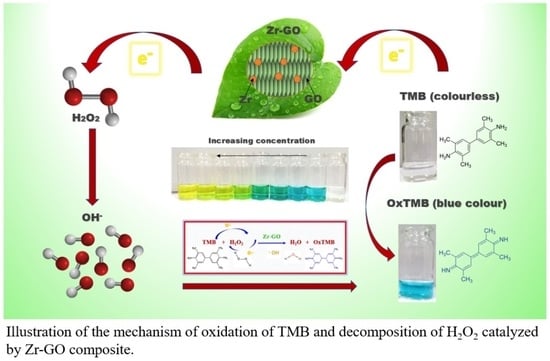
Decorating Zirconium on Graphene Oxide to Design a Multifunctional Nanozyme for Eco-Friendly Detection of Hydrogen Peroxide
Abstract
:1. Introduction
2. Results and discussion
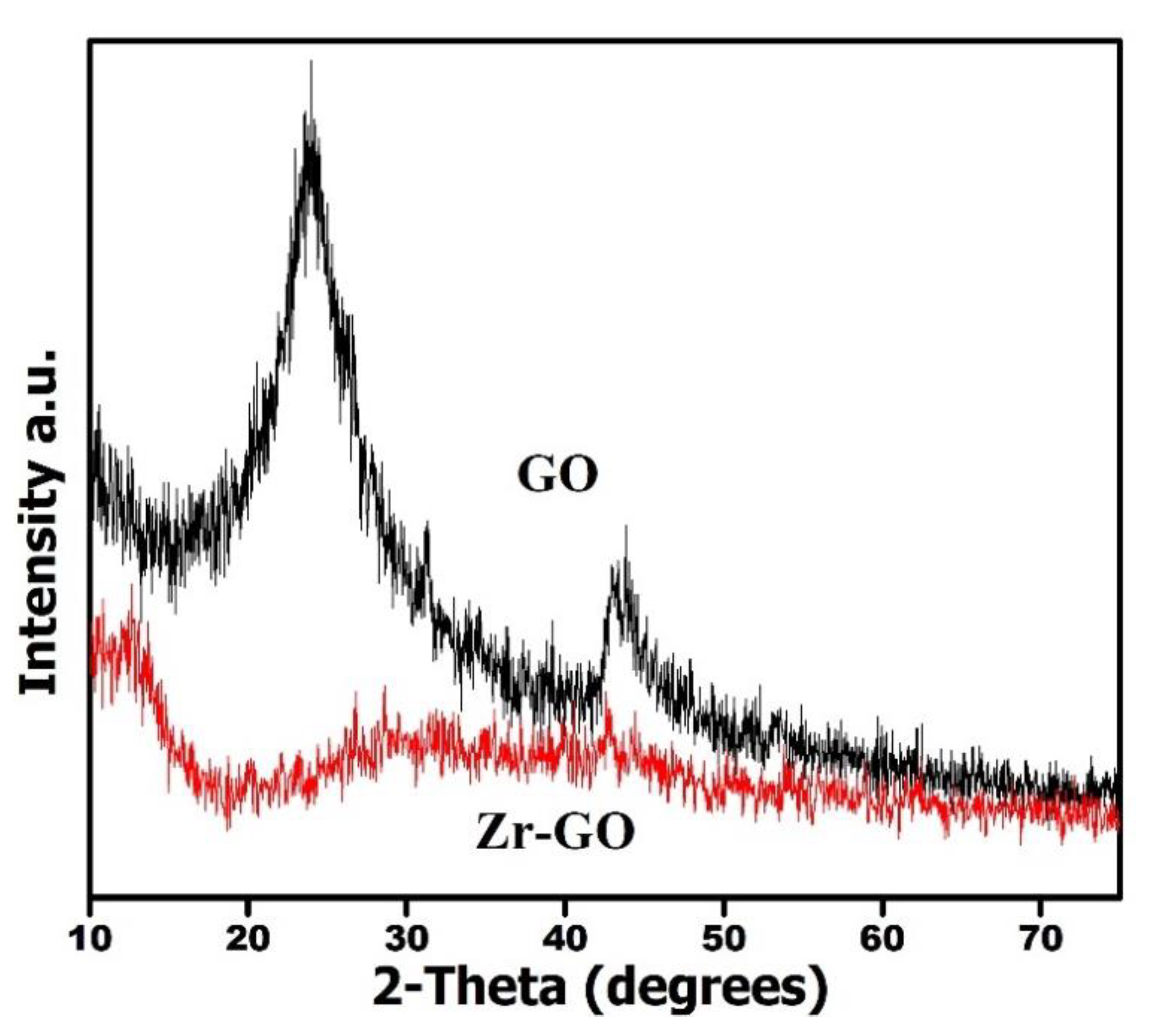
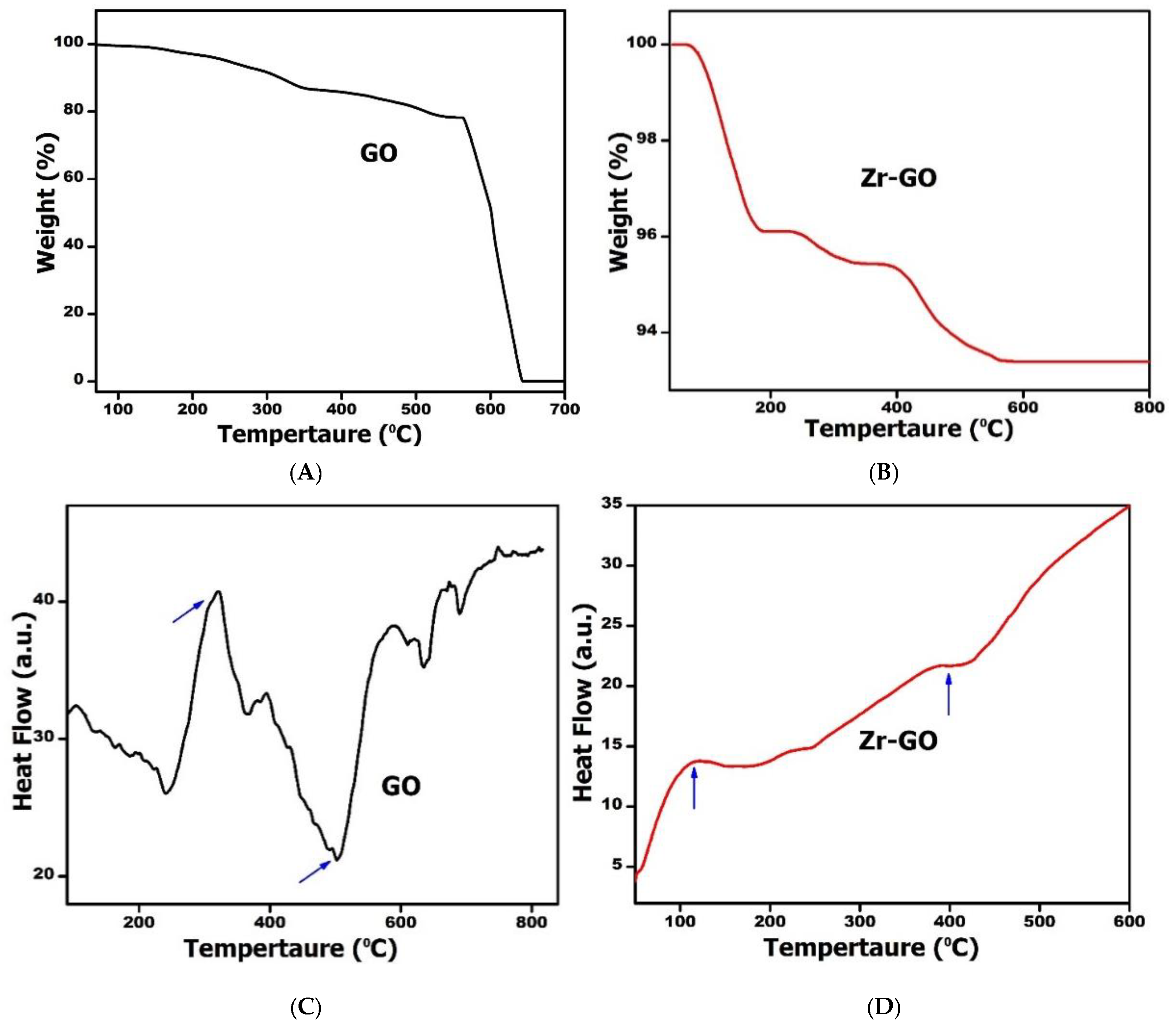
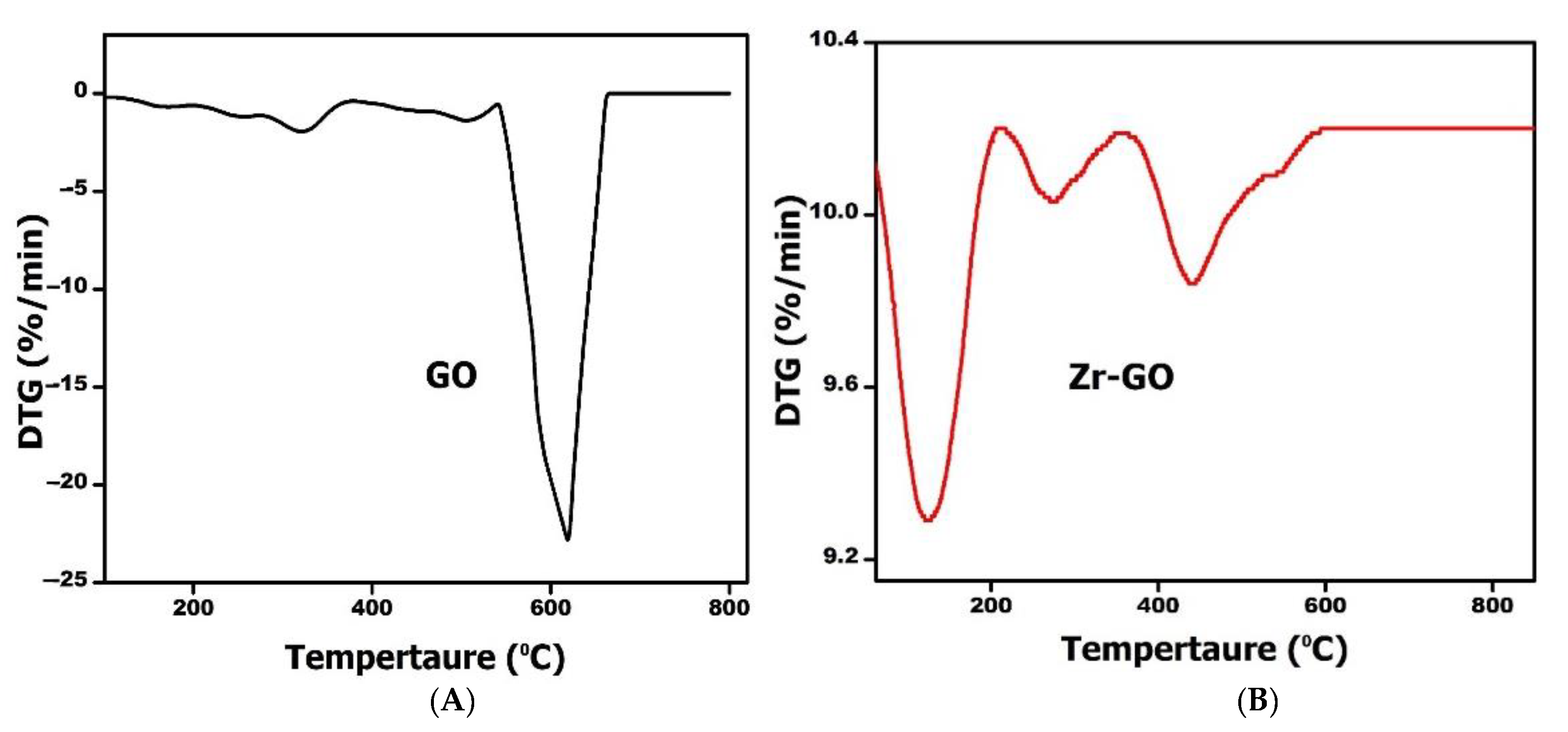
2.1. Characterization
2.2. Peroxidase-Mimicking Activity of Zr
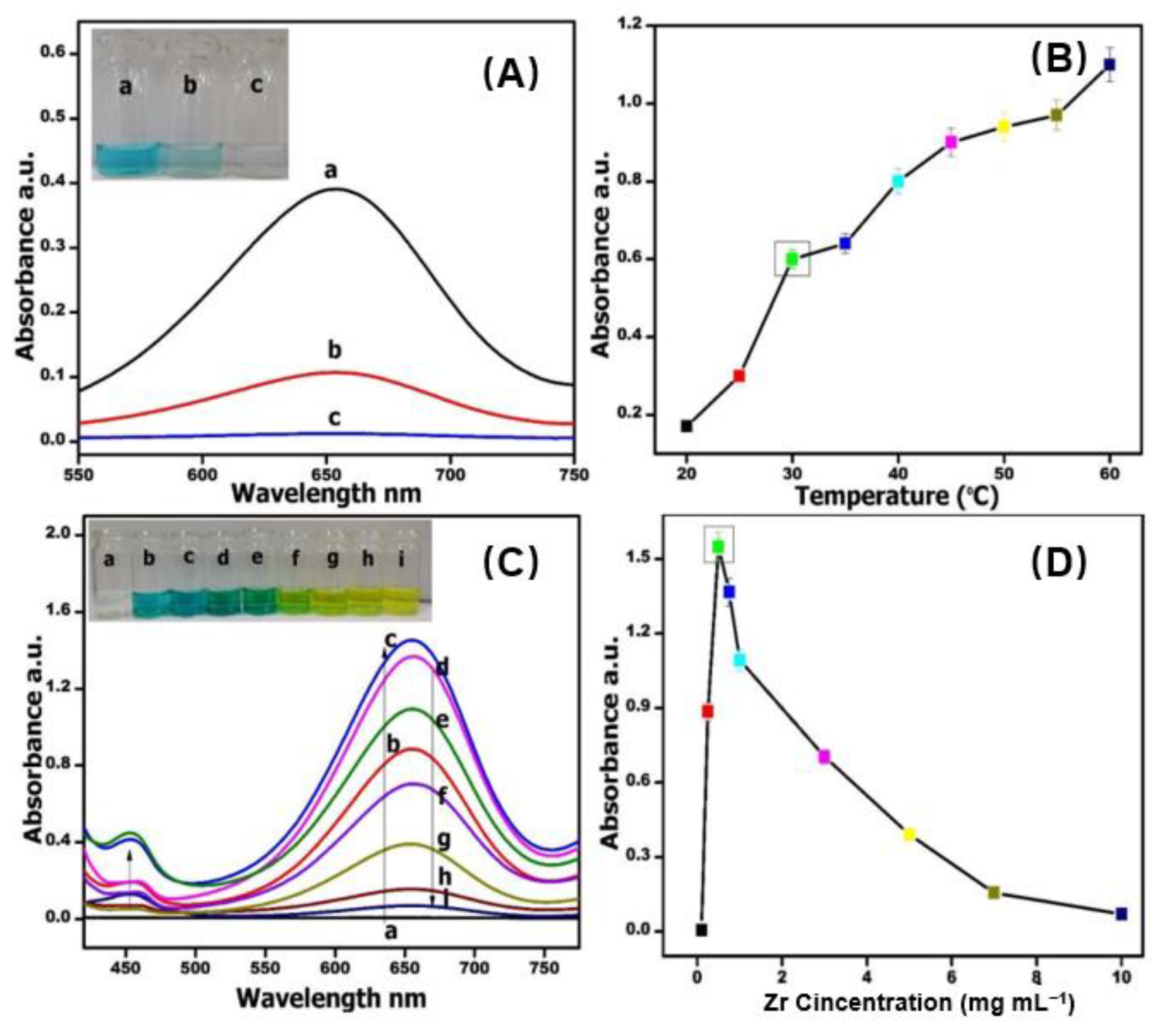
2.3. Optimization of Reaction Parameters
- Effect of Medium:
- Effect of Temperature:
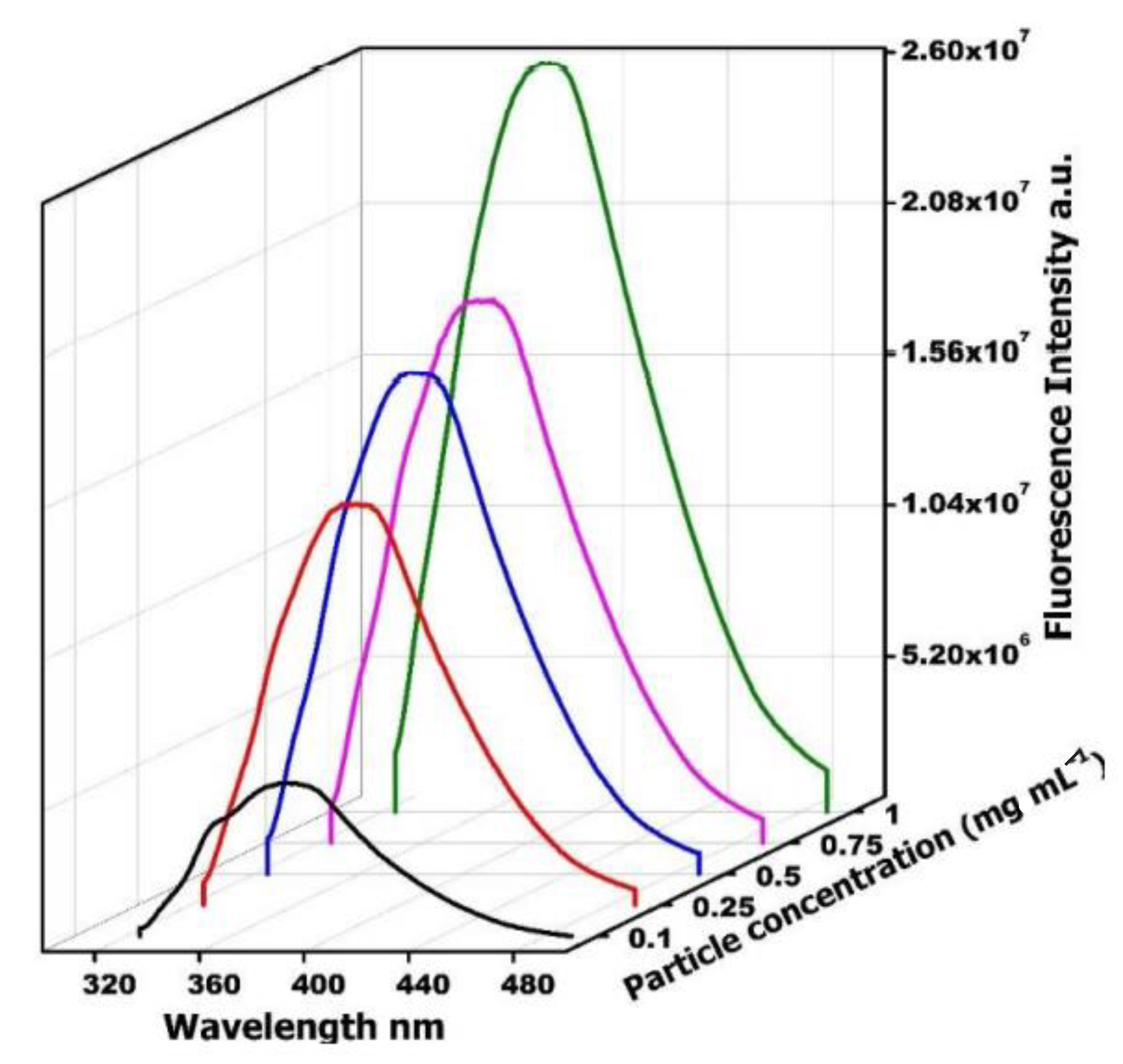
- Concentration of GO:
- Concentration of Zr:
- Concentration of TMB:
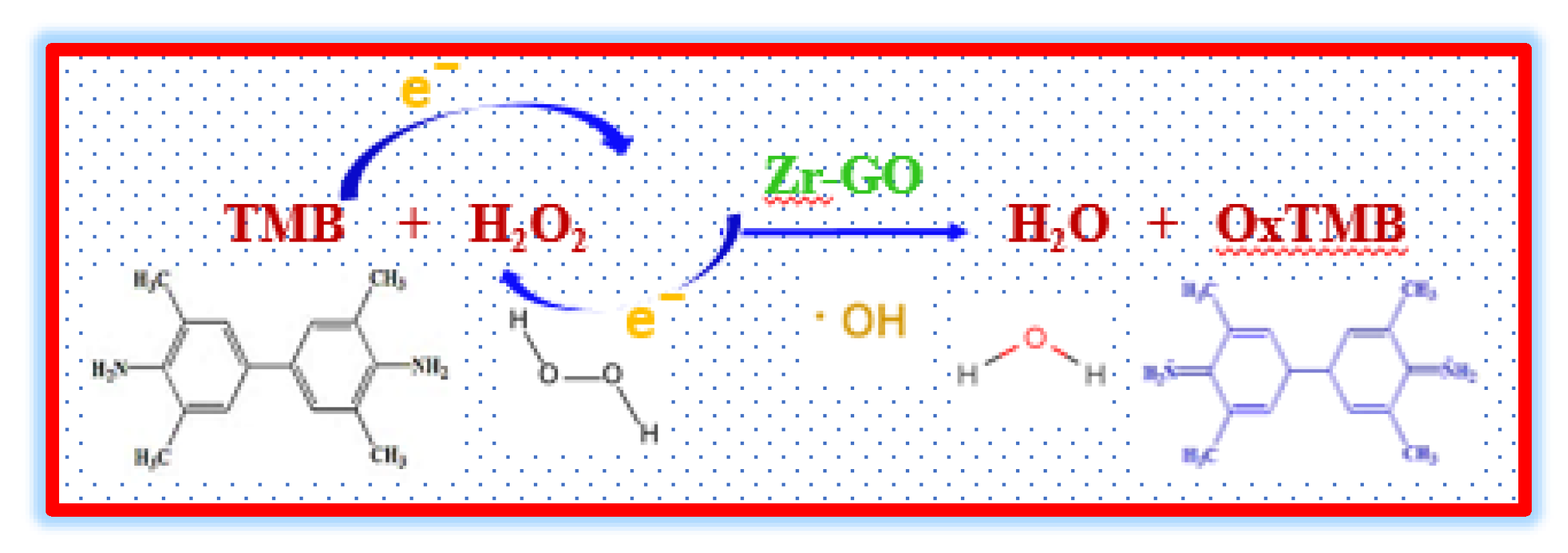
2.4. Catalytic Mechanism of the Peroxidase Mimicking Zr
 2H2O+A
2H2O+A
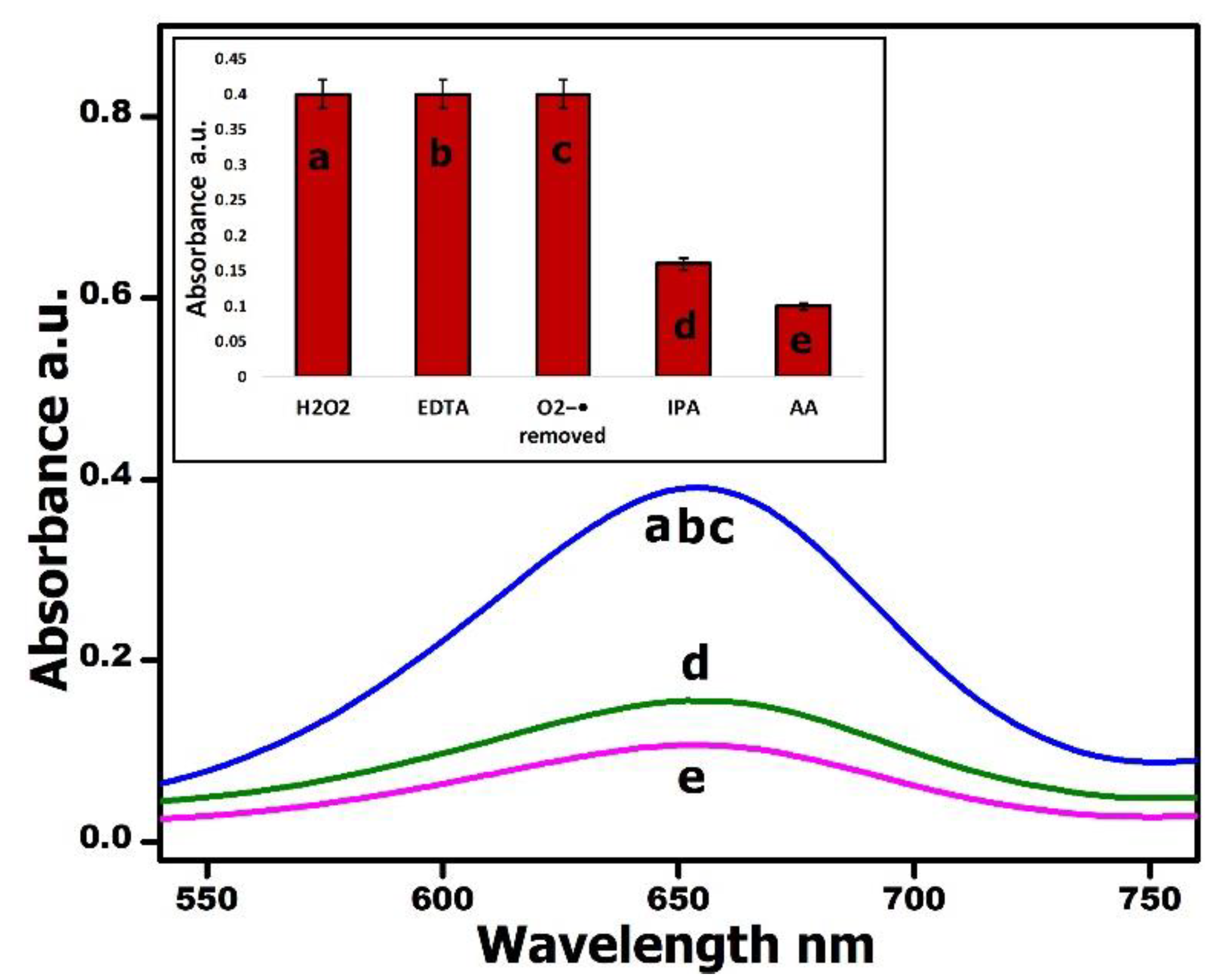
- Scavenger Experiments
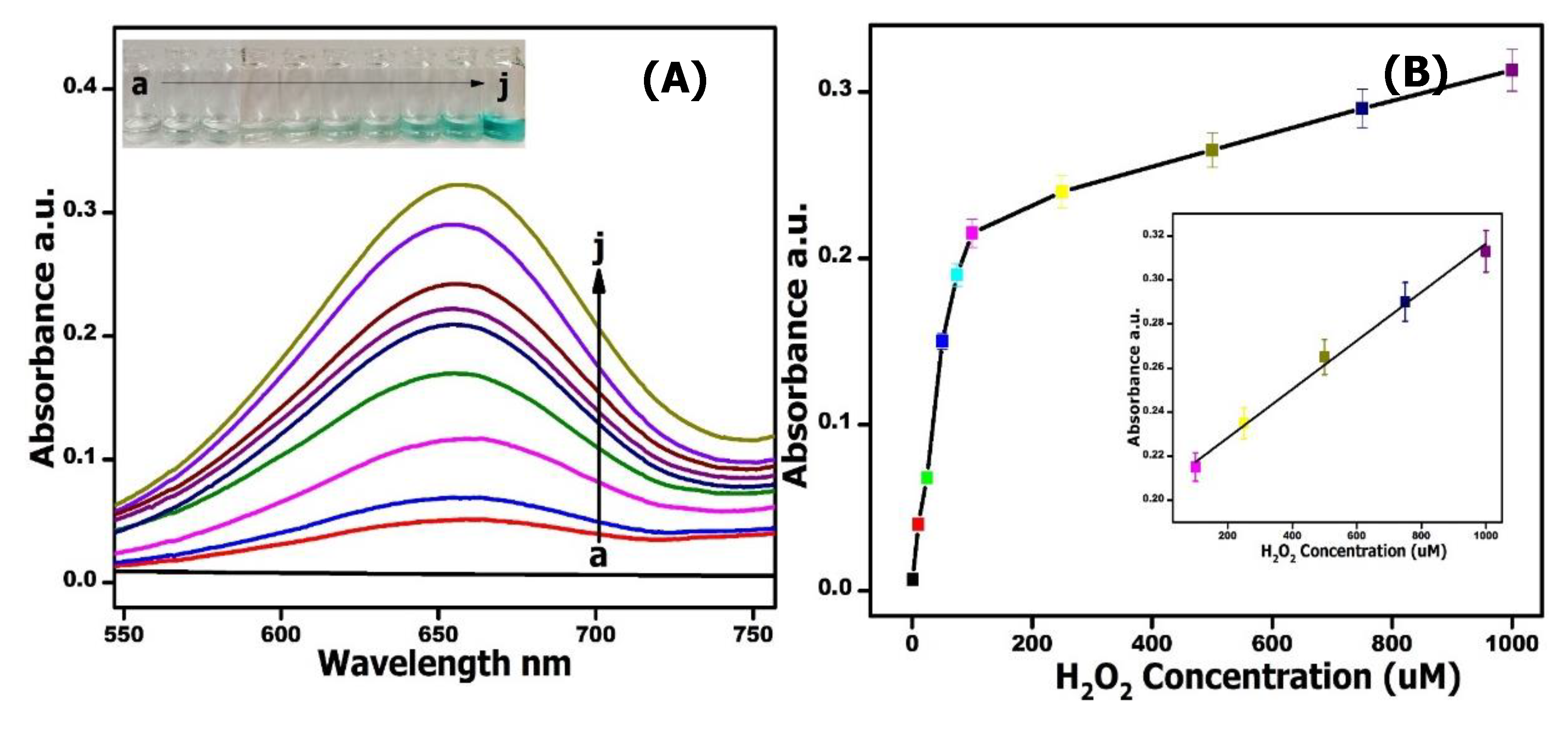
2.5. Colorimetric Detection of H2O2
2.6. Interference Experiments
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Instruments
3.3. Synthesis of Graphene Oxide
3.4. Preparation of Zr-GO Composite
3.5. Peroxidase-Like Activity Measurements
3.6. Investigation of Catalytic Mechanism
3.7. Colorimetric Detection of H2O2
3.8. Determination of Selectivity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, Z.; Dai, Z.; Hu, X.; Chen, K.; Gao, C.; Zheng, X.; Yu, Y. Synthesis of PB@ FePt hybrid nanoparticles with peroxidase-mimicking activity for colorimetric determination of hydrogen peroxide in living cells. Anal. Methods 2019, 11, 677–683. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, Y.; Yang, Y.; Chen, N.; Feng, C.; Huang, X.; Yu, C. Fast Capture of Fluoride by Anion-Exchange Zirconium–Graphene Hybrid Adsorbent. Langmuir 2019, 35, 6861–6869. [Google Scholar] [CrossRef] [PubMed]
- Gul, U.; Kanwal, S.; Tabassum, S.; Gilani, M.A.; Rahim, A. Microwave-assisted synthesis of carbon dots as reductant and stabilizer for silver nanoparticles with enhanced-peroxidase like activity for colorimetric determination of hydrogen peroxide and glucose. Microchim. Acta 2020, 187, 135. [Google Scholar] [CrossRef] [PubMed]
- Rauf, S.; Ali, N.; Tayyab, Z.; Shah, M.Y.; Yang, C.P.; Hu, J.; Kong, W.; Huang, Q.; Hayat, A.; Muhammad, N. Ionic liquid coated zerovalent manganese nanoparticles with stabilized and enhanced peroxidase-like catalytic activity for colorimetric detection of hydrogen peroxide. Mater. Res. Express 2020, 7, 035018. [Google Scholar] [CrossRef]
- Liu, M.-M.; Li, S.-H.; Huang, D.-D.; Xu, Z.-W.; Wu, Y.-W.; Lei, Y.; Liu, A.-L. MoOx quantum dots with peroxidase-like activity on microfluidic paper-based analytical device for rapid colorimetric detection of H2O2 released from PC12 cells. Sens. Actuators B Chem. 2020, 305, 127512. [Google Scholar] [CrossRef]
- Singh, S. Nanomaterials exhibiting enzyme-like properties (Nanozymes): Current advances and future perspectives. Front. Chem. 2019, 7, 46. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, K.; Lyu, H.; Zhang, X.; Liu, Z.; Fan, G.; Zhang, X.; Zhu, X.; Liu, Q. Porphyrin functionalized Co(OH) 2/GO nanocomposites as an excellent peroxidase mimic for colorimetric biosensing. Analyst 2019, 144, 5284–5291. [Google Scholar] [CrossRef]
- Dong, W.; Huang, Y. CeO2/C nanowire derived from a cerium (III) based organic framework as a peroxidase mimic for colorimetric sensing of hydrogen peroxide and for enzymatic sensing of glucose. Microchim. Acta 2020, 187, 11. [Google Scholar] [CrossRef]
- Ai, Y.; Hu, Z.; Liang, X.; Sun, H.; Xin, H.; Liang, Q. Recent advances in Nanozymes: From matters to bioapplications. Adv. Funct. Mater. 2022, 32, 2110432. [Google Scholar] [CrossRef]
- Lee, J.; Liao, H.; Wang, Q.; Han, J.; Han, J.-H.; Shin, H.; Ge, M.; Park, W.; Li, F. Exploration of nanozymes in viral diagnosis and therapy. In Exploration; Wiley Online Library: Hoboken, NJ, USA, 2022. [Google Scholar]
- Deng, H.-H.; Luo, B.-Y.; He, S.-B.; Chen, R.-T.; Lin, Z.; Peng, H.-P.; Xia, X.-H.; Chen, W. Redox recycling-triggered peroxidase-like activity enhancement of bare gold nanoparticles for ultrasensitive colorimetric detection of rare-earth Ce3+ ion. Anal. Chem. 2019, 91, 4039–4046. [Google Scholar] [CrossRef]
- Song, C.; Ding, W.; Zhao, W.; Liu, H.; Wang, J.; Yao, Y.; Yao, C. High peroxidase-like activity realized by facile synthesis of FeS2 nanoparticles for sensitive colorimetric detection of H2O2 and glutathione. Biosens. Bioelectron. 2020, 151, 111983. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, L.; Xie, W. Rapid and sensitive colorimetric sensor for H2O2 and Hg2+ detection based on homogeneous iodide with high peroxidase-mimicking activity. Microchem. J. 2019, 147, 75–82. [Google Scholar] [CrossRef]
- Nikoofar, K.; Khademi, Z. A review on green Lewis acids: Zirconium (IV) oxydichloride octahydrate (ZrOCl2 8H2O) and zirconium (IV) tetrachloride (ZrCl4) in organic chemistry. Res. Chem. Intermed. 2016, 42, 3929–3977. [Google Scholar] [CrossRef]
- Moghaddam, F.M.; Ismaili, H.; Bardajee, G.R. Zirconium (IV) oxide chloride and anhydrous copper (II) sulfate mediated synthesis of 2-substituted benzothiazoles. Heteroat. Chem. Int. J. Main Group Elem. 2006, 17, 136–141. [Google Scholar]
- Nielsen, R.H.; Schlewitz, J.H.; Neilson, H. Zirconium and zirconium compounds. Kirk-Othmer Encycl. Chem. Technol. 2000, 1–46. [Google Scholar] [CrossRef]
- Abdollahi-Alibeik, M.; Shabani, E. Synthesis of 2,3-dihydroquinazolin-4(1H)-ones catalyzed by zirconium (IV) chloride as a mild and efficient catalyst. Chin. Chem. Lett. 2011, 22, 1163–1166. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, K.; Zhou, B.; Wang, J.; Zheng, C.; Qu, L.; Chai, H.; Zhang, X. Magnetic zirconium-based Prussian blue analog nanozyme: Enhanced peroxidase-mimicking activity and colorimetric sensing of phosphate ion. Microchim. Acta 2022, 189, 220. [Google Scholar] [CrossRef]
- Yang, J.; Dai, H.; Sun, Y.; Wang, L.; Qin, G.; Zhou, J.; Chen, Q.; Sun, G. 2D material–based peroxidase-mimicking nanozymes: Catalytic mechanisms and bioapplications. Anal. Bioanal. Chem. 2022, 414, 2971–2989. [Google Scholar] [CrossRef]
- Menon, S.; Sam, S.; Keerthi, K.; Kumar, K.G. Carbon nanomaterial-based sensors: Emerging trends, markets, and concerns. Carbon Nanomater.-Based Sens. 2022, 347–379. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, X.; Li, J.; Chen, L.; Wang, N.; Yu, S.; Li, G.; Xiong, L.; Ju, H. Colorimetric Detection of Nucleic Acids through Triplex-Hybridization Chain Reaction and DNA Controlled Growth of Platinum Nanoparticles on Graphene Oxide. Anal. Chem. 2020, 92, 2714–2721. [Google Scholar] [CrossRef]
- Youssef, K.; Hashim, A.; Roberto, S.; Hamed, S.F.; Abd Elsalam, K. Graphene-based nanocomposites: Synthesis, characterizations, and their agri-food applications. In Carbon Nanomaterials for Agri-Food and Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 33–57. [Google Scholar]
- Qi, Y.; Chen, Y.; He, J.; Gao, X. Highly sensitive and simple colorimetric assay of hydrogen peroxide and glucose in human serum via the smart synergistic catalytic mechanism. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 234, 118233. [Google Scholar] [CrossRef] [PubMed]
- Qiu, N.; Liu, Y.; Guo, R. Electrodeposition-assisted Rapid Preparation of Pt Nanocluster/3D Graphene Hybrid Nanozyme with Outstanding Multiple Oxidase-Like Activity for Distinguishing Colorimetric Determination of Dihydroxybenzene Isomers. ACS Appl. Mater. Interfaces 2020, 12, 15553–15561. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhang, L.; Guan, Q.; Ning, P.; Ye, D. Preparation of α-zirconium phosphate-pillared reduced graphene oxide with increased adsorption towards methylene blue. Chem. Eng. J. 2014, 258, 77–84. [Google Scholar] [CrossRef]
- Nataraj, N. An Efficient Electrochemical Sensor Based on Zirconium Molybdate Decorated Reduced Graphene Oxide for the Detection of Hydroquinone. Int. J. Electrochem. Sci. 2020, 15, 8321–8335. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Z.; Li, X.; Zhou, J. A nano graphene oxide/α-zirconium phosphate hybrid for rigid polyvinyl chloride foams with simultaneously improved mechanical strengths, smoke suppression, flame retardancy and thermal stability. Compos. Part A Appl. Sci. Manuf. 2019, 121, 180–188. [Google Scholar] [CrossRef]
- Ye, K.; Li, Y.; Yang, H.; Li, M.; Huang, Y.; Zhang, S.; Ji, H. An ultrathin carbon layer activated CeO2 heterojunction nanorods for photocatalytic degradation of organic pollutants. Appl. Catal. B Environ. 2019, 259, 118085. [Google Scholar] [CrossRef]
- Li, Y.; Xia, Y.; Liu, K.; Ye, K.; Wang, Q.; Zhang, S.; Huang, Y.; Liu, H. Constructing Fe-MOF-derived Z-scheme photocatalysts with enhanced charge transport: Nanointerface and carbon sheath synergistic effect. ACS Appl. Mater. Interfaces 2020, 12, 25494–25502. [Google Scholar] [CrossRef]
- Biira, S.; Hlatshwayo, T.T.; Crouse, P.L.; Bissett, H.; Thabethe, T.T.; Mlambo, M.; Malherbe, J.B. Effect of the ZrCl4 static vaporiser system and deposition time on growth characteristics of chemical vapour deposited zirconium carbide layers. Appl. Phys. A 2019, 125, 185. [Google Scholar] [CrossRef]
- Marin, P.; Módenes, A.N.; Bergamasco, R.; Paraíso, P.R.; Hamoudi, S. Synthesis, characterization and application of ZrCl4-graphene composite supported on activated carbon for efficient removal of fluoride to obtain drinking water. Water Air Soil Pollut. 2016, 227, 479. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, D.; Zhang, J.; Balogun, M.T.; Wang, P.; Tong, Y.; Huang, Y. Charge Relays via Dual Carbon-Actions on Nanostructured BiVO4 for High Performance Photoelectrochemical Water Splitting. Adv. Funct. Mater. 2022, 32, 2112738. [Google Scholar] [CrossRef]
- Pourbeyram, S. Effective removal of heavy metals from aqueous solutions by graphene oxide–zirconium phosphate (GO–Zr-P) nanocomposite. Ind. Eng. Chem. Res. 2016, 55, 5608–5617. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Ramin, S. Effective removal of copper from aqueous solutions by modified magnetic chitosan/graphene oxide nanocomposites. Int. J. Biol. Macromol. 2018, 113, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Lee, D.S. Three-dimensional barium-sulfate-impregnated reduced graphene oxide aerogel for removal of strontium from aqueous solutions. J. Nucl. Mater. 2018, 504, 206–214. [Google Scholar] [CrossRef]
- Vu, T.H.T.; Tran, T.T.T.; Le, H.N.T.; Nguyen, P.H.T.; Bui, N.Q.; Essayem, N. A new green approach for the reduction of graphene oxide nanosheets using caffeine. Bull. Mater. Sci. 2015, 38, 667–671. [Google Scholar]
- Kumar, R.; Singh, R.; Gurjar, A.; Kashyap, R.; Kumar, M.; Kumar, D. Study the thermal stability of functionalized graphene oxide. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2019. [Google Scholar]
- Du, R.; Shi, Z.; Yu, C.; Rao, W.; Xu, C.; Wang, J. Synthesis of graphene oxide/sulfur composites for advanced lithium-sulfur batteries. Ionics 2021, 27, 4269–4279. [Google Scholar] [CrossRef]
- Cui, S.; Suo, H.; Jing, F.; Yu, S.; Xue, J.; Shen, X.; Lin, B.; Jiang, S.; Liu, Y. Facile preparation of ZrCO composite aerogel with high specific surface area and low thermal conductivity. J. Sol-Gel Sci. Technol. 2018, 86, 383–390. [Google Scholar] [CrossRef]
- Hussein, A.; Sarkar, S.; Oh, D.; Lee, K.; Kim, B. Epoxy/p-phenylenediamine functionalized graphene oxide composites and evaluation of their fracture toughness and tensile properties. J. Appl. Polym. Sci. 2016, 133, 43821. [Google Scholar] [CrossRef]
- Hussein, A.; Sarkar, S.; Kim, B. Low temperature reduction of graphene oxide using hot-plate for nanocomposites applications. J. Mater. Sci. Technol. 2016, 32, 411–418. [Google Scholar] [CrossRef]
- Farivar, F.; Yap, P.L.; Karunagaran, R.; Losic, D. Thermogravimetric analysis (TGA) of graphene materials: Effect of particle size of graphene, graphene oxide and graphite on thermal parameters. J. Carbon Res. 2021, 7, 41. [Google Scholar] [CrossRef]
- Fares, M.M.; Al-Rub, F.A.A.; Mohammad, A.R. Ultimate eradication of the ciprofloxacin antibiotic from the ecosystem by nanohybrid GO/O-CNTs. ACS Omega 2020, 5, 4457–4468. [Google Scholar] [CrossRef]
- Elganzory, H.H.; Hassan, S.; Aly, S.A.; Abdalla, E.M. Synthesis, Characterization, PXRD Studies, Theoretical Calculation, and Antitumor Potency Studies of a Novel N, O-Multidentate Chelating Ligand and Its Zr (IV), V (IV), Ru (III), and Cd (II) Complexes. Bioinorg. Chem. Appl. 2022, 2022, 2006451. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Gao, Y. Cerium (III)-doped MoS2 nanosheets with expanded interlayer spacing and peroxidase-mimicking properties for colorimetric determination of hydrogen peroxide. Microchim. Acta 2020, 187, 111. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lv, J.; Wang, S.; Wang, J.; Lin, Y. Expanded mesoporous silica-encapsulated ultrasmall Pt nanoclusters as artificial enzymes for tracking hydrogen peroxide secretion from live cells. Anal. Chim. Acta 2020, 1104, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Pourbeyram, S.; Alizadeh, S.; Gholizadeh, S. Simultaneous removal of arsenate and arsenite from aqueous solutions by graphene oxide-zirconium (GO-Zr) nanocomposite. J. Environ. Chem. Eng. 2016, 4, 4366–4373. [Google Scholar] [CrossRef]
- Pidenko, P.S.; Pidenko, S.A.; Skibina, Y.S.; Zacharevich, A.M.; Drozd, D.; Goryacheva, I.Y.; Burmistrova, N.A. Molecularly imprinted polyaniline for detection of horseradish peroxidase. Anal. Bioanal. Chem. 2020, 412, 6509–6517. [Google Scholar] [CrossRef]
- Harpaz, D.; Eltzov, E.; Ng, T.S.E.; Marks, R.S.; Tok, A.I.Y. Enhanced Colorimetric Signal for Accurate Signal Detection in Paper-Based Biosensors. Diagnostics 2020, 10, 28. [Google Scholar] [CrossRef]
- Oloketuyi, S.; Annovi, G.; de Marco, A. Peroxidase zymograms obtained by agarose native gel electrophoresis have unmet resolution and completeness. Int. J. Biol. Macromol. 2020, 156, 869–873. [Google Scholar] [CrossRef]
- Li, Z.; Liu, X.; Liang, X.-H.; Zhong, J.; Guo, L.; Fu, F. Colorimetric determination of xanthine in urine based on peroxidase-like activity of WO3 nanosheets. Talanta 2019, 204, 278–284. [Google Scholar] [CrossRef]
- Ragavan, K.; Ahmed, S.R.; Weng, X.; Neethirajan, S. Chitosan as a peroxidase mimic: Paper based sensor for the detection of hydrogen peroxide. Sens. Actuators B Chem. 2018, 272, 8–13. [Google Scholar] [CrossRef]
- Fu, B.; Liu, T.; Chen, J.; Li, K. CaF2 nanoparticles as peroxidase mimics for rapid and sensitive detection of aldosterone. Anal. Chim. Acta 2019, 1078, 119–124. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Y.; Zhang, C.; Ye, C.; Bian, Q.; Cheng, X.; Xia, H.; Zheng, J.; Liu, H. A novel colorimetric method for H2O2 sensing and its application: Fe2+-catalyzed H2O2 prevents aggregation of AuNPs by oxidizing cysteine (FeHOAuC). Anal. Chim. Acta 2022, 1207, 339840. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Dong, H.; Liu, L.; Li, M.; Xiao, K.; Xu, M. Selective and sensitive colorimetric sensor of mercury (II) based on gold nanoparticles and 4-mercaptophenylboronic acid. Sens. Actuators B Chem. 2014, 196, 106–111. [Google Scholar] [CrossRef]
- Ilyas, Q.; Waseem, M.T.; Junaid, H.M.; Khan, Z.A.; Munir, F.; Shaikh, A.J.; Shahzad, S.A. Fluorescein based fluorescent and colorimetric sensors for sensitive detection of TNP explosive in aqueous medium: Application of logic gate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 272, 120994. [Google Scholar] [CrossRef]
- Rasheed, Q.; Ajab, H.; Farooq, M.; Shahzad, S.A.; Yaqub, A. Fabrication of colorimetric sensor using Fe3O4@ Musa paradisiaca L. nanoparticles for detecting hydrogen peroxide: An application in environmental and biological samples. Appl. Nanosci. 2022, 12, 1–15. [Google Scholar] [CrossRef]
- Rathinam, R.; Singh, D.P.; Dutta, A.; Rudresha, S.; Ali, S.R.; Chatterjee, P. TiO2 Nanoparticles Based Peroxidase Mimics for Colorimetric Sensing of Cholesterol and Hydrogen Peroxide. In Advances in Science and Technology; Trans Tech Publication: Bäch SZ, Switzerland, 2022; Volume 117. [Google Scholar]
- Abd El-Salam, F.H.; Taher, F.A.; Shaban, S. Synthesis of Silver Decorated Magnetic Fe3o4/Alginate Polymeric Surfactant with Controllable Catalytic and Enzymatic-Mimic Activity for Solid-Colorimetric H2O2 Detection. SSRN Soc. Sci. Res. Netw. 2022. [Google Scholar] [CrossRef]
- Wang, D.; Dang, X.; Tan, B.; Zhan, Q.; Zhao, H. 3D V2O5-MoS2/rGO nanocomposites with enhanced peroxidase mimicking activity for sensitive colorimetric determination of H2O2 and glucose. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 269, 120750. [Google Scholar] [CrossRef]
- Ye, H.; Ding, Y.; Liu, T.; Li, J.; Wang, Q.; Li, Y.; Gu, J.; Zhang, Z.; Wang, X. Colorimetric assay based on NiCo2S4@ N, S-rGO nanozyme for sensitive detection of H2O2 and glucose in serum and urine samples. RSC Adv. 2022, 12, 20838–20849. [Google Scholar] [CrossRef]
- Wei, X.; Li, Y.; Qi, S.; Chen, Y.; Yin, M.; Zhang, L.; Tian, X.; Gong, S.; Wang, F.; Zhu, Y.; et al. Ce-MOF Nanosphere as Colorimetric Sensor with High Oxidase Mimicking Activity for Sensitive Detection of H2O2. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1–6. [Google Scholar] [CrossRef]
- Wang, S.; Hu, Z.; Wei, Q.; Zhang, H.; Tang, Y.; Sun, Y.; Duan, H.; Dai, Z.; Liu, Q.; Zheng, X. Diatomic active sites nanozymes: Enhanced peroxidase-like activity for dopamine and intracellular H2O2 detection. Nano Res. 2022, 15, 4266–4273. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Zeng, M.; Zhao, Y.; Zuo, X.; Meng, F.; Lv, F.; Lu, Y. Zr (IV)-based metal-organic framework nanocomposites with enhanced peroxidase-like activity as a colorimetric sensing platform for sensitive detection of hydrogen peroxide and phenol. Environ. Res. 2022, 203, 111818. [Google Scholar] [CrossRef]
- Tan, E.; Kahyaoğlu, İ.M.; Karakuş, S. A sensitive and smartphone colorimetric assay for the detection of hydrogen peroxide based on antibacterial and antifungal matcha extract silver nanoparticles enriched with polyphenol. Polym. Bull. 2022, 79, 7363–7389. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Rong, Q.; Bao, T.; Jiao, M.; Mao, L.; Xue, X.; Wen, W.; Wu, Z.; Zhang, X.; Wang, S. Synthesis of dual-functional CuO nanotubes for high-efficiently photoelectrochemical and colorimetric sensing of H2O2. Anal. Chim. Acta 2022, 1199, 339598. [Google Scholar] [CrossRef] [PubMed]

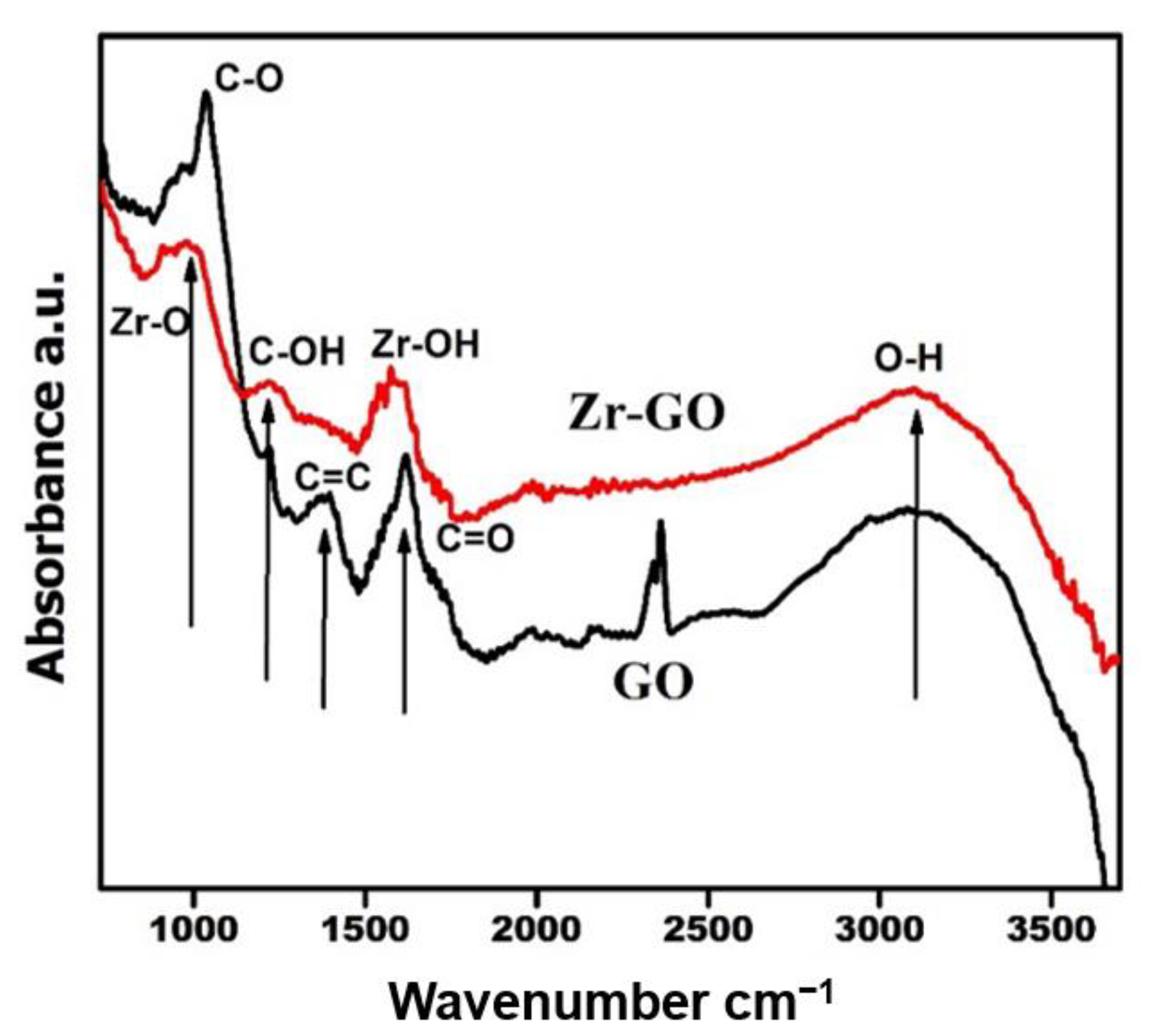
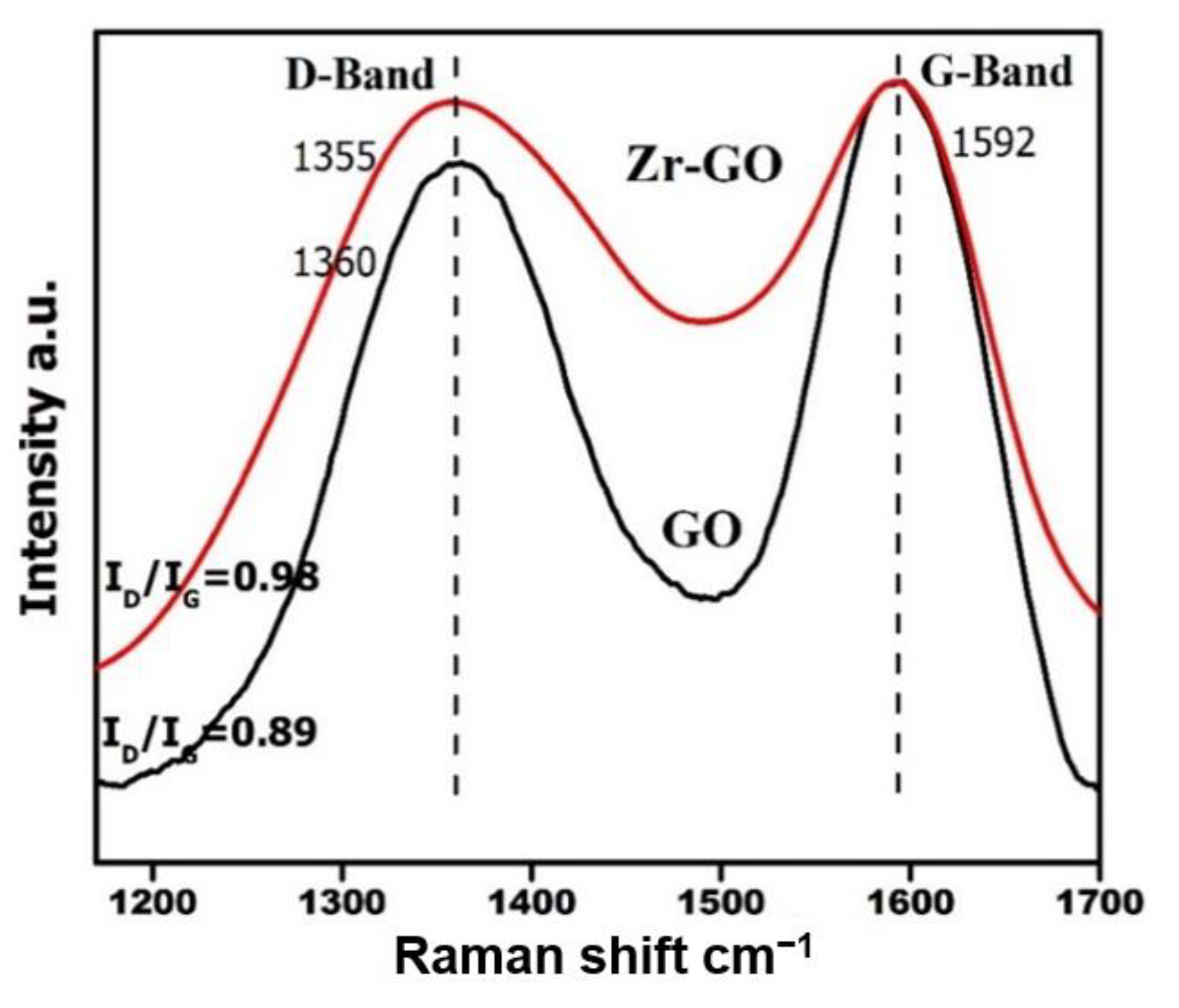
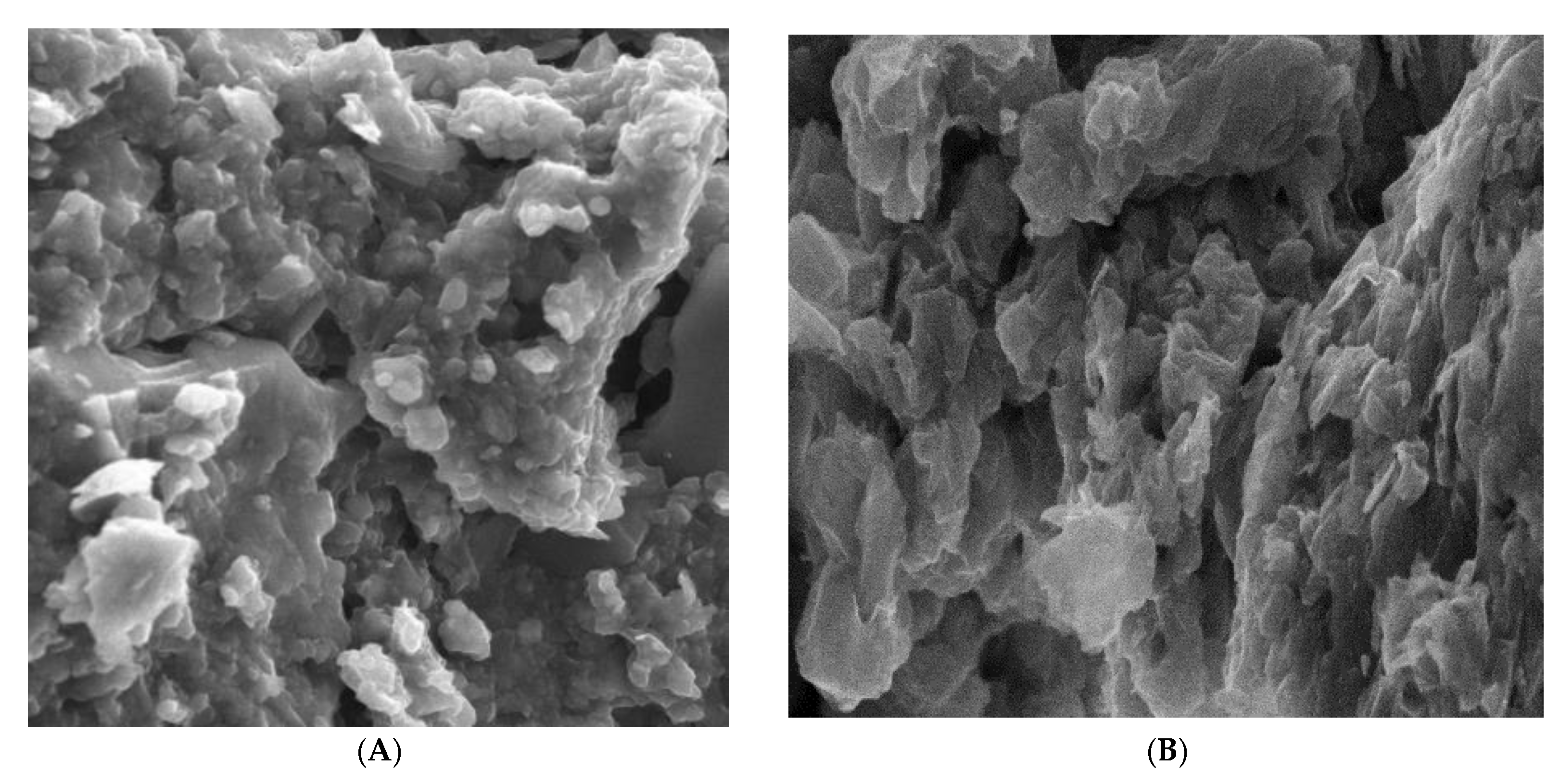



| Sample | Surface Area (m2 g−1) | Average Pore Diameter (nm) | Pore Volume (cm3 g−1) |
|---|---|---|---|
| GO | 37.22 | 3.52 | 0.017 |
| Zr-GO | 14.57 | 2.13 | 0.001 |
| Analyte | Linear Range | Regression Equation Slope | Intercept | Correction Co-Efficient | LOD |
|---|---|---|---|---|---|
| (µM) | (µM) | ||||
| H2O2 | 10–1000 | 0.002 | 0.005 | 0.996 | 0.57 |
| Nanomaterial | Linear Range (μM) | Detection Limit (μM) | References |
|---|---|---|---|
| TiO2 Nanoparticles | 100 to 50,000 | 61 | [58] |
| Ag@Amalg12 @ Fe3O4 | 0 to 1250 | 14 | [59] |
| V2o5-MoS2/Rgo | 20 to 800 | 12.4 | [60] |
| NiCo2S4@N,S-Rgo | 40 to 50,000 | 12 | [61] |
| Ce-Mof Nanosphere | 4000 to 16,000 | 10 | [62] |
| Fen3/Ptn4-Single-Atom Nanozymes (Sazyme) | 10 to 1000 | 7.97 | [63] |
| Zr-Mof-Pvp Nanocomposites | 10 to 800 | 2.76 | [64] |
| Me-Ag Nps | 0.05 to 20 | 0.82 | [65] |
| CuO Nanotubes | 2 to 150 | 0.62 | [66] |
| Zr-GO | 10 to 1000 | 0.57 | Present study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddiqui, A.S.; Ahmad, M.A.; Nawaz, M.H.; Hayat, A.; Nasir, M. Decorating Zirconium on Graphene Oxide to Design a Multifunctional Nanozyme for Eco-Friendly Detection of Hydrogen Peroxide. Catalysts 2022, 12, 1105. https://doi.org/10.3390/catal12101105
Siddiqui AS, Ahmad MA, Nawaz MH, Hayat A, Nasir M. Decorating Zirconium on Graphene Oxide to Design a Multifunctional Nanozyme for Eco-Friendly Detection of Hydrogen Peroxide. Catalysts. 2022; 12(10):1105. https://doi.org/10.3390/catal12101105
Chicago/Turabian StyleSiddiqui, Ayesha Saleem, Muhammad Ashfaq Ahmad, Mian Hasnain Nawaz, Akhtar Hayat, and Muhammad Nasir. 2022. "Decorating Zirconium on Graphene Oxide to Design a Multifunctional Nanozyme for Eco-Friendly Detection of Hydrogen Peroxide" Catalysts 12, no. 10: 1105. https://doi.org/10.3390/catal12101105
APA StyleSiddiqui, A. S., Ahmad, M. A., Nawaz, M. H., Hayat, A., & Nasir, M. (2022). Decorating Zirconium on Graphene Oxide to Design a Multifunctional Nanozyme for Eco-Friendly Detection of Hydrogen Peroxide. Catalysts, 12(10), 1105. https://doi.org/10.3390/catal12101105