Development of Silicon Carbide-Supported Palladium Catalysts and Their Application as Semihydrogenation Catalysts for Alkynes under Batch- and Continuous-Flow Conditions
Abstract
1. Introduction
2. Results
3. Materials and Methods
3.1. Materials
3.2. Preparation of Catalyst
3.3. General Procedure for Hydrogenation Reactions
3.4. Spectroscopic Data of Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swamy, K.C.K.; Reddy, A.S.; Sandeep, K.; Kalyani, A. Advances in chemoselective and/or stereoselective semihydrogenation of alkynes. Tetrahedron Lett. 2018, 59, 419–429. [Google Scholar] [CrossRef]
- Sharma, D.M.; Punji, B. 3 d Transition Metal-Catalyzed Hydrogenation of Nitriles and Alkynes. Chem. Asian J. 2020, 15, 690–708. [Google Scholar] [CrossRef]
- Lindlar, H. Ein neuer Katalysator für selektive Hydrierungen. Helv. Chim. Acta 1952, 35, 446–450. [Google Scholar] [CrossRef]
- Lindlar, H.; Dubuis, R. Palladium Catalyst for Partial Reduction of Acetylenes. Org. Synth. Coll. 1973, 5, 880–883. [Google Scholar]
- Thiel, N.O.; Kaewmee, B.; Ngoc, T.T.; Teichert, J.F. A Simple Nickel Catalyst Enabling an E-Selective Alkyne Semihydrogenation. Chem. Eur. J. 2020, 26, 1597–1603. [Google Scholar] [CrossRef]
- Zhang, Y.; Karunananda, M.K.; Yu, H.-C.; Clark, K.J.; Williams, W.; Mankad, N.P.; Ess, D.H. Dynamically Bifurcating Hydride Transfer Mechanism and Origin of Inverse Isotope Effect for Heterodinuclear AgRu-Catalyzed Alkyne Semihydrogenation. ACS Catal. 2019, 9, 2657–2663. [Google Scholar] [CrossRef]
- Gramigna, K.M.; Dickie, D.A.; Foxman, B.M.; Thomas, C.M. Cooperative H2 Activation across a Metal–Metal Multiple Bond and Hydrogenation Reactions Catalyzed by a Zr/Co Heterobimetallic Complex. ACS Catal. 2019, 9, 3153–3164. [Google Scholar] [CrossRef]
- Murugesan, K.; Bheeter, C.B.; Linnebank, P.R.; Spannenberg, A.; Reek, J.N.H.; Jagadeesh, R.V.; Beller, M. Nickel-Catalyzed Stereodivergent Synthesis of E- and Z-Alkenes by Hydrogenation of Alkynes. ChemSusChem 2019, 12, 3363–3369. [Google Scholar] [CrossRef] [PubMed]
- Hancker, S.; Neumann, H.; Beller, M. Development of a Palladium-Catalyzed Process for the Synthesis of Z-Alkenes by Sequential Sonogashira–Hydrogenation Reaction. Eur. J. Org. Chem. 2018, 2018, 5253–5259. [Google Scholar] [CrossRef]
- Gorgas, N.; Brünig, J.; Stöger, B.; Vanicek, S.; Tilset, M.; Veiros, L.F.; Kirchner, K. Efficient Z-Selective Semihydrogenation of Internal Alkynes Catalyzed by Cationic Iron(II) Hydride Complexes. J. Am. Chem. Soc. 2019, 141, 17452–17458. [Google Scholar] [CrossRef]
- Duan, Y.; Ji, G.; Zhang, S.; Chen, X.; Yang, Y. Additive-modulated switchable reaction pathway in the addition of alkynes with organosilanes catalyzed by supported Pd nanoparticles: Hydrosilylation versus semihydrogenation. Catal. Sci. Technol. 2018, 8, 1039–1050. [Google Scholar] [CrossRef]
- Wen, X.; Shi, X.; Qiao, X.; Wu, Z.; Bai, G. Ligand-free nickel-catalyzed semihydrogenation of alkynes with sodium borohydride: A highly efficient and selective process for cis-alkenes under ambient conditions. Chem. Commun. 2017, 53, 5372–5375. [Google Scholar] [CrossRef]
- Gong, D.; Hu, B.; Yang, W.; Kong, D.; Xia, H.; Chen, D. A Bidentate Ru(II)-NC Complex as a Catalyst for Semihydrogenation of Alkynes to (E)-Alkenes with Ethanol. Organometallics 2020, 39, 862–869. [Google Scholar] [CrossRef]
- Ekebergh, A.; Begon, R.; Kann, N. Ruthenium-Catalyzed E-Selective Alkyne Semihydrogenation with Alcohols as Hydrogen Donors. J. Org. Chem. 2020, 85, 2966–2975. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, C.; Sun, Y.; Man, X.; Li, J.; Sun, F. Ligand-controlled iridium-catalyzed semihydrogenation of alkynes with ethanol: Highly stereoselective synthesis of E- and Z-alkenes. Chem. Commun. 2019, 55, 1903–1906. [Google Scholar] [CrossRef]
- Kaicharla, T.; Zimmermann, B.M.; Oestreich, M.; Teichert, J.F. Using alcohols as simple H2-equivalents for copper-catalysed transfer semihydrogenations of alkynes. Chem. Commun. 2019, 55, 13410–13413. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Zhang, B.; Ge, H.; Gu, X.; Zhang, S.; Qin, Y. Porous TiO2/Pt/TiO2 Sandwich Catalyst for Highly Selective Semihydrogenation of Alkyne to Olefin. ACS Catal. 2017, 7, 6567–6572. [Google Scholar] [CrossRef]
- Shibahara, F.; Mizuno, T.; Shibata, Y.; Murai, T. Transfer Semihydrogenation of Alkynes Catalyzed by Imidazo [1,5-a]pyrid-3-ylidene–Pd Complexes: Positive Effects of Electronic and Steric Features on N-Heterocyclic Carbene Ligands. Bull. Chem. Soc. Jpn. 2020, 93, 332–337. [Google Scholar] [CrossRef]
- Liang, S.; Hammond, G.B.; Xu, B. Supported gold nanoparticles catalyzed cis-selective semihydrogenation of alkynes using ammonium formate as the reductant. Chem. Commun. 2016, 52, 6013–6016. [Google Scholar] [CrossRef]
- Tian, W.-F.; He, Y.-Q.; Song, X.-R.; Ding, H.-X.; Ye, J.; Guo, W.-J.; Xiao, Q. cis-Selective Transfer Semihydrogenation of Alkynes by Merging Visible-Light Catalysis with Cobalt Catalysis. Adv. Synth. Catal. 2020, 362, 1032–1038. [Google Scholar] [CrossRef]
- Das, M.; Kaicharla, T.; Teichert, J.F. Stereoselective Alkyne Hydrohalogenation by Trapping of Transfer Hydrogenation Intermediates. Org. Lett. 2018, 20, 4926–4929. [Google Scholar] [CrossRef] [PubMed]
- Brzozowska, A.; Azofra, L.M.; Zubar, V.; Atodiresei, I.; Cavallo, L.; Rueping, M.; El-Sepelgy, O. Highly Chemo- and Stereoselective Transfer Semihydrogenation of Alkynes Catalyzed by a Stable, Well-Defined Manganese(II) Complex. ACS Catal. 2018, 8, 4103–4109. [Google Scholar] [CrossRef]
- Zhao, C.-Q.; Chen, Y.-G.; Qiu, H.; Wei, L.; Fang, P.; Mei, T.-S. Water as a Hydrogenating Agent: Stereodivergent Pd-Catalyzed Semihydrogenation of Alkynes. Org. Lett. 2019, 21, 1412–1416. [Google Scholar] [CrossRef]
- Bao, H.; Zhou, B.; Jin, H.; Liu, Y. Diboron-Assisted Copper-Catalyzed Z-Selective Semihydrogenation of Alkynes Using Ethanol as a Hydrogen Donor. J. Org. Chem. 2019, 84, 3579–3589. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Hu, J.; Chen, C.; Yuan, Y.; Shi, Z. Copper-catalysed, diboron-mediated cis-dideuterated semihydrogenation of alkynes with heavy water. Chem. Commun. 2019, 55, 6922–6925. [Google Scholar] [CrossRef] [PubMed]
- Decker, D.; Drexler, H.-J.; Heller, D.; Beweries, T. Homogeneous catalytic transfer semihydrogenation of alkynes—An overview of hydrogen sources, catalysts and reaction mechanisms. Catal. Sci. Technol. 2020, 10, 6449–6463. [Google Scholar] [CrossRef]
- Garduño, J.A.; Arévalo, A.; García, J.J. Bond and small-molecule activation with low-valent nickel complexes. Dalton Trans. 2015, 44, 13419–13438. [Google Scholar] [CrossRef]
- Sorella, G.L.; Sperni, L.; Canton, P.; Coletti, L.; Fabris, F.; Strukul, G.; Scarso, A. Selective Hydrogenations and Dechlorinations in Water Mediated by Anionic Surfactant-Stabilized Pd Nanoparticles. J. Org. Chem. 2018, 83, 7438–7446. [Google Scholar] [CrossRef]
- Choe, K.; Zheng, F.; Wang, H.; Yuan, Y.; Zhao, W.; Xue, G.; Qiu, X.; Ri, M.; Shi, X.; Wang, Y.; et al. Fast and Selective Semihydrogenation of Alkynes by Palladium Nanoparticles Sandwiched in Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2020, 59, 3650–3657. [Google Scholar] [CrossRef]
- Liu, H.-J.; Lo, H.-K.; Copéret, C. Silica-Supported Cu Nanoparticle Catalysts for Alkyne Semihydrogenation: Effect of Ligands on Rates and Selectivity. J. Am. Chem. Soc. 2016, 138, 16502–16507. [Google Scholar]
- Kaeffer, N.; Liu, H.-J.; Lo, H.-K.; Fedorov, A.; Copéret, C. An N-heterocyclic carbene ligand promotes highly selective alkyne semihydrogenation with copper nanoparticles supported on passivated silica. Chem. Sci. 2018, 9, 5366–5371. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Nakanishi, K.; Tanaka, A.; Kominami, H. Accelerated Semihydrogenation of Alkynes over a Copper/Palladium/Titanium (IV) Oxide Photocatalyst Free from Poison and H2 Gas. ChemCatChem 2020, 12, 1609–1616. [Google Scholar] [CrossRef]
- Gregori, B.J.; Schwarzhuber, F.; Pöllath, S.; Zweck, J.; Fritsch, L.; Schoch, R.; Bauer, M.; von Wangelin, A.J. Stereoselective Alkyne Hydrogenation by using a Simple Iron Catalyst. ChemSusChem 2019, 12, 3864–3870. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Lin, Y.; Huang, L.; Sun, Z.; Yang, Y.; Zhou, X.; Vovk, E.; Liu, X.; Huang, X.; Sun, M.; et al. Copper Catalysts in Semihydrogenation of Acetylene: From Single Atoms to Nanoparticles. ACS Catal. 2020, 10, 3495–3504. [Google Scholar] [CrossRef]
- García-Calvo, J.; Calvo-Gredilla, P.; Vallejos, S.; García, J.M.; Cuevas-Vicario, J.V.; García-Herbosa, G.; Avellab, M.; Torroba, T. Palladium nanodendrites uniformly deposited on the surface of polymers as an efficient and recyclable catalyst for direct drug modification via Z-selective semihydrogenation of alkynes. Green Chem. 2018, 20, 3875–3883. [Google Scholar] [CrossRef]
- Lu, Y.; Feng, X.; Takale, B.S.; Yamamoto, Y.; Zhang, W.; Bao, M. Highly Selective Semihydrogenation of Alkynes to Alkenes by Using an Unsupported Nanoporous Palladium Catalyst: No Leaching of Palladium into the Reaction Mixture. ACS Catal. 2017, 7, 8296–8303. [Google Scholar] [CrossRef]
- Fukazawa, A.; Minoshima, J.; Tanaka, K.; Hashimoto, Y.; Kobori, Y.; Sato, Y.; Atobe, M. A New Approach to Stereoselective Electrocatalytic Semihydrogenation of Alkynes to Z-Alkenes using a Proton-Exchange Membrane Reactor. ACS Sustain. Chem. Eng. 2019, 7, 11050–11055. [Google Scholar] [CrossRef]
- Desai, S.P.; Ye, J.; Islamoglu, T.; Farha, O.K.; Lu, C.C. Mechanistic Study on the Origin of the Trans Selectivity in Alkyne Semihydrogenation by a Heterobimetallic Rhodium–Gallium Catalyst in a Metal–Organic Framework. Organometallics 2019, 38, 3466–3473. [Google Scholar] [CrossRef]
- Long, Y.; Li, J.; Wu, L.; Wang, Q.; Liu, Y.; Wang, X.; Song, S.; Zhang, H. Construction of trace silver modified core@shell structured Pt-Ni nanoframe@CeO2 for semihydrogenation of phenylacetylene. Nano Res. 2019, 12, 869–875. [Google Scholar] [CrossRef]
- Yang, L.; Yu, S.; Peng, C.; Fang, X.; Cheng, Z.; Zhou, Z. Semihydrogenation of phenylacetylene over nonprecious Ni-based catalysts supported on AlSBA-15. J. Catal. 2019, 370, 310–320. [Google Scholar] [CrossRef]
- Lomelí-Rosales, D.A.; Delgado, J.A.; de los Bernardos, M.D.M.; Pérez-Rodríguez, S.; Gual, A.; Claver, C.; Godard, C. A General One-Pot Methodology for the Preparation of Mono- and Bimetallic Nanoparticles Supported on Carbon Nanotubes: Application in the Semi-hydrogenation of Alkynes and Acetylene. Chem. Eur. J. 2019, 25, 8321–8331. [Google Scholar] [CrossRef] [PubMed]
- Dang-Bao, T.; Pradel, C.; Favier, I.; Gómez, M. Bimetallic Nanocatalysts in Glycerol for Applications in Controlled Synthesis. A Structure–Reactivity Relationship Study. ACS Appl. Nano Mater. 2019, 2, 1033–1044. [Google Scholar] [CrossRef]
- da Silva, F.P.; Fiorio, J.L.; Gonçalves, R.V.; Teixeira-Neto, E.; Rossi, L.M. Synergic Effect of Copper and Palladium for Selective Hydrogenation of Alkynes. Ind. Eng. Chem. Res. 2018, 57, 16209–16216. [Google Scholar] [CrossRef]
- Desai, S.P.; Ye, J.; Zheng, J.; Ferrandon, M.S.; Webber, T.E.; Platero-Prats, A.E.; Duan, J.; Garcia-Holley, P.; Camaioni, D.M.; Chapman, K.W.; et al. Well-Defined Rhodium–Gallium Catalytic Sites in a Metal–Organic Framework: Promoter-Controlled Selectivity in Alkyne Semihydrogenation to E-Alkenes. J. Am. Chem. Soc. 2018, 140, 15309–15318. [Google Scholar] [CrossRef] [PubMed]
- Wissing, M.; Niehues, M.; Ravoo, B.J.; Studer, A. Mixed AuPd Nanoparticles as Highly Active Catalysts for Alkyne Z-Semihydrogenation. Eur. J. Org. Chem. 2018, 2018, 3403–3409. [Google Scholar] [CrossRef]
- Delgado, J.A.; Benkirane, O.; Claver, C.; Curulla-Ferré, D.; Godard, C. Advances in the preparation of highly selective nanocatalysts for the semi-hydrogenation of alkynes using colloidal approaches. Dalton Trans. 2017, 46, 12381–12403. [Google Scholar] [CrossRef] [PubMed]
- Reina, A.; Favier, I.; Pradel, C.; Gómeza, M. Stable Zero-Valent Nickel Nanoparticles in Glycerol: Synthesis and Applications in Selective Hydrogenations. Adv. Synth. Catal. 2018, 360, 3544–3552. [Google Scholar] [CrossRef]
- Mäsing, F.; Nüsse, H.; Klingauf, J.; Studer, A. Light Mediated Preparation of Palladium Nanoparticles as Catalysts for Alkyne cis-Semihydrogenation. Org. Lett. 2017, 19, 2658–2661. [Google Scholar] [CrossRef] [PubMed]
- Verho, O.; Zheng, H.; Gustafson, K.P.J.; Nagendiran, A.; Zou, X.; Bäckvall, J.-E. Application of Pd Nanoparticles Supported on Mesoporous Hollow Silica Nanospheres for the Efficient and Selective Semihydrogenation of Alkynes. ChemCatChem 2016, 8, 773–778. [Google Scholar] [CrossRef]
- Sajiki, H.; Mori, S.; Ohkubo, T.; Ikawa, T.; Kume, A.; Maegawa, T.; Monguchi, Y. Partial Hydrogenation of Alkynes to cis-Olefins by Using a Novel Pd0–Polyethyleneimine Catalyst. Chem. Eur. J. 2008, 14, 5109–5111. [Google Scholar] [CrossRef]
- Mori, S.; Ohkubo, T.; Ikawa, T.; Kume, A.; Maegawa, T.; Monguchi, Y.; Sajiki, H. Pd(0)–polyethyleneimine complex as a partial hydrogenation catalyst of alkynes to alkenes. J. Mol. Catal. A 2009, 307, 77–87. [Google Scholar] [CrossRef]
- Yabe, Y.; Yamada, T.; Nagata, S.; Sawama, Y.; Monguchi, Y.; Sajiki, H. Development of a Palladium on Boron Nitride Catalyst and its Application to the Semihydrogenation of Alkynes. Adv. Synth. Catal. 2012, 354, 1264–1268. [Google Scholar] [CrossRef]
- Yabe, Y.; Sawama, Y.; Yamada, T.; Nagata, S.; Monguchi, Y.; Sajiki, H. Easily-Controlled Chemoselective Hydrogenation by using Palladium on Boron Nitride. ChemCatChem 2013, 5, 2360–2366. [Google Scholar] [CrossRef]
- Sajiki, H.; Hattori, K.; Hirota, K. Pd/C(en)-catalyzed regioselective hydrogenolysis of terminal epoxides to secondary alcohols. Chem. Commun. 1999, 1041–1042. [Google Scholar] [CrossRef]
- Sajiki, H.; Hattori, K.; Hirota, K. Easy and partial hydrogenation of aromatic carbonyls to benzyl alcohols using Pd/C(en)-catalyst. J. Chem. Soc. Perkin Trans. 1998, 1, 4043–4044. [Google Scholar] [CrossRef]
- Sajiki, H.; Hattori, K.; Hirota, K. The Formation of a Novel Pd/C−Ethylenediamine Complex Catalyst: Chemoselective Hydrogenation without Deprotection of the O-Benzyl and N-Cbz Groups. J. Org. Chem. 1998, 63, 7990–7992. [Google Scholar] [CrossRef]
- Hattori, K.; Sajiki, H.; Hirota, K. Undesirable deprotection of O-TBDMS groups by Pd/C-catalyzed hydrogenation and chemoselective hydrogenation using a Pd/C(en) catalyst. Tetrahedron 2001, 57, 2109–2114. [Google Scholar] [CrossRef]
- Hattori, K.; Sajiki, H.; Hirota, K. Chemoselective control of hydrogenation among aromatic carbonyl and benzyl alcohol derivatives using Pd/C(en) catalyst. Tetrahedron 2001, 57, 4817–4824. [Google Scholar] [CrossRef]
- Hattori, K.; Sajiki, H.; Hirota, K. The undesirable lability of tert-butyldimethylsilyl ethers under Pd/C-catalyzed hydrogenation conditions and solution of the problem by using a Pd/C(en) catalyst. Tetrahedron Lett. 2000, 41, 5711–5714. [Google Scholar] [CrossRef]
- Hattori, K.; Sajiki, H.; Hirota, K. Pd/C(en)-catalyzed chemoselective hydrogenation with retention of the N-Cbz protective group and its scope and limitations. Tetrahedron 2000, 56, 8433–8441. [Google Scholar] [CrossRef]
- Xia, L.; Li, D.; Long, J.; Huang, F.; Yang, L.; Guo, Y.; Jia, Z.; Xiao, J.; Liu, H. N-doped graphene confined Pt nanoparticles for efficient semi-hydrogenation of phenylacetylene. Carbon 2019, 145, 47–52. [Google Scholar] [CrossRef]
- Li, X.; Pan, Y.; Yi, H.; Hu, J.; Yang, D.; Lv, F.; Li, W.; Zhou, J.; Wu, X.; Lei, A.; et al. Mott–Schottky Effect Leads to Alkyne Semihydrogenation over Pd-Nanocube@N-Doped Carbon. ACS Catal. 2019, 9, 4632–4641. [Google Scholar] [CrossRef]
- Ji, G.; Duan, Y.; Zhang, S.; Fei, B.; Chen, X.; Yang, Y. Selective Semihydrogenation of Alkynes Catalyzed by Pd Nanoparticles Immobilized on Heteroatom-Doped Hierarchical Porous Carbon Derived from Bamboo Shoots. ChemSusChem 2017, 10, 3427–3434. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Kreyenschulte, C.; Radnik, J.; Lund, H.; Surkus, A.-E.; Junge, K.; Beller, M. Selective Semihydrogenation of Alkynes with N-Graphitic-Modified Cobalt Nanoparticles Supported on Silica. ACS Catal. 2017, 7, 1526–1532. [Google Scholar] [CrossRef]
- Yoshii, T.; Umemoto, D.; Kuwahara, Y.; Mori, K.; Yamashita, H. Engineering of Surface Environment of Pd Nanoparticle Catalysts on Carbon Support with Pyrene–Thiol Ligands for Semihydrogenation of Alkynes. ACS Appl. Mater. Interfaces 2019, 11, 37708–37719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wen, X.; Shi, Y.; Yue, R.; Bai, L.; Liu, Q.; Ba, X. Sulfur-Containing Polymer As a Platform for Synthesis of Size-Controlled Pd Nanoparticles for Selective Semihydrogenation of Alkynes. Ind. Eng. Chem. Res. 2019, 58, 1142–1149. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, L.; Zhang, W.; Hu, C.; Dai, L.; Ren, L.; Wu, B.; Fu, G.; Zheng, N. Thiol Treatment Creates Selective Palladium Catalysts for Semihydrogenation of Internal Alkynes. Chem 2018, 4, 1080–1091. [Google Scholar] [CrossRef]
- Mori, A.; Mizusaki, T.; Kawase, M.; Maegawa, T.; Monguchi, Y.; Takao, S.; Takagi, Y.; Sajiki, H. Novel Palladium-on-Carbon/Diphenyl Sulfide Complex for Chemoselective Hydrogenation: Preparation, Characterization, and Application. Adv. Synth. Catal. 2008, 350, 406–410. [Google Scholar] [CrossRef]
- Mori, A.; Miyakawa, Y.; Ohashi, E.; Haga, T.; Maegawa, T.; Sajiki, H. Pd/C-Catalyzed Chemoselective Hydrogenation in the Presence of Diphenylsulfide. Org. Lett. 2006, 8, 3279–3281. [Google Scholar] [CrossRef]
- Yabe, Y.; Sawama, Y.; Monguchi, Y.; Sajiki, H. New aspect of chemoselective hydrogenation utilizing heterogeneous palladium catalysts supported by nitrogen- and oxygen-containing macromolecules. Catal. Sci. Technol. 2014, 4, 260–271. [Google Scholar] [CrossRef]
- Monguchi, Y.; Ichikawa, T.; Sajiki, H. Recent Development of Palladium-supported Catalysts for Chemoselective Hydrogenation. Chem. Pharm. Bull. 2017, 65, 2–9. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, Y.; Liu, Y.; Chu, W. Promising SiC support for Pd catalyst in selective hydrogenation of acetylene to ethylene. Appl. Surf. Sci. 2018, 442, 736–741. [Google Scholar] [CrossRef]
- Ichikawa, T.; Netsu, M.; Mizuno, M.; Mizusaki, T.; Takagi, Y.; Sawama, Y.; Monguchi, Y.; Sajiki, H. Development of a Unique Heterogeneous Palladium Catalyst for the Suzuki–Miyaura Reaction using (Hetero)aryl Chlorides and Chemoselective Hydrogenation. Adv. Synth. Catal. 2017, 359, 2269–2279. [Google Scholar] [CrossRef]
- Ding, Z.; Li, C.; Chen, J.; Zeng, J.; Tang, H.; Ding, Y.; Zhan, Z. Palladium/Phosphorus-Doped Porous Organic Polymer as Recyclable Chemoselective and Efficient Hydrogenation Catalyst under Ambient Conditions. Adv. Synth. Catal. 2017, 359, 2280–2287. [Google Scholar] [CrossRef]
- Qi, H.L.; Chen, D.S.; Ye, J.S.; Hung, J.M. Electrochemical Technique and Copper-Promoted Transformations: Selective Hydroxylation and Amination of Arylboronic Acids. J. Org. Chem. 2013, 78, 7482–7487. [Google Scholar] [CrossRef] [PubMed]
- Das, B.G.; Ghorai, P. The direct reductive amination of electron-deficient amines with aldehydes: The unique reactivity of the Re2O7 catalyst. Chem. Commun. Chem. 2012, 48, 8276–8278. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Miao, C.; Xu, D.; Xia, C.; Sun, W. Highly Efficient Oxidation of Secondary Alcohols to Ketones Catalyzed by Manganese Complexes of N4 Ligands with H2O2. Org. Lett. 2015, 17, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, C.; Cui, Y.; Sun, M.; Jia, X.; Li, J. Bu4NI-Catalyzed C–C Bond Cleavage and Oxidative Esterification of Allyl Alcohols with Toluene Derivatives. Synthesis 2019, 51, 3667–3674. [Google Scholar] [CrossRef]
- Mao, R.; Balon, J.; Hu, X. Decarboxylative C(sp3)−O Cross-Coupling. Angew. Chem. Int. Ed. 2018, 57, 13624–13628. [Google Scholar] [CrossRef] [PubMed]
- Malapit, C.A.; Ichiishi, N.; Sanford, M.S. Pd-Catalyzed Decarbonylative Cross-Couplings of Aroyl Chlorides. Org. Lett. 2017, 19, 4142–4145. [Google Scholar] [CrossRef] [PubMed]
- Sloan, M.E.; Staubitz, A.; Lee, K.; Manners, I. Scope and Selectivity of Heterogeneous Rh0-Catalyzed Tandem Dehydrocoupling/Hydrogenation Using Me2NH·BH3 as a Stoichiometric H2 Source. Eur. J. Org. Chem. 2011, 2011, 672–675. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, X.; Zhao, L.; Pi, C.; Ji, J.; Cui, X.; Wu, Y. Generalized Chemoselective Transfer Hydrogenation/Hydrodeuteration. Adv. Synth. Catal. 2020, 362, 4119–4129. [Google Scholar] [CrossRef]
- Richmond, E.; Moran, J. Ligand Control of E/Z Selectivity in Nickel-Catalyzed Transfer Hydrogenative Alkyne Semireduction. J. Org. Chem. 2015, 80, 6922–6929. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.; Judkele, L.; Bruffaerts, J.; Marek, I. Metal-Catalyzed Remote Functionalization of ω-Ene Unsaturated Ethers: Towards Functionalized Vinyl Species. Angew. Chem. Int. Ed. 2018, 57, 8012–8016. [Google Scholar] [CrossRef]
- Broggi, J.; Jurčík, V.; Songis, O.; Poater, A.; Cavallo, L.; Slawin, A.M.Z.; Cazin, C.S.J. The Isolation of [Pd{OC(O)H}(H)(NHC)(PR3)] (NHC = N-Heterocyclic Carbene) and Its Role in Alkene and Alkyne Reductions Using Formic Acid. J. Am. Chem. Soc. 2013, 135, 4588–4591. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Kim, U.; Sung, D.; Kim, W. A Synthetic Approach to N-Aryl Carbamates via Copper-Catalyzed Chan–Lam Coupling at Room Temperature. J. Org. Chem. 2015, 80, 1856–1865. [Google Scholar] [CrossRef]
- Shen, G.; Liu, H.; Chen, J.; He, Z.; Zhou, Y.; Wang, L.; Luo, Y.; Su, Z.; Fan, B. Zinc salt-catalyzed reduction of α-aryl imino esters, diketones and phenylacetylenes with water as hydrogen source. Org. Biomol. Chem. 2021, 19, 3601–3610. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Hosoya, H.; Ikeda, H.; Nishi, K.; Tsurugi, H.; Mashima, K. Metal-Free Deoxygenation and Reductive Disilylation of Nitroarenes by Organosilicon Reducing Reagents. Chem. Eur. J. 2018, 24, 11278–11282. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Han, J.; Liu, J.; Li, Y.; Zhang, F.; Yu, H.; Hu, S.; Wang, X. Pd-Catalyzed Vinylation of Aryl Halides with Inexpensive Organosilicon Reagents Under Mild Conditions. Chem. Eur. J. 2018, 24, 10324–10328. [Google Scholar] [CrossRef] [PubMed]
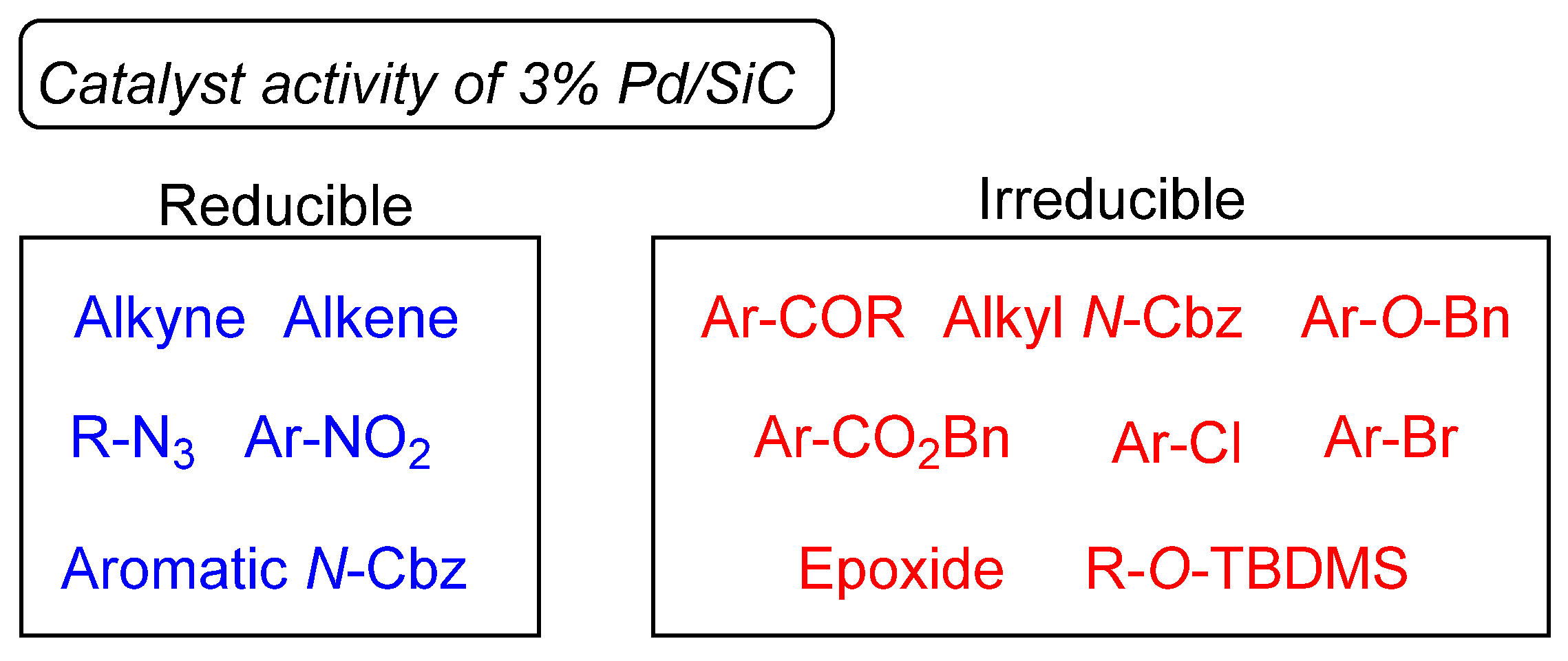


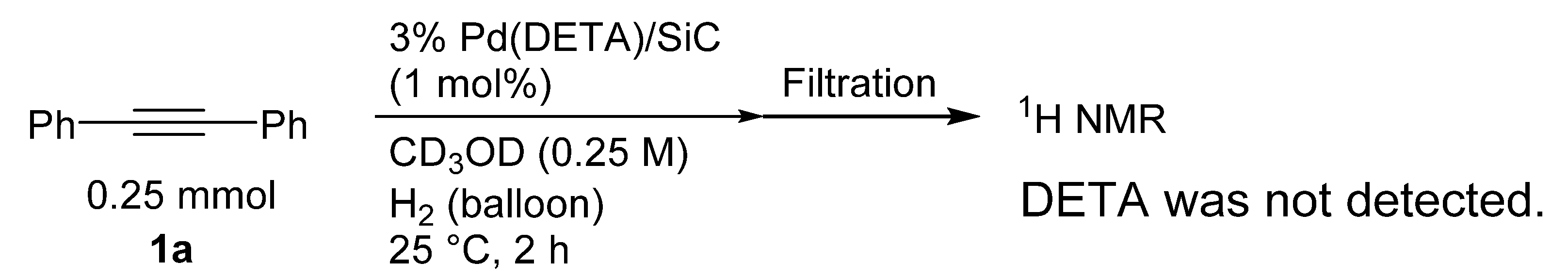
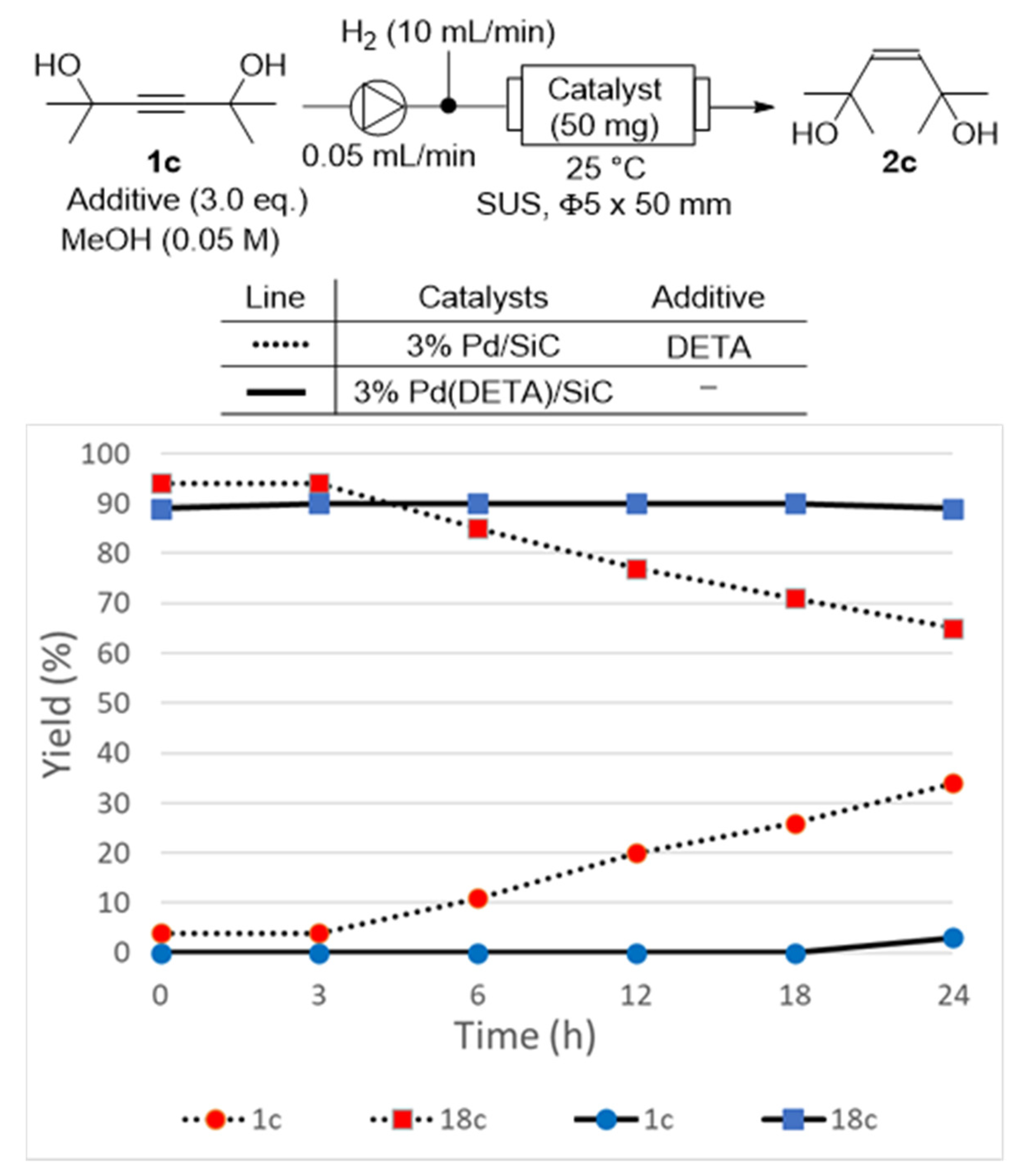
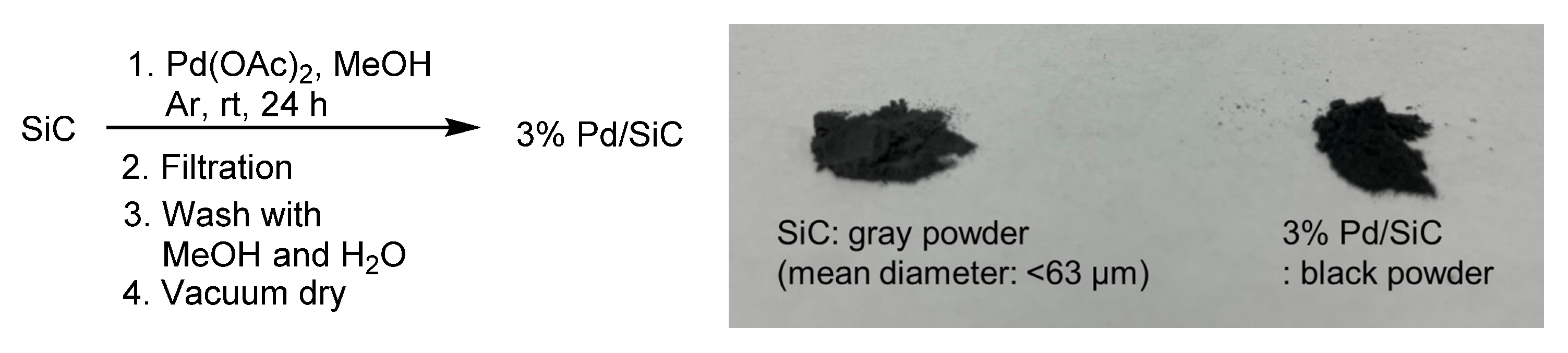
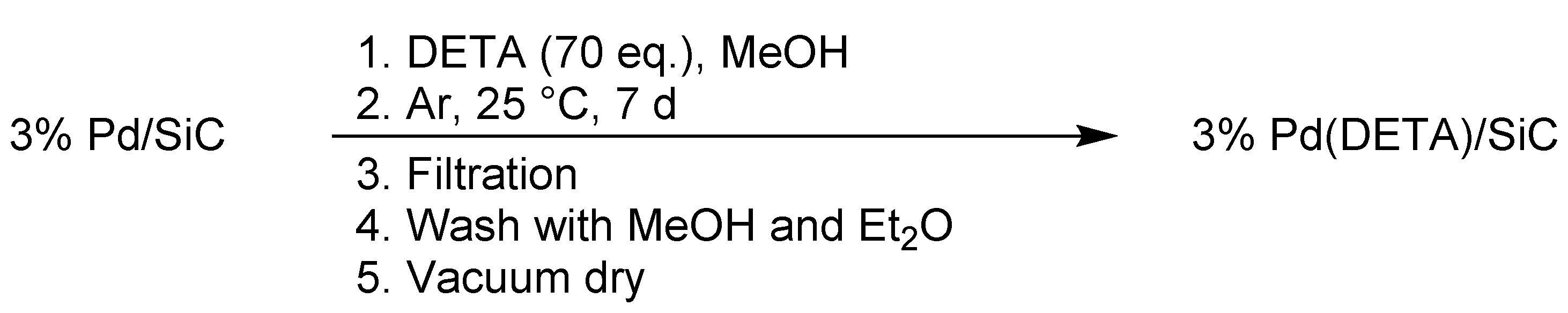
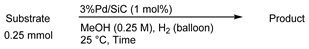
| Entry | Substrate | Product | Yield a (Time) |
|---|---|---|---|
| 1 | 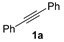 | 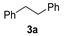 | quant. (3 h) |
| 2 | 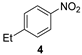 | 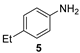 | quant. (6 h) |
| 3 | 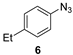 | 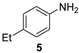 | quant. (4 h) |
| 4 b | 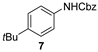 | 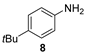 | quant. (4 h) |
| 5 c | 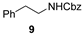 | n.r. | (quant.) (24 h) |
| 6 c |  | n.r. | (quant.) (24 h) |
| 7 c |  | n.r. | (quant.) (24 h) |
| 8 | 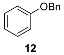 | n.r. | (quant.) (24 h) |
| 9 | 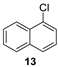 | n.r. | (quant.) (24 h) |
| 10 | 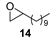 | n.r. | (97%) (24 h) |
| 11 | 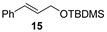 | 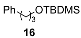 | quant. (1 h) |
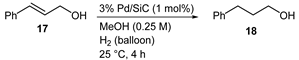
| Run | 1st | 2nd | 3rd |
|---|---|---|---|
| Yield (%) | 99 | quant. | 97 |
| Catalyst recovery yield (%) | 99 | quant. | 95 |

| Entry | Product | Time (h) | Conv. (%) b | Yield (%) b (E/Z) |
|---|---|---|---|---|
| 1 |  | 2 | 98 | 93 [52] c (2/98) |
| 2 | 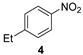 | 24 | 100 | 93 (1/99) |
| 3 | 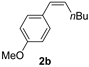 | 4 | 100 | 95 (2/98) |
| 4 | 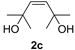 | 4 | 99 | 97 (85) c (2/98) |
| 5 | 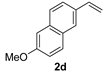 | 2 | 100 | 96 |
| 6 | 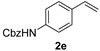 | 1.5 | 100 | 90 |
| 7 d |  | 6 | 99 | 99 |
| 8 | 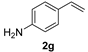 | 2 | 100 | 67 |
| 9 e | 1 | 98 | 92 | |
| 10 | 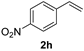 | 0.75 | 85 | 84 |
| 11 | 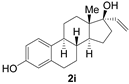 | 1 | 98 | 96 |
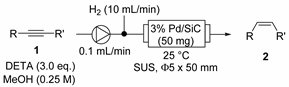
| Entry | Product | Residence Time | Conv. (%) b | Yield (%) b (E/Z) |
|---|---|---|---|---|
| 1 |  | 1 min | 97 | 90 [44] c (2/98) |
| 2 d | 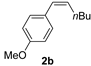 | 2 min | 97 | 91 (2/98) |
| 3 d | 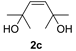 | 2 min | 10 | 95 (83) c (3/97) |
| 4 | 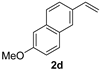 | 1 min | 96 | 90 |
| 5 | 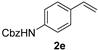 | 1 min | 100 | 82 |
| 6 d | 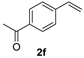 | 2 min | 100 | 80 |
| 7 e | 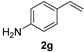 | 1 min | 100 | 87 |
| 8 | 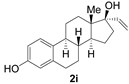 | 1 min | 93 | 92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, T.; Yamamoto, H.; Kawai, K.; Park, K.; Aono, N.; Sajiki, H. Development of Silicon Carbide-Supported Palladium Catalysts and Their Application as Semihydrogenation Catalysts for Alkynes under Batch- and Continuous-Flow Conditions. Catalysts 2022, 12, 1253. https://doi.org/10.3390/catal12101253
Yamada T, Yamamoto H, Kawai K, Park K, Aono N, Sajiki H. Development of Silicon Carbide-Supported Palladium Catalysts and Their Application as Semihydrogenation Catalysts for Alkynes under Batch- and Continuous-Flow Conditions. Catalysts. 2022; 12(10):1253. https://doi.org/10.3390/catal12101253
Chicago/Turabian StyleYamada, Tsuyoshi, Haruka Yamamoto, Kanon Kawai, Kwihwan Park, Norihiko Aono, and Hironao Sajiki. 2022. "Development of Silicon Carbide-Supported Palladium Catalysts and Their Application as Semihydrogenation Catalysts for Alkynes under Batch- and Continuous-Flow Conditions" Catalysts 12, no. 10: 1253. https://doi.org/10.3390/catal12101253
APA StyleYamada, T., Yamamoto, H., Kawai, K., Park, K., Aono, N., & Sajiki, H. (2022). Development of Silicon Carbide-Supported Palladium Catalysts and Their Application as Semihydrogenation Catalysts for Alkynes under Batch- and Continuous-Flow Conditions. Catalysts, 12(10), 1253. https://doi.org/10.3390/catal12101253








