Tailoring Structure: Current Design Strategies and Emerging Trends to Hierarchical Catalysts
Abstract
1. Introduction
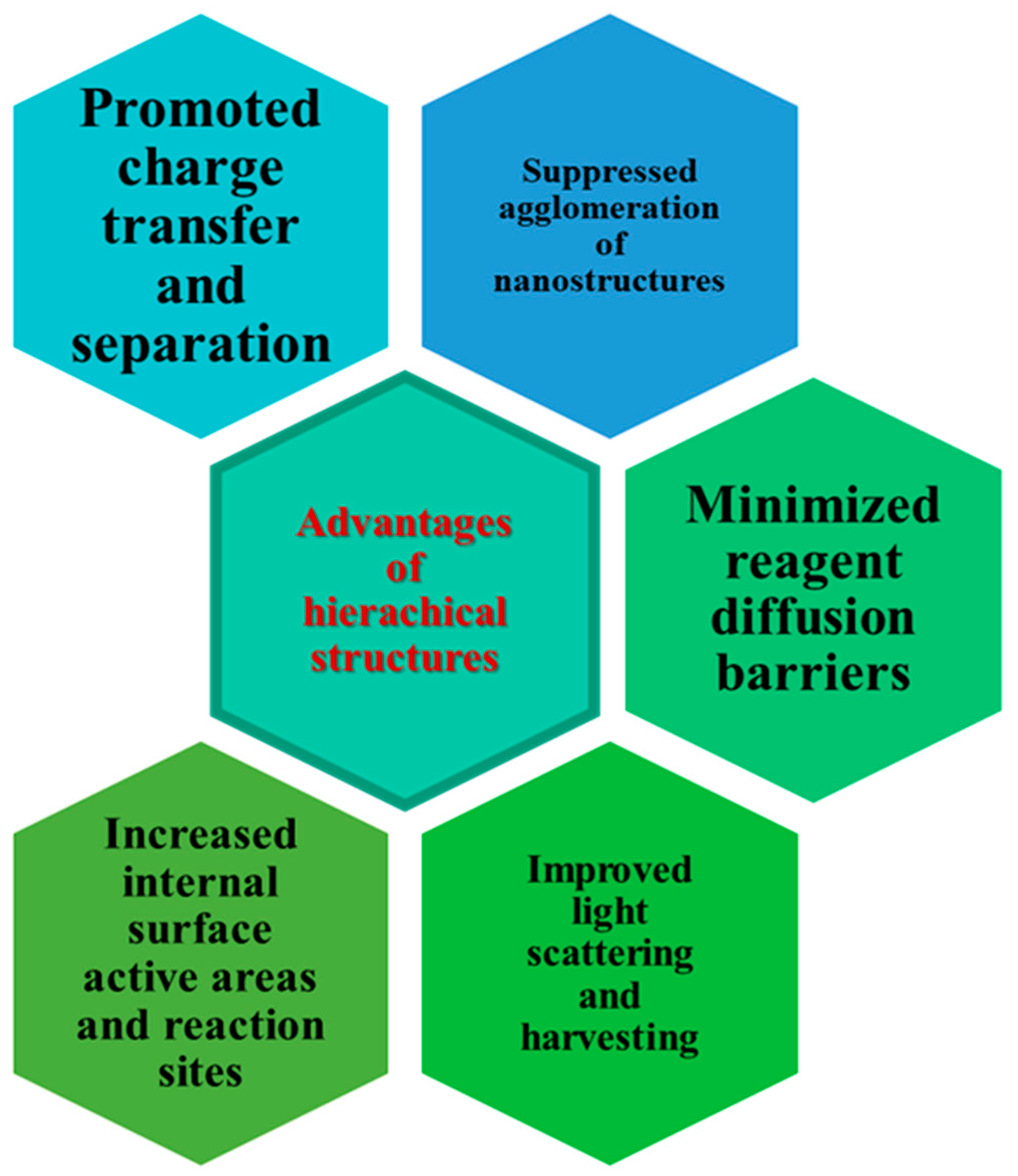
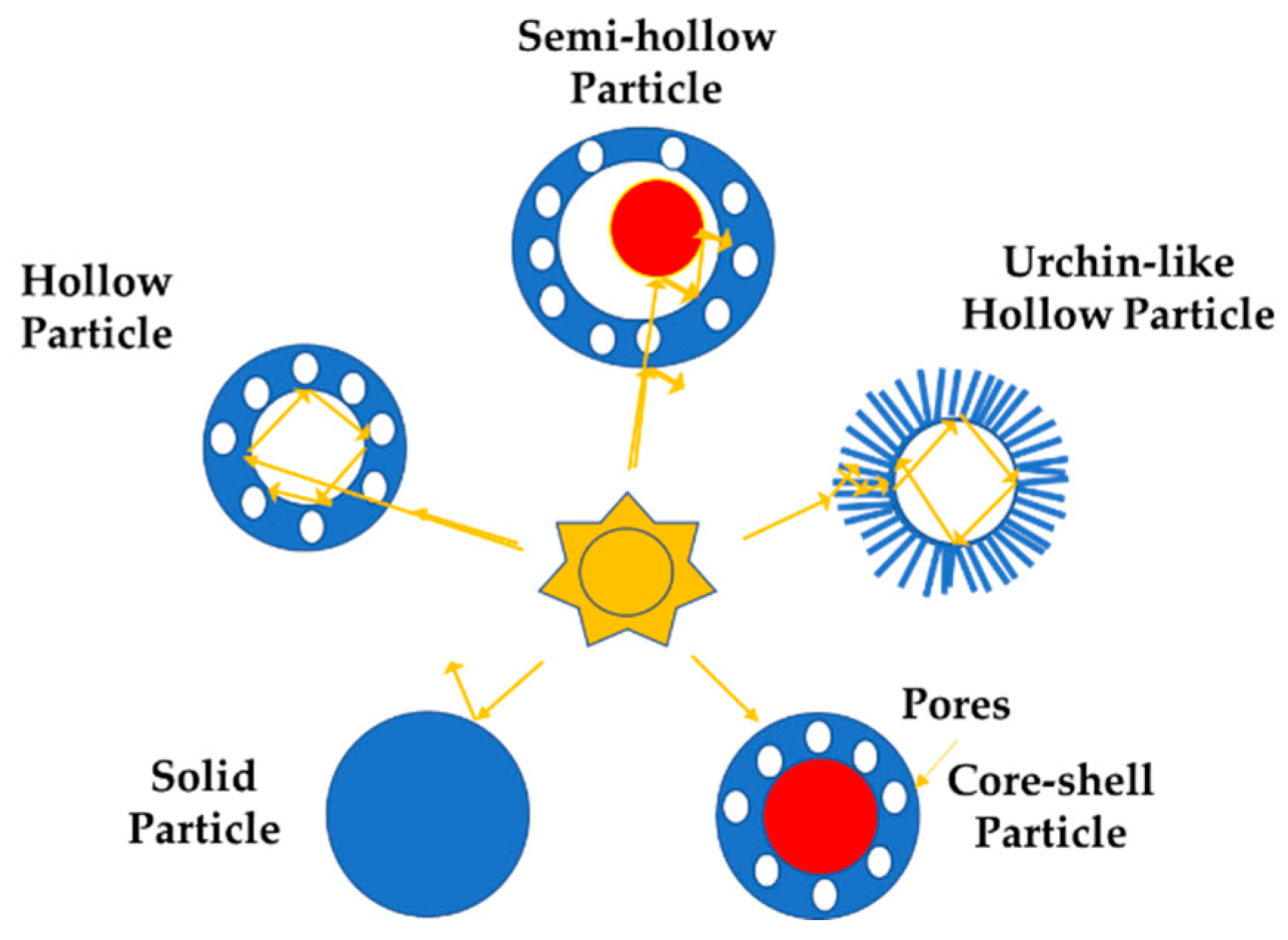
2. Hierarchical Photocatalysts
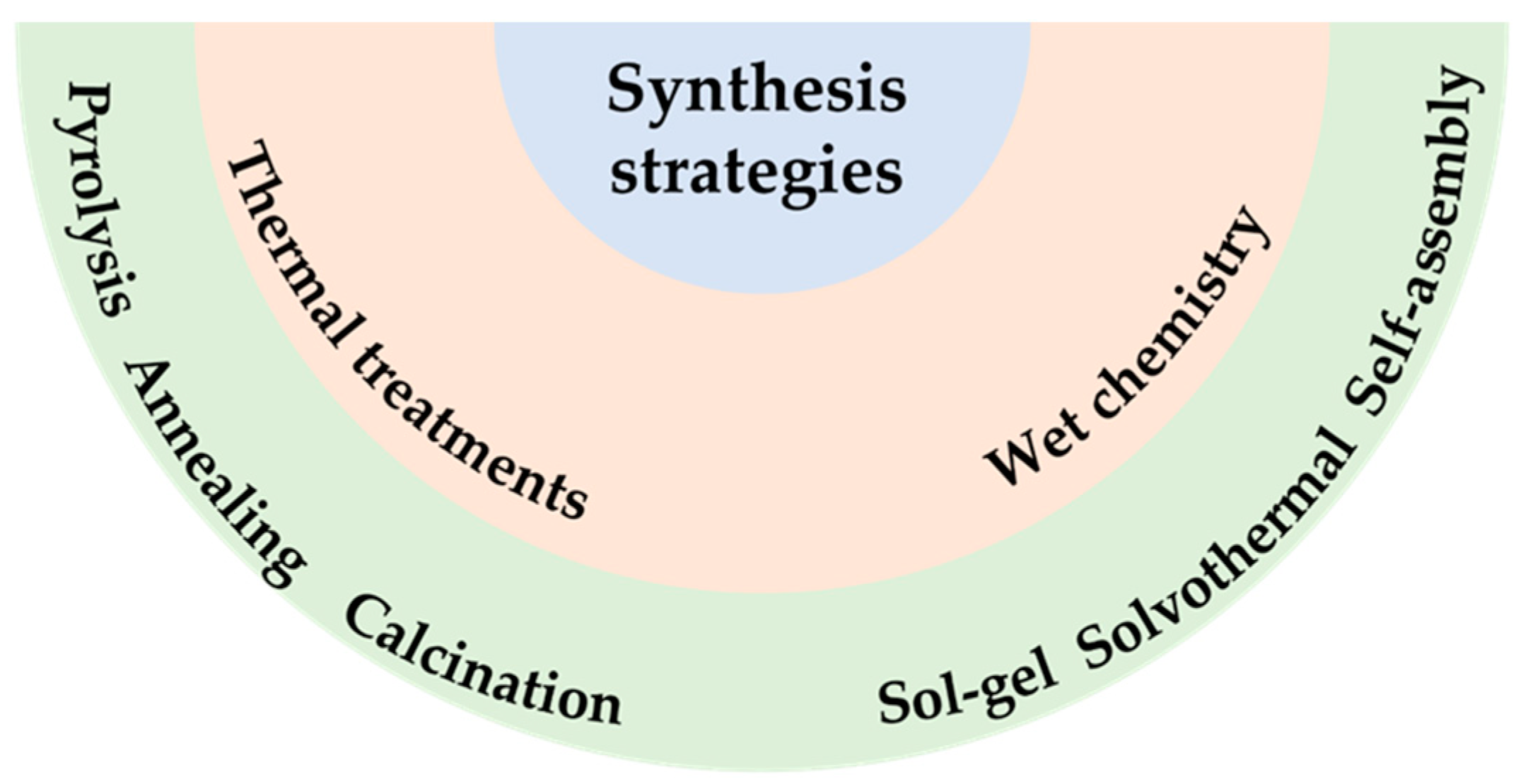
Synthesis Approaches to Hierarchical Structures
3. Inorganic Templates
4. Organic Templates
| Composition | Synthesis Strategy | Morphology | SSA (m2/g) | Application | Activity | Irradiation | Template(s) | Template Nature | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Anatase TiO2 | Sol-gel | Hierarchical ordered macro-meso | 125.91 | photodegradation of aqueous Rhodamine B (RhB) | 90% | 365 nm | copolymer P12/oyster shell | Soft/hard | [98] |
| Dimethylglyoxime (DMG)/TiO2/polyacrylonitrile (PAN) nanofiber | Electrospinning | Nanofibers | 50–60 | Photocatalytic MB degradation efficiency | 97% | Visible Light | PAN/PVP | Soft | [103] |
| Bi2WO6/WO3/PAN | Electrospinning and solvothermal process | Nanofibrous membrane | - | Degradation of cationic pollutants | 85% of RhB, 87.8% for BQ and 95.7% for IPA, 96% of MB, 77.4% of chlortetracycline hydrochloride and 90.37% of tetracycline hydrochloride | UV-Vis | Triton-X | Soft | [104] |
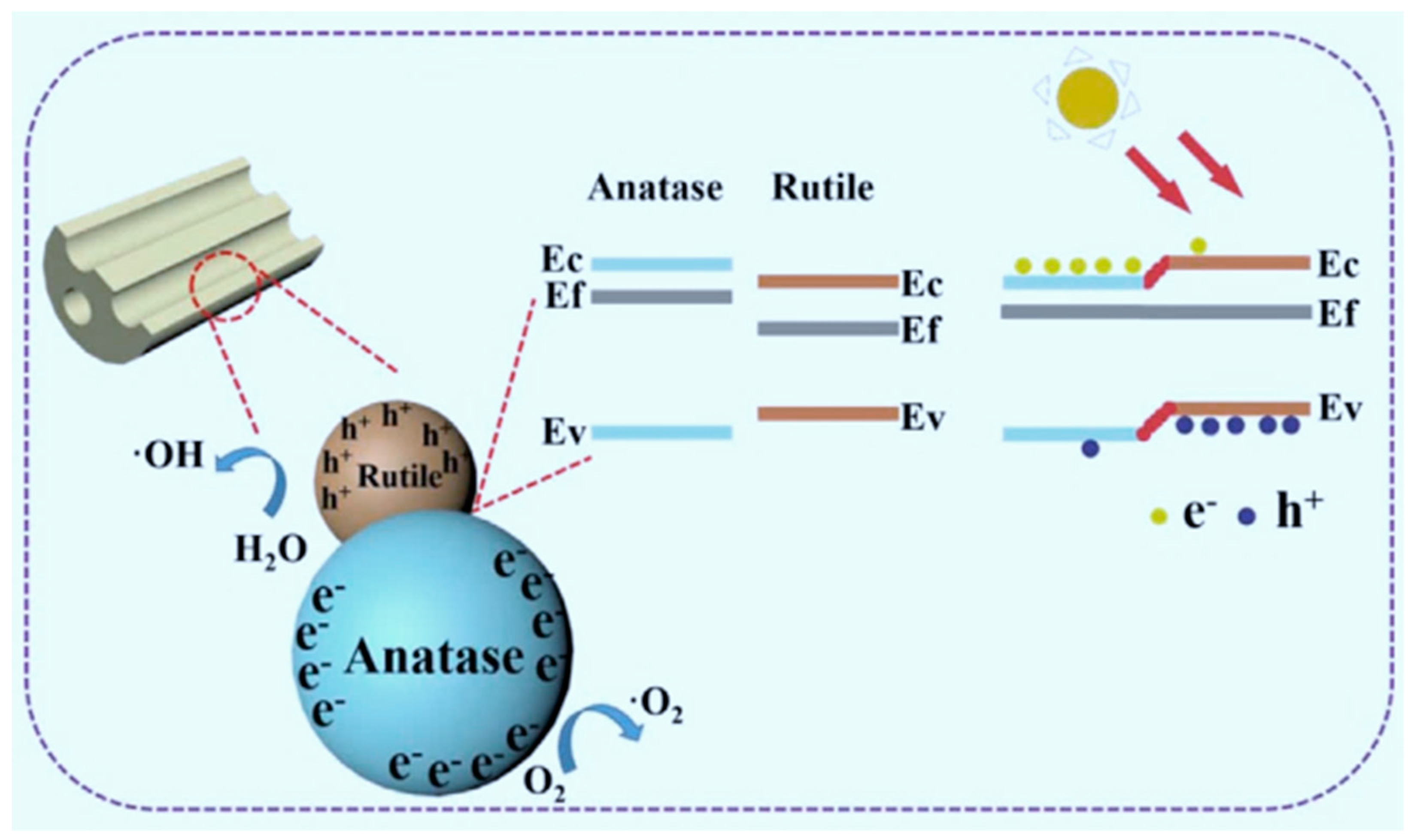
| Anatase/rutile TiO2 | microemulsion electrospinning (ME-ES)/pyrolysis | multi-channel irregular mesoporous TiO2 nanofibers (hundreds of nm in diameter) | / | Photodegradation of aqueous methylene Blue | 100% | 365 nm | CTAB | soft | [105] |
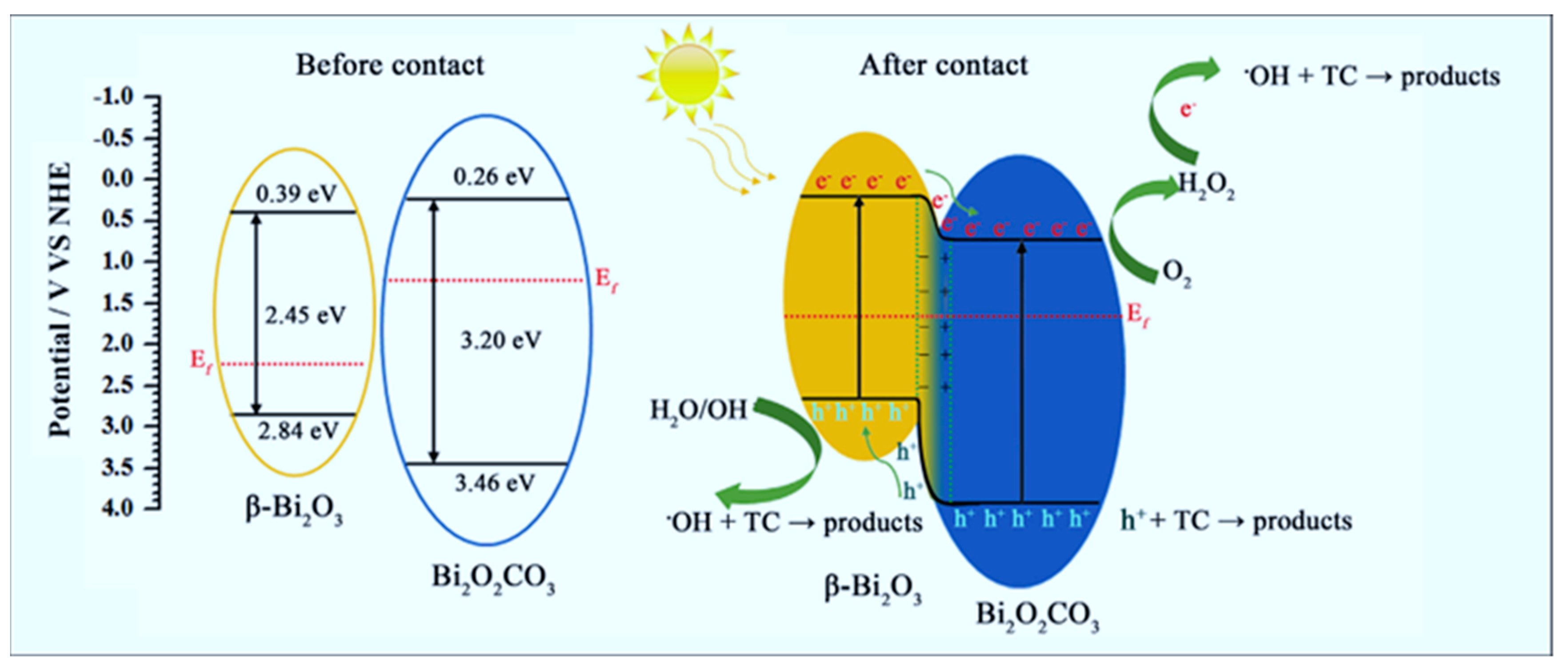
| β-Bi2O3/Bi2O2CO3 | Sol-gel with reflux | Mesoporous Micrometric flower-like structures | 27.78 | Degradation of tetracycline | 98.79% | Sunlight | DL-aspartic acid (DLAA)/Pluronic F123 | soft | [106] |
| Composition | Synthesis Strategy | Morphology | SSA (m2/g) | Application | Activity | Irradiation | Template(s) | Template Nature | Reference |
|---|---|---|---|---|---|---|---|---|---|
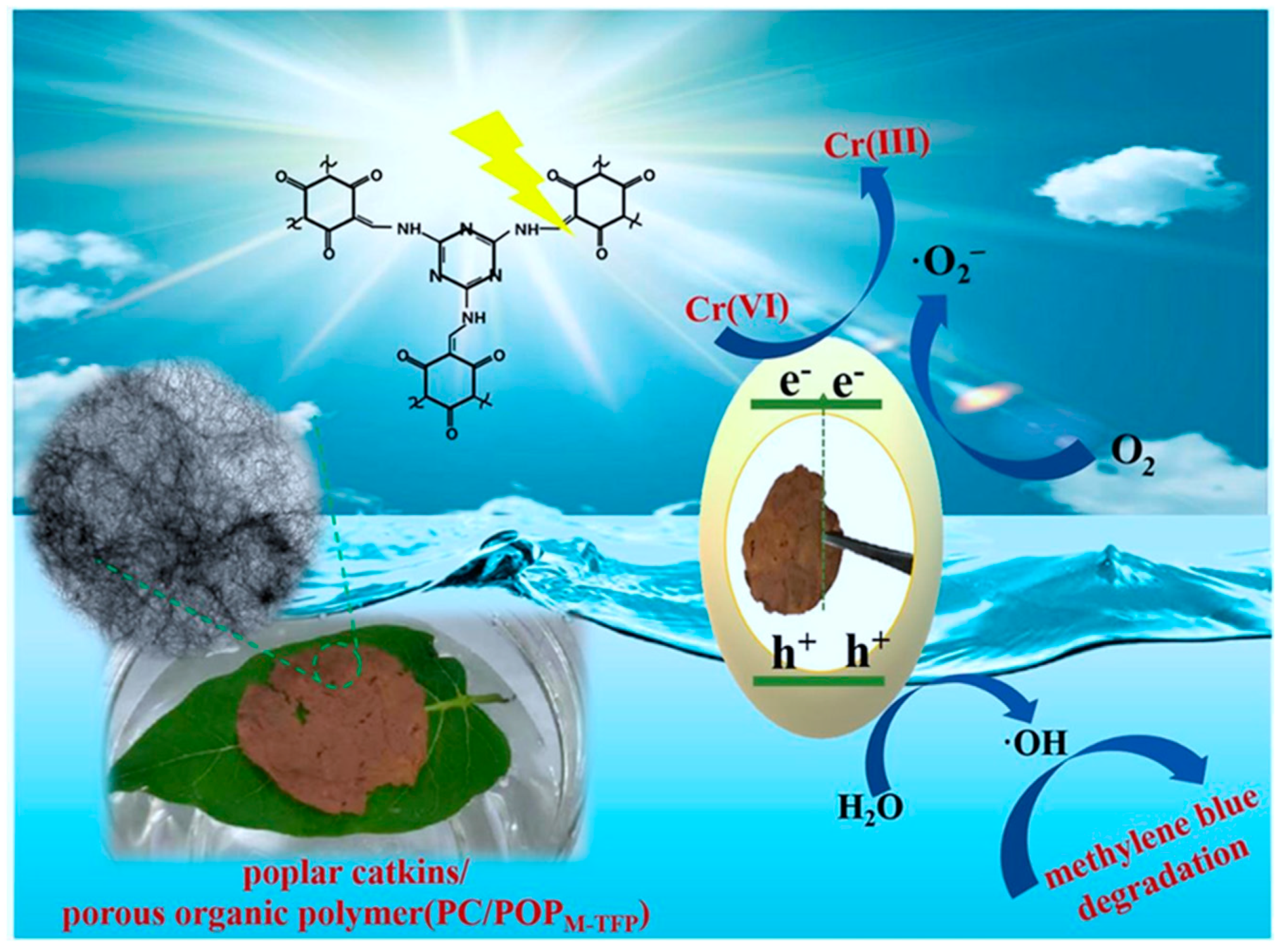
| PC/POP | hydrothermal | Self-supporting film-like structures | 122.5 | Cr(IV) photoreduction/methylene blue photodegradation | 100% Cr (IV) reduction/100% methylen blue photodegradation | Visible light | Poplar catckins | Hard | [100] |
| α-Fe2O3 | Sol-gel | hierarchical porous and fibrous structures (15 μm diameter, hundreds μm length) | 51.3 | Photodegradation of aqueous methylene Blue | 96.8% | Visible light | Cotton | hard | [102] |
| H3PW12O40/TiO2 | Sol-gel & calcination | nanotubes | 80.7 | Methylene blue photodegradation | 95% methylene blue degradation | UV light | Cellulose | soft | [107] |
| Cellulose-TiO2 | non-solvent induced phase separation (NIPS) technique | Macro-Mesoporous composite monoliths | 16.96 | photodegradation of aqueous methylene Blue | 99% | UV lamp | Cellulose | soft | [108] |
| - | - |
5. Hybrid Templates
| Composition | Synthesis Strategy | Morphology | SSA (m2/g) | Application | Activity | Irradiation | Template(s) | Reference |
|---|---|---|---|---|---|---|---|---|
| Rutile/anatase TiO2 | Sol-gel | Submicrometric parallelepiped with rounded corners | 19 | Photocatalytic reduction of water | 1394 μmol h−1 g−1 H2 production rate | UV-vis | MIL-125 | [120] |
| Co3O4 | Oil bath method | 2D hierarchical nanosheets | 24.96 | Efficient photocatalytic conversion of CO2 | 39.70 μmol h−1 CO generation | Visible light | Co/1,4-H2NDC | [110] |
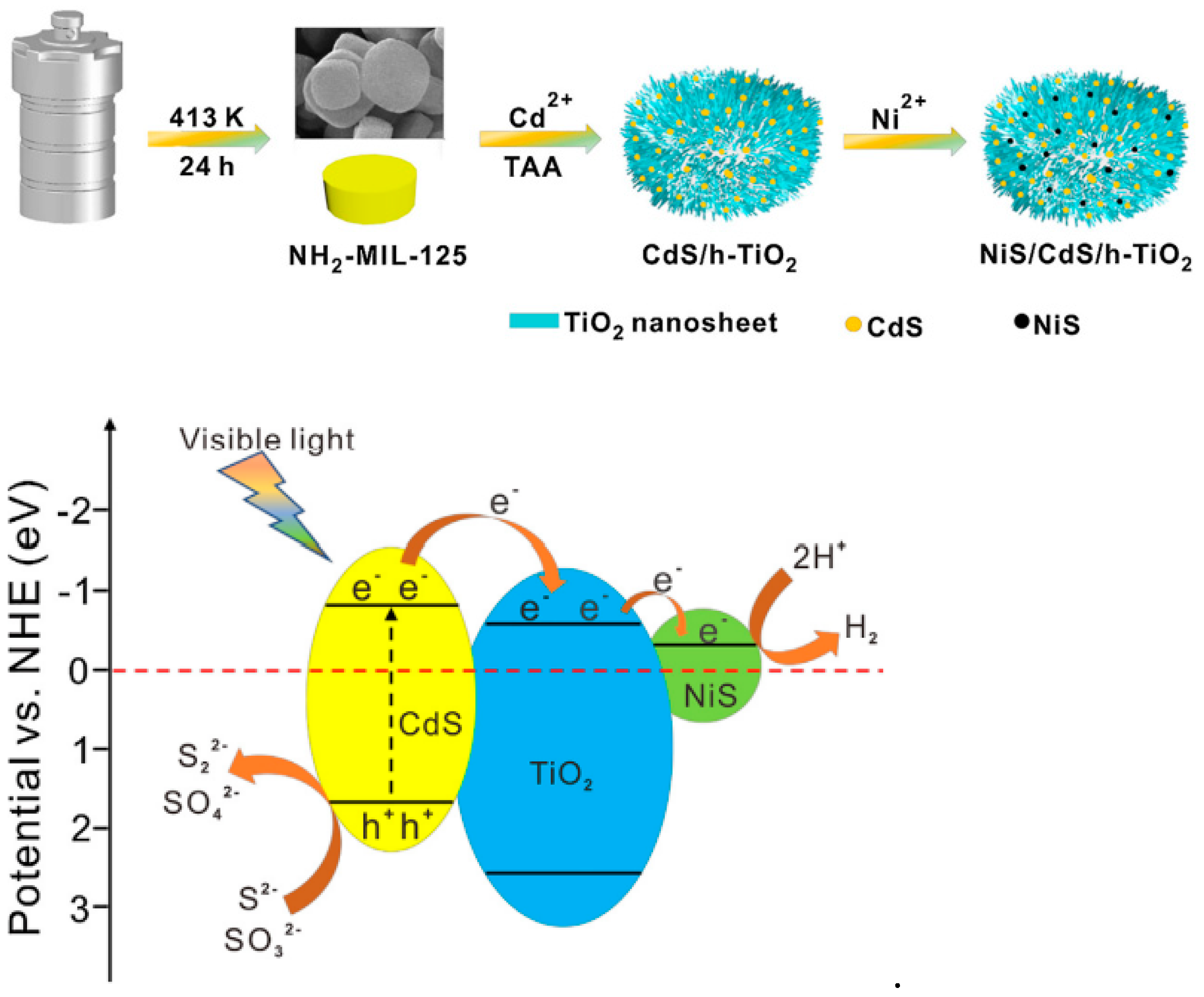
| NiS/CdS/TiO2 | Hydrolysis + sulfidation | Porous/hollow structure | Photocatalytic reduction of water | H2 production rate of 2149.15 µmol g−1 h−1 | Visible light | NH2-MIL-125 | [121] | |
| CdS | Impregnation + pyrolysis | Microporous nanoparticles | 119 | Photocatalytic reduction of water | 634.0 µmol g−1 h−1 | Visible light | MIL-53 (Al) | [109] |
| C-CuO | pyrolysis | Hollow spheres | 72.8 | Photocatalytic reduction of water | 67.3 mmol/g/h H2 production | Visible light | Cu/benzoic acid/1,4 dicarboxybenzene | [111] |
| C-ZnO | Pyrolysis under N2 atmosphere | ZnO crystals embedded within a porous carbonaceous matrix | 500 | Adsorption and photodegradation of RhB | 100% adsorption efficiency | // | MOF-5 | [123] |
| Pd-Anatase TiO2 | pyrolysis | Submicron TiO2 tablets | Photocatalytic reduction of water | 979.7/112.7 mol h−1 H2 production | UV/solar light | NH2-MIL-125 | [114] | |
| Ni/g-C3N4 | Pyrolysis under Ar atmosphere | layered platelets with curled edges | 64.9 | Photocatalytic reduction of water | H2 production rate of 2989.5 μmol g−1 h−1 | Visible light | 2D Co-MOF | [125] |
| W/Co3S4 | Hydrothermal treatment | 10 mm square sheet arrangement | 10.93 | Photocatalytic reduction of water | 85.7 μmol/h H2 production rate | Visible light | Co-ZIF-9 | [126] |
| Cu/TiO2 | Photolysis under N2 atmosphere | Mesoporous core-shell Cu/TiO2 hybrid nanoplatforms | - | Photocatalytic reduction of water | 334 μmol g−1 h−1 H2 production | Simulated light | Cu-MOF | [127] |
| TiO2–NiCoS-PC | Annealing under Ar atmpsphere | Spherical porous carbon shell Embedding TiO2 | 93.5 | Photocatalytic reduction of water | 1.29 mmol h−1 g−1 | Visible light | NiCo-ZIF | [129] |
| MoS2−Zn0.5Co0.5S | Hydrothermal | Hollow rhombic dodecahedra | 57 | Photocatalytic reduction of water | 15.47 mmol h−1 g−1 | UV-Vis | ZnxCo1−x-ZIF | [122] |
| MnS/In2S3 | Solvothermal | Sub-micro rods | / | photocatalytic CO2 reduction | 58 μmol g−1 h−1 CO production rate | Xenon lamp 300 W (credo UV-vis) | MIL-68 (In) | [131] |
| NiO/CeO2 | Hydrothermal and calcination | Porous microsphere | 31.1 | Dye photodegradation/water splitting | 100% MO and MB degradation/71.5 μmol g−1 H2 production rate | UV | Ni/Ce mixed-metal MOF | [112] |
| Cu/CuO-TiO2 | Sol-gel + calcination | nanoparticles | 45.3 | Photocatalytic reduction of water | 286 mmol g−1 h−1 H2 production rate | Solar (Sun) | Cu-MOF | [132] |
| CdS/ ZnxCo3-xO4 | Hydrothermal method | Hollow polyhedra | 29.6 | Photocatalytic reduction of water | 3978.6 μmol g−1 h−1 H2 production rate | Visible | ZnCo-ZIF | [135] |
| In2O3/CuO@N-C | calcination | Pagoda-like heteroepitaxial micro-rods | 147.1 | cross-dehydrogenative coupling (CDC) | 88–99% yield of reaction | Blue light | MIL-68-In | [136] |
| CuInSe2/NiFe2O4 | hydrothermal | Irregular-shaped nanoparticles | 27.54 | Bisphenol-A and resorcinol degradation | 95–95% removal rate | Visible light | Ni–Fe MOF | [137] |
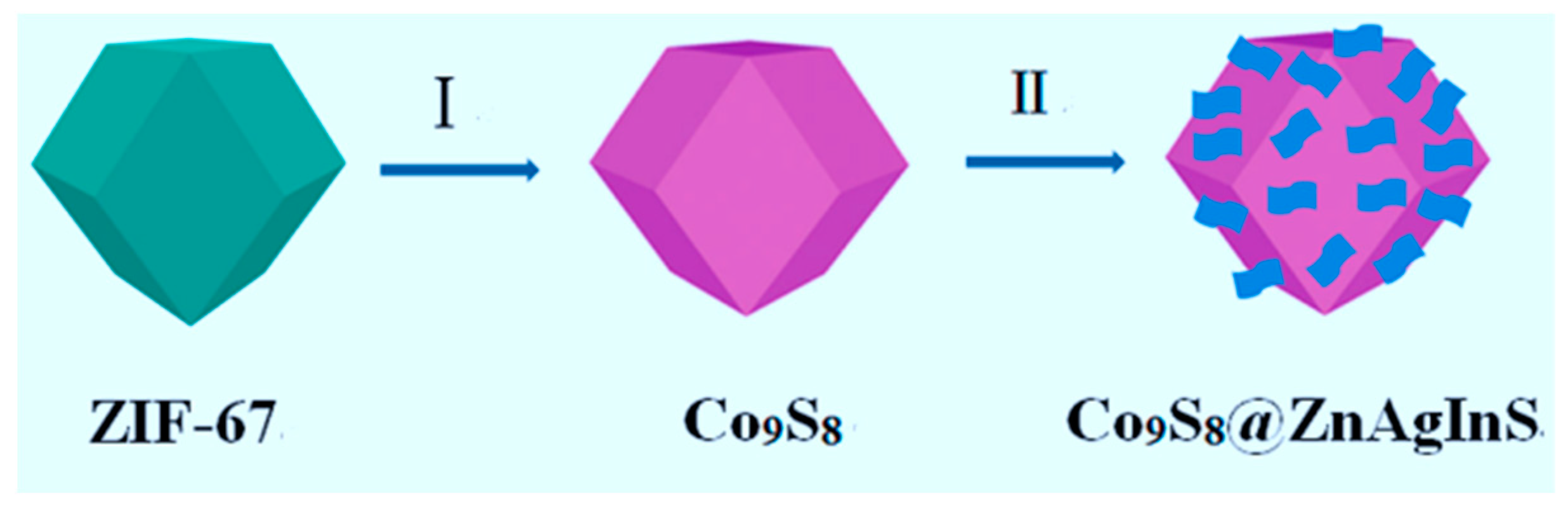
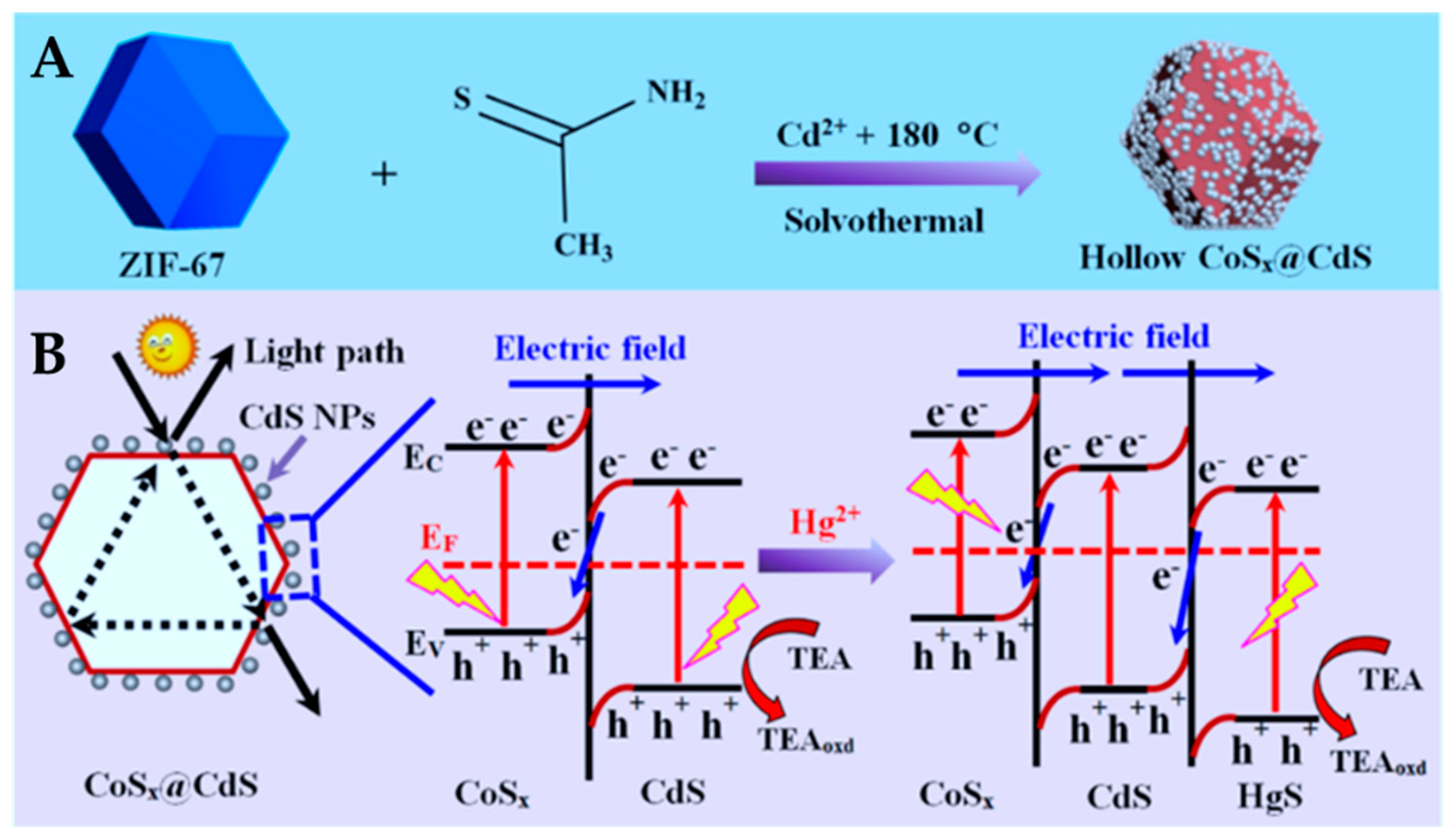
| CoSx/CdS | solvothermal | CdS NPs on the surface of CoSx polyhedrons | / | Hg2+ detection | 0.010 to 1000 nM Hg2+ detection | Visible light | ZIF-67 | [138] |
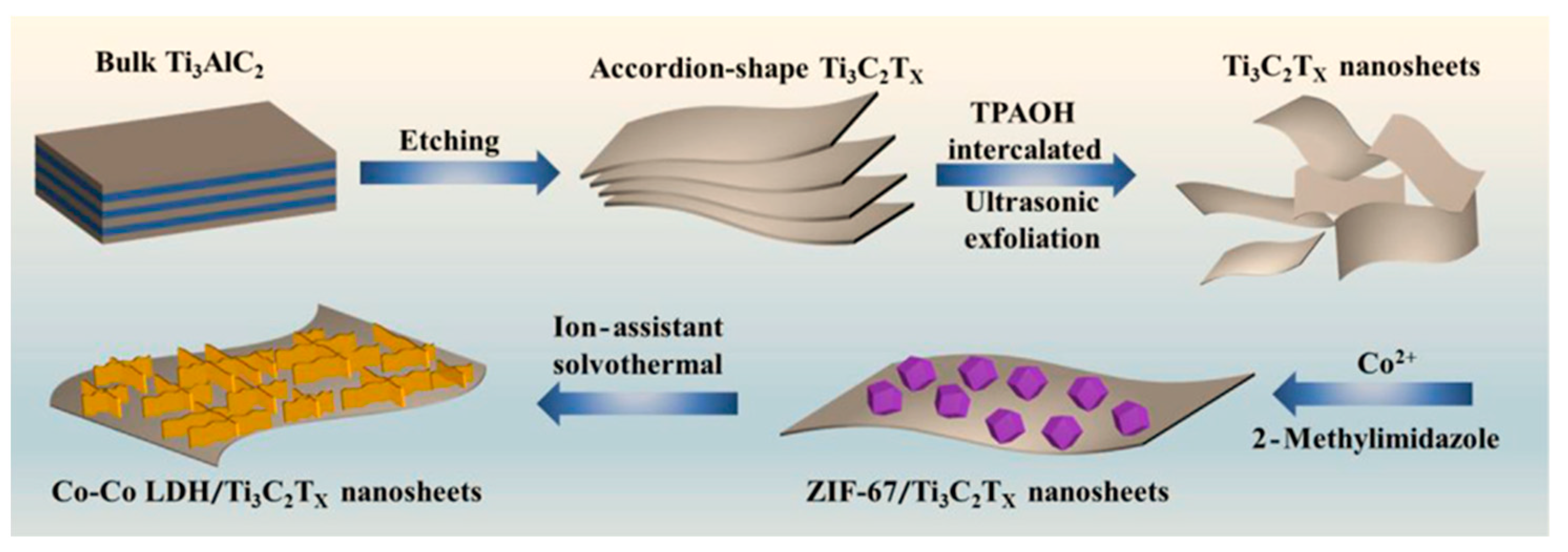
| Co-Co LDH/Ti3C2TX | solvothermal | 3D nanosheets nanoarray | / | CO2 photoreduction | 1.25 × 104 µmol h−1 g−1 CO production rate | Visible light | ZIF-67 | [113] |
| ZIF-NC/g-C3N4 | Thermal treatment | ZIF-NC dodecahedra deposited onto g-C3N4 layers | / | bisphenol A degradation | 95% removal | Visible light | ZIF-8 | [117] |
| MoS2@Cu/Cu2O@C | Pyrolysis | Hierarchical rough polyhedra | 94 | Selective oxidation of cyclohexane to KA oil | 1.31% conversion rate; 98% selectivity | Visible light | Cu-MOF | [116] |
| ZnO/ZnFe2O4 | calcination | Spherical assemble of flake-like nanosheets | 87.74 | RhB and MB degradation | 100% degradation | UV-vis | Zn(Fe)-MOF | [119] |
6. Template Free Approach
| Composition | Synthesis Strategy | Morphology | SSA (m2/g) | Application | Activity | Irradiation | Ref |
|---|---|---|---|---|---|---|---|
| ZnCuCo layered double hydroxide | Hydrothermal | 3D flower-like hierarchical morphologies with in- terlaced petal-like nanosheet | 86–179 | photocatalytic H2 production and degradation of SMZ | H2 production rate (3700 μmol g−1 h−1, 95% sulfamethazine (SMZ) degradation | Visible light | [43] |
| Ti3C2/Bi2WO6 | hydrothermal | 2D/2D hetero junction i | 33.5–58.3 | Photocatalysis degradation of tetracycline hydrochloride (TC-HCl) | 0.430 min−1 | Visible light | [44] |
| ZnFe2O4 modified Cu2S | in-situ self-assembly method | Dendritic fractal structure similar to a snowflake | - | photocatalytic reduction of nitrobenzene with and degradation of methyl orange and methylene blue dyes proficiently | 98% yield of degradation of nitrobenzene, 94.3 and 86% fordegradation of methyl orange and methylene blue, respectively | Visible light | [45] |
| ZnO-graphene nanocomposite | Solution route | hollow microspheres | 29.7–37.6 | Adsorption/photocatalytic activity towards degradation of water-soluble organic pollutants (such as Rhodamine B, methyl orange, phenol) | 90% adsorption capacity | UV (254 nm) | [141] |
| ZnO | Solvothermal route | Monodisperse microspheres | 18 | Photocatalytic activity for degradation of methylene blue | 100% | UV-Vis | [142] |
| ZnO | Hydrothermal and calcination | Hierarchically porous microspheres composed of nanosheets | 46–91 | Photocatalytic RhB degradation activity | Up to 100% | solar | [60] |
| TiO2 | Hydrothermal route | (i) long and well oriented macrochannels (ii) surface macropores of 0.8–9.3 μm in size, and (iii) porous walls with pores mostly smaller than 0.5 μm. | 82.9–216.1 | Decomposition of methyl ethyl ketone (MEK) in a continuous flow photoreactor | Up to 37% | UV (254 nm) | [144] |
| TiO2 | Hydrothermal route | Partial spherical like structure | 30–43.7 | RhB photodegradation | Up to 98% | Visible light | [145] |
| TiO2 | Hydrothermal route | Nanoflowers with a spherical hierarchical structure | 20–80 | Aqueous methylene blue photo-oxidation | Up to 50% | solar | [146] |
| CeO2 | Hydrothermal route | Mesoporous nanosphere | 42.1–68.2 | photocatalytic activity of rhodamine B (RhB) dye degradation | 97.8–92% RhB dye degradation | UV-Vis and acidic condition | [147] |
| Cu2O | Low temperature route | Spherical, cuboctahedral or cubic nanoparticles | 7–13 | Photocatalytic degradation of the antibiotic trimethoprim | Up to 48% | Visible light | [148] |
| WO3 | Hydrothermal route | Regular-shaped nanosheets with an average thickness of approximately 30–40 nm | 2–18 | Photocatalytic activity towards an aqueous solution of tetracycline (TC) and possess good stability and reusability | Up to 94% | Visible light | [149] |
| Bi2WO6 | Hydrothermal method and calcining process | Mesoporous nanoplate multi-directional | 53.5 | Photocatalytic oxidation of NO | Up to 90% | Solar | [150] |
| Sm, Y, La and Nd-doped CeO2 | hydrothermal | broom-like hierarchical structure | - | BPA degradation and on CO2 evolution from CH3CHO decomposition | Up to 99% | UV | [151] |
| Yttria (Y2O3) nanosphere decorated ceria (CeO2) | Hydrothermal route | Yttrium nanoparticles are anchored on the surface of CeO2 nanorod with a particle size of 10 nm | - | Photocatalytic decomposition of aqueous Rhodamine B | Up to 96% | Solar light | [62] |
| SnS2/TiO2 | Ultrasonic treatment | Ordered channels with a size about 1–3 μm were formed in the particles, and lots of holes appeared on the wall of the channels. | 28 | Photocatalytic degradation of Methyl Orange (MO) | Up to 90.9% | Solar light | [152] |
| ZnO/g-C3N4 | Solvothermal | Porous microsphere with a size of about 700nm. | 9.9–32-7 | Photocatalytic degradation on rhodamine B and phenol | Up to 100% | Solar | [133] |
| ZnO/graphene | Solvothermal | Core-shell structure | 65–201 | Photocatalytic degradation of rhodamine B | Up to 98.5% | Visible light | [153] |
| TiO2 Microspheres with Carbonaceous Species | Solvothermal | Porous structure | 337 | Photodegradation of rhodamine B | Up to 100% | Visible light | [154] |
| Carbon-coated TiO2 | solvothermal | Hierarchical nanotubes | Up to 244.4 | Photocatalysts activity for water oxidation | Up to 705 µmol h−1 g−1 | Solar light | [155] |
| S-deficient CoS/CdS | Solvothermal | Hexagonal nanoplates | Up to 84.32 | Photocatalytic water-splitting | Up to 14.5% | Visible light | [156] |
| BiVO4/Bi2WO6 | Solvothermal | Self-assembled hierarchical BiVO4/Bi2WO6 heterostructured composites | 5.16 | Photocatalytic activities for methylene blue (MB) degradation and photoelectrochemical performance | Up to 50% | Visible light | [157] |
| 3D LaPO4 | Solution route using citric acid (CA) | Urchin-like hollow sphere | 51–124 | Photocatalytic CO2-reduction performance | 6.8-fold enhancement of the AQY | UV | [159] |
| ZnO | Hydrothermal-calcinatio n | Mesoporous multi-shelled ZnO microspheres | 3–20 | Photocatalysis for NO oxidation | Up to 77% | UV | [161] |
| ZnO/CeO2 | Hydrothermal/calcination | Spherically hierarchical structure | 41–56 | photocatalytic rhodamine B (RhB) | Up to 96% | Visible light | [162] |
| TiO2/graphene/MoS2 | Hydrothermal | 2D rGO sheets assembled into macroporous 3D structures | 124 | CO2 Reduction Photocatalyst | Up to 97% | UV-Vis | [165] |
| Ag,Au/TiO2 | Solvothermal | Mesoporous spherical shape | 153–173 | Degradation of Textile Dyes | Up to 85% | Solar and visible light | [163] |
| Au-H-ZnO | Low temperature aqueous reaction and heat treatment | Nanosheets | - | Gas sensing and photocatalytic properties | Up to 94.8% | Visible light | [166] |
| PDA-modified Zn | Self-assembly | Rough microstructures | 32–38 | Photocatalytic CO2 reduction | Up to 0.95 µmol h−1 g−1 of CH3OH | UV | [167] |
| ZnIn2S4 marigold flower/Bi2WO6 (ZIS/BW) | Hydrothermal method followed by wet-impregnation | Hierarchical marigold flower and flower-like morphologies | 14–73 | Decomposition of metronidazole | Up to 56% | Visible light | [168] |
| BiOCl/BiVO4 | Coprecipitation-hydrothermal method | Micro-nanosheet | 1.53–2.83 | Photo- degradation rate of rhodamine B (RhB) | Up to 96% | Visible light | [169] |
| CuS | Microwave-assisted wet chemical process | Spherical monodispersed submicron particles | 17.8–26.4 | Decomposition of organic dyes including rhodamine B, methylene blue and malachite green | Up to 100% | Visible light | [180] |
| CuS | Microwave irradiation | Aggregates of roughly spherical nanoparticles | 14.74 | Degradation of methylene blue, methyl orange, and 4- chlorophenol | Up to 100% | Solar light | [181] |
| 3D- Fe2O3 | Ultrasound irradiation method | Large quantity of 3D sea urchin-like structures combining a 1D rod-like structure on spherical support | 129.4–282.7 | Adsorption for heavy metals and photocatalytic activities toward the dyes (methylene blue and phenol) | Up to 100% | Solar light | [160] |
| g-C3N4 | Ultrasound-assisted molecular rearrangement strategy | Hierarchical Rodlike | 88.6 | RhB degradation and H2 evolution | Up to 23% | Visible light | [182] |
| CdMoO4 | Low temperature oil bath method | Uniform and porous spheres | - | Photocatalytic removal of mixed dye aqueous solutions | Up to 100% | UV-Vis | [170] |
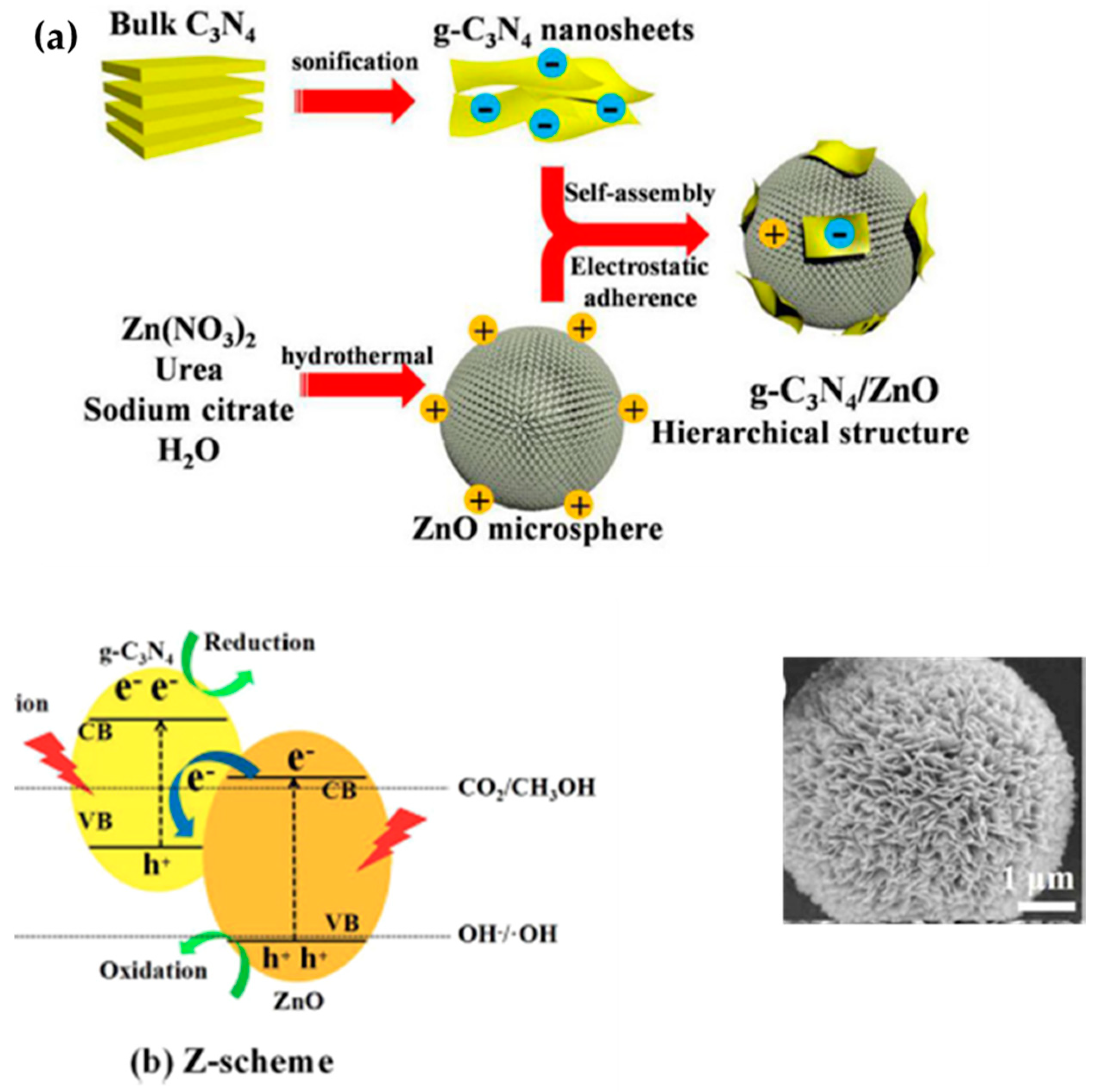
| g-C3N4/ZnO | Electrostatic self-assembly method | nanosheets | 156 | Photocatalytic CO2 reduction activity | 0.64 µmol h−1 g−1 | Solar light | [171] |
| BiOI | Solid-state reaction with subsequent hydrolysis at room temperature. | Hierarchical microspheres assembled by nanoplates | 3.1–13.8 | Photocatalytic activity for phenol degradation. | Up to 100% | Visible light | [172] |
| SnS2 | Heating the mixture of SnCl2·2H2O and thiourea in air at 170 °C for 2 | Porous flower-like hierarchical nanostructure | 36.15–82.4 | Adsorption and photocatalytic reduction of aqueous Cr(VI) | Up to 79.4% | Visible light | [173] |
| ZnO | Annealing of zinc oxalate. | Mesoporous nanostructured microlumps | 1.7–29−9 | Discoloration of the Methyl violet 2B | Up to 100% | UV | [174] |
| O-Doped g-C3N4 | Successive thermal oxidation exfoliation and curling-condensation of bulk g-C3N4 | Uniform porous network | 36 | Photocatalytic CO2 Reduction Activity | Up to 0.88 μmol g−1 h−1 | Visible light | [175] |
| Yttrium-doped g-C3N4 | Pyrolysis method | Sheet-like morphology with worm-like pores | 39–106 | Photocatalytic performance in rhodamine B degradation | Up to 100% | Visible light | [176] |
| 3D g-C3N4 | Cold quenching | Self-assembled nanoscrolls | 76 | Photocatalytic CO2 reduction | Up to 11.2 μmol g−1 h−1 | UV-Vis | [177] |
| Bismuth oxychlorides (BOC) | One-pot sorbitol-nitrate solution auto-combustion method | Mesoporous-mixed-phase of grain-like | 93 | Photocatalytic application in treatment of antibiotic effluents | Up to 80% | Visible light | [178] |
| Fe2O3 | Pulsed Laser Deposition and thermal oxidation | Spherical particulates to an urchin-like struc- ture with evolution of nanowires | - | Photocatalytic water purification | Up to 100% | H2O2 and visible light | [179] |
| AgO | Oxidizing solid Ag films in an environment of reactive magnetron sputtering deposition of NiO | Porous AgO nanorod films. | - | Photocatalyst and all solid-state thin film battery | Up to 100% | UV-Vis | [183] |
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mukherjee, A. Biomimetics Learning from Nature; IntechOpen: London, UK, 2012; ISBN 9789533070254. [Google Scholar]
- Bhushan, B. Biomimetics: Lessons from Nature—An overview. Philos. Trans. R. Soc. A 2009, 367, 1445–1486. [Google Scholar] [CrossRef] [PubMed]
- Wegst, U.G.K.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired structural materials. Nat. Mater. 2015, 14, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.F.; Yang, H.B.; Han, Z.M.; Ling, Z.C.; Yu, S.H. An all-natural bioinspired structural material for plastic replacement. Nat. Commun. 2020, 11, 5401. [Google Scholar] [CrossRef] [PubMed]
- Martin-Martinez, F.J.; Jin, K.; López Barreiro, D.; Buehler, M.J. The rise of hierarchical nanostructured materials from renewable sources: Learning from nature. ACS Nano 2018, 12, 7425–7433. [Google Scholar] [CrossRef]
- Fratzl, P.; Weinkamer, R. Nature’s hierarchical materials. Prog. Mater. Sci. 2007, 52, 1263–1334. [Google Scholar] [CrossRef]
- Dan, N. Synthesis of hierarchical materials. Trends Biotechnol. 2000, 18, 370–374. [Google Scholar] [CrossRef]
- Koch, K.; Bhushan, B.; Jung, Y.C.; Barthlott, W. Fabrication of artificial Lotus leaves and significance of hierarchical structure for superhydrophobicity and low adhesion. Soft Matter 2009, 5, 1386–1393. [Google Scholar] [CrossRef]
- Latthe, S.S.; Terashima, C.; Nakata, K.; Fujishima, A. Superhydrophobic surfaces developed by mimicking hierarchical surface morphology of lotus leaf. Molecules 2014, 19, 4256–4283. [Google Scholar] [CrossRef]
- Lingham-Soliar, T. Microstructural tissue-engineering in the rachis and barbs of bird feathers. Sci. Rep. 2017, 7, 45162. [Google Scholar] [CrossRef]
- Sullivan, T.N.; Wang, B.; Espinosa, H.D.; Meyers, M.A. Extreme lightweight structures: Avian feathers and bones. Mater. Today 2017, 20, 377–391. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, W.; Zhang, D. Fabrication of Sensor Materials Inspired by Butterfly Wings. Adv. Mater. Technol. 2017, 2, 1600209. [Google Scholar] [CrossRef]
- Niu, S.; Li, B.; Mu, Z.; Yang, M.; Zhang, J.; Han, Z.; Ren, L. Excellent structure-based multifunction of morpho butterfly wings: A review. J. Bionic Eng. 2015, 12, 170–189. [Google Scholar] [CrossRef]
- Su, B.-L.; Sanchez, C.; Yang, X.-Y. Hierarchically Structured Porous Materials: From Nanoscience to Catalysis, Separation, Optics, Energy, and Life Science; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 3527639594. [Google Scholar]
- Li, X.; Yu, J.; Jaroniec, M. Hierarchical photocatalysts. Chem. Soc. Rev. 2016, 45, 2603–2636. [Google Scholar] [CrossRef] [PubMed]
- Trogadas, P.; Ramani, V.; Strasser, P.; Fuller, T.F.; Coppens, M. Hierarchically structured nanomaterials for electrochemical energy conversion. Angew. Chem. Int. Ed. 2016, 55, 122–148. [Google Scholar] [CrossRef]
- Shi, Y.; Ye, G.; Yang, C.; Tang, Y.; Peng, C.; Qian, G.; Yuan, W.; Duan, X.; Zhou, X. Pore engineering of hierarchically structured hydrodemetallization catalyst pellets in a fixed bed reactor. Chem. Eng. Sci. 2019, 202, 336–346. [Google Scholar] [CrossRef]
- Hsu, M.-H.; Chang, C.-J. S-doped ZnO nanorods on stainless-steel wire mesh as immobilized hierarchical photocatalysts for photocatalytic H2 production. Int. J. Hydrog. Energy 2014, 39, 16524–16533. [Google Scholar] [CrossRef]
- Miao, Y.-E.; Wang, R.; Chen, D.; Liu, Z.; Liu, T. Electrospun self-standing membrane of hierarchical SiO2@ γ-AlOOH (Boehmite) core/sheath fibers for water remediation. ACS Appl. Mater. Interfaces 2012, 4, 5353–5359. [Google Scholar] [CrossRef]
- Brady, R.; Woonton, B.; Gee, M.L.; O’Connor, A.J. Hierarchical mesoporous silica materials for separation of functional food ingredients—A review. Innov. Food Sci. Emerg. Technol. 2008, 9, 243–248. [Google Scholar] [CrossRef]
- Nayak, A.K.; Ghosh, R.; Santra, S.; Guha, P.K.; Pradhan, D. Hierarchical nanostructured WO3-SnO2 for selective sensing of volatile organic compounds. Nanoscale 2015, 7, 12460–12473. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Y.; Ji, G.; Cui, R.; Liu, Z. One-pot synthesis of hierarchical-pore metal–organic frameworks for drug delivery and fluorescent imaging. CrystEngComm 2018, 20, 1087–1093. [Google Scholar] [CrossRef]
- Hartmann, M. Hierarchical zeolites: A proven strategy to combine shape selectivity with efficient mass transport. Angew. Chemie Int. Ed. 2004, 43, 5880–5882. [Google Scholar] [CrossRef] [PubMed]
- Su, B.-L.; Sanchez, C.; Yang, X.-Y. Zeolite Molecular Sieves Chemistry of Zeolites and Related Porous Materials Ordered Mesoporous Materials; John Wiley & Sons: Chichester, UK, 2012; ISBN 9780470577578. [Google Scholar]
- Holm, M.S.; Taarning, E.; Egeblad, K.; Christensen, C.H. Catalysis with hierarchical zeolites. Catal. Today 2011, 168, 3–16. [Google Scholar] [CrossRef]
- Parlett, C.M.A.; Wilson, K.; Lee, A.F. Hierarchical porous materials: Catalytic applications. Chem. Soc. Rev. 2013, 42, 3876–3893. [Google Scholar] [CrossRef] [PubMed]
- Costantini, A.; Venezia, V.; Pota, G.; Bifulco, A.; Califano, V.; Sannino, F. Adsorption of cellulase on wrinkled silica nanoparticles with enhanced inter-wrinkle distance. Nanomaterials 2020, 10, 1799. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wee, P.; Lai, Y.; Wang, X.; Gong, D.; Kanhere, P.D.; Lim, T.T.; Dong, Z.; Chen, Z. Hierarchical TiO2 nanoflakes and nanoparticles hybrid structure for improved photocatalytic activity. J. Phys. Chem. C 2012, 116, 2772–2780. [Google Scholar] [CrossRef]
- Gao, C.; Wei, T.; Zhang, Y.; Song, X.; Huan, Y.; Liu, H.; Zhao, M.; Yu, J.; Chen, X. A Photoresponsive Rutile TiO2 Heterojunction with Enhanced Electron–Hole Separation for High-Performance Hydrogen Evolution. Adv. Mater. 2019, 31, 1806596. [Google Scholar] [CrossRef] [PubMed]
- Yahya, N.; Aziz, F.; Jamaludin, N.A.; Mutalib, M.A.; Ismail, A.F.; Salleh, W.N.W.; Jaafar, J.; Yusof, N.; Ludin, N.A. A review of integrated photocatalyst adsorbents for wastewater treatment. J. Environ. Chem. Eng. 2018, 6, 7411–7425. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Low, J.; Fang, Y.; Xiao, J.; Chen, X. Engineering heterogeneous semiconductors for solar water splitting. J. Mater. Chem. A 2015, 3, 2485–2534. [Google Scholar] [CrossRef]
- Fang, M.; Dong, G.; Wei, R.; Ho, J.C. Hierarchical Nanostructures: Hierarchical Nanostructures: Design for Sustainable Water Splitting (Adv. Energy Mater. 23/2017). Adv. Energy Mater. 2017, 7, 1770135. [Google Scholar] [CrossRef]
- Stein, A.; Rudisill, S.G.; Petkovich, N.D. Perspective on the influence of interactions between hard and soft templates and precursors on morphology of hierarchically structured porous materials. Chem. Mater. 2014, 26, 259–276. [Google Scholar] [CrossRef]
- Wang, P.; Xuan, J.; Zhang, R.; Zhang, H.; Wang, Q.; Wang, H.; Liu, H.; Zhang, L. Hierarchically Structured Components: Design, Additive Manufacture, and Their Energy Applications. Adv. Mater. Technol. 2022, 7, 2100672. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Zang, S.; Lou, X.W. Hierarchical hollow heterostructures for photocatalytic CO2 reduction and water splitting. Small Methods 2020, 4, 1900586. [Google Scholar] [CrossRef]
- Sun, C.; Yang, J.; Xu, M.; Cui, Y.; Ren, W.; Zhang, J.; Zhao, H.; Liang, B. Recent intensification strategies of SnO2-based photocatalysts: A review. Chem. Eng. J. 2022, 427, 131564. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, H.; Yang, H.; Sun, N.; Huang, Z.; Pang, H. Applications of hierarchical metal–organic frameworks and their derivatives in electrochemical energy storage and conversion. J. Energy Storage 2022, 55, 105354. [Google Scholar] [CrossRef]
- Janani, R.; Preethi, V.R.; Singh, S.; Rani, A.; Chang, C.-T. Hierarchical ternary sulfides as effective photocatalyst for hydrogen generation through water splitting: A review on the performance of ZnIn2S4. Catalysts 2021, 11, 277. [Google Scholar] [CrossRef]
- Meng, S.; Wu, H.; Cui, Y.; Zheng, X.; Wang, H.; Chen, S.; Wang, Y.; Fu, X. One-step synthesis of 2D/2D-3D NiS/Zn3In2S6 hierarchical structure toward solar-to-chemical energy transformation of biomass-relevant alcohols. Appl. Catal. B Environ. 2020, 266, 118617. [Google Scholar] [CrossRef]
- Tahir, M.B.; Asiri, A.M.; Nabi, G.; Rafique, M.; Sagir, M. Fabrication of heterogeneous photocatalysts for insight role of carbon nanofibre in hierarchical WO3/MoSe2 composite for enhanced photocatalytic hydrogen generation. Ceram. Int. 2019, 45, 5547–5552. [Google Scholar] [CrossRef]
- Tan, H.; Li, J.; He, M.; Li, J.; Zhi, D.; Qin, F.; Zhang, C. Global evolution of research on green energy and environmental technologies: A bibliometric study. J. Environ. Manage. 2021, 297, 113382. [Google Scholar] [CrossRef]
- Jiang, Y.; Liao, J.-F.; Xu, Y.-F.; Chen, H.-Y.; Wang, X.-D.; Kuang, D.-B. Hierarchical CsPbBr3 nanocrystal-decorated ZnO nanowire/macroporous graphene hybrids for enhancing charge separation and photocatalytic CO2 reduction. J. Mater. Chem. A 2019, 7, 13762–13769. [Google Scholar] [CrossRef]
- Gholami, P.; Khataee, A.; Ritala, M. Template-free hierarchical trimetallic oxide photocatalyst derived from organically modified ZnCuCo layered double hydroxide. J. Clean. Prod. 2022, 366, 132761. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, X.; Zuo, Y.; Dong, H.; Ren, H. Decorating 2D Ti3C2 on flower-like hierarchical Bi2WO6 for the 2D/2D heterojunction construction towards photodegradation of tetracycline antibiotics. Sep. Purif. Technol. 2022, 299, 121715. [Google Scholar] [CrossRef]
- Kaushik, B.; Rana, P.; Solanki, K.; Rawat, D.; Yadav, S.; Naikwadi, D.R.; Biradar, A.V.; Sharma, R.K. In-situ synthesis of 3-D hierarchical ZnFe2O4 modified Cu2S snowflakes: Exploring their bifunctionality in selective photocatalytic reduction of nitroarenes and methyl orange degradation. J. Photochem. Photobiol. A Chem. 2022, 433, 114165. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, J.; Fu, L.; Chen, C.; Mao, S.; Pu, Z.; Yang, J.; Shi, J.-W.; Wu, K. Fabrication of heterostructural Ru-SrTiO3 fibers through in-situ exsolution for visible-light-induced photocatalysis. J. Alloys Compd. 2022, 925, 166747. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jaroniec, M. Hierarchical porous photocatalysts. Interface Sci. Technol. 2020, 31, 63–102. [Google Scholar]
- Shi, J.W.; Zong, X.; Wu, X.; Cui, H.J.; Xu, B.; Wang, L.; Fu, M.L. Carbon-doped Titania Hollow Spheres with Tunable Hierarchical Macroporous Channels and Enhanced Visible Light-induced Photocatalytic Activity. ChemCatChem 2012, 4, 488–491. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, L.; Cheng, B.; Su, Y. Hydrothermal preparation and photocatalytic activity of hierarchically sponge-like macro-/mesoporous Titania. J. Phys. Chem. C 2007, 111, 10582–10589. [Google Scholar] [CrossRef]
- Wang, X.; Yu, J.C.; Ho, C.; Hou, Y.; Fu, X. Photocatalytic activity of a hierarchically macro/mesoporous titania. Langmuir 2005, 21, 2552–2559. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Huang, B.; Qin, X.; Zhang, X.; Dai, Y. Strategic synthesis of hierarchical TiO2 microspheres with enhanced photocatalytic activity. Chem. A Eur. J. 2010, 16, 11266–11270. [Google Scholar] [CrossRef]
- Yu, J.; Su, Y.; Cheng, B. Template-free fabrication and enhanced photocatalytic activity of hierarchical macro-/mesoporous titania. Adv. Funct. Mater. 2007, 17, 1984–1990. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, J.C. A sonochemical approach to hierarchical porous titania spheres with enhanced photocatalytic activity. Chem. Commun. 2003, 3, 2078–2079. [Google Scholar] [CrossRef]
- Shao, G.S.; Wang, F.Y.; Ren, T.Z.; Liu, Y.; Yuan, Z.Y. Hierarchical mesoporous phosphorus and nitrogen doped titania materials: Synthesis, characterization and visible-light photocatalytic activity. Appl. Catal. B Environ. 2009, 92, 61–67. [Google Scholar] [CrossRef]
- Dong, G.; Wang, Y.; Lei, H.; Tian, G.; Qi, S.; Wu, D. Hierarchical mesoporous titania nanoshell encapsulated on polyimide nanofiber as flexible, highly reactive, energy saving and recyclable photocatalyst for water purification. J. Clean. Prod. 2020, 253, 120021. [Google Scholar] [CrossRef]
- Ta, Q.T.H.; Cho, E.; Sreedhar, A.; Noh, J.S. Mixed-dimensional, three-level hierarchical nanostructures of silver and zinc oxide for fast photocatalytic degradation of multiple dyes. J. Catal. 2019, 371, 1–9. [Google Scholar] [CrossRef]
- Yukhnovets, O.; Semenova, A.A.; Levkevich, E.A.; Maximov, A.I.; Moshnikov, V.A. Zinc oxide hierarchical nanostructures for photocatalysis. J. Phys. Conf. Ser. 2018, 993, 012009. [Google Scholar] [CrossRef]
- Kim, J.H.; Joshi, M.K.; Lee, J.; Park, C.H.; Kim, C.S. Polydopamine-assisted immobilization of hierarchical zinc oxide nanostructures on electrospun nanofibrous membrane for photocatalysis and antimicrobial activity. J. Colloid Interface Sci. 2018, 513, 566–574. [Google Scholar] [CrossRef]
- Adhyapak, P.V.; Meshram, S.P.; Tomar, V.; Amalnerkar, D.P.; Mulla, I.S. Effect of preparation parameters on the morphologically induced photocatalytic activities of hierarchical zinc oxide nanostructures. Ceram. Int. 2013, 39, 7367–7378. [Google Scholar] [CrossRef]
- Wang, S.; Kuang, P.; Cheng, B.; Yu, J.; Jiang, C. ZnO hierarchical microsphere for enhanced photocatalytic activity. J. Alloys Compd. 2018, 741, 622–632. [Google Scholar] [CrossRef]
- Yin, Q.; Qiao, R.; Li, Z.; Zhang, X.L.; Zhu, L. Hierarchical nanostructures of nickel-doped zinc oxide: Morphology controlled synthesis and enhanced visible-light photocatalytic activity. J. Alloys Compd. 2015, 618, 318–325. [Google Scholar] [CrossRef]
- Magdalane, C.M.; Kaviyarasu, K.; Priyadharsini, G.M.A.; Bashir, A.K.H.; Mayedwa, N.; Matinise, N.; Isaev, A.B.; Al-Dhabi, N.A.; Arasu, M.V.; Arokiyaraj, S. Improved photocatalytic decomposition of aqueous Rhodamine-B by solar light illuminated hierarchical yttria nanosphere decorated ceria nanorods. J. Mater. Res. Technol. 2019, 8, 2898–2909. [Google Scholar] [CrossRef]
- Qian, J.; Chen, Z.; Sun, H.; Chen, F.; Xu, X.; Wu, Z.; Li, P.; Ge, W. Enhanced photocatalytic H2 production on three-dimensional porous CeO2/carbon nanostructure. ACS Sustain. Chem. Eng. 2018, 6, 9691–9698. [Google Scholar] [CrossRef]
- Xiang, Q.; Cheng, F.; Lang, D. Hierarchical layered WS2/graphene-modified CdS nanorods for efficient photocatalytic hydrogen evolution. ChemSusChem 2016, 9, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yu, J.; Xiang, Q.; Cheng, B. Enhanced photocatalytic activity of hierarchical macro/mesoporous TiO2–graphene composites for photodegradation of acetone in air. Appl. Catal. B Environ. 2012, 119, 109–116. [Google Scholar] [CrossRef]
- Luo, Q.-P.; Yu, X.-Y.; Lei, B.-X.; Chen, H.-Y.; Kuang, D.-B.; Su, C.-Y. Reduced graphene oxide-hierarchical ZnO hollow sphere composites with enhanced photocurrent and photocatalytic activity. J. Phys. Chem. C 2012, 116, 8111–8117. [Google Scholar] [CrossRef]
- Sin, J.-C.; Lam, S.-M.; Satoshi, I.; Lee, K.-T.; Mohamed, A.R. Sunlight photocatalytic activity enhancement and mechanism of novel europium-doped ZnO hierarchical micro/nanospheres for degradation of phenol. Appl. Catal. B Environ. 2014, 148, 258–268. [Google Scholar] [CrossRef]
- Chen, J.; Wang, H.; Huang, G.; Zhang, Z.; Han, L.; Song, W.; Li, M.; Zhang, Y. Facile synthesis of urchin-like hierarchical Nb2O5 nanospheres with enhanced visible light photocatalytic activity. J. Alloys Compd. 2017, 728, 19–28. [Google Scholar] [CrossRef]
- Sang, Y.; Cao, X.; Dai, G.; Wang, L.; Peng, Y.; Geng, B. Facile one-pot synthesis of novel hierarchical Bi2O3/Bi2S3 nanoflower photocatalyst with intrinsic p-n junction for efficient photocatalytic removals of RhB and Cr(VI). J. Hazard. Mater. 2020, 381, 120942. [Google Scholar] [CrossRef]
- Sharma, S.; Khare, N. Hierarchical Bi2S3 nanoflowers: A novel photocatalyst for enhanced photocatalytic degradation of binary mixture of Rhodamine B and Methylene blue dyes and degradation of mixture of p-nitrophenol and p-chlorophenol. Adv. Powder Technol. 2018, 29, 3336–3347. [Google Scholar] [CrossRef]
- Deas, R.; Pearce, S.; Goss, K.; Wang, Q.; Chen, W.T.; Waterhouse, G.I.N. Hierarchical Au/TiO2 nanoflower photocatalysts with outstanding performance for alcohol photoreforming under UV irradiation. Appl. Catal. A Gen. 2020, 602, 39–41. [Google Scholar] [CrossRef]
- Cao, F.; Shi, W.; Zhao, L.; Song, S.; Yang, J.; Lei, Y.; Zhang, H. Hydrothermal synthesis and high photocatalytic activity of 3D wurtzite ZnSe hierarchical nanostruetures. J. Phys. Chem. C 2008, 112, 17095–17101. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, C. Effective solar absorption and radial microchannels of SnO2 hierarchical structure for high photocatalytic activity. Catal. Commun. 2011, 14, 32–36. [Google Scholar] [CrossRef]
- Li, D.; Fang, M.; Jiang, C.; Lin, H.; Luo, C.; Qi, R.; Huang, R.; Peng, H. Size-controlled synthesis of hierarchical bismuth selenide nanoflowers and their photocatalytic performance in the presence of H2O2. J. Nanoparticle Res. 2018, 20, 228. [Google Scholar] [CrossRef]
- Song, J.M.; Mao, C.J.; Niu, H.L.; Shen, Y.H.; Zhang, S.Y. Hierarchical structured bismuth oxychlorides: Self-assembly from nanoplates to nanoflowers via a solvothermal route and their photocatalytic properties. CrystEngComm 2010, 12, 3875–3881. [Google Scholar] [CrossRef]
- Liang, Y.; Ding, M.; Yang, Y.; Xu, K.; Luo, X.; Yu, T.; Zhang, W.; Liu, W.; Yuan, C. Highly dispersed Pt nanoparticles on hierarchical titania nanoflowers with {010} facets for gas sensing and photocatalysis. J. Mater. Sci. 2019, 54, 6826–6840. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.K.; Rana, P.S.; Nehra, S.P.; Duhan, S. Surfactant assisted hydrothermal synthesis of porous 3-D hierarchical SnO2 nanoflowers for photocatalytic degradation of Rose Bengal. Mater. Lett. 2015, 154, 124–127. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Tu, W.; Liu, Y.; Wu, S.; Tan, Y.Z.; Chew, J.W. Construction of hierarchical 2D-2D Zn3In2S6/fluorinated polymeric carbon nitride nanosheets photocatalyst for boosting photocatalytic degradation and hydrogen production performance. Appl. Catal. B Environ. 2018, 233, 58–69. [Google Scholar] [CrossRef]
- Jin, Z.; Dong, W.; Yang, M.; Wang, J.; Gao, H.; Wang, G. One-Pot Preparation of Hierarchical Nanosheet-Constructed Fe3O4/MIL-88B(Fe) Magnetic Microspheres with High Efficiency Photocatalytic Degradation of Dye. ChemCatChem 2016, 8, 3510–3517. [Google Scholar] [CrossRef]
- Jia, Y.; Ma, Y.; Tang, J.; Shi, W. Hierarchical nanosheet-based Bi2MoO6 microboxes for efficient photocatalytic performance. Dalt. Trans. 2018, 47, 5542–5547. [Google Scholar] [CrossRef]
- Tanveer, M.; Cao, C.; Ali, Z.; Aslam, I.; Idrees, F.; Khan, W.S.; But, F.K.; Tahir, M.; Mahmood, N. Template free synthesis of CuS nanosheet-based hierarchical microspheres: An efficient natural light driven photocatalyst. CrystEngComm 2014, 16, 5290–5300. [Google Scholar] [CrossRef]
- Taheri, M.; Abdizadeh, H.; Golobostanfard, M.R. Hierarchical ZnO nanoflowers and urchin-like shapes synthesized via sol-gel electrophoretic deposition with enhanced photocatalytic performance. Mater. Chem. Phys. 2018, 220, 118–127. [Google Scholar] [CrossRef]
- Li, H.; Fei, G.T.; Fang, M.; Cui, P.; Guo, X.; Yan, P.; Zhang, L. De Synthesis of urchin-like Co3O4 hierarchical micro/nanostructures and their photocatalytic activity. Appl. Surf. Sci. 2011, 257, 6527–6530. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, J.; Xiao, T.; Tang, Z.; Shen, J.; Li, H.; Zhou, Y.; Zou, Z. Urchin-like hierarchical CoZnAl-LDH/RGO/g-C3N4 hybrid as a Z-scheme photocatalyst for efficient and selective CO2 reduction. Appl. Catal. B Environ. 2019, 255, 117771. [Google Scholar] [CrossRef]
- Liu, J.; Guo, Z.; Wang, W.; Huang, Q.; Zhu, K.; Chen, X. Heterogeneous ZnS hollow urchin-like hierarchical nanostructures and their structure-enhanced photocatalytic properties. Nanoscale 2011, 3, 1470–1473. [Google Scholar] [CrossRef]
- Xiang, L.; Zhao, X.; Yin, J.; Fan, B. Well-organized 3D urchin-like hierarchical TiO2 microspheres with high photocatalytic activity. J. Mater. Sci. 2012, 47, 1436–1445. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, Y.; Xu, L.; Sun, P.; Su, Z.; Jin, F.; Liu, F.; Sun, Y.; Lu, G. High specific surface area urchin-like hierarchical ZnO-TiO2 architectures: Hydrothermal synthesis and photocatalytic properties. Mater. Lett. 2016, 175, 52–55. [Google Scholar] [CrossRef]
- Xiao, X.; Xing, C.; He, G.; Zuo, X.; Nan, J.; Wang, L. Solvothermal synthesis of novel hierarchical Bi4O5I2 nanoflakes with highly visible light photocatalytic performance for the degradation of 4-tert-butylphenol. Appl. Catal. B Environ. 2014, 148, 154–163. [Google Scholar] [CrossRef]
- Ong, W.L.; Natarajan, S.; Kloostra, B.; Ho, G.W. Metal nanoparticle-loaded hierarchically assembled ZnO nanoflakes for enhanced photocatalytic performance. Nanoscale 2013, 5, 5568–5575. [Google Scholar] [CrossRef]
- Sun, M.; Chen, C.; Chen, L.; Su, B. Hierarchically porous materials: Synthesis strategies and emerging applications. Front. Chem. Sci. Eng. 2016, 10, 301–347. [Google Scholar] [CrossRef]
- Yang, Q.; Hao, J. Synthesis of metal sulfides via ionic liquid-mediated assembly strategy and their photocatalytic degradation of dyes in water. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127848. [Google Scholar] [CrossRef]
- Sang, Y.; Ding, G.; Guo, Z.; Xue, Y.; Li, G.; Zhang, R. Facile synthesis of amorphous bimetallic hydroxide on Fe-doped Ni3S2 as an active electrocatalyst for oxygen evolution reaction. J. Alloys Compd. 2022, 919, 165855. [Google Scholar] [CrossRef]
- Xu, T.; Wang, S.; Li, L.; Liu, X. Dual templated synthesis of tri-modal porous SrTiO3/TiO2@ carbon composites with enhanced photocatalytic activity. Appl. Catal. A Gen. 2019, 575, 132–141. [Google Scholar] [CrossRef]
- Xu, T.; Liu, X.; Wang, S.; Li, L. Ferroelectric oxide nanocomposites with trimodal pore structure for high photocatalytic performance. Nano-micro Lett. 2019, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Wang, H.; Zhang, Y.; Wang, S.; Liu, X. Fabrication of N doped TiO2/C nanocomposites with hierarchical porous structure and high photocatalytic activity. Microporous Mesoporous Mater. 2019, 288, 109604. [Google Scholar] [CrossRef]
- Liu, H.; Liu, X.; Mu, S.; Wang, S.; Wang, S.; Li, L.; Giannelis, E.P. A novel fabrication approach for three-dimensional hierarchical porous metal oxide/carbon nanocomposites for enhanced solar photocatalytic performance. Catal. Sci. Technol. 2017, 7, 1965–1970. [Google Scholar] [CrossRef]
- Lyu, J.; Shao, J.; Wang, Y.; Qiu, Y.; Li, J.; Li, T.; Peng, Y.; Liu, F. Construction of a porous core-shell homojunction for the photocatalytic degradation of antibiotics. Chem. Eng. J. 2019, 358, 614–620. [Google Scholar] [CrossRef]
- Zhao, J.; Liao, C.; Chen, X.; Song, W. Hierarchically ordered macro–mesoporous anatase TiO2 prepared by pearl oyster shell and triblock copolymer dual templates for high photocatalytic activity. RSC Adv. 2018, 8, 38461–38469. [Google Scholar] [CrossRef]
- Rana, A.; Sudhaik, A.; Raizada, P.; Nguyen, V.-H.; Xia, C.; Khan, A.A.P.; Thakur, S.; Nguyen-Tri, P.; Nguyen, C.C.; Kim, S.Y. Graphitic carbon nitride based immobilized and non-immobilized floating photocatalysts for environmental remediation. Chemosphere 2022, 297, 134229. [Google Scholar] [CrossRef]
- Xu, M.; Wu, J.; Wang, J.; Mao, Y.; Liu, M.; Yang, Y.; Yang, C.; Sun, L.; Du, Y.; Li, Y. The integration of Triazine-based porous organic polymer with bio-waste poplar catkin as water-floatable photocatalyst. Appl. Surf. Sci. 2022, 581, 152409. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, L.; He, X.; Huang, Z.; Wang, G.; Fang, W.; Li, W. Highly enhanced photocatalytic degradation of dye rhodamine B on the biogenic hierarchical porous TiO2/SiO2 with 1D/3D-chain. Clean Technol. Environ. Policy 2018, 20, 887–897. [Google Scholar] [CrossRef]
- CHEN, F.; CHEN, X.; CHEN, Z. Artificial Synthesis and Improved Photo Catalysis Performance of Bionic Hierarchical Fibers Alpha-Fe2O3; 2nd International Seminar on Applied Physics, Optoelectronics and Photonics (APOP): Suzhou, China, 2017; ISBN 978-1-60595-522-3. [Google Scholar]
- Lv, H.; Zhang, M.; Wang, P.; Xu, X.; Liu, Y.; Yu, D.-G. Ingenious construction of Ni(DMG)2/TiO2-decorated porous nanofibers for the highly efficient photodegradation of pollutants in water. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129561. [Google Scholar] [CrossRef]
- He, D.; Ma, Y.; Zhao, R.; Qiu, J.; Sun, B.; Wang, H.; Yang, M.; Jia, X.; Li, X.; Li, Y. Complete-lifecycle-available, lightweight and flexible hierarchical structured Bi2WO6/WO3/PAN nanofibrous membrane for X-Ray shielding and photocatalytic degradation. Adv. Mater. Interfaces 2021, 8, 2002131. [Google Scholar] [CrossRef]
- Zhang, J.; Hou, X.; Pang, Z.; Cai, Y.; Zhou, H.; Lv, P.; Wei, Q. Fabrication of hierarchical TiO2 nanofibers by microemulsion electrospinning for photocatalysis applications. Ceram. Int. 2017, 43, 15911–15917. [Google Scholar] [CrossRef]
- Zhou, H.; Zhong, S.; Shen, M.; Yao, Y. Composite soft template-assisted construction of a flower-like β-Bi2O3/Bi2O2CO3 heterojunction photocatalyst for the enhanced simulated sunlight photocatalytic degradation of tetracycline. Ceram. Int. 2019, 45, 15036–15047. [Google Scholar] [CrossRef]
- Lin, Z.; Huang, J. A hierarchical H3PW12O40/TiO2 nanocomposite with cellulose as scaffold for photocatalytic degradation of organic pollutants. Sep. Purif. Technol. 2021, 264, 118427. [Google Scholar] [CrossRef]
- Sun, X.; Wang, K.; Shu, Y.; Zou, F.; Zhang, B.; Sun, G.; Uyama, H.; Wang, X. One-pot route towards active TiO2 doped hierarchically porous cellulose: Highly efficient photocatalysts for methylene blue degradation. Materials 2017, 10, 373. [Google Scholar] [CrossRef]
- Xiao, J.; Jiang, H. Thermally Stable Metal-Organic Framework-Templated Synthesis of Hierarchically Porous Metal Sulfides: Enhanced Photocatalytic Hydrogen Production. Small 2017, 13, 1700632. [Google Scholar] [CrossRef]
- Ren, J.-T.; Zheng, Y.-L.; Yuan, K.; Zhou, L.; Wu, K.; Zhang, Y.-W. Self-templated synthesis of Co3O4 hierarchical nanosheets from a metal–organic framework for efficient visible-light photocatalytic CO2 reduction. Nanoscale 2020, 12, 755–762. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, L.; Chen, J.; Chen, L.; Fang, R.; Li, Y. Regulating the Electronic Structure and Water Adsorption Capability by Constructing Carbon-Doped CuO Hollow Spheres for Efficient Photocatalytic Hydrogen Evolution. ChemSusChem 2020, 13, 5711–5721. [Google Scholar] [CrossRef]
- Li, P.; Zhang, M.; Li, X.; Wang, C.; Wang, R.; Wang, B.; Yan, H. MOF-derived NiO/CeO2 heterojunction: A photocatalyst for degrading pollutants and hydrogen evolution. J. Mater. Sci. 2020, 55, 15930–15944. [Google Scholar] [CrossRef]
- Chen, W.; Han, B.; Xie, Y.; Liang, S.; Deng, H.; Lin, Z. Ultrathin Co-Co LDHs nanosheets assembled vertically on MXene: 3D nanoarrays for boosted visible-light-driven CO2 reduction. Chem. Eng. J. 2020, 391, 123519. [Google Scholar] [CrossRef]
- Yan, B.; Zhang, L.; Tang, Z.; Al-Mamun, M.; Zhao, H.; Su, X. Palladium-decorated hierarchical titania constructed from the metal-organic frameworks NH2-MIL-125 (Ti) as a robust photocatalyst for hydrogen evolution. Appl. Catal. B Environ. 2017, 218, 743–750. [Google Scholar] [CrossRef]
- Zhao, F.; Yin, D.; Khaing, K.K.; Liu, B.; Chen, T.; Deng, L.; Li, L.; Guo, X.; Wang, J.; Xiao, S. Fabrication of hierarchical Co9S8@ ZnAgInS heterostructured cages for highly efficient photocatalytic hydrogen generation and pollutants degradation. Inorg. Chem. 2020, 59, 7027–7038. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Zhang, Y.; Xu, G.; Tian, Y.; Ma, D.; Zhang, Y.; Qiu, P. Synthesis of multilevel structured MoS2@ Cu/Cu2O@ C visible-light-driven photocatalyst derived from MOF–guest polyhedra for cyclohexane oxidation. ACS Sustain. Chem. Eng. 2020, 8, 6622–6633. [Google Scholar] [CrossRef]
- Gong, Y.; Zhao, X.; Zhang, H.; Yang, B.; Xiao, K.; Guo, T.; Zhang, J.; Shao, H.; Wang, Y.; Yu, G. MOF-derived nitrogen doped carbon modified g-C3N4 heterostructure composite with enhanced photocatalytic activity for bisphenol A degradation with peroxymonosulfate under visible light irradiation. Appl. Catal. B Environ. 2018, 233, 35–45. [Google Scholar] [CrossRef]
- He, X.; Nguyen, V.; Jiang, Z.; Wang, D.; Zhu, Z.; Wang, W.-N. Highly-oriented one-dimensional MOF-semiconductor nanoarrays for efficient photodegradation of antibiotics. Catal. Sci. Technol. 2018, 8, 2117–2123. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, S.; Li, X.; Huang, Y.; Wang, Z.; Han, Y.; Wu, J.; Meng, H.; Zhang, X. Synthesis, characterization, and photocatalytic degradation properties of ZnO/ZnFe2O4 magnetic heterostructures. New J. Chem. 2017, 41, 15433–15438. [Google Scholar] [CrossRef]
- Kampouri, S.; Ireland, C.P.; Valizadeh, B.; Oveisi, E.; Schouwink, P.A.; Mensi, M.; Stylianou, K.C. Mixed-phase MOF-derived titanium dioxide for photocatalytic hydrogen evolution: The impact of the templated morphology. ACS Appl. Energy Mater. 2018, 1, 6541–6548. [Google Scholar] [CrossRef]
- Li, N.; Huang, H.; Bibi, R.; Shen, Q.; Ngulube, R.; Zhou, J.; Liu, M. Noble-metal-free MOF derived hollow CdS/TiO2 decorated with NiS cocatalyst for efficient photocatalytic hydrogen evolution. Appl. Surf. Sci. 2019, 476, 378–386. [Google Scholar] [CrossRef]
- Mao, Q.; Chen, J.; Chen, H.; Chen, Z.; Chen, J.; Li, Y. Few-layered 1T-MoS2-modified ZnCoS solid-solution hollow dodecahedra for enhanced photocatalytic hydrogen evolution. J. Mater. Chem. A 2019, 7, 8472–8484. [Google Scholar] [CrossRef]
- Yang, S.J.; Im, J.H.; Kim, T.; Lee, K.; Park, C.R. MOF-derived ZnO and ZnO@C composites with high photocatalytic activity and adsorption capacity. J. Hazard. Mater. 2011, 186, 376–382. [Google Scholar] [CrossRef]
- Dolgopolova, E.A.; Shustova, N.B. Metal–organic framework photophysics: Optoelectronic devices, photoswitches, sensors, and photocatalysts. MRS Bull. 2016, 41, 890–896. [Google Scholar] [CrossRef]
- Li, M.; Song, S.; Su, C.; Li, L.; Yan, Z.; Cao, X. MOF-templated in situ fabrication of surface-modified Ni/graphitic carbon nitride with enhanced photocatalytic hydrogen evolution. Catal. Sci. Technol. 2019, 9, 3828–3835. [Google Scholar] [CrossRef]
- Wang, H. Rational design W-doped Co-ZIF-9 based Co3S4 composite photocatalyst for efficient visible-light-driven photocatalytic H 2 evolution. Sustain. Energy Fuels 2019, 3, 173–183. [Google Scholar] [CrossRef]
- Mondal, I.; Gonuguntla, S.; Pal, U. Photoinduced fabrication of Cu/TiO2 core–shell heterostructures derived from Cu-MOF for solar hydrogen generation: The size of the Cu nanoparticle matters. J. Phys. Chem. C 2019, 123, 26073–26081. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Chen, Y.; Sun, X.; Xu, X.; Qiu, L.; Duo, S.; Li, P. Ternary photocatalysts based on MOF-derived TO2 co-decorated with ZnIn2S4 nanosheets and CdS nanoparticles for effective visible light degradation of organic pollutants. New J. Chem. 2022, 46, 7195–7201. [Google Scholar] [CrossRef]
- Gao, L.-M.; Zhao, J.-H.; Li, T.; Li, R.; Xie, H.-Q.; Zhu, P.-L.; Niu, X.-Y.; Li, K. High-performance TO2 photocatalyst produced by the versatile functions of the tiny bimetallic MOF-derived NiCoS-porous carbon cocatalyst. CrystEngComm 2019, 21, 3686–3693. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, B.; Cheng, X.; Guo, Z.; Zhuang, T.; Lv, Z. Noble-Metal-Free CdS Decorated Porous NixCo1–xO Skeleton Derived from Metal–Organic Framework for Efficient Visible-Light H2 Production. ACS Appl. Energy Mater. 2019, 3, 852–860. [Google Scholar] [CrossRef]
- Tan, J.; Yu, M.; Cai, Z.; Lou, X.; Wang, J.; Li, Z. MOF-derived synthesis of MnS/In2S3 pn heterojunctions with hierarchical structures for efficient photocatalytic CO2 reduction. J. Colloid Interface Sci. 2021, 588, 547–556. [Google Scholar] [CrossRef]
- Mondal, I.; Pal, U. Synthesis of MOF templated Cu/CuO@ TO2 nanocomposites for synergistic hydrogen production. Phys. Chem. Chem. Phys. 2016, 18, 4780–4788. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhao, H.-J.; Li, C.-F.; Liu, J.; Dong, W.; Zhao, H.; Wang, C.; Liu, Y.; Hu, Z.-Y.; Chen, L. Type II heterojunction in hierarchically porous zinc oxide/graphitic carbon nitride microspheres promoting photocatalytic activity. J. Colloid Interface Sci. 2019, 538, 99–107. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.-J.; Chen, X.; Cai, S.-C.; Yu, E.-Q.; Chen, J.; Jia, H. MOF-Templated Approach for Hollow NiOx/Co3O4 Catalysts: Enhanced Light-Driven Thermocatalytic Degradation of Toluene. ACS Appl. Nano Mater. 2018, 1, 2971–2981. [Google Scholar] [CrossRef]
- Chen, W.; Fang, J.; Zhang, Y.; Chen, G.; Zhao, S.; Zhang, C.; Xu, R.; Bao, J.; Zhou, Y.; Xiang, X. CdS nanosphere-decorated hollow polyhedral ZCO derived from a metal–organic framework (MOF) for effective photocatalytic water evolution. Nanoscale 2018, 10, 4463–4474. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, L.; Zhan, W.; Wang, X.; Han, X. Separated redox site strategies for engineering highly efficient photocatalysts: A pagoda-like In2O3/CuO heteroepitaxial structure coated with a N-doped C layer. J. Mater. Chem. A 2021, 9, 4310–4316. [Google Scholar] [CrossRef]
- Jiang, Z.; Feng, L.; Zhu, J.; Liu, B.; Li, X.; Chen, Y.; Khan, S. Construction of a hierarchical NiFe2O4/CuInSe2 (pn) heterojunction: Highly efficient visible-light-driven photocatalyst in the degradation of endocrine disruptors in an aqueous medium. Ceram. Int. 2021, 47, 8996–9007. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, L.; Li, P.; Chen, X.; Jiang, J.; Zhang, S.; Zhang, C.; Zhang, A.; Chen, G.; Wang, H. Direct Z-scheme photocatalyst of hollow CoSx@ CdS polyhedron constructed by ZIF-67-templated one-pot solvothermal route: A signal-on photoelectrochemical sensor for mercury (II). Chem. Eng. J. 2020, 395, 125072. [Google Scholar] [CrossRef]
- Al-Hajji, L.A.; Ismail, A.A.; Bumajdad, A.; Alsaidi, M.; Ahmed, S.A.; Almutawa, F.; Al-Hazza, A. Construction of Au/TiO2 Heterojunction with high photocatalytic performances under UVA illumination. Ceram. Int. 2020, 46, 20155–20162. [Google Scholar] [CrossRef]
- Li, T.; Cui, J.-D.; Xu, M.-L.; Li, R.; Gao, L.-M.; Zhu, P.-L.; Xie, H.-Q.; Li, K. Engineering a hetero-MOF-derived TiO2–Co3O4 heterojunction decorated with nickel nanoparticles for enhanced photocatalytic activity even in pure water. CrystEngComm 2020, 22, 5620–5627. [Google Scholar] [CrossRef]
- Bera, S.; Pal, M.; Naskar, A.; Jana, S. Hierarchically structured ZnO-graphene hollow microspheres towards effective reusable adsorbent for organic pollutant via photodegradation process. J. Alloys Compd. 2016, 669, 177–186. [Google Scholar] [CrossRef]
- Zhou, L.; Han, Z.; Li, G.-D.; Zhao, Z. Template-free synthesis and photocatalytic activity of hierarchical hollow ZnO microspheres composed of radially aligned nanorods. J. Phys. Chem. Solids 2021, 148, 109719. [Google Scholar] [CrossRef]
- Bian, Z.; Zhu, J.; Li, H. Solvothermal alcoholysis synthesis of hierarchical TiO2 with enhanced activity in environmental and energy photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2016, 28, 72–86. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.-S. Photocatalytic oxidation of MEK over hierarchical TiO2 catalysts: Effect of photocatalyst features and operating conditions. Appl. Catal. B Environ. 2019, 251, 1–16. [Google Scholar] [CrossRef]
- Hiremath, V.; Deonikar, V.G.; Kim, H.; Seo, J.G. Hierarchically assembled porous TiO2 nanoparticles with enhanced photocatalytic activity towards Rhodamine-B degradation. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 586, 124199. [Google Scholar] [CrossRef]
- Harris, J.; Silk, R.; Smith, M.; Dong, Y.; Chen, W.-T.; Waterhouse, G.I.N. Hierarchical TiO2 nanoflower photocatalysts with remarkable activity for aqueous methylene blue photo-oxidation. ACS Omega 2020, 5, 18919–18934. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Lo, S.-L. Single-phase cerium oxide nanospheres: An efficient photocatalyst for the abatement of rhodamine B dye. Environ. Sci. Pollut. Res. 2018, 25, 6532–6544. [Google Scholar] [CrossRef]
- Sekar, K.; Chuaicham, C.; Balijapalli, U.; Li, W.; Wilson, K.; Lee, A.F.; Sasaki, K. Surfactant-and template-free hydrothermal assembly of Cu2O visible light photocatalysts for trimethoprim degradation. Appl. Catal. B Environ. 2021, 284, 119741. [Google Scholar] [CrossRef]
- Rong, R.; Wang, L. Synthesis of hierarchical hollow nest-like WO3 micro/nanostructures with enhanced visible light-driven photocatalytic activity. J. Alloys Compd. 2021, 850, 156742. [Google Scholar] [CrossRef]
- Wan, J.; Du, X.; Wang, R.; Liu, E.; Jia, J.; Bai, X.; Hu, X.; Fan, J. Mesoporous nanoplate multi-directional assembled Bi2WO6 for high efficient photocatalytic oxidation of NO. Chemosphere 2018, 193, 737–744. [Google Scholar] [CrossRef]
- Xu, B.; Yang, H.; Zhang, Q.; Yuan, S.; Xie, A.; Zhang, M.; Ohno, T. Design and Synthesis of Sm, Y, La and Nd-doped CeO2 with a broom-like hierarchical structure: A photocatalyst with enhanced oxidation performance. ChemCatChem 2020, 12, 2638–2646. [Google Scholar] [CrossRef]
- Dai, G.; Qin, H.; Zhou, H.; Wang, W.; Luo, T. Template-free fabrication of hierarchical macro/mesoporpous SnS2/TiO2 composite with enhanced photocatalytic degradation of Methyl Orange (MO). Appl. Surf. Sci. 2018, 430, 488–495. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, Z.; Xia, P.; Li, H.; Xie, Y. Synthesis of hierarchical ZnO&Graphene composites with enhanced photocatalytic activity. Ceram. Int. 2018, 44, 849–856. [Google Scholar]
- Liu, W.; Xu, Y.; Zhou, W.; Zhang, X.; Cheng, X.; Zhao, H.; Gao, S.; Huo, L. A facile synthesis of hierarchically porous TiO2 microspheres with carbonaceous species for visible-light photocatalysis. J. Mater. Sci. Technol. 2017, 33, 39–46. [Google Scholar] [CrossRef]
- Liang, Z.; Bai, X.; Hao, P.; Guo, Y.; Xue, Y.; Tian, J.; Cui, H. Full solar spectrum photocatalytic oxygen evolution by carbon-coated TiO2 hierarchical nanotubes. Appl. Catal. B Environ. 2019, 243, 711–720. [Google Scholar] [CrossRef]
- Li, Z.; Chen, H.; Li, Y.; Wang, H.; Liu, Y.; Li, X.; Lin, H.; Li, S.; Wang, L. Porous direct Z-scheme heterostructures of S-deficient CoS/CdS hexagonal nanoplates for robust photocatalytic H2 generation. CrystEngComm 2022, 24, 404–416. [Google Scholar] [CrossRef]
- Chen, L.; Meng, D.; Wu, X.; Wang, A.; Wang, J.; Yu, M.; Liang, Y. Enhanced visible light photocatalytic performances of self-assembled hierarchically structured BiVO4/Bi2WO6 heterojunction composites with different morphologies. RSC Adv. 2016, 6, 52300–52309. [Google Scholar] [CrossRef]
- Zhen, M.; Li, K.; Guo, S.-Q.; Li, H.; Shen, B. Template-free construction of hollow TiO2 microspheres for long-life and high-capacity lithium storage. J. Alloys Compd. 2021, 859, 157761. [Google Scholar] [CrossRef]
- Pan, B.; Zhou, Y.; Su, W.; Wang, X. Self-assembly synthesis of LaPO4 hierarchical hollow spheres with enhanced photocatalytic CO2-reduction performance. Nano Res. 2017, 10, 534–545. [Google Scholar] [CrossRef]
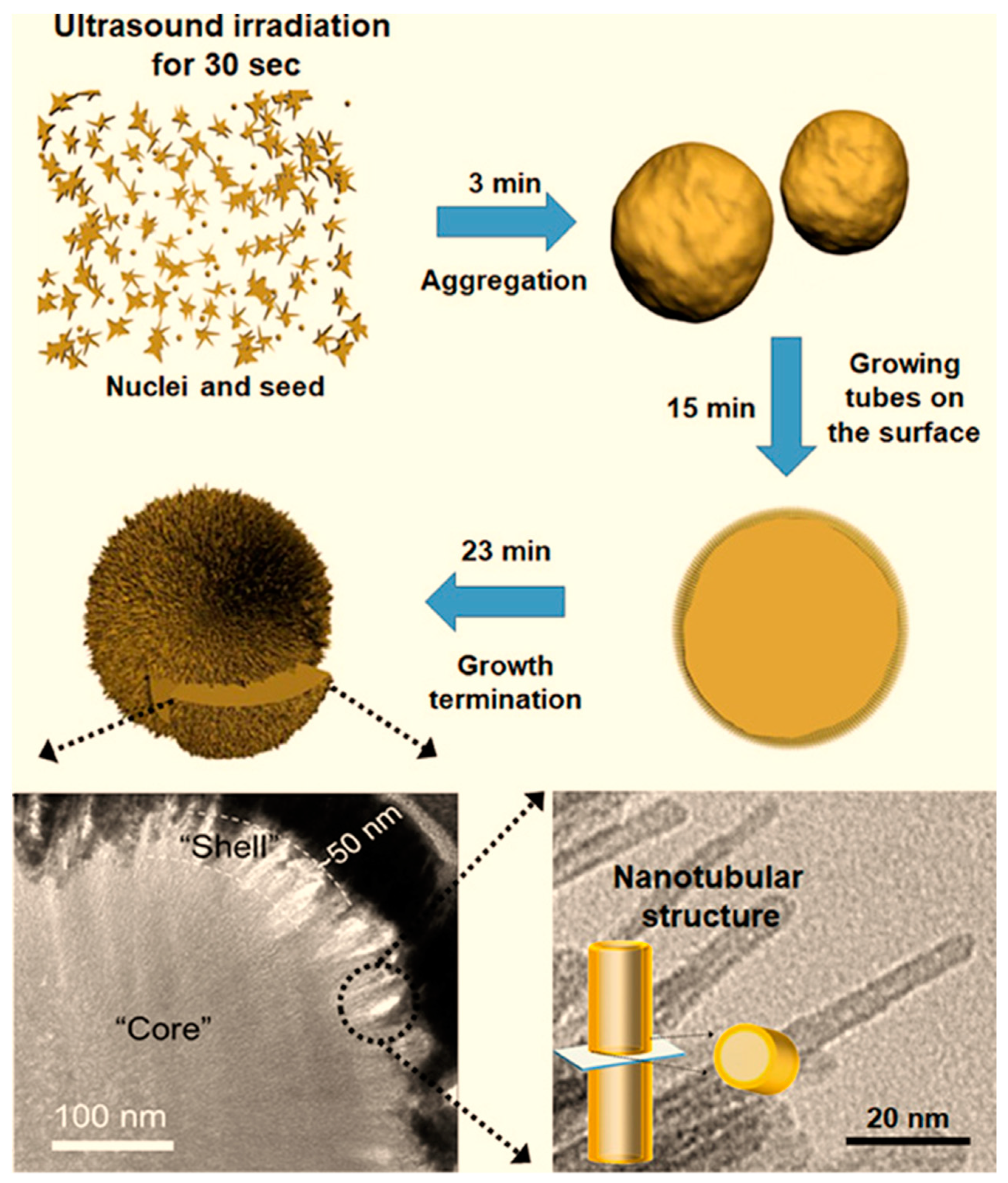
- Lee, S.C.; Jeong, Y.; Kim, Y.J.; Kim, H.; Lee, H.U.; Lee, Y.-C.; Lee, S.M.; Kim, H.J.; An, H.-R.; Ha, M.G. Hierarchically three-dimensional (3D) nanotubular sea urchin-shaped iron oxide and its application in heavy metal removal and solar-induced photocatalytic degradation. J. Hazard. Mater. 2018, 354, 283–292. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Zhang, D.; Miao, Y.; Li, G. Controllable synthesis of mesoporous multi-shelled ZnO microspheres as efficient photocatalysts for NO oxidation. Appl. Surf. Sci. 2018, 435, 468–475. [Google Scholar] [CrossRef]
- Zhu, L.; Li, H.; Xia, P.; Liu, Z.; Xiong, D. Hierarchical ZnO decorated with CeO2 nanoparticles as the direct Z-scheme heterojunction for enhanced photocatalytic activity. ACS Appl. Mater. Interfaces 2018, 10, 39679–39687. [Google Scholar] [CrossRef] [PubMed]
- Anjugam Vandarkuzhali, S.A.; Pugazhenthiran, N.; Mangalaraja, R.V.; Sathishkumar, P.; Viswanathan, B.; Anandan, S. Ultrasmall plasmonic nanoparticles decorated hierarchical mesoporous TiO2 as an efficient photocatalyst for photocatalytic degradation of textile dyes. ACS Omega 2018, 3, 9834–9845. [Google Scholar] [CrossRef]
- Cai, Y.; Song, J.; Liu, X.; Yin, X.; Li, X.; Yu, J.; Ding, B. Soft BiOBr@ TiO2 nanofibrous membranes with hierarchical heterostructures as efficient and recyclable visible-light photocatalysts. Environ. Sci. Nano 2018, 5, 2631–2640. [Google Scholar] [CrossRef]
- Jung, H.; Cho, K.M.; Kim, K.H.; Yoo, H.-W.; Al-Saggaf, A.; Gereige, I.; Jung, H.-T. Highly efficient and stable CO2 reduction photocatalyst with a hierarchical structure of mesoporous TiO2 on 3D graphene with few-layered MoS2. ACS Sustain. Chem. Eng. 2018, 6, 5718–5724. [Google Scholar] [CrossRef]
- Qu, X.; Yang, R.; Tong, F.; Zhao, Y.; Wang, M.-H. Hierarchical ZnO microstructures decorated with Au nanoparticles for enhanced gas sensing and photocatalytic properties. Powder Technol. 2018, 330, 259–265. [Google Scholar] [CrossRef]
- Nie, N.; He, F.; Zhang, L.; Cheng, B. Direct Z-scheme PDA-modified ZnO hierarchical microspheres with enhanced photocatalytic CO2 reduction performance. Appl. Surf. Sci. 2018, 457, 1096–1102. [Google Scholar] [CrossRef]
- Jo, W.-K.; Lee, J.Y.; Natarajan, T.S. Fabrication of hierarchically structured novel redox-mediator-free ZnIn2S4 marigold flower/Bi2WO6 flower-like direct Z-scheme nanocomposite photocatalysts with superior visible light photocatalytic efficiency. Phys. Chem. Chem. Phys. 2016, 18, 1000–1016. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Pang, Y.; Zheng, Y.; Chen, C.; Ge, L. Design, preparation and enhanced photocatalytic activity of porous BiOCl/BiVO4 microspheres via a coprecipitation-hydrothermal method. J. Alloys Compd. 2017, 710, 375–382. [Google Scholar] [CrossRef]
- Madhusudan, P.; Zhang, J.; Yu, J.; Cheng, B.; Xu, D.; Zhang, J. One-pot template-free synthesis of porous CdMoO4 microspheres and their enhanced photocatalytic activity. Appl. Surf. Sci. 2016, 387, 202–213. [Google Scholar] [CrossRef]
- Nie, N.; Zhang, L.; Fu, J.; Cheng, B.; Yu, J. Self-assembled hierarchical direct Z-scheme g-C3N4/ZnO microspheres with enhanced photocatalytic CO2 reduction performance. Appl. Surf. Sci. 2018, 441, 12–22. [Google Scholar] [CrossRef]
- He, R.; Zhang, J.; Yu, J.; Cao, S. Room-temperature synthesis of BiOI with tailorable (0 0 1) facets and enhanced photocatalytic activity. J. Colloid Interface Sci. 2016, 478, 201–208. [Google Scholar] [CrossRef]
- Wei, H.; Hou, C.; Zhang, Y.; Nan, Z. Scalable low temperature in air solid phase synthesis of porous flower-like hierarchical nanostructure SnS2 with superior performance in the adsorption and photocatalytic reduction of aqueous Cr (VI). Sep. Purif. Technol. 2017, 189, 153–161. [Google Scholar] [CrossRef]
- Sedlak, J.; Kuritka, I.; Masar, M.; Machovsky, M.; Urbanek, P.; Bazant, P.; Janota, P.; Dvorackova, M. Contributions of morphological and structural parameters at different hierarchical morphology levels to photocatalytic activity of mesoporous nanostructured ZnO. Appl. Surf. Sci. 2020, 513, 145773. [Google Scholar] [CrossRef]
- Fu, J.; Zhu, B.; Jiang, C.; Cheng, B.; You, W.; Yu, J. Hierarchical porous O-doped g-C3N4 with enhanced photocatalytic CO2 reduction activity. Small 2017, 13, 1603938. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Bai, X.; Cai, Q.; Liu, C.; Zuo, Y.; Kang, S.; Cui, L. Facile synthesis of Y-doped graphitic carbon nitride with enhanced photocatalytic performance. Catal. Commun. 2016, 84, 179–182. [Google Scholar] [CrossRef]
- Liu, M.; Wageh, S.; Al-Ghamdi, A.A.; Xia, P.; Cheng, B.; Zhang, L.; Yu, J. Quenching induced hierarchical 3D porous gC3N4 with enhanced photocatalytic CO2 reduction activity. Chem. Commun. 2019, 55, 14023–14026. [Google Scholar] [CrossRef]
- Shabani, M.; Haghighi, M.; Kahforoushan, D.; Haghighi, A. Mesoporous-mixed-phase of hierarchical bismuth oxychlorides nanophotocatalyst with enhanced photocatalytic application in treatment of antibiotic effluents. J. Clean. Prod. 2019, 207, 444–457. [Google Scholar] [CrossRef]
- Edla, R.; Tonezzer, A.; Orlandi, M.; Patel, N.; Fernandes, R.; Bazzanella, N.; Date, K.; Kothari, D.C.; Miotello, A. 3D hierarchical nanostructures of iron oxides coatings prepared by pulsed laser deposition for photocatalytic water purification. Appl. Catal. B Environ. 2017, 219, 401–411. [Google Scholar] [CrossRef]
- Hu, H.; Wang, J.; Deng, C.; Niu, C.; Le, H. Microwave-assisted controllable synthesis of hierarchical CuS nanospheres displaying fast and efficient photocatalytic activities. J. Mater. Sci. 2018, 53, 14250–14261. [Google Scholar] [CrossRef]
- Nethravathi, C.; Rajamathi, J.T.; Rajamathi, M. Microwave-assisted synthesis of porous aggregates of CuS nanoparticles for sunlight photocatalysis. ACS Omega 2019, 4, 4825–4831. [Google Scholar] [CrossRef]
- Huang, Z.; Yan, F.-W.; Yuan, G. Ultrasound-assisted fabrication of hierarchical rodlike graphitic carbon nitride with fewer defects and enhanced visible-light photocatalytic activity. ACS Sustain. Chem. Eng. 2018, 6, 3187–3195. [Google Scholar] [CrossRef]
- Xu, W.; Wang, S.Q.; Zhang, Q.Y.; Ma, C.Y.; Wang, Q.; Wen, D.H.; Li, X.N. Hierarchically structured AgO films with nano-porosity for photocatalyst and all solid-state thin film battery. J. Alloys Compd. 2019, 802, 210–216. [Google Scholar] [CrossRef]
- Zhong, G.; Liu, D. Biomorphic CdS/Cd Photocatalyst Derived from Butterfly Wing for Efficient Hydrogen Evolution Reaction. Adv. Mater. Interfaces 2022, 9, 2102423. [Google Scholar] [CrossRef]




| Composition | Synthesis Strategy | Morphology | SSA (m2/g) | Application | Activity | Irradiation | Template(s) | Reference |
|---|---|---|---|---|---|---|---|---|
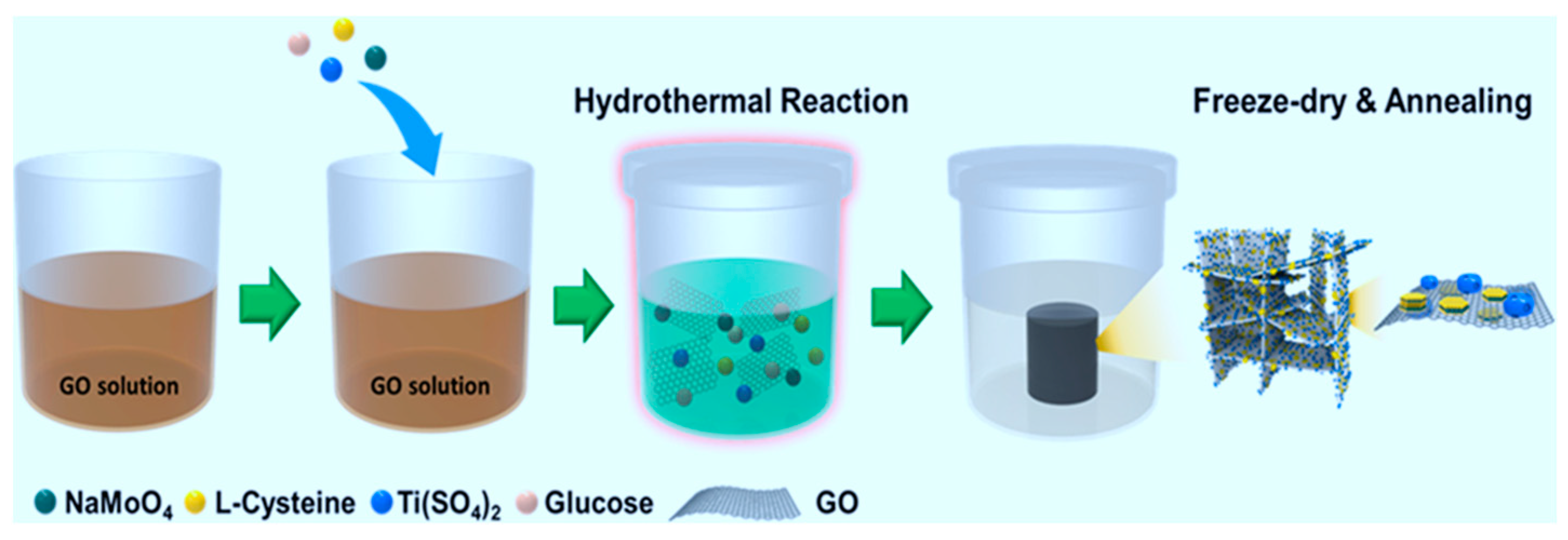
| SrTiO3/TiO2@carbon | hydrothermal | heterostructure with tri-modal (micro-, meso-, macro-) pores | 93–417 | photocatalytic hydrogen production from water splitting, methylene blue degradation | 2.52 mmol h−1 g−1 100% Degradation | UV | KOH | [93] |
| PbTiO3/TiO2/carbon | dual template | quasi-1D nanoneedle | 277–374 | photocatalytic and photoelectrochemical performances | MB degradation r 100% Under UV, 75% Under Visible+ ultrasound radiation the rates of hydrogen generation are 2360 and 9.6 μmol−1 g−1 | UV-Vis | ice/silica hard templates | [94] |
| N–TiO2/C | dropping | flower-like in hierarchical porous structure | 217–407 | MB degradation and hydrogen production | 2.0 and 48 times of benchmark P25 for MB degradation: 95% under UV and 92% under Vis irradiation and the hydrogen production rates are as high as 2.832 and 0.038 mmol g−1 h−1 | UV-Vis | ice/silica hard templates | [95] |
| Metal oxide/C | Dual-template method followed by heat treatment | hollow “dragon-bone” structure | 14–375 | methylene blue degradation | 100% (UV) 90% (Vis) | UV-Vis | ice/silica hard templates | [96] |
| TiO2 | hydrothermal | 3D hierarchical porous core-shell | 13.5–42.3 | Adsorption and mineralization of tetracycline hydrochloride | up to 70% | UV-Vis | silica | [97] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venezia, V.; Pota, G.; Silvestri, B.; Costantini, A.; Vitiello, G.; Luciani, G. Tailoring Structure: Current Design Strategies and Emerging Trends to Hierarchical Catalysts. Catalysts 2022, 12, 1152. https://doi.org/10.3390/catal12101152
Venezia V, Pota G, Silvestri B, Costantini A, Vitiello G, Luciani G. Tailoring Structure: Current Design Strategies and Emerging Trends to Hierarchical Catalysts. Catalysts. 2022; 12(10):1152. https://doi.org/10.3390/catal12101152
Chicago/Turabian StyleVenezia, Virginia, Giulio Pota, Brigida Silvestri, Aniello Costantini, Giuseppe Vitiello, and Giuseppina Luciani. 2022. "Tailoring Structure: Current Design Strategies and Emerging Trends to Hierarchical Catalysts" Catalysts 12, no. 10: 1152. https://doi.org/10.3390/catal12101152
APA StyleVenezia, V., Pota, G., Silvestri, B., Costantini, A., Vitiello, G., & Luciani, G. (2022). Tailoring Structure: Current Design Strategies and Emerging Trends to Hierarchical Catalysts. Catalysts, 12(10), 1152. https://doi.org/10.3390/catal12101152










