Semiconductors Application Forms and Doping Benefits to Wastewater Treatment: A Comparison of TiO2, WO3, and g-C3N4
Abstract
1. Introduction
2. General Features of Catalysts for Photo-based Treatment Processes
3. Catalyst Doping
3.1. TiO2
3.1.1. Transition Metals Doping
| Catalyst | Precursor | Method | Ebg (eV) | Radiation Source | Contaminant | Removal (Time) | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Ti | Dopant | |||||||
| Ag-TiO2 | - | Silver Nitrate | Precipitation | 3.65 | UV | Rhodamine B, 5 mg L−1 | 97% (20 min) | [41] |
| - | Silver Nitrate | Ion-exchange | - | UV-Visible | Methyl Orange, 1 mg L−1 | 80% (150 min) | [42] | |
| S. aureus, 106 CFU mL−1 | 100% (2 h) | |||||||
| E. coli, 106 CFU mL−1 | 100% (1 h) | |||||||
| Au-TiO2 | TBOT | Gold (III) Chloride | Solvothermal | 3.70 | UV-Visible | Methylene Blue and Diuron 0.03 mM each | 65% MB (180 min) and 95% DIU (120 min) | [43] |
| Ce-TiO2 | - | Cerium Nitrate | EDTA-Citrate | 2.50 | Solar | Ciprofloxacin and Norfloxacin, 10 mg L−1 each | 93.2% CPR and 93.6% NOR (180 min) | [44] |
| E. coli, 108 CFU mL−1 | 95.0% (180 min) | |||||||
| Cu-TiO2 | TTIP | Copper Nitrate | Sol-gel | 3.70 | Visible | Methylene Blue, 0.05 M | 27.5% (60 min) | [38] |
| TBOT | Copper Nitrate | Sol-gel and Ion-exchange | 2.38 | Visible | Phenol, 5 mg L−1 | 100% (4 h) | [39] | |
| Eu-TiO2 | TTIP | Europium Oxide | Sol-gel | 2.86 | Visible | Methylene Blue and Methyl Orange, 5 mg L−1 | 72.1% MB and 71.8% MO (180 min) | [45] |
| Fe-TiO2 | TTIP | Iron Acetylacetonate | Sol-gel | 2.80 | Visible | Acid Orange Azo Dye, 10 mg L−1 | 80% (60 min) | [46] |
| TEOT | Iron Nitrate | Sol-gel | - | UV-Visible | Nitrobenzene, 2.45 × 10−4 M | 97.3% (240 min) | [47] | |
| La-TiO2 | TTIP | Lanthanum Nitrate | Electrospinning | 2.68 | Visible | Ciprofloxacin and Methylene Blue, 10 mg L−1 each | 91% MB and 99.5% CIP (300 min) | [48] |
| Mn-TiO2 | TTIP | Manganese Acetate | Microwave- assisted Hydrothermal | 1.65 | UV | Prozac®, 10 mg L−1 | 95% (30 min) | [40] |
| Pr-TiO2 | TTIP | Praseodymium Nitrate | Sol-gel | 3.00 | Visible | Acid Orange Azo Dye, 10 mg L−1 | 53% (60 min) | [46] |
| Zn-TiO2 | TTIP | Zinc Nitrate | Sol-gel | 2.83 | Visible | Methylene Blue, 0.05 M | 99.6 (60 min) | [38] |
| Zr-TiO2 | TTIP | Zirconium Nitrate | Sol-gel | 3.30 | Visible | Methylene Blue, 0.05 M | 81.9% (60 min) | [38] |
3.1.2. Noble and Rare-Earth Metals Doping
3.1.3. Non-Metals Doping
| Catalyst | Precursor | Method | Ebg (eV) | Radiation Source | Contaminant | Removal (Time) | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Ti | Dopant | |||||||
| B-TiO2 | * P25 | Boric Acid | EDTA-Citrate | 2.87 | Solar | Ciprofloxacin and Norfloxacin, 10 mg L−1 | 93.2% CPR and 93.0% NOR (180 min) | [44] |
| E. coli, 108 CFU mL−1 | 99.9% (180 min) | |||||||
| TBOT | Boric Acid | Sol-gel | 3.01 | Visible | Catechol, 10 mg L−1 | 100% (60 min) | [75] | |
| TTIP | Boric Acid | Sol-gel | 2.98 | UV | Diclofenac, 50 mg L−1 | 98% (180 min) | [67] | |
| F-TiO2 | TiCl4 | Ammonium Fluoride | Nebulizer Spray | 2.79 | Visible | Malachite Green, 300 mg L−1 | 90% (60 min) | [76] |
| I-TiO2 | TBOT | Iodic Acid | Sol-gel | 3.18 | Solar | Methylene Blue, 4.8 mg L−1 | 30% (60 min) | [77] |
| N-TiO2 | TTIP | Urea | Sol-gel | 2.27 | UV | Diclofenac, 50 mg L−1 | 95% (180 min) | [67] |
| TBOT | Guanidinium Chloride | Sol-gel | 2.91 | Visible | Methylene Blue, 10 mg L−1 | 97% (100 min) | [71] | |
| TTIP | Urea | Sol-gel | - | Solar | Methylene Blue, 10 mg L−1 | 99% (100 min) | [68] | |
| TTIP | N,N’-dimethyl urea | Sol-gel | - | 98% (120 min) | ||||
| TTIP | Semicarbazide | Sol-gel | - | 98% (80 min) | ||||
| TBOT | Urea | Sol-gel | 2.58 | Visible | Microcystis aeruginosa, 3 × 106 cells mL−1 | 99.1% (12 h) | [78] | |
| TTIP | Ammonium Hydroxide | Sol-gel | 2.31 | Visible | E. coli, 105 CFU mL−1 | ~100% (12 h) | [79] | |
| S. aureus, 105 CFU mL−1 | ~100% (12 h) | |||||||
| Mycobacterium avium, 105 CFU mL−1 | ~100% (12 h) | |||||||
| Candida albicans, 105 CFU mL−1 | 99.9% (12 h) | |||||||
| S-TiO2 | TTIP | Sulfuric Acid | Flame Spray Pyrolysis | 2.78 | Visible | Acetaldehyde, 0.5 mM | 65% (300 min) | [80] |
| TTIP | Hydrogen Sulfide | Chemical Vapor Deposition | - | Visible | Methyl Orange, 5 mg L−1 | 38% (300 min) | [81] | |
3.1.4. Co-Doping
3.2. WO3
3.2.1. Transition Metals Doping
3.2.2. Noble and Rare-Earth Metals Doping
3.2.3. Non-Metals Doping
3.2.4. Co-Doping
3.3. g-C3N4
3.3.1. Metals Doping
3.3.2. Noble and Rare-Earth Metals Doping
| Catalyst | Precursor | Method | Ebg (eV) | Radiation Source | Contaminant | Removal (Time) | Ref. | |
|---|---|---|---|---|---|---|---|---|
| C3N4 | Dopant | |||||||
| Ag-g-C3N4 | Melamine and Urea | Silver Nitrate | Thermal Polymerization and Ion-exchange | 2.19 | Solar | Tetracycline, 20 mg L−1 | 96.8% (120 min) | [107] |
| Melamine | Silver Nitrate | Thermal Polymerization | 2.46 | Visible | Oxytetracycline, 10 mg L−1 and | 98.6% (120 min) | [110] | |
| Antibiotics Wastewater: Oxytetracycline, 101.5 mg L−1; Tetracycline, 85.3 mg L−1; Gatifloxacin, 89.4 mg L−1 | 94.5% OTC, 81.8% TC, 67.3% GFA (120 min) | |||||||
| Au-g-C3N4 | Melamine | Au NPs | Thermal Polymerization | 2.86 | Visible | Arsenazo, 4 mg L−1 | 100% (60 min) | [108] |
| Co-g-C3N4 | Melamine | Cobalt Nitrate | Thermal Polymerization | 2.38 | Visible | Paracetamol, 10 mg L−1 | 96.3% (120 min) | [104] |
| Er-g-C3N4 | Melamine | Europium Nitrate | Thermal Polymerization | 2.50 | Solar | Tetracycline, 25 mg L−1 | 85% (90 min) | [109] |
| Tylosin, 25 mg L−1 | 70% (90 min) | |||||||
| Rhodamine B, 5 mg L−1 | 90% (30 min) | |||||||
| Cyanuric Acid and Melamine | Europium Chloride | Thermal Polymerization | 2.70 | Visible | Rhodamine B, 10 µg L−1 | 100% (90 min) | [111] | |
| Fe-g-C3N4 | Melamine | Iron Nitrate | Thermal Polymerization | 2.73 | Visible | Rhodamine B, 10 mg L−1 | 100% (150 min) | [103] |
| K-g-C3N4 | Melamine | Potassium Chloride | Thermal Polymerization and Ion-exchange | 2.50 | Solar | Phenolic Effluent, Ph = 980 mg L−1 and COD = 6300 mg L−1 | 56.5% (300 min) | [106] |
| Thiourea | Potassium Bromide | Thermal Polymerization | 2.15 | Visible | Nitric Oxide, 600 ppb | 36.8% (30 min) | [112] | |
| Ni-g-C3N4 | Melamine | Nickel Nitrate | Thermal Polymerization | 2.25 | Solar | Toluene and Nitrobenzene, 5 mg L−1 | 85.8% TOL 98.6% NBZ (120 min) | [105] |
| V-g-C3N4 | Melamine | Ammonium Metavanadate | Thermal Polymerization | 2.63 | Solar | Tetracycline, 10 mg L−1 | 81.9% (240 min) | [113] |
3.3.3. Non-Metals Doping
| Catalyst | Precursor | Method | Ebg (eV) | Radiation Source | Contaminant | Removal (Time) | Ref. | |
|---|---|---|---|---|---|---|---|---|
| C3N4 | Dopant | |||||||
| B-P-g-C3N4 | Melamine | Boric and Phosphoric Acid | Thermal Polymerization and Hydrothermal | 2.66 | Visible | Diclofenac, 10 mg L−1 | 100% (90 min) | [119] |
| Cl-g-C3N4 | Dicyandiamide | Cyanuric Chloride | Solvothermal | 1.78 | Visible | Rhodamine B, 10 mg L−1 | 99.6% (125 min) | [120] |
| Melamine | Ammonium Chloride | Thermal Polymerization | 2.71 | Visible | Rhodamine B, 10 mg L−1 | 57.8% (30 min) | [121] | |
| O-g-C3N4 | Semicarbazide Hydrochloride | Molten-salt | 2.25 | Visible | Naproxen, 100 mg L−1 | 100% (5 h) | [122] | |
| Urea | Hydrogen Peroxide | Thermal Polymerization and Solvothermal | 2.94 | Solar | Rhodamine B, 10 mg L−1 | 95% (20 min) | [114] | |
| Methyl Orange, 10 mg L−1 | 70% (4 h) | |||||||
| Urea | Cyanuric Acid | Thermal Polymerization | 2.62 | Visible | Rhodamine B, 10 mg L−1 | 40% (120 min) | [115] | |
| Ofloxacin, 20 mg L−1 | 97% (120 min) | |||||||
| P-g-C3N4 | Melamine | Phosphoric Acid | Thermal Polymerization | - | Visible | Dinotefuran, 2 mg L−1 | 40.6% (5 h) | [123] |
| Melamine | Phosphoric Acid | Solvothermal | 1.66 | Visible | p-Hydroxybenzoic Acid, 1 mg L−1 | 77.3% (120 min) | [117] | |
| S-g-C3N4 | Melamine | Thiourea | Thermal Polymerization | 2.51 | Visible | Rhodamine B, 10 mg L−1 | 29.7% (30 min) | [121] |
| S-Cl-g-C3N4 | Melamine | Thiourea and Ammonium Chloride | Thermal Polymerization | 2.55 | Visible | Rhodamine B, 10 mg L−1 | 100% (30 min) | [121] |
| Si-g-C3N4 | Urea | Ammonium Fluorosilicate | Thermal Polymerization | 2.75 | Visible | Rhodamine B, 10 mg L−1 | 75% (50 min) | [118] |
3.3.4. Co-Doping
3.4. Overall Considerations
4. Composite Catalysts
| Composite | SBET (m2 g−1) | Ebg (eV) | Radiation Source | Contaminant | Removal (Time) | Ref. |
|---|---|---|---|---|---|---|
| g-C3N4/TiO2 | 200.0 | 2.70 | UV | Formic Acid, 50 mg L−1 | 90.0% (5.5 h) | [138] |
| 40.2 | 2.81 | Solar | Methylene Blue, 10 mg L−1 | 94.9% (80 min) | [137] | |
| Rhodamine B, 15 mg L−1 | 93.1% (80 min) | |||||
| - | - | Visible | E. coli, 107 CFU mL−1 | 100% (180 min) | [139] | |
| - | 2.58 | Solar | E. coli, 103 CFU mL−1 | 96.8% (30 min) | [140] | |
| WO3/TiO2 | 88.4 | 3.06 | Visible | Malachite Green, 50 mg L−1 | 99.0% (60 min | [131] |
| 11.7 | 2.40 | Visible | Methylene Blue, 10 mg L−1 | 87.8% (150 min) | [128] | |
| Metoprolol, 2 mg L−1 | 67.1% (150 min | |||||
| 103.9 | 2.95 | Visible | Diclofenac, 10 mg L−1 | 100% (150 min) | [126] | |
| WO3/g-C3N4 | - | - | UV-A + Visible + NIR | Ciprofloxacin, 10 mg L−1 | 98.6% (180 min) | [141] |
| Tetracycline, 10 mg L−1 | 98.5% (180 min) | |||||
| 28.6 | 2.53 | UV + Visible | Tartrazine, 25 mg L−1 | 95.0% (20 min) | [142] | |
| S-doped g-C3N4/TiO2 | - | 3.00 | Visible | Tetracycline, 10 mg L−1 | 98.1% (60 min) | [143] |
| N-doped CHS/g-C3N4/TiO2 | 78.0 | - | Visible | Tetracycline, 20 mg L−1 | 85.0% (120 min) | [144] |
| Ag3PO4/ g-C3N4/TiO2 | - | 2.07 | Visible | Metronidazole, 8.2 mg L−1 | 97.2% (60 min) | [145] |
| C-doped WO3/TiO2 | 93.0 | 2.98 | Solar | Diclofenac, 10 mg L−1 | 100% (250 min) | [133] |
| Ag-doped WO3/TiO2 | - | 3.07 | Visible | Methylene Blue, 3.2 g L−1 | 72% (60 min) | [134] |
| GO/WO3/TiO2 | - | 2.89 | Solar | Rhodamine B, 143.7 mg L−1 | 98.2% (5 h) | [146] |
| - | - | Solar | E. coli, 2 × 103 CFU mL−1 | 97.3% (80 min) | [147] | |
| CQDs/WO3/TiO2 | 96.2 | 2.61 | Solar | Cephalexin, 10 mg L−1 | 100% (90 min) | [148] |
| GO/CQDs/WO3/TiO2/SiO2 | 202.6 | - | Solar | Rhodamine B, 14.4 mg L−1 | 98% (60 min) | [135] |
| Cd-doped WO3/g-C3N4 | 8.5 | 1.53 | Visible | Methylene Blue, 10 mg L−1 | 96.0 (80 min) | [93] |
4.1. TiO2
| Composite | SBET (m2 g−1) | Ebg (eV) | Radiation Source | Contaminant | Removal (Time) | Ref. |
|---|---|---|---|---|---|---|
| ZnO/TiO2 | - | 3.15 | UV | Eriochrome Black T, 6.4 × 103 mg L−1 | 82% (6 h) | [155] |
| 84.7 | 3.15 | Solar | Methylene Blue, 6.4 mg L−1 | 95% (60 min) | [151] | |
| Methyl Orange, 6.5 mg L−1 | 99% (60 min) | |||||
| BiVO4/N-TiO2 | 92.0 | 2.56 | Visible | Ofloxacin, 20 mg L−1 | 98% (90 min) | [152] |
| Rhodamine B, 20 mg L−1 | 92% (90 min) | |||||
| Bi2O3/TiO2 | 102.9 | - | Visible | Rhodamine B, 10 mg L−1 | 100% (100 min) | [153] |
| CuO/Bi2O3/TiO2 | 83.6 | - | Visible | Rhodamine B, 10 mg L−1 | 100% (60 min) | |
| Fe2O3/TiO2 | - | 2.49 | Solar | Methylene Blue, 10 mg L−1 | 92% (180 min) | [156] |
| - | Phenol, 10 mg L−1 | 52% (180 min) | ||||
| 56.9 | 3.08 | Visible | Naproxen and Ibuprofen, 10 mg L−1 each | 100% NPX (15 min) and 91% IBF (240 min) | [157] | |
| Au/Fe2O3/TiO2 | - | 1.55 | Visible | 2,4 Dichlorophenol, 10 mg L−1 | 94% (90 min) | [158] |
| - | 1.55 | Visible | 4-Bromophenol, 10 mg L−1 | 97% (60 min) | ||
| SiO2/Fe3O4/Sn-TiO2 | - | 1.32 | UV | Tetracycline, 10 mg L−1 | 98.2% (40 min) | [159] |
| P/Ag/Ag2O/Ag3PO4/TiO2 | 307.2 | 2.98 | Visible | E.coli, 107 CFU mL−1 | 100% (20 min) | [160] |
| Salmonella, 107 CFU mL−1 | 100% (30 min) | |||||
| Enterococcus sp., 107 CFU mL−1 | 100% (6 h) | |||||
| S. aureus, 107 CFU mL−1 | 100% (3 h) | |||||
| CNT/Au-TiO2 | - | 1.95 | Solar | Methylene Blue, 3 mg L−1 | 80% (30 min) | [161] |
| GO/TiO2 | - | 3.02 | Solar | E. coli, 107 CFU mL−1 | 99.9% (30 min) | [162] |
| Chitosan/N-TiO2 | 52.0 | 2.82 | UV | Patulin, 500 µg kg−1 | 100% (35 min) | [163] |
| Perlite/F-Ce-TiO2 | 14.8 | 2.96 | Visible | Microcystis aeruginosa, 2.7 × 106 cell mL−1 | 98.1% (9 h) | [164] |
4.2. WO3
| Composite | SBET (m2 g−1) | Ebg (eV) | Radiation Source | Contaminant | Removal (Time) | Ref. |
|---|---|---|---|---|---|---|
| Ag3PO4/WO3 | 23.9 | - | Visible | Rhodamine B, 20 mg L−1 | 98% (90 min) | [170] |
| Ag3PO4/WO3·H2O | - | 2.43 | Visible | Methylene Blue, 10 mg L−1 and Tetracycline 20 mg L−1 | 98.9% MB and 70.4% (35 min) | [171] |
| Ag3PO4/NG/WO3 | - | 2.36 | Visible | Indomethacin, 5 mg L−1 | 99.3% (50 min) | [172] |
| NaNbO3/WO3 | 7.2 | 2.60 | Visible | 2,4-Dichlorophenoxyacetic acid, 10 mg L−1 | 60% (210 min) | [173] |
| Bi2S3/WO3 | 53.8 | 1.90 | Visible | Rhodamine B, 10 mg L−1 | 90.7% (100 min) | [175] |
| Ag/ZnWO4/WO3 | - | - | UV-Visible | Methylene Blue, 200 mg L−1 | 94% (80 min) | [174] |
| GO/WO3 | 18.7 | 2.32 | Visible | Rhodamine B, 20 mg L−1 | 96% (120 min) | [176] |
| Ciprofloxacin, 20 mg L−1 | 90% (120 min) | |||||
| Ag/GO/Chitosan/WO3 | 26.4 | 2.40 | Visible | Methylene Blue, 10 mg L−1 | 99% (8 min) | [177] |
| SBA-15/Ag-WO3 | 208 | 1.70 | Visible | Atrazine, 20 mg L−1 | 68% (18 min) | [178] |
4.3. g-C3N4
| Composite | Radiation Source | Contaminant | Oxidant | Removal (Time) | References |
|---|---|---|---|---|---|
| GO/g-C3N4 | Solar | Oxalic Acid, 10 mg L−1 | O2 | 49.5% (40 min) | [192] |
| O3 | 93.2% (40 min) | ||||
| - | 82% (40 min) | ||||
| SBA-15/Ag-g-C3N4 | Solar | Oxalic Acid, 10 mg L−1 | O2 | 16.8% (11 min) | [193] |
| O3 | 100% (11 min) | ||||
| - | 4% (11 min) | ||||
| Fe2O3/S-g-C3N4 | Visible | Bisphenol A, 50 mg L−1 | O2 | 41.1% TOC (3 h) | [190] |
| O3 | 97.8% TOC (3 h) | ||||
| 40.1% TOC (3 h) |
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gittins, J.R.; Hemingway, J.R.; Dajka, J.C. How a Water-Resources Crisis Highlights Social-Ecological Disconnects. Water Res. 2021, 194, 116937. [Google Scholar] [CrossRef]
- Gleick, P.H. The World’s Water; Island Press: Washington, DC, USA, 2015; Volume 8. [Google Scholar]
- McCance, W.; Jones, O.A.H.; Cendón, D.I.; Edwards, M.; Surapaneni, A.; Chadalavada, S.; Wang, S.; Currell, M. Combining Environmental Isotopes with Contaminants of Emerging Concern (CECs) to Characterise Wastewater Derived Impacts on Groundwater Quality. Water Res. 2020, 182, 1–15. [Google Scholar] [CrossRef]
- Rizzo, L.; Malato, S.; Antakyali, D.; Beretsou, V.G.; Đolić, M.B.; Gernjak, W.; Heath, E.; Ivancev-Tumbas, I.; Karaolia, P.; Lado Ribeiro, A.R.; et al. Consolidated vs New Advanced Treatment Methods for the Removal of Contaminants of Emerging Concern from Urban Wastewater. Sci. Total Environ. 2019, 655, 986–1008. [Google Scholar] [CrossRef]
- Valbonesi, P.; Pro, M.; Vasumini, I.; Fabbri, E. Contaminants of Emerging Concern in Drinking Water: Quality Assessment by Combining Chemical and Biological Analysis. Sci. Total Environ. 2021, 758, 143624. [Google Scholar] [CrossRef]
- Olalla, A.; Moreno, L.; Valcárcel, Y. Prioritisation of Emerging Contaminants in the Northern Antarctic Peninsula Based on Their Environmental Risk. Sci. Total Environ. 2020, 742, 140417. [Google Scholar] [CrossRef]
- Lencioni, V.; Bellamoli, F.; Paoli, F. Multi-Level Effects of Emerging Contaminants on Macroinvertebrates in Alpine Streams: From DNA to the Ecosystem. Ecol. Indic. 2020, 117, 106660. [Google Scholar] [CrossRef]
- Llamas-dios, M.I.; Vadillo, I.; Jiménez-gavilán, P.; Candela, L.; Corada-fernández, C. Assessment of a Wide Array of Contaminants of Emerging Concern in a Mediterranean Water Basin (Guadalhorce River, Spain): Motivations for an Improvement of Water Management and Pollutants Surveillance. Sci. Total Environ. 2021, 788, 147822. [Google Scholar] [CrossRef]
- Tian, Z.; Wark, D.A.; Bogue, K.; James, C.A. Suspect and Non-Target Screening of Contaminants of Emerging Concern in Streams in Agricultural Watersheds. Sci. Total Environ. 2021, 795, 148826. [Google Scholar] [CrossRef]
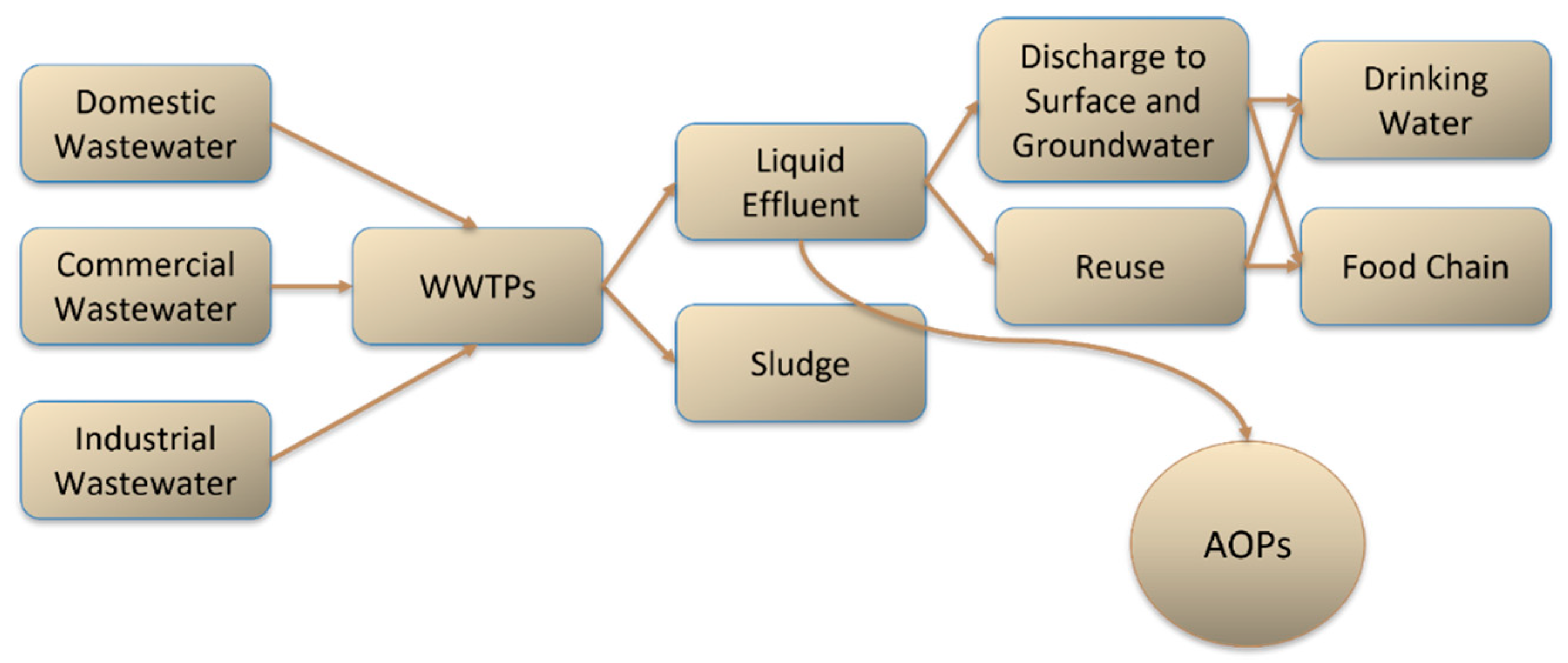
- Shah, A.I.; Din Dar, M.U.; Bhat, R.A.; Singh, J.P.; Singh, K.; Bhat, S.A. Prospectives and Challenges of Wastewater Treatment Technologies to Combat Contaminants of Emerging Concerns. Ecol. Eng. 2020, 152, 105882. [Google Scholar] [CrossRef]
- Liu, Z.; Demeestere, K.; Hulle, S. Van Comparison and Performance Assessment of Ozone-Based AOPs in View of Trace Organic Contaminants Abatement in Water and Wastewater: A Review. J. Environ. Chem. Eng. 2021, 9, 105599. [Google Scholar] [CrossRef]
- Giwa, A.; Yusuf, A.; Balogun, H.A.; Sambudi, N.S.; Bilad, M.R.; Adeyemi, I.; Chakraborty, S.; Curcio, S. Recent Advances in Advanced Oxidation Processes for Removal of Contaminants from Water: A Comprehensive Review. Process Saf. Environ. Prot. 2021, 146, 220–256. [Google Scholar] [CrossRef]
- Sgroi, M.; Snyder, S.; Roccaro, P. Comparison of AOPs at Pilot Scale: Energy Costs for Micro-Pollutants Oxidation, Disinfection by-Products Formation and Pathogens Inactivation. Chemosphere 2021, 273, 128527. [Google Scholar] [CrossRef]
- Khan, J.A.; Sayed, M.; Khan, S.; Shah, N.S.; Dionysiou, D.D.; Boczkaj, G. Advanced Oxidation Processes for the Treatment of Contaminants of Emerging Concern. In Contaminants of Emerging Concern in Water and Wastewater: Advanced Treatment Processes; Elsevier Inc.: Cambridge, MA, USA, 2019; pp. 299–365. ISBN 9780128135617. [Google Scholar]
- Itzel, F.; Gehrmann, L.; Bielak, H.; Ebersbach, P.; Boergers, A.; Herbst, H.; Maus, C.; Simon, A.; Dopp, E.; Hammers-Wirtz, M.; et al. Investigation of Full-Scale Ozonation at a Municipal Wastewater Treatment Plant Using a Toxicity-Based Evaluation Concept. J. Toxicol. Environ. Health—Part A Curr. Issues 2017, 80, 1242–1258. [Google Scholar] [CrossRef] [PubMed]
- Irani, R.; Khoshfetrat, A.B.; Forouzesh, M. Real Municipal Wastewater Treatment Using Simultaneous Pre and Post-Ozonation Combined Biological Attached Growth Reactor: Energy Consumption Assessment. J. Environ. Chem. Eng. 2021, 9, 104595. [Google Scholar] [CrossRef]
- Gulde, R.; Rutsch, M.; Clerc, B.; Schollée, J.E.; von Gunten, U.; McArdell, C.S. Formation of Transformation Products during Ozonation of Secondary Wastewater Effluent and Their Fate in Post-Treatment: From Laboratory- to Full-Scale. Water Res. 2021, 200, 117200. [Google Scholar] [CrossRef]
- Fernandes, E.; Contreras, S.; Medina, F.; Martins, R.C.; Gomes, J. N-Doped Titanium Dioxide for Mixture of Parabens Degradation Based on Ozone Action and Toxicity Evaluation: Precursor of Nitrogen and Titanium Effect. Process Saf. Environ. Prot. 2020, 138, 80–89. [Google Scholar] [CrossRef]
- Sgroi, M.; Anumol, T.; Vagliasindi, F.G.A.; Snyder, S.A.; Roccaro, P. Comparison of the New Cl2/O3/UV Process with Different Ozone- and UV-Based AOPs for Wastewater Treatment at Pilot Scale: Removal of Pharmaceuticals and Changes in Fluorescing Organic Matter. Sci. Total Environ. 2021, 765, 142720. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.Q.; Jothinathan, L.; Deng, S.H.; Ong, S.L.; Ng, H.Y.; Hu, J.Y. Fenton- and Ozone-Based AOP Processes for Industrial Effluent Treatment; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9780128210116. [Google Scholar]
- Fernandes, E.; Martins, R.C.; Gomes, J. Photocatalytic Ozonation of Parabens Mixture Using 10% N-TiO2 and the Effect of Water Matrix. Sci. Total Environ. 2020, 718, 137321. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Xi, X.; Hou, G. Typical Non–TiO2-Based Visible-Light Photocatalysts. In Semiconductor Photocatalysis—Materials, Mechanisms and Applications; BoD–Books on Demand: Norderstedt, Germany, 2016; pp. 209–229. [Google Scholar]
- Barakat, M.A.; Kumar, R. Photocatalytic Activity Enhancement of Titanium Dioxide Nanoparticles; Springer: Cham, Switzerland, 2016; ISBN 9783319242699. [Google Scholar]
- Gomes, J.; Roccamante, M.; Contreras, S.; Medina, F.; Oller, I.; Martins, R.C. Scale-up Impact over Solar Photocatalytic Ozonation with Benchmark-P25 and N-TiO2 for Insecticides Abatement in Water. J. Environ. Chem. Eng. 2021, 9, 104915. [Google Scholar] [CrossRef]
- Huang, F.; Yan, A.; Zhao, H. Influences of Doping on Photocatalytic Properties of TiO2 Photocatalyst. In Semiconductor Photocatalysis: Materials, Mechanisms and Applications; InTech: Osaka, Japan, 2016; pp. 31–80. [Google Scholar]
- García-López, E.I.; Palmisano, L. (Eds.) Material Science in Photocatalysis; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9781119130536. [Google Scholar]
- Nguyen, T.K.A.; Pham, T.T.; Nguyen-Phu, H.; Shin, E.W. The Effect of Graphitic Carbon Nitride Precursors on the Photocatalytic Dye Degradation of Water-Dispersible Graphitic Carbon Nitride Photocatalysts. Appl. Surf. Sci. 2021, 537, 148027. [Google Scholar] [CrossRef]
- Fernandes, R.A.; Sampaio, M.J.; Dražić, G.; Faria, J.L.; Silva, C.G. Efficient Removal of Parabens from Real Water Matrices by a Metal-Free Carbon Nitride Photocatalyst. Sci. Total Environ. 2020, 716, 135346. [Google Scholar] [CrossRef] [PubMed]
- Orge, C.A.; Sampaio, M.J.; Faria, J.L.; Pereira, M.F.R.; Silva, C.G. Efficiency and Stability of Metal-Free Carbon Nitride in the Photocatalytic Ozonation of Oxamic Acid under Visible Light. J. Environ. Chem. Eng. 2020, 8, 104172. [Google Scholar] [CrossRef]
- Liu, H.; Ma, S.; Shao, L.; Liu, H.; Gao, Q.; Li, B.; Fu, H.; Fu, S.; Ye, H.; Zhao, F.; et al. Defective Engineering in Graphitic Carbon Nitride Nanosheet for Efficient Photocatalytic Pathogenic Bacteria Disinfection. Appl. Catal. B Environ. 2020, 261, 118201. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Shuai, D.; Naraginti, S.; Wang, D.; Zhang, W. Visible-Light-Driven Photocatalytic Inactivation of MS2 by Metal-Free g-C3N4: Virucidal Performance and Mechanism. Water Res. 2016, 106, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Nagy, D.; Nagy, D.; Szilágyi, I.M.; Fan, X. Effect of the Morphology and Phases of WO3 Nanocrystals on Their Photocatalytic Efficiency. RSC Adv. 2016, 6, 33743–33754. [Google Scholar] [CrossRef]
- Palanisamy, G.; Bhuvaneswari, K.; Pazhanivel, T.; Bharathi, G. Enriched Photocatalytic Activity of Rhodamine B Dye from Aqueous Solution Using Hollow Sphere Tungsten Trioxide Nanoparticles. Optik 2020, 204, 164171. [Google Scholar] [CrossRef]
- Mena, E.; Rey, A.; Contreras, S.; Beltrán, F.J. Visible Light Photocatalytic Ozonation of DEET in the Presence of Different Forms of WO3. Catal. Today 2015, 252, 100–106. [Google Scholar] [CrossRef]
- Razali, N.A.M.; Salleh, W.N.W.; Rosman, N.; Ismail, N.H.; Ahmad, S.Z.N.; Aziz, F.; Jye, L.W.; Ismail, A.F. Palm Oil Mill Effluent Treatment Using Tungsten Trioxide: Adsorption and Photocatalytic Degradation. Mater. Today Proc. 2021, 42, 22–27. [Google Scholar] [CrossRef]
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Reddy, K.R.; Raghu, A.V.; Reddy, C.V. Recent Progress in Metal-Doped TiO2, Non-Metal Doped/Codoped TiO2 and TiO2 Nanostructured Hybrids for Enhanced Photocatalysis. Int. J. Hydrogen Energy 2020, 45, 7764–7778. [Google Scholar] [CrossRef]
- Varma, K.S.; Tayade, R.J.; Shah, K.J.; Joshi, P.A.; Shukla, A.D.; Gandhi, V.G. Photocatalytic Degradation of Pharmaceutical and Pesticide Compounds (PPCs) Using Doped TiO2 Nanomaterials: A Review. Water-Energy Nexus 2020, 3, 46–61. [Google Scholar] [CrossRef]
- Karuppasamy, P.; Ramzan Nilofar Nisha, N.; Pugazhendhi, A.; Kandasamy, S.; Pitchaimuthu, S. An Investigation of Transition Metal Doped TiO2 photocatalysts for the Enhanced Photocatalytic Decoloration of Methylene Blue Dye under Visible Light Irradiation. J. Environ. Chem. Eng. 2021, 9, 105254. [Google Scholar] [CrossRef]
- Lee, H.; Jang, H.S.; Kim, N.Y.; Joo, J.B. Cu-Doped TiO2 Hollow Nanostructures for the Enhanced Photocatalysis under Visible Light Conditions. J. Ind. Eng. Chem. 2021, 99, 352–363. [Google Scholar] [CrossRef]
- Moreira, A.J.; Malafatti, J.O.D.; Giraldi, T.R.; Paris, E.C.; Pereira, E.C.; de Mendonça, V.R.; Mastelaro, V.R.; Freschi, G.P.G. Prozac® Photodegradation Mediated by Mn-Doped TiO2 Nanoparticles: Evaluation of by-Products and Mechanisms Proposal. J. Environ. Chem. Eng. 2020, 8, 104543. [Google Scholar] [CrossRef]
- Ellouzi, I.; Bouddouch, A.; Bakiz, B.; Benlhachemi, A.; Abou Oualid, H. Glucose-Assisted Ball Milling Preparation of Silver-Doped Biphasic TiO2 for Efficient Photodegradation of Rhodamine B: Effect of Silver-Dopant Loading. Chem. Phys. Lett. 2021, 770, 138456. [Google Scholar] [CrossRef]
- Wu, M.C.; Lin, T.H.; Hsu, K.H.; Hsu, J.F. Photo-Induced Disinfection Property and Photocatalytic Activity Based on the Synergistic Catalytic Technique of Ag Doped TiO2 Nanofibers. Appl. Surf. Sci. 2019, 484, 326–334. [Google Scholar] [CrossRef]
- Saber, N.B.; Mezni, A.; Alrooqi, A.; Altalhi, T. Fabrication of Efficient Au@TiO2/RGO Heterojunction Nanocomposite: Boosted Photocatalytic Activity under Ultraviolet and Visible Light Irradiation. J. Mater. Res. Technol. 2021, 12, 2238–2246. [Google Scholar] [CrossRef]
- Manasa, M.; Chandewar, P.R.; Mahalingam, H. Photocatalytic Degradation of Ciprofloxacin & Norfloxacin and Disinfection Studies under Solar Light Using Boron & Cerium Doped TiO2 Catalysts Synthesized by Green EDTA-Citrate Method. Catal. Today 2021, 375, 522–536. [Google Scholar] [CrossRef]
- Komaraiah, D.; Radha, E.; Sivakumar, J.; Reddy, M.V.R.; Sayanna, R. Influence of Fe3+ Ion Doping on the Luminescence Emission Behavior and Photocatalytic Activity of Fe3+, Eu3+-Codoped TiO2 Thin Films. J. Alloys Compd. 2021, 868, 159109. [Google Scholar] [CrossRef]
- Mancuso, A.; Sacco, O.; Vaiano, V.; Sannino, D.; Pragliola, S.; Venditto, V.; Morante, N. Visible Light Active Fe-Pr Co-Doped TiO2 for Water Pollutants Degradation. Catal. Today 2021. [Google Scholar] [CrossRef]
- Crişan, M.; Rəileanu, M.; Drəgan, N.; Crişan, D.; Ianculescu, A.; Niţoi, I.; Oancea, P.; Şoməcescu, S.; Stənicə, N.; Vasile, B.; et al. Sol-Gel Iron-Doped TiO2 Nanopowders with Photocatalytic Activity. Appl. Catal. A Gen. 2015, 504, 130–142. [Google Scholar] [CrossRef]
- Pascariu, P.; Cojocaru, C.; Homocianu, M.; Samoila, P.; Dascalu, A.; Suchea, M. New La3+ Doped TiO2 Nanofibers for Photocatalytic Degradation of Organic Pollutants: Effects of Thermal Treatment and Doping Loadings. Ceram. Int. 2022, 48, 4953–4964. [Google Scholar] [CrossRef]
- Hernández-Ramírez, A.; Medina-Ramírez, I.; Bustos, E.; Manríquez, J.; Peralta-Hernández, J.M. Photocatalytic Semiconductors; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 9783319109985. [Google Scholar]
- Coronado, J.M.; Fresno, F.; Hernández-Alonso, M.D.; Portela, R. Design of Advanced Photocatalytic Materials for Energy and Environmental Applications; Springer: London, UK, 2013; Volume 71, ISBN 9781447150602. [Google Scholar]
- Kayani, Z.N.; Intizar, T.; Riaz, S.; Naseem, S. Antibacterial, Magnetic and Dielectric Properties of Nano-Structured V Doped TiO2 Thin Films Deposited by Dip Coating Technique. Mater. Chem. Phys. 2021, 267, 124659. [Google Scholar] [CrossRef]
- Lashuk, B.; Yargeau, V. A Review of Ecotoxicity Reduction in Contaminated Waters by Heterogeneous Photocatalytic Ozonation. Sci. Total Environ. 2021, 787, 147645. [Google Scholar] [CrossRef]
- Asgari, E.; Sheikhmohammadi, A.; Nourmoradi, H.; Nazari, S.; Aghanaghad, M. Degradation of Ciprofloxacin by Photocatalytic Ozonation Process under Irradiation with UVA: Comparative Study, Performance and Mechanism. Process Saf. Environ. Prot. 2021, 147, 356–366. [Google Scholar] [CrossRef]
- Mecha, A.C.; Onyango, M.S.; Ochieng, A.; Momba, M.N.B. Ultraviolet and Solar Photocatalytic Ozonation of Municipal Wastewater: Catalyst Reuse, Energy Requirements and Toxicity Assessment. Chemosphere 2017, 186, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Umbuzeiro, G. de A. Effects of a Textile Azo Dye on Mortality, Regeneration, and Reproductive Performance of the Planarian, Girardia Tigrina. Environ. Sci. Eur. 2014, 26, 1–8. [Google Scholar] [CrossRef]
- Gomes, J.F.; Leal, I.; Bednarczyk, K.; Gmurek, M.; Stelmachowski, M.; Diak, M.; Emília Quinta-Ferreira, M.; Costa, R.; Quinta-Ferreira, R.M.; Martins, R.C. Photocatalytic Ozonation Using Doped TiO2 Catalysts for the Removal of Parabens in Water. Sci. Total Environ. 2017, 609, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Prakash, J.; Samriti; Kumar, A.; Dai, H.; Janegitz, B.C.; Krishnan, V.; Swart, H.C.; Sun, S. Novel Rare Earth Metal–Doped One-Dimensional TiO2 Nanostructures: Fundamentals and Multifunctional Applications. Mater. Today Sustain. 2021, 13, 100066. [Google Scholar] [CrossRef]
- Cerrato, E.; Gaggero, E.; Calza, P.; Paganini, M.C. The Role of Cerium, Europium and Erbium Doped TiO2 Photocatalysts in Water Treatment: A Mini-Review. Chem. Eng. J. Adv. 2022, 10, 100268. [Google Scholar] [CrossRef]
- Mancuso, A.; Sacco, O.; Sannino, D.; Pragliola, S.; Vaiano, V. Enhanced Visible-Light-Driven Photodegradation of Acid Orange 7 Azo Dye in Aqueous Solution Using Fe-N Co-Doped TiO2. Arab. J. Chem. 2020, 13, 8347–8360. [Google Scholar] [CrossRef]
- Lin, L.; Wang, H.; Luo, H.; Xu, P. Enhanced Photocatalysis Using Side-Glowing Optical Fibers Coated with Fe-Doped TiO2 Nanocomposite Thin Films. J. Photochem. Photobiol. A Chem. 2015, 307–308, 88–98. [Google Scholar] [CrossRef]
- Gusain, R.; Kumar, N.; Ray, S.S. Factors Influencing the Photocatalytic Activity of Photocatalysts in Wastewater Treatment. In Photocatalysts in Advanced Oxidation Processes for Wastewater Treatment; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 229–270. [Google Scholar]
- Mirzaei, A.; Eddah, M.; Roualdes, S.; Ma, D.; Chaker, M. Multiple-Homojunction Gradient Nitrogen Doped TiO2 for Photocatalytic Degradation of Sulfamethoxazole, Degradation Mechanism, and Toxicity Assessment. Chem. Eng. J. 2021, 422, 130507. [Google Scholar] [CrossRef]
- Ramezanisani, S.; Rajabi, M.; Mohseni, F. Influence of Nitrogen Doping on Visible Light Photocatalytic Activity of TiO2 Nanowires with Anatase-Rutile Junction. Chem. Phys. Lett. 2020, 744, 137217. [Google Scholar] [CrossRef]
- Suwannaruang, T.; Kamonsuangkasem, K.; Kidkhunthod, P.; Chirawatkul, P.; Saiyasombat, C.; Chanlek, N.; Wantala, K. Influence of Nitrogen Content Levels on Structural Properties and Photocatalytic Activities of Nanorice-like N-Doped TiO2 with Various Calcination Temperatures. Mater. Res. Bull. 2018, 105, 265–276. [Google Scholar] [CrossRef]
- Erdogan, N.; Bouziani, A.; Park, J.; Micusik, M.; Kim, S.Y.; Majkova, E.; Omastova, M.; Ozturk, A. Synthesis and Enhanced Photocatalytic Activity of Nitrogen-Doped Triphasic TiO2 Nanoparticles. J. Photochem. Photobiol. A Chem. 2019, 377, 92–100. [Google Scholar] [CrossRef]
- Toe, E.D.; Kurniawan, W.; Mariquit, E.G.; Hinode, H. Synthesis of N-Doped Mesoporous TiO2 by Facile One-Step Solvothermal Process for Visible Light Photocatalytic Degradation of Organic Pollutant. J. Environ. Chem. Eng. 2018, 6, 5125–5134. [Google Scholar] [CrossRef]
- Yadav, V.; Saini, V.K.; Sharma, H. How Different Dopants Leads to Difference in Photocatalytic Activity in Doped TiO2? Ceram. Int. 2020, 46, 27308–27317. [Google Scholar] [CrossRef]
- Bakre, P.V.; Tilve, S.G.; Shirsat, R.N. Influence of N Sources on the Photocatalytic Activity of N-Doped TiO2. Arab. J. Chem. 2020, 13, 7637–7651. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, S.; Zhang, S.; Wang, R.; Wang, K. Preparation and Visible-Light Photocatalytic Activity of N-Doped TiO2 by Plasma-Assisted Sol-Gel Method. Catal. Today 2019, 337, 37–43. [Google Scholar] [CrossRef]
- Gao, H.; Zhou, J.; Dai, D.; Qu, Y. Photocatalytic Activity and Electronic Structure Analysis of N-Doped Anatase TiO2: A Combined Experimental and Theoretical Study. Chem. Eng. Technol. 2009, 32, 867–872. [Google Scholar] [CrossRef]
- Assayehegn, E.; Solaiappan, A.; Chebude, Y.; Alemayehu, E. Fabrication of Tunable Anatase/Rutile Heterojunction N/TiO2 Nanophotocatalyst for Enhanced Visible Light Degradation Activity. Appl. Surf. Sci. 2020, 515, 145966. [Google Scholar] [CrossRef]
- Chaturvedi, R.; Singh, P.K. Materials Today: Proceedings Synthesis and Characterization of Nano Crystalline Nitrogen Doped Titanium Dioxide. Mater. Today Proc. 2021, 45, 3666–3669. [Google Scholar] [CrossRef]
- Wafi, A.; Szabó-Bárdos, E.; Horváth, O.; Makó, É.; Jakab, M.; Zsirka, B. Coumarin-Based Quantification of Hydroxyl Radicals and Other Reactive Species Generated on Excited Nitrogen-Doped TiO2. J. Photochem. Photobiol. A Chem. 2021, 404, 112913. [Google Scholar] [CrossRef]
- Huang, J.; Dou, L.; Li, J.; Zhong, J.; Li, M.; Wang, T. Excellent Visible Light Responsive Photocatalytic Behavior of N-Doped TiO2 toward Decontamination of Organic Pollutants. J. Hazard. Mater. 2021, 403, 123857. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, M.; Zhao, H.; Zhang, L.r.; Wei, G.; Zhao, G. Preferential and Efficient Degradation of Phenolic Pollutants with Cooperative Hydrogen-Bond Interactions in Photocatalytic Process. Chemosphere 2021, 269, 129404. [Google Scholar] [CrossRef]
- Ravidhas, C.; Anitha, B.; Venkatesh, R.; Monica, S.E.S.; Gopalakrishna, D.; Raj, A.M.E.; Ravichandran, K. Role of Fluorine Doping on Luminescence Centers and Enhanced Photocatalytic Performance of Nebulizer Sprayed TiO2 Films under Visible Light. J. Lumin. 2018, 198, 272–283. [Google Scholar] [CrossRef]
- Hwang, J.; Kalanur, S.S.; Seo, H. Identification of Visible Photocatalytic and Photoelectrochemical Properties of I-TiO2 via Electronic Band Structure. Electrochim. Acta 2017, 252, 482–489. [Google Scholar] [CrossRef]
- Zhou, L.; Cai, M.; Zhang, X.; Cui, N.; Chen, G.; Zou, G. yan In-Situ Nitrogen-Doped Black TiO2 with Enhanced Visible-Light-Driven Photocatalytic Inactivation of Microcystis Aeruginosa Cells: Synthesization, Performance and Mechanism. Appl. Catal. B Environ. 2020, 272, 119019. [Google Scholar] [CrossRef]
- Tzeng, J.H.; Weng, C.H.; Yen, L.T.; Gaybullaev, G.; Chang, C.J.; de Luna, M.D.G.; Lin, Y.T. Inactivation of Pathogens by Visible Light Photocatalysis with Nitrogen-Doped TiO2 and Tourmaline-Nitrogen Co-Doped TiO2. Sep. Purif. Technol. 2021, 274, 118979. [Google Scholar] [CrossRef]
- Boningari, T.; Inturi, S.N.R.; Suidan, M.; Smirniotis, P.G. Novel One-Step Synthesis of Sulfur Doped-TiO2 by Flame Spray Pyrolysis for Visible Light Photocatalytic Degradation of Acetaldehyde. Chem. Eng. J. 2018, 339, 249–258. [Google Scholar] [CrossRef]
- Bento, R.T.; Correa, O.V.; Pillis, M.F. Photocatalytic Activity of Undoped and Sulfur-Doped TiO2 Films Grown by MOCVD for Water Treatment under Visible Light. J. Eur. Ceram. Soc. 2019, 39, 3498–3504. [Google Scholar] [CrossRef]
- Portela, R. Non-Metal Doping for Band-Gap Engineering Raquel. In Design of Advanced Photocatalytic Materials for Energy and Environmental Applications. In Green Energy and Technology; Coronado, J.M., Fresno, F., Hernández-Alonso, M.D., Portela, R., Eds.; Springer: London, UK, 2013; Volume 71, pp. 287–309. ISBN 9781447150602. [Google Scholar]
- He, J.; Kumar, A.; Khan, M.; Lo, I.M.C. Critical Review of Photocatalytic Disinfection of Bacteria: From Noble Metals- and Carbon Nanomaterials-TiO2 Composites to Challenges of Water Characteristics and Strategic Solutions. Sci. Total Environ. 2021, 758, 143953. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Yang, H.; Xue, X.; Liu, Z. Doping TiO2 with Boron or/and Cerium Elements: Effects on Photocatalytic Antimicrobial Activity. Vacuum 2016, 131, 58–64. [Google Scholar] [CrossRef]
- Jahdi, M.; Mishra, S.B.; Nxumalo, E.N.; Mhlanga, S.D.; Mishra, A.K. Smart Pathways for the Photocatalytic Degradation of Sulfamethoxazole Drug Using F-Pd Co-Doped TiO2 Nanocomposites. Appl. Catal. B Environ. 2020, 267, 118716. [Google Scholar] [CrossRef]
- Khedr, T.M.; El-Sheikh, S.M.; Hakki, A.; Ismail, A.A.; Badawy, W.A.; Bahnemann, D.W. Highly Active Non-Metals Doped Mixed-Phase TiO2 for Photocatalytic Oxidation of Ibuprofen under Visible Light. J. Photochem. Photobiol. A Chem. 2017, 346, 530–540. [Google Scholar] [CrossRef]
- Venkatesan, A.; Al-onazi, W.A.; Elshikh, M.S.; Pham, T.H.; Suganya, S.; Boobas, S.; Priyadharsan, A. Study of Synergistic Effect of Cobalt and Carbon Codoped with TiO2 Photocatalyst for Visible Light Induced Degradation of Phenol. Chemosphere 2022, 305, 135333. [Google Scholar] [CrossRef]
- Ariza-Tarazona, M.C.; Villarreal-Chiu, J.F.; Hernández-López, J.M.; Rivera De la Rosa, J.; Barbieri, V.; Siligardi, C.; Cedillo-González, E.I. Microplastic Pollution Reduction by a Carbon and Nitrogen-Doped TiO2: Effect of PH and Temperature in the Photocatalytic Degradation Process. J. Hazard. Mater. 2020, 395, 122632. [Google Scholar] [CrossRef]
- Thongpool, V.; Phunpueok, A.; Jaiyen, S.; Sornkwan, T. Results in Physics Synthesis and Photocatalytic Activity of Copper and Nitrogen Co-Doped Titanium Dioxide Nanoparticles. Results Phys. 2020, 16, 102948. [Google Scholar] [CrossRef]
- Wu, Y.; Dong, Y.; Xia, X.; Liu, X.; Li, H. Facile Synthesis of N-F Codoped and Molecularly Imprinted TiO2 for Enhancing Photocatalytic Degradation of Target Contaminants. Appl. Surf. Sci. 2016, 364, 829–836. [Google Scholar] [CrossRef]
- Razali, N.A.M.; Salleh, W.N.W.; Aziz, F.; Jye, L.W.; Yusof, N.; Ismail, A.F. Review on Tungsten Trioxide as a Photocatalysts for Degradation of Recalcitrant Pollutants. J. Clean. Prod. 2021, 309, 127438. [Google Scholar] [CrossRef]
- Palharim, P.H.; Fusari, B.L.D.d.R.; Ramos, B.; Otubo, L.; Teixeira, A.C.S.C. Effect of HCl and HNO3 on the Synthesis of Pure and Silver-Based WO3 for Improved Photocatalytic Activity under Sunlight. J. Photochem. Photobiol. A Chem. 2022, 422, 113550. [Google Scholar] [CrossRef]
- Hanif, M.A.; Akter, J.; Islam, M.A.; Sapkota, K.P.; Hahn, J.R. Visible-Light-Driven Enhanced Photocatalytic Performance Using Cadmium-Doping of Tungsten (VI) Oxide and Nanocomposite Formation with Graphitic Carbon Nitride Disks. Appl. Surf. Sci. 2021, 565, 150541. [Google Scholar] [CrossRef]
- Mehmood, F.; Iqbal, J.; Jan, T.; Gul, A.; Mansoor, Q.; Faryal, R. Structural, Photoluminescence, Electrical, Anti Cancer and Visible Light Driven Photocatalytic Characteristics of Co Doped WO3 Nanoplates. Vib. Spectrosc. 2017, 93, 78–89. [Google Scholar] [CrossRef]
- Quyen, V.T.; Kim, J.T.; Park, P.M.; Huong, P.T.; Viet, N.M.; Thang, P.Q. Enhanced the Visible Light Photocatalytic Decomposition of Antibiotic Pollutant in Wastewater by Using Cu Doped WO3. J. Environ. Chem. Eng. 2021, 9, 104737. [Google Scholar] [CrossRef]
- Luxmi, V.; Kumar, A. Enhanced Photocatalytic Performance of m-WO3 and m-Fe-Doped WO3 Cuboids Synthesized via Sol-Gel Approach Using Egg Albumen as a Solvent. Mater. Sci. Semicond. Process. 2019, 104, 104690. [Google Scholar] [CrossRef]
- Govindaraj, T.; Mahendran, C.; Marnadu, R.; Shkir, M.; Manikandan, V.S. The Remarkably Enhanced Visible-Light-Photocatalytic Activity of Hydrothermally Synthesized WO3 Nanorods: An Effect of Gd Doping. Ceram. Int. 2021, 47, 4267–4278. [Google Scholar] [CrossRef]
- Yazdanbakhsh, A.R.; Eslami, A.; Massoudinejad, M.; Avazpour, M. Enhanced Degradation of Sulfamethoxazole Antibiotic from Aqueous Solution Using Mn-WO3/LED Photocatalytic Process: Kinetic, Mechanism, Degradation Pathway and Toxicity Reduction. Chem. Eng. J. 2020, 380, 122497. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, G.; Yu, Y.; Zhou, Y.; He, F. Synthesis of Carbon Doped WO3·0.33H2O Hierarchical Photocatalyst with Improved Photocatalytic Activity. Appl. Surf. Sci. 2016, 362, 182–190. [Google Scholar] [CrossRef]
- Tijani, J.O.; Ugochukwu, O.; Fadipe, L.A.; Bankole, M.T.; Abdulkareem, A.S.; Roos, W.D. Photocatalytic Degradation of Local Dyeing Wastewater by Iodine-Phosphorus Co-Doped Tungsten Trioxide Nanocomposites under Natural Sunlight Irradiation. J. Environ. Manag. 2019, 236, 519–533. [Google Scholar] [CrossRef]
- Han, F.; Li, H.; Fu, L.; Yang, J.; Liu, Z. Synthesis of S-Doped WO3 Nanowires with Enhanced Photocatalytic Performance towards Dye Degradation. Chem. Phys. Lett. 2016, 651, 183–187. [Google Scholar] [CrossRef]
- Tahir, M.B.; Sagir, M. Carbon Nanodots and Rare Metals (RM = La, Gd, Er) Doped Tungsten Oxide Nanostructures for Photocatalytic Dyes Degradation and Hydrogen Production. Sep. Purif. Technol. 2019, 209, 94–102. [Google Scholar] [CrossRef]
- Ma, T.; Shen, Q.; Xue, B.Z.J.; Guan, R.; Liu, X.; Jia, H.; Xu, B. Facile Synthesis of Fe-Doped g-C3N4 for Enhanced Visible-Light Photocatalytic Activity. Inorg. Chem. Commun. 2019, 107, 107451. [Google Scholar] [CrossRef]
- Pham, T.H.; Park, J.W.; Kim, T.Y. Enhanced Photodegradation of Paracetamol from Water by Cobalt Doped Graphitic Carbon Nitride. Sol. Energy 2021, 215, 151–156. [Google Scholar] [CrossRef]
- Pham, T.H.; Jung, S.H.; Kim, T.Y. Enhanced Photodegradation of Toxic Volatile Organic Pollutants Using Ni-Doped Graphitic Carbon Nitride under Natural Solar Light. Sol. Energy 2021, 224, 18–26. [Google Scholar] [CrossRef]
- Tripathi, A.; Narayanan, S. Potassium Doped Graphitic Carbon Nitride with Extended Optical Absorbance for Solar Light Driven Photocatalysis. Appl. Surf. Sci. 2019, 479, 1–11. [Google Scholar] [CrossRef]
- Tri, N.L.M.; Kim, J.; Giang, B.L.; Tahtamouni, T.M.A.; Huong, P.T.; Lee, C.; Viet, N.M.; Trung, D.Q. Ag-Doped Graphitic Carbon Nitride Photocatalyst with Remarkably Enhanced Photocatalytic Activity towards Antibiotic in Hospital Wastewater under Solar Light. J. Ind. Eng. Chem. 2019, 80, 597–605. [Google Scholar] [CrossRef]
- Bawazeer, T.M.; Al-Shehri, B.M.; Alsoufi, M.S.; Shkir, M.; Hamdy, M.S. Solvent-Free Facile Fabrication of Gold Nanoparticles Loaded Carbon Nitride and Their Photocatalytic Performance under Visible Light Illumination. Optik 2021, 241, 167205. [Google Scholar] [CrossRef]
- Li, G.; Wang, B.; Zhang, J.; Wang, R.; Liu, H. Er-Doped g-C3N4 for Photodegradation of Tetracycline and Tylosin: High Photocatalytic Activity and Low Leaching Toxicity. Chem. Eng. J. 2020, 391, 123500. [Google Scholar] [CrossRef]
- Viet, N.M.; Trung, D.Q.; Giang, B.L.; Tri, N.L.M.; Thao, P.; Pham, T.H.; Kamand, F.Z.; Al Tahtamouni, T.M. Noble Metal -Doped Graphitic Carbon Nitride Photocatalyst for Enhancement Photocatalytic Decomposition of Antibiotic Pollutant in Wastewater under Visible Light. J. Water Process Eng. 2019, 32, 100954. [Google Scholar] [CrossRef]
- Xu, J.; Brenner, T.J.K.; Chen, Z.; Neher, D.; Antonietti, M.; Shalom, M. Upconversion-Agent Induced Improvement of g-C3N4 Photocatalyst under Visible Light. ACS Appl. Mater. Interfaces 2014, 6, 16481–16486. [Google Scholar] [CrossRef]
- Xiong, T.; Cen, W.; Zhang, Y.; Dong, F. Bridging the g-C3N4 Interlayers for Enhanced Photocatalysis. ACS Catal. 2016, 6, 2462–2472. [Google Scholar] [CrossRef]
- Liu, J.; Liang, H.; Li, C.; Bai, J. Construction of V-Doped Graphitic Carbon Nitride with Nanotube Structure for Sustainable Photodegradation of Tetracycline. Vacuum 2022, 204, 111342. [Google Scholar] [CrossRef]
- Zhang, B.; Li, X.; Zhao, Y.; Song, H.; Wang, H. Facile Synthesis of Oxygen Doped Mesoporous Graphitic Carbon Nitride with High Photocatalytic Degradation Efficiency under Simulated Solar Irradiation. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 580, 123736. [Google Scholar] [CrossRef]
- Praus, P.; Smýkalová, A.; Foniok, K.; Novák, V.; Hrbáč, J. Doping of Graphitic Carbon Nitride with Oxygen by Means of Cyanuric Acid: Properties and Photocatalytic Applications. J. Environ. Chem. Eng. 2021, 9, 105498. [Google Scholar] [CrossRef]
- Krumova, K.; Cosa, G.; Aubry, J.; Kanofsky, J.R. Overview of Reactive Oxygen Species Katerina. In Singlet Oxygen: Applications in Biosciences and Nanosciences; The Royal Society of Chemistry: Cambridge, UK, 2016; pp. 3–18. ISBN 9781412916837. [Google Scholar]
- Wang, S.; He, F.; Zhao, X.; Zhang, J.; Ao, Z.; Wu, H.; Yin, Y.; Shi, L.; Xu, X.; Zhao, C.; et al. Phosphorous Doped Carbon Nitride Nanobelts for Photodegradation of Emerging Contaminants and Hydrogen Evolution. Appl. Catal. B Environ. 2019, 257, 117931. [Google Scholar] [CrossRef]
- Liang, Z.; Ba, G.; Li, H.; Du, N.; Hou, W. Facile Synthesis of Silicon-Doped Polymeric Carbon Nitride with Enhanced Photocatalytic Performance. J. Alloys Compd. 2020, 815, 152488. [Google Scholar] [CrossRef]
- Ganganboina, A.B.; Nguyen, M.D.; Nguyen, T.H.L.; Kuncoro, E.P.; Doong, R.-A. Boron and Phosphorus Co-Doped One-Dimensional Graphitic Carbon Nitride for Enhanced Visible-Light-Driven Photodegradation of Diclofenac. Chem. Eng. J. 2021, 425, 131520. [Google Scholar] [CrossRef]
- Cao, M.; Wang, K.; Tudela, I.; Fan, X. Improve Photocatalytic Performance of g-C3N4 through Balancing the Interstitial and Substitutional Chlorine Doping. Appl. Surf. Sci. 2021, 536, 147784. [Google Scholar] [CrossRef]
- Yi, F.; Gan, H.; Jin, H.; Zhao, W.; Zhang, K.; Jin, H.; Zhang, H.; Qian, Y.; Ma, J. Sulfur- and Chlorine-Co-Doped g-C3N4 Nanosheets with Enhanced Active Species Generation for Boosting Visible-Light Photodegradation Activity. Sep. Purif. Technol. 2020, 233, 115997. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, J.; Wang, F.; Xie, Y.; Liu, S.; Ao, Z.; Li, C. Hydrogen Generation from Photocatalytic Treatment of Wastewater Containing Pharmaceuticals and Personal Care Products by Oxygen-Doped Crystalline Carbon Nitride. Sep. Purif. Technol. 2022, 296, 121425. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Ma, Y.; Fang, S.; Kong, F.; Pang, X. Photocatalytic Degradation of Dinotefuran by Layered Phosphorus-Doped Carbon Nitride and Its Mechanism. J. Photochem. Photobiol. A Chem. 2021, 414, 113287. [Google Scholar] [CrossRef]
- Pandit, B.B.; Sonawane, S.; Aniruddha, V.P. (Eds.) Handbook of Nanomaterials for Wastewater Treatment: Fundamental and Scale Up Issues, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9789896540821. [Google Scholar]
- Khan, M.M.; Pradhan, D.; Sohn, Y. (Eds.) Nanocomposites for Visible Light-Induced Photocatalysis; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 9783319624457. [Google Scholar]
- Mugunthan, E.; Saidutta, M.B.; Jagadeeshbabu, P.E. Visible Light Assisted Photocatalytic Degradation of Diclofenac Using TiO2-WO3 Mixed Oxide Catalysts. Environ. Nanotechnol. Monit. Manag. 2018, 10, 322–330. [Google Scholar] [CrossRef]
- El-Yazeed, W.S.A.; Ahmed, A.I. Photocatalytic Activity of Mesoporous WO3/TiO2 Nanocomposites for the Photodegradation of Methylene Blue. Inorg. Chem. Commun. 2019, 105, 102–111. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, W.; Hu, X.; Xu, L.; Chen, G.; Li, X. Hollow Spherical WO3/TiO2 Heterojunction for Enhancing Photocatalytic Performance in Visible-Light. J. Water Process Eng. 2021, 40, 101943. [Google Scholar] [CrossRef]
- Su, X.; Liu, C.j.; Liu, Y.; Yang, Y.; Liu, X.; Chen, S. Construction of BiVO4 Nanosheets@WO3 Arrays Heterojunction Photoanodes by Versatile Phase Transformation Strategy. Trans. Nonferrous Met. Soc. China 2021, 31, 533–544. [Google Scholar] [CrossRef]
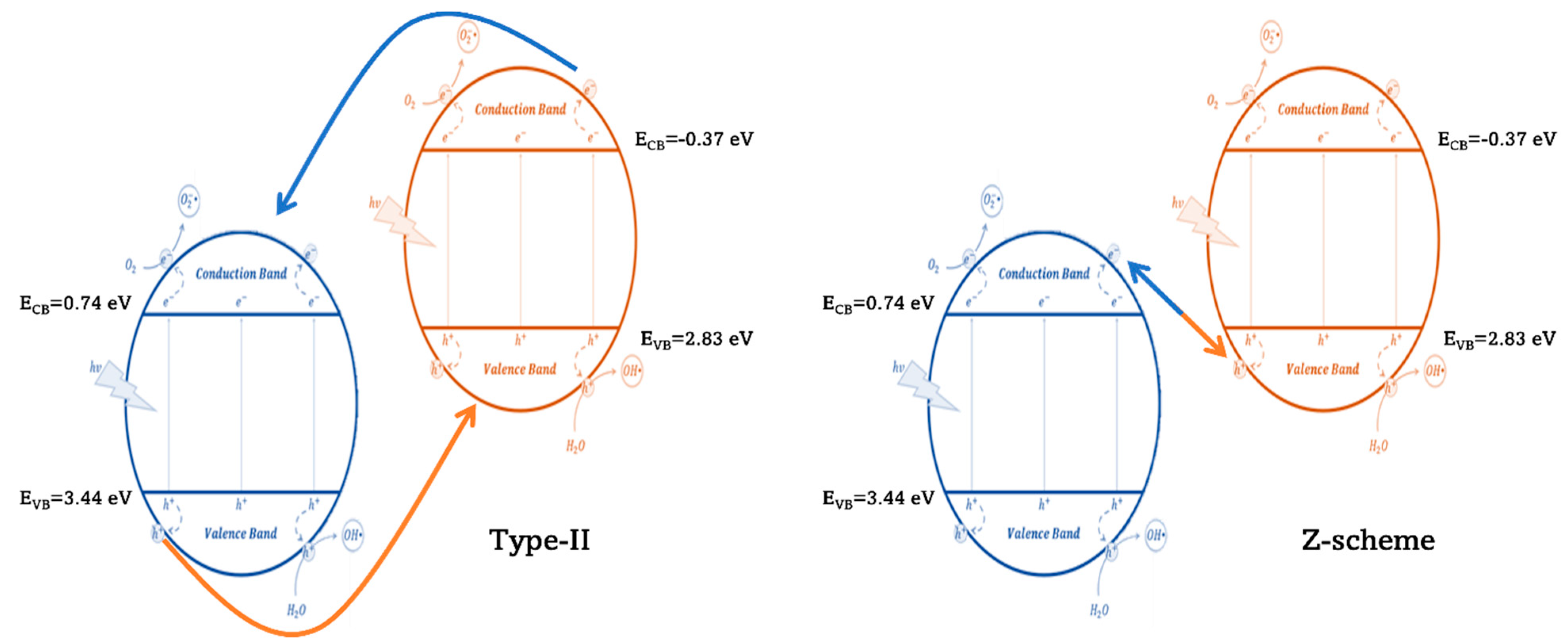
- Meng, S.; Sun, W.; Zhang, S.; Zheng, X.; Fu, X.; Chen, S. Insight into the Transfer Mechanism of Photogenerated Carriers for WO3/TiO2 Heterojunction Photocatalysts: Is It the Transfer of Band-Band or Z-Scheme? Why? J. Phys. Chem. C 2018, 122, 26326–26336. [Google Scholar] [CrossRef]
- Wei, Y.; Huang, Y.; Fang, Y.; Zhao, Y.; Luo, D.; Guo, Q.; Fan, L.; Wu, J. Hollow Mesoporous TiO2/WO3 Sphere Heterojunction with High Visible-Light-Driven Photocatalytic Activity. Mater. Res. Bull. 2019, 119, 110571. [Google Scholar] [CrossRef]
- Zhou, Z.; Niu, X.; Zhang, Y.; Wang, J. Janus MoSSe/WSeTe Heterostructures: A Direct Z-Scheme Photocatalyst for Hydrogen Evolution. J. Mater. Chem. A 2019, 7, 21835–21842. [Google Scholar] [CrossRef]
- Cordero-García, A.; Guzmán-Mar, J.L.; Hinojosa-Reyes, L.; Ruiz-Ruiz, E.; Hernández-Ramírez, A. Effect of Carbon Doping on WO3/TiO2 Coupled Oxide and Its Photocatalytic Activity on Diclofenac Degradation. Ceram. Int. 2016, 42, 9796–9803. [Google Scholar] [CrossRef]
- Basumatary, B.; Basumatary, R.; Ramchiary, A.; Konwar, D. Evaluation of Ag@TiO2/WO3 Heterojunction Photocatalyst for Enhanced Photocatalytic Activity towards Methylene Blue Degradation. Chemosphere 2022, 286, 131848. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, X.; Long, H.; Ding, F.; Liu, Q.; Chen, X. Preparation and Photocatalytic Performance of Graphene Oxide/WO3 Quantum Dots/TiO2@SiO2 Microspheres. Vacuum 2019, 164, 66–71. [Google Scholar] [CrossRef]
- Deng, M.; Cao, X.; Li, Z.; Tang, B. Hybrid Triazine-Based g-C3N4(001)/ Anatase TiO2(001) Heterojunction: Insights into Enhanced Photocatalytic Mechanisms Via DFT Calculation. J. Photochem. Photobiol. A Chem. 2022, 423, 113577. [Google Scholar] [CrossRef]
- Barzegar, M.H.; Sabzehmeidani, M.M.; Ghaedi, M.; Avargani, V.M.; Moradi, Z.; Roy, V.A.L.; Heidari, H. S-Scheme Heterojunction g-C3N4/TiO2 with Enhanced Photocatalytic Activity for Degradation of a Binary Mixture of Cationic Dyes Using Solar Parabolic Trough Reactor. Chem. Eng. Res. Des. 2021, 174, 307–318. [Google Scholar] [CrossRef]
- Gahlot, S.; Dappozze, F.; Mishra, S.; Guillard, C. High Surface Area g-C3N4 and g-C3N4-TiO2 Photocatalytic Activity under UV and Visible Light: Impact of Individual Component. J. Environ. Chem. Eng. 2021, 9, 105587. [Google Scholar] [CrossRef]
- Li, G.; Nie, X.; Chen, J.; Jiang, Q.; An, T.; Wong, P.K.; Zhang, H.; Zhao, H.; Yamashita, H. Enhanced Visible-Light-Driven Photocatalytic Inactivation of Escherichia Coli Using g-C3N4/TiO2 Hybrid Photocatalyst Synthesized Using a Hydrothermal-Calcination Approach. Water Res. 2015, 86, 17–24. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, X.; Hu, X.; Hu, J.; Wang, Z.; Yin, Y.; Sun, C.; Zhang, X. Two-Dimensional g-C3N4/TiO2 Nanocomposites as Vertical Z-Scheme Heterojunction for Improved Photocatalytic Water Disinfection. Catal. Today 2019, 335, 243–251. [Google Scholar] [CrossRef]
- Huang, J.; Wang, B.; Hao, Z.; Zhou, Z.; Qu, Y. Boosting Charge Separation and Broadening NIR Light Response over Defected WO3 Quantum Dots Coupled g-C3N4 Nanosheets for Photocatalytic Degrading Antibiotics. Chem. Eng. J. 2021, 416, 129109. [Google Scholar] [CrossRef]
- Cadan, F.M.; Ribeiro, C.; Azevedo, E.B. Improving g-C3N4:WO3 Z-Scheme Photocatalytic Performance under Visible Light by Multivariate Optimization of g-C3N4 Synthesis. Appl. Surf. Sci. 2021, 537, 147904. [Google Scholar] [CrossRef]
- Divakaran, K.; Baishnisha, A.; Balakumar, V.; Perumal, K.N.; Meenakshi, C.; Kannan, R.S. Photocatalytic Degradation of Tetracycline under Visible Light Using TiO2@sulfur Doped Carbon Nitride Nanocomposite Synthesized via In-Situ Method. J. Environ. Chem. Eng. 2021, 9, 105560. [Google Scholar] [CrossRef]
- Dehkordi, A.B.; Badiei, A. Insight into the Activity of TiO2@nitrogen-Doped Hollow Carbon Spheres Supported on g-C3N4 for Robust Photocatalytic Performance. Chemosphere 2022, 288, 132392. [Google Scholar] [CrossRef] [PubMed]
- Abbasi-Asl, H.; Sabzehmeidani, M.M.; Ghaedi, M. Efficient Degradation of Metronidazole Antibiotic by TiO2/Ag3PO4/g–C3N4 Ternary Composite Photocatalyst in a Continuous Flow-Loop Photoreactor. J. Environ. Chem. Eng. 2021, 9, 105963. [Google Scholar] [CrossRef]
- Wang, C.; Luo, S.; Liu, C.; Chen, C. WO3 Quantum Dots Enhanced the Photocatalytic Performances of Graphene Oxide/TiO2 Films under Flowing Dye Solution. Inorg. Chem. Commun. 2020, 115, 107875. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, Z.; Wang, G.; Gengenbach, T.R.; McCarthy, D.T.; Deletic, A.; Yu, J.; Zhang, X. Highly Dispersed TiO2 Nanocrystals and WO3 Nanorods on Reduced Graphene Oxide: Z-Scheme Photocatalysis System for Accelerated Photocatalytic Water Disinfection. Appl. Catal. B Environ. 2017, 218, 163–173. [Google Scholar] [CrossRef]
- Sun, X.; He, W.; Yang, T.; Ji, H.; Liu, W.; Lei, J.; Liu, Y.; Cai, Z. Ternary TiO2/WO3/CQDs Nanocomposites for Enhanced Photocatalytic Mineralization of Aqueous Cephalexin: Degradation Mechanism and Toxicity Evaluation. Chem. Eng. J. 2021, 412, 128679. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, F.; Yuan, B.; Xiao, S.; Kuang, A.; Zhong, M.; Dang, S.; Long, X.; Zhang, W. Strain-Tunable Visible-Light-Responsive Photocatalytic Properties of Two-Dimensional Cds/g-C3N4: A Hybrid Density Functional Study. Nanomaterials 2019, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Someswararao, M.V.; Dubey, R.S.; Subbarao, P.S.V. Electrospun Composite Nanofibers Prepared by Varying Concentrations of TiO2/ZnO Solutions for Photocatalytic Applications. J. Photochem. Photobiol. 2021, 6, 100016. [Google Scholar] [CrossRef]
- Das, A.; Kumar, P.M.; Bhagavathiachari, M.; Nair, R.G. Hierarchical ZnO-TiO2 Nanoheterojunction: A Strategy Driven Approach to Boost the Photocatalytic Performance through the Synergy of Improved Surface Area and Interfacial Charge Transport. Appl. Surf. Sci. 2020, 534, 147321. [Google Scholar] [CrossRef]
- Cipagauta-Díaz, S.; Estrella-González, A.; Navarrete-Magaña, M.; Gómez, R. N Doped-TiO2 Coupled to BiVO4 with High Performance in Photodegradation of Ofloxacin Antibiotic and Rhodamine B Dye under Visible Light. Catal. Today 2021, 394, 445–457. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, N.; Mari, B.; Chauhan, N.S.; Mittal, A.; Maken, S.; Kumari, K. Solution Combustion Synthesized TiO2/Bi2O3/CuO Nano-Composites and Their Photocatalytic Activity Using Visible LEDs Assisted Photoreactor. Inorg. Chem. Commun. 2021, 125, 108418. [Google Scholar] [CrossRef]
- Chawla, H.; Chandra, A.; Ingole, P.P.; Garg, S. Recent Advancements in Enhancement of Photocatalytic Activity Using Bismuth-Based Metal Oxides Bi2MO6 (M = W, Mo, Cr) for Environmental Remediation and Clean Energy Production. J. Ind. Eng. Chem. 2021, 95, 1–15. [Google Scholar] [CrossRef]
- Munguti, L.; Dejene, F. Influence of Annealing Temperature on Structural, Optical and Photocatalytic Properties of ZnO–TiO2 Composites for Application in Dye Removal in Water. Nano-Struct. Nano-Objects 2020, 24, 100594. [Google Scholar] [CrossRef]
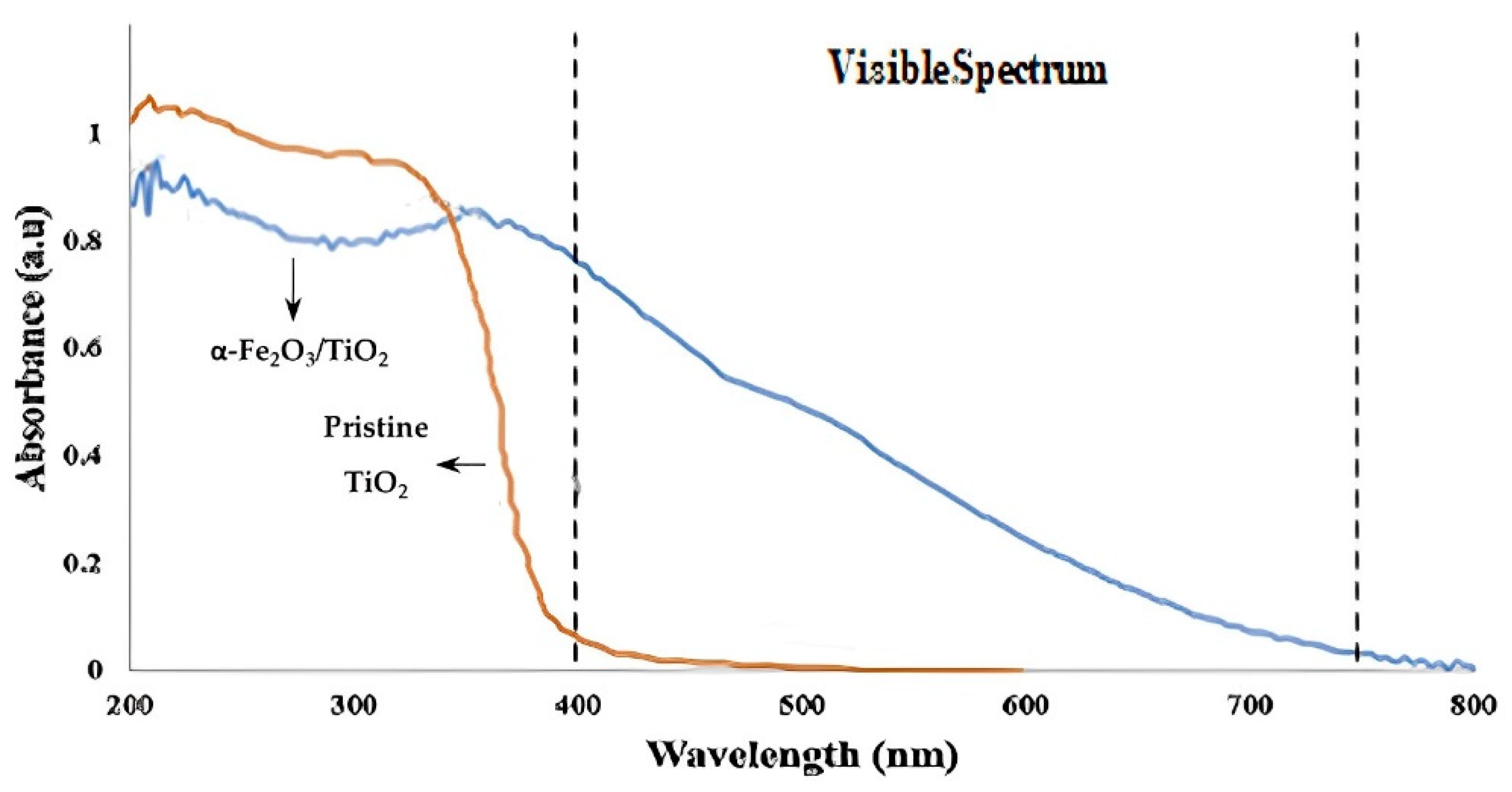
- Bouziani, A.; Park, J.; Ozturk, A. Synthesis of α-Fe2O3/TiO2 Heterogeneous Composites by the Sol-Gel Process and Their Photocatalytic Activity. J. Photochem. Photobiol. A Chem. 2020, 400, 112718. [Google Scholar] [CrossRef]
- Peña-Velasco, G.; Hinojosa-Reyes, L.; Morán-Quintanilla, G.A.; Hernández-Ramírez, A.; Villanueva-Rodríguez, M.; Guzmán-Mar, J.L. Synthesis of Heterostructured Catalyst Coupling MOF Derived Fe2O3 with TiO2 for Enhanced Photocatalytic Activity in Anti-Inflammatory Drugs Mixture Degradation. Ceram. Int. 2021, 47, 24632–24640. [Google Scholar] [CrossRef]
- Li, Y.; Yang, B.; Liu, B. MOF Assisted Synthesis of TiO2/Au/Fe2O3 Hybrids with Enhanced Photocatalytic Hydrogen Production and Simultaneous Removal of Toxic Phenolic Compounds. J. Mol. Liq. 2021, 322, 114815. [Google Scholar] [CrossRef]
- Lu, F.; Chen, K.; Feng, Q.; Cai, H.; Ma, D.; Wang, D.; Li, X.; Zuo, C.; Wang, S. Insight into the Enhanced Magnetic Separation and Photocatalytic Activity of Sn-Doped TiO2 Core-Shell Photocatalyst. J. Environ. Chem. Eng. 2021, 9, 105840. [Google Scholar] [CrossRef]
- Liu, N.; Ming, J.; Sharma, A.; Sun, X.; Kawazoe, N.; Chen, G.; Yang, Y. Sustainable Photocatalytic Disinfection of Four Representative Pathogenic Bacteria Isolated from Real Water Environment by Immobilized TiO2-Based Composite and Its Mechanism. Chem. Eng. J. 2021, 426, 131217. [Google Scholar] [CrossRef]
- Mohammed, M.K.A. Sol-Gel Synthesis of Au-Doped TiO2 Supported SWCNT Nanohybrid with Visible-Light-Driven Photocatalytic for High Degradation Performance toward Methylene Blue Dye. Optik 2020, 223, 165607. [Google Scholar] [CrossRef]
- Thomas, C.-T.; Manisekaran, R.; Santoyo-Salazar, J.; Schoefs, B.; Velumani, S.; Castaneda, H.; Jantrania, A. Graphene Oxide Decorated TiO2 and BiVO4 Nanocatalysts for Enhanced Visible-Light-Driven Photocatalytic Bacterial Inactivation. J. Photochem. Photobiol. A Chem. 2021, 418, 113374. [Google Scholar] [CrossRef]
- Huang, C.; Peng, B. Photocatalytic Degradation of Patulin in Apple Juice Based on Nitrogen-Doped Chitosan-TiO2 Nanocomposite Prepared by a New Approach. Lwt 2021, 140, 110726. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Zhao, J.; Song, J.; Wang, J.; Ma, R.; Ma, J. Solar Light-Driven Photocatalytic Destruction of Cyanobacteria by F-Ce-TiO2/Expanded Perlite Floating Composites. Chem. Eng. J. 2017, 320, 253–263. [Google Scholar] [CrossRef]
- Asadi, A.M.S.; Malakootian, M.; Kowsari, E.; Alidadi, H. Ionic Liquid-Assisted Sol-Gel Synthesis of Fe2O3-TiO2 for Enhanced Photocatalytic Degradation of Bisphenol a under UV Illumination: Modeling and Optimization Using Response Surface Methodology. Optik 2020, 204, 164229. [Google Scholar] [CrossRef]
- Orge, C.A.; Soares, O.S.G.P.; Faria, J.L.; Pereira, M.F.R. Synthesis of TiO2-Carbon Nanotubes through Ball-Milling Method for Mineralization of Oxamic Acid (OMA) by Photocatalytic Ozonation. J. Environ. Chem. Eng. 2017, 5, 5599–5607. [Google Scholar] [CrossRef]
- Chávez, A.M.; Quiñones, D.H.; Rey, A.; Beltrán, F.J.; Álvarez, P.M. Simulated Solar Photocatalytic Ozonation of Contaminants of Emerging Concern and Effluent Organic Matter in Secondary Effluents by a Reusable Magnetic Catalyst. Chem. Eng. J. 2020, 398, 125642. [Google Scholar] [CrossRef]
- Asgari, E.; Farzadkia, M.; Esrafili, A.; Badi, M.Y.; Jokandan, S.F.; Sobhi, H.R. Application of a Photocatalytic Ozonation Process Using TiO2 Magnetic Nanoparticles for the Removal of Ceftazide from Aqueous Solutions: Evaluation of Performance, Comparative Study and Mechanism. Optik 2020, 212, 164667. [Google Scholar] [CrossRef]
- Chávez, A.M.; Solís, R.R.; Beltrán, F.J. Magnetic Graphene TiO2-Based Photocatalyst for the Removal of Pollutants of Emerging Concern in Water by Simulated Sunlight Aided Photocatalytic Ozonation. Appl. Catal. B Environ. 2020, 262, 118275. [Google Scholar] [CrossRef]
- Liu, X.; Xu, J.; Ni, Z.; Wang, R.; You, J.; Guo, R. Adsorption and Visible-Light-Driven Photocatalytic Properties of Ag3PO4/WO3 Composites: A Discussion of the Mechanism. Chem. Eng. J. 2019, 356, 22–33. [Google Scholar] [CrossRef]
- Tang, J.; Meng, R.; Xue, Y.; Zhang, S.; Li, Q. Fabrication of a Novel Ag3PO4/WO3·H2O Composite with Enhanced Visible Light Photocatalytic Performance for the Degradation of Methylene Blue and Oxytetracycline. Inorg. Chem. Commun. 2021, 132, 108792. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Zhang, Q.; Wang, Y.; Fan, Y.; Gao, X.; Niu, J. Boosting Visible-Light Photocatalytic Degradation of Indomethacin by an Efficient and Photostable Ag3PO4/NG/WO3 Composites. Appl. Surf. Sci. 2019, 490, 481–491. [Google Scholar] [CrossRef]
- Hernández-Moreno, E.J.; Martínez de la Cruz, A.; Hinojosa-Reyes, L.; Guzmán-Mar, J.; Gracia-Pinilla, M.A.; Hernández-Ramírez, A. Synthesis, Characterization, and Visible Light–Induced Photocatalytic Evaluation of WO3/NaNbO3 Composites for the Degradation of 2,4-D Herbicide. Mater. Today Chem. 2021, 19, 100406. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, H.; Cao, J. Electrospinning of Ag/ZnWO4/WO3 Composite Nanofibers with High Visible Light Photocatalytic Activity. Mater. Lett. 2019, 236, 171–174. [Google Scholar] [CrossRef]
- Rong, J.; Zhang, T.; Qiu, F.; Rong, X.; Zhu, X.; Zhang, X. Preparation of Hierarchical Micro/Nanostructured Bi2S3-WO3 composites for Enhanced Photocatalytic Performance. J. Alloys Compd. 2016, 685, 812–819. [Google Scholar] [CrossRef]
- Govindaraj, T.; Mahendran, C.; Manikandan, V.S.; Archana, J.; Shkir, M.; Chandrasekaran, J. Fabrication of WO3 Nanorods/RGO Hybrid Nanostructures for Enhanced Visible-Light-Driven Photocatalytic Degradation of Ciprofloxacin and Rhodamine B in an Ecosystem. J. Alloys Compd. 2021, 868, 159091. [Google Scholar] [CrossRef]
- Elhakim, A.A.; El-Kemary, M.; Ibrahim, M.M.; El-Mehasseb, I.M.; El-Sheshtawy, H.S. Direct Z-Scheme of WO3/GO Decorated with Silver Nanoparticles for Synergetic Adsorption and Photocatalytic Activity for Organic and Inorganic Water Pollutants Removal. Appl. Surf. Sci. 2021, 564, 150410. [Google Scholar] [CrossRef]
- Gondal, M.A.; Suliman, M.A.; Dastageer, M.A.; Chuah, G.K.; Basheer, C.; Yang, D.; Suwaiyan, A. Visible Light Photocatalytic Degradation of Herbicide (Atrazine) Using Surface Plasmon Resonance Induced in Mesoporous Ag-WO3/SBA-15 Composite. J. Mol. Catal. A Chem. 2016, 425, 208–216. [Google Scholar] [CrossRef]
- Yan, L.; Hou, J.; Li, T.; Wang, Y.; Liu, C.; Zhou, T.; Jiang, W.; Wang, D.; Che, G. Tremella-like Integrated Carbon Nitride with Polyvinylimine-Doped for Enhancing Photocatalytic Degradation and Hydrogen Evolution Performances. Sep. Purif. Technol. 2021, 279, 119766. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, Y.; Xia, Y.; Uddin, M.H.; Xia, D.; McCarthy, D.T.; Deletic, A.; Yu, J.; Zhang, X. Cooperatively Modulating Reactive Oxygen Species Generation and Bacteria-Photocatalyst Contact over Graphitic Carbon Nitride by Polyethylenimine for Rapid Water Disinfection. Appl. Catal. B Environ. 2020, 274, 119095. [Google Scholar] [CrossRef]
- Alias, N.H.; Jaafar, J.; Samitsu, S.; Yusof, N.; Othman, M.H.D.; Rahman, M.A.; Ismail, A.F.; Aziz, F.; Salleh, W.N.W.; Othman, N.H. Photocatalytic Degradation of Oilfield Produced Water Using Graphitic Carbon Nitride Embedded in Electrospun Polyacrylonitrile Nanofibers. Chemosphere 2018, 204, 79–86. [Google Scholar] [CrossRef]
- Jing, L.; Xu, Y.; Liu, J.; Zhou, M.; Xu, H.; Xie, M.; Li, H.; Xie, J. Direct Z-Scheme Red Carbon Nitride/Rod-like Lanthanum Vanadate Composites with Enhanced Photodegradation of Antibiotic Contaminants. Appl. Catal. B Environ. 2020, 277, 119245. [Google Scholar] [CrossRef]
- Dong, S.; Lee, G.J.; Zhou, R.; Wu, J.J. Synthesis of g-C3N4/BiVO4 Heterojunction Composites for Photocatalytic Degradation of Nonylphenol Ethoxylate. Sep. Purif. Technol. 2020, 250, 117202. [Google Scholar] [CrossRef]
- Hu, H.; Kong, W.; Wang, J.; Liu, C.; Cai, Q.; Kong, Y.; Zhou, S.; Yang, Z. Engineering 2D Compressed Layered G-C3N4 Nanosheets by the Intercalation of BiVO4-Bi2WO6 Composites for Boosting Photocatalytic Activities. Appl. Surf. Sci. 2021, 557, 149796. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, W.; Hou, F.; Guo, W.; Zhang, Q.; Wang, T.; Wei, A. Visible-Light-Driven Photocatalytic Disinfection of Aspergillus Fumigatus under Germinative Biomorph: Efficiency and Mechanism. Sep. Purif. Technol. 2021, 265, 1–10. [Google Scholar] [CrossRef]
- Iqbal, S.; Bahadur, A.; Javed, M.; Hakami, O.; Irfan, R.M.; Ahmad, Z.; AlObaid, A.; Al-Anazy, M.M.; Baghdadi, H.B.; Abd-Rabboh, H.S.M.; et al. Design Ag-Doped ZnO Heterostructure Photocatalyst with Sulfurized Graphitic C3N4 Showing Enhanced Photocatalytic Activity. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2021, 272, 115320. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, M.; Li, Y.; Shuai, D. Visible-Light-Driven Photocatalytic Disinfection of Human Adenovirus by a Novel Heterostructure of Oxygen-Doped Graphitic Carbon Nitride and Hydrothermal Carbonation Carbon. Appl. Catal. B Environ. 2019, 248, 11–21. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, G.; Shao, C.; Li, X.; Jiang, X.; Liu, Y. Fabrication of g-C3N4/SiO2-Au Composite Nanofibers with Enhanced Visible Photocatalytic Activity. Ceram. Int. 2017, 43, 15699–15707. [Google Scholar] [CrossRef]
- Akulinkin, A.; Bolgaru, K.; Reger, A. Facile Synthesis of Porous g-C3N4/β-SiAlON Material with Visible Light Photocatalytic Activity. Mater. Lett. 2021, 305, 130788. [Google Scholar] [CrossRef]
- Jourshabani, M.; Dominic, J.A.; Achari, G.; Shariatinia, Z. Synergetic Photocatalytic Ozonation Using Modified Graphitic Carbon Nitride for Treatment of Emerging Contaminants under UVC, UVA and Visible Irradiation. Chem. Eng. Sci. 2019, 209, 115181. [Google Scholar] [CrossRef]
- Wang, T.; Sun, M.; Sun, H.; Shang, J.; Wong, P.K. Efficient Z-Scheme Visible-Light-Driven Photocatalytic Bacterial Inactivation by Hierarchical MoS2-Encapsulated Hydrothermal Carbonation Carbon Core-Shell Nanospheres. Appl. Surf. Sci. 2019, 464, 43–52. [Google Scholar] [CrossRef]
- Yin, J.; Liao, G.; Zhu, D.; Lu, P.; Li, L. Photocatalytic Ozonation of Oxalic Acid by g-C3N4/Graphene Composites under Simulated Solar Irradiation. J. Photochem. Photobiol. A Chem. 2016, 315, 138–144. [Google Scholar] [CrossRef]
- Ling, Y.; Liao, G.; Feng, W.; Liu, Y.; Li, L. Excellent Performance of Ordered Ag-g-C3N4/SBA-15 for Photocatalytic Ozonation of Oxalic Acid under Simulated Solar Light Irradiation. J. Photochem. Photobiol. A Chem. 2017, 349, 108–114. [Google Scholar] [CrossRef]
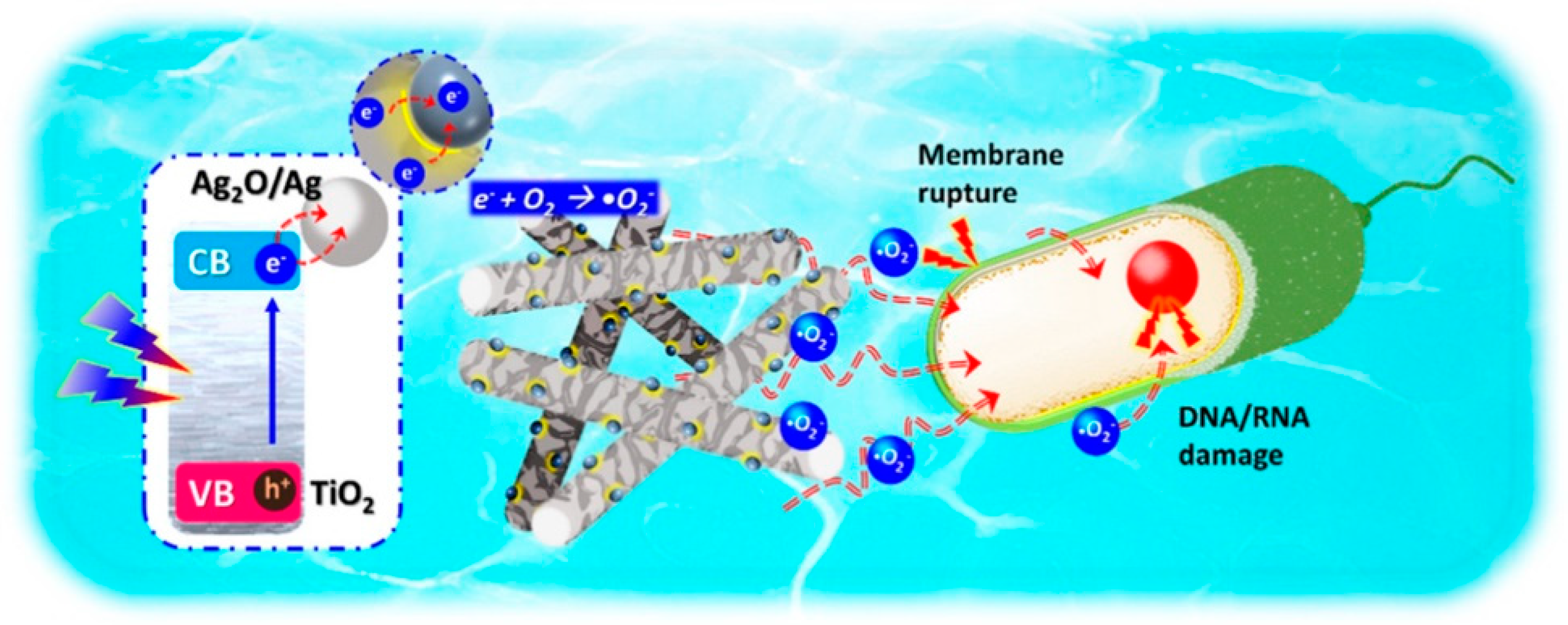
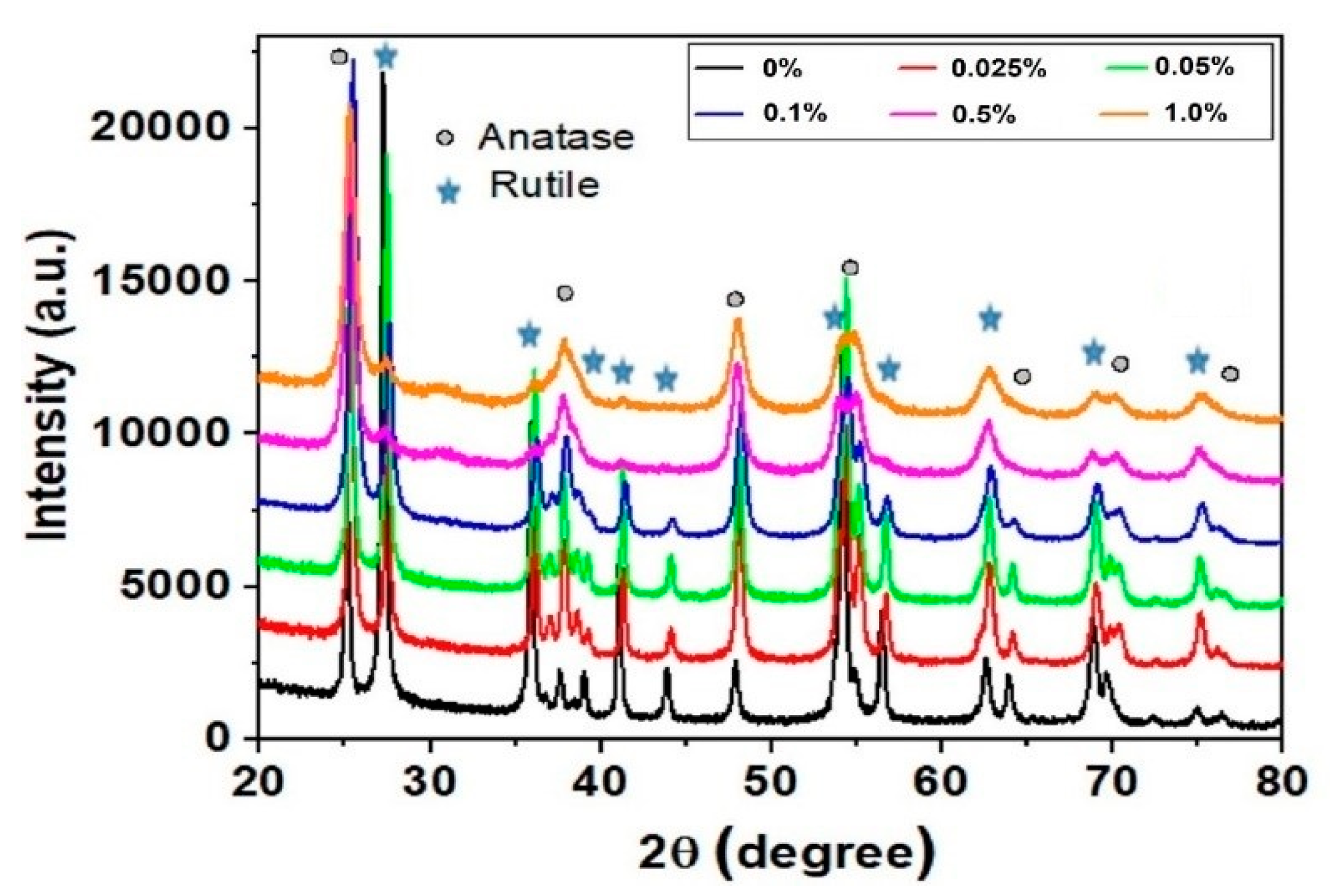
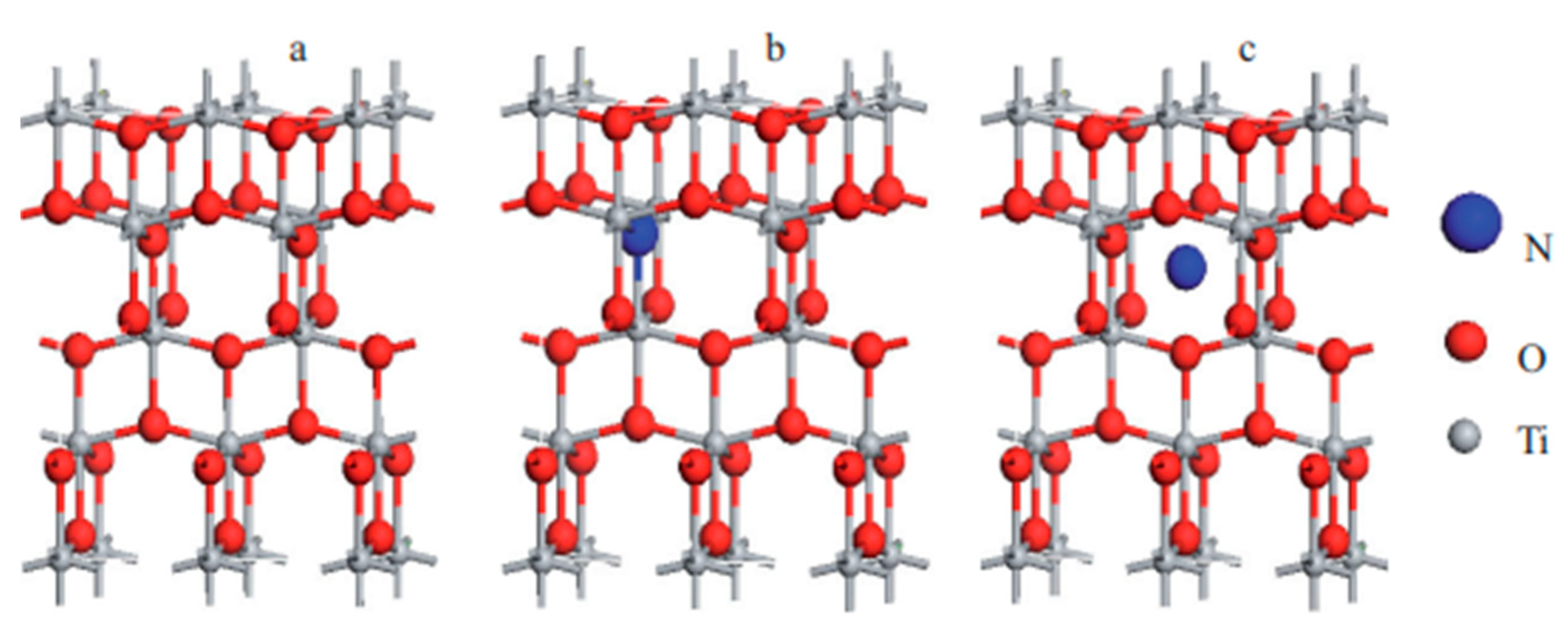
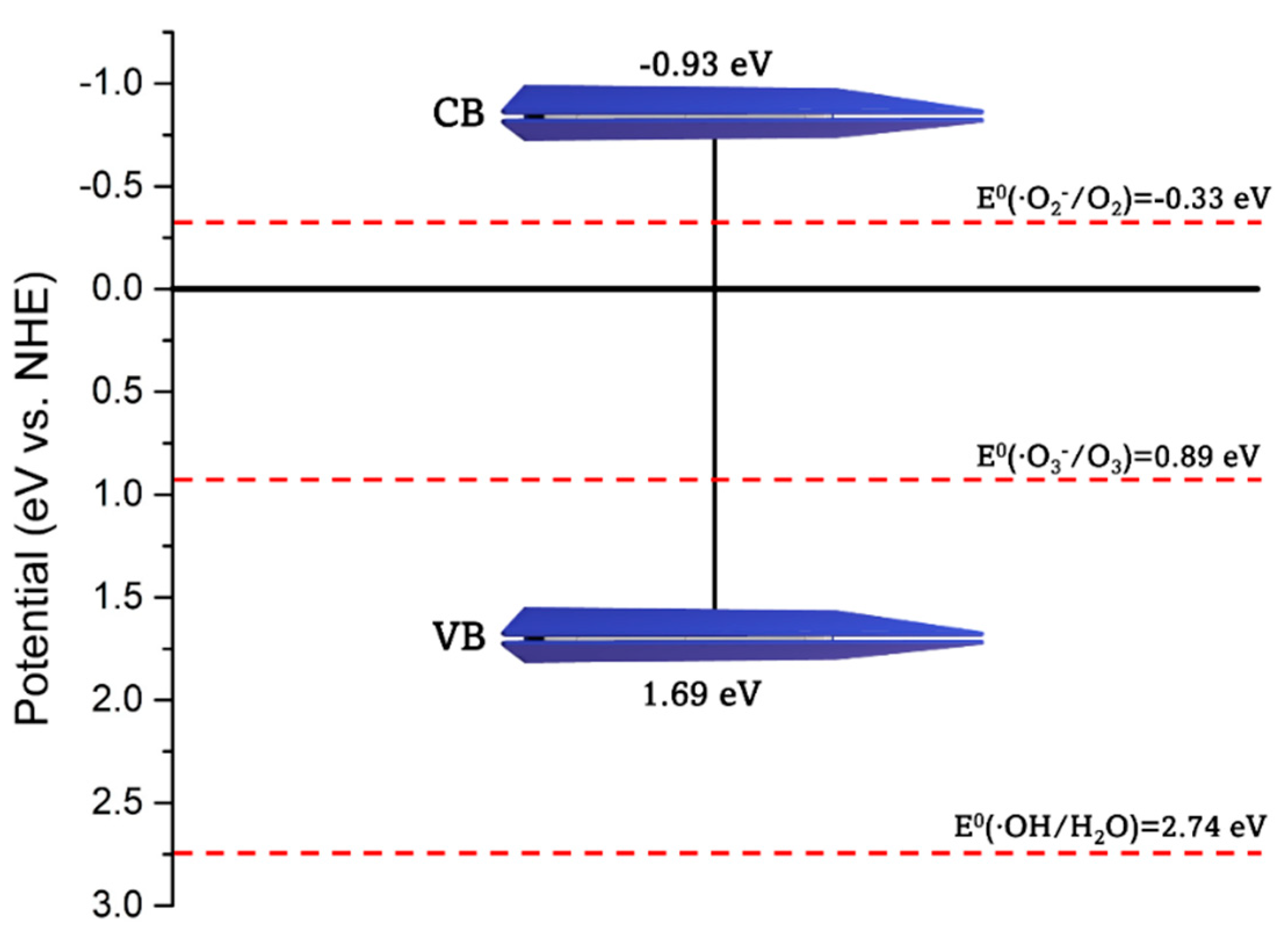
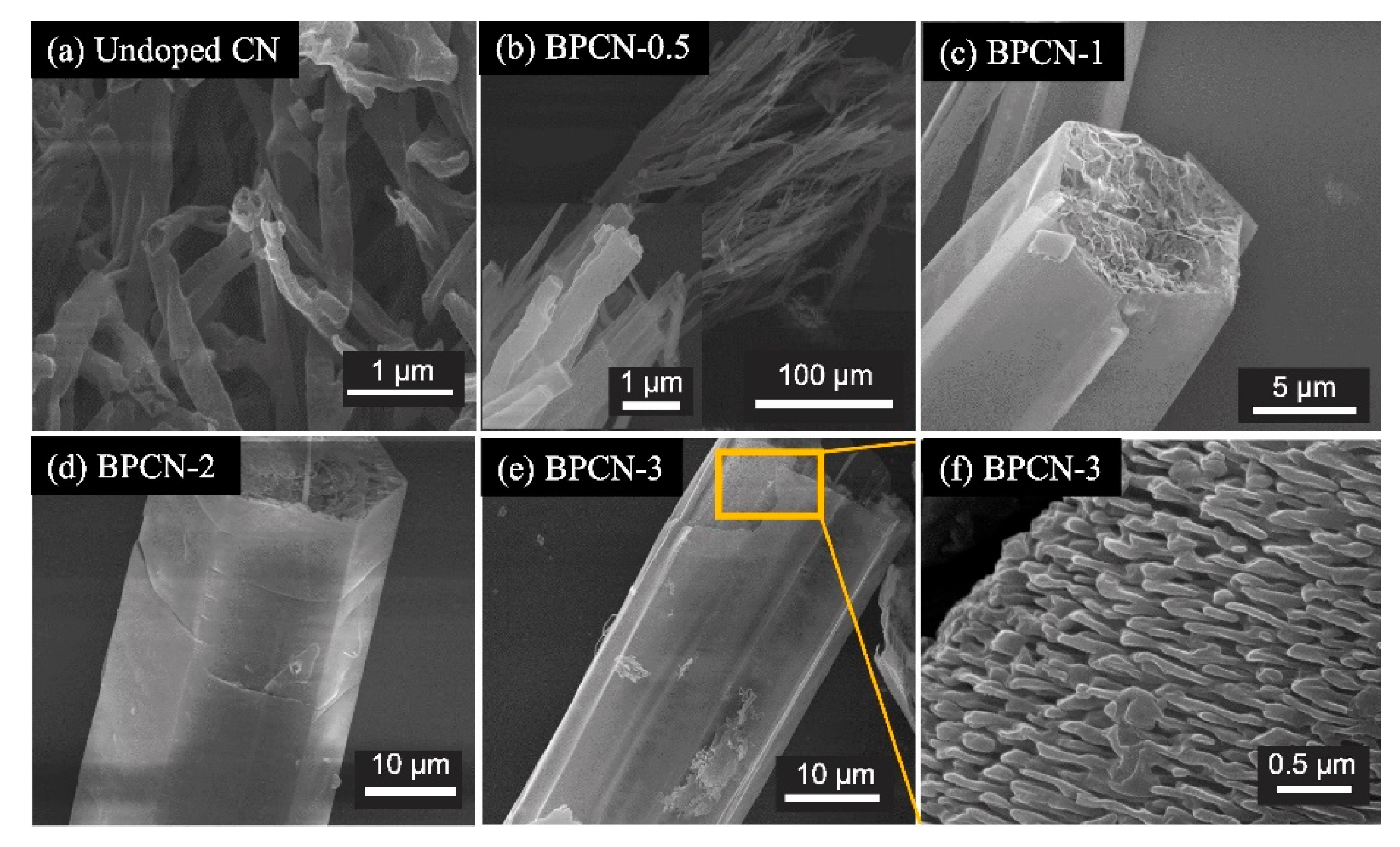
| Catalyst | Precursor | Method | Ebg (eV) | Radiation Source | Contaminant | Removal (Time) | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Ti | Dopant | |||||||
| C-Co-TiO2 | TTIP | Glucose and Cobalt Chloride | Co-precipitation | 2.81 | Visible | Phenol, 100 mg L−1 | 100% (120 min) | [87] |
| C-N-TiO2 | TBOT | Extrapallial Fluid of Mussels | Chemical Deposition | 2.90 | Visible | High-density Polyethylene, 0.4% w/v | 72.0% (50 h) | [88] |
| C-N-S-TiO2 | Ti2(SO4)3 | Thiourea | Hydrothermal | 2.90 | Visible | Ibuprofen, 20 mg L−1 | 100% (5 h) | [86] |
| Cu-N-TiO2 | TTIP | Urea and Copper (III) Nitrate | Sol-gel | - | Visible | Methylene Blue, 12.5 mg L−1 | 56.3% (90 min) | [89] |
| F-N-TiO2 | TBOT | Ammonium Fluoride and 2-nitrophenol | Solvothermal | - | Solar | 2-nitrophenol, 10 mg L−1 | ~98.1% (75 min) | [90] |
| Ammonium Fluoride and 4-nitrophenol | - | Solar | 4-nitrophenol, 10 mg L−1 | ~93.9% (75 min) | ||||
| F-Pd-TiO2 | TBOT | Trifluoroacetic Acid and Palladium Chloride | Microwave- assisted Hydrothermal | 0.54 | Solar | Sulfamethoxazole, 30 mg L−1 | 98.4% (40 min) | [85] |
| Fe-Eu-TiO2 | TTIP | Europium Oxide and Iron Nitrate | Sol-gel | 2.78 | Visible | Methylene Blue and Methyl Orange, 5 mg L−1 | 97.9% MB and 99.7% MO (180 min) | [45] |
| Fe-N-TiO2 | TTIP | Urea and Iron Acetylacetonate | Sol-gel | 2.70 | Visible | Acid Orange Azo Dye, 10 mg L−1 | 90% (60 min) | [59] |
| Fe-Pr-TiO2 | TTIP | Praseodymium Nitrate and Iron Acetylacetonate | Sol-gel | 2.70 | Visible | Acid Orange Azo Dye, 10 mg L−1 | 87% (60 min) | [46] |
| Catalyst | Precursor | Method | Ebg (eV) | Radiation Source | Contaminant | Removal (Time) | Ref. | |
|---|---|---|---|---|---|---|---|---|
| W | Dopant | |||||||
| Ag-WO3 | Sodium Tungstate | Silver Nitrate | Hydrothermal, HCl | 2.63 | Solar | Acetaminophen, 5 mg L−1 | 75.4% (120 min) | [92] |
| Cd-WO3 | - | Cadmium Nitrate | Ion-exchange | 1.85 | Visible | Methylene Blue, 10 mg L−1 | 75.5% (80 min) | [93] |
| Co-WO3 | Sodium Tungstate | Cobalt Chloride | Co-precipitation | - | Visible | Methyl Red, 10 mg L−1 | 90% (120 min) | [94] |
| Cu-WO3 | - | Copper Nitrate | Precipitation | 2.60 | Visible | Tetracycline, 50 mg L−1 | 96.7% (120 min) | [95] |
| Fe-WO3 | Ammonium Paratungstate | Iron Chloride | Sol-gel | 2.39 | Visible | Methylene Blue, 10 mg L−1 | 95% (120 min) | [96] |
| Gd-WO3 | Sodium Tungstate | Gadolinium Nitrate | Hydrothermal | 2.64 | Visible | Rhodamine B, 20 mg L−1 | 94% (100 min) | [97] |
| Mn-WO3 | Tungstic Acid | Manganese Chloride | Microwave- assisted Precipitation | 2.00 | Visible | Sulfamethoxazole, 1 mg L−1 | 100% (70 min) | [98] |
| C-WO3 | Sodium Tungstate | Carbonized Glucose | Hydrothermal | - | UV-Visible | Rhodamine B, 20 mg L−1 | 95% (180 min) | [99] |
| I-WO3 | Ammonium Paratungstate and Spondias mombin leaves extract | Ammonium Iodide | Hydrolysis and Precipitation | 2.17 | Solar | Dyeing Wastewater, TOC = 576.8 mg L−1 COD = 991 mg L−1 | 88.2% TOC 89.1% COD (240 min) | [100] |
| P-WO3 | Ammonium Phosphate | 2.41 | 86.8% TOC 86.6% COD (240 min) | |||||
| P-I-WO3 | Ammonium Phosphate and Ammonium Iodide | 2.02 | 93.4% TOC 95.1% COD (240 min) | |||||
| S-WO3 | Sodium Tungstate | Thiourea | Hydrothermal | - | Visible | Methyl Orange, 20 mg L−1 | 97% (3 h) | [101] |
| Composite | Radiation Source | Contaminant | Oxidant | Results | Ref. |
|---|---|---|---|---|---|
| CNT/TiO2 | UV | Oxamic acid, 89 mg L−1 | O2 | 70% removal in 60 min | [166] |
| O3 | 100% removal in 60 min | ||||
| - | 24% removal in 60 min | ||||
| Fe3O4/TiO2 | UV-A | Ceftazide, 10 mg L−1 | O2 | 34.6% removal in 15 min | [168] |
| O3 | 100% removal in 15 min | ||||
| - | 86.7% removal in 15 min | ||||
| AC/Fe3O4/ TiO2 | Solar | Metoprolol, Ibuprofen, Clofibric acid and DEET, 2 mg L−1 each | O2 | 56% and 45% removals of contaminants mixture in 120 min in, respectively, synthetic and real secondary effluent, and up to 40% of DOC removal | [167] |
| O3 | 100% removal of contaminants mixture in 15 min and up to 70% of DOC removal | ||||
| - | 100% removal of contaminants mixture in 30 min and up to 25% of DOC removal | ||||
| GO/Fe3O4/TiO2 | Solar | Cotinine, Caffeine, Ciproflaxin, Metoprolol, Sulfamethoxazole, Bezafibrate, Tritosulfuron, Ibuprofen, Clofibric acid, and DEET, 0.5 mg L−1 each | O3 | 70% of TOC removal in 120 min at pH = 4 in urban wastewater | [169] |
| - | 63% of TOC removal in 120 min at pH = 4 in urban wastewater |
| Composite | SBET (m2 g−1) | Ebg (eV) | Radiation Source | Contaminant | Removal (Time) | Ref. |
|---|---|---|---|---|---|---|
| Bi4O7/g-C3N4 | 109 | - | Visible | Aspergillus fumigatus, 106 CFU mL−1 | 81% (6 h) | [179] |
| BiVO4/g-C3N4 | 7 | 2.43 | Visible | Nonylphenol Ethoxylate, 50 ppm | 100% (120 min) | [180] |
| BiVO4/Bi2O6/g-C3N4 | 95.9 | - | Visible | Rhodamine B, 20 mg L−1 | 100% (60 min) | [181] |
| Tetracycline, 20 mg L−1 | 100% (60 min) | |||||
| LaVO4/g-C3N4 | - | - | Visible | Tetracycline, 20 mg L−1 | 83.4% (30 min) | [182] |
| Naproxen, 20 mg L−1 | 80% (120 min) | |||||
| Ag-ZnO/S-g-C3N4 | 57.2 | 2.51 | Solar | Methylene Blue, 10 mg L−1 | 97% (40 min) | [183] |
| HTCC/O-g-C3N4 | - | 0.95 | Visible | Human Adenovirus Type 2, 105 MPN mL−1 | 100% (120 min) | [184] |
| PAN/g-C3N4 | 13.3 | - | UV | Oilfield Produced Water | 96.6% (8 h) | [185] |
| Visible | 85.4% (8 h) | |||||
| PEI/g-C3N4 | 70.2 | 1.74 | Visible | Tetracycline, 40 mg L−1 | 80% (120 min) | [186] |
| PEI/g-C3N4 | - | - | Solar | E. coli, 2 × 106 CFU mL−1 | 100% (45 min) | [187] |
| Enterococcus faecalis, 2 × 106 CFU mL−1 | 67.7% (60 min) | |||||
| Au-SiO2/g-C3N4 | 365 | - | Visible | Rhodamine B, 10 mg L−1 | 99.8% (90 min) | [188] |
| β-SiAlON/g-C3N4 | - | - | Visible | Murexide, 250 mg L−1 | 90% (8 h) | [189] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, E.; Gomes, J.; Martins, R.C. Semiconductors Application Forms and Doping Benefits to Wastewater Treatment: A Comparison of TiO2, WO3, and g-C3N4. Catalysts 2022, 12, 1218. https://doi.org/10.3390/catal12101218
Fernandes E, Gomes J, Martins RC. Semiconductors Application Forms and Doping Benefits to Wastewater Treatment: A Comparison of TiO2, WO3, and g-C3N4. Catalysts. 2022; 12(10):1218. https://doi.org/10.3390/catal12101218
Chicago/Turabian StyleFernandes, Eryk, João Gomes, and Rui C. Martins. 2022. "Semiconductors Application Forms and Doping Benefits to Wastewater Treatment: A Comparison of TiO2, WO3, and g-C3N4" Catalysts 12, no. 10: 1218. https://doi.org/10.3390/catal12101218
APA StyleFernandes, E., Gomes, J., & Martins, R. C. (2022). Semiconductors Application Forms and Doping Benefits to Wastewater Treatment: A Comparison of TiO2, WO3, and g-C3N4. Catalysts, 12(10), 1218. https://doi.org/10.3390/catal12101218