propagating High-Temperature Synthesis Catalysts in Dry Reforming of Methane to Hydrogen-Enriched Fuel Mixtures. Catalysts, 12(10), 1270. https://doi.org/10.3390/catal12101270