Solvents for extractions and chromatography were of technical grade and were distilled prior to use. Extracts were dried over technical grade anhydrous Na2SO4. Melting points were determined on a Kofler micro hot stage (Laica Galen III, Leica, Germany). The NMR spectra were obtained on a Bruker Avance DPX 300 at 300 MHz for 1H nucleus and Bruker UltraShield 500 plus (Bruker, Billerica, MA, USA) at 500 MHz for 1H and 126 MHz for 13C nucleus, using DMSO-d6 and CDCl3 with TMS as the internal standard, as solvents. Mass spectra were recorded on an Agilent 6224 Accurate Mass TOF LC/MS (Agilent Technologies, Santa Clara, CA, USA), IR spectra on a Perkin-Elmer Spectrum BX FTIR spectrophotometer (PerkinElmer, Waltham, MA, USA). CD spectra were recorded on a J-1500 Circular Dichroism Spectrophotometer (JASCO corporation, Tokyo, Japan). Column chromatography (CC) was performed on silica gel (Silica gel 60, particle size: 0.035–0.070 mm (Sigma-Aldrich, St. Louis, MO, USA)). HPLC analyses were performed on an Agilent 1260 Infinity LC (Agilent Technologies, Santa Clara, CA, USA) using CHIRALPAK AD-H (0.46 cm ø × 25 cm), as chiral column (CHIRAL TECHNOLOGIES, INC., West Chester, PE, USA). All the commercially available chemicals used were purchased from Sigma-Aldrich (St. Louis, MO, USA).
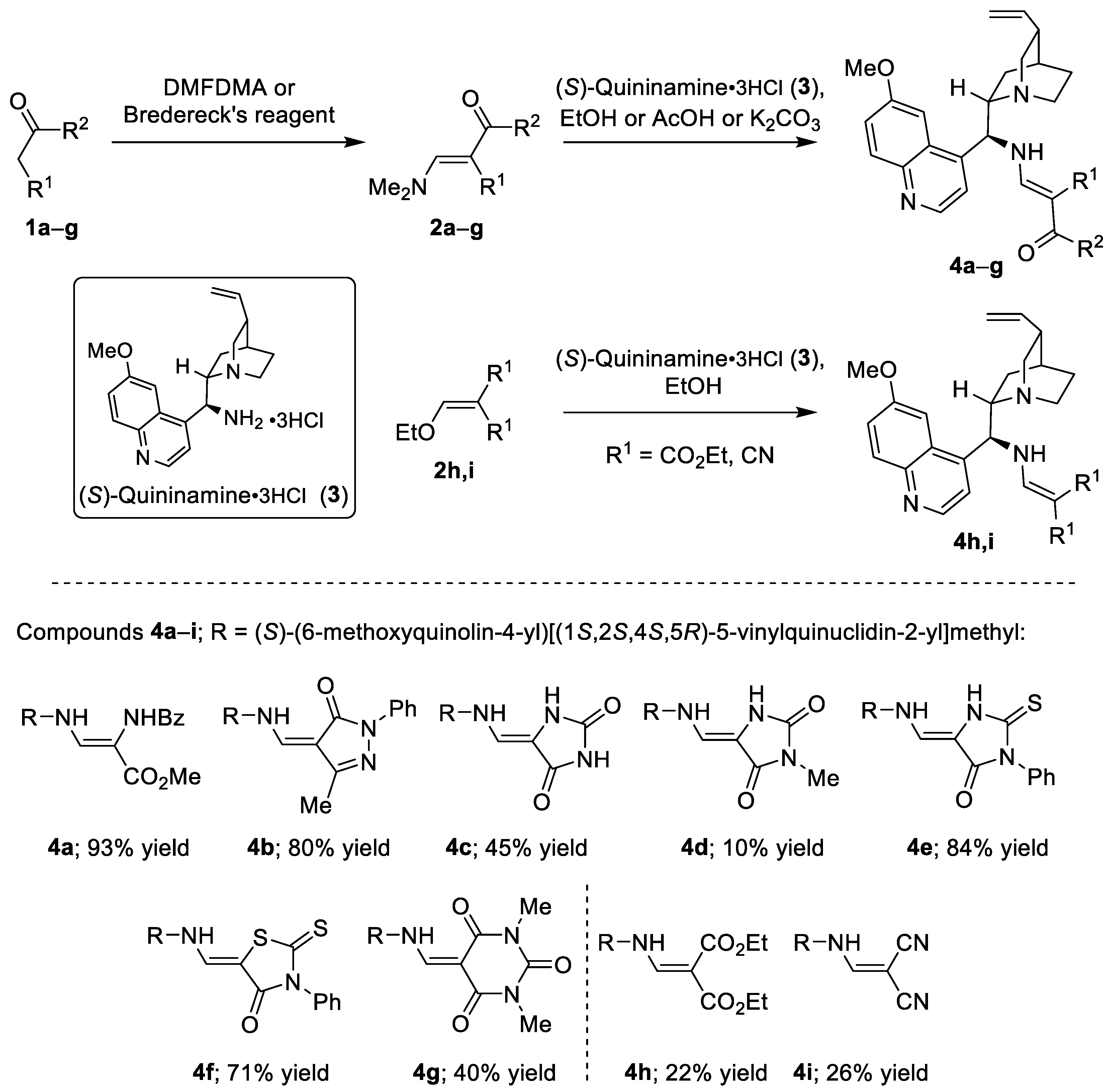
3.2. Synthesis of Compounds 4a –i
General procedure 1 (GP1): To a solution of enaminone 2 in AcOH, (S)-quininamine trihydrochloride (3) (1 equivalent) was added and the reaction mixture was stirred at 25 °C for 24 h. Acetic acid was evaporated in vacuo and the residue was purified by CC. Fractions containing the product 4 were combined and volatile components evaporated in vacuo.
General procedure 2 (GP2): To a solution of enaminone or ethoxymethylene compound 2 in EtOH, (S)-quininamine trihydrochloride (3) (1 equivalent) was added and the reaction mixture was stirred at 25 °C for 24 h. Ethanol was evaporated in vacuo and the residue was purified by CC. Fractions containing the product 4 were combined and volatile components evaporated in vacuo.
3.2.1. Methyl 2-Benzamido-3-({(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}amino)acrylate (4a)
Following GP1. Prepared from 3 (1 mmol, 433 mg), and methyl 2-benzamido-3-(dimethylamino)acrylate (2a) (1 mmol, 248 mg), AcOH (2 mL), 25 °C, 24 h, CC (EtOAc). Yield: 490 mg (0.93 mmol, 93%) of white solid, ratio of isomers: 1:8, mp = 92.9–94.9 °C. [α]Dr.t. = −119 (0.067, MeOH). EI-HRMS: m/z = 264.1360 (M + 2H)+2; C31H36N4O4 requires: m/z = 264.1363 (M + 2H)+2; νmax 3245, 2944, 1693, 1644, 1617, 1579, 1507, 1476, 1433, 1359, 1227, 1186, 1140, 1025, 915, 853, 830, 760, 707, 641, 613 cm−1. 1H-NMR (500 MHz, DMSO-d6): δ major isomer 0.65–0.79 (m, 1H), 1.11–1.26 (m, 1H), 1.44–1.64 (m, 3H), 2.16–2.25 (m, 1H), 2.52–2.54 (m, 1H), 2.57–2.68 (m, 2H), 3.10 (dd, J = 13.7, 10.0 Hz, 1H), 3.17–3.30 (m, 1H), 3.45 (s, 3H), 3.98 (s, 3H), 4.85–4.99 (m, 2H), 5.15–5.40 (m, 1H), 5.72–5.89 (m, 1H), 6.73 (dd, J = 5.3, 13.8 Hz, 1H), 7.32 (br s, 1H), 7.43–7.52 (m, 3H), 7.53–7.58 (m, 1H), 7.63 (dd, J = 7.4, 4.5 Hz, 1H), 7.74–7.83 (m, 1H), 7.90–8.02 (m, 3H), 8.77 (d, J = 4.6 Hz, 1H), 8.93 (s, 1H); minor isomer 2.25–2.30 (m, 1H), 2.68–2.78 (m, 2H), 3.23 (dd, J = 9.9, 13.6 Hz, 1H), 3.55 (s, 3H), 3.94 (s, 3H), 7.38 (dd, J = 7.0, 8.4 Hz, 1H), 8.56 (br d, J = 12.5 Hz, 1H). 13C-NMR (126 MHz, DMSO-d6): δ 25.46, 27.04, 27.31, 40.31, 50.40, 50.40, 55.19, 55.67, 55.76, 78.95, 96.95, 102.60, 114.19, 121.61, 127.33, 127.71, 128.09, 128.15, 131.16, 131.38, 134.56, 142.01, 142.07, 143.75, 144.20, 147.68, 157.52, 165.39, 165.92.
3.2.2. (Z)-4-[({(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}amino)methylene]-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one (4b)
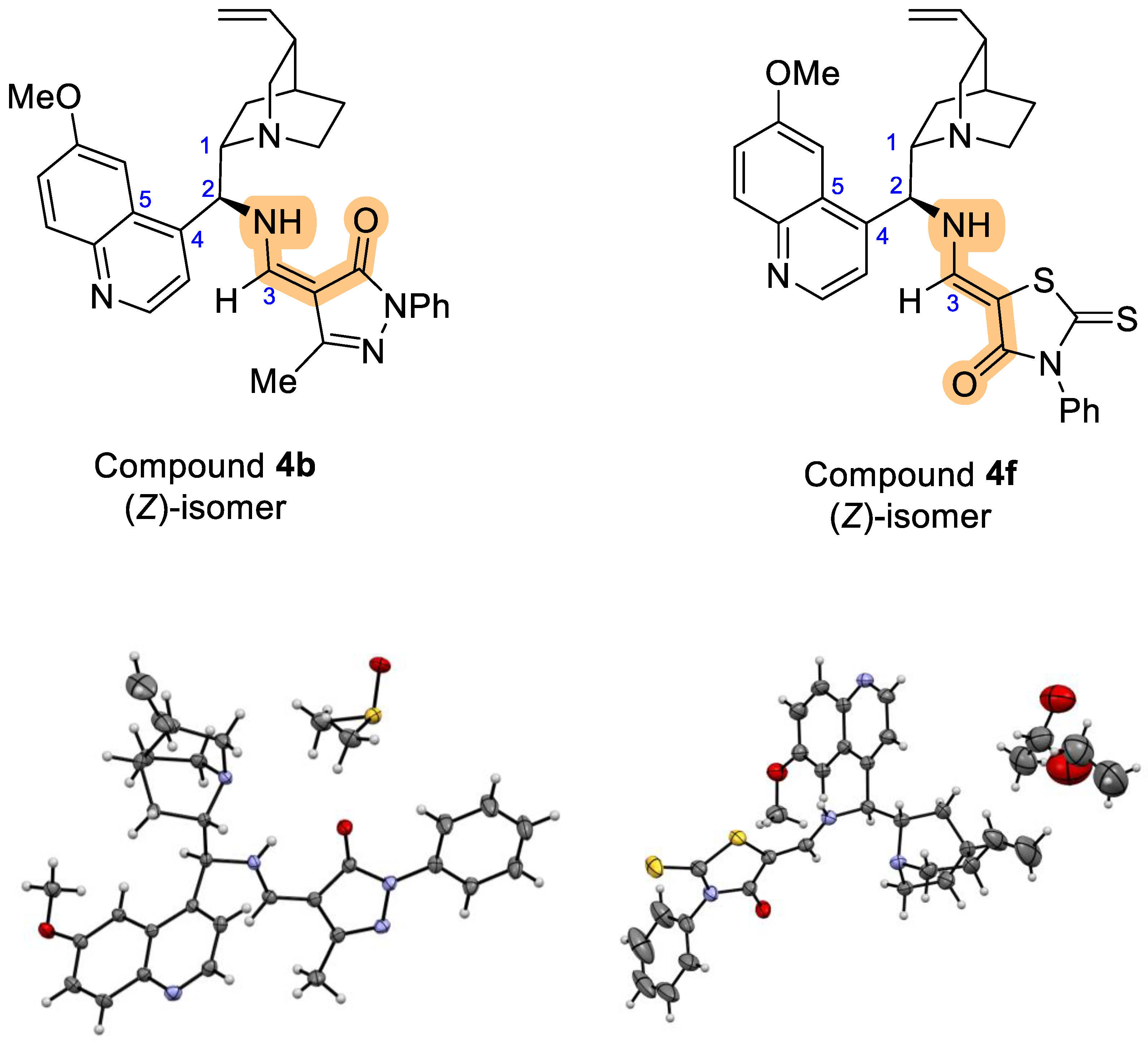
Following GP2. Prepared from 3 (0.50 mmol, 215 mg), and (E)-4-[(dimethylamino)methylene]-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one (2b) (0.50 mmol, 115 mg), EtOH (1 mL), 25 °C, 24 h; CC (EtOAc). Yield: 203 mg (0.40 mmol, 80%) of pale orange solid, mp = 157.7–158.0 °C. [α]Dr.t. = −564 (0.185, MeOH). EI-HRMS: m/z = 254.6388 (M + 2H)+2; C31H35N5O2 requires: m/z = 254.6390 (M + 2H)+2; νmax 3377, 2934, 2604, 1663, 1618, 1598, 1545, 1499, 1458, 1383, 1347, 1250, 1161, 1117, 1092, 1025, 923, 853, 759, 715, 694, 670, 42 cm−1. 1H-NMR (500 MHz, DMSO-d6): δ 1.03–1.11 (m, 1H), 1.66–1.79 (m, 1H), 1.88–2.05 (m, 3H), 2.16 (s, 3H), 2.76–2.84 (m, 1H), 3.24–3.36 (m, 2H), 4.11 (s, 3H), 4.24 (br s, 1H), 4.80 (q, J = 9.6 Hz, 1H), 5.18 (d, J = 10.5 Hz, 1H), 5.25 (d, J = 17.2 Hz, 1H), 5.89–6.07 (m, 2H), 7.08 (t, J = 7.4 Hz, 1H), 7.34 (t, J = 7.8 Hz, 2H), 7.62 (d, J = 9.2 Hz, 1H), 7.92 (d, J = 8.2 Hz, 2H), 7.95 (d, J = 5.0 Hz, 1H), 8.03 (s, 1H), 8.12 (d, J = 9.2 Hz, 1H), 8.62 (br s, 1H), 8.99 (d, J = 4.8 Hz, 1H), 9.56 (s, 1H), 9.97 (br s, 1H). 13C-NMR (126 MHz, DMSO-d6): δ 12.54, 21.18, 25.38, 27.09, 33.89, 40.46, 54.99, 56.19, 58.61, 79.38, 99.61, 102.43, 114.60, 117.42, 120.89, 121.84, 123.35, 127.58, 128.64, 131.60, 139.32, 142.16, 144.32, 147.86, 148.22, 152.27, 157.94, 164.91, 172.01.
3.2.3. (Z)-5-[({(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}amino)methylene]imidazolidine-2,4-dione (4c)
Following GP1. Prepared from 3 (1.2 mmol, 517 mg), and (Z)-5-[(dimethylamino)methylene]imidazolidine-2,4-dione (2c) (1.2 mmol, 186 mg), AcOH (2.5 mL), 25 °C, 24 h, CC (EtOAc:MeOH = 4:1). Yield: 234 mg (0.54 mmol, 45%) of white solid, mp = 232–234 °C. [α]Dr.t. = −153 (0.027, MeOH). EI-HRMS: m/z = 434.2181 (M + H)+; C24H28N5O3 requires: m/z = 434.2187 (M + H)+; νmax 3292, 2970, 2945, 2882, 1697, 1655, 1619, 1559, 1509, 1473, 1225, 1090, 1025, 918, 855, 717, 674 cm−1. 1H-NMR (500 MHz, DMSO-d6): δ 0.63 (s, 1H), 1.08–1.30 (m, 1H), 1.40–1.60 (m, 4H), 2.23 (s, 1H), 2.57–2.77 (m, 2H), 3.09–3.17 (m, 2H), 3.98 (s, 3H), 4.88–5.04 (m, 2H), 5.12 (br s, 1H), 5.82 (ddd, J = 17.5, 10.3, 7.6, 1H), 6.55 (br s, 1H), 7.42 (dd, J = 9.2, 2.7, 1H), 7.49 (dd, J = 13.3, 6.4, 1H), 7.68 (d, J = 4.6, 1H), 7.74 (s, 1H), 7.95 (d, J = 9.2, 1H), 8.73 (d, J = 4.6, 1H), 9.59 (s, 1H), 10.09 (s, 1H). 13C-NMR (126 MHz, DMSO-d6): δ 22.53, 25.57, 27.21, 27.46, 40.40, 45.51, 55.26, 55.96, 55.97, 102.46, 104.17, 114.25, 121.66, 131.39, 131.48, 132.04, 141.13, 142.17, 144.14, 147.72, 152.70, 157.52, 164.22, 174.05.
3.2.4. (Z)-5-[({(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}amino)methylene]-1-methylimidazolidine-2,4-dione (4d)
Following GP2. Prepared from 3 (1.2 mmol, 517 mg), and (Z)-5-[(dimethylamino)methylene]-3-methylimidazolidine-2,4-dione (2d) (1.2 mmol, 203 mg), EtOH (2.5 mL), 25 °C, 24 h, CC (EtOAc:MeOH = 4:1). Yield: 56 mg (0.125 mmol, 10%) of colorless solid, mp = 120–133.3 °C. [α]Dr.t. = −170 (0.11, MeOH). EI-HRMS: m/z = 224.6209 (M + 2H)+2; C25H31N5O3 requires: m/z = 224.6208 (M + 2H)+2; νmax 3367, 3011, 2784, 2452, 1730, 1620, 1509, 1466, 1435, 1392, 1229, 1122, 1022, 833 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.97 (dd, J = 14.2, 6.6 Hz, 1H), 1.49 (br s, 1H), 1.67–1.87 (m, 3H), 2.45 (br s, 1H), 2.91 (s, 3H), 2.92–3.13 (m, 2H), 3.26–3.55 (m, 2H), 3.67 (br s, 1H), 3.85–4.01 (m, 4H), 4.95–5.17 (m, 1H), 5.05 (dd, J = 13.9, 9.8 Hz, 2H), 5.61–5.83 (m, 1H), 6.53 (br s, 1H), 7.34–7.58 (m, 4H), 8.05 (d, J = 9.3 Hz, 1H), 8.76 (d, J = 4.8 Hz, 1H). 13C-NMR (126 MHz, CDCl3): δ 23.91, 24.29, 26.98, 38.17, 41.05, 54.66, 55.96, 76.91, 77.16, 77.36, 77.42, 101.35, 105.95, 116.26, 121.97, 123.78, 132.29, 136.85, 139.13, 144.93, 147.77, 154.03, 158.58, 164.08, 179.10.
3.2.5. (Z)-5-[({(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}amino)methylene]-3-phenyl-2-thioxoimidazolidin-4-one (4e)
Following GP1. Prepared from 3 (0.98 mmol, 424 mg), and (Z)-5-[(dimethylamino)methylene]-3-phenyl-2-thioxoimidazolidin-4-one (2e) (0.98 mmol, 243 mg), AcOH (5 mL), 60 °C, 24 h. Isolation by filtration. Yield: 430 mg (0.82 mmol, 84%) of red solid, mp = 191–193 (m°C. [α]Dr.t. = –792 (0.02, MeOH). EI-HRMS: m/z = 263.6170 (M + 2H)+2; C30H33N5O2S requires: m/z = 263.6172 (M + 2H)+2; νmax 3330, 3090, 2921, 2359, 2042, 1964, 1707, 1648, 1593, 1543, 1478, 1387, 1366, 1329, 1302, 1262, 1211, 1175, 1097, 1031, 1004, 983, 933, 917, 868, 823, 773, 750, 706, 678, 646 cm−1. 1H-NMR (500 MHz, DMSO-d6): δ 0.98–1.07 (m, 1H), 1.73–1.83 (m, 1H), 1.91–2.01 (m, 3H), 2.76–2.85 (m, 1H), 3.23–3.39 (m, 2H), 3.90–4.01 (m, 1H), 4.06 (s, 3H), 4.43 (br s, 1H), 5.16 (d, J=10.4, 1H), 5.25 (d, J = 17.2, 1H), 5.73 (br s, 1H), 5.91–6.02 (m, 1H), 7.18–7.23 (m, 2H), 7.35 (br s, 1H), 7.37–7.41 (m, 1H), 7.45 (dd, J = 8.3, 6.7, 2H), 7.63 (dd, J = 9.3, 2.5, 1H), 7.90 (s, 1H), 7.95 (d, J = 4.9, 1H), 8.13 (d, J = 9.3, 1H), 8.83 (br s, 1H), 9.00 (d, J = 4.9, 1H), 9.74–9.79 (m, 1H), 11.54 (s, 1H). 13C-NMR (126 MHz, DMSO-d6): δ 23.43, 23.71, 25.51, 35.85, 41.68, 47.71, 52.01, 56.36, 58.89, 87.42, 89.78, 102.48, 116.65, 120.85, 123.40, 127.85, 128.62, 128.75, 130.24, 133.38, 133.98, 138.30, 146.65, 158.76, 159.10, 169.62, 172.76, 182.99.
3.2.6. (Z)-5-[({(S)-(6-methoxyquinolin-4-yl)((1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}amino)methylene]-3-phenyl-2-thioxothiazolidin-4-one (4f)
Following GP1. Prepared from 3 (1.2 mmol, 517 mg), and (Z)-5-[(dimethylamino)methylene]-3-phenyl-2-thioxoimidazolidin-4-one (2f) (1.2 mmol, 320 mg), AcOH (3 mL), 25 °C, 24 h. Isolation by filtration followed by CC (EtOAc). Yield: 464 mg (0.85 mmol, 71%) of yellow solid, mp = 153.1–155.6 °C. [α]Dr.t. = −475.3 (0.235, MeOH). EI-HRMS: m/z = 272.0976 (M + 2H)+2; C30H32N4O2S2 requires: m/z = 272.0978 (M + 2H)+2; νmax 3274, 3067, 2940, 2865, 1674, 1608, 1508, 1475, 1455, 1431, 1354, 1228, 1157, 1109, 1030, 984, 917, 854, 834, 744, 690, 641 cm−1. 1H-NMR (500 MHz, DMSO-d6): δ 0.57–0.83 (m, 1H), 1.26–1.41 (m, 1H), 1.48–1.71 (m, 3H), 2.21–2.34 (m, 1H), 2.61–2.83 (m, 2H), 3.11–3.25 (m, 1H), 3.34–3.43 (m, 1H), 3.45–3.60 (m, 1H), 3.99 (s, 3H), 4.88–5.08 (m, 2H), 5.50 (br s, 1H), 5.88 (ddd, J = 17.6, 10.3, 7.5, 1H), 7.02–7.13 (m, 1H), 7.29–7.37 (m, 2H), 7.49 (dd, J = 9.2, 2.6, 1H), 7.72 (d, J = 4.6, 2H), 7.86–7.96 (m, 3H), 8.01 (d, J = 9.2, 1H), 8.82 (d, J = 4.6, 1H), 10.17 (br s, 1H). 13C-NMR (126 MHz, DMSO-d6): δ 25.72, 27.28, 27.28, 40.31, 55.12, 55.42, 56.05, 58.79, 89.06, 102.54, 114.27, 120.06, 121.60, 127.49, 131.47, 131.54, 135.84, 136.41, 141.93, 142.12, 143.64, 144.20, 144.46, 147.73, 147.82, 157.81, 166.46, 191.03.
3.2.7. 5-[({(S)-(6-methoxyquinolin-4-yl)((1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}amino)methylene]-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione (4g)
Prepared from 3 (1 mmol, 433 mg), and 5-[(dimethylamino)methylene]-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione (2g) (1 mmol, 215 mg), K2CO3 (3 mmol, 415 mg), MeOH (2 mL), 25 °C, 24 h. Isolation by filtration followed by CC (EtOAc). Yield: 197 mg (0.40 mmol, 40%) of yellowish solid, mp = 113.3–115.8 °C. [α]Dr.t. = −88.5 (0.135, MeOH). EI-HRMS: m/z = 245.6258 (M + 2H)+2; C27H33N5O4 requires: m/z = 245.6261 (M + 2H)+2; νmax 3551, 3216, 2947, 2890, 1714, 1650, 1591, 1508, 1478, 1334, 1261, 1230, 1097, 1054, 1028, 915, 855, 834, 779, 757 cm−1. 1H-NMR (500 MHz, DMSO-d6): δ 0.68 (br s, 1H), 1.40–1.28 (m, 1H), 1.62–1.46 (m, 3H), 2.27 (d, J = 8.1 Hz, 1H), 2.79–2.62 (m, 2H), 3.06 (s, 3H), 3.12 (s, 3H), 3.18 (dd, J = 13.6, 9.9 Hz, 1H), 3.46–3.37 (m, 1H), 3.50 (q, J = 9.4 Hz, 1H), 3.97 (s, 3H), 4.94 (dt, J = 10.5, 1.3 Hz, 1H), 5.02 (dt, J = 17.2, 1.5 Hz, 1H), 5.94–5.81 (br s, 1H), 5.64 (s, 1H), 7.48 (dd, J = 9.2, 2.6 Hz, 1H), 7.71 (d, J = 4.6 Hz, 1H), 7.83 (br s, 1H), 8.01 (d, J = 9.2 Hz, 1H), 8.10 (bd, J = 14.7 Hz, 1H), 8.81 (d, J = 4.5 Hz, 1H), 10.84 (br s, 1H). 13C-NMR (126 MHz, DMSO-d6): δ 25.50, 26.79, 27.18, 27.29, 27.36, 40.23, 55.20, 55.94, 58.82, 79.25, 90.16, 102.24, 114.41, 120.66, 121.90, 127.90, 131.65, 142.06, 144.32, 147.89, 151.51, 157.86, 157.97, 162.05, 163.90.
3.2.8. Diethyl 2-[({(S)-(6-methoxyquinolin-4-yl)((1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}amino)methylene]malonate (4h)
Following GP2. Prepared from 3 (1.2 mmol, 517 mg), and diethyl 2-(ethoxymethylene)malonate (2h) (1.2 mmol, 258 mg), EtOH (2 mL), 25 °C, 24 h, CC (EtOAc). Yield: 131 mg (0.26 mmol, 22%) of colorless semisolid. [α]Dr.t. = –255 (0.067, MeOH). EI-HRMS: m/z = 247.6358 (M + 2H)+2; C28H37N3O5 requires: m/z = 247.6361 (M + 2H)+2; νmax 3266, 2936, 2867, 1683, 1646, 1620, 1597, 1507, 1475, 1430, 1344, 1222, 1170, 1074, 1030, 913, 855, 801, 718 cm−1. 1H-NMR (500 MHz, DMSO-d6): δ 0.64–0.77 (m, 1H), 1.03 (t, J = 7.1 Hz, 3H), 1.20 (t, J = 7.1 Hz, 3H), 1.30 (br s, 1H), 1.46–1.64 (m, 3H), 2.27 (br s, 1H), 2.62–2.81 (m, 2H), 3.20 (br t, J = 11.7 Hz, 1H), 3.25–3.55 (br s, 2H, overlapped by the signal for H2O), 3.86–3.93 (m, 2H), 3.95 (s, 3H), 4.09 (q, J = 7.1 Hz, 2H), 4.93 (d, J = 10.4 Hz, 1H), 5.01 (d, J = 17.0 Hz, 1H), 5.46 (br s, 1H), 5.85 (quintet, J = 8.8 Hz, 1H), 7.48 (dd, J = 9.2, 2.6 Hz, 1H), 7.63 (d, J = 4.6 Hz, 1H), 7.68–7.86 (m, 2H), 8.00 (d, J = 9.2 Hz, 1H), 8.79 (d, J = 4.5 Hz, 1H), 9.72 (d, J = 13.7 Hz, 1H). 13C-NMR (126 MHz, DMSO-d6): δ 14.19, 14.23, 25.46, 27.09, 27.29, 40.25, 55.17, 55.82, 58.81, 59.04, 89.19, 102.31, 114.41, 121.05, 121.82, 127.91, 131.63, 142.03, 142.50, 144.35, 147.88, 157.71, 157.82, 165.36, 167.42.
3.2.9. 2-[({(S)-(6-methoxyquinolin-4-yl)((1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}amino)methylene]malononitrile (4i)
Following GP2. Prepared from 3 (0.761 mmol, 328 mg), 2-(ethoxymethylene)malononitrile (2i) (0.761 mmol, 77.4 mg), EtOH (2 mL), 25 °C, 24 h, CC (EtOAc). Yield: 79 mg (0.20 mmol, 26%) of colorless semisolid. [α]Dr.t. = −264 (0.13, MeOH). EI-HRMS: m/z = 200.6102 (M + 2H)+2; C24H27N5O requires: m/z = 200.6102 (M + 2H)+2; νmax 3286, 3233, 2931, 2861, 2203, 1644,1619, 1592, 1552, 1506, 1474, 1432, 1338, 1281, 1258, 1241, 1226, 1170, 1135, 1088, 1030, 988, 900, 846, 818, 781, 758, 714, 687, 665, 636 cm−1. 1H-NMR (500 MHz, DMSO-d6): δ 0.59–0.51 (m, 1H), 1.39–1.30 (m, 1H), 1.53–1.46 (m, 2H), 1.58–1.53 (m, 1H), 2.29–2.22 (m, 1H), 2.74–2.64 (m, 2H), 3.18 (dd, J = 13.7, 10.0 Hz, 1H), 3.32–3.24 (m, 1H), 3.54–3.45 (m, 1H), 3.99 (s, 3H), 4.98 (dt, J = 10.3, 1.4 Hz, 1H), 5.03 (dt, J = 17.1, 1.6 Hz, 1H), 5.30 (br d, J = 10.9 Hz, 1H), 5.88 (ddd, J = 17.5, 10.4, 7.5 Hz, 1H), 7.47 (dd, J = 9.2, 2.6 Hz, 1H), 7.66 (d, J = 2.7 Hz, 1H), 7.71 (d, J = 4.6 Hz, 1H), 7.99 (d, J = 9.2 Hz, 1H), 8.19 (br s, 1H), 8.79 (d, J = 4.6 Hz, 1H), 9.58 (br s, 1H). 13C-NMR (126 MHz, DMSO-d6): δ 21.10, 25.65, 27.20, 27.28, 46.52, 55.18, 55.98, 57.70, 58.50, 102.06, 114.38, 114.88, 117.20, 120.36, 121.57, 127.47, 131.60, 142.07, 142.17, 144.15, 147.80, 157.93, 160.86.
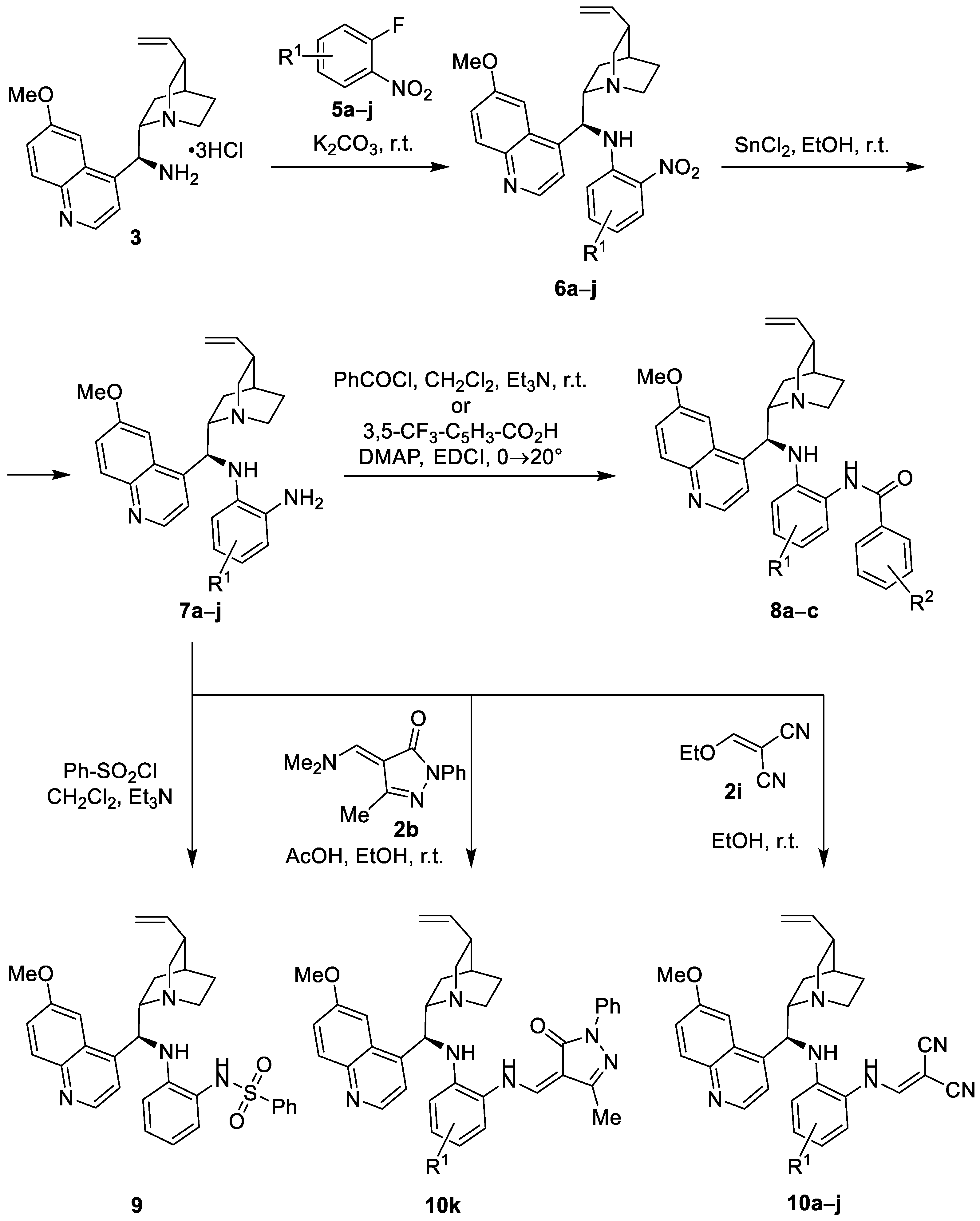
3.3. Synthesis of Compounds 6a –j
General procedure 3 (GP3): To a solution of ortho-fluoronitrobenzene derivative 5 and Na2CO3 in DMF, (S)-quininamine trihydrochloride (3) was added. The reaction mixture was stirred at 25 °C for 168 h. The reaction mixture was evaporated in vacuo and the residue was purified by column chromatography (CC). Fractions containing the product 6 were combined and volatile components evaporated in vacuo.
3.3.1. N-{[(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl)methyl]}-2-nitroaniline (6a) [23]
Following
GP3. Prepared from
3 (2.3 mmol, 1 g), 3-fluoro-2-nitrobenzene (
5a) (2.30 mmol, 239 mL), K
2CO
3 (6.15 mmol, 0.85 g), and DMF (5 mL). Isolation by CC (petroleum ether:EtOAc = 2:1). Yield: 759 mg (1.71 mmol, 74%) of yellowish semisolid.
1H-NMR (500 MHz, CDCl
3): δ 1.13–1.22 (
m, 1H), 1.35–1.47 (
m, 1H), 1.58–1.68 (
m, 2H), 1.69–1.76 (
m, 1H), 2.26–2.36 (
m, 1H), 2.74–2.89 (
m, 2H), 3.00 (
br s, 1H), 3.36 (
dd, 1H), 3.96 (
br s, 3H), 4.91 (
d, 1H), 4.97 (
d, 1H), 5.64–5.74 (
m, 1H), 6.35 (
br s, 1H), 6.52 (
ddd,
J = 8.4, 7.0, 1.3 Hz, 1H), 7.05 (
br t, 1H), 7.43 (
br s, 1H), 7.56 (
d,
J = 4.5 Hz, 1H), 7.73 (
br s, 1H), 8.08 (
s, 1H), 8.15–8.10 (
m, 2H), 8.75 (
d,
J = 4.5 Hz, 1H), 9.32 (
s, 1H). Spectral data are in agreement with the literature data [
23]. The product was used in next step as it is, without further purification.
3.3.2. N-{[(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}-2-nitro-4-(trifluoromethyl)aniline (6b) [23]
Following GP3. Prepared from 3 (1.15 mmol, 0.5 g), 1-fluoro-2-nitro-4-(trifluoromethyl)benzene (5b) (1.15 mmol, 298 mL), K2CO3 (6.15 mmol, 0.85 g), and DMF (5 mL). Isolation by CC (petroleum ether:EtOAc = 2:1). Yield: 562 mg (1.10 mmol, 96%) of yellowish semisolid. The product was used in next step as it is, without further purification.
3.3.3. 4-({(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}amino)-3-nitrobenzonitrile (6c)
Following GP3. Prepared from 3 (2.31 mmol, 1 g), 4-fluoro-3-nitrobenzonitrile (5c) (2.30 mmol, 384 mg), K2CO3 (6.15 mmol, 0.85 g), and DMF (5 mL). Isolation by CC (EtOAc). Yield: 1.06 g (2.26 mmol, 98%) of yellowish semisolid. EI-HRMS: m/z = 235.6126 (M + 2H)+2; C27H29N5O3 requires: m/z = 235.6130 (M + 2H)+2. 1H-NMR (500 MHz, CDCl3): δ 1.08–1.20 (m, 1H), 1.35–1.53 (m, 1H), 1.56–1.68 (m, 2H), 1.74 (br s, 1H), 2.25–2.38 (m, 1H), 3.20–2.73 (m, 4H), 3.25–3.42 (m, 1H), 3.97 (br s, 3H), 4.81–5.17 (m, 3H), 5.58–5.75 (m, 1H), 6.68–6.32 (m, 1H), 6.76 (dd, J = 8.6, 1.6 Hz, 1H), 7.47 (br s, 1H), 7.51 (d, J = 4.5 Hz, 1H), 7.62 (br s, 1H), 8.09 (br s, 1H), 8.17 (d, J = 8.7 Hz, 1H), 8.76 (s, 1H), 9.36 (br s, 1H). 13C-NMR (126 MHz, DMSO): δ 14.14, 27.36, 27.96, 39.53, 40.75, 53.67, 55.60, 55.84, 57.49, 63.03, 100.41, 114.64, 117.08, 117.71, 118.50, 119.20, 121.95, 123.08, 127.75, 132.51, 134.77, 141.17, 143.25, 143.95, 144.81, 147.95, 158.51. The product was used in next step as it is, without further purification.
3.3.4. 3-({(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}amino)-4-nitrobenzonitrile (6d)
Following GP3. Prepared from 3 (1.15 mmol, 0.5 g), 3-fluoro-4-nitrobenzonitrile (5d) (1.15 mmol, 191 mg), K2CO3 (6.15 mmol, 0.85 g), and DMF (5 mL). Isolation by CC (EtOAc). Yield: 498 mg (1.06 mmol, 92%) of yellowish semisolid. 1H-NMR (500 MHz, CDCl3): δ 1.08–1.18 (m, 1H), 1.41 (br s, 1H), 1.57–1.68 (m, 2H), 1.73 (br s, 1H), 2.04 (s, 1H), 2.27–2.36 (m, 1H), 2.73–2.87 (m, 2H), 2.97 (br s, 1H), 3.33 (dd, J = 13.9, 10.1 Hz, 1H), 3.95 (s, 3H), 4.84–5.00 (m, 2H), 4.99–5.24 (m, 1H), 5.66 (ddd, J = 17.4, 10.4, 7.4 Hz, 1H), 6.57 (br s, 1H), 6.74 (dd, J = 8.7, 1.7 Hz, 1H), 7.29–7.49 (m, 1H), 7.54 (d, J = 4.6 Hz, 1H), 7.58–7.69 (m, 1H), 8.10 (br s, 1H), 8.15 (d, J = 8.7 Hz, 1H), 8.76 (s, 1H), 9.29 (br s, 1H). 13C-NMR (126 MHz, CDCl3): δ 21.39, 25.43, 27.41, 27.93, 39.47, 40.83, 53.69, 55.75, 57.64, 62.94, 77.36, 100.51, 114.89, 117.14, 117.95, 118.66, 119.14, 122.18, 127.87, 132.26, 134.94, 141.05, 143.28, 144.34, 147.71, 158.60, 174.87. The product was used in next step as it is, without further purification.
3.3.5. 4-Bromo-N-{(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}-2-nitroaniline (6e)
Following GP3. Prepared from 3 (1.15 mmol, 0.5 g), 4-bromo-1-fluoro-2-nitrobenzene (5e) (1.15 mmol, 253 mg), K2CO3 (6.15 mmol, 0.85 g), and DMF (5 mL). Isolation by CC (petroleum ether:EtOAc = 2:1). Yield: 550 mg (1.05 mmol, 91%) of yellowish semisolid. EI-HRMS: m/z = 262.0705 (M + 2H)+2; C26H29BrN4O3 requires: m/z = 262.0706 (M + 2H)+2. 1H-NMR (500 MHz, CDCl3): δ 1.11–1.22 (m, 1H), 1.35–1.51 (br m, 1H), 1.64 (br t, J = 7.1 Hz, 2H), 1.73 (s, 1H), 2.25–2.38 (br m, 1H), 2.73–2.88 (m, 2H), 2.97 (br s, 1H), 3.13 (br s, 1H), 3.37 (br dd, J = 10.5, 13.6 Hz, 1H), 3.67–4.19 (br m, 3H), 4.93 (br d, J = 10.3 Hz, 1H), 4.98 (br d, J = 17.1 Hz, 1H), 5.09 (br s, 1H), 5.70 (ddd, J = 17.1, 10.3, 7.3 Hz, 1H), 6.30 (br s, 1H), 7.19–7.30 (br m, 1H), 7.46 (br s, 1H), 7.51 (d, J = 4.4 Hz, 1H), 7.62 (br s, 1H), 8.10 (br s, 1H), 8.42 (br s, 1H), 8.75 (br d, J = 4.6 Hz, 1H), 9.58 (br s, 1H). 13C-NMR (126 MHz, CDCl3): δ 25.62, 27.56, 28.24, 39.70, 40.93, 53.89, 55.71, 56.00, 63.13, 100.78, 114.84, 115.64, 119.15, 121.72, 122.48, 124.63, 124.94, 127.60, 131.92, 132.16, 132.78, 141.31, 144.46, 145.51, 148.15, 158.32. The product was used in next step as it is, without further purification.
3.3.6. 5-Bromo-N-{(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}-2-nitroaniline (6f)
Following GP3. Prepared from 3 (1.15 mmol, 0.5 g), 4-bromo-2-fluoro-1-nitrobenzene (5f) (1.15 mmol, 253 mg), K2CO3 (6.15 mmol, 0.85 g), and DMF (5 mL). Isolation by CC (petroleum ether:EtOAc = 2:1). Yield: 450 mg (0.860 mmol, 75%) of yellowish semisolid. EI-HRMS: m/z = 262.0706 (M + 2H)+2; C26H29BrN4O3 requires: m/z = 262.0706 (M + 2H)+2. 1H-NMR (500 MHz, CDCl3): δ 1.11 (br q, J = 6.1 Hz, 1H), 1.44 (br t, J = 11.9 Hz, 1H), 1.66 (br t, J = 6.7 Hz, 2H), 1.75 (br s, 1H), 2.28–2.37 (br m, 1H), 2.75–2.90 (m, 2H), 2.91–3.08 (m, 1H), 3.36 (dd, J = 10.1, 14.0 Hz, 1H), 3.81–4.10 (br m, 3H), 4.95 (br d, J = 10.4 Hz, 1H), 4.77–5.24 (br m, 1H), 4.99 (br d, J = 17.1 Hz, 1H), 5.69 (ddd, J = 7.5, 10.4, 14.4 Hz, 1H), 6.51–6.74 (br m, 1H), 6.66 (dd, J = 9.0, 1.9 Hz, 1H), 7.46 (br m, 2H), 7.58 (d, J = 4.6 Hz, 1H), 7.66 (br m, 1H), 7.96 (d, J = 9.1 Hz, 1H), 8.12 (br s, 1H), 8.78 (d, J = 4.6 Hz, 1H), 9.22 (br s, 1H). 13C-NMR (126 MHz, CDCl3): δ 20.99, 27.45, 28.00, 39.48, 40.79, 45.03, 55.75, 63.02, 73.73, 100.59, 104.80, 104.98, 112.05, 112.07, 114.86, 117.64, 119.27, 122.34, 128.17, 131.08, 131.87, 132.06, 141.08, 144.22, 147.57, 158.33. The product was used in next step as it is, without further purification.
3.3.7. N-{(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}-4-methyl-2-nitroaniline (6g)
Following GP3. Prepared from 3 (1.15 mmol, 0.5 g), 1-fluoro-4-methyl-2-nitrobenzene (5g) (1.15 mmol, 178 mg), K2CO3 (6.15 mmol, 0.85 g), and DMF (5 mL). Isolation by CC (petroleum ether: EtOAc = 2:1). Yield: 251 mg (0.55 mmol, 48%) of yellowish semisolid. EI-HRMS: m/z = 230.1229 (M + 2H)+2; C27H32N4O3 requires: m/z = 230.1232 (M + 2H)+2. 1H-NMR (500 MHz, CDCl3): δ 1.10–1.19 (m, 1H), 1.32–1.45 (m, 1H), 1.57–1.67 (br m, 2H), 1.71 (br s, 1H), 2.13 (s, 3H), 2.22–2.35 (m, 1H), 2.88–2.74 (m, 2H), 2.98 (br s, 1H), 3.06–3.29 (br s, 1H), 3.42–3.30 (m, 1H), 3.94 (br s, 3H), 5.19–4.74 (m, 3H), 5.76–5.61 (m, 1H), 6.25 (br s, 1H), 6.88 (d, J = 8.5 Hz, 1H), 7.41 (br s, 1H), 7.54 (d, J = 4.6 Hz, 1H), 7.80–7.62 (m, 1H), 7.92 (br s, 1H), 8.07 (br s, 1H), 8.73 (d, J = 4.5 Hz, 1H), 9.17 (br s, 1H). 13C-NMR (126 MHz, CDCl3): δ 19.97, 24.04, 26.40, 27.69, 28.35, 39.82, 40.95, 54.19, 55.73, 56.09, 63.16, 77.37, 101.21, 114.70, 115.02, 121.80, 125.51, 126.36, 127.79, 132.39, 132.81, 137.29, 141.52, 142.20, 145.01, 147.85, 158.02. The product was used in next step as it is, without further purification.
3.3.8. N-{(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}-5-methyl-2-nitroaniline (6h)
Following GP3. Prepared from 3 (1.15 mmol, 0.5 g), 2-fluoro-4-methyl-1-nitrobenzene (5h) (1.15 mmol, 178 mg), K2CO3 (6.15 mmol, 0.85 g), and DMF (5 mL). Isolation by CC (petroleum ether:EtOAc = 2:1). Yield: 430 mg (0.938 mmol, 82%) of yellowish semisolid. EI-HRMS: m/z = 230.1225 (M + 2H)+2; C27H32N4O3 requires: m/z = 230.1232 (M + 2H)+2. 1H-NMR (500 MHz, CDCl3): δ 1.08–1.17 (m, 1H), 1.34–1.44 (m, 1H), 1.581.65 (m, 2H), 1.66–1.75 (m, 1H), 1.93 (s, 3H), 2.25–2.35 (m, 1H), 2.72–2.86 (m, 2H), 2.98 (d, J = 15.5 Hz, 1H), 3.34 (dd, J = 10.0, 14.0 Hz, 1H), 3.13–3.42 (m, 1H), 3.93 (s, 3H), 5.12–4.64–5.12 (m, 3H), 5.68 (ddd, J = 17.4, 10.4, 7.5 Hz, 1H), 6.20 (br s, 1H), 6.33 (dd, J = 8.7, 1.6 Hz, 1H), 7.41 (br s, 1H), 7.56 (d, J = 4.5 Hz, 1H), 7.73 (br s, 1H), 7.99 (d, J = 8.7 Hz, 1H), 8.07 (d, J = 10.1 Hz, 1H), 8.74 (d, J = 4.5 Hz, 1H), 9.27 (br s, 1H). 13C-NMR (126 MHz, CDCl3): δ 21.40, 22.07, 26.27, 27.63, 28.23, 39.71, 40.88, 55.74, 55.98, 63.21, 77.36, 101.02, 114.68, 114.85, 117.50, 121.95, 126.91, 127.76, 131.14, 132.20, 141.41, 144.02, 144.81, 147.20, 147.74, 158.04, 174.42. The product was used in next step as it is, without further purification.
3.3.9. 5-Methoxy-N-{(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}-2-nitroaniline (6i)
Following GP3. Prepared from 3 (1.15 mmol, 0.5 g), 2-fluoro-4-methoxy-1-nitrobenzene (5i) (1.15 mmol, 197 mg), K2CO3 (6.15 mmol, 0.85 g), and DMF (5 mL). Isolation by CC (petroleum ether:EtOAc = 2:1). Yield: 476 mg (1.00 mmol, 87%) of yellowish semisolid. EI-HRMS: m/z = 238.1204 (M + 2H)+2; C27H32N4O4 requires: m/z = 238.1206 (M + 2H)+2. 1H-NMR (500 MHz, CDCl3): δ 0.79–0.89 (m, 1H), 1.08–1.16 (m, 1H), 1.21–1.34 (m, 1H), 1.37–1.47 (m, 1H), 1.55–1.67 (m, 2H), 1.68–1.76 (m, 1H), 2.26–2.35 (m, 1H), 2.74–2.88 (m, 2H), 3.02 (br s, 3H), 3.34 (dd, J = 14.0, 10.0 Hz, 1H), 3.82–4.08 (m, 3H), 4.87–5.00 (m, 2H), 5.00–5.23 (m, 1H), 5.60 (br s, 1H), 5.69 (ddd, J = 17.4, 10.8, 7.6 Hz, 1H), 6.06 (dd, J = 9.5, 2.5 Hz, 1H), 7.44 (br s, 1H), 7.57 (d, J = 4.5 Hz, 1H), 7.70 (br s, 1H), 8.04 (d, J = 9.5 Hz, 1H), 8.07 (br s, 1H), 8.75 (d, J = 4.5 Hz, 1H), 9.49 (br s, 1H). 13C-NMR (126 MHz, CDCl3): δ 21.32, 27.58, 28.22, 39.63, 40.90, 53.79, 55.14, 55.76, 55.91, 62.60, 77.36, 96.65, 100.77, 105.56, 114.74, 119.71, 121.89, 127.22, 129.15, 132.35, 141.34, 144.38, 146.20, 148.25, 158.22, 165.30, 174.66. The product was used in next step as it is, without further purification.
3.3.10. N-{(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}-2-nitropyridin-3-amine (6j) [23]
Following GP3. Prepared from 3 (2.3 mmol, 1 g), 3-fluoro-2-nitropyridine (5j) (2.30 mmol, 328 mg), K2CO3 (6.15 mmol, 0.85 g), and DMF (5 mL). Isolation by extraction followed by CC (petroleum ether:EtOAc = 2:1). Yield: 874 mg (1.96 mmol, 85%) of brownish semisolid. The product was used in next step as it is, without further purification.
3.4. Synthesis of Compounds 7a –j
General procedure 4 (GP4): To a solution of nitroaniline 6 in EtOH, SnCl2·2H2O (4 equivalents) was added and the mixture was stirred at 25 °C for 24 h. Then, the reaction mixture was diluted with 2 M NaOH(aq) until pH 12. Dichloromethane was added and the mixture was stirred vigorously for 30 min. The aqueous phase and the organic phase were separated. The organic phase was dried over anhydrous Na2SO4, filtered, and volatile components evaporated in vacuo. The product was used in next step as it is, without further purification.
3.4.1. N1-{(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}benzene-1,2-diamine (7a) [23]
Following
GP4. Prepared from
6a (1.71 mmol, 759 mg) and SnCl
2·2H
2O (6.84 mmol, 1.54 g) in EtOH (10 mL). Yield: 330 mg (0.67 mmol, 76%) of brownish semisolid.
1H-NMR (500 MHz, CDCl
3): δ 1.10–1.19 (
m, 1H), 1.23–1.36 (
m, 1H), 1.52–1.71 (
m, 3H), 2.28 (
br s, 1H), 2.68–2.80 (
m, 2H), 2.98–3.15 (
m, 2H), 3.28 (
dd,
J = 13.9, 10.0, 1H), 3.38–3.63 (
br s, 2H), 3.99 (
s, 3H), 4.84 (
br s, 1H), 4.90–5.00 (
m, 2H), 5.41 (
br s, 1H), 5.70 (
ddd,
J = 17.5, 10.3, 7.6, 1H), 6.17 (
br s, 1H), 6.41–6.48 (
m, 1H), 6.56 (
td,
J = 7.5, 1.3, 1H), 6.67 (
dd,
J = 7.6, 1.5, 1H), 7.42 (
dd,
J = 9.3, 2.7, 1H), 7.69 (
d,
J = 4.4, 1H), 8.02 (
s, 1H), 8.07 (
d,
J = 9.3, 1H), 8.71 (
d,
J = 4.6, 1H). Spectral data are in agreement with the literature data [
23]. The product was used in next step as it is, without further purification.
3.4.2. N1-{(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}-4-(trifluoromethyl)benzene-1,2-diamine (7b) [23]
Following GP4. Prepared from 6b (0.79 mmol, 404 mg) and SnCl2·2H2O (3.16 mmol, 711 mg) in EtOH (10 mL). Yield: 352 mg (0.73 mmol, 92%) of yellowish solid. The product was used in next step as it is, without further purification.
3.4.3. 3-Amino-4-({(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}amino)benzonitrile (7c)
Following GP4. Prepared from 6c (1.89 mmol, 889 mg) and SnCl2·2H2O (7.56 mmol, 1.70 g) in EtOH (10 mL). Yield: 795 mg (1.81 mmol, 96%) of yellowish solid. EI-HRMS: m/z = 220.6255 (M + 2H)+2; C27H31N5O requires: m/z = 220.6259 (M + 2H)+2. 1H-NMR (500 MHz, CDCl3) δ 1.09 (dd, J = 14.3, 6.3, 1H), 1.39 (dt, J = 10.6, 7.1, 1H), 1.72 (d, J = 11.7, 3H), 2.27 (t, J = 8.1, 1H), 2.55–2.73 (m, 2H), 2.86 (d, J = 14.4, 1H), 3.10 (d, J = 13.6, 1H), 3.39 (s, 1H), 4.02 (s, 3H), 5.02 (dd, J = 32.1, 13.7, 2H), 5.08–5.20 (m, 1H), 5.43 (br s, 2H), 6.25 (s, 1H), 6.34 (s, 1H), 7.02–7.10 (m, 1H), 7.11–7.17 (m, 1H), 7.42–7.60 (m, 4H), 8.13 (d, J = 9.2, 1H), 8.79 (d, J = 4.6, 1H). 13C-NMR (126 MHz, CDCl3) δ 18.56, 24.48, 26.87, 27.29, 38.70, 40.79, 52.90, 55.23, 55.89, 61.61, 100.74, 111.61, 114.88, 115.96, 118.67, 119.81, 121.17, 126.39, 127.23, 128.04, 132.21, 133.10, 139.54, 143.57, 144.47, 148.49, 158.58, 158.69. The product was used in next step as it is, without further purification.
3.4.4. 4-Amino-3-({(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}amino)benzonitrile (7d)
Following GP4. Prepared from 6d (0.84 mmol, 394 mg) and SnCl2·2H2O (3.36 mmol, 757 mg) in EtOH (10 mL). Yield: 231 mg (0.53 mmol, 63%) of colorless solid. EI-HRMS: m/z = 220.6259 (M + 2H)+2; C27H31N5O requires: m/z = 220.6259 (M + 2H)+2. 1H-NMR (500 MHz, CDCl3): δ 1.09–1.17 (m, 1H), 1.26–1.35 (m, 1H), 1.53–1.69 (m, 3H), 2.23–2.32 (m, 1H), 2.66–2.77 (m, 2H), 2.92–3.04 (m, 1H), 3.13 (br s, 1H), 3.20–3.28 (m, 1H), 3.95 (s, 3H), 4.24 (br s, 2H), 4.81 (br s, 1H), 4.86–4.96 (m, 2H), 5.44 (s, 1H), 5.64 (ddd, J = 14.3, 10.4, 7.4 Hz, 1H), 6.41 (br s, 1H), 6.56 (dd, J = 7.8, 2.6 Hz, 1H), 6.83 (dd, J = 8.0, 1.8 Hz, 1H), 7.38–7.46 (m, 1H), 7.60 (d, J = 4.6 Hz, 1H), 7.78 (br s, 1H), 8.07 (d, J = 9.2 Hz, 1H), 8.71 (d, J = 4.5 Hz, 1H). 13C-NMR (126 MHz, CDCl3): δ 14.20, 21.07, 25.34, 27.32, 27.86, 39.51, 40.82, 53.88, 55.76, 62.17, 77.36, 100.89, 101.30, 114.35, 114.75, 115.39, 121.47, 124.76, 128.29, 132.23, 135.07, 140.76, 140.81, 141.07, 144.71, 147.87, 158.02. The product was used in next step as it is, without further purification.
3.4.5. 4-Bromo-N1-{(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}benzene-1,2-diamine (7e)
Following GP4. Prepared from 6e (1.05 mmol, 550 mg) and SnCl2·2H2O (4.20 mmol, 950 mg) in EtOH (10 mL). Yield: 372 mg (0.75 mmol, 71%) of yellowish solid. EI-HRMS: m/z = 247.0841 (M + 2H)+2; C26H31BrN4O requires: m/z = 247.0835 (M + 2H)+2. 1H-NMR (500 MHz, CDCl2): δ 1.03–1.15 (m, 1H), 1.22–1.31 (m, 1H), 1.45–1.66 (m, 3H), 2.17–2.29 (m, 1H), 2.61–2.74 (m, 2H), 2.91–2.99 (m, 2H), 3.21 (dd, J = 13.9, 10.0, 1H), 3.73 (br s, 2H), 3.94 (s, 3H), 4.61–4.82 (m, 1H), 4.83–4.97 (m, 2H), 5.32 (s, 1H), 5.54–5.70 (m, 1H), 5.99 (s, 1H), 6.46 (dd, J = 8.5, 2.2, 1H), 6.72 (d, J = 2.3, 1H), 7.34–7.46 (m, 1H), 7.63 (d, 1H), 7.79 (br s, 1H), 8.05 (d, J = 9.2, 1H), 8.67 (d, J = 4.5, 1H). 13C-NMR (126 MHz, CDCl3): δ 27.35, 28.06, 31.34, 36.40, 39.63, 40.73, 55.55, 55.91, 62.34, 101.46, 110.85, 113.79, 114.44, 117.91, 119.87, 121.13, 121.92, 128.37, 132.07, 134.97, 137.00, 141.34, 145.85, 147.98, 157.66, 162.47. The product was used in next step as it is, without further purification.
3.4.6. 5-Bromo-N1-{(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}benzene-1,2-diamine (7f)
Following GP4. Prepared from 6f (0.88 mmol, 460 mg) and SnCl2·2H2O (3.52 mmol, 794 mg) in EtOH (10 mL). Isolation by extraction. Yield: 330 mg (0.67 mmol, 76%) of yellowish solid. EI-HRMS: m/z = 247.0836 (M + 2H)+2; C26H31BrN4O requires: m/z = 247.0835 (M + 2H)+2. 1H-NMR (500 MHz, CDCl3): δ 1.03–1.12 (m, 1H), 1.27–1.34 (m, 1H), 1.52–1.69 (m, 3H), 2.23–2.30 (m, 1H), 2.66–2.77 (m, 2H), 2.92–3.01 (m, 1H), 3.10 (br s, 1H), 3.26 (dd, J = 13.9, 10.0 Hz, 1H), 3.46–3.63 (m, 2H), 3.99 (s, 3H), 4.75 (s, 1H), 4.86–4.98 (m, 2H), 5.48 (s, 1H), 5.68 (ddd, J = 17.6, 10.3, 7.6 Hz, 1H), 6.36 (s, 1H), 6.49 (d, J = 8.1 Hz, 1H), 6.64 (dd, J = 8.2, 2.1 Hz, 1H), 7.43 (dd, J = 9.2, 2.6 Hz, 1H), 7.64 (dd, J = 5.2, 3.0 Hz, 1H), 7.74–7.90 (m, 1H), 8.07 (d, J = 9.2 Hz, 1H), 8.73 (d, J = 4.5 Hz, 1H). 13C-NMR (126 MHz, CDCl3): δ 25.52, 27.46, 28.14, 31.44, 36.49, 39.77, 40.83, 55.75, 56.07, 77.30, 101.14, 111.91, 114.57, 115.68, 116.68, 120.39, 121.37, 128.48, 132.21, 134.30, 137.81, 141.43, 144.75, 147.99, 157.88, 162.55. The product was used in next step as it is, without further purification.
3.4.7. N1-{(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}-4-methylbenzene-1,2-diamine (7g)
Following GP4. Prepared from 6g (0.47 mmol, 216 mg) and SnCl2·2H2O (1.88 mmol, 425 mg) in EtOH (5 mL). Yield: 155 mg (0.361 mmol, 77%) of brownish solid. EI-HRMS: m/z = 215.1365 (M + 2H)+2; C27H34N4O requires: m/z = 215.1361 (M + 2H)+2. 1H-NMR (500 MHz, CDCl3): δ 1.10–1.19 (m, 1H), 1.24–1.35 (m, 1H), 1.54–1.63 (m, 2H), 1.6–1.70 (m, 1H), 2.10 (s, 3H), 2.23–2.31 (m, 1H), 2.68–2.80 (m, 2H), 2.97–3.17 (m, 3H), 3.28 (dd, J = 13.9, 10.0, 1H), 3.98 (s, 3H), 4.83 (br s, 1H), 4.90–4.99 (m, 2H), 5.70 (ddd, J = 17.1, 10.3, 7.5, 1H), 6.10 (br s, 1H), 6.22–6.28 (m, 1H), 6.50 (d, J = 1.9, 1H), 7.42 (dd, J = 9.2, 2.7, 1H), 7.69 (s, 1H), 7.82 (br s, 1H), 8.06 (d, J = 9.2, 1H), 8.71 (d, J = 4.5, 1H). 13C-NMR (126 MHz, CDCl3) δ 20.63, 25.53, 27.66, 28.36, 39.98, 40.98, 55.69, 56.23, 62.37, 77.37, 101.74, 113.12, 114.59, 116.79, 120.18, 120.55, 121.26, 128.72, 132.24, 133.88, 135.56, 141.69, 141.71, 144.80, 146.66, 148.31, 157.69. The product was used in next step as it is, without further purification.
3.4.8. N1-{(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}-5-methylbenzene-1,2-diamine (7h)
Following GP4. Prepared from 6h (0.64 mmol, 295 mg) and SnCl2·2H2O (2.56 mmol, 580 mg) in EtOH (5 mL). Yield: 176 mg (0.41 mmol, 64%) of brownish solid. EI-HRMS: m/z = 215.1361 (M + 2H)+2; C27H34N4O requires: m/z = 215.1361 (M + 2H)+2. 1H-NMR (500 MHz, CDCl3): δ 1.13 (dd, J = 14.1, 7.1, 1H), 1.25 (s, 1H), 1.32 (t, J = 12.0, 1H), 1.55–1.70 (m, 3H), 1.89 (s, 1H), 2.17 (d, J = 1.0, 1H), 2.23–2.33 (m, 1H), 2.69–2.82 (m, 2H), 2.99–3.08 (m, 1H), 3.14 (br s, 1H), 3.30 (dd, J = 13.9, 10.1, 1H), 3.62–3.73 (m, 1H), 3.99 (s, 3H), 4.83 (br s, 1H), 4.90–5.00 (m, 2H), 5.70 (ddd, J = 17.5, 10.4, 7.6, 1H), 6.06 (s, 1H), 6.37 (d, J = 7.7, 1H), 6.56 (d, J = 7.7, 1H), 7.42 (d, J = 9.1, 1H), 7.69 (s, 1H), 7.87 (br s, 1H), 8.07 (d, J = 9.3, 1H), 8.73 (d, J = 4.5, 1H), two protons are missing. 13C-NMR (126 MHz, CDCl3): δ 21.09, 25.58, 25.59, 27.66, 28.24, 29.85, 31.10, 39.88, 40.98, 55.77, 56.14, 76.77, 113.94, 114.71, 116.10, 119.24, 121.43, 128.65, 129.44, 132.23, 132.27, 132.59, 136.64, 141.49, 141.53, 148.28, 157.82. The product was used in next step as it is, without further purification.
3.4.9. 5-Methoxy-N1-{(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}benzene-1,2-diamine (7i)
Following GP4. Prepared from 6i (0.88 mmol, 390 mg) and SnCl2·2H2O (3.52 mmol, 794 mg) in EtOH (10 mL). Yield: 367 mg (0.77 mmol, 88%) of white solid. EI-HRMS: m/z = 223.1331 (M + 2H)+2; C27H34N4O2 requires: m/z = 223.1335 (M + 2H)+2. 1H-NMR (500 MHz, CDCl3): δ 1.11–1.17 (m, 1H), 1.29–1.37 (m, 1H), 1.56–1.63 (m, 2H), 1.65–1.69 (m, 1H), 2.25–2.32 (m, 1H), 2.70–2.80 (m, 2H), 2.83–3.07 (m, 3H), 3.13 (br s, 1H), 3.24–3.35 (m, 4H), 3.98 (s, 3H), 4.81 (br s, 1H), 4.89–4.99 (m, 2H), 5.62 (s, 1H), 5.71 (ddd, J = 17.5, 10.3, 7.6 Hz, 1H), 5.81 (br s, 1H), 6.08 (dd, J = 8.3, 2.7 Hz, 1H), 6.58 (d, J = 8.3 Hz, 1H), 7.41 (dd, J = 9.2, 2.7 Hz, 1H), 7.68 (d, J = 4.6 Hz, 1H), 7.85 (br s, 1H), 8.07 (d, J = 9.2 Hz, 1H), 8.73 (d, J = 4.5 Hz, 1H). 13C-NMR (126 MHz, CDCl3): δ 25.57, 27.62, 28.32, 39.91, 40.95, 55.09, 55.72, 56.18, 62.47, 77.36, 99.91, 101.54, 102.57, 114.61, 117.10, 120.59, 121.33, 127.90, 128.56, 132.32, 138.48, 141.60, 144.80, 146.43, 148.28, 154.39, 157.76. The product was used in next step as it is, without further purification.
3.4.10. N3-{(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}pyridine-2,3-diamine (7j) [23]
Following
GP4. Prepared from
6j (1.95 mmol, 872 mg) and SnCl
2·2H
2O (7.8 mmol, 1.76 g) in EtOH (10 mL). Yield: 760 mg (1.83 mmol, 94%) of brown solid.
1H-NMR (500 MHz, CDCl
3) δ 1.08–1.19 (
m, 1H), 1.29–1.37 (
m, 2H), 1.58–1.77 (
m, 3H), 2.29 (
s, 1H), 2.71–2.81 (
m, 2H), 2.97–3.17 (
m, 1H), 3.28 (
dd,
J = 13.9, 10.0, 1H), 4.00 (
s, 3H), 4.79 (
brs, 1H), 4.90–5.00 (
m, 2H), 5.64–5.76 (
m, 1H), 6.23–6.35 (
m, 2H), 7.41–7.50 (
m, 2H), 7.59–7.68 (
m, 1H), 8.02 (
d,
J = 2.9, 1H), 8.09 (
d,
J = 9.2, 1H), 8.73 (
dd,
J = 4.5, 1.1, 1H), three protons are missing. Spectral data are in agreement with the literature data [
23]. The product was used in next step as it is, without further purification.
3.5. Amidation of Benzenediamines 7
General procedure 5 (GP5): To a solution of diamine 7 in anhydrous CH2Cl2, Et3N (1 equivalent) was added. Aroyl chloride (1 equivalent) or benzenesulphonyl chloride (1.1 equivalent) was slowly added and the mixture was stirred at 25 °C for 24 h. The reaction mixture was evaporated in vacuo and the residue was purified by CC. Fractions containing the products 8 or 9 were combined and volatile components evaporated in vacuo. Alternatively, DMAP (1 equivalent) and EDC·HCl (1 equivalent) were added to a stirred solution of carboxylic acid in anhydrous CH2Cl2 at 0 °C (ice-bath). The reaction mixture was stirred at 0 °C for 1 h and then the ice-bath was removed and the reaction mixture was stirred at room temperature for 24 h. The reaction mixture was diluted with CH2Cl2 (10 mL) and washed with distilled water (2 × 5 mL). The organic phase was dried over anhydrous Na2SO4, filtered, volatile components were evaporated in vacuo, and the residue was purified by CC. Fractions containing the product were combined and volatile components evaporated in vacuo.
3.5.1. N-[2-({(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}amino)phenyl]benzamide (8a)
Following GP5. Prepared from 7a (0.24 mmol, 100 mg), benzoyl chloride (0.24 mmol, 28 µL), and Et3N (0.24 mmol, 33 µL) in CH2Cl2 (5 mL), 25 °C, 24 h, CC (EtOAc). Yield: 82 mg (0.16 mmol, 66%) of colorless semisolid. [α]Dr.t. = −62.3 (0.13, MeOH). EI-HRMS: m/z = 260.1413 (M + 2H)+2; C33H36N4O2 requires: m/z = 260.1414 (M + 2H)+2; νmax 3286, 2937, 2864, 1655, 1620, 1604, 1508, 1473, 1454, 1259, 1241, 1227, 1136, 1029, 915, 857, 746, 710 cm−1. 1H-NMR (500 MHz, CDCl3): δ 1.07–1.13 (m, 1H), 1.31–1.38 (m, 1H), 1.53–1.62 (m, 2H), 1.64–1.69 (m, 1H), 1.84 (br s, 2H), 2.23–2.30 (m, 1H), 2.62–2.75 (m, 2H), 2.97–3.11 (m, 1H), 3.12–3.20 (m, 1H), 3.95 (s, 3H), 4.79 (br s, 1H), 4.89–4.98 (m, 2H), 5.68 (ddd, J = 17.6, 10.3, 7.6 Hz, 1H), 5.84 (br s, 1H), 6.28 (d, J = 7.8 Hz, 1H), 6.76 (t, J = 7.6 Hz, 1H), 6.79–6.84 (m, 1H), 7.42 (dd, J = 9.2, 2.6 Hz, 1H), 7.52 (t, J = 7.5 Hz, 2H), 7.58 (t, J = 7.2 Hz, 1H), 7.60–7.67 (m, 2H), 7.71 (br s, 1H), 7.88–7.97 (m, 2H), 8.07 (d, J = 9.2 Hz, 1H), 8.71 (d, J = 4.5 Hz, 1H). 13C-NMR (126 MHz, CDCl3): δ 25.70, 27.56, 28.18, 39.89, 40.89, 55.72, 56.04, 62.63, 77.37, 101.59, 114.76, 119.39, 120.36, 121.45, 125.48, 125.61, 126.99, 127.38, 128.39, 128.87, 131.82, 132.30, 135.40, 141.47, 141.51, 144.83, 146.06, 148.21, 157.90, 166.66.
3.5.2. N-[5-Cyano-2-({(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}amino)phenyl]benzamide (8b)
Following GP5. Prepared from 7c (0.23 mmol, 100 mg), benzoyl chloride (0.23 mmol, 27 µL), and Et3N (0.23 mmol, 32 µL) in CH2Cl2 (2 mL), 0 °C, 24 h, CC (EtOAc). Yield: 60 mg (0.11 mmol, 48%) of brown semisolid. [α]Dr.t. = −55.1 (0.058, MeOH). EI-HRMS: m/z = 272.6383 (M + 2H)+2; C34H35N5O2 requires: m/z = 272.6390 (M + 2H)+2; νmax 3275, 2936, 2215, 1655, 1607, 1508, 1474, 1432, 1345, 1289, 1261, 1229, 1145, 1080, 1028, 917, 857, 815, 753, 711, 666, 622 cm−1. 1H-NMR (500 MHz, CDCl3): δ 1.13–1.21 (m, 1H), 1.34–1.41 (m, 1H), 1.57–1.66 (m, 2H), 1.67–1.72 (m, 1H), 2.12–2.31 (m, 2H), 2.61–2.75 (m, 2H), 3.03 (br s, 2H), 3.14 (dd, J = 13.8, 10.1 Hz, 1H), 3.97 (s, 3H), 4.82–4.98 (m, 3H), 5.63 (ddd, J = 17.5, 10.3, 7.5 Hz, 1H), 6.13 (br s, 1H), 6.76 (s, 1H), 7.01 (dd, J = 8.5, 1.9 Hz, 1H), 7.45 (dd, J = 9.3, 2.6 Hz, 1H), 7.52 (t, J = 7.6 Hz, 2H), 7.55–7.62 (m, 3H), 7.66 (br s, 1H), 7.95 (d, J = 7.4 Hz, 2H), 8.09 (d, J = 9.2 Hz, 1H), 8.72 (d, J = 4.5 Hz, 1H). 13C-NMR (126 MHz, CDCl3): δ 25.47, 27.27, 27.79, 39.52, 40.68, 55.60, 55.74, 62.94, 76.77, 99.86, 101.19, 112.78, 114.92, 119.66, 119.96, 121.58, 123.86, 127.40, 127.94, 128.83, 130.22, 132.03, 132.17, 132.34, 134.28, 140.87, 141.09, 144.69, 145.89, 148.04, 158.12, 166.92.
3.5.3. N-[2-({(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}amino)-5-trifluoromethyl]phenyl}-3,5-bis(trifluoromethyl)benzamide (8c)
A solution of EDCI·HCl (138 mg, 0.72 mmol) in CH2Cl2 (3 mL) was slowly added to a stirred mixture of compound 7b (0.6 mmol, 248 mg), 3,5-bis(trifluoromethyl)benzoic acid (0.6 mmol, 155 mg), DMAP (0.06 mmol, 7.3 mg), and CH2Cl2 (3 mL) at 0 °C (an ice bath). The ice bath was then removed and stirring was continued at 25 °C for 24 h. The reaction mixture was diluted with CH2Cl2 (25 mL) and the combined organic phase was washed with distilled water (5 mL), dried over anhydrous Na2SO4, filtered and the filtrate was evaporated in vacuo. The residue was purified by CC (EtOAc). Fractions containing the products were combined and the volatile components were evaporated in vacuo to give 8c. Yield: 353 mg (0.49 mmol, 82%) of colorless solid, mp = 107–109 °C. [α]Dr.t. = –119 (0.083, MeOH). EI-HRMS: m/z = 362.1222 (M + 2H)+2; C36H33F9N4O2 requires: m/z = 362.1224 (M + 2H)+2; νmax 3241, 2931, 2870, 1652, 1618, 1509, 1453, 1327, 1276, 1170, 1127, 1032, 907, 817, 765, 701, 680, 629 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.82–0.89 (m, 1H), 1.08–1.15 (m, 1H), 1.22–1.31 (m, 1H), 1.32–1.38 (m, 1H), 1.53–1.64 (m, 2H), 1.68 (br s, J = 3.2, 1H), 2.22–2.30 (m, 1H), 2.60–2.69 (m, 2H), 2.8– 3.17 (m, 2H), 3.96 (s, 3H), 4.72–4.98 (m, 3H), 5.62 (ddd, J = 17.4, 10.4, 7.3, 1H), 6.22 (br s, 1H), 6.29 (s, 1H), 7.06 (dd, J = 8.6, 2.1, 1H), 7.43 (dd, J = 9.3, 2.6, 1H), 7.53 (d, J = 4.5, 1H), 7.63 (br s, 1H), 8.05 (d, J = 9.2 Hz, 1H), 8.09 (s, 1H), 8.41 (s, 2H), 8.57 (s, 1H), 8.66 (d, J = 4.5, 1H). 13C-NMR (126 MHz, CDCl3): δ 25.58, 27.29, 28.03, 39.49, 40.92, 51.08, 55.71, 62.71, 77.37, 101.33, 113.20, 114.98, 119.84, 121.61, 121.93, 123.09, 124.10, 125.51, 126.27, 127.86, 128.15, 128.91, 131.06, 132.08, 132.33, 132.62, 132.89, 136.86, 140.98, 144.71, 145.06, 148.03, 158.22, 164.31.
3.5.4. N-[2-({(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}amino)phenyl]benzenesulfonamide (9)
Following GP5. Prepared from 7a (0.24 mmol, 100 mg), benzenesulfonyl chloride (0.24 mmol, 30 mL), and Et3N (0.24 mmol, 33 µL) in CH2Cl2 (5 mL), 25 °C, 24 h, CC (EtOAc). Yield: 47 mg (0.085 mmol, 35%) of colorless solid, mp = 85.1–86.4 °C. [α]Dr.t. = –152 (0.027, MeOH). EI-HRMS: m/z = 278.1249 (M + 2H)+2; C32H36N4O3S requires: m/z = 278.1249 (M + 2H)+2; νmax 3068, 2942, 2865, 1621, 1602, 1507, 1473, 1446, 1431, 1325, 1258, 1240, 1228, 1157, 1092, 1029, 987, 918, 857, 830, 748, 688, 666 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.98–1.05 (m, 1H), 1.28–1.36 (m, 1H), 1.5–1.64 (m, 2H), 1.65–1.69 (m, 1H), 2.28–2.36 (m, 1H), 2.70–2.84 (m, 2H), 2.90–3.02 (m, 2H), 3.34 (dd, J = 13.9, 10.0 Hz, 1H), 3.96 (s, 3H), 4.70 (br s, 1H), 4.93–5.03 (m, 2H), 5.29 (br s, 1H), 5.45 (d, J = 15.6 Hz, 1H), 5.71 (ddd, J = 17.5, 10.4, 7.4 Hz, 1H), 6.06 (s, 1H), 6.51–6.57 (m, 1H), 6.68–6.74 (m, 1H), 6.99 (s, 1H), 7.22 (d, J = 4.6 Hz, 1H), 7.41 (dd, J = 9.3, 2.6 Hz, 1H), 7.49 (t, J = 7.9 Hz, 2H), 7.54–7.66 (m, 2H), 7.82–7.88 (m, 2H), 8.06 (d, J = 9.2 Hz, 1H), 8.65 (d, J = 4.5 Hz, 1H). 13C-NMR (126 MHz, CDCl3): δ 25.54, 27.53, 28.06, 39.59, 40.84, 55.72, 88.82, 62.17, 77.36, 101.39, 114.83, 114.89, 118.75, 119.93, 121.48, 123.85, 127.06, 127.52, 127.83, 128.43, 129.06, 129.07, 132.22, 132.75, 140.67, 141.29, 142.73, 144.61, 145.89, 148.03, 148.09, 157.96.
3.6. Synthesis of Benzenediamines 10
General procedure 6 (GP6): To a solution of diamine 7 in anhydrous CH2Cl2, 2-(ethoxymethylene)malononitrile (2i) was added. The reaction mixture was stirred at 25 °C for 24 h, volatile components were evaporated in vacuo, and the residue was purified by CC. Fractions containing the product 10 were combined and volatile components evaporated in vacuo.
3.6.1. 2-({[2-({(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}amino)phenyl]amino}methylene)malononitrile (10a)
Following GP6. Prepared from 7a (0.63 mmol, 263 mg) and 2i (0.761 mmol, 77.4 mg) in CH2Cl2 (1 mL), 25 °C, 24 h, CC (EtOAc). Yield: 178 mg (0.36 mmol, 57%) of colorless solid, mp = 121–122 °C. [α]Dr.t. = −87.8 (0.067, MeOH). EI-HRMS: m/z = 246.1312 (M + 2H)+2; C30H32N6O requires: m/z = 246.1313 (M + 2H)+2; νmax 3365, 2929, 2214, 1993, 1620, 1598, 1506, 1454, 1431, 1314, 1257, 1225, 1136, 1081, 1028, 987, 911, 855, 824, 728, 683, 645 cm−1. 1H-NMR (500 MHz, CDCl3): δ 1.02–1.12 (m, 1H), 1.37–1.46 (m, 1H), 1.62–1.75 (m, 3H), 2.27–2.38 (m, 1H), 2.69–2.79 (m, 1H), 2.74 (br d, J = 12.2 Hz, 1H), 2.83 (br quintet, J = 8.0 Hz, 1H), 3.05–3.17 (m, 1H), 3.17–3.29 (m, 2H), 4.00 (s, 3H), 4.88–5.03 (m, 3H), 5.39 (br s, 1H), 5.70 (ddd, J = 19.3, 10.0, 7.2 Hz, 1H), 6.25 (br s, 1H), 6.72 (t, J = 7.8 Hz, 1H), 6.85 (t, J = 7.7 Hz, 1H), 6.90 (dd, J = 7.8, 1.4 Hz, 1H), 7.46 (dd, J = 9.2, 2.6 Hz, 1H), 7.52 (d, J = 4.6 Hz, 1H), 7.59 (s, 1H), 7.62 (s, 1H), 8.11 (d, J = 9.3 Hz, 1H), 8.76 (d, J = 4.5 Hz, 1H). 13C-NMR (126 MHz, CDCl3): δ 27.24, 27.66, 39.05, 40.76, 55.16, 55.31, 55.83, 64.57, 77.36, 101.31, 114.24, 115.35, 115.47, 119.81, 120.33, 121.40, 121.67, 121.71, 128.25, 128.40, 132.63, 132.93, 138.70, 139.39, 140.52, 144.71, 144.75, 148.23, 157.39, 158.29.
3.6.2. 2-[({[2-({(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}amino)-5-trifluoromethyl]phenyl}amino)methylene]malononitrile (10b)
Following GP6. Prepared from 7b (0.49 mmol, 236 mg) and 2i (0.50 mmol, 60.9 mg) in CH2Cl2 (2 mL), 25°C, 24 h, CC (EtOAc). Yield: 100 mg (0.18 mmol, 36%) of colorless solid, mp = 107–109 °C. [α]Dr.t. = –141.5 (0.092, MeOH). EI-HRMS: m/z = 280.1248 (M + 2H)+2; C31H31F3N6O requires: m/z = 280.1250 (M + 2H)+2; νmax 3348, 2930, 2869, 2218, 1614, 1507, 1433, 1326, 1265, 1109, 1029, 919, 813, 716, 616 cm−1. 1H-NMR (500 MHz, CDCl3): δ 1.04–1.12 (m, 1H), 1.36–1.45 (m, 1H), 1.61–1.75 (m, 3H), 2.17–2.24 (m, 1H), 2.53–2.69 (m, 2H), 2.81 (d, J = 12.8 Hz, 1H), 3.06 (d, J = 12.8 Hz, 1H), 3.37 (br s, 1H), 4.02 (s, 3H), 4.93 (d, J = 16.8 Hz, 1H), 5.00 (d, J = 10.4, 1.2 Hz, 1H), 5.04–5.18 (m, 1H), 5.67 (ddd, J = 17.5, 10.4, 7.5 Hz, 1H), 6.16 (d, J = 6.2 Hz, 1H), 6.31 (d, J = 8.6 Hz, 1H), 7.04 (d, J = 2.1 Hz, 1H), 7.14 (dd, J = 8.3, 2.1 Hz, 1H), 7.48 (dd, J = 9.3, 2.5 Hz, 1H), 7.54–7.59 (m, 2H), 7.61 (s, 1H), 7.98 (br s, 1H), 8.12 (d, J = 9.2 Hz, 1H), 8.80 (d, J = 4.6 Hz, 1H). 13C-NMR (126 MHz, CDCl3): δ 24.67, 26.94, 27.48, 38.89, 40.73, 53.21, 55.44, 55.82, 61.50, 77.36, 100.83, 111.61, 114.92, 115.07, 115.64, 119.77, 120.01, 121.17, 122.90, 125.06, 125.96, 126.84, 128.13, 133.01, 139.69, 142.65, 144.53, 144.58, 148.52, 158.49, 158.55.
3.6.3. 2-({[5-Cyano-2-({(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}amino)phenyl]amino}methylene)malononitrile (10c)
Following GP6. Prepared from 7c (0.63 mmol, 279 mg) and 2i (0.77 mmol, 78 mg) in CH2Cl2 (1 mL), 25 °C, 24 h, CC (EtOAc). Yield: 280 mg (0.54 mmol, 86%) of colorless solid, mp = 138–140 °C. [α]Dr.t. = –142.7 (0.075, MeOH). EI-HRMS: m/z = 258.6286 (M + 2H)+2; C31H31N7O requires: m/z = 258.6290 (M + 2H)+2; νmax 3565, 2922, 2862, 2215, 2196, 2163, 2023, 1952, 1729, 1605, 1589, 1555, 1506, 1455, 1431, 1311, 1256, 1226, 1129, 1083, 1026, 985, 947, 915, 854, 809, 717, 683, 610 cm−1. 1H-NMR (500 MHz, CDCl3): δ 1.05–1.14 (m, 1H), 1.35–1.45 (m, 1H), 1.56–1.79 (m, 4H), 2.21–2.30 (m, 1H), 2.58–2.72 (m, 2H), 2.80–2.90 (m, 1H), 3.05–3.15 (m, 1H), 3.32–3.47 (m, 1H), 4.02 (s, 3H), 4.94–5.08 (m, 2H), 5.12 (br s, 1H), 5.68 (ddd, J = 17.4, 10.4, 7.3 Hz, 1H), 6.24 (br s, 1H), 6.34 (br s, 1H), 7.05–7.10 (m, 1H), 7.11–7.16 (m, 1H), 7.37–7.52 (br m, 1H), 7.49 (d, J = 9.4 Hz, 1H), 7.53–7.59 (m, 2H), 8.13 (d, J = 9.2 Hz, 1H), 8.79 (d, J = 4.6 Hz, 1H). 13C-NMR (126 MHz, CDCl3): δ 24.41, 26.87, 27.29, 38.69, 40.79, 52.96, 55.23, 55.89, 56.26, 61.61, 77.36, 99.99, 100.73, 111.62, 114.88, 114.99, 115.96, 118.67, 119.92, 121.17, 126.42, 127.23, 128.04, 132.21, 133.11, 139.54, 143.57, 144.47, 148.50, 158.58, 158.70.
3.6.4. 2-({[4-Cyano-2-({(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}amino)phenyl]amino}methylene)malononitrile (10d)
Following GP6. Prepared from 7d (0.31 mmol, 136 mg) and 2i (0.50 mmol, 60.9 mg) in CH2Cl2 (2 mL), 25 °C, 24 h, CC (EtOAc). Yield: 75 mg (0.145 mmol, 47%) of white solid, mp = 117–120 °C. [α]Dr.t. = –218 (0.11, MeOH). EI-HRMS: m/z = 258.6289 (M + 2H)+2; C31H31N7O requires: m/z = 258.6290 (M + 2H)+2; νmax 3330, 2928, 2864, 2220, 2194, 2171, 1980, 1621, 1587, 1547, 1509, 1462, 1432, 1359, 1312, 1257, 1090, 1029, 921, 834, 787, 721, 621 cm−1. 1H-NMR (500 MHz, CDCl3): δ 1.01–1.12 (m, 1H), 1.37–1.47 (m, 1H), 1.64–1.78 (m, 3H), 2.22–2.32 (m, 1H), 2.56–2.68 (m, 1H), 2.68–2.79 (m, 1H), 2.86–2.96 (m, 1H), 3.05–3.17 (m, 1H), 3.29 –3.44 (m, 1H), 4.02 (s, 3H), 4.91–5.10 (m, 3H), 5.68 (ddd, J = 17.3, 10.4, 7.2 Hz, 1H), 5.89 (br s, 1H), 6.53 (br s, 1H), 6.84–7.00 (m, 3H), 7.49 (dd, J = 9.4, 2.5 Hz, 1H), 7.51 (d, J = 4.5 Hz, 1H), 7.57 (br s, 1H), 7.63 (s, 1H), 8.13 (d, J = 9.3 Hz, 1H), 8.80 (d, J = 4.5 Hz, 1H). 13C-NMR (126 MHz, CDCl3): δ 24.85, 26.97, 27.21, 38.65, 40.81, 55.10, 55.99, 56.62, 61.31, 77.37, 100.70, 111.07, 114.93, 115.19, 115.70, 116.22, 118.41, 121.65, 121.92, 122.89, 128.11, 132.15, 132.50, 133.00, 139.75, 139.80, 143.56, 144.69, 148.25, 157.61, 158.83.
3.6.5. 2-({[5-Bromo-2-({(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}amino)phenyl]amino}methylene)malononitrile (10e)
Following GP9. Prepared from 7e (0.5 mmol, 246 mg) and 2i (0.50 mmol, 60.9 mg) in CH2Cl2 (2 mL), 25 °C, 24 h, CC (EtOAc). Yield: 256 mg (0.45 mmol, 90%) of colorless solid, mp = 121–123 °C. [α]Dr.t. = –67.7 (0.12, MeOH). EI-HRMS: m/z = 285.0863 (M + 2H)+2; C30H31BrN6O requires: m/z = 258.0866 (M + 2H)+2; νmax 3305, 2933, 2864, 2833, 2191, 1727, 1620, 1588, 1546, 1505, 1452, 1311, 1240, 1138, 1083, 1028, 916, 854, 827, 716, 649 cm−1. 1H-NMR (500 MHz, CDCl3): δ 1.10–1.25 (m, 1H), 1.39–1.54 (m, 1H), 1.72–1.90 (m, 3H), 2.36–2.52 (m, 1H), 2.76–2.91 (m, 1H), 2.91–3.08 (m, 1H), 3.21–3.42 (m, 2H), 3.48–3.66 (m, 1H), 4.01 (s, 3H), 4.98–5.11 (m, 2H), 5.17 (s, 1H), 5.69 (ddd, J = 17.3, 10.4, 7.0 Hz, 1H), 5.89 (s, 1H), 6.22 (s, 1H), 6.93–7.01 (m, 2H), 7.41–7.46 (m, 1H), 7.47–7.53 (m, 2H), 7.55–7.60 (m, 1H), 8.06 (d, J = 9.2 Hz, 1H), 8.74 (d, J = 4.5 Hz, 1H), 9.66 (s, 1H). 13C-NMR (126 MHz, CDCl3): δ 24.20, 26.17, 37.80, 40.87, 53.04, 54.29, 54.51, 61.30, 77.36, 100.70, 110.27, 114.91, 115.08, 115.75, 116.38, 119.66, 120.00, 121.58, 125.83, 128.27, 129.43, 131.04, 132.17, 132.53, 138.69, 143.70, 144.29, 148.08, 158.67, 178.38.
3.6.6. 2-({[4-Bromo-2-({(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}amino)phenyl]amino}methylene)malononitrile (10f)
Following GP9. Prepared from 7f (0.5 mmol, 246 mg) and 2i (0.5 mmol, 60.9 mg), CH2Cl2 (2 mL), 25 °C, 24 h, CC (EtOAc). Yield: 214 mg (0.38 mmol, 75%) of yellowish solid, mp = 116.1–118.9 °C. [α]Dr.t. = –118 (0.083, MeOH). EI-HRMS: m/z = 285.0862 (M + 2H)+2; C30H31BrN6O requires: m/z = 285.0866 (M + 2H)+2; νmax 3344, 2949, 2877, 2359, 2219, 2163, 2034, 1960, 1622, 1593, 1508, 1474, 1421, 1322, 1258, 1175, 1030, 922, 827, 788, 717, 685 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.93–1.02 (m, 1H), 1.31–1.42 (m, 1H), 1.58–1.74 (m, 3H), 2.15–2.23 (m, 1H), 2.47–2.64 (m, 2H), 2.69–2.80 (m, 1H), 3.03–3.13 (m, 1H), 3.28–3.43 (m, 1H), 4.04 (s, 3H), 4.89–5.08 (m, 3H), 5.67 (ddd, J = 17.4, 10.4, 7.2 Hz, 1H), 5.88 (d, J = 6.9 Hz, 1H), 6.51 (s, 1H), 6.62 (d, J = 8.3 Hz, 1H), 6.68 (dd, J = 8.3, 2.0 Hz, 1H), 7.45 (dd, J = 9.2, 2.5 Hz, 1H), 7.51 (s, 1H), 7.55–7.59 (m, 2H), 8.09 (d, J = 9.2 Hz, 1H), 8.36 (br s, 1H), 8.79 (d, J = 4.6 Hz, 1H). 13C-NMR (126 MHz, CDCl3): δ 24.78, 26.94, 27.39, 38.63, 40.67, 54.78, 55.13, 55.86, 61.17, 77.36, 99.97, 115.14, 115.17, 115.32, 115.50, 120.15, 120.50, 121.87, 121.93, 123.89, 126.37, 128.14, 132.75, 140.03, 141.03, 144.47, 144.64, 148.34, 158.24, 158.64.
3.6.7. 2-({[2-({(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}amino)-4-methylphenyl]amino}methylene)malononitrile (10g)
Following GP9. Prepared from 7g (0.31 mmol, 130 mg) and 2i (0.33 mmol, 40.0 mg) in CH2Cl2 (2 mL), 25 °C, 24 h, CC (EtOAc). Yield: 77 mg (0.149 mmol, 48%) of brownish solid, mp = 102.9–107.2 °C. [α]Dr.t. = –143 (0.1, MeOH). EI-HRMS: m/z = 253.1392 (M + 2H)+2; C31H34N6O requires: m/z = 253.1392 (M + 2H)+2; νmax 3305, 2942, 2873, 2844, 2358, 2217, 2193, 2087, 2036, 1962, 1613, 1548, 1510, 1474, 1433, 1329, 1260, 1240, 1139, 1030, 921, 856, 717, 668, 621 cm−1. 1H-NMR (500 MHz, CDCl3): δ 1.15 (dd, J = 13.6, 7.0, 1H), 1.41 (s, 1H), 1.61–1.70 (m, 2H), 1.73 (s, 1H), 2.33 (s, 1H), 2.71–2.90 (m, 3H), 2.99 (s, 1H), 3.36 (dd, J = 14.0, 10.1, 1H), 3.79–4.11 (m, 3H), 4.90–5.07 (m, 2H), 5.62–5.78 (m, 1H), 6.69 (s, 1H), 7.36–7.70 (m, 3H), 8.08 (s, 2H), 8.50–8.58 (m, 1H), 8.75 (d, J = 4.6, 1H). 13C-NMR (126 MHz, CDCl3): δ 21.20, 27.56, 28.21, 39.64, 40.97, 55.82, 58.63, 89.46, 111.85, 114.95, 115.94, 116.89, 117.49, 140.41, 141.21, 144.32, 152.73, 153.20, 175.10.
3.6.8. 2-({[2-({(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}amino)-5-methylphenyl]amino}methylene)malononitrile (10h)
Following GP9. Prepared from 7h (0.33 mmol, 140 mg) and 2i (0.50 mmol, 60.9 mg) in CH2Cl2 (2 mL), 25 °C, 24 h, CC (EtOAc). Yield: 105 mg (0.20 mmol, 62%) of brownish solid, mp = 110–112 °C. [α]Dr.t. = –230 (0.125, MeOH). EI-HRMS: m/z = 253.1388 (M + 2H)+2; C31H34N6O requires: m/z = 253.1392 (M + 2H)+2; νmax 3363, 2943, 2358, 2215, 2035, 1970, 1622, 1590, 1508, 1473, 1432, 1314, 1259, 1228, 1139, 1030, 989, 919, 854, 717, 683 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.96–1.04 (m, 1H), 1.19–1.33 (m, 1H), 1.38–1.48 (m, 1H), 1.61–1.68 (m, 2H), 1.69–1.72 (m, 1H), 1.97 (s, 3H), 2.29–2.36 (m, 1H), 2.69–2.77 (m, 1H), 2.79–2.87 (m, 1H), 3.01–3.09 (m, 1H), 3.16–3.30 (m, 2H), 4.00 (s, 3H), 4.88 (br s, 1H), 4.96–5.02 (m, 2H), 5.23 (br s, 1H), 5.71 (ddd, J = 17.4, 10.1, 7.2 Hz, 1H), 6.10 (br s, 1H), 6.55 (dd, J = 8.1, 1.8 Hz, 1H), 6.79 (d, J = 7.9 Hz, 1H), 7.46 (dd, J = 9.2, 2.6 Hz, 1H), 7.49 (d, J = 4.5 Hz, 1H), 7.56 (s, 1H), 7.65 (br s, 1H), 8.11 (d, J = 9.2 Hz, 1H), 8.78 (d, J = 4.6 Hz, 1H). 13C-NMR (126 MHz, CDCl3): δ 21.42, 25.69, 27.31, 27.79, 39.19, 40.74, 54.73, 55.45, 55.84, 60.83, 77.37, 101.18, 114.23, 115.20, 115.52, 117.16, 120.43, 120.82, 121.40, 121.51, 126.19, 128.40, 132.58, 138.29, 139.31, 140.77, 143.09, 144.80, 148.17, 157.28, 158.24.
3.6.9. 2-({[4-Methoxy-2-({(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}amino)phenyl]amino}methylene)malononitrile (10i)
Following GP9. Prepared from 7i (0.675 mmol, 300 mg) and 2i (0.742 mmol, 90.2 mg) in CH2Cl2 (3 mL), 25 °C, 24 h, CC (EtOAc). Yield: 251 mg (0.48 mmol, 71%) of yellowish solid, decompose >250 °C. [α]Dr.t. = –177 (0.092, MeOH). EI-HRMS: m/z = 261.1361 (M + 2H)+2; C31H34N6O2 requires: m/z = 261.1366 (M + 2H)+2; νmax 3369, 2942, 2872, 2358, 2200, 2012, 1980, 1621, 1592, 1548, 1509, 1455, 1433, 1319, 1261, 1215, 1173, 1138, 1033, 991, 918, 856, 825, 719 cm−1. 1H-NMR (500 MHz, CDCl3): δ 1.02–1.09 (m, 1H), 1.39–1.48 (m, 1H), 1.60–1.70 (m, 2H), 1.70–1.75 (m, 1H), 2.33 (s, 1H), 2.70–2.86 (m, 2H), 3.01–3.12 (m, 1H), 3.17–3.29 (m, 2H), 3.33 (s, 3H), 4.00 (s, 3H), 4.91 (s, 1H), 4.96–5.03 (m, 2H), 5.49 (s, 1H), 5.71 (ddd, J= 17.3, 10.0, 7.2, 1H), 5.78 (s, 1H), 6.20 (dt, J = 8.7, 2.2, 1H), 6.81 (dd, J = 8.6, 1.7, 1H), 7.26 (s, 1H), 7.45 (dd, J = 9.3, 2.6, 1H), 7.51 (s, 1H), 7.54 (d, J = 4.5, 1H), 7.66 (s, 1H), 8.11 (d, J=9.2, 1H), 8.78 (d, J = 4.6, 1H). 13C-NMR (126 MHz, CDCl3): δ 14.28, 22.81, 25.59, 27.30, 27.87, 31.74, 39.23, 40.79, 54.44, 55.12, 55.50, 55.84, 100.96, 104.52, 115.20, 115.44, 120.90, 121.44, 123.73, 128.33, 128.61, 132.21, 132.29, 132.70, 133.07, 140.79, 141.50, 144.80, 148.30, 158.21, 159.88.
3.6.10. 2-({[3-({(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}amino)pyridin-2-yl]amino}methylene)malononitrile (10j)
Following GP9. Prepared from 7j (0.65 mmol, 270 mg) and 2i (0.77 mmol, 78 mg) in CH2Cl2 (1 mL), 25 °C, 24 h, CC (EtOAc). Yield: 88 mg (0.18 mmol, 27%) of yellowish solid, decompose 243 °C. [α]Dr.t. = –30.4 (0.092, MeOH). EI-HRMS: m/z = 246.6286 (M + 2H)+2; C29H31N7O requires: m/z = 246.6290 (M + 2H)+2; νmax 3326, 3213, 2944, 2223, 2035, 1621, 1579, 1508, 1475, 1432, 1361, 1231, 1027, 916, 831 cm−1. 1H-NMR (500 MHz, CDCl3): δ 1.12–1.19 (m, 1H), 1.27–1.37 (m, 1H), 1.57–1.65 (m, 2H), 1.65–1.72 (m, 1H), 2.24–2.33 (m, 1H), 2.99–3.09 (m, 1H), 3.13 (br s, 1H), 3.30 (dd, J = 13.8, 10.0, 1H), 3.99 (s, 3H), 4.86 (br s, 1H), 4.90–5.00 (m, 2H), 5.70 (ddd, J = 17.6, 10.3, 7.5, 1H), 6.19 (br s, 1H), 6.44 (td, J = 7.7, 1.5, 1H), 6.57 (td, J = 7.5, 1.3, 1H), 6.66 (dd, J = 7.5, 1.5, 1H), 7.40–7.48 (m, 1H), 7.68–7.74 (m, 1H), 7.83 (br s, 1H), 8.08 (d, J = 9.2, 1H), 8.72 (d, J = 4.4, 1H). 13C-NMR (126 MHz, CDCl3): δ 25.45, 27.60, 28.23, 39.84, 40.94, 55.69, 56.09, 77.37, 101.66, 112.81, 114.67, 115.88, 119.08, 120.05, 121.28, 128.57, 128.63, 128.67, 132.17, 132.24, 135.32, 136.35, 141.50, 144.74, 146.39, 148.26, 157.75.
3.6.11. (E)-4-({[2-({(S)-(6-methoxyquinolin-4-yl)[(1S,2S,4S,5R)-5-vinylquinuclidin-2-yl]methyl}amino)phenyl]amino}methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one (10k)
Prepared from 7a (0.24 mmol, 100 mg), 2b (0.24 mmol, 55 mg), and AcOH (5 µL) in EtOH (1 mL), 25 °C, 24 h. The precipitate was collected by filtration and washed with cold EtOH (1 mL) to give 10k. Yield: 126 mg (0.21 mmol, 88%) of yellow solid, mp = 167.1–169.2 °C. [α]Dr.t. = –517 (0.027, MeOH). EI-HRMS: m/z = 300.1596 (M + 2H)+2; C37H40N6O2 requires: m/z = 300.1601 (M + 2H)+2; νmax 3312, 3075, 2912, 2863, 1672, 1621, 1596, 1552, 1510, 1496, 1474, 1455, 1416, 1363, 1335, 1320, 1296, 1259, 1204, 1174, 1157, 1109, 1083, 1037, 1004, 952, 906, 878, 864, 822, 788, 734, 712, 686, 670, 636, 621 cm−1. 1H-NMR (500 MHz, CDCl3): δ 1.05–1.16 (m, 1H), 1.28–1.40 (m, 1H), 1.53–1.66 (m, 2H), 1.67–1.75 (m, 1H), 2.24–2.36 (m, 4H), 2.66–2.81 (m, 1H), 2.91–3.06 (m, 2H), 3.23 (dd, J = 13.9, 10.0 Hz, 2H), 3.99 (s, 3H), 4.65–5.07 (m, 3H), 5.74 (ddd, J = 17.4, 10.4, 7.3 Hz, 1H), 5.96 (s, 1H), 6.39 (s, 1H), 6.74 (td, J = 7.6, 1.3 Hz, 1H), 6.83 (t, J = 7.7 Hz, 1H), 7.10–7.21 (m, 2H), 7.36–7.47 (m, 3H), 7.69 (s, 1H), 7.87 (s, 2H), 7.99–8.16 (m, 3H), 8.72 (d, J = 4.5 Hz, 1H), one proton is missing. 13C-NMR (126 MHz, CDCl3): δ 12.87, 25.63, 27.62, 28.33, 39.91, 40.89, 55.66, 55.94, 77.36, 101.63, 103.51, 114.54, 114.60, 117.71, 118.70, 119.19, 121.47, 124.27, 126.94, 128.05, 128.43, 128.87, 132.21, 139.06, 139.29, 141.67, 144.46, 144.95, 147.96, 148.23, 157.88, 166.01, three carbons are missing.