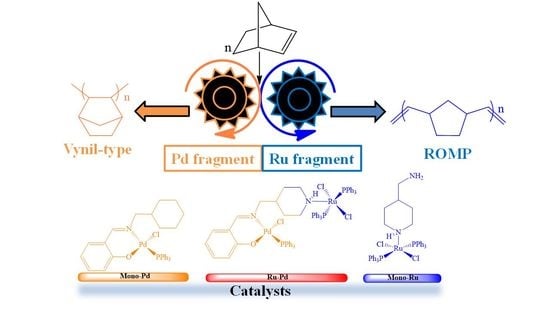
Ru/Pd Complex and Its Monometallic Fragments as Catalysts for Norbornene Polymerization via ROMP and Addition
Abstract
1. Introduction
2. Results and Discussion
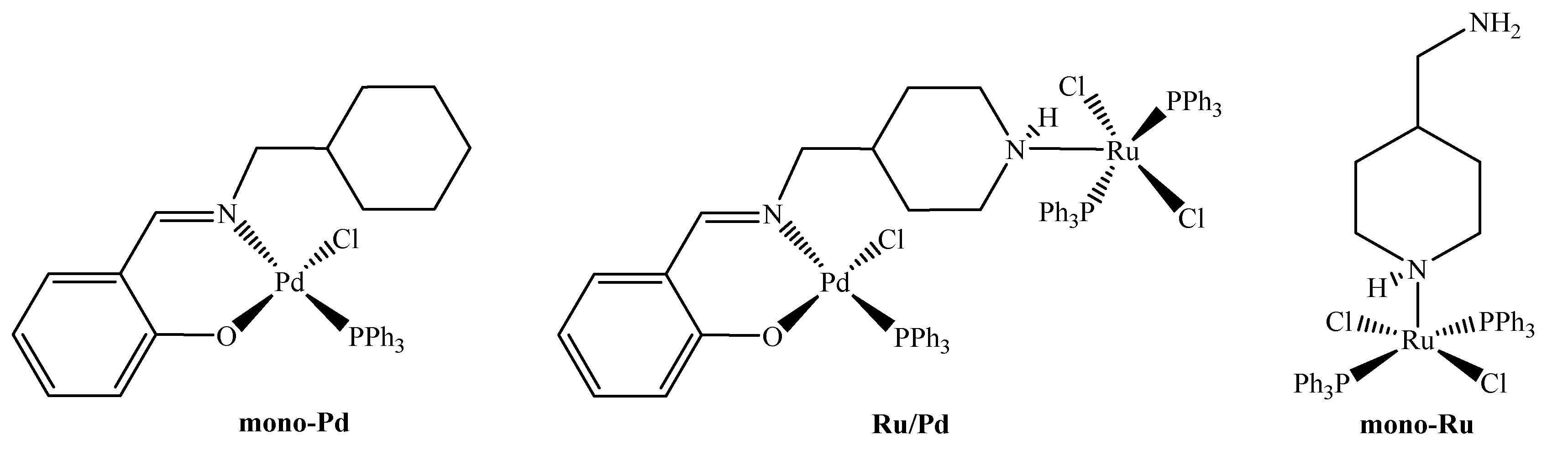
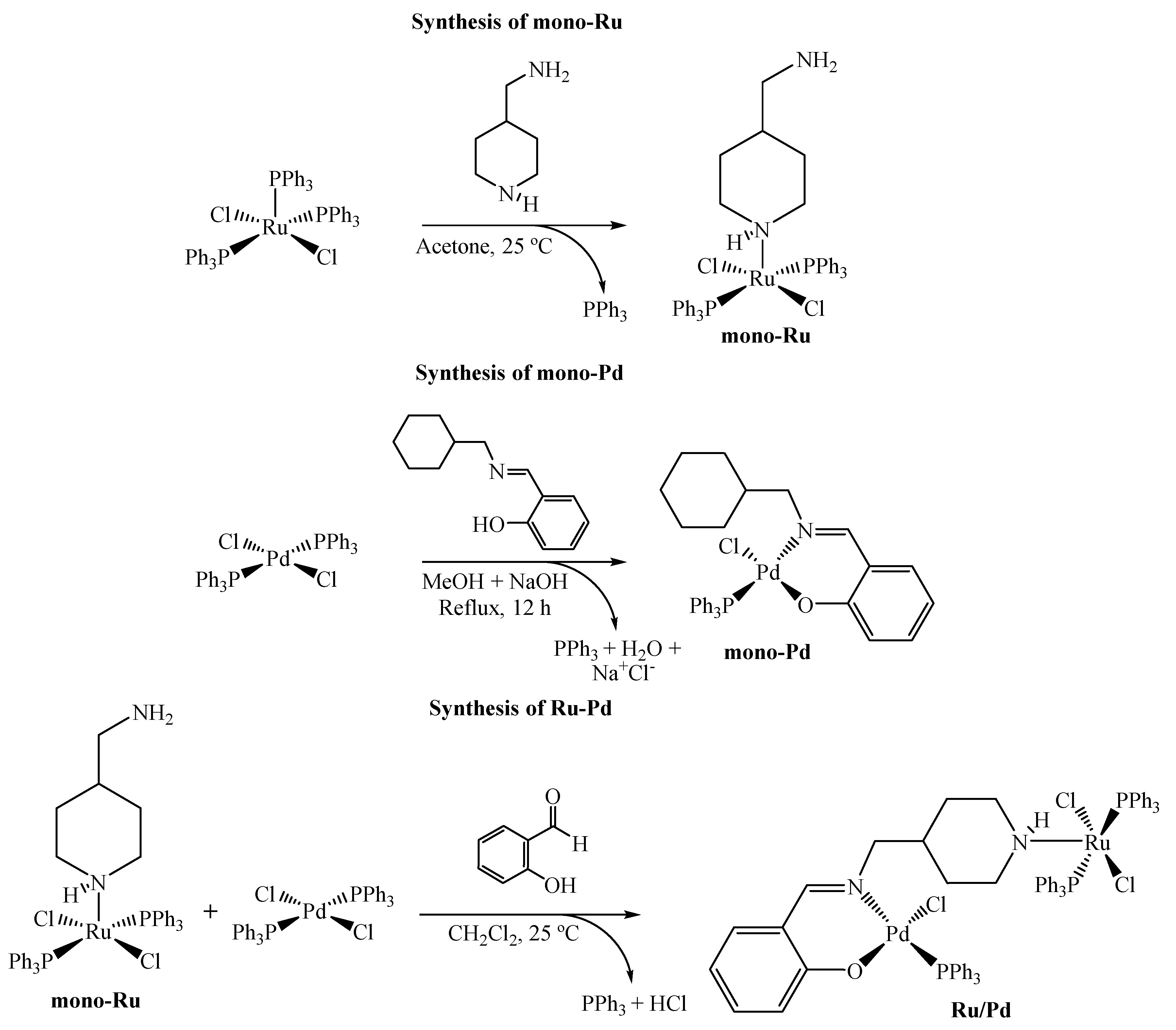
2.1. Synthesis and Characterization
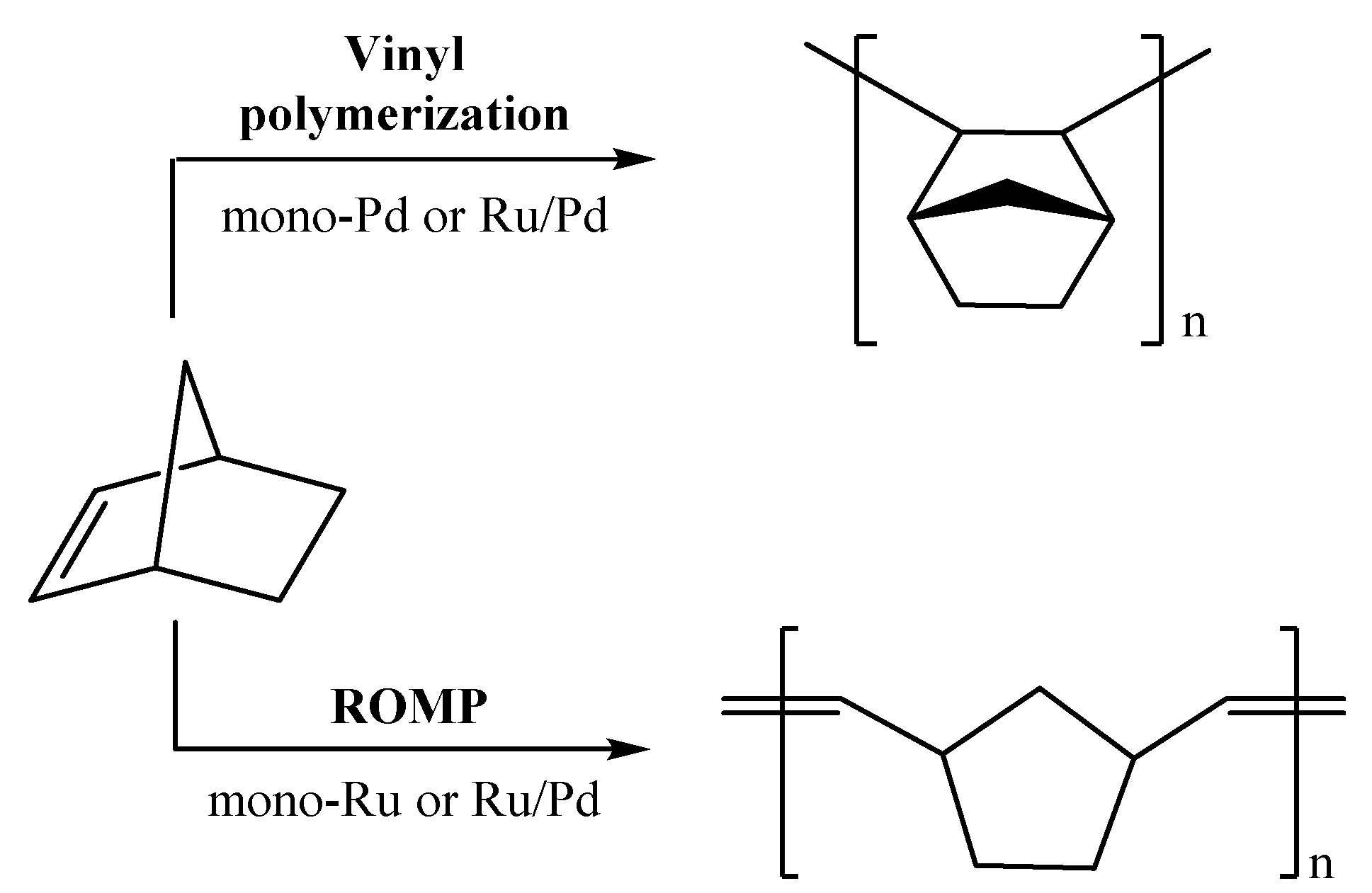
2.2. Polymerization Reactions
2.2.1. Vinyl Polymerization Reactions
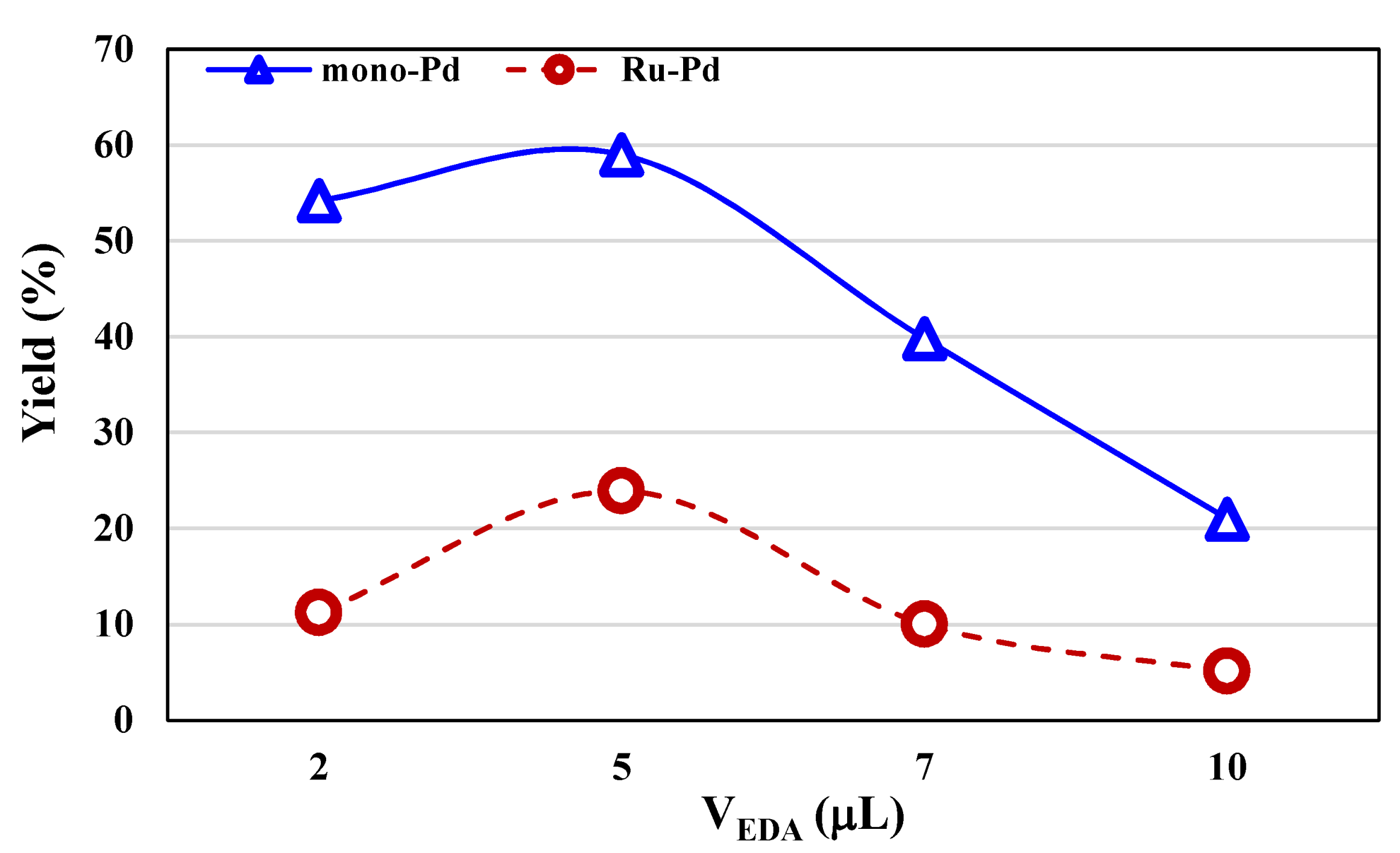
2.2.2. ROMP Reactions
3. Experimental Section
3.1. General Remarks
3.2. Analyses
3.3. Computation Details
3.4. ROMP Procedure
3.5. Vinyl Polymerization Procedure
3.6. Synthesis of [RuCl2(PPh3)2(piperidine-4(aminomethyl)] (mono-Ru)
3.7. Synthesis of [PdCl(PPh3)(N,O-Schiff)] (mono-Pd)
3.8. Synthesis of [RuCl2(PPh3)2](μ-Schiff)Pd(PPh3)Cl] (Ru/Pd)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bender, M.L. Mechanism of Homogeneous Catalysis from Proton to Proteins; Wiley: New York, NY, USA, 1971; pp. 19–320. [Google Scholar]
- Collman, J.P. Patterns of organometallic reactions related to homogeneous catalysis. Acc. Chem. Res. 1968, 1, 136–143. [Google Scholar] [CrossRef]
- Singh, K.S. Recent advances in C–H bond functionalization with ruthenium-based catalysts. Catalysts 2019, 9, 173. [Google Scholar] [CrossRef]
- Montgomery, T.P.; Johns, A.M.; Grubbs, R.H. Recent advancements in stereoselective olefin metathesis using ruthenium catalysts. Catalysts 2017, 7, 87. [Google Scholar] [CrossRef]
- Clapham, S.E.; Hadzovic, A.; Morris, R.H. Mechanisms of the H2-hydrogenation and transfer hydrogenation of polar bonds catalyzed by ruthenium hydride complexes. Coord. Chem. Rev. 2004, 248, 2201–2237. [Google Scholar] [CrossRef]
- Bellina, F.; Carpita, A.; Rossi, R. Palladium catalysts for the Suzuki cross-coupling reaction: An overview of recent advances. Synthesis 2004, 2004, 2419–2440. [Google Scholar] [CrossRef]
- Seki, M. Recent advances in Pd/C-catalyzed coupling reactions. Synthesis 2006, 2006, 2975–2992. [Google Scholar] [CrossRef]
- Kim, A.N.; Stoltz, B.M. Recent advances in homogeneous catalysts for the asymmetric hydrogenation of heteroarenes. ACS Catal. 2020, 10, 13834–13851. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.D.; Moorhouse, R.A.; Urbin, S.A.; White, P.S.; Brookhart, M. γ-agostic species as key intermediates in the vinyl addition polymerization of norbornene with cationic (allyl) Pd catalysts: Synthesis and mechanistic insights. J. Am. Chem. Soc. 2009, 131, 9055–9069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J. Recent developments in Pd-catalyzed reactions of diazo compounds. Eur. J. Organ. Chem. 2011, 2011, 1015–1026. [Google Scholar] [CrossRef]
- Gois, P.D.D.S.; Maia, J.I.P.; Masson, G.H.C.; Martins, D.M.; Machado, A.E.D.H.; Goi, B.E.; Carvalho, V.P.D., Jr. Monometallic and heterobimetallic ruthenium (II) and palladium (II) complexes based on a pyridine-hydrazone ligand as bifunctional catalysts for ROMP of norbornene and ethylene polymerization. Appl. Organomet. Chem. 2022, 36, 6491. [Google Scholar] [CrossRef]
- Lin, S.-C.A.; Liu, Y.-H.; Peng, S.-M.; Liu, S.-T. Hetero-bimetallic complexes based on an anthyridine ligand preparation and catalytic activity. Organometallics 2019, 39, 123–131. [Google Scholar] [CrossRef]
- Bitzer, M.J.; Kühn, F.E.; Baratta, W. Tandem Suzuki–Miyaura/transfer hydrogenation reaction catalyzed by a Pd–Ru complex bearing an anionic dicarbene. J. Catal. 2016, 338, 222–226. [Google Scholar] [CrossRef]
- Sabater, S.; Mata, J.A.; Peris, E. Hydrodefluorination of carbon–fluorine bonds by the synergistic action of a ruthenium–palladium catalyst. Nat. Commun. 2013, 4, 1–7. [Google Scholar] [CrossRef]
- Mata, J.A.; Hahn, F.E.; Peris, E. Heterometallic complexes, tandem catalysis and catalytic cooperativity. Chem. Sci. 2014, 5, 1723. [Google Scholar] [CrossRef]
- Sun, W.-H.; Yang, H.; Li, Z.; Li, Y. Vinyl polymerization of norbornene with neutral salicylaldiminato nickel (II) complexes. Organometallics 2003, 22, 3678–3683. [Google Scholar] [CrossRef]
- Calderon, N. Ring-opening polymerization of cycloolefins. J. Macromol. Sci. Rev. Macromol. Chem. 1972, 7, 105–159. [Google Scholar] [CrossRef]
- Grubbs, R.H. Handbook of Metathesis; Wiley-VCH: Weinheim, Germany, 2003; Volume 3. [Google Scholar]
- Sutthasupa, S.; Shiotsuki, M.; Sanda, F. Recent advances in ring-opening metathesis polymerization, and application to synthesis of functional materials. Polym. J. 2010, 42, 905–915. [Google Scholar] [CrossRef]
- Janiak, C.; Paul, G.L. The vinyl homopolymerization of norbornene. Macromol. Rapid Commun. 2001, 22, 479–493. [Google Scholar] [CrossRef]
- Mathew, J.P.; Reinmuth, A.; Melia, J.; Swords, A.N.; Risse, W. (η3-allyl) palladium (II) and palladium (II) nitrile catalysts for the addition polymerization of norbornene derivatives with functional groups. Macromolecules 1996, 29, 2755–2763. [Google Scholar] [CrossRef]
- Liu, H.; Haibin, Y.; Xiaochao, S. Synthesis of nickel and palladium complexes with diarylamido-based unsymmetrical pincer ligands and application for norbornene polymerization. Dalton Transact. 2019, 48, 609–617. [Google Scholar] [CrossRef]
- Fonseca, L.R.; Sá, J.L.S.; Carvalho, V.P.; Lima-Neto, B.S. Cross-link in norbornadiene-based polymers from ring-opening metathesis polymerization with pyrrolidine-based Ru complex. Polym. Bull. 2018, 75, 3705–3721. [Google Scholar] [CrossRef]
- Silva, R.A.N.; Borim, P.; Fonseca, L.R.; Lima-Neto, B.S.; Sá, J.L.S.; Carvalho, V., Jr. Non-carbene complex [RuCl2(PPh3)2(azocane)] as active catalyst precursor for ROMP and ATRP. Catal. Lett. 2017, 147, 1144–1152. [Google Scholar] [CrossRef]
- Afonso, M.B.A.; Cruz, T.R.; Silva, Y.F.; Pereira, J.C.A.; Machado, A.E.; Goi, B.E.; Carvalho, V.P., Jr. Ruthenium (II) complexes of Schiff base derived from cycloalkylamines as pre-catalysts for ROMP of norbornene and ATRP of methyl methacrylate. J. Organomet. Chem. 2017, 851, 225–234. [Google Scholar] [CrossRef]
- Chaves, H.K.; Ferraz, C.P.; Carvalho, V., Jr.; Lima-Neto, B.S. Tuning the activity of alternative Ru-based initiators for ring-opening metathesis polymerization of norbornene and norbornadiene by the substituent in 4-CH2R-piperidine. J. Mol. Catal. A Chem. 2014, 385, 46–53. [Google Scholar] [CrossRef]
- Cruz, T.R.; Silva, R.A.; Machado, A.E.; Lima-Neto, B.S.; Goi, B.E.; Carvalho, V.P. New dmso–ruthenium catalysts bearing N-heterocyclic carbene ligands for the ring-opening metathesis of norbornene. New J. Chem. 2019, 43, 6220–6227. [Google Scholar] [CrossRef]
- Masson, G.H.C.; Cruz, T.R.; Gois, P.D.S.; Martins, D.M.; Lima-Neto, B.S.; Oliveira, G.S.; Machado, A.E.H.; Bernardo-Gusmão, K.; Goi, B.E.; Carvalho, V.P., Jr. Ruthenium–nickel heterobimetallic complex as a bifunctional catalyst for ROMP of norbornene and ethylene polymerization. New J. Chem. 2021, 45, 11466–11473. [Google Scholar] [CrossRef]
- Matos, J.M.E.; Lima-Neto, B.S. Piperidine as ancillary ligand in the novel [RuCl2(PPh3)2(piperidine)] complex for metathesis polymerization of norbornene and norbornadiene. J. Mol. Catal. A Chem. 2004, 222, 81–85. [Google Scholar] [CrossRef]
- Fernandes, R.J.; Silva, T.B.; Lima-Neto, B.S.; Haiduke, R.L. Structural and kinetic insights into the mechanism for ring opening metathesis polymerization of norbornene with [RuCl2(PPh3)2(piperidine)] as initiator complex. J. Mol. Catal. A Chem. 2015, 410, 58–65. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, M.; Zhang, Y. Amino-salicylaldimine–palladium(II) complexes: New and efficient catalysts for Suzuki and Heck reactions. Inorg. Chem. Commun. 2010, 13, 81–85. [Google Scholar] [CrossRef]
- Agrahari, B.; Layek, S.; Ganguly, R.; Pathak, D.D. Synthesis, crystal structures, and application of two new pincer type palladium (II)-Schiff base complexes in CC cross-coupling reactions. Inorg. Chim. Acta 2018, 471, 345–354. [Google Scholar] [CrossRef]
- Kennedy, J.P.; Makowski, H.S.J. Carbonium ion polymerization of norbornene and its derivatives. J. Macromol. Sci. Chem. 1967, 1, 345. [Google Scholar] [CrossRef]
- Barnes, D.A.; Benedikt, G.M.; Goodall, B.L.; Hung, S.S.; Kalamarides, H.A.; Lenhard, S.; McIntosh, L.H.; Selvy, K.T.; Shick, R.A.; Rhodes, L.F. Addition polymerization of norbornene-type monomers using neutral nickel complexes containing fluorinated aryl ligands. Macromolecules 2003, 36, 2623–2632. [Google Scholar] [CrossRef]
- Patil, A.O.; Zushma, S.; Stibrany, R.T.; Rucker, S.P.; Wheeler, L.M. Vinyl-type polymerization of norbornene by nickel(II) bisbenzimidazole catalysts. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 2095–2106. [Google Scholar] [CrossRef]
- Zhao, C.; Ribeiro, M.R.; de Pinho, M.N.; Subrahmanyam, V.S.; Gil, C.L.; de Lima, A.P. Structural characteristics and gas permeation properties of polynorbornenes with retained bicyclic structure. Polymer 2001, 42, 2455–2462. [Google Scholar] [CrossRef]
- Carvalho-Jr, V.; Ferraz, C.P.; Lima-Neto, B.S. Electronic synergism in [RuCl2(PPh3)2(amine)] complexes differing the reactivity for ROMP of norbornene and norbornadiene. J. Mol. Catal. A Chem. 2010, 333, 46–53. [Google Scholar] [CrossRef]
- Grela, K. (Ed.) Olefin Metathesis: Theory and Practice, 1st ed.; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Slugovc, C. The ring opening metathesis polymerisation toolbox. Macromol. Rapid Commun. 2004, 25, 1283–1297. [Google Scholar] [CrossRef]
- Hallman, P.S.; Stephenson, T.A.; Wilkinson, G. Tetrakis(triphenylphosphine)dichlororuthenium(II) and Tris(triphenylphosphine)dichlororuthenium(II). Inorg. Synth. 1970, 12, 237. [Google Scholar]
- Zahrtmann, N.; Claver, C.; Godard, C.; Riisager, A.; Garcia-Suarez, E.J. Selective oxidative carbonylation of aniline to diphenylurea with ionic liquids. Chem. Cat. Chem. 2018, 10, 2450–2457. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Godbout, N.; Salahub, D.R.; Andzelm, J.; Wimmer, E. Optimization of Gaussian-type basis sets for local spin density functional calculations. Part I. Boron through neon, optimization technique and validation. Can. J. Chem. 1992, 70, 560. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cancès, E. The IEF version of the PCM solvation method: An overview of a new method addressed to study molecular solutes at the QM ab initio level. J. Mol. Struct. Theochem. 1999, 464, 211–226. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Tenderholt, A.L.; Langner, K.M. cclib: A library for package-independent computational chemistry algorithms. Comp. Chem. 2007, 29, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Falivene, L.; Cao, Z.; Petta, A.; Serra, L.; Poater, A.; Oliva, R.; Cavallo, L. Towards the online computer-aided design of catalytic pockets. Nat. Chem. 2019, 1, 872–879. [Google Scholar] [CrossRef] [PubMed]
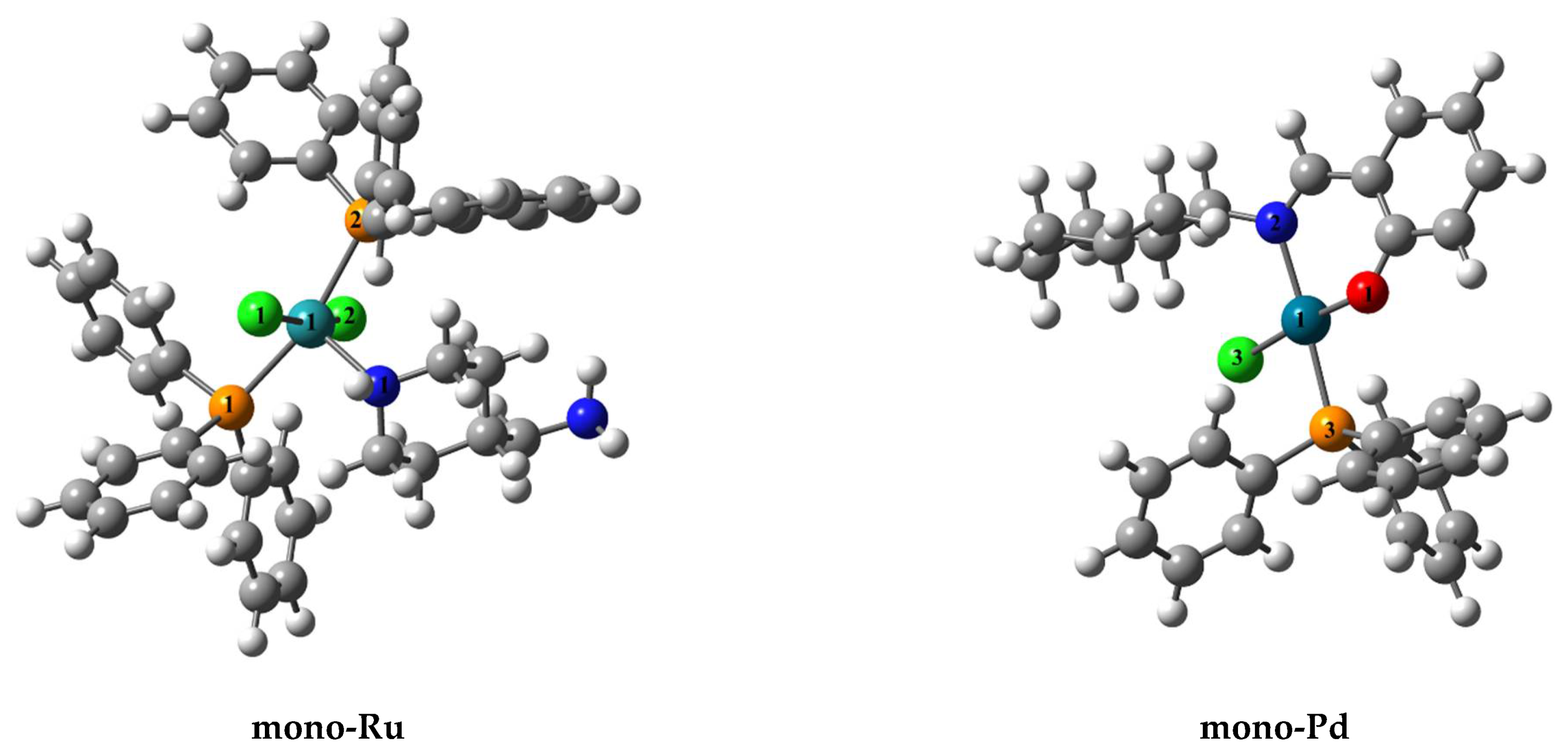
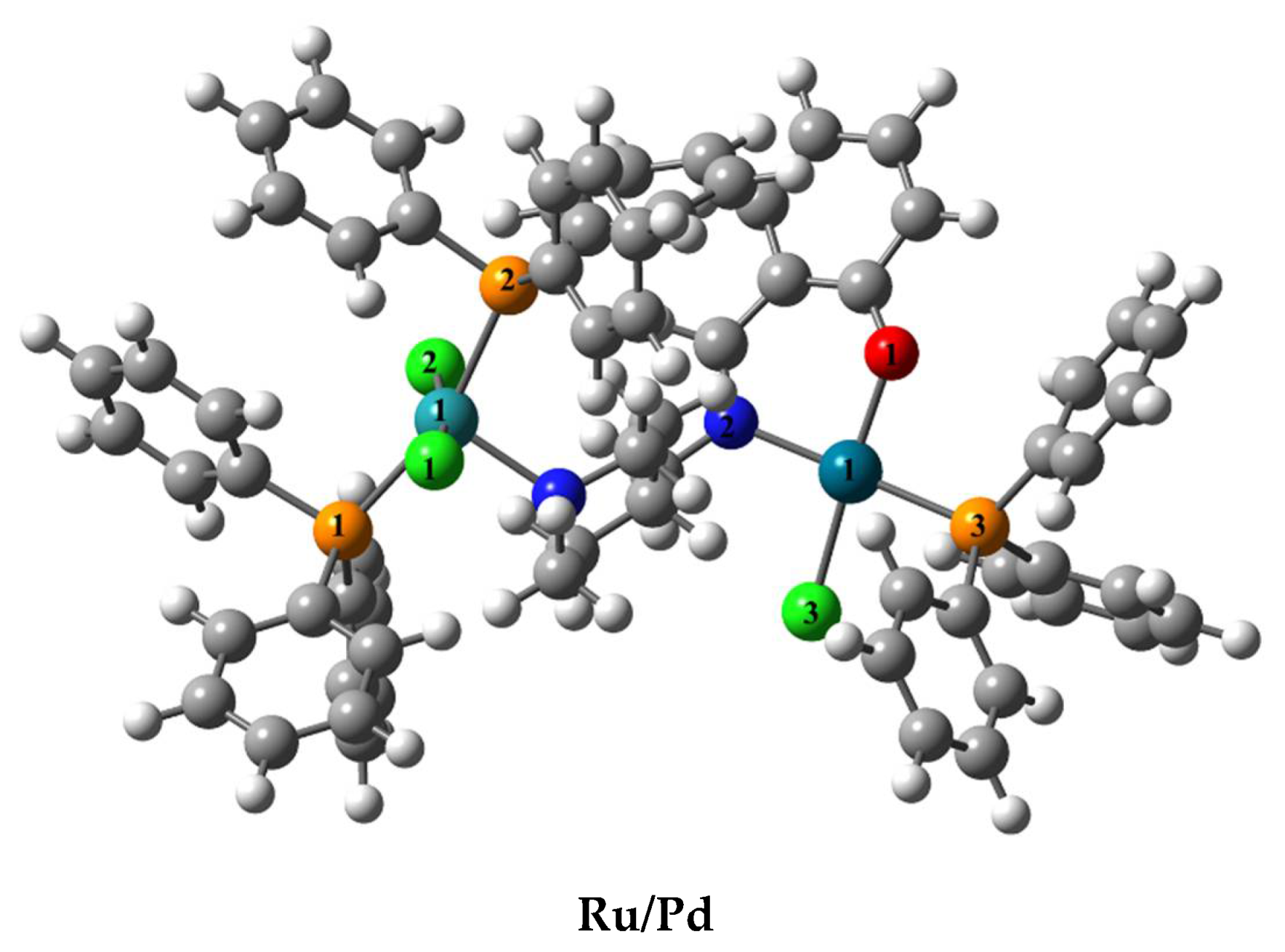
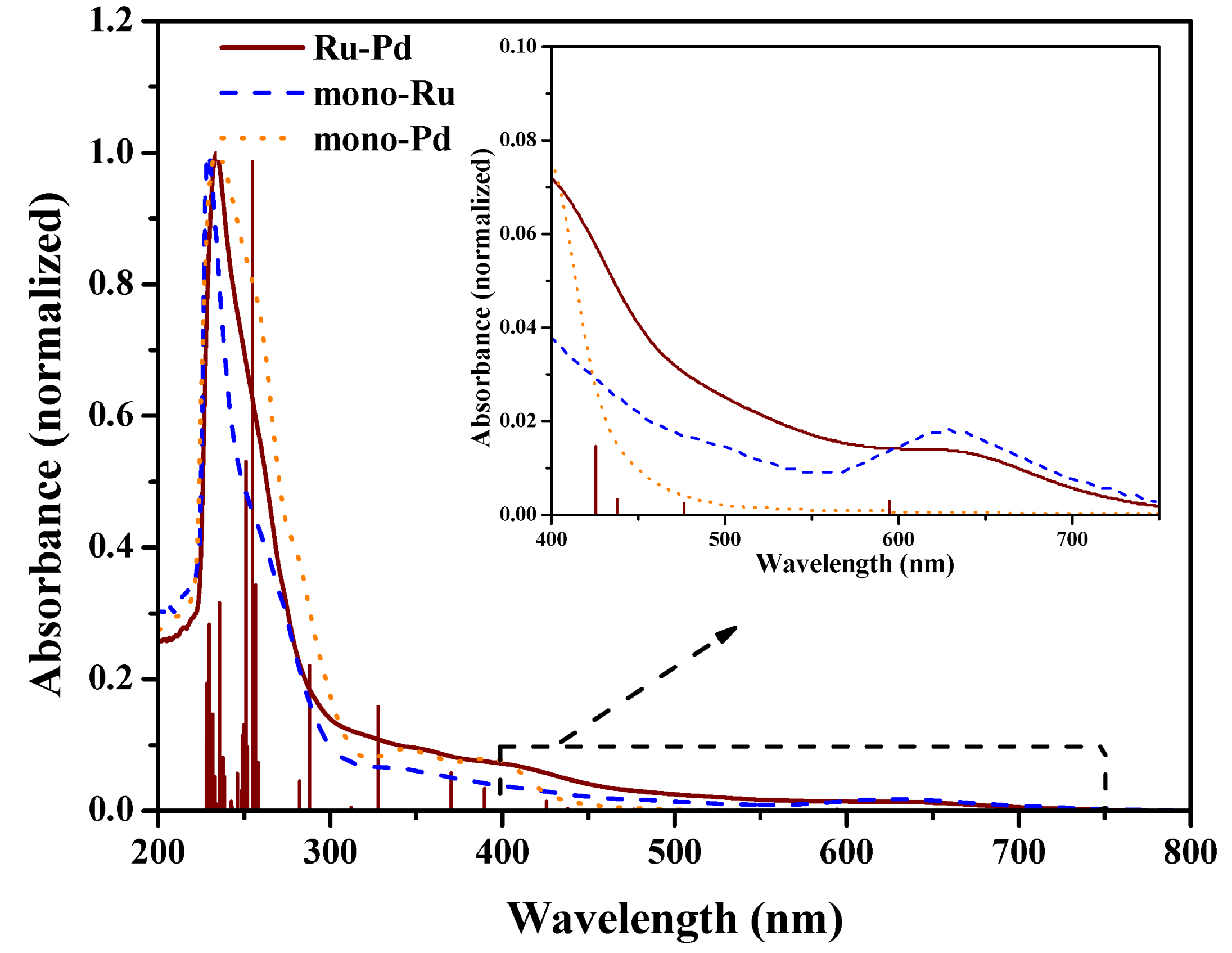
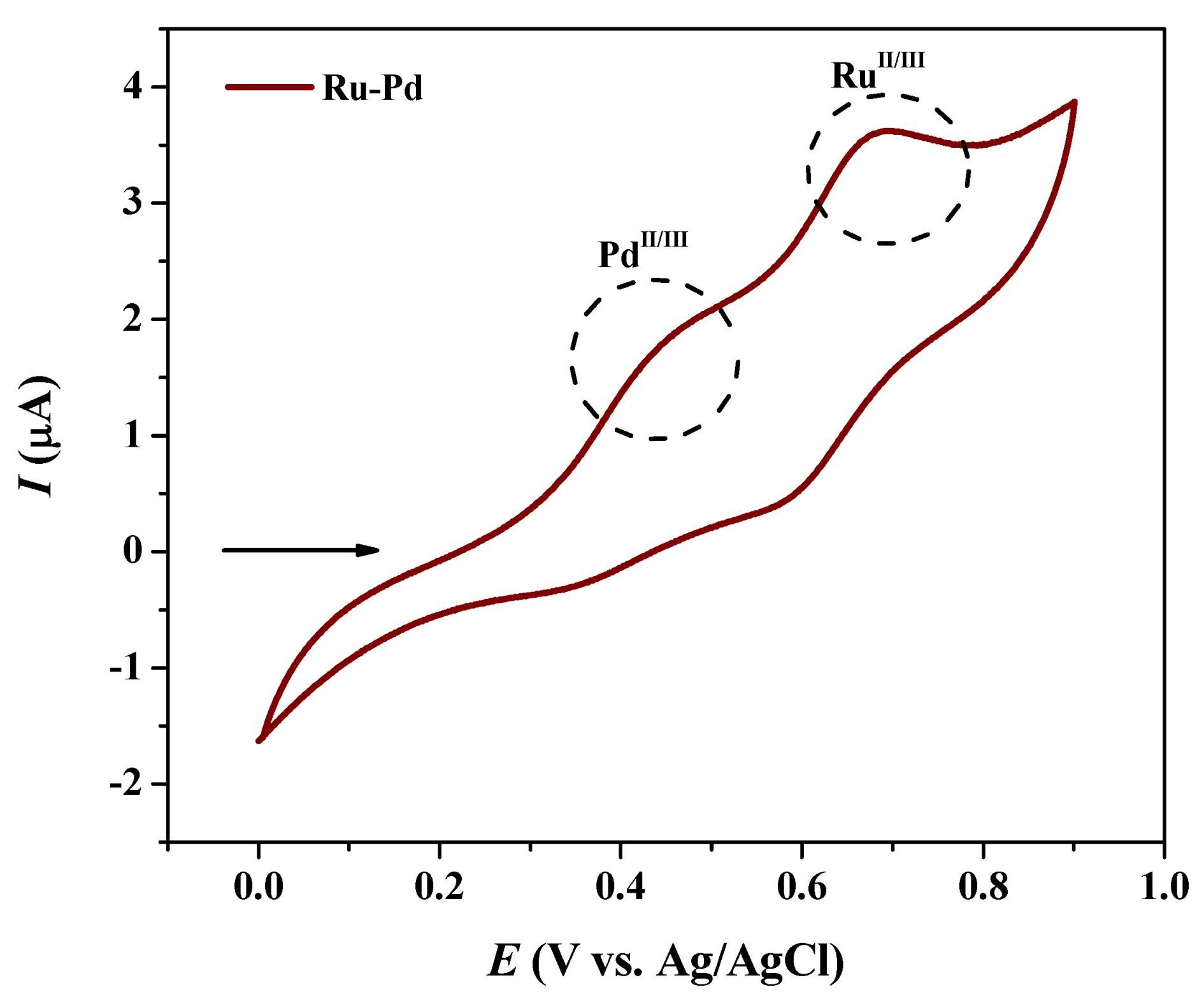

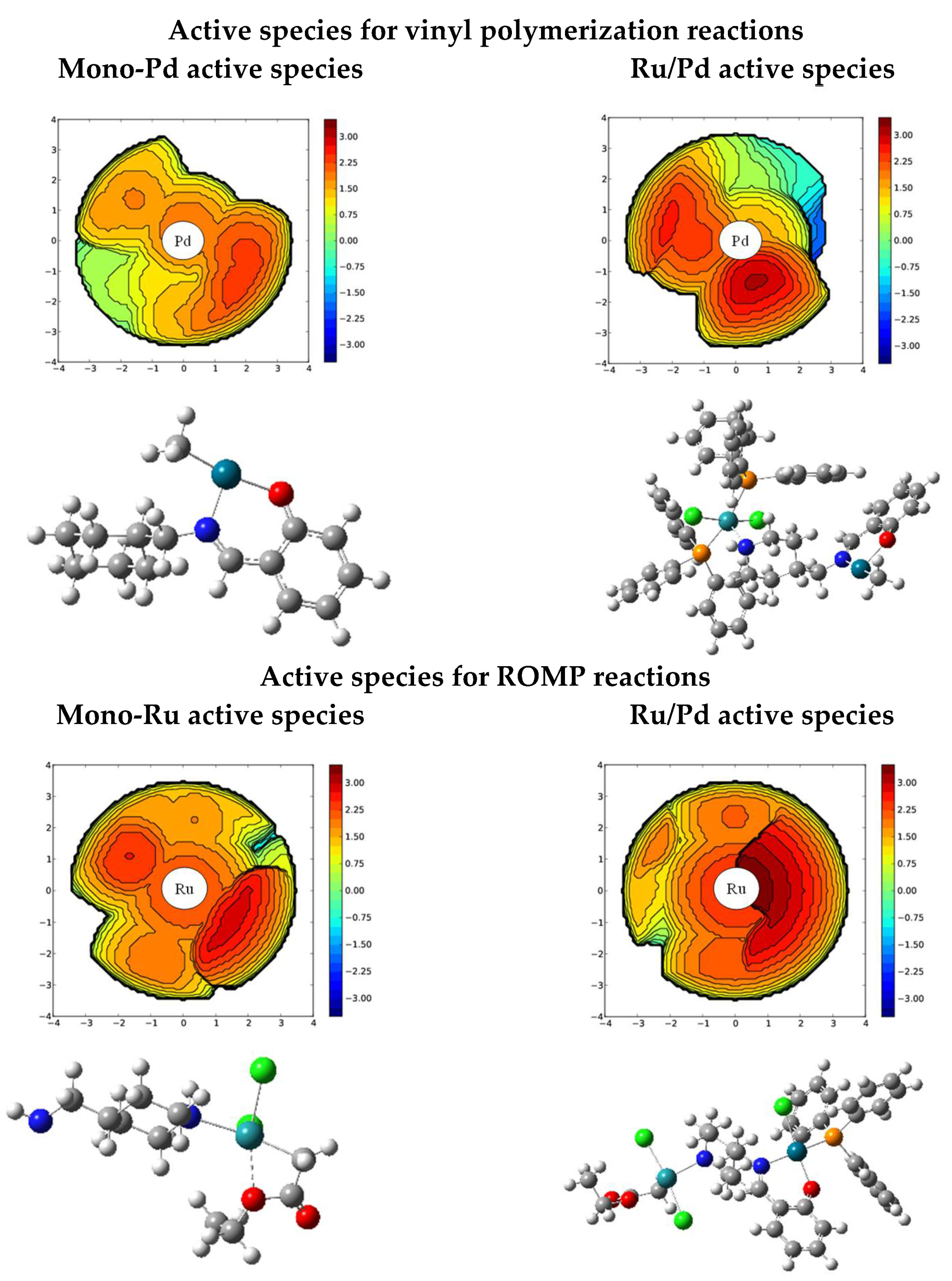
| Geometric Parameters | mono-Ru | Ru/Pd | mono-Pd |
|---|---|---|---|
| Ru(1)−Cl(1) | 2.4492 | 2.4528 | - |
| Ru(1)−Cl(2) | 2.4510 | 2.4623 | - |
| Ru(1)-P(1) | 2.4497 | 2.4539 | - |
| Ru(1)-P(2) | 2.4583 | 2.4308 | - |
| Ru(1)-N(1) | 2.1425 | 2.1361 | - |
| Cl(1)−Ru(1)−Cl(2) | 168.420 | 170.521 | - |
| Cl(1)−Ru(1)−N(1) | 85.655 | 85.857 | - |
| Cl(2)−Ru(1)−N(1) | 98.720 | 103.557 | - |
| P(1)−Ru(1)−P(2) | 163.994 | 162.371 | - |
| Pd(1)−Cl(3) | - | 2.3583 | 2.3580 |
| Pd(1)−N(2) | - | 2.0755 | 2.0773 |
| Pd(1)−O(1) | - | 2.0224 | 2.0172 |
| Pd(1)−P(3) | - | 2.2953 | 2.2971 |
| N(2)−Pd(1)−O(1) | - | 89.251 | 89.664 |
| P(3)−Pd(1)−Cl(3) | - | 86.590 | 86.369 |
| Complex | Entry | Al/Pd | [NBE]/[Pd] | T (°C) | Activity b | Yield (%) |
|---|---|---|---|---|---|---|
| mono-Pd | 1 | 0 | 20,000 | 30 | - | - |
| 2 | 1000 | 20,000 | 30 | 2.4 | 31.9 | |
| 3 | 1500 | 20,000 | 30 | 3.4 | 44.7 | |
| 4 | 2000 | 20,000 | 30 | 5.4 | 72.34 | |
| 5 | 2500 | 20,000 | 30 | 7.5 | 100 | |
| 6 | 3000 | 20,000 | 30 | 6.6 | 87.2 | |
| 7 | 2500 | 10,000 | 30 | 3.1 | 83.0 | |
| 8 | 2500 | 30,000 | 30 | 6.7 | 59.7 | |
| 9 | 2500 | 20,000 | 60 | 7.5 | 100 | |
| 10 | 2500 | 20,000 | 90 | 4.0 | 52.8 | |
| Ru/Pd | 11 | 0 | 20,000 | 30 | - | - |
| 12 | 1000 | 20,000 | 30 | 0.4 | 5.1 | |
| 13 | 1500 | 20,000 | 30 | 0.8 | 10.4 | |
| 14 | 2000 | 20,000 | 30 | 1.1 | 14.2 | |
| 15 | 2500 | 20,000 | 30 | 1.7 | 23.2 | |
| 16 | 3000 | 20,000 | 30 | 1.6 | 21.7 | |
| 17 | 2500 | 10,000 | 30 | 0.9 | 23.0 | |
| 18 | 2500 | 30,000 | 30 | 1.0 | 8.7 | |
| 19 | 2500 | 20,000 | 60 | 3.5 | 47.0 | |
| 20 | 2500 | 20,000 | 90 | 0.3 | 3.6 |
| Complex | Entry | VEDA (μL) | [NBE]/[Ru] | T (°C) | Yield (%) | Mn b (104 g mol−1) | Ð b |
|---|---|---|---|---|---|---|---|
| mono-Ru | 1 | 0 | 3000 | 25 | - | - | - |
| 2 | 2 | 3000 | 25 | 54.2 | 2.1 | 1.65 | |
| 3 | 5 | 3000 | 25 | 59.0 | 5.9 | 1.53 | |
| 4 | 7 | 3000 | 25 | 39.8 | 6.8 | 1.62 | |
| 5 | 10 | 3000 | 25 | 21.6 | 3.5 | 1.76 | |
| 6 | 5 | 1000 | 25 | 19.5 | 2.1 | 1.48 | |
| 7 | 5 | 5000 | 25 | 28.7 | 2.7 | 1.54 | |
| 8 | 5 | 7000 | 50 | 22.6 | 3.1 | 1.48 | |
| 9 | 5 | 3000 | 50 | 68.0 | 5.9 | 1.61 | |
| Ru/Pd | 10 | 0 | 3000 | 25 | - | - | - |
| 11 | 2 | 3000 | 25 | 11.2 | 2.1 | 1.63 | |
| 12 | 5 | 3000 | 25 | 23.9 | 2.9 | 1.58 | |
| 13 | 7 | 3000 | 25 | 10.4 | 2.2 | 1.71 | |
| 14 | 10 | 3000 | 25 | 5.1 | 1.9 | 1.79 | |
| 15 | 5 | 1000 | 25 | 8.6 | 1.8 | 1.48 | |
| 16 | 5 | 5000 | 25 | 9.9 | 2.1 | 1.54 | |
| 17 | 5 | 7000 | 50 | 22.6 | 3.0 | 1.48 | |
| 18 | 5 | 3000 | 50 | 31.2 | 4.5 | 1.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, T.R.; Masson, G.H.C.; Amorim, K.A.E.; Machado, A.E.H.; Goi, B.E.; Carvalho-Jr., V.P. Ru/Pd Complex and Its Monometallic Fragments as Catalysts for Norbornene Polymerization via ROMP and Addition. Catalysts 2022, 12, 1111. https://doi.org/10.3390/catal12101111
Cruz TR, Masson GHC, Amorim KAE, Machado AEH, Goi BE, Carvalho-Jr. VP. Ru/Pd Complex and Its Monometallic Fragments as Catalysts for Norbornene Polymerization via ROMP and Addition. Catalysts. 2022; 12(10):1111. https://doi.org/10.3390/catal12101111
Chicago/Turabian StyleCruz, Thaís R., Gustavo H. C. Masson, Kelly A. E. Amorim, Antonio E. H. Machado, Beatriz E. Goi, and Valdemiro P. Carvalho-Jr. 2022. "Ru/Pd Complex and Its Monometallic Fragments as Catalysts for Norbornene Polymerization via ROMP and Addition" Catalysts 12, no. 10: 1111. https://doi.org/10.3390/catal12101111
APA StyleCruz, T. R., Masson, G. H. C., Amorim, K. A. E., Machado, A. E. H., Goi, B. E., & Carvalho-Jr., V. P. (2022). Ru/Pd Complex and Its Monometallic Fragments as Catalysts for Norbornene Polymerization via ROMP and Addition. Catalysts, 12(10), 1111. https://doi.org/10.3390/catal12101111