Design and Microwave-Assisted Synthesis of TiO2-Lanthanides Systems and Evaluation of Photocatalytic Activity under UV-LED Light Irradiation
Abstract
:1. Introduction
2. Results
2.1. Crystal Structure
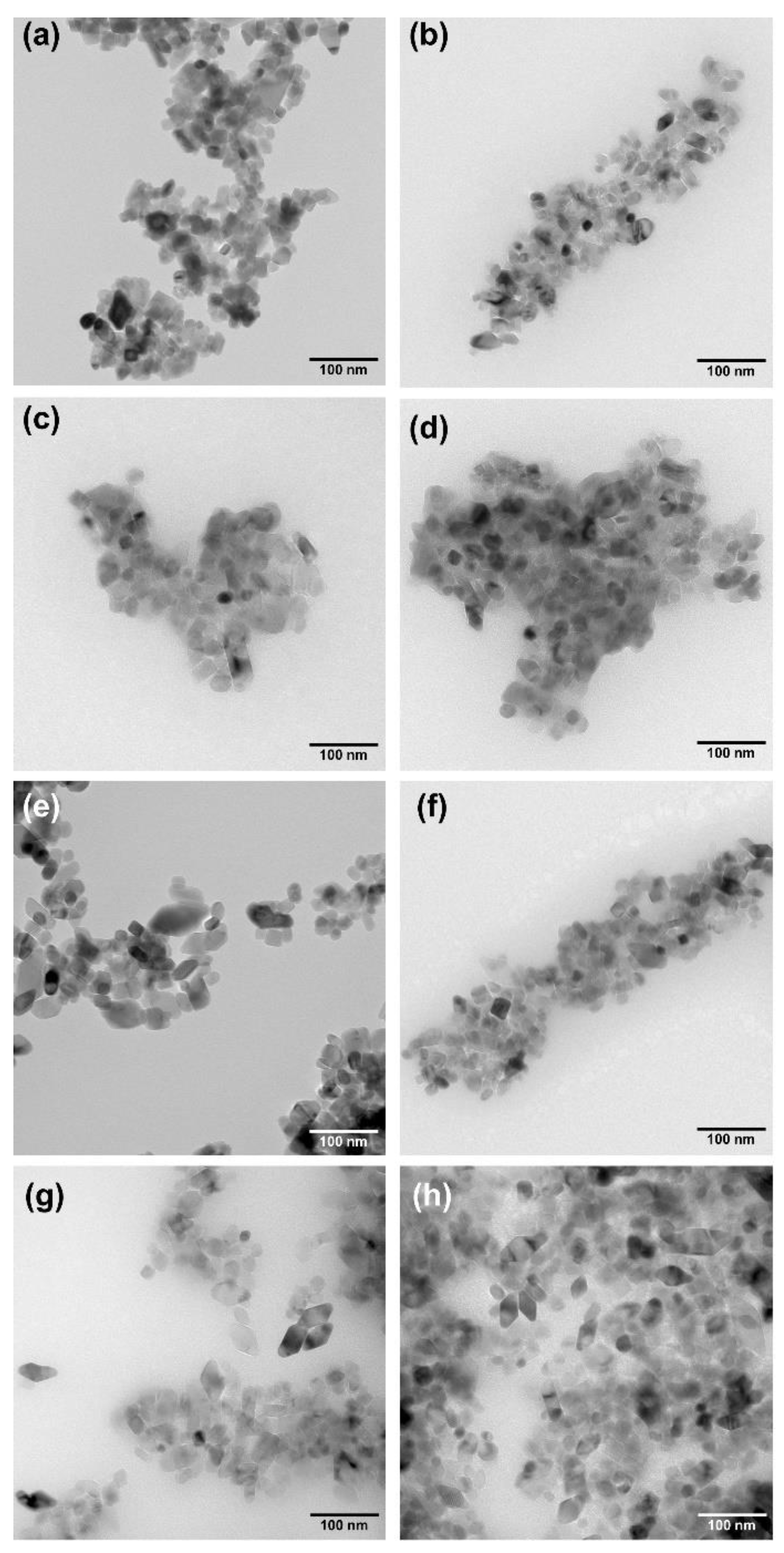
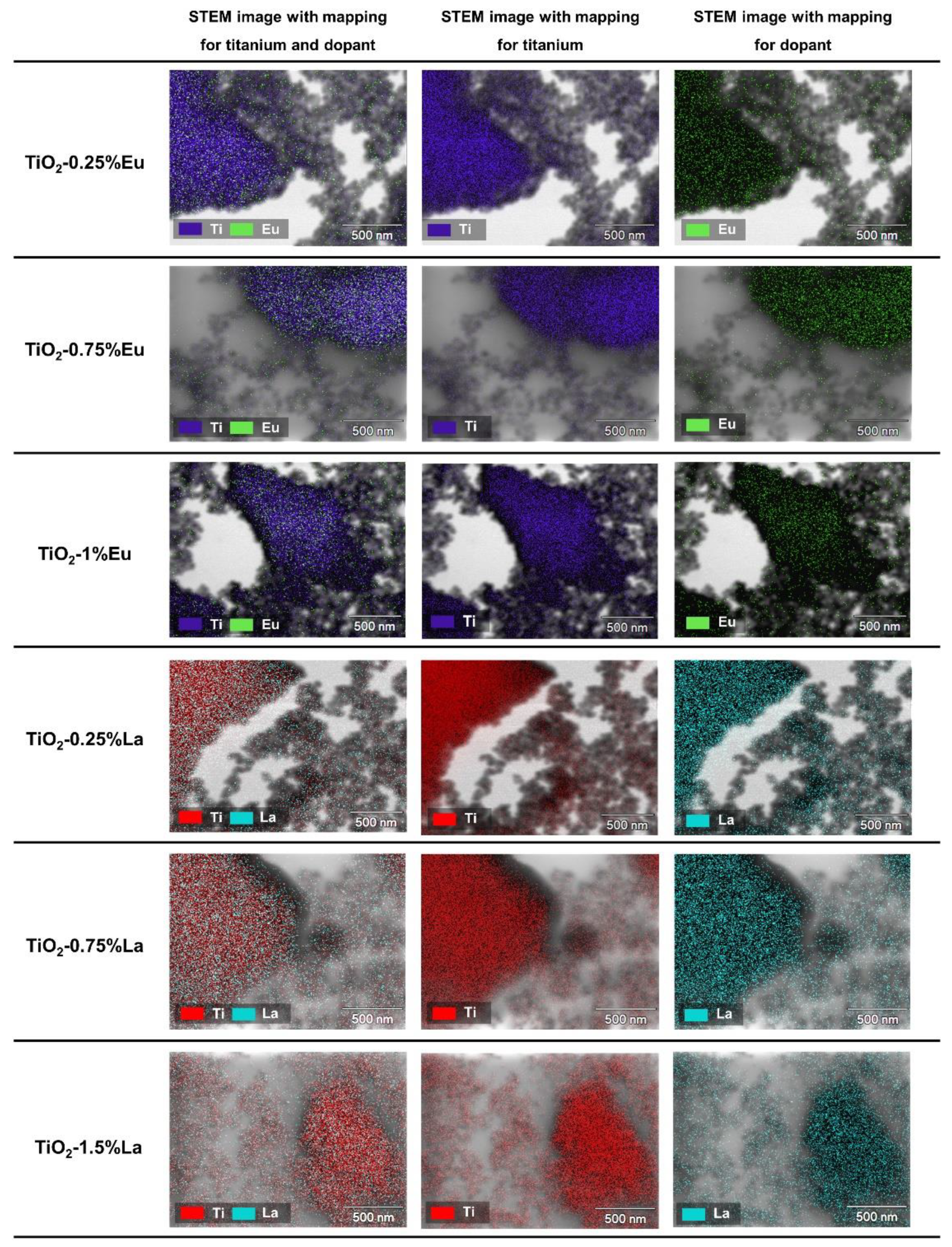
2.2. High-Resolution Transmission Electron Microscopy (HR-TEM)
2.3. X-ray Photoelectron Spectroscopy
2.4. Morphology and Surface Properties
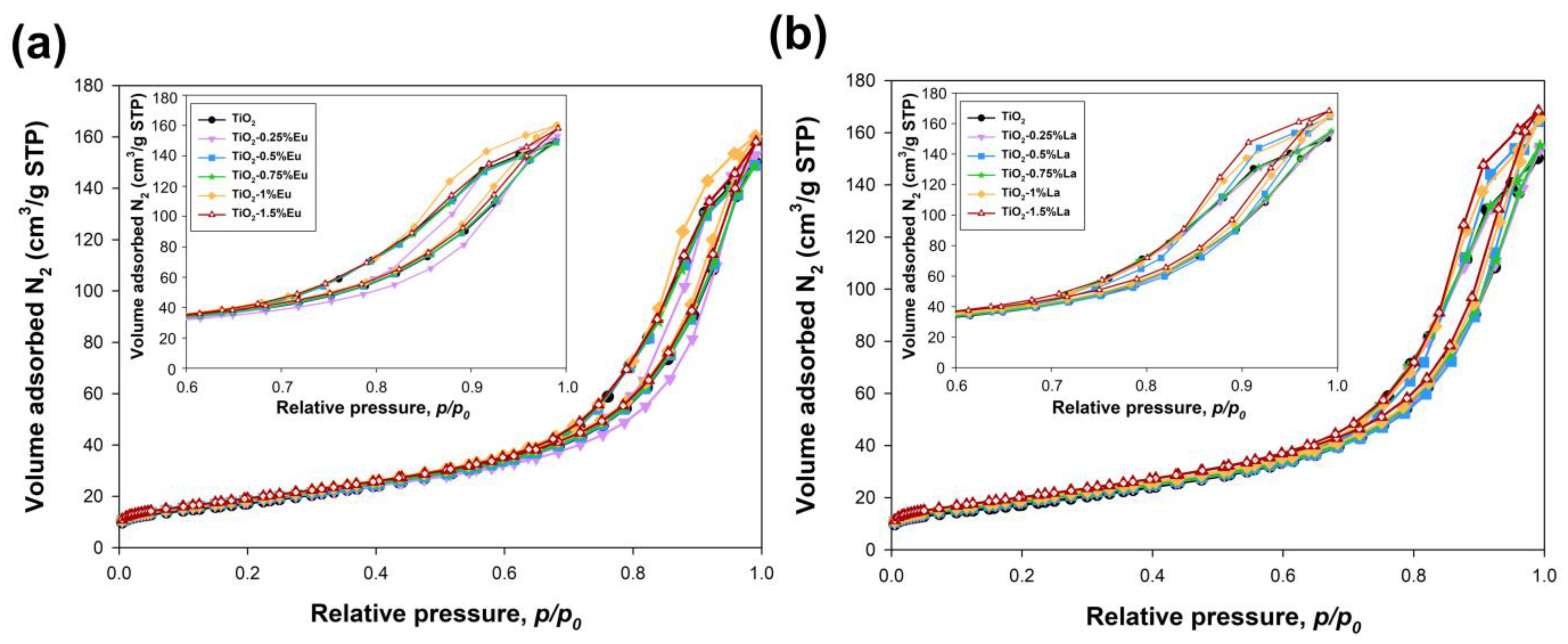
2.5. Parameters of the Porous Structure
2.6. Optical Properties
2.7. Thermal Stability
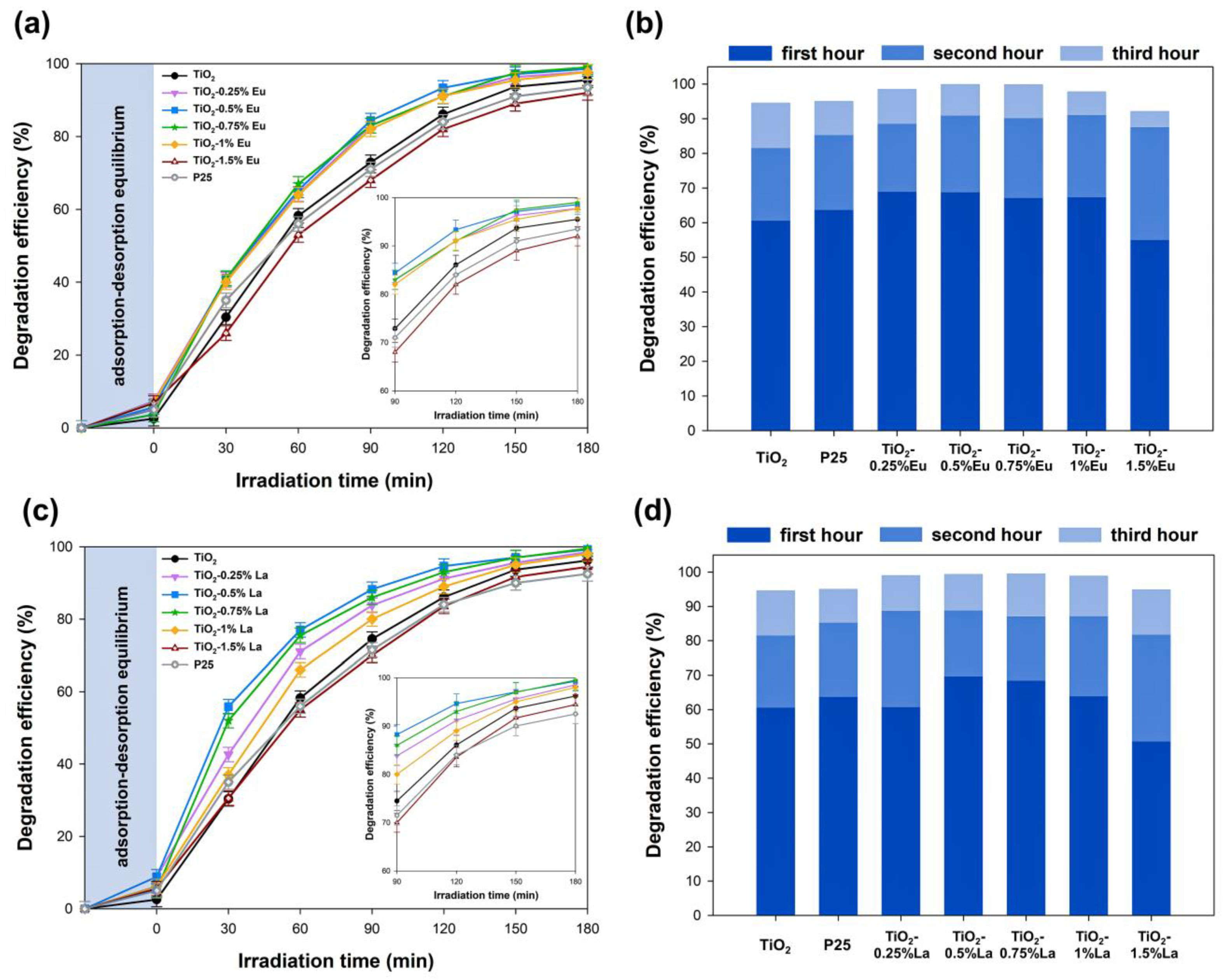
2.8. Photocatalytic Activity
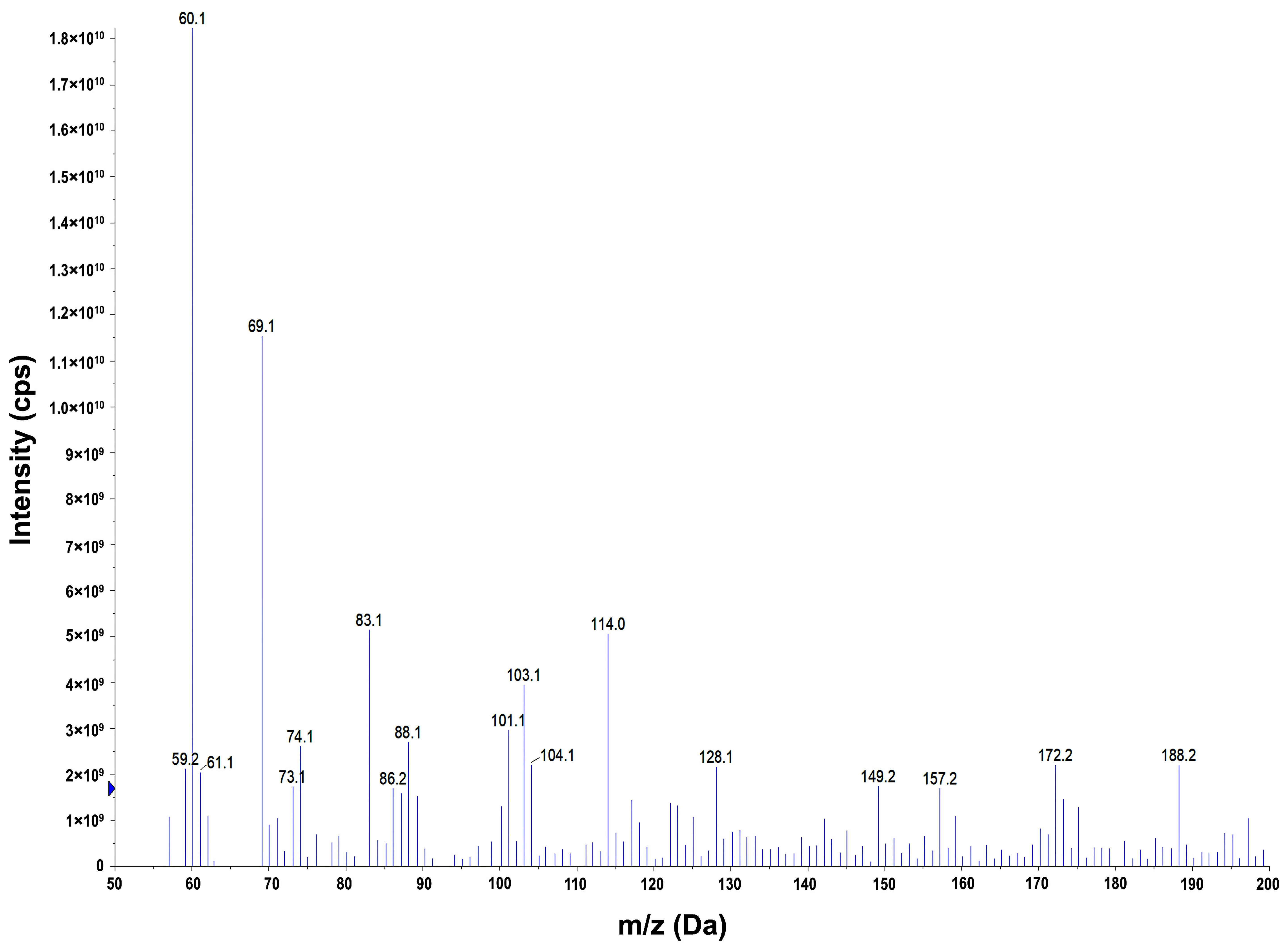
2.9. Identification of Degradation Products
3. Materials and Methods
3.1. Materials
3.2. Synthesis of TiO2-Eu and TiO2-La Systems
3.3. Characteristics of Synthesized Systems
3.4. Photocatalytic Activity of TiO2-Eu and TiO2-La Systems
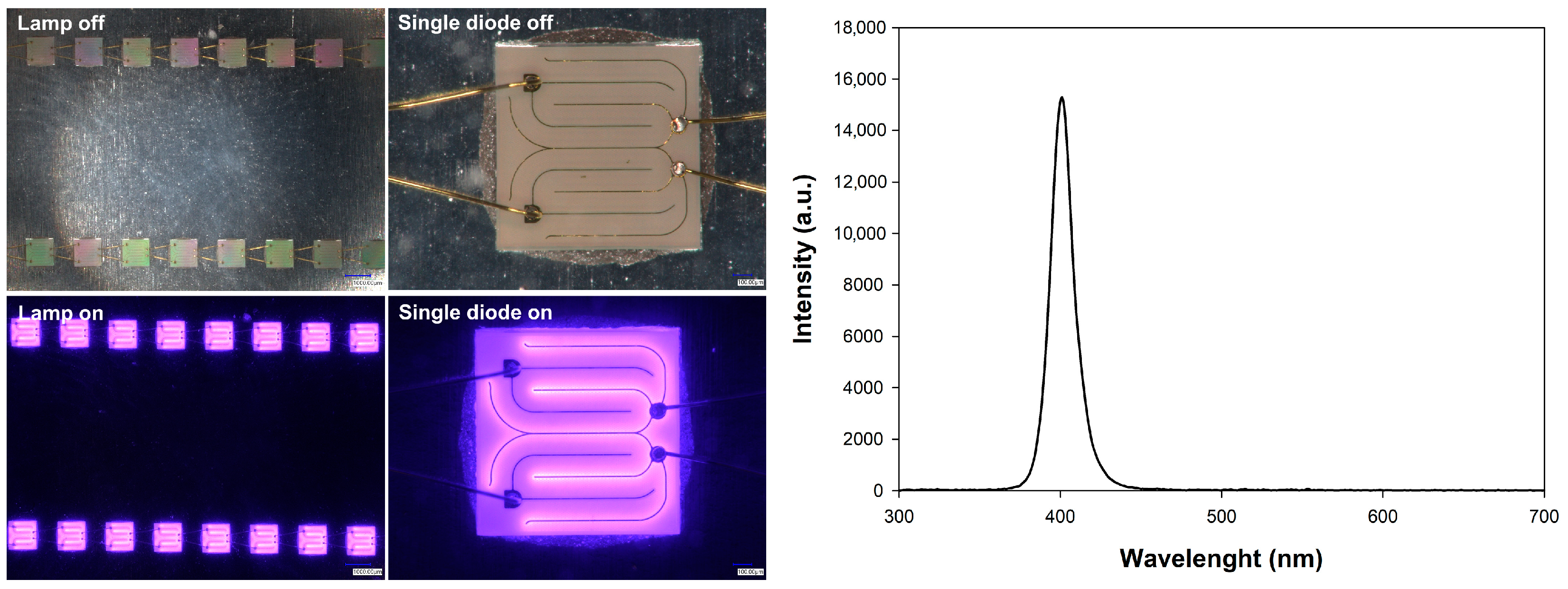
3.4.1. The Used Light Source
3.4.2. The Photo-Oxidation Measurement
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lienert, J.; Bürki, T.; Escher, B.I. Reducing micropollutants with source control: Substance flow analysis of 212 pharmaceuticals in faeces and urine. Water Sci. Technol. 2007, 56, 87–96. [Google Scholar] [CrossRef]
- Jjemba, P.K. Excretion and ecotoxicity of pharmaceutical and personal care products in the environment. Ecotoxicol. Environ. Saf. 2006, 63, 113–130. [Google Scholar] [CrossRef]
- Hanna, N.; Sun, P.; Sun, Q.; Li, X.; Yang, X.; Ji, X.; Zou, H.; Ottoson, J.; Nilsson, L.E.; Berglund, B.; et al. Presence of antibiotic residues in various environmental compartments of Shandong province in eastern China: Its potential for resistance development and ecological and human risk. Environ. Int. 2018, 114, 131–142. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Igwegbe, C.A.; Adeniyi, A.G.; Adeyanju, C.A.; Ogunniyi, S. Mitigation of Metronidazole (Flagyl) pollution in aqueous media by adsorption: A review. Environ. Technol. Rev. 2020, 9, 137–148. [Google Scholar] [CrossRef]
- Dong, S.; Sun, J.; Li, Y.; Yu, C.; Li, Y.; Sun, J. ZnSnO3 hollow nanospheres/reduced graphene oxide nanocomposites as high-performance photocatalysts for degradation of metronidazole. Appl. Catal. B Environ. 2014, 144, 386–393. [Google Scholar] [CrossRef]
- Gómez, M.J.; Malato, O.; Ferrer, I.; Agüera, A.; Fernández-Alba, A.R. Solid-phase extraction followed by liquid chromatography-time-of-flight-mass spectrometry to evaluate pharmaceuticals in effluents. A pilot monitoring study. J. Environ. Monit. 2007, 9, 719–729. [Google Scholar] [CrossRef]
- Dantas, R.F.; Rossiter, O.; Teixeira, A.K.R.; Simões, A.S.M.; da Silva, V.L. Direct UV photolysis of propranolol and metronidazole in aqueous solution. Chem. Eng. J. 2010, 158, 143–147. [Google Scholar] [CrossRef]
- Vulliet, E.; Cren-Olivé, C. Screening of pharmaceuticals and hormones at the regional scale, in surface and groundwaters intended to human consumption. Environ. Pollut. 2011, 159, 2929–2934. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.B.; Mehrvar, M. Aqueous metronidazole degradation by UV/H2O2 process in single-and multi-lamp tubular photoreactors: Kinetics and reactor design. Ind. Eng. Chem. Res. 2008, 47, 6525–6537. [Google Scholar] [CrossRef]
- Aarab, N.; Hsini, A.; Essekri, A.; Laabd, M.; Lakhmiri, R.; Albourine, A. Removal of an emerging pharmaceutical pollutant (metronidazole) using PPY-PANi copolymer: Kinetics, equilibrium and DFT identification of adsorption mechanism. Groundw. Sustain. Dev. 2020, 11, 100416. [Google Scholar] [CrossRef]
- Manjunath, S.V.; Kumar, M. Evaluation of single-component and multi-component adsorption of metronidazole, phosphate and nitrate on activated carbon from Prosopıs julıflora. Chem. Eng. J. 2018, 346, 525–534. [Google Scholar] [CrossRef]
- Asgari, E.; Sheikhmohammadi, A.; Yeganeh, J. Application of the Fe3O4-chitosan nano-adsorbent for the adsorption of metronidazole from wastewater: Optimization, kinetic, thermodynamic and equilibrium studies. Int. J. Biol. Macromol. 2020, 164, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Aarab, N.; Hsini, A.; Laabd, M.; Essekri, A.; Laktif, T.; Haki, M.A.; Lakhmiri, R.; Albourine, A. Theoretical study of the adsorption of sodium salicylate and metronidazole on the PANi. Mater. Today Proc. 2020, 22, 100–103. [Google Scholar] [CrossRef]
- Alamgir; Talha, K.; Wang, B.; Liu, J.H.; Ullah, R.; Feng, F.; Yu, J.; Chen, S.; Li, J.R. Effective adsorption of metronidazole antibiotic from water with a stable Zr(IV)-MOFs: Insights from DFT, kinetics and thermodynamics studies. J. Environ. Chem. Eng. 2020, 8, 103642. [Google Scholar] [CrossRef]
- Farzadkia, M.; Bazrafshan, E.; Esrafili, A.; Yang, J.K.; Shirzad-Siboni, M. Photocatalytic degradation of Metronidazole with illuminated TiO2 nano-particles. J. Environ. Health Sci. Eng. 2015, 13, 35. [Google Scholar] [CrossRef] [Green Version]
- Tran, M.L.; Fu, C.C.; Juang, R.S. Removal of metronidazole by TiO2 and ZnO photocatalysis: A comprehensive comparison of process optimization and transformation products. Environ. Sci. Pollut. Res. 2018, 25, 28285–28295. [Google Scholar] [CrossRef] [PubMed]
- Ahammad, S.Z.; Gomes, J.; Sreekrishnan, T.R. Wastewater treatment forproductionof H2S-free biogas. J. Chem. Technol. Biotechnol. 2008, 83, 1163–1169. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Titanium dioxide photocatalysis for pharmaceutical wastewater treatment. Environ. Chem. Lett. 2014, 12, 27–47. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Goud, B.S.; Koyyada, G.; Jung, J.H.; Reddy, G.R.; Shim, J.; Nam, N.D.; Vattikuti, S.V.P. Surface oxygen vacancy facilitated Z-scheme MoS2/Bi2O3 heterojunction for enhanced visible-light driven photocatalysis-pollutant degradation and hydrogen production. Int. J. Hydrogen Energy 2020, 45, 18961–18975. [Google Scholar] [CrossRef]
- Raizada, P.; Kumar, A.; Hasija, V.; Singh, P.; Thakur, V.K.; Khan, A.A.P. An overview of converting reductive photocatalyst into all solid-state and direct Z-scheme system for water splitting and CO2 reduction. J. Ind. Eng. Chem. 2021, 93, 1–27. [Google Scholar] [CrossRef]
- Yao, X.; Hu, X.; Liu, Y.; Wang, X.; Hong, X.; Chen, X.; Pillai, S.C.; Dionysiou, D.D.; Wang, D. Simultaneous photocatalytic degradation of ibuprofen and H2 evolution over Au/sheaf-like TiO2 mesocrystals. Chemosphere 2020, 261, 127759. [Google Scholar] [CrossRef]
- Zhang, M.; Lai, C.; Li, B.; Xu, F.; Huang, D.; Liu, S.; Qin, L.; Fu, Y.; Liu, X.; Yi, H.; et al. Unravelling the role of dual quantum dots cocatalyst in 0D/2D heterojunction photocatalyst for promoting photocatalytic organic pollutant degradation. Chem. Eng. J. 2020, 396, 125343. [Google Scholar] [CrossRef]
- Raizada, P.; Soni, V.; Kumar, A.; Singh, P.; Khan, A.A.P.; Asiri, A.M.; Thakur, V.K.; Nguyen, V.H. Surface defect engineering of metal oxides photocatalyst for energy application and water treatment. J. Metar. 2021, 7, 388–418. [Google Scholar] [CrossRef]
- Zhou, J.; Ding, J.; Wan, H.; Guan, G. Boosting photocatalytic degradation of antibiotic wastewater by synergy effect of heterojunction and phosphorus doping. J. Colloid Interface Sci. 2021, 582, 961–968. [Google Scholar] [CrossRef]
- Ismael, M. A review and recent advances in solar-to-hydrogen energy conversion based on photocatalytic water splitting over doped-TiO2 nano-particles. Sol. Energy 2020, 211, 522–546. [Google Scholar] [CrossRef]
- Singh, J.; Soni, R.K. Controlled synthesis of CuO decorated defect enriched ZnO nanoflakes for improved sunlight-induced photocatalytic degradation of organic pollutants. Appl. Surf. Sci. 2020, 521, 146420. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Q.; Tian, X.; Hong, Y.; Nie, Y.; Su, N.; Jin, G.; Zhai, Z.; Fu, C. Highly efficient photocatalytic degradation of oil pollutants by oxygen deficient SnO2 quantum dots for water remediation. Chem. Eng. J. 2021, 404, 127146. [Google Scholar] [CrossRef]
- Ding, J.; Wang, H.; Luo, Y.; Xu, Y.; Liu, J.; Lin, R.; Gao, Y.; Lin, Y. Carbon quantum dots modified (002) oriented Bi2O2CO3 composites with enhanced photocatalytic removal of toluene in air. Nanomaterials 2020, 10, 1795. [Google Scholar] [CrossRef] [PubMed]
- Grzegórska, A.; Głuchowski, P.; Karczewski, J.; Ryl, J.; Wysocka, I.; Siuzdak, K.; Trykowski, G.; Grochowska, K.; Zielińska-Jurek, A. Enhanced photocatalytic activity of accordion-like layered Ti3C2 (MXene) coupled with Fe-modified decahedral anatase particles exposing {101} and {001} facets. Chem. Eng. J. 2021, 426, 130801. [Google Scholar] [CrossRef]
- Saqib, N.U.; Adnan, R.; Shah, I. A mini-review on rare earth metal-doped TiO2 for photocatalytic remediation of wastewater. Environ. Sci. Pollut. Res. 2016, 23, 15941–15951. [Google Scholar] [CrossRef]
- Huo, Y.; Zhu, J.; Li, J.; Li, G.; Li, H. An active La/TiO2 photocatalyst prepared by ultrasonication-assisted sol-gel method followed by treatment under supercritical conditions. J. Mol. Catal. A Chem. 2007, 278, 237–243. [Google Scholar] [CrossRef]
- Khade, G.V.; Gavade, N.L.; Suwarnkar, M.B.; Dhanavade, M.J.; Sonawane, K.D.; Garadkar, K.M. Enhanced photocatalytic activity of europium doped TiO2 under sunlight for the degradation of methyl orange. J. Mater. Sci. Mater. Electron. 2017, 28, 11002–11011. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, T.; Wang, H. Preparation and photocatalytic activity of La3+ and Eu3+ co-doped TiO2 nano-particles: Photo-assisted degradation of methylene blue. J. Rare Earths 2011, 29, 746–752. [Google Scholar] [CrossRef]
- Tahir, M. La-modified TiO2/carbon nanotubes assembly nanocomposite for efficient photocatalytic hydrogen evolution from glycerol-water mixture. Int. J. Hydrogen Energy 2019, 44, 3711–3725. [Google Scholar] [CrossRef]
- Huang, C.Y.; Guo, R.T.; Pan, W.G.; Tang, J.Y.; Zhou, W.G.; Qin, H.; Liu, X.Y.; Jia, P.Y. Eu-doped TiO2 nano-particles with enhanced activity for CO2 phpotcatalytic reduction. J. CO2 Util. 2018, 26, 487–495. [Google Scholar] [CrossRef]
- Kubiak, A.; Wojciechowska, W.; Kurc, B.; Pigłowska, M.; Synoradzki, K.; Gabała, E.; Moszyński, D.; Szybowicz, M.; Siwińska-Ciesielczyk, K.; Jesionowski, T. Highly crystalline TiO2-MoO3 composite materials synthesized via a template-assisted microwave method for electrochemical application. Crystals 2020, 10, 493. [Google Scholar] [CrossRef]
- Kubiak, A.; Bielan, Z.; Kubacka, M.; Gabała, E.; Zgoła-Grześkowiak, A.; Janczarek, M.; Zalas, M.; Zielińska-Jurek, A.; Siwińska-Ciesielczyk, K.; Jesionowski, T. Microwave-assisted synthesis of a TiO2-CuO heterojunction with enhanced photocatalytic activity against tetracycline. Appl. Surf. Sci. 2020, 520, 146344. [Google Scholar] [CrossRef]
- Kubiak, A.; Bielan, Z.; Bartkowiak, A.; Gabała, E.; Piasecki, A.; Zalas, M.; Zielińska-Jurek, A.; Janczarek, M.; Siwińska-Ciesielczyk, K.; Jesionowski, T. Synthesis of titanium dioxide via surfactantassisted microwave method for photocatalytic and dye-sensitized solar cells applications. Catalysts 2020, 10, 586. [Google Scholar] [CrossRef]
- Fagan, R.; McCormack, D.E.; Dionysiou, D.D.; Pillai, S.C. A review of solar and visible light active TiO2 photocatalysis for treating bacteria, cyanotoxins and contaminants of emerging concern. Mater. Sci. Semicond. Process. 2016, 42, 2–14. [Google Scholar] [CrossRef] [Green Version]
- Nian, J.N.; Teng, H. Hydrothermal synthesis of single-crystalline anatase TiO2 nanorods with nanotubes as the precursor. J. Phys. Chem. B 2006, 110, 4193–4198. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Luo, C.; Fu, Q.; Pan, C. Hydrothermal synthesis of the novel rutile-mixed anatase TiO2 nanosheets with dominant {001} facets for high photocatalytic activity. RSC Adv. 2016, 6, 84035–84041. [Google Scholar] [CrossRef]
- Zhou, J.; Lei, R.; Wang, H.; Hua, Y.; Li, D.; Yang, Q.; Deng, D.; Xu, S. A new generation of dual-mode optical thermometry based on ZrO2:Eu3+ nanocrystals. Nanophotonics 2019, 8, 2347–2358. [Google Scholar] [CrossRef]
- Zeng, C.H.; Zheng, K.; Lou, K.L.; Meng, X.T.; Yan, Z.Q.; Ye, Z.N.; Su, R.R.; Zhong, S. Synthesis of porous europium oxide particles for photoelectrochemical water splitting. Electrochim. Acta 2015, 165, 396–401. [Google Scholar] [CrossRef]
- Kim, M.S.; Chung, S.H.; Yoo, C.J.; Lee, M.S.; Cho, I.H.; Lee, D.W.; Lee, K.Y. Catalytic reduction of nitrate in water over Pd-Cu/TiO2 catalyst: Effect of the strong metal-support interaction (SMSI) on the catalytic activity. Appl. Catal. B Environ. 2013, 142–143, 354–361. [Google Scholar] [CrossRef]
- Olowoyo, J.O.; Kumar, M.; Dash, T.; Saran, S.; Bhandari, S.; Kumar, U. Self-organized copper impregnation and doping in TiO2 with enhanced photocatalytic conversion of H2O and CO2 to fuel. Int. J. Hydrogen Energy 2018, 43, 19468–19480. [Google Scholar] [CrossRef]
- Singh, D.; Tanwar, V.; Simantilleke, A.P.; Mari, B.; Kadyan, P.S.; Singh, I. Rapid synthesis and enhancement in down conversion emission properties of BaAl2O4:Eu2+,Re3+ (Re3+ = Y, Pr) nanophosphors. J. Mater. Sci. Mater. Electron. 2016, 27, 2260–2266. [Google Scholar] [CrossRef]
- Tian, B.; Li, C.; Gu, F.; Jiang, H.; Hu, Y.; Zhang, J. Flame sprayed V-doped TiO2 nano-particles with enhanced photocatalytic activity under visible light irradiation. Chem. Eng. J. 2009, 151, 220–227. [Google Scholar] [CrossRef]
- Ola, O.; Maroto-Valer, M.M. Synthesis, characterization and visible light photocatalytic activity of metal based TiO2 monoliths for CO2 reduction. Chem. Eng. J. 2016, 283, 1244–1253. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Chen, F.; Zhang, J.; Chen, H.; Anpo, M. Fe3+-TiO2 photocatalysts prepared by combining sol-gel method with hydrothermal treatment and their characterization. J. Photochem. Photobiol. A Chem. 2006, 180, 196–204. [Google Scholar] [CrossRef]
- Liqiang, J.; Xiaojun, S.; Baifu, X.; Baiqi, W.; Weimin, C.; Honggang, F. The preparation and characterization of La doped TiO2 nano-particles and their photocatalytic activity. J. Solid State Chem. 2004, 177, 3375–3382. [Google Scholar] [CrossRef]
- Kibombo, H.S.; Weber, A.S.; Wu, C.M.; Raghupathi, K.R.; Koodali, R.T. Effectively dispersed europium oxide dopants in TiO2 aerogel supports for enhanced photocatalytic pollutant degradation. J. Photochem. Photobiol. A Chem. 2013, 269, 49–58. [Google Scholar] [CrossRef]
- Anandan, S.; Ikuma, Y.; Murugesan, V. Highly active rare-earth-metal La-doped photocatalysts: Fabrication, characterization, and their photocatalytic activity. Int. J. Photoenergy 2012, 2012, 921412. [Google Scholar] [CrossRef]
- Huang, J.; Tian, B.; Wang, J.; Wang, Y.; Lu, W.; Li, Q.; Jin, L.; Li, C.; Wang, Z. Controlled synthesis of 3D flower-like MgWO4:Eu3+ hierarchical structures and fluorescence enhancement through introduction of carbon dots. CrystEngComm 2018, 20, 608–614. [Google Scholar] [CrossRef]
- Siah, W.R.; Lintang, H.O.; Yuliati, L. Role of lanthanum species in improving the photocatalytic activity of titanium dioxide. Catal. Sci. Technol. 2017, 7, 159–167. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.S.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Kubiak, A.; Żółtowska, S.; Gabała, E.; Szybowicz, M.; Siwińska-Ciesielczyk, K.; Jesionowski, T. Controlled microwave-assisted and pH-affected growth of ZnO structures and their photocatalytic performance. Powder Technol. 2021, 386, 221–235. [Google Scholar] [CrossRef]
- Janczarek, M.; Kowalska, E. On the origin of enhanced photocatalytic activity of copper-modified titania in the oxidative reaction systems. Catalysts 2017, 7, 317. [Google Scholar] [CrossRef] [Green Version]
- Juan, J.L.X.; Maldonado, C.S.; Sánchez, R.A.L.; Díaz, O.J.E.; Ronquillo, M.R.R.; Sandoval-Rangel, L.; Aguilar, N.P.; Delgado, N.A.T.; Martínez-Vargas, D.X. TiO2 doped with europium (Eu): Synthesis, characterization and catalytic performance on pesticide degradation under solar irradiation. Catal. Today, 2021; in press. [Google Scholar] [CrossRef]
- Wang, R.; Wang, F.; An, S.; Song, J.; Zhang, Y. Y/Eu co-doped TiO2: Synthesis and photocatalytic activities under UV-light. J. Rare Earths 2015, 33, 154–159. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, G.; Cui, Z.; Zhang, K.; Feng, Y.; Meng, S. Influence of difference quantity La-doped TiO2 photoanodes on the performance of dye-sensitized solar cells: A strategy for choosing an appropriate doping quantity. J. Solid State Chem. 2016, 237, 242–247. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, J.; Yang, Y.; Lu, Z.; Shi, W. Understanding electronic and optical properties of La and Mn co-doped anatase TiO2. Comput. Condens. Matter 2016, 6, 5–17. [Google Scholar] [CrossRef] [Green Version]
- Borlaf, M.; Moreno, R.; Ortiz, A.L.; Colomer, M.T. Synthesis and photocatalytic activity of Eu3+-doped nanoparticulate TiO2 sols and thermal stability of the resulting xerogels. Mater. Chem. Phys. 2014, 144, 8–16. [Google Scholar] [CrossRef]
- Armaković, S.J.; Grujić-Brojčin, M.; Šćepanović, M.; Armaković, S.; Golubović, A.; Babić, B.; Abramović, B.F. Efficiency of La-doped TiO2 calcined at different temperatures in photocatalytic degradation of β-blockers. Arab. J. Chem. 2019, 12, 5355–5369. [Google Scholar] [CrossRef]
- Šćepanović, M.; Aškrabić, S.; Berec, V.; Golubović, A.; Dohčević-Mitrović, Z.; Kremenović, A.; Popović, Z.V. Characterization of La-doped TiO2 nanopowders by Raman spectroscopy. Acta Phys. Pol. A 2009, 115, 771–774. [Google Scholar] [CrossRef]
- Kubiak, A.; Siwińska-Ciesielczyk, K.; Bielan, Z.; Zielińska-Jurek, A.; Jesionowski, T. Synthesis of highly crystalline photocatalysts based on TiO2 and ZnO for the degradation of organic impurities under visible-light irradiation. Adsorption 2019, 25, 309–325. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Yuan, C. Characterization and photocatalysis of Eu3+-TiO2 sol in the hydrosol reaction system. Mater. Res. Bull. 2004, 39, 533–543. [Google Scholar] [CrossRef]
- Weidner, E.; Siwińska-Ciesielczyk, K.; Moszyński, D.; Jesionowski, T.; Ciesielczyk, F. A comprehensive method for tetracycline removal using a lanthanum-enriched titania–zirconia oxide system with tailored physicochemical properties. Environ. Technol. Innov. 2021, 24, 102016. [Google Scholar] [CrossRef]
- Xiaohong, W.; Wei, Q.; Xianbo, D.; Yang, W.; Huiling, L.; Zhaohua, J. Photocatalytic activity of Eu-doped TiO2 ceramic films prepared by microplasma oxidation method. J. Phys. Chem. Solids 2007, 68, 2387–2393. [Google Scholar] [CrossRef]
- Wagil, M.; Maszkowska, J.; Białk-Bielińska, A.; Caban, M.; Stepnowski, P.; Kumirska, J. Determination of metronidazole residues in water, sediment and fish tissue samples. Chemosphere 2015, 119, S28–S34. [Google Scholar] [CrossRef]
- Freeman, C.D.; Klutman, N.E.; Lamp, K.C. Metronidazole. A therapeutic review and update. Drugs 1997, 54, 679–708. [Google Scholar] [CrossRef]
- Sánchez-Polo, M.; Rivera-Utrilla, J.; Prados-Joya, G.; Ferro-García, M.A.; Bautista-Toledo, I. Removal of pharmaceutical compounds, nitroimidazoles, from waters by using the ozone/carbon system. Water Res. 2008, 42, 4163–4171. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qian, Y.; Liu, H.; Huang, T. Oxidative degradation of diclofenac by thermally activated persulfate: Implication for ISCO. Environ. Sci. Pollut. Res. 2016, 23, 3824–3833. [Google Scholar] [CrossRef] [PubMed]
- Zarezadeh, S.; Habibi-Yangjeh, A.; Mousavi, M. BiOBr and AgBr co-modified ZnO photocatalyst: A novel nanocomposite with p-n-n heterojunctions for highly effective photocatalytic removal of organic contaminants. J. Photochem. Photobiol. A Chem. 2019, 379, 11–23. [Google Scholar] [CrossRef]
- Cheng, W.; Yang, M.; Xie, Y.; Liang, B.; Fang, Z.; Tsang, E.P. Enhancement of mineralization of metronidazole by the electro-Fenton process with a Ce/SnO2-Sb coated titanium anode. Chem. Eng. J. 2013, 220, 214–220. [Google Scholar] [CrossRef]
- Ghribi, F.; Sehailia, M.; Aoudjit, L.; Touahra, F.; Zioui, D.; Boumechhour, A.; Halliche, D.; Bachari, K.; Benmaamar, Z. Solar-light promoted photodegradation of metronidazole over ZnO-ZnAl2O4 heterojunction derived from 2D-layered double hydroxide structure. J. Photochem. Photobiol. A Chem. 2020, 397, 112510. [Google Scholar] [CrossRef]
- Li, J.; Zhao, W.; Guo, Y.; Wei, Z.; Han, M.; He, H.; Yang, S.; Sun, C. Facile synthesis and high activity of novel BiVO4/FeVO4 heterojunction photocatalyst for degradation of metronidazole. Appl. Surf. Sci. 2015, 351, 270–279. [Google Scholar] [CrossRef]
- Boxi, S.S.; Paria, S. Effect of silver doping on TiO2, CdS, and ZnS nano-particles for the photocatalytic degradation of metronidazole under visible light. RSC Adv. 2014, 4, 37752–37760. [Google Scholar] [CrossRef]
- Farzadkia, M.; Esrafili, A.; Baghapour, M.A.; Shahamat, Y.D.; Okhovat, N. Degradation of metronidazole in aqueous solution by nano-ZnO/UV photocatalytic process. Desalination Water Treat. 2014, 52, 4947–4952. [Google Scholar] [CrossRef]
- Malakootian, M.; Olama, N.; Malakootian, M.; Nasiri, A. Photocatalytic degradation of metronidazole from aquatic solution by TiO2-doped Fe3+ nano-photocatalyst. Int. J. Environ. Sci. Technol. 2019, 16, 4275–4284. [Google Scholar] [CrossRef]
- Salimi, M.; Esrafili, A.; Sobhi, H.R.; Behbahani, M.; Gholami, M.; Farzadkia, M.; Jafari, A.J.; Kalantary, R.R. Photocatalytic degradation of metronidazole using D-g-C3N4-Bi5O7I composites under visible light irradiation: Degradation product, and mechanisms. ChemistrySelect 2019, 4, 10288–10295. [Google Scholar] [CrossRef]
- Tran, M.L.; Fu, C.C.; Juang, R.S. Effects of water matrix components on degradation efficiency and pathways of antibiotic metronidazole by UV/TiO2 photocatalysis. J. Mol. Liq. 2019, 276, 32–38. [Google Scholar] [CrossRef]
- Ding, C.; Fu, K.; Pan, Y.; Liu, J.; Deng, H.; Shi, J. Comparison of Ag and Agi-modified zno as heterogeneous photocatalysts for simulated sunlight driven photodegradation of metronidazole. Catalysts 2020, 10, 1097. [Google Scholar] [CrossRef]
- Kubiak, A.; Żółtowska, S.; Bartkowiak, A.; Gabała, E.; Sacharczuk, N.; Zalas, M.; Siwińska-Ciesielczyk, K.; Jesionowski, T. The TiO2-ZnO systems with multifunctional applications in photoactive processes—efficient photocatalyst under UV-LED light and electrode materials in DSSCs. Materials 2021, 14, 6063. [Google Scholar] [CrossRef] [PubMed]
- Zia, J.; Farhat, S.M.; Aazam, E.S.; Riaz, U. Highly efficient degradation of metronidazole drug using CaFe2O4/PNA nanohybrids as metal-organic catalysts under microwave irradiation. Environ. Sci. Pollut. Res. 2021, 28, 4125–4135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.H.; Gao, L.; Guo, J.K. Preparation and characterization of nanosized TiO2 powders from aqueous TiCl4 solution. Nanostruct. Mater. 1999, 11, 1293–1300. [Google Scholar] [CrossRef]
- Fairley, N.; Fernandez, V.; Richard-Plouet, M.; Guillot-Deudon, C.; Walton, J.; Smith, E.; Flahaut, D.; Greiner, M.; Biesinger, M.; Tougaard, S.; et al. Systematic and collaborative approach to problem solving using X-ray photoelectron spectroscopy. Appl. Surf. Sci. Adv. 2021, 5, 100112. [Google Scholar] [CrossRef]







| Sample | Crystallite Size (nm) | Phase Composition (%) | Lattice Parameters | |||||
|---|---|---|---|---|---|---|---|---|
| Anatase | Rutile | Anatase | Rutile | Anatase | Rutile | |||
| a (Å) | c (Å) | a (Å) | c (Å) | |||||
| TiO2 | 8.1 | - | 100 | - | 3.78020 | 9.48188 | - | - |
| TiO2-0.25%Eu | 17.0 | 13.7 | 99.5 | 0.5 | 3.79107 | 9.52264 | 4.58045 | 2.94155 |
| TiO2-0.5%Eu | 16.7 | 14.9 | 99.0 | 1.0 | 3.79081 | 9.52052 | 4.60394 | 2.97229 |
| TiO2-0.75%Eu | 16.3 | 15.7 | 97.9 | 2.1 | 3.78916 | 9.51984 | 4.61782 | 2.97654 |
| TiO2-1%Eu | 16.0 | 19.5 | 96.6 | 3.4 | 3.78776 | 9.51701 | 4.62014 | 2.97741 |
| TiO2-1.5%Eu | 15.9 | 22.0 | 95.9 | 4.1 | 3.78615 | 9.48887 | 4.62526 | 2.97845 |
| Sample | Crystallite Size (nm) | Phase Composition (%) | Lattice Parameters | |||||
|---|---|---|---|---|---|---|---|---|
| Anatase | Rutile | Anatase | Rutile | Anatase | Rutile | |||
| a (Å) | c (Å) | a (Å) | c (Å) | |||||
| TiO2 | 8.1 | - | 100 | - | 3.78020 | 9.48188 | - | - |
| TiO2-0.25%La | 17.1 | 10.6 | 99.3 | 0.7 | 3.79101 | 9.52252 | 4.61981 | 2.95819 |
| TiO2-0.5%La | 16.9 | 11.1 | 99.1 | 0.9 | 3.78883 | 9.51708 | 4.62610 | 2.96073 |
| TiO2-0.75%La | 16.9 | 12.1 | 98.0 | 2.0 | 3.78729 | 9.51484 | 4.62274 | 2.96199 |
| TiO2-1%La | 16.5 | 16.3 | 97.9 | 2.1 | 3.78786 | 9.51481 | 2.96073 | 2.96241 |
| TiO2-1.5%La | 16.5 | 17.2 | 97.5 | 2.5 | 3.78741 | 9.51126 | 4.62329 | 2.96435 |
| Sample | TiO2 (wt.%) | Eu-Doped (wt.%) | La-Doped (wt.%) |
|---|---|---|---|
| TiO2-0.25%Eu | 99.71 | 0.29 | - |
| TiO2-0.5%Eu | 99.44 | 0.56 | - |
| TiO2-0.75%Eu | 99.28 | 0.72 | - |
| TiO2-1%Eu | 98.92 | 1.08 | - |
| TiO2-1.5%Eu | 98.42 | 1.58 | - |
| TiO2-0.25%La | 99.79 | - | 0.21 |
| TiO2-0.5%La | 99.40 | - | 0.60 |
| TiO2-0.75%La | 99.15 | - | 0.85 |
| TiO2-1%La | 98.95 | - | 1.05 |
| TiO2-1.5%La | 98.55 | - | 1.45 |
| Sample | ABET (m2/g) | Vp (cm3/g) | Sp (nm) |
| TiO2 | 63 | 0.24 | 13.7 |
| TiO2-0.25%Eu | 65 | 0.24 | 12.5 |
| TiO2-0.5%Eu | 67 | 0.23 | 12.6 |
| TiO2-0.75%Eu | 68 | 0.23 | 12.3 |
| TiO2-1%Eu | 69 | 0.24 | 12.3 |
| TiO2-1.5%Eu | 71 | 0.23 | 12.2 |
| TiO2-0.25%La | 67 | 0.24 | 12.9 |
| TiO2-0.5%La | 67 | 0.24 | 13.1 |
| TiO2-0.75%La | 68 | 0.25 | 13.3 |
| TiO2-1%La | 70 | 0.26 | 13.5 |
| TiO2-1.5%La | 73 | 0.26 | 13.0 |
| Sample | Metronidazole Concentration (mg/dm3) | Amount of Photocatalyst (mg/100 cm3) | Degradation Efficiency (%) | Irradiation Time (min) | Type and Power of the Light Source | Literature |
|---|---|---|---|---|---|---|
| TiO2-0.25%Eu TiO2-0.25%La | 20 | 100 | 99 | 180 | UV-LED (20 W) | this work |
| BiVO4-FeVO4 | 10 | 200 | 91 | 90 | Xe-lamp (500 W) | [77] |
| Ag-CdS | 15 | 100 | 95 | 120 | Hg-lamp (125 W) | [78] |
| ZnO | 80 | 1500 | 97 | 180 | Hg-lamp (125 W) | [79] |
| TiO2-Fe3+ | 80 | 500 | 97 | 120 | Hg-lamp (125 W) | [80] |
| D-g-C3N4-Bi5O7I | 15 | 80 | 80 | 360 | Xe-lamp (300 W) | [81] |
| TiO2-Fe3+ | 20 | 1500 | 50 | - | Hg-lamp (100 W) | [82] |
| TiO2 | 80 | 300 | 95 | 180 | Hg-lamp (125 W) | [15] |
| Ag/ZnO | 10 | 100 | 80 | 30 | Xe-lamp (300 W) | [83] |
| Type of Lamp | Power of Lamp (W) | Power Consumption Per Hour (kWh) | Power Consumption Per Year (kWh) | Cost of Electricity Consumed Assuming Continuous Operation for 365 Days (EUR) * |
|---|---|---|---|---|
| UV-LED | 20 | 0.021 | 184 | 40 |
| Hg high-pressure | 150 | 0.195 | 1708 | 374 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubiak, A.; Grzegórska, A.; Zembrzuska, J.; Zielińska-Jurek, A.; Siwińska-Ciesielczyk, K.; Janczarek, M.; Krawczyk, P.; Jesionowski, T. Design and Microwave-Assisted Synthesis of TiO2-Lanthanides Systems and Evaluation of Photocatalytic Activity under UV-LED Light Irradiation. Catalysts 2022, 12, 8. https://doi.org/10.3390/catal12010008
Kubiak A, Grzegórska A, Zembrzuska J, Zielińska-Jurek A, Siwińska-Ciesielczyk K, Janczarek M, Krawczyk P, Jesionowski T. Design and Microwave-Assisted Synthesis of TiO2-Lanthanides Systems and Evaluation of Photocatalytic Activity under UV-LED Light Irradiation. Catalysts. 2022; 12(1):8. https://doi.org/10.3390/catal12010008
Chicago/Turabian StyleKubiak, Adam, Anna Grzegórska, Joanna Zembrzuska, Anna Zielińska-Jurek, Katarzyna Siwińska-Ciesielczyk, Marcin Janczarek, Piotr Krawczyk, and Teofil Jesionowski. 2022. "Design and Microwave-Assisted Synthesis of TiO2-Lanthanides Systems and Evaluation of Photocatalytic Activity under UV-LED Light Irradiation" Catalysts 12, no. 1: 8. https://doi.org/10.3390/catal12010008
APA StyleKubiak, A., Grzegórska, A., Zembrzuska, J., Zielińska-Jurek, A., Siwińska-Ciesielczyk, K., Janczarek, M., Krawczyk, P., & Jesionowski, T. (2022). Design and Microwave-Assisted Synthesis of TiO2-Lanthanides Systems and Evaluation of Photocatalytic Activity under UV-LED Light Irradiation. Catalysts, 12(1), 8. https://doi.org/10.3390/catal12010008












