Enzymatic Formation of Protectin Dx and Its Production by Whole-Cell Reaction Using Recombinant Lipoxygenases
Abstract
1. Introduction
2. Results and Discussion
2.1. Identification of Products Obtained from the Conversion of 10S-HDHA, 8S-HETE, and 8S-HEPE by BT 15SLOX
2.2. Substrate Specificity of BT 15SLOX for PUFAs and HFAs
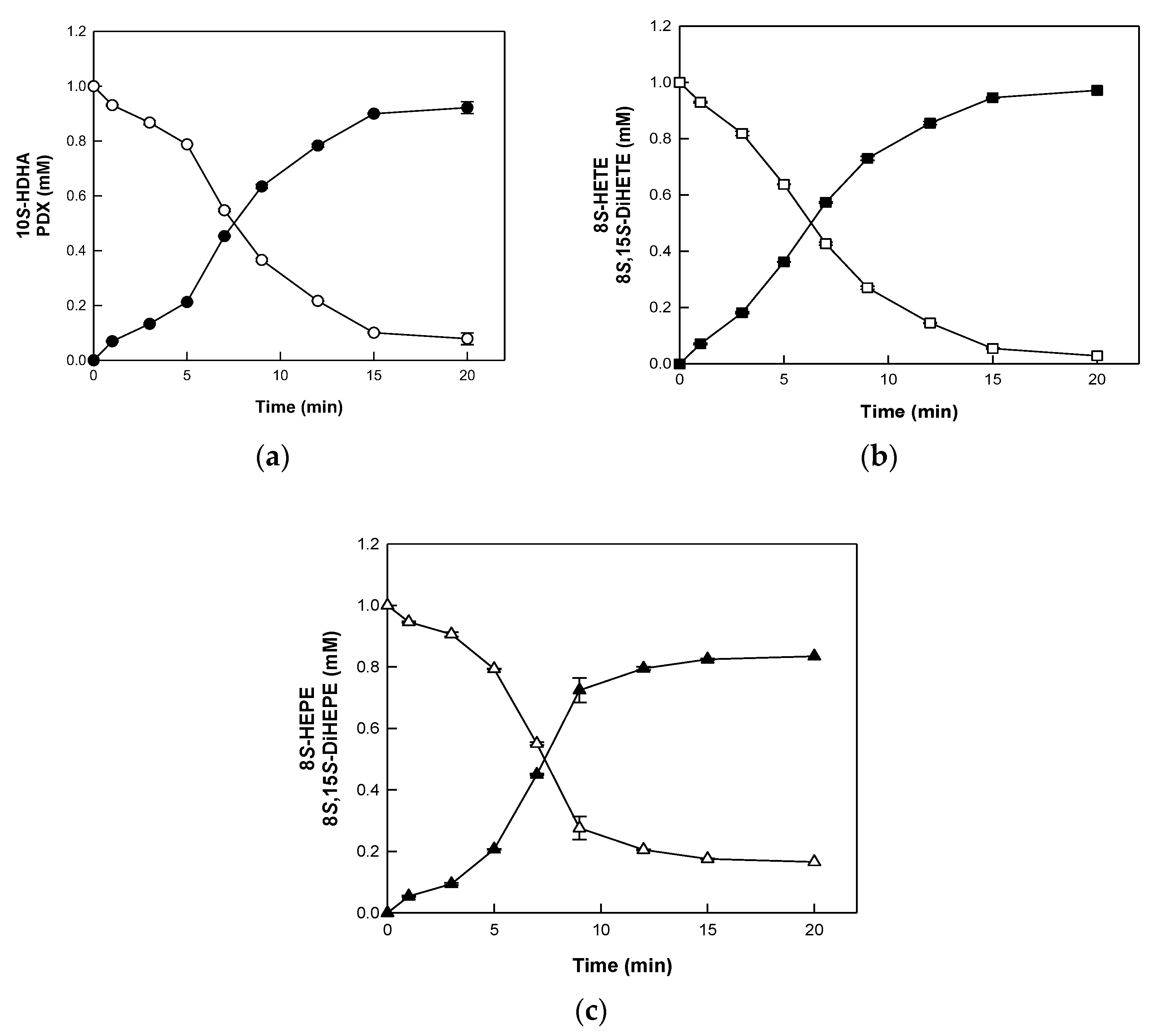
2.3. Biotransformation of HFAs to DiHFAs by BT 15SLOX
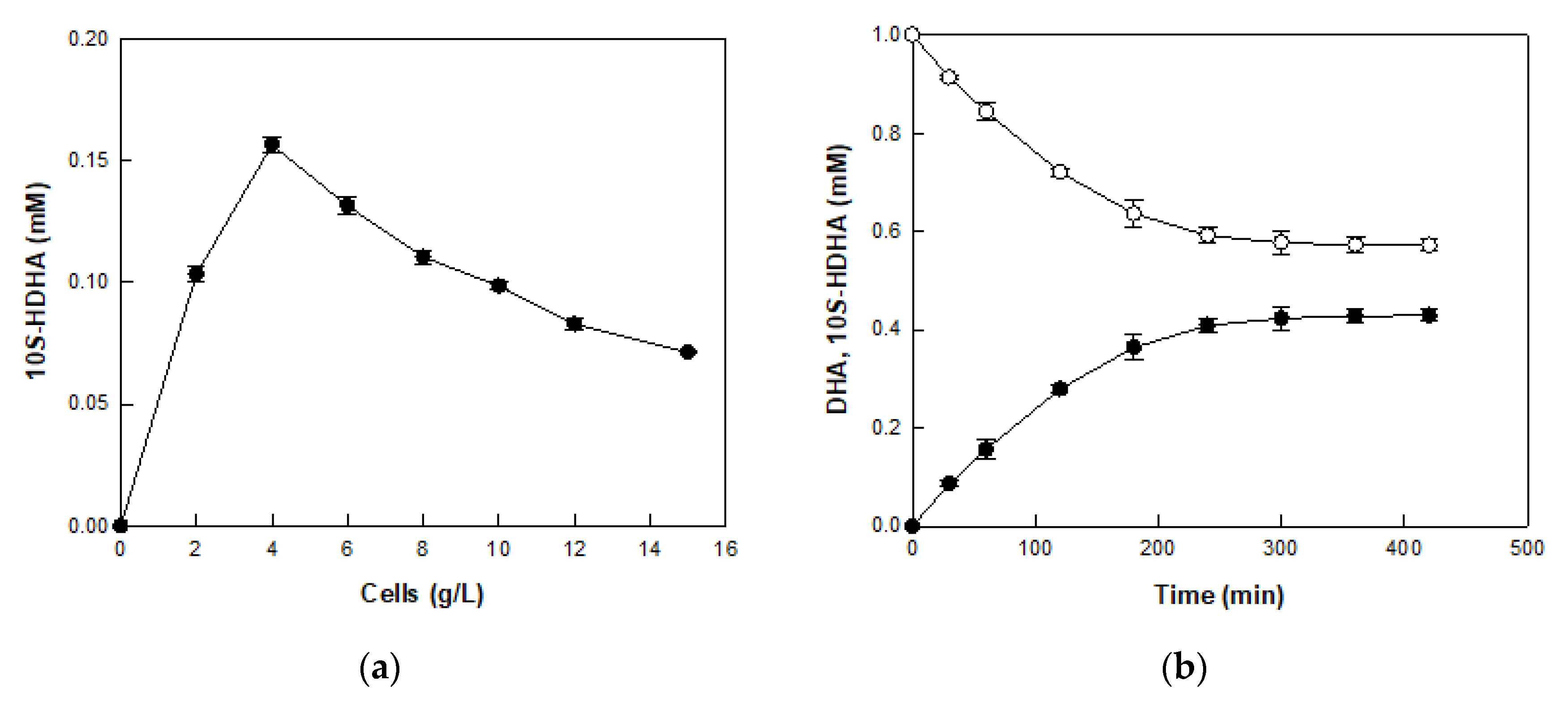
2.4. Production of 10S-HDHA from DHA-Enriched Fish Oil via DHA in DFOH by E. coli Cells Expressing MO 8SLOX
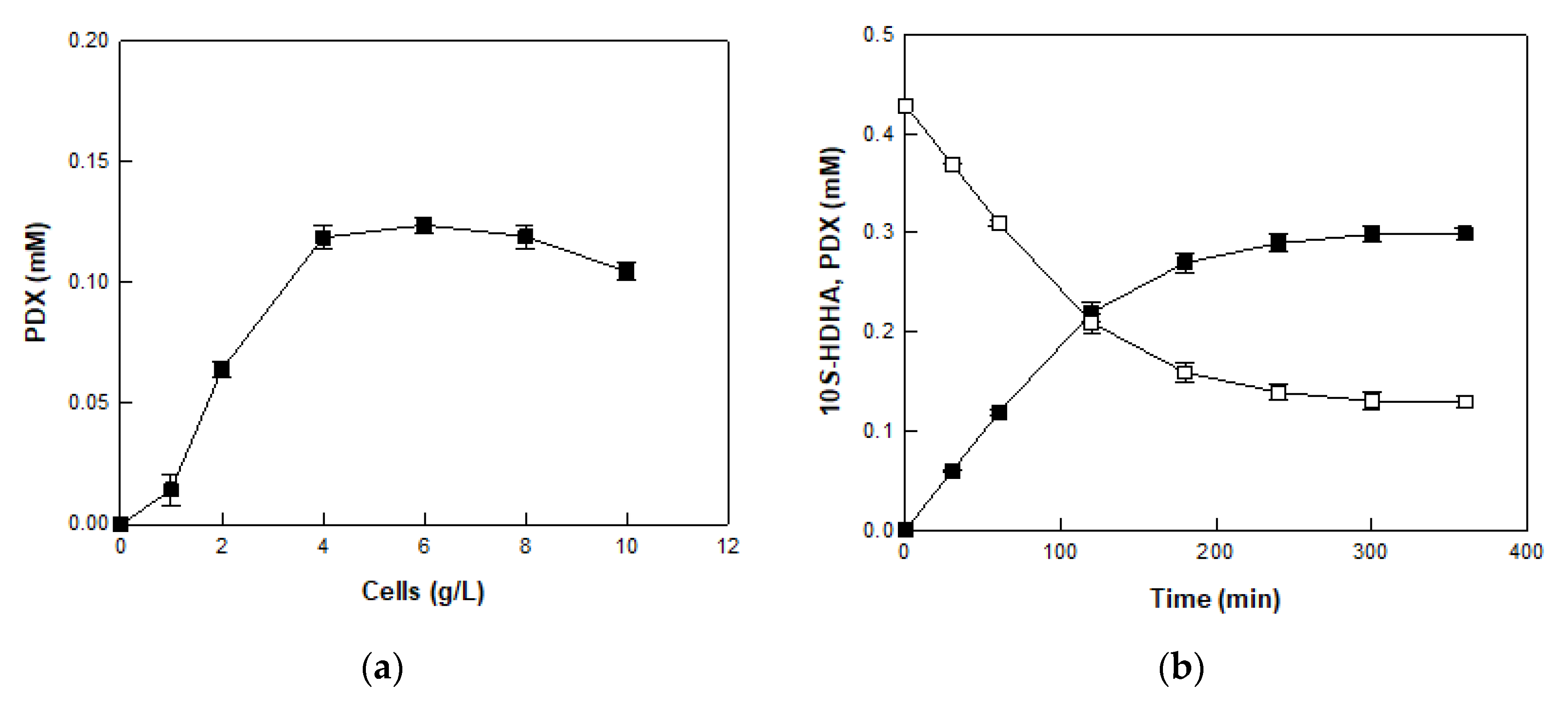
2.5. Production of PDX from 10S-HDHA in MO 8SLOX-Treated DFOH by E. coli Cells Expressing BT 15SLOX
3. Materials and Methods
3.1. Materials
3.2. Gene Cloning and Culture Conditions
3.3. Enzyme Purification
3.4. Enzyme Reaction
3.5. Whole-Cell Reaction
3.6. HPLC Analysis
3.7. LC-MS/MS and NMR Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Venugopalan, V.K.; Gopakumar, L.R.; Kumaran, A.K.; Chatterjee, N.S.; Soman, V.; Peeralil, S.; Mathew, S.; McClements, D.J.; Nagarajarao, R.C. Encapsulation and protection of omega-3-rich fish oils using food-grade delivery systems. Foods 2021, 10, 1566. [Google Scholar] [CrossRef] [PubMed]
- Ghelichi, S.; Shabanpour, B.; Pourashouri, P.; Hajfathalian, M.; Jacobsen, C. Extraction of unsaturated fatty acid-rich oil from common carp (Cyprinus carpio) roe and production of defatted roe hydrolysates with functional, antioxidant, and antibacterial properties. J. Sci. Food Agric. 2018, 98, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.K.; Tsai, M.F.; Huang, C.Y.; Lee, C.L.; Lin, C.; Shieh, C.J.; Kuo, C.H. Chitosan-based anti-oxidation delivery nano-platform: Applications in the encapsulation of DHA-enriched fish oil. Mar. Drugs 2021, 19, 470. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, H.; Su, J.; Wang, W.; Chen, H.; Yang, B.; Wang, Y. Highly efficient and enzyme-recoverable method for enzymatic concentrating omega-3 fatty acids generated by hydrolysis of fish oil in a substrate-constituted three-liquid-phase system. J. Agric. Food Chem. 2019, 67, 2570–2580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, F.; Ma, X.; Huang, H.; Wang, Y. Two-stage enzymatic preparation of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) enriched fish oil triacylglycerols. J. Agric. Food Chem. 2018, 66, 218–227. [Google Scholar] [CrossRef]
- Nordgren, T.M.; Anderson Berry, A.; Van Ormer, M.; Zoucha, S.; Elliott, E.; Johnson, R.; McGinn, E.; Cave, C.; Rilett, K.; Weishaar, K.; et al. Omega-3 fatty acid supplementation, pro-resolving mediators, and clinical outcomes in maternal-infant pairs. Nutrients 2019, 11, 98. [Google Scholar] [CrossRef]
- Grenon, S.M.; Owens, C.D.; Nosova, E.V.; Hughes-Fulford, M.; Alley, H.F.; Chong, K.; Perez, S.; Yen, P.K.; Boscardin, J.; Hellmann, J.; et al. Short-term, high-dose fish oil supplementation increases the production of omega-3 fatty acid-derived mediators in patients with peripheral artery disease (the OMEGA-PAD I Trial). J. Am. Heart Assoc. 2015, 4, e002034. [Google Scholar] [CrossRef]
- Ramirez, J.L.; Gasper, W.J.; Khetani, S.A.; Zahner, G.J.; Hills, N.K.; Mitchell, P.T.; Sansbury, B.E.; Conte, M.S.; Spite, M.; Grenon, S.M. Fish oil increases specialized pro-resolving lipid mediators in PAD (The OMEGA-PAD II Trial). J. Surg. Res. 2019, 238, 164–174. [Google Scholar] [CrossRef]
- Basil, M.C.; Levy, B.D. Specialized pro-resolving mediators: Endogenous regulators of infection and inflammation. Nat. Rev. Immunol. 2016, 16, 51–67. [Google Scholar] [CrossRef]
- Sugimoto, M.A.; Vago, J.P.; Perretti, M.; Teixeira, M.M. Mediators of the resolution of the inflammatory response. Trends Immunol. 2019, 40, 212–227. [Google Scholar] [CrossRef]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Chiang, N.; Fredman, G.; Bäckhed, F.; Oh, S.F.; Vickery, T.; Schmidt, B.A.; Serhan, C.N. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 2012, 484, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Leuti, A.; Maccarrone, M.; Chiurchiù, V. Proresolving lipid mediators: Endogenous modulators of oxidative stress. Oxidative Med. Cell Longev. 2019, 2019, 8107265. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Castillo, M.; Ortega, Á.; Cudris-Torres, L.; Duran, P.; Rojas, M.; Manzano, A.; Garrido, B.; Salazar, J.; Silva, A.; Rojas-Gomez, D.M.; et al. Specialized pro-resolving lipid mediators: The future of chronic pain therapy? Int. J. Mol. Sci. 2021, 22, 10370. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, T.; Yoshida, M.; Arita, M. Omega-3 fatty acid-derived mediators that control inflammation and tissue homeostasis. Int. Immunol. 2019, 31, 559–567. [Google Scholar] [CrossRef]
- White, P.J.; St-Pierre, P.; Charbonneau, A.; Mitchell, P.L.; St-Amand, E.; Marcotte, B.; Marette, A. Protectin DX alleviates insulin resistance by activating a myokine-liver glucoregulatory axis. Nat. Med. 2014, 20, 664–669. [Google Scholar] [CrossRef]
- Gobbetti, T.; Dalli, J.; Colas, R.A.; Federici Canova, D.; Aursnes, M.; Bonnet, D.; Alric, L.; Vergnolle, N.; Deraison, C.; Hansen, T.V.; et al. Protectin D1(n-3 DPA) and resolvin D5(n-3 DPA) are effectors of intestinal protection. Proc. Natl. Acad. Sci. USA 2017, 114, 3963–3968. [Google Scholar] [CrossRef]
- Bäck, M.; Yurdagul, A., Jr.; Tabas, I.; Öörni, K.; Kovanen, P.T. Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019, 16, 389–406. [Google Scholar] [CrossRef]
- Ramon, S.; Gao, F.; Serhan, C.N.; Phipps, R.P. Specialized proresolving mediators enhance human B cell differentiation to antibody-secreting cells. J. Immunol. 2012, 189, 1036–1042. [Google Scholar] [CrossRef]
- Spite, M.; Norling, L.V.; Summers, L.; Yang, R.; Cooper, D.; Petasis, N.A.; Flower, R.J.; Perretti, M.; Serhan, C.N. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 2009, 461, 1287–1291. [Google Scholar] [CrossRef]
- Morita, M.; Kuba, K.; Ichikawa, A.; Nakayama, M.; Katahira, J.; Iwamoto, R.; Watanebe, T.; Sakabe, S.; Daidoji, T.; Nakamura, S.; et al. The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell 2013, 153, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Iwuchukwu, I.; Nguyen, D.; Shirazian, A.; Asatryan, A.; Jun, B.; Bazan, N.G. Neuroprotectin D1, a lipid anti-inflammatory mediator, in patients with intracerebral hemorrhage. Biochimie 2022, 195, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Antony, R.; Lukiw, W.J.; Bazan, N.G. Neuroprotectin D1 induces dephosphorylation of Bcl-xL in a PP2A-dependent manner during oxidative stress and promotes retinal pigment epithelial cell survival. J. Biol. Chem. 2010, 285, 18301–18308. [Google Scholar] [CrossRef]
- Belayev, L.; Hong, S.H.; Menghani, H.; Marcell, S.J.; Obenaus, A.; Freitas, R.S.; Khoutorova, L.; Balaszczuk, V.; Jun, B.; Oriá, R.B.; et al. Docosanoids promote neurogenesis and angiogenesis, blood-brain barrier integrity, penumbra protection, and neurobehavioral recovery after experimental ischemic stroke. Mol. Neurobiol. 2018, 55, 7090–7106. [Google Scholar] [CrossRef] [PubMed]
- Asatryan, A.; Bazan, N.G. Molecular mechanisms of signaling via the docosanoid neuroprotectin D1 for cellular homeostasis and neuroprotection. J. Biol. Chem. 2017, 292, 12390–12397. [Google Scholar] [CrossRef]
- Chen, P.; Fenet, B.; Michaud, S.; Tomczyk, N.; Véricel, E.; Lagarde, M.; Guichardant, M. Full characterization of PDX, a neuroprotectin/protectin D1 isomer, which inhibits blood platelet aggregation. FEBS Lett. 2009, 583, 3478–3484. [Google Scholar] [CrossRef]
- An, J.U.; Kim, B.J.; Hong, S.H.; Oh, D.K. Characterization of an omega-6 linoleate lipoxygenase from Burkholderia thailandensis and its application in the production of 13-hydroxyoctadecadienoic acid. Appl. Microbiol. Biotechnol. 2015, 99, 5487–5497. [Google Scholar] [CrossRef]
- Lagarde, M.; Guichardant, M.; Bernoud-Hubac, N. Anti-inflammatory and anti-virus potential of poxytrins, especially protectin DX. Biochimie 2020, 179, 281–284. [Google Scholar] [CrossRef]
- Hwang, H.-J.; Jung, T.W.; Kim, J.W.; Kim, J.A.; Lee, Y.B.; Hong, S.H.; Roh, E.; Choi, K.M.; Baik, S.H.; Yoo, H.J. Protectin DX prevents H2O2-mediated oxidative stress in vascular endothelial cells via an AMPK-dependent mechanism. Cell. Signal. 2019, 53, 14–21. [Google Scholar] [CrossRef]
- Liu, M.; Boussetta, T.; Makni-Maalej, K.; Fay, M.; Driss, F.; El-Benna, J.; Lagarde, M.; Guichardant, M. Protectin DX, a double lipoxygenase product of DHA, inhibits both ROS production in human neutrophils and cyclooxygenase activities. Lipids 2014, 49, 49–57. [Google Scholar] [CrossRef]
- Yang, J.X.; Li, M.; Hu, X.; Lu, J.C.; Wang, Q.; Lu, S.Y.; Gao, F.; Jin, S.W.; Zheng, S.X. Protectin DX promotes epithelial injury repair and inhibits fibroproliferation partly via ALX/PI3K signalling pathway. J. Cell. Mol. Med. 2020, 24, 14001–14012. [Google Scholar] [CrossRef] [PubMed]
- Sancéau, J.Y.; Maltais, R.; Poirier, D.; Marette, A. Total synthesis of the antidiabetic (type 2) lipid mediator protectin DX/PDX. J. Org. Chem. 2019, 84, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Snajdrova, R.; Moore, J.C.; Baldenius, K.; Bornscheuer, U.T. Biocatalysis: Enzymatic Synthesis for Industrial Applications. Angew. Chem. Int. Ed. Engl. 2021, 60, 88–119. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.L.; Finnigan, W.; France, S.P.; Green, A.P.; Hayes, M.A.; Hepworth, L.J.; Lovelock, S.L.; Niikura, H.; Osuna, S.; Romero, E. Biocatalysis. Nat. Rev. Methods Primers 2021, 1, 1–21. [Google Scholar] [CrossRef]
- Serhan, C.N.; Gotlinger, K.; Hong, S.; Lu, Y.; Siegelman, J.; Baer, T.; Yang, R.; Colgan, S.P.; Petasis, N.A. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: Assignments of dihydroxy-containing docosatrienes. J. Immunol. 2006, 176, 1848–1859. [Google Scholar] [CrossRef]
- Lee, J.; An, J.U.; Kim, T.H.; Ko, Y.J.; Park, J.B.; Oh, D.K. Discovery and engineering of a microbial double-oxygenating lipoxygenase for synthesis of dihydroxy fatty acids as specialized proresolving mediators. ACS Sustain. Chem. Eng. 2020, 8, 16172–16183. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, J.; Kim, S.E.; Oh, D.K. Biocatalytic synthesis of dihydroxy fatty acids as lipid mediators from polyunsaturated fatty acids by double dioxygenation of the microbial 12S-lipoxygenase. Biotechnol. Bioeng. 2021, 118, 3094–3104. [Google Scholar] [CrossRef]
- Shin, K.C.; Kang, W.R.; Seo, M.J.; Kim, D.W.; Oh, D.K. Production of 8S- and 10S-hydroxy polyunsaturated fatty acids by recombinant Escherichia coli cells expressing mouse arachidonate 8S-lipoxygenase. Biotechnol. Lett. 2019, 41, 575–582. [Google Scholar] [CrossRef]
- Abbott, K.; Burrows, T.L.; Acharya, S.; Thota, R.N.; Garg, M.L. Dietary supplementation with docosahexaenoic acid rich fish oil increases circulating levels of testosterone in overweight and obese men. Prostaglandins Leukot. Essent. Fatty Acids 2020, 163, 102204. [Google Scholar] [CrossRef]
- Shin, K.C.; Seo, M.J.; Kang, S.H.; Oh, D.K. Production of 8,11-dihydroxy fatty acids from oleic and palmitoleic acids by Escherichia coli cells expressing variant 6,8-linoleate diol synthases from Penicillium oxalicum. Biotechnol. Prog. 2022, 7, e3267. [Google Scholar] [CrossRef]
- Tsai, W.C.; Kalyanaraman, C.; Yamaguchi, A.; Holinstat, M.; Jacobson, M.P.; Holman, T.R. In Vitro biosynthetic pathway investigations of neuroprotectin D1 (NPD1) and protectin DX (PDX) by human 12-lipoxygenase, 15-lipoxygenase-1, and 15-lipoxygenase-2. Biochemistry 2021, 60, 1741–1754. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.J.; Shin, K.C.; Oh, D.K. Production of 5,8-dihydroxy-9,12(Z,Z)-octadecadienoic acid from linoleic acid by whole recombinant Escherichia coli cells expressing diol synthase from Aspergillus nidulans. Appl. Microbiol. Biotechnol. 2014, 98, 7447–7456. [Google Scholar] [CrossRef] [PubMed]

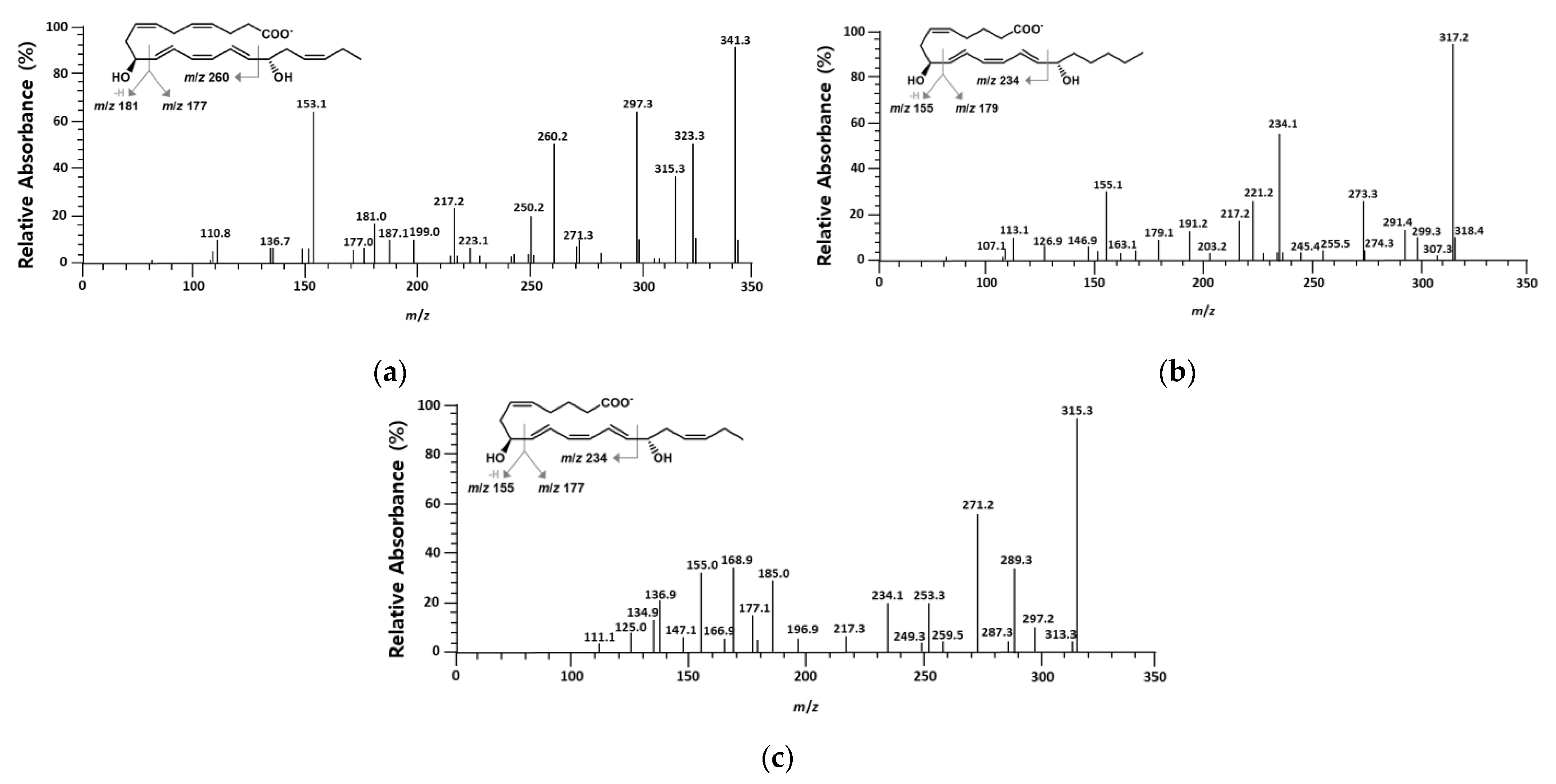
| Proton Number | 1H (δ) | Multiplet | J (Hz) | Protons | 13C (δ) |
|---|---|---|---|---|---|
| 1 | 177.4 | ||||
| 2 | 2.29 | m | 2H | 35.3 | |
| 3 | 2.23 | m | 2H | 24.1 | |
| 4 | 5.35 | m | 1H | 129.4 | |
| 5 | 5.35 | m | 1H | 130.2 | |
| 6 | 2.80 | m | 2H | 26.8 | |
| 7 | 5.40 | m | 1H | 131.1 | |
| 8 | 5.40 | m | 1H | 126.6 | |
| 9 | 2.31, 2.29 | m | 2H | 36.5 | |
| 10 | 4.14 | td | 6.53, 6.12 | 1H | 73.2 |
| 11 | dd | 15.05, 6.12 | 1H | 138.2 | |
| 12 | dd | 15.05 | 1H | 126.6 | |
| 13 | d | 9.83 | 1H | 130.1 | |
| 14 | d | 9.83 | 1H | 130.1 | |
| 15 | dd | 15.07 | 1H | 126.6 | |
| 16 | ddd | 15.07, 6.54, 2.65 | 1H | 138.2 | |
| 17 | 4.12 | td | 6.58, 6.54 | 1H | 73.3 |
| 18 | 2.28, 2.24 | m | 2H | 36.3 | |
| 19 | 5.33 | m | 1H | 125.6 | |
| 20 | 5.43 | m | 1H | 134.8 | |
| 21 | 2.02 | td | 7.50, 7.15 | 2H | 21.8 |
| 22 | 0.92 | t | 7.50 | 3H | 14.7 |
| Substrate | Product | Specific Activity (μmol/min/mg) |
|---|---|---|
| DHA | 17S-HDHA | 2.74 ± 0.06 |
| AA | 15S-HETE | 4.89 ± 0.19 |
| EPA | 15S-HEPE | 3.09 ± 0.06 |
| 10S-HDHA | PDX | 1.17 ± 0.01 |
| 8S-HETE | 8S,15S-DiHETE | 0.38 ± 0.01 |
| 8S-HEPE | 8S,15S-DiHEPE | 0.61 ± 0.02 |
| Step (Biocatalyst) | Substrate (mg/L) [mM] | Product (mg/L) [mM] | Productivity (mg/L/h) [μM/h] | Step Yield (g/g) [mol/mol] | Total Yield (g/g) |
|---|---|---|---|---|---|
| Hydrolysis (lipase from Thermomyces lanuginose) | DHA-enriched fish oil (455) | DHA (328 ± 11) [1.00 ± 0.03] | (328 ± 11) [1000 ± 34] | (0.72) | 0.72 |
| 1st hydroxylation (E. coli cells expressing MO 8S-LOX) | DHA in DFOH (328) [1.00] | 10S-HDHA (148 ± 4.1) [0.43 ± 0.01] | (24.7 ± 0.68) [72 ± 2.0] | (0.45) [0.43] | 0.33 |
| 2nd hydroxylation (E. coli cells expressing BT 15S-LOX) | 10S-HDHA in MO 8S-LOX treated DFOH (148) [0.43] | PDX (108 ± 1.8) [0.30 ± 0.01] | (21.6 ± 0.36) [60 ± 1.0] | (0.73) [0.70] | 0.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, K.-C.; Lee, T.-E.; Kim, S.-E.; Ko, Y.-J.; Seo, M.-J.; Oh, D.-K. Enzymatic Formation of Protectin Dx and Its Production by Whole-Cell Reaction Using Recombinant Lipoxygenases. Catalysts 2022, 12, 1145. https://doi.org/10.3390/catal12101145
Shin K-C, Lee T-E, Kim S-E, Ko Y-J, Seo M-J, Oh D-K. Enzymatic Formation of Protectin Dx and Its Production by Whole-Cell Reaction Using Recombinant Lipoxygenases. Catalysts. 2022; 12(10):1145. https://doi.org/10.3390/catal12101145
Chicago/Turabian StyleShin, Kyung-Chul, Tae-Eui Lee, Su-Eun Kim, Yoon-Joo Ko, Min-Ju Seo, and Deok-Kun Oh. 2022. "Enzymatic Formation of Protectin Dx and Its Production by Whole-Cell Reaction Using Recombinant Lipoxygenases" Catalysts 12, no. 10: 1145. https://doi.org/10.3390/catal12101145
APA StyleShin, K.-C., Lee, T.-E., Kim, S.-E., Ko, Y.-J., Seo, M.-J., & Oh, D.-K. (2022). Enzymatic Formation of Protectin Dx and Its Production by Whole-Cell Reaction Using Recombinant Lipoxygenases. Catalysts, 12(10), 1145. https://doi.org/10.3390/catal12101145








