Regio- and Stereoselective One-Pot Synthesis of New Heterocyclic Compounds with Two Selenium Atoms Based on 2-Bromomethyl-1,3-thiaselenole Using Phase Transfer Catalysis
Abstract
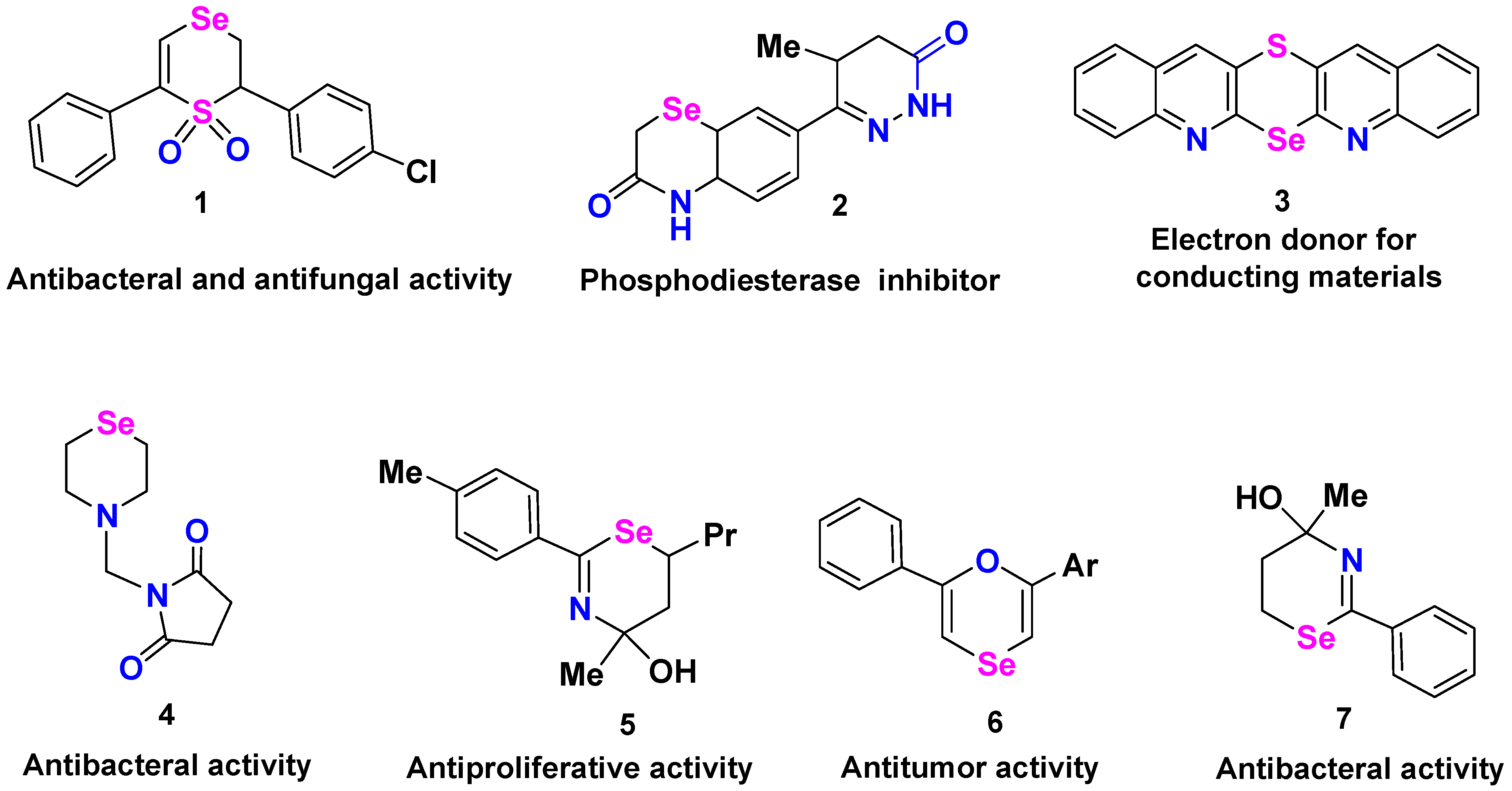
1. Introduction
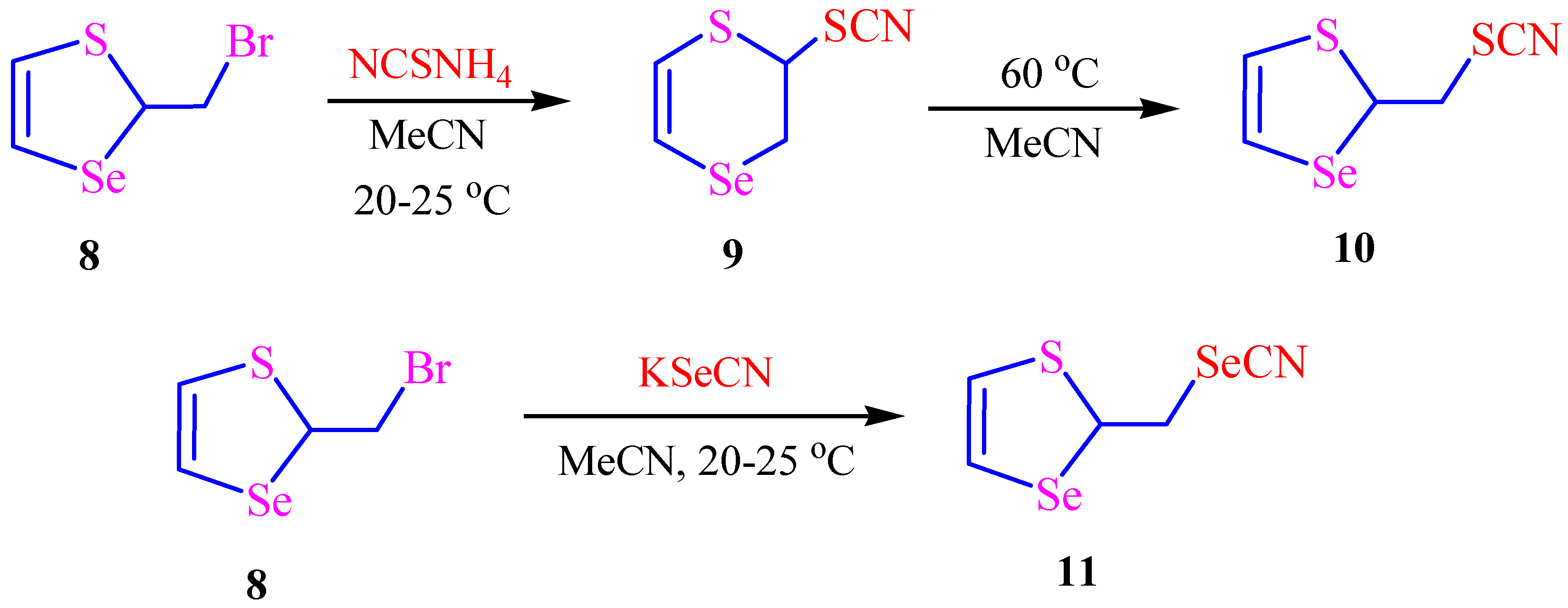
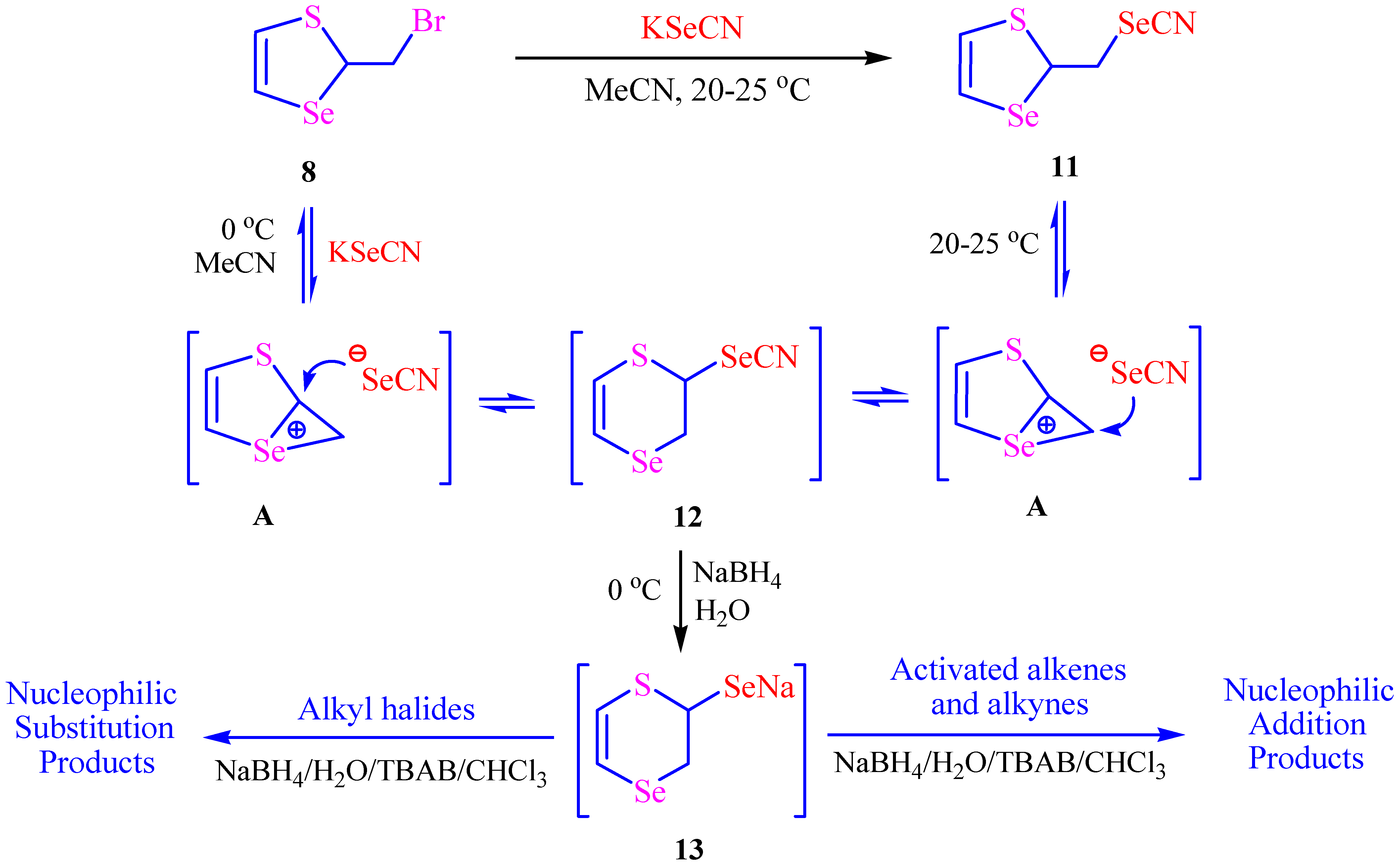
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Modified Procedure for the Preparation of Starting 2-Bromomethyl-1,3-thiaselenole
3.3. Synthesis of Selenocyanates 11 and 12
3.4. Synthesis of 2,3-Dihydro-1,4-thiaselenin-2-yl Alkyl Selenides
3.5. Synthesis of 2,3-Dihydro-1,4-thiaselenin-2-yl Allyl and 2-Propynyl Selenides 17 and 18
3.6. Synthesis of the Products 19–21
3.7. Synthesis of Alkyl (Z)-3-(2,3-Dihydro-1,4-thiaselenin-2-ylselanyl)acrylates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santi, C. (Ed.) Organoselenium Chemistry: Between Synthesis and Biochemistry; Bentham Science Publishers: Sharjah, United Arab Emirates, 2014; p. 563. [Google Scholar]
- Tiekink, E.R.T. Therapeutic potential of selenium and tellurium compounds: Opportunities yet unrealized. Dalton Trans. 2012, 41, 6390–6395. [Google Scholar] [CrossRef]
- Braga, A.L.; Rafique, J. Synthesis of biologically relevant small molecules containing selenium. Part B. Anti-infective and anticancer compounds. In Patai’s Chemistry of Functional Groups. Organic Selenium and Tellurium Compounds; Rappoport, Z., Ed.; John Wiley and Sons: Chichester, UK, 2013; Volume 4, pp. 1053–1117. [Google Scholar]
- Mugesh, G.; du Mont, W.W.; Sies, H. Chemistry of biologically important synthetic organoselenium compounds. Chem. Rev. 2001, 101, 2125–2179. [Google Scholar] [CrossRef]
- Nogueira, C.W.; Zeni, G.; Rocha, J.B.T. Organoselenium and organotellurium compounds: Toxicology and pharmacology. Chem. Rev. 2004, 104, 6255–6286. [Google Scholar] [CrossRef]
- Azad, G.K.; Tomar, R.S. Ebselen, a promising antioxidant drug: Mechanisms of action and targets of biological pathways. Mol. Biol. Rep. 2014, 41, 4865–4879. [Google Scholar] [CrossRef]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef]
- Weglarz-Tomczak, E.; Tomczak, J.M.; Talma, M.; Burda-Grabowska, M.; Giurg, M.; Brul, S. Identification of ebselen and its analogues as potent covalent inhibitors of papain-like protease from SARS-CoV-2. Sci. Rep. 2021, 11, 3640. [Google Scholar] [CrossRef]
- Seliman, A.A.A.; Altaf, M.; Onawole, A.T.; Ahmad, S.; Ahmed, M.Y.; Al-Saadi, A.A.; Altuwaijri, S.; Bhatia, G.; Singh, J.; Isab, A.A. Synthesis, X-ray structures and anticancer activity of gold(I)-carbene complexes with selenones as co-ligands and their molecular docking studies with thioredoxin reductase. J. Organomet. Chem. 2017, 848, 175–183. [Google Scholar] [CrossRef]
- Al-Rubaie, A.Z.; Al-Jadaan, S.A.S.; Muslim, S.K.; Saeed, E.A.; Ali, E.T.; Al-Hasani, A.K.J.; Al-Salman, H.N.K.; Al-Fadal, S.A.M. Synthesis, characterization and antibacterial activity of some new ferrocenyl selenazoles and 3,5-diferrocenyl-1,2,4-selenadiazole. J. Organomet. Chem. 2014, 774, 43–47. [Google Scholar] [CrossRef]
- Dhau, J.S.; Singh, A.; Singh, A.; Sooch, B.S.; Brandão, P.; Félix, V. Synthesis and antibacterial activity of pyridylselenium compounds: Self-assembly of bis(3-bromo-2-pyridyl)diselenide via intermolecular secondary and π⋯π stacking interactions. J. Organomet. Chem. 2014, 766, 57–66. [Google Scholar] [CrossRef]
- Angeli, A.; Tanini, D.; Capperucci, A.; Supuran, C.T. Synthesis of novel selenides bearing benzenesulfonamide moieties as carbonic anhydrase I, II, IV, VII, and IX inhibitors. ASC Med. Chem. Lett. 2017, 8, 1213–1217. [Google Scholar] [CrossRef]
- Banerjee, B.; Koketsy, M. Recent developments in the synthesis of biologically relevant selenium-containing scaffolds. Coord. Chem. Rev. 2017, 339, 104–127. [Google Scholar] [CrossRef]
- Elsherbini, M.; Hamama, W.S.; Zoorob, H.H. Recent advances in the chemistry of selenium-containing heterocycles: Five-membered ring systems. Coord. Chem. Rev. 2016, 312, 149–177. [Google Scholar] [CrossRef]
- Ninomiya, M.; Garud, D.R.; Koketsu, M. Biologically significant selenium-containing heterocycles. Coord. Chem. Rev. 2011, 255, 2968–2990. [Google Scholar] [CrossRef]
- Sonawane, A.D.; Sonawane, R.A.; Ninomiya, M.N.; Koketsu, M. Synthesis of seleno-heterocycles via electrophilic/radical cyclization of alkyne containing heteroatoms. Adv. Synth. Catal. 2020, 362, 3485–3515. [Google Scholar] [CrossRef]
- Koketsu, M.; Yang, H.; Kim, Y.M.; Ichihash, M.; Ishihara, H. Preparation of 1,4-Oxaselenin from AgNO3/LDA-Assisted Reaction of 3-Selena-4-pentyn-1-one as Potential Antitumor Agents. Organic Lett. 2001, 3, 1705–1707. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.B.; Babu, N.C.; Padmavathi, V.; Padmaja, A. 2-Arylethenyl-2’-Arylethynyl Sulfones: A Potential Source for New Heterocycles. Phosphorus, Sulfur, Silicon Relat. Elements 2000, 165, 237–242. [Google Scholar] [CrossRef]
- Nowak, M.; Pluta, K.; Suwinska, K. Synthesis of novel heteropentacenes containing nitrogen, sulfur and oxygen or selenium. New J. Chem. 2002, 26, 1216–1220. [Google Scholar] [CrossRef]
- Takimiya, K.; Takamori, A.; Aso, Y.; Otsubo, T.; Kawamoto, T.; Mori, T. Organic superconductors based on a new electron donor, methylenedithio-diselenadithiafulvalene (MDT-ST). Chem. Mater. 2003, 15, 1225–1227. [Google Scholar] [CrossRef]
- Takimiya, K.; Kodani, M.; Niihara, N.; Aso, Y.; Otsubo, T.; Bando, Y.; Kawamoto, T.; Mori, T. Pressure-induced superconductivity in (MDT-TS)(AuI2)0.441 [MDT-TS) = 5H-2-(1,3-diselenol-2-ylidene)-1,3,4,6-tetrathiapentalene]: A new organic superconductor possessing an incommensurate anion lattice. Chem. Mater. 2004, 16, 5120–5123. [Google Scholar] [CrossRef]
- Ashizawa, M.; Yamamoto, H.M.; Nakao, A.; Kato, R. The first methyl antimony linked dimeric tetrathiafulvalene and tetraselenafulvalenes. Tetrahedron. Lett. 2006, 47, 8937–8941. [Google Scholar] [CrossRef]
- Imakubo, T.; Shirahata, T.; Kibune, M.; Yoshino, H. Hybrid organic/inorganic supramolecular conductors D2[Au(CN)4] [D = Diiodo(ethylenedichalcogeno)tetrachalcogenofulvalene], including a new ambient pressure superconductor. Eur. J. Inorg. Chem. 2007, 2007, 4727–4735. [Google Scholar] [CrossRef]
- Ohki, D.; Yoshimi, K.; Kobayashi, A. Interaction-induced quantum spin Hall insulator in the organic Dirac electron system α-(BEDT-TSeF)2I3. Phys. Rev. B 2022, 105, 205123. [Google Scholar] [CrossRef]
- Ashizawa, M.; Akutsu, A.; Noda, B.; Nii, H.; Kawamoto, T.; Mori, T.; Nakayashiki, T.; Misaki, Y.; Tanaka, K.; Takimiya, K.; et al. Synthesis and structures of highly conducting charge-transfer salts of selenium containing TTM-TTP derivatives. Bull. Chem. Soc. Jpn. 2004, 77, 1449–1458. [Google Scholar] [CrossRef]
- Ashizawa, M.; Nakao, A.; Yamamoto, H.M.; Kato, R. Development of the first methyl antimony bridged tetrachalcogenafulvalene systems. J. Low Temp. Phys. 2006, 142, 449–452. [Google Scholar] [CrossRef]
- Mori, T. Organic conductors with unusual band fillings. Chem. Rev. 2004, 104, 4947–4969. [Google Scholar] [CrossRef]
- Yamada, J.-i.; Akutsu, H. New trends in the synthesis of π -electron donors for molecular conductors and superconductors. Chem. Rev. 2004, 104, 5057–5083. [Google Scholar] [CrossRef]
- Fabre, J.M. Synthesis strategies and chemistry of nonsymmetrically substituted tetrachalcogenafulvalenes. Chem. Rev. 2004, 104, 5133–5150. [Google Scholar] [CrossRef]
- Potapov, V.A.; Amosova, S.V. New methods for preparation of organoselenium and organotellurium compounds from elemental chalcogens. Russ. J. Org. Chem. 2003, 39, 1373–1380. [Google Scholar] [CrossRef]
- Abakumov, G.A.; Piskunov, A.V.; Cherkasov, V.K.; Fedushkin, I.L.; Ananikov, V.P.; Eremin, D.B.; Gordeev, E.G.; Beletskaya, I.P.; Averin, A.D.; Bochkarev, M.N.; et al. Organoelement chemistry: Promising growth areas and challenges. Russ. Chem. Rev. 2018, 87, 393–507. [Google Scholar] [CrossRef]
- Woollins, J.D.; Laitinen, R.S. (Eds.) Selenium and Tellurium Chemistry. From Small Molecules to Biomolecules and Materials; Springer: Heidelberg, Germany, 2011; p. 334. [Google Scholar]
- Potapov, V.A.; Musalov, M.V.; Musalova, M.V.; Amosova, S.V. Recent Advances in Organochalcogen Synthesis Based on Reactions of Chalcogen Halides with Alkynes and Alkenes. Curr. Org. Chem. 2016, 20, 136–145. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A. Selenium dihalides: New possibilities for the synthesis of selenium-containing heterocycles. Chem. Heterocycl. Comp. 2017, 53, 150–152. [Google Scholar] [CrossRef]
- Amosova, S.V.; Penzik, M.V.; Albanov, A.I.; Potapov, V.A. Addition of selenium dibromide to divinyl sulfide: Spontaneous rearrangement of 2,6-dibromo-1,4-thiaselenane to 5-bromo-2-bromomethyl-1,3-thiaselenolane. Tetrahedron Lett. 2009, 50, 306–308. [Google Scholar] [CrossRef]
- Amosova, S.V.; Filippov, A.A.; Makhaeva, N.A.; Albanov, A.I.; Potapov, V.A. Regio- and stereoselective synthesis of new ensembles of diversely functionalized 1,3-thiaselenol-2-ylmethyl selenides by a double rearrangement reaction. Beilstein J. Org. Chem. 2020, 16, 515–523. [Google Scholar] [CrossRef]
- Potapov, V.A.; Amosova, S.V.; Volkova, K.A.; Penzik, M.V.; Albanov, A.I. Reactions of selenium dichloride and dibromide with divinyl selenide: Synthesis of novel selenium heterocycles and rearrangement of 2,6-dihalo-1,4-diselenanes. Tetrahedron Lett. 2010, 51, 89–92. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalov, M.V.; Musalova, M.V.; Rusakov, Y.Y.; Khabibulina, A.G.; Rusakova, I.L.; Amosova, S.V. Stereoselective synthesis of E-2-halovinyl tellanes, ditellanes and selenides based on tellurium tetrahalides, selenium dihalides and internal alkynes. J. Organomet. Chem. 2018, 867, 300–305. [Google Scholar] [CrossRef]
- Musalov, M.V.; Yakimov, V.A.; Potapov, V.A.; Amosova, S.V.; Borodina, T.N.; Zinchenko, S.V. A novel methodology for the synthesis of condensed selenium heterocycles based on the annulation and annulation–methoxylation reactions of selenium dihalides. New J. Chem. 2019, 43, 18476–18483. [Google Scholar] [CrossRef]
- Potapov, V.A.; Amosova, S.V.; Abramova, E.V.; Musalov, M.V.; Lyssenko, K.A.; Finn, M.G. 2,6-Dihalo-9-selenabicyclo[3.3.1]nonanes and their complexes with selenium dihalides: Synthesis and structural characterisation. New J. Chem. 2015, 39, 8055–8059. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalov, M.V.; Kurkutov, E.O.; Yakimov, V.A.; Khabibulina, A.G.; Musalova, M.V.; Amosova, S.V.; Borodina, T.N.; Albanov, A.I. Remarkable alkene-to-alkene and alkene-to-alkyne transfer reactions of selenium dibromide and PhSeBr. Stereoselective addition of selenium dihalides to cycloalkenes. Molecules 2020, 25, 194. [Google Scholar] [CrossRef]
- Braverman, S.; Cherkinsky, M.; Kalendar, Y.; Jana, R.; Sprecher, M.; Goldberg, I. Synthesis of water-soluble vinyl selenides and their high glutathione peroxidase (GPx)-like antioxidant activity. Synthesis 2014, 46, 119–125. [Google Scholar] [CrossRef]
- Sarbu, L.G.; Hopf, H.; Jones, P.G.; Birsa, L.M. Selenium halide-induced bridge formation in [2.2]paracyclophanes. Beilstein J. Org. Chem. 2014, 10, 2550–2555. [Google Scholar] [CrossRef]
- Arsenyan, P. A simple method for the preparation of selenopheno[3,2-b] and [2,3-b]thiophenes. Tetrahedron Lett. 2014, 55, 2527–2529. [Google Scholar] [CrossRef]
- Arsenyan, P.; Petrenko, A.; Belyakov, S. Improved conditions for the synthesis and transformations of aminomethyl selenophenothiophenes. Tetrahedron 2015, 71, 2226–2233. [Google Scholar] [CrossRef]
- Volkova, Y.M.; Makarov, A.Y.; Zikirin, S.B.; Genaev, A.M.; Bagryanskaya, I.Y.; Zibarev, A.V. 3,1,2,4-Benzothiaselenadiazine and related heterocycles. Mendeleev Commun. 2017, 27, 19–22. [Google Scholar] [CrossRef]
- Amosova, S.V.; Rykunova, Y.I.; Filippov, A.S.; Penzik, M.V.; Makhaeva, N.A.; Albanov, A.I.; Potapov, V.A. Cascade regio- and stereoselective reactions of 2-bromomethyl-1,3-thiaselenole with water and ethylene glycol: En roote to the first representatives of polyfunctional 2,3-dihydro-1,4-thiaselenines. J. Organomet. Chem. 2018, 867, 398–403. [Google Scholar] [CrossRef]
- Amosova, S.V.; Penzik, M.V.; Potapov, V.A.; Filippov, A.S.; Shagun, V.A.; Albanov, A.I.; Borodina, T.N.; Smirnov, V.I. Unexpected Regioselective Reactions of 2-Bromomethyl-1,3-thiaselenole with Dithiocarbamates: The First Example of nucleophilic attack at selenium atom of seleniranium intermediate. Synlett 2016, 27, 1653–1658. [Google Scholar] [CrossRef]
- Amosova, S.V.; Filippov, A.S.; Potapov, V.A.; Penzik M., V.; Albanov, A.I. Unexpected Reaction of 2-Bromomethyl-1,3-thiaselenole with Formation of Bis[(Z)-2-(vinylsulfanyl)ethenyl] Diselenide. Russ. J. Org. Chem. 2017, 53, 1878–1880. [Google Scholar] [CrossRef]
- Amosova, S.V.; Novokshonova, I.A.; Penzik, M.V.; Filippov, A.S.; Albanov, A.I.; Potapov, V.A. Reaction of 2-bromomethyl-1,3-thiaselenole with thiourea: En route to the first representatives of 2-(organylsulfanyl)-2,3-dihydro-1,4-thiaselenines. Tetrahedron Lett. 2017, 58, 4381–4383. [Google Scholar] [CrossRef]
- Amosova, S.V.; Filippov, A.S.; Makhaeva, N.A.; Albanov, A.I.; Potapov, V.A. New methodology of nucleophilic substitution at three different centers of a seleniranium intermediate in reactions of 2-bromomethyl-1,3-thiaselenole with mercapto benzazoles. New. J. Chem. 2019, 43, 11189–11199. [Google Scholar] [CrossRef]
- Amosova, S.V.; Filippov, A.S.; Potapov, V.A.; Penzik, M.V.; Makhaeva, N.A.; Albanov, A.I. Regio- and stereoselective synthesis of a novel family of unsaturated compounds with the S–Se bond and their cyclization to 2,3-dihydro-1,4-thiaselenines. Synthesis 2019, 8, 1832–1840. [Google Scholar] [CrossRef]
- Amosova, S.V.; Shagun, V.A.; Makhaeva, N.A.; Novokshonova, A.I.; Potapov, V.A. Quantum Chemical and Experimental Studies of an Unprecedented Reaction Pathway of Nucleophilic Substitution of 2-Bromomethyl-1,3-thiaselenole with 1,3-Benzothiazole-2-thiol Proceeding Stepwise at Three Different Centers of Seleniranium Intermediates. Molecules. 2021, 26, 6685. [Google Scholar] [CrossRef]
- Accurso, A.A.; Cho, S.-H.; Amin, A.; Potapov, V.A.; Amosova, S.V.; Finn, M.G. Thia-, aza-, and selena[3.3.1]bicyclononane dichlorides: Rates vs internal nucleophile in anchimeric assistance. J. Org. Chem. 2011, 76, 4392–4395. [Google Scholar] [CrossRef] [PubMed]
- Amosova, S.V.; Penzik, M.V.; Potapov, V.A.; Albanov, A.I. Unexpected reaction of 2-(bromomethyl)-1,3-thiaselenole with ammonium thiocyanate. Russ. J. Org. Chem. 2015, 51, 287–289. [Google Scholar] [CrossRef]
- Potapov, V.A.; Filippov, A.S.; Amosova, S.V. Selective Synthesis of 1,3-Thiaselenol-2-ylmethyl Selenocyanate. Russ. J. Org. Chem. 2018, 54, 957–958. [Google Scholar] [CrossRef]
- Sukhai, R.S.; Jong, R.; Verkruijsse, H.D.; Brandsma, L. Synthesis of six- and seven-membered heterocycles by reaction of 1-(2-chloroethylthio)-1-alkynes and 1-(3-chloropropylthio)-1-alkynes with alkali metal sulfide, selenide and telluride. Recueil 1981, 100, 368–372. [Google Scholar] [CrossRef]
- Amosova, S.V.; Penzik, M.V.; Potapov, V.A.; Albanov, A.I. A rearrangement in the reaction of 2-bromomethyl-1,3-thiaselenole with ethanol: Synthesis of 2-ethoxy-2,3-dihydro-1,4-thiaselenine. Russ. Chem. Bull. 2011, 60, 766. [Google Scholar] [CrossRef][Green Version]
- Perin, G.; Lenardão, E.J.; Jacob, R.G.; Panatieri, R.B. Synthesis of Vinyl Selenides. Chem. Rev. 2009, 109, 1277–1301. [Google Scholar] [CrossRef]
- Menezes, P.H.; Zeni, G. Vinyl Selenides. In Patai’s Chemistry of Functional Groups. Organic Selenium and Tellurium Compounds; Rappoport, Z., Ed.; John Wiley and Sons: Chichester, UK, 2012. [Google Scholar] [CrossRef]
- Duddeck, H. Selenium-77 nuclear magnetic resonance spectroscopy. Prog. Nucl. Magn. Reson. Spectrosc. 1995, 27, 1–323. [Google Scholar] [CrossRef]
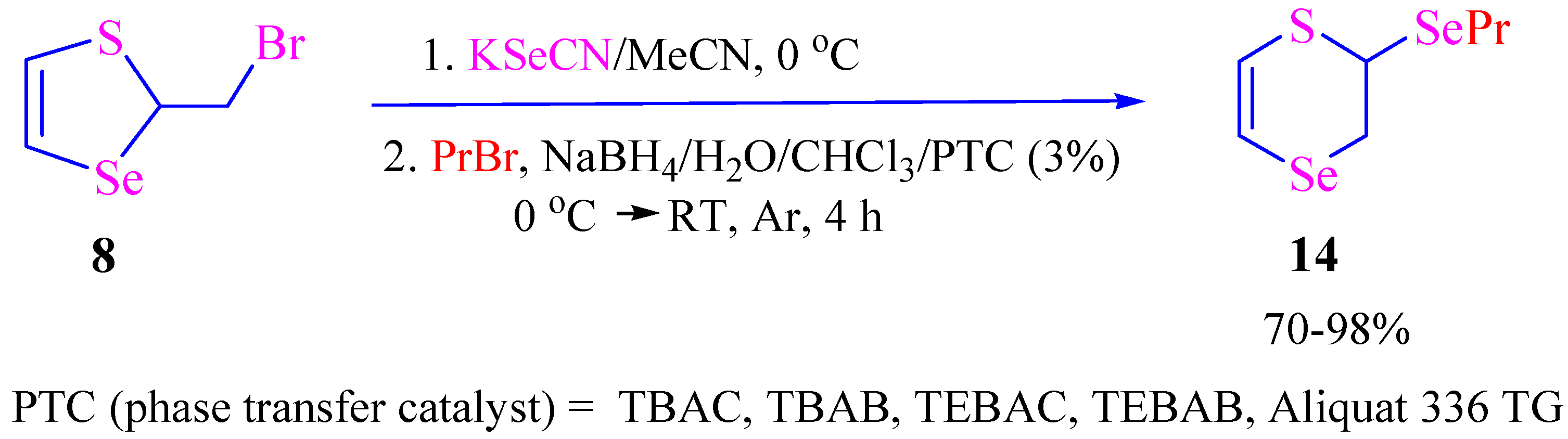
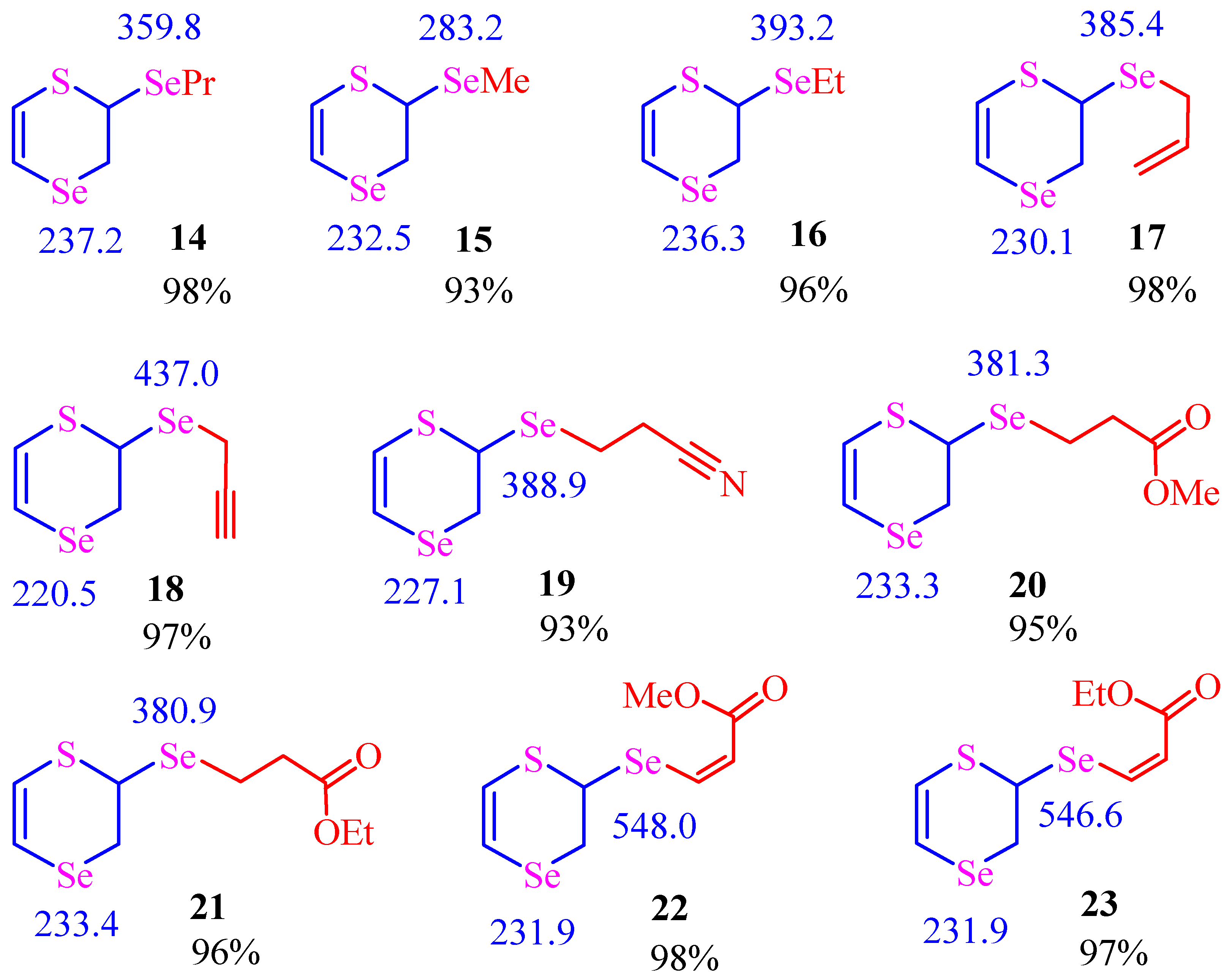
 | |||||
|---|---|---|---|---|---|
| Runs | Starting Compound | Duration (h) | TBAB (mol%) | Product | Yield (%) |
| 1 | Propyl bromide | 4 | 3 | 14 | 98% |
| 2 | Methyl iodide | 4 | 3 | 15 | 93% |
| 3 | Ethyl bromide | 4 | 3 | 16 | 96% |
| 4 | Allyl bromide | 3 | 3 | 17 | 98% |
| 5 | Propargyl bromide | 3 | 3 | 18 | 97% |
| 6 | Acrylonitrile | 6 | 6 | 19 | 93% |
| 7 | Methyl acrylate | 5 | 6 | 20 | 95% |
| 8 | Ethyl acrylate | 5 | 6 | 21 | 96% |
| 9 | Methyl propiolate | 4 | 6 | 22 | 98% |
| 10 | Ethyl propiolate | 4 | 6 | 23 | 97% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amosova, S.V.; Filippov, A.S.; Potapov, V.A.; Albanov, A.I. Regio- and Stereoselective One-Pot Synthesis of New Heterocyclic Compounds with Two Selenium Atoms Based on 2-Bromomethyl-1,3-thiaselenole Using Phase Transfer Catalysis. Catalysts 2022, 12, 1236. https://doi.org/10.3390/catal12101236
Amosova SV, Filippov AS, Potapov VA, Albanov AI. Regio- and Stereoselective One-Pot Synthesis of New Heterocyclic Compounds with Two Selenium Atoms Based on 2-Bromomethyl-1,3-thiaselenole Using Phase Transfer Catalysis. Catalysts. 2022; 12(10):1236. https://doi.org/10.3390/catal12101236
Chicago/Turabian StyleAmosova, Svetlana V., Andrey S. Filippov, Vladimir A. Potapov, and Alexander I. Albanov. 2022. "Regio- and Stereoselective One-Pot Synthesis of New Heterocyclic Compounds with Two Selenium Atoms Based on 2-Bromomethyl-1,3-thiaselenole Using Phase Transfer Catalysis" Catalysts 12, no. 10: 1236. https://doi.org/10.3390/catal12101236
APA StyleAmosova, S. V., Filippov, A. S., Potapov, V. A., & Albanov, A. I. (2022). Regio- and Stereoselective One-Pot Synthesis of New Heterocyclic Compounds with Two Selenium Atoms Based on 2-Bromomethyl-1,3-thiaselenole Using Phase Transfer Catalysis. Catalysts, 12(10), 1236. https://doi.org/10.3390/catal12101236







