2-(Arylimino)benzylidene-8-arylimino-5,6,7-trihydroquinoline Cobalt(II) Dichloride Polymerization Catalysts for Polyethylenes with Narrow Polydispersity
Abstract
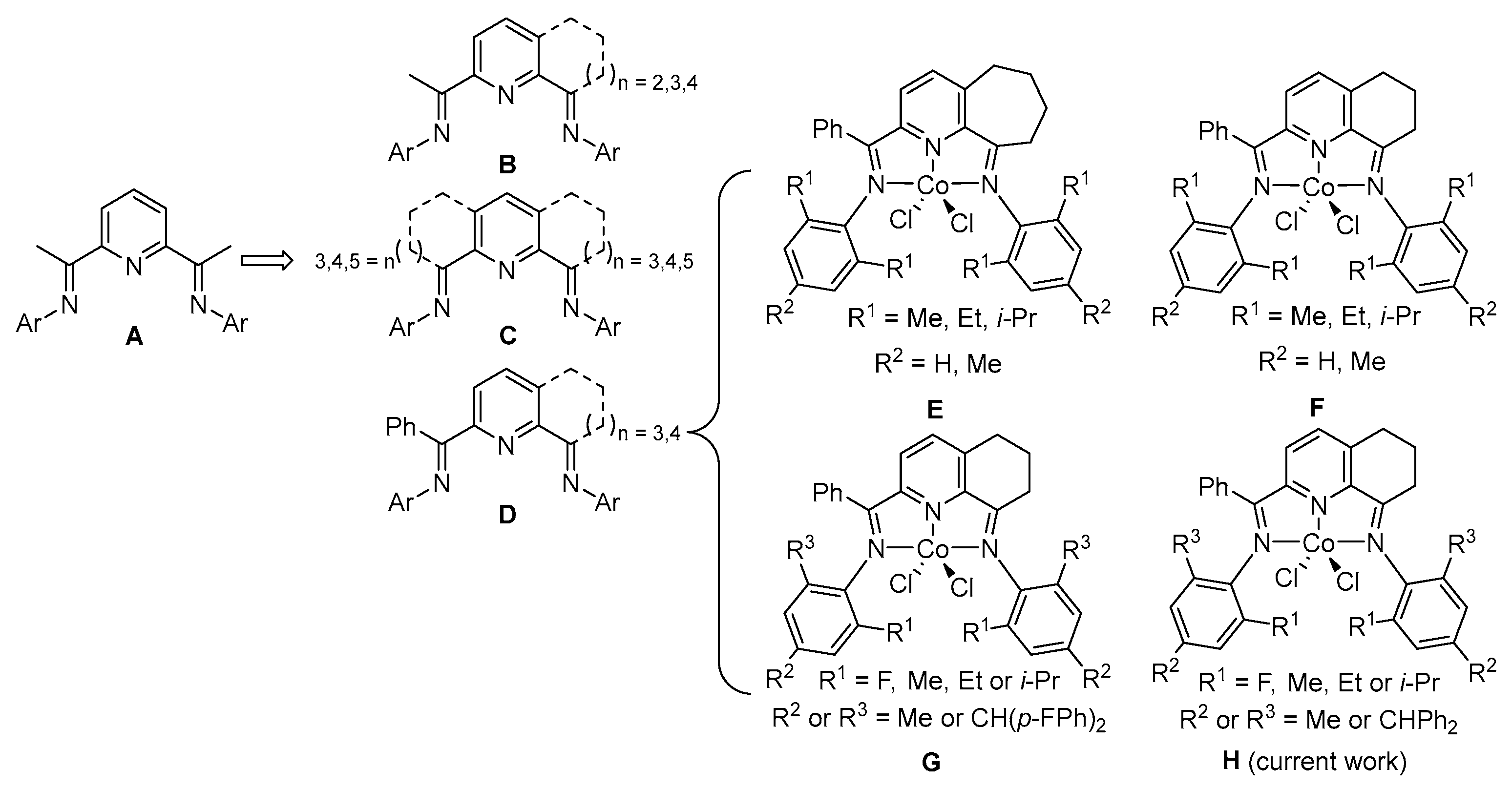
1. Introduction
2. Results and Discussion
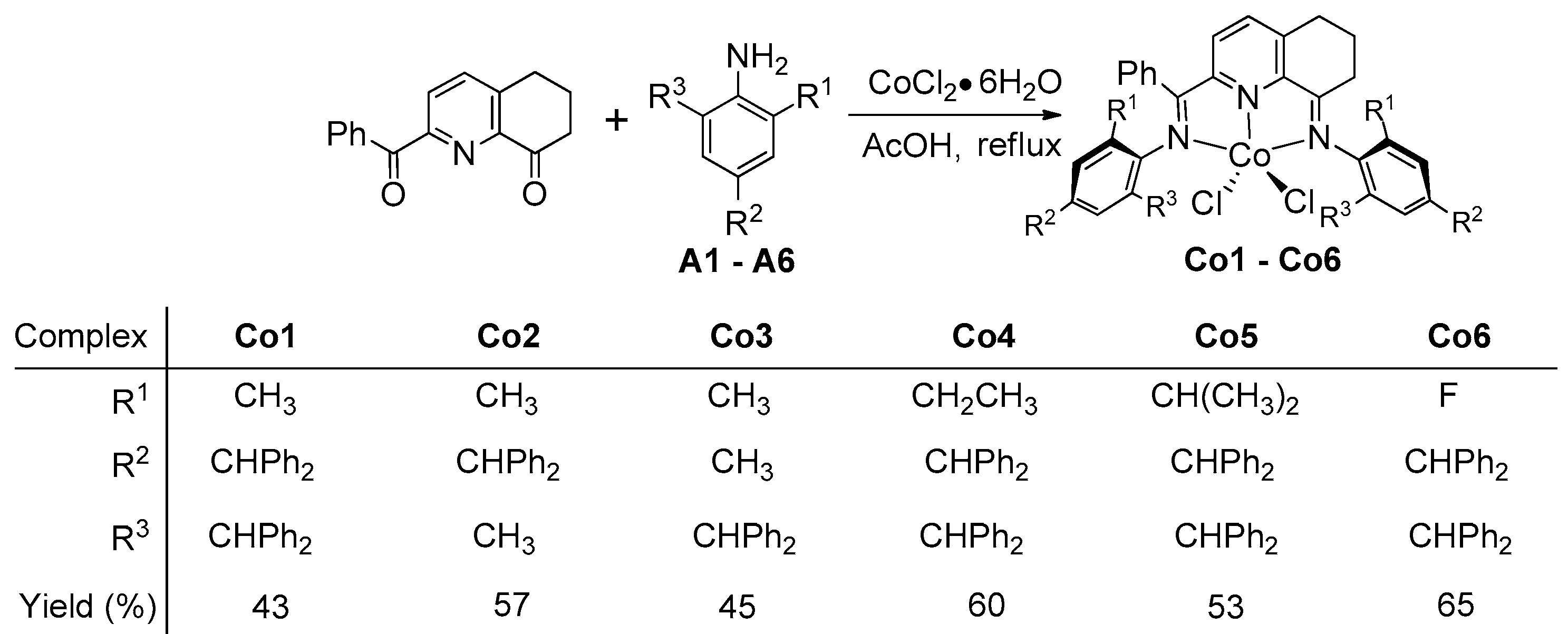
2.1. Preparation and Characterization of Co1–Co6
2.2. Catalytic Evaluation of Co1–Co6 for Ethylene Polymerization
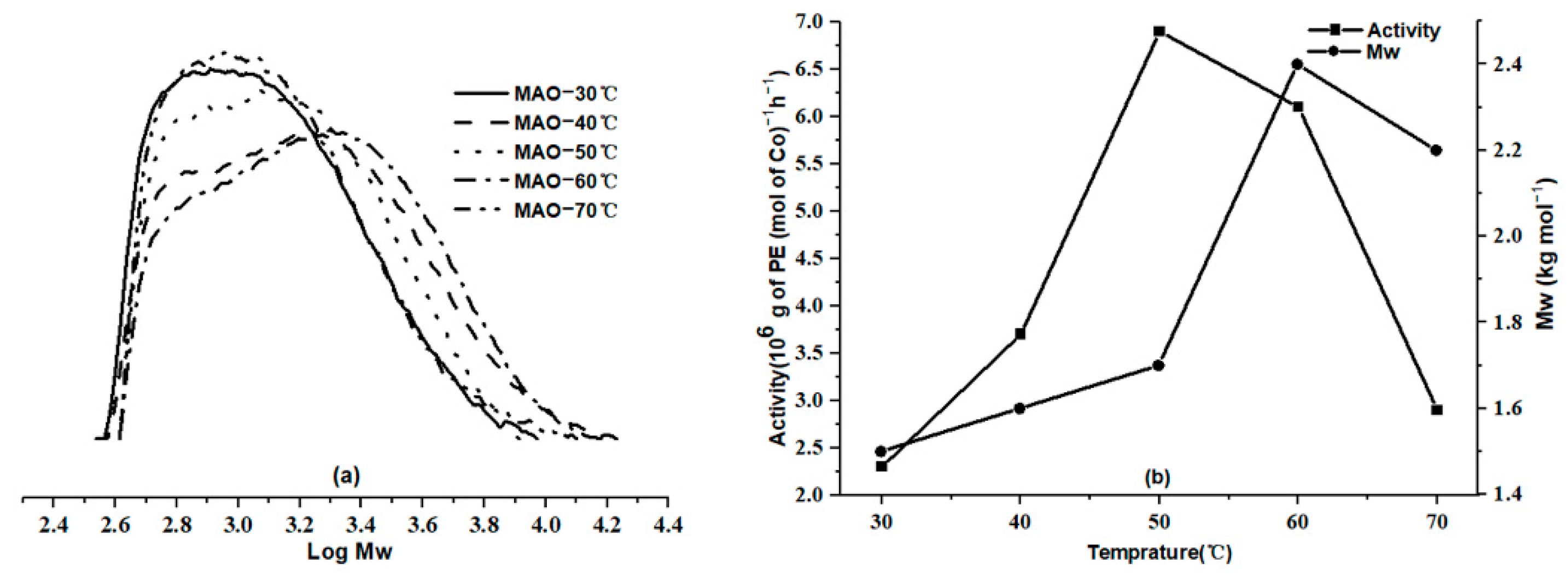
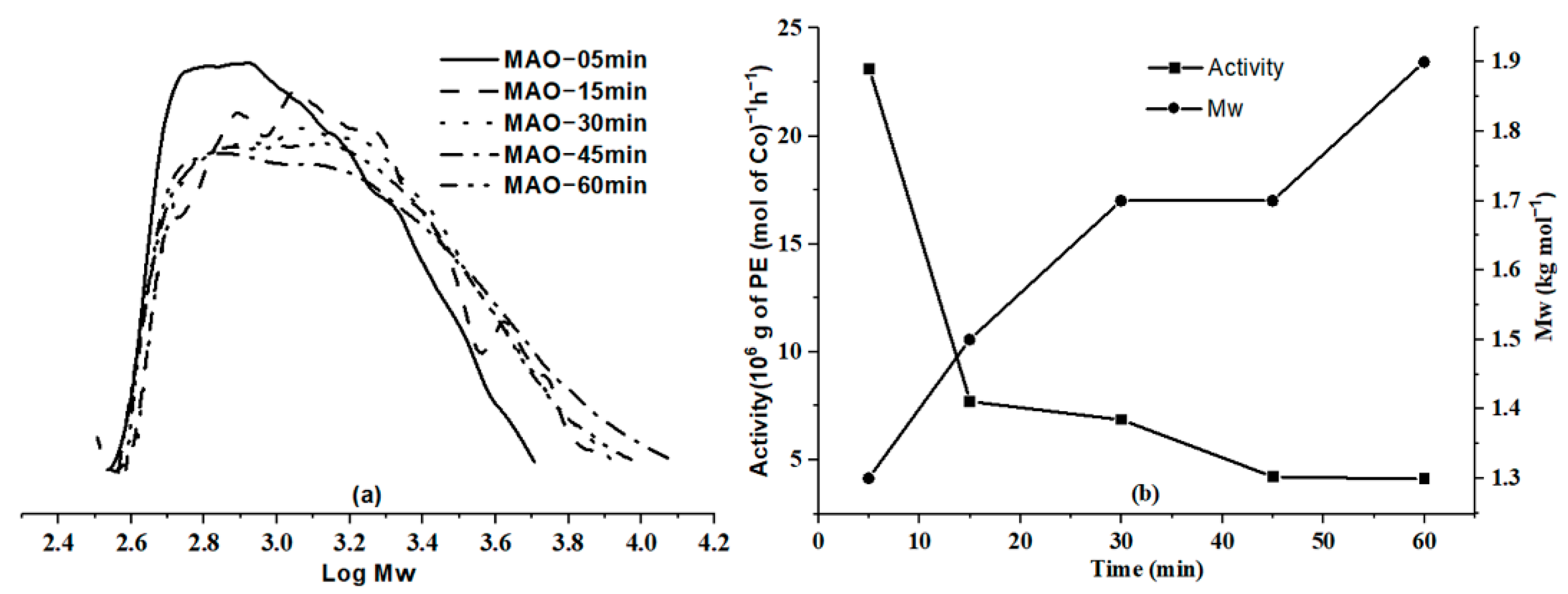
2.2.1. Optimization of the Polymerization Conditions Using Co6/MAO
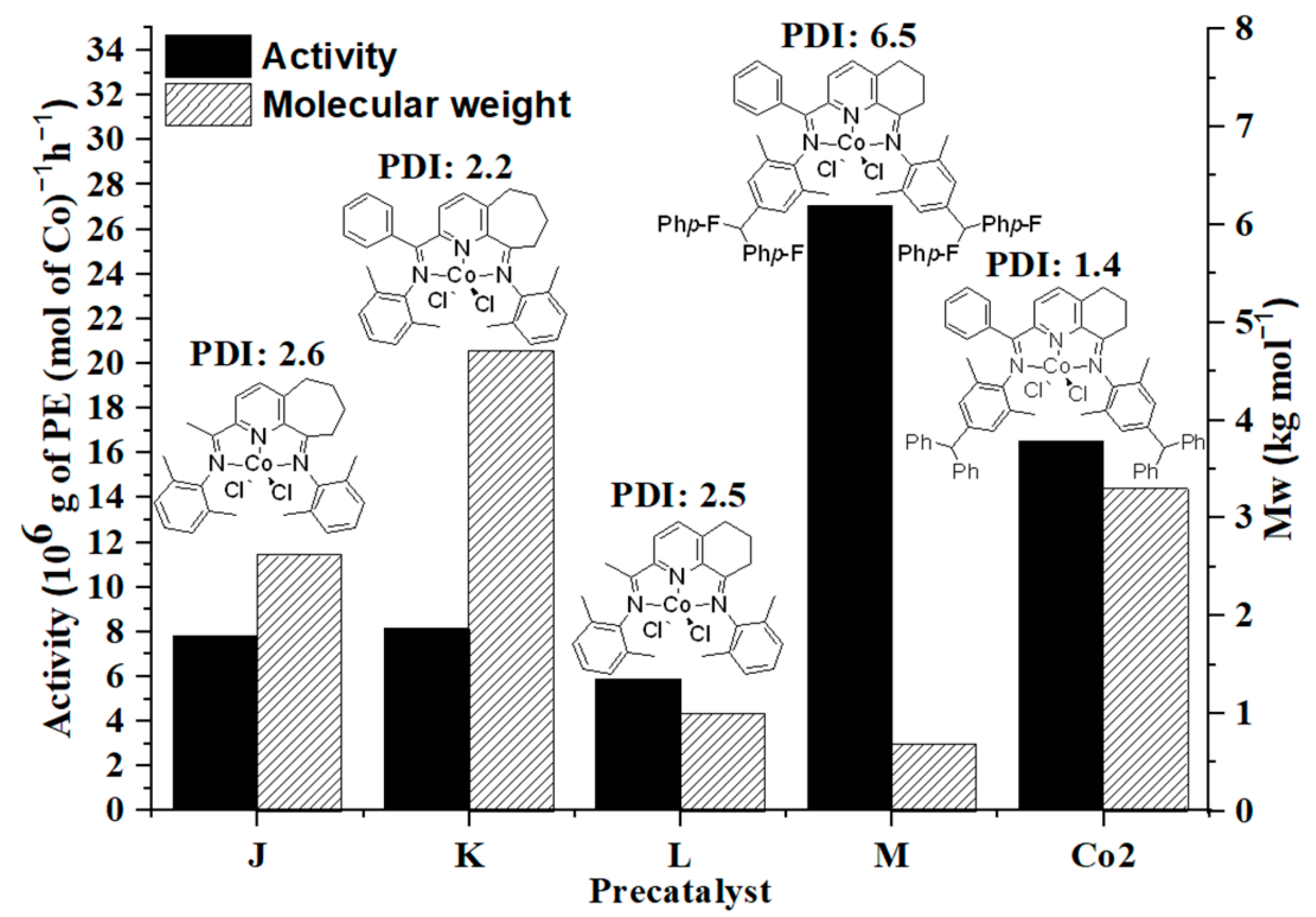
2.2.2. Catalytic Evaluation of Co1–Co6 Using MAO as Co-Catalyst under the Optimal Conditions
2.2.3. Optimization of the Polymerization Conditions Using Co6/MMAO
2.2.4. Catalytic Evaluation of Co1–Co6 Using MMAO as Co-Catalyst under the Optimal Conditions
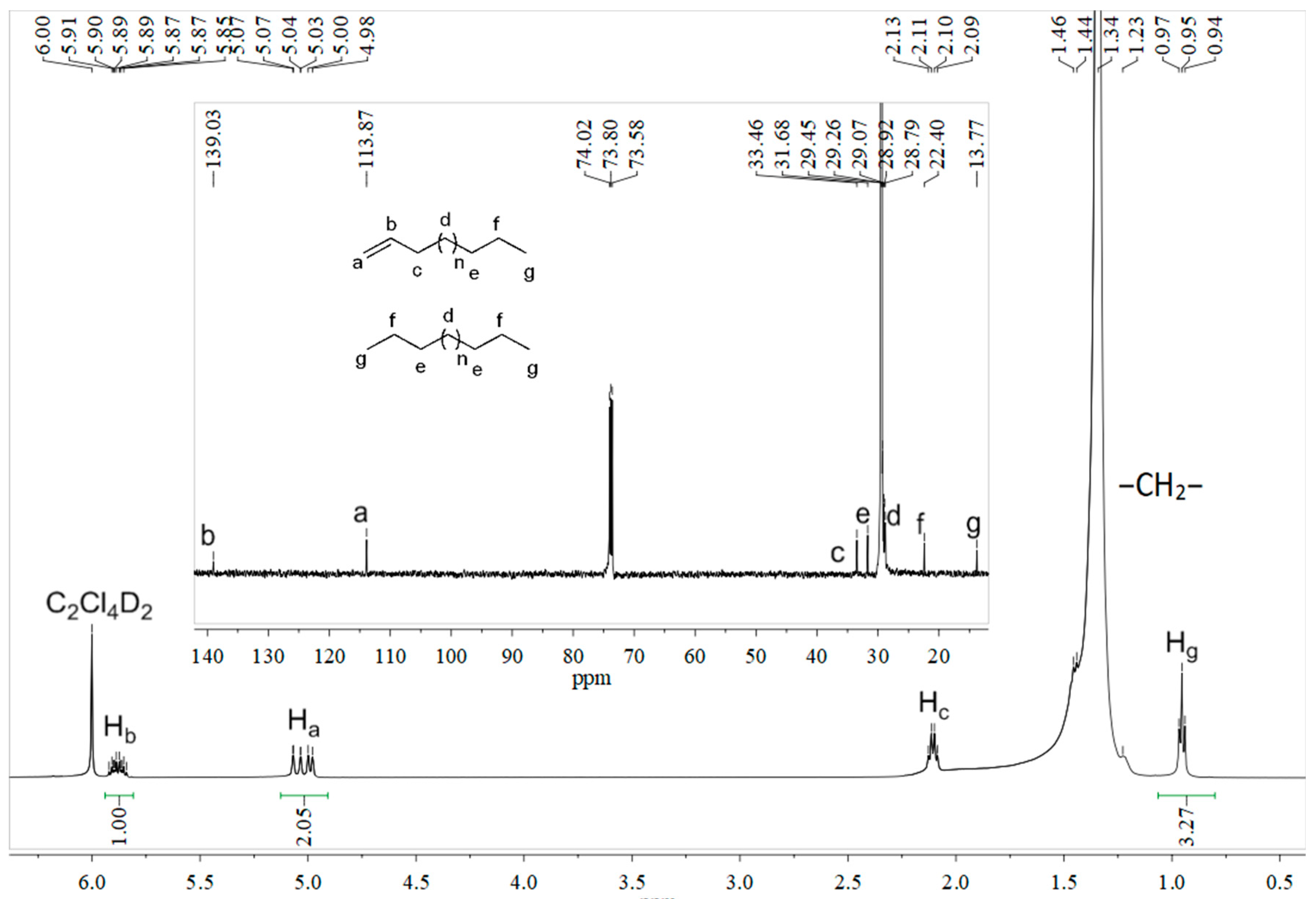
2.3. Microstructural Properties of Resultant Polyethylenes
3. Experimental Section
3.1. General Considerations
3.2. Synthesis of [2-(ArN=CPh)-8-(NAr)-C9H8N]CoCl2 (Co1–Co6)
3.3. X-ray Crystallographic Studies
3.4. Procedures for Ethylene Polymerization
3.4.1. Ethylene Polymerization under 1 atm Ethylene
3.4.2. Ethylene Polymerization under 5 or 10 atm Ethylene
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gibson, V.C.; Redshaw, C.; Solan, G.A. Bis(imino)pyridines: Surprisingly reactive ligands and a gateway to new families of catalysts. Chem. Rev. 2007, 107, 1745–1776. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, C.; Giambastiani, G.; Luconi, L.; Meli, A. Olefin oligomerization, homopolymerization and copolymerization by late transition metals supported by (imino)pyridine ligands. Coord. Chem. Rev. 2010, 254, 431–455. [Google Scholar] [CrossRef]
- Small, B.L. Discovery and development of pyridine-bis(imine) and related catalysts for olefin polymerization and oligomerization. Acc. Chem. Res. 2015, 48, 2599–2611. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Yang, W.; Sun, W.-H. Recent progress on transition metal (Fe, Co, Ni, Ti and V) complex catalysts in olefin polymerization with high thermal stability. Chin. J. Chem. 2017, 35, 531–540. [Google Scholar] [CrossRef]
- Wang, Z.; Solan, G.A.; Zhang, W.; Sun, W.-H. Carbocyclic-fused N,N,N-pincer ligands as ring-strain adjustable supports for iron and cobalt catalysts in ethylene oligo-/polymerization. Coord. Chem. Rev. 2018, 363, 92–108. [Google Scholar] [CrossRef]
- Bariashir, C.; Jiang, S.; Ma, Y.; Solan, G.A.; Sun, Y.; Sun, W.-H. Recent advances in homogeneous chromium catalyst design for ethylene tri-, tetra-, oligo- and polymerization. Coord. Chem. Rev. 2019, 385, 208–229. [Google Scholar] [CrossRef]
- Small, B.L.; Brookhart, M. Iron-based catalysts with exceptionally high activities and selectivities for oligomerization of ethylene to linear α-Olefins. J. Am. Chem. Soc. 1998, 120, 7143–7144. [Google Scholar] [CrossRef]
- Liu, M.; Brookhart, M. CF3O-Functionalized bis(arylimino)pyridine cobalt ethylene polymerization catalysts: Harnessing solvent effects on performance and polymer properties. Organometallics 2022. [Google Scholar] [CrossRef]
- Liu, T.; Liu, M.; Ma, Y.; Solan, G.A.; Liang, T.; Sun, W.-H. Cobalt catalysts bearing ortho-(4,4′-dichlorobenzhydryl) substituents and their use in generating narrowly dispersed polyethylene of high linearity. Eur J. Inorg. Chem. 2022, e202200396. [Google Scholar] [CrossRef]
- Bianchini, C.; Giambastiani, G.; Rios, I.G.; Mantovani, G.; Meli, A.; Segarra, A.M. Ethylene oligomerization, homopolymerization and copolymerization by iron and cobalt catalysts with 2,6-(bis-organylimino)pyridyl ligands. Coord. Chem. Rev. 2006, 250, 1391–1418. [Google Scholar] [CrossRef]
- Flisak, Z.; Sun, W.-H. Progression of diiminopyridines: From single application to catalytic versatility. ACS. Catal. 2015, 5, 4713–4724. [Google Scholar] [CrossRef]
- Ma, J.; Feng, C.; Wang, S.; Zhao, K.-Q.; Sun, W.-H.; Redshaw, C.; Solan, G.A. Bi- and tridentate imino-based iron and cobalt pre-catalysts for ethylene oligo-/polymerization. Inorg. Chem. Front. 2014, 1, 14–34. [Google Scholar] [CrossRef]
- Gao, R.; Sun, W.-H.; Redshaw, C. Nickel complex pre-catalysts in ethylene polymerization: New approaches to elastomeric materials. Catal. Sci. Technol. 2013, 3, 1172–1179. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, W.-H.; Redshaw, C. Tailoring iron complexes for ethylene oligomerization and/or polymerization. Dalton. Trans. 2013, 42, 8988–8997. [Google Scholar] [CrossRef]
- Jie, S.; Sun, W.-H.; Xiao, T. Prospects and crucial problems in oligomerization and polymerization with iron and cobalt complex catalysts. Chin. J. Polym. Sci. 2010, 28, 299–304. [Google Scholar] [CrossRef]
- Xiao, T.; Zhang, W.; Lai, J.; Sun, W.-H. Iron-oriented ethylene oligomerization and polymerization: The iron age or a flash in the pan. Comptes Rendus Chim. 2011, 14, 851–855. [Google Scholar] [CrossRef]
- Sun, W.-H.; Zhang, S.; Zuo, W. Our variations on iron and cobalt catalysts toward ethylene oligomerization and polymerization. Comptes Rendus Chim. 2008, 11, 307–316. [Google Scholar] [CrossRef]
- Sun, W.-H.; Zhao, W.; Yu, J.; Zhang, W.; Hao, X.; Redshaw, C. Enhancing the activity and thermal stability of iron precatalysts using 2-(1-{2,6-bis[bis(4-fluorophenyl)methyl]-4-methylphenylimino}ethyl)-6-[1-(arylimino)ethyl]pyridines. Macromol. Chem. Phys. 2012, 213, 1266–1273. [Google Scholar] [CrossRef]
- Xing, Q.; Zhao, T.; Du, S.; Yang, W.; Liang, T.; Redshaw, C.; Sun, W.-H. Biphenyl-bridged 6-(1-aryliminoethyl)-2-iminopyridyl cobalt complexes: Synthesis, characterization, and ethylene polymerization behavior. Organometallics 2014, 33, 1382–1388. [Google Scholar] [CrossRef]
- Cao, X.; He, F.; Zhao, W.; Cai, Z.; Hao, X.; Shiono, T.; Redshawd, C.; Sun, W.-H. 2-[1-(2,6-dibenzhydryl-4-chlorophenylimino) ethyl]-6-[1-(arylimino)ethyl]pyridyliron(II) dichlorides: Synthesis, characterization and ethylene polymerization behavior. Polymer 2012, 53, 1870–1880. [Google Scholar] [CrossRef]
- Wang, S.; Li, B.; Liang, T.; Redshaw, C.; Li, Y.; Sun, W.-H. Synthesis, characterization and catalytic behavior toward ethylene of 2-[1-(4,6-dimethyl-2-benzhydrylphenylimino)ethyl]-6-[1-(arylimino)ethyl]pyridylmetal (iron or cobalt) chlorides. Dalton. Trans. 2013, 42, 9188–9197. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liu, H.; Zhang, W.; Hao, X.; Sun, W.-H. Access to highly active and thermally stable iron procatalysts using bulky 2-[1-(2,6-dibenzhydryl-4-methylphenylimino)ethyl]-6-[1-(arylimino)ethyl]pyridine ligands. Chem. Commun. 2011, 47, 3257–3259. [Google Scholar] [CrossRef] [PubMed]
- Small, B.L.; Brookhart, M.; Bennett, A.M.A. Highly active iron and cobalt catalysts for the polymerization of ethylene. J. Am. Chem. Soc. 1998, 120, 4049–4050. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Gibson, V.C.; Kimberley, B.S.; Maddox, P.J.; McTavish, S.J.; Solan, G.A.; White, A.J.P.; Williams, D.J. Novel olefin polymerization catalysts based on iron and cobalt. Chem. Commun. 1998, 849–850. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Bruce, M.; Gibson, V.C.; Kimberley, B.S.; Maddox, P.J.; Mastroianni, S.; McTavish, S.J.; Redshaw, C.; Solan, G.A.; Stromberg, S.; et al. Iron and cobalt ethylene polymerization catalysts bearing 2,6-bis(imino)pyridyl ligands: Synthesis, structures, and polymerization studies. J. Am. Chem. Soc. 1999, 121, 8728–8740. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Mastroianni, S.; Solan, G.A.; Baugh, S.P.D.; Redshaw, C.; Gibson, V.C.; White, A.J.P.; Williams, D.J.; Elsegood, M.R.J. Oligomerisation of ethylene by bis(imino)pyridyliron and -cobalt complexes. Chem. Eur. J. 2000, 6, 2221–2231. [Google Scholar] [CrossRef]
- Knijnenburg, Q.; Gambarotta, S.; Budzelaar, P.H.M. Ligand-centred reactivity in diiminepyridine complexes. Dalton. Trans. 2006, 5442–5448. [Google Scholar] [CrossRef]
- Scott, J.; Gambarotta, S.; Korobkov, I.; Budzelaar, P.H.M. Metal versus ligand alkylation in the reactivity of the (bis-iminopyridinato) Fe catalyst. J. Am. Chem. Soc. 2005, 127, 13019–13029. [Google Scholar] [CrossRef]
- Sugiyama, H.; Aharonian, G.; Gambarotta, S.; Yap, G.P.A.; Budzelaar, P.H.M. Participation of the α,α′-diiminopyridine ligand system in reduction of the metal center during alkylation. J. Am. Chem. Soc. 2002, 124, 12268–12274. [Google Scholar] [CrossRef]
- Bouwkamp, M.W.; Lobkovsky, E.; Chirik, P.J. Bis(imino)pyridine ligand deprotonation promoted by a transient iron amide. Inorg. Chem. 2006, 45, 2–4. [Google Scholar] [CrossRef]
- Smit, T.M.; Tomov, A.K.; Britovsek, G.J.P.; Gibson, V.C.; White, A.J.P.; Williams, D.J. The effect of imine-carbon substituents in bis(imino)pyridine-based ethylene polymerisation catalysts across the transition series. Catal. Sci. Technol. 2012, 2, 643–655. [Google Scholar] [CrossRef]
- McTavish, S.; Britovsek, G.J.P.; Smit, T.M.; Gibson, V.C.; White, A.J.P.; Williams, D.J. Iron-based ethylene polymerization catalysts supported by bis(imino)pyridine ligands: Derivatization via deprotonation/alkylation at the ketimine methyl position. J. Mol. Catal. A-Chem. 2007, 261, 293–300. [Google Scholar] [CrossRef]
- Sun, W.-H.; Kong, S.; Chai, W.; Shiono, T.; Redshaw, C.; Hu, X.; Guo, C.; Hao, X. 2-(1-(Arylimino)ethyl)-8-arylimino-5,6,7- trihydroquinolylcobalt dichloride: Synthesis and polyethylene wax formation. Appl. Catal. A-Gen. 2012, 447–448, 67–73. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, W.; Yue, E.; Liang, T.; Hu, X.; Sun, W.-H. Controlling the molecular weights of polyethylene waxes using the highly active precatalysts of 2-(1-aryliminoethyl)-9-arylimino-5,6,7,8- tetrahydrocycloheptapyridylcobalt chlorides: Synthesis, characterization, and catalytic behavior. Dalton. Trans. 2016, 45, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Ba, J.; Du, S.; Yue, E.; Hu, X.; Flisak, Z.; Sun, W.-H. Constrained formation of 2-(1-(arylimino)ethyl)-7-arylimino-6,6-dimethyl cyclopentapyridines and their cobalt(II) chloride complexes: Synthesis, characterization and ethylene polymerization. RSC. Adv. 2015, 5, 32720–32729. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, W.; Sun, Y.; Hu, X.; Solan, G.A.; Sun, W.-H. Bis(imino)-6,7-dihydro-5H-quinoline-cobalt complexes as highly active catalysts for the formation of vinyl-terminated PE waxes; steps towards inhibiting deactivation pathways through targeted ligand design. New. J. Chem. 2016, 40, 8012–8023. [Google Scholar] [CrossRef]
- Han, M.; Zuo, Z.; Ma, Y.; Solan, G.A.; Hu, X.; Liang, T.; Sun, W.-H. Thermally stable and highly active cobalt precatalysts for vinyl-polyethylenes with narrow polydispersities: Integrating fused-ring and imino-carbon protection into ligand design. RSC. Adv. 2021, 11, 39869–39878. [Google Scholar] [CrossRef]
- Zuo, Z.; Han, M.; Ma, Y.; Solan, G.A.; Hu, X.; Liang, T.; Sun, W.-H. Fluorinated bis(arylimino)-6,7-dihydro-5H-quinoline-cobalt polymerization catalysts: Electronic versus steric modulation in the formation of vinyl-terminated linear PE waxes. Appl. Organomet. Chem. 2022, 36, e6500. [Google Scholar] [CrossRef]
- Du, S.; Zhang, W.; Yue, E.; Huang, F.; Liang, T.; Sun, W.-H. α,α′-Bis(arylimino)-2,3:5,6-bis(pentamethylene)pyridylcobalt chlorides: Synthesis, characterization, and ethylene polymerization behavior. Eur. J. Inorg. Chem. 2016, 11, 1748–1755. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, Y.; Guo, J.; Liu, Q.; Solan, G.A.; Liang, T.; Sun, W.-H. Bis(imino)pyridines fused with 6- and 7-membered carbocylic rings as N,N,N-scaffolds for cobalt ethylene polymerization catalysts. Dalton Trans. 2019, 48, 2582–2591. [Google Scholar] [CrossRef]
- Wang, K.; Wedeking, K.; Zuo, W.; Zhang, D.; Sun, W.-H. Iron(II) and cobalt(II) complexes bearing N-((pyridin-2-yl)methylene) quinolin-8-amine derivatives: Synthesis and application to ethylene oligomerization. J. Organomet. Chem. 2008, 693, 1073–1080. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, S.; Du, S.; Guo, C.-Y.; Hao, X.; Sun, W.-H. 2-(1-(2,4-Bis((di(4-fluorophenyl)methyl)-6- methylphenylimino) ethyl)-6-(1-(arylimino)ethyl)pyridylmetal (iron or cobalt) complexes: Synthesis, characterization, and ethylene polymerization behavior. Macromol. Chem. Phys. 2014, 215, 1797–1809. [Google Scholar] [CrossRef]
- Zhao, W.; Yu, J.; Song, S.; Yang, W.; Liu, H.; Hao, X.; Redshaw, C.; Sun, W.-H. Controlling the ethylene polymerization parameters in iron pre-catalysts of the type 2-[1-(2,4-dibenzhydryl-6-methylphenylimino)ethyl]-6-[1-(arylimino)ethyl] pyridyliron dichloride. Polymer 2012, 53, 130–137. [Google Scholar] [CrossRef]
- Lai, J.; Zhao, W.; Yang, W.; Redshaw, C.; Liang, T.; Liu, Y.; Sun, W.-H. 2-[1-(2,4-dibenzhydryl-6-methylphenylimino)ethyl]- 6-[1-(arylimino)ethyl] pyridylcobalt(II) dichlorides: Synthesis, characterization and ethylene polymerization behavior. Polym. Chem. 2012, 3, 787–793. [Google Scholar] [CrossRef]
- Guo, L.; Zada, M.; Zhang, W.; Vignesh, A.; Zhu, D.; Ma, Y.; Liang, T.; Sun, W.-H. Highly linear polyethylenes tailored with 2,6- bis [1-(p-dibenzo-cycloheptylarylimino)ethyl]- pyridylcobalt dichlorides. Dalton Trans. 2019, 48, 5604–5613. [Google Scholar] [CrossRef]
- Zhang, R.; Han, M.; Oleynik, I.V.; Solan, G.A.; Oleynik, I.I.; Ma, Y.; Liang, T.; Sun, W.-H. Boosting activity, thermostability, and lifetime of iron ethylene polymerization catalysts through gem-dimethyl substitution and incorporation of ortho-cycloalkyl substituents. Appl. Organomet. Chem. 2021, 35, e6376. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, R.; Hao, X.; Sun, W.-H. 2-Oxazoline/benzoxazole-1,10-phenanthrolinylmetal (iron, cobalt or nickel) dichloride: Synthesis, characterization and their catalytic reactivity for the ethylene oligomerization. J. Organomet. Chem. 2008, 693, 3867–3877. [Google Scholar] [CrossRef]
- Kulkarni, N.V.; Elkin, T.; Tumaniskii, B.; Botoshansky, M.; Shimon, L.J.; Eisen, M.S. Asymmetric Bis (formamidinate) Group 4 Complexes: Synthesis, Structure and Their Reactivity in the Polymerization of α-Olefins. Organometallics 2014, 33, 3119–3136. [Google Scholar] [CrossRef]
- Elkin, T.; Kulkarni, N.V.; Tumanskii, B.; Botoshansky, M.; Shimon, L.J.; Eisen, M.S. Synthesis and structure of Group 4 symmetric amidinate complexes and their reactivity in the polymerization of α-olefins. Organometallics 2013, 32, 6337–6352. [Google Scholar] [CrossRef]
- Zhang, Y.; Suo, H.; Huang, F.; Liang, T.; Hu, X.; Sun, W.-H. Thermo-sTable 2-(arylimino)benzylidene-9-arylimino-5,6,7,8- tetrahydrocyclohepta[b]pyridyliron(II) precatalysts toward ethylene polymerization and highly linear polyethylenes. J. Polym. Sci. Pol. Chem. 2016, 55, 830–842. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, N.; Xiang, J.; Solan, G.A.; Suo, H.; Ma, Y.; Liang, T.; Sun, W.-H. Bis-cycloheptyl-fused bis(imino)pyridine-cobalt catalysts for PE wax formation: Positive effects of fluoride substitution on catalytic performance and thermal stability. Dalton Trans. 2020, 49, 9425–9437. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Oleynik, I.I.; Liu, M.; Ma, Y.; Oleynik, I.V.; Solan, G.A.; Liang, T.; Sun, W.-H. Ring size enlargement in an ortho-cycloalkyl-substituted bis(imino)pyridine-cobalt ethylene polymerization catalyst and its impact on performance and polymer properties. Appl. Organomet. Chem. 2022, 36, e6529. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, W.; Cao, F.; Jiang, Y.; Zhang, R.; Ma, Y.; Solan, G.A.; Sun, Y.; Sun, W.-H. Remote dibenzocycloheptyl substitution on a bis(arylimino)pyridyl-iron ethylene polymerization catalyst; enhanced thermal stability and unexpected effects on polymer properties. Polym. Chem. 2021, 12, 4214–4225. [Google Scholar] [CrossRef]
- Martinez-Romo, A.; Gonzalez-Mota, R.; Soto-Bernal, J.J.; Rosales-Candelas, I. Investigating the degradability of HDPE, LDPE, PE-BIO, and PE-OXO films under UV-B radiation. J. Spectrosc. 2015, 2015, 586514. [Google Scholar] [CrossRef]
- Glenz, W.; Peterlin, A. IR-studies of drawn polyethylene part III. The orientation of vinyl and methyl end-groups. Makromol. Chem. 1971, 150, 163–177. [Google Scholar] [CrossRef]
- Jung, M.R.; Horgen, F.D.; Orski, S.V.; Rodriguez, V.; Beers, C.K.L.; Balazs, G.H.; Jones, T.T.; Work, T.M.; Brignac, K.C.; Royer, S.-J.; et al. Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar. Pollut. Bull. 2018, 127, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Kennedy, A.R.; Nelson, D.J. Synthesis and characterisation of an N-heterocyclic carbene with spatially-defined steric impact. Dalton Trans. 2016, 45, 11772–11780. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, W.; Oleynik, I.I.; Solan, G.A.; Oleynik, I.V.; Liang, T.; Sun, W.-H. Probing the effect of ortho-cycloalkyl ring size on activity and thermostability in cycloheptyl-fused N,N,N-iron ethylene polymerization catalysts. Dalton Trans. 2020, 49, 136–146. [Google Scholar] [CrossRef]
- Huang, F.; Xing, Q.; Liang, T.; Flisak, Z.; Ye, B.; Hu, X.; Yang, W.; Sun, W.-H. 2-(1-aryliminoethyl)-9-arylimino-5,6,7,8- tetrahydrocycloheptapyridyl iron(II) dichloride: Synthesis, characterization, and the highly active and tunable active species in ethylene polymerization. Dalton Trans. 2014, 43, 16818–16829. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal- structure determination. Acta. Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta. Cryst. 2015, C71, 3–8. [Google Scholar]
- Spek, L. Bis-cycloheptyl-fused bis(imino)pyridine-cobalt catalysts for PE wax formation: Positive effects of fluoride substitution on catalytic performance and thermal stability. Acta. Cryst. D-Biol. Cryst. 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
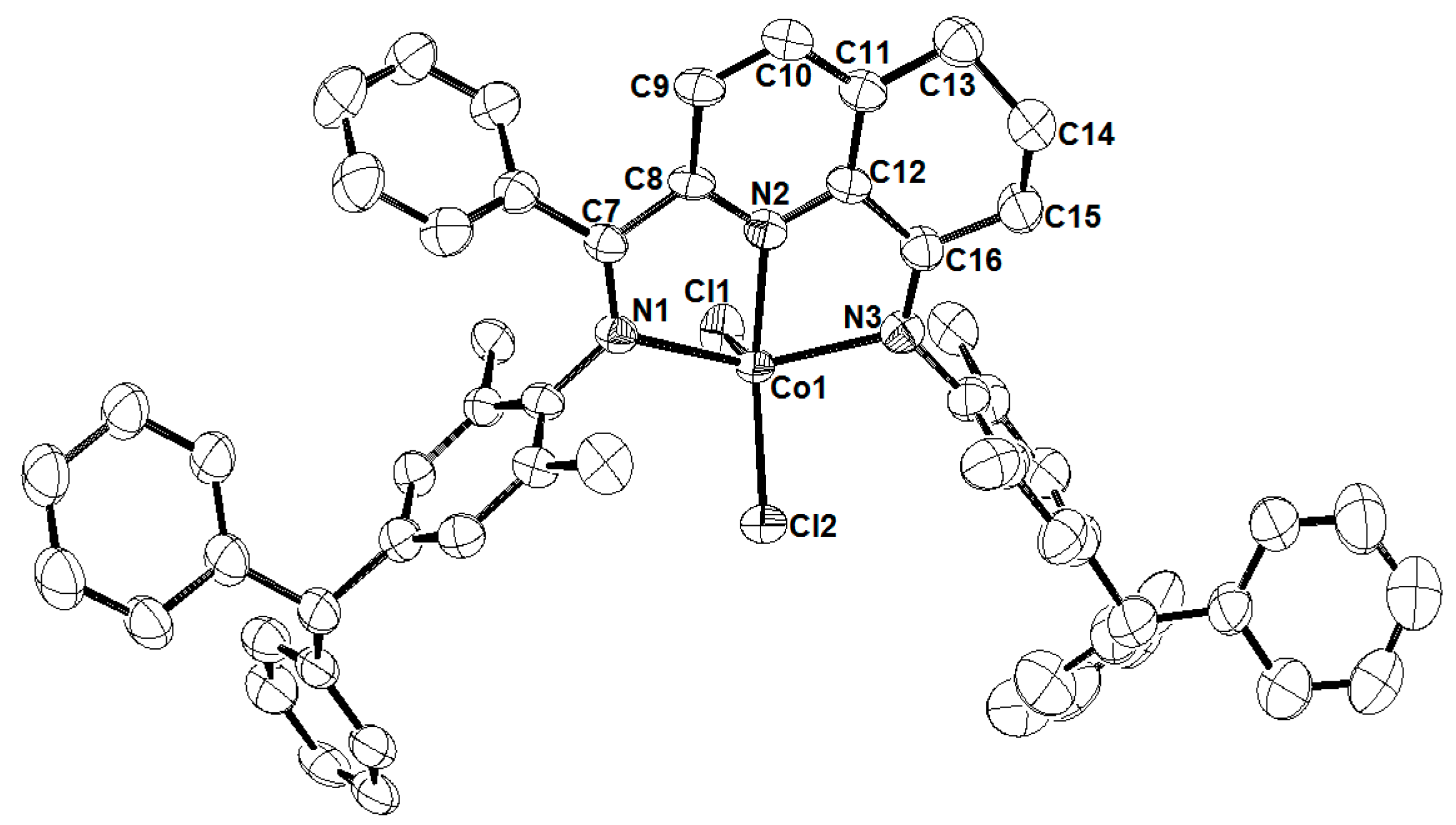



| Co2 | Co5 | |
|---|---|---|
| Bond lengths (Å) | ||
| Co(1)–Cl(2) | 2.239(3) | 2.1984(10) |
| Co(1)–Cl(1) | 2.256(3) | 2.2914(10) |
| Co(1)–N(2) | 2.051(8) | 2.051(2) |
| Co(1)–N(1) | 2.295(7) | 2.310(3) |
| Co(1)–N(3) | 2.258(8) | 2.214(2) |
| N(2)–C(12) | 1.368(12) | 1.343(4) |
| N(2)–C(8) | 1.349(11) | 1.335(4) |
| N(1)–C(7) | 1.286(11) | 1.288(4) |
| N(3)–C(16) | 1.242(12) | 1.285(4) |
| Bond angles (°) | ||
| Cl(1)–Co(1)–Cl(2) | 117.10(13) | 117.32(4) |
| N(1)–Co(1)–N(3) | 145.4(3) | 146.07(9) |
| N(1)–Co(1)–Cl(1) | 99.1(2) | 99.37(7) |
| N(1)–Co(1)–Cl(2) | 97.35(2) | 99.04(7) |
| N(2)–Co(1)–Cl(1) | 103.6(2) | 92.64(8) |
| N(2)–Co(1)–Cl(2) | 139.3(2) | 150.02(8) |
| N(2)–Co(1)–N(3) | 74.7(3) | 75.42(9) |
| N(1)–Co(1)–N(2) | 73.5(3) | 73.10(9) |
| N(3)–Co(1)–Cl(1) | 101.2(2) | 94.35(7) |
| N(3)–Co(1)–Cl(2) | 97.9(2) | 101.91(7) |
| Entry | T (°C) | Al:Co | t (min) | Mass of PE (g) | Activity b | Tm c (°C) | Mw d | Mw/Mn d | Ri e | Rt f |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 30 | 2000 | 30 | 2.3 | 2.3 | 123.9 | 1.5 | 1.5 | 164.0 | 4600.0 |
| 2 | 40 | 2000 | 30 | 3.7 | 3.7 | 123.6 | 1.6 | 1.8 | 263.8 | 8325.0 |
| 3 | 50 | 2000 | 30 | 6.9 | 6.9 | 122.6 | 1.7 | 1.5 | 492.0 | 12,176.5 |
| 4 | 60 | 2000 | 30 | 6.1 | 6.1 | 120.2 | 2.4 | 1.7 | 434.9 | 8641.7 |
| 5 | 70 | 2000 | 30 | 2.9 | 2.9 | 122.8 | 2.2 | 1.5 | 206.8 | 3954.5 |
| 6 | 50 | 1750 | 30 | 6.3 | 6.3 | 121.9 | 1.5 | 2.1 | 449.2 | 17,640.0 |
| 7 | 50 | 2250 | 30 | 6.5 | 6.5 | 122.1 | 1.6 | 1.5 | 463.5 | 12,187.5 |
| 8 | 50 | 2500 | 30 | 6.5 | 6.5 | 121.5 | 1.6 | 1.5 | 463.5 | 12,187.5 |
| 9 | 50 | 2750 | 30 | 6.6 | 6.6 | 121.8 | 1.6 | 1.6 | 470.6 | 13,200.0 |
| 10 | 50 | 3000 | 30 | 5.6 | 5.6 | 121.8 | 1.5 | 2.0 | 399.3 | 14,933.3 |
| 11 | 50 | 4000 | 30 | 5.5 | 5.5 | 122.5 | 1.3 | 1.4 | 392.2 | 11,846.2 |
| 12 | 50 | 2000 | 5 | 3.9 | 23.1 | 118.9 | 1.3 | 1.4 | 1668.4 | 50,400.0 |
| 13 | 50 | 2000 | 15 | 4.8 | 7.7 | 120.5 | 1.5 | 1.5 | 684.5 | 19,200.0 |
| 14 | 50 | 2000 | 45 | 6.3 | 4.2 | 121.1 | 1.7 | 1.6 | 299.5 | 7905.9 |
| 15 | 50 | 2000 | 60 | 8.2 | 4.1 | 123.2 | 1.9 | 1.7 | 292.3 | 7336.8 |
| 16 g | 50 | 2000 | 30 | 2.1 | 2.1 | 122.4 | 1.1 | 1.3 | 149.7 | 4963.6 |
| 17 h | 50 | 2000 | 30 | - | - | - | - | - | - | - |
| Entry | Precat. | Mass of PE (g) | Activity b | Tm c (°C) | Mw d | Mw/Mn d | Ri e | Rt f |
|---|---|---|---|---|---|---|---|---|
| 1 | Co1 | 5.1 | 5.1 | 133.9 | 68.2 | 2.7 | 363.6 | 403.8 |
| 2 | Co2 | 16.5 | 16.5 | 123.9 | 3.3 | 1.4 | 1176.5 | 14,000.0 |
| 3 | Co3 | 5.0 | 5.0 | 132.5 | 55.4 | 1.4 | 356.5 | 252.7 |
| 4 | Co4 | 3.9 | 3.9 | 132.4 | 62.8 | 2.8 | 278.1 | 347.8 |
| 5 | Co5 | 3.8 | 3.8 | 134.7 | 198.7 | 1.7 | 270.9 | 65.0 |
| 6 | Co6 | 6.9 | 6.9 | 122.6 | 1.7 | 1.5 | 492.0 | 12,176.5 |
| Entry | T (°C) | Al:Co | t (min) | Mass of PE (g) | Activity b | Tm c (°C) | Mw d | Mw/Mn d | Ri e | Rt f |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 30 | 2000 | 30 | 1.9 | 1.9 | 123.1 | 3.7 | 1.1 | 135.5 | 1129.7 |
| 2 | 40 | 2000 | 30 | 2.4 | 2.4 | 121.7 | 2.4 | 1.3 | 171.1 | 2600.0 |
| 3 | 50 | 2000 | 30 | 2.2 | 2.2 | 121.5 | 2.1 | 1.3 | 156.9 | 2723.8 |
| 4 | 60 | 2000 | 30 | 1.5 | 1.5 | 120.6 | 5.0 | 1.7 | 107.0 | 1020.0 |
| 5 | 40 | 2250 | 30 | 5.4 | 5.4 | 122.0 | 2.7 | 1.3 | 385.0 | 5200.0 |
| 6 | 40 | 2500 | 30 | 2.5 | 2.5 | 121.9 | 2.4 | 1.3 | 178.3 | 2708.3 |
| 7 | 40 | 2750 | 30 | 1.5 | 1.5 | 122.9 | 2.6 | 1.3 | 107.0 | 1500.0 |
| 8 | 40 | 3000 | 30 | 1.1 | 1.1 | 122.4 | 2.8 | 1.4 | 78.4 | 1100.0 |
| 9 | 40 | 2250 | 5 | 3.3 | 19.8 | 119.9 | 1.8 | 1.3 | 1411.8 | 28,600.0 |
| 10 | 40 | 2250 | 15 | 4.2 | 8.4 | 121.6 | 2.6 | 1.3 | 598.9 | 8400.0 |
| 11 | 40 | 2250 | 45 | 7.9 | 5.2 | 121.8 | 2.8 | 1.3 | 375.5 | 4890.5 |
| 12 | 40 | 2250 | 60 | 10.2 | 5.1 | 122.2 | 6.1 | 2.1 | 363.6 | 3511.5 |
| 13 g | 40 | 2250 | 30 | 1.2 | 1.2 | 121.5 | 1.1 | 1.3 | 85.6 | 2836.4 |
| 14 h | 40 | 2250 | 30 | - | - | - | - | - | - | - |
| Entry | Precat. | Mass of PE (g) | Activity b | Tm c (°C) | Mw d | Mw/Mn d | Ri e | Rt f |
|---|---|---|---|---|---|---|---|---|
| 1 | Co1 | 4.3 | 4.3 | 133.5 | 81.6 | 1.8 | 306.6 | 189.7 |
| 2 | Co2 | 11.1 | 11.1 | 123.4 | 3.6 | 1.5 | 791.4 | 9250.0 |
| 3 | Co3 | 4.1 | 4.1 | 134.1 | 138.5 | 3.1 | 292.3 | 183.5 |
| 4 | Co4 | 3.7 | 3.7 | 134.4 | 150.2 | 2.9 | 263.8 | 142.9 |
| 5 | Co5 | 3.1 | 3.1 | 134.7 | 386.6 | 2.3 | 221.0 | 36.9 |
| 6 | Co6 | 5.4 | 5.4 | 122.0 | 2.7 | 1.3 | 385.0 | 5200.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, Z.; Zhang, Q.; Han, M.; Liu, M.; Sun, Y.; Ma, Y.; Sun, W.-H. 2-(Arylimino)benzylidene-8-arylimino-5,6,7-trihydroquinoline Cobalt(II) Dichloride Polymerization Catalysts for Polyethylenes with Narrow Polydispersity. Catalysts 2022, 12, 1119. https://doi.org/10.3390/catal12101119
Zuo Z, Zhang Q, Han M, Liu M, Sun Y, Ma Y, Sun W-H. 2-(Arylimino)benzylidene-8-arylimino-5,6,7-trihydroquinoline Cobalt(II) Dichloride Polymerization Catalysts for Polyethylenes with Narrow Polydispersity. Catalysts. 2022; 12(10):1119. https://doi.org/10.3390/catal12101119
Chicago/Turabian StyleZuo, Zheng, Qiuyue Zhang, Mingyang Han, Ming Liu, Yang Sun, Yanping Ma, and Wen-Hua Sun. 2022. "2-(Arylimino)benzylidene-8-arylimino-5,6,7-trihydroquinoline Cobalt(II) Dichloride Polymerization Catalysts for Polyethylenes with Narrow Polydispersity" Catalysts 12, no. 10: 1119. https://doi.org/10.3390/catal12101119
APA StyleZuo, Z., Zhang, Q., Han, M., Liu, M., Sun, Y., Ma, Y., & Sun, W.-H. (2022). 2-(Arylimino)benzylidene-8-arylimino-5,6,7-trihydroquinoline Cobalt(II) Dichloride Polymerization Catalysts for Polyethylenes with Narrow Polydispersity. Catalysts, 12(10), 1119. https://doi.org/10.3390/catal12101119










