Highly Photoactive Titanium Dioxide Supported Platinum Catalyst: Synthesis Using Cleaner Ultrasound Approach
Abstract
1. Introduction
2. Results and Discussion
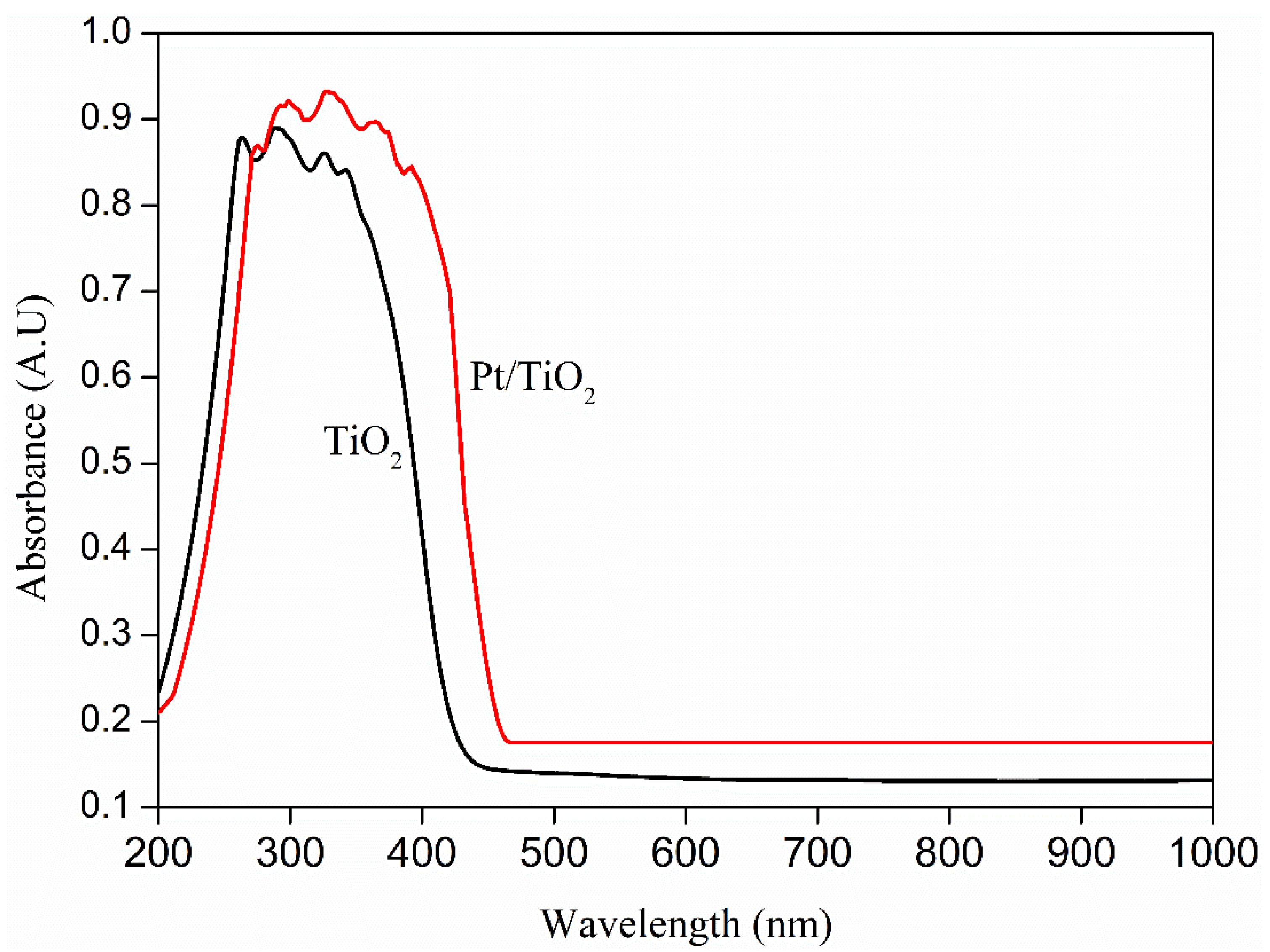
2.1. Photocatalytic Activity of TiO2 and Pt/TiO2
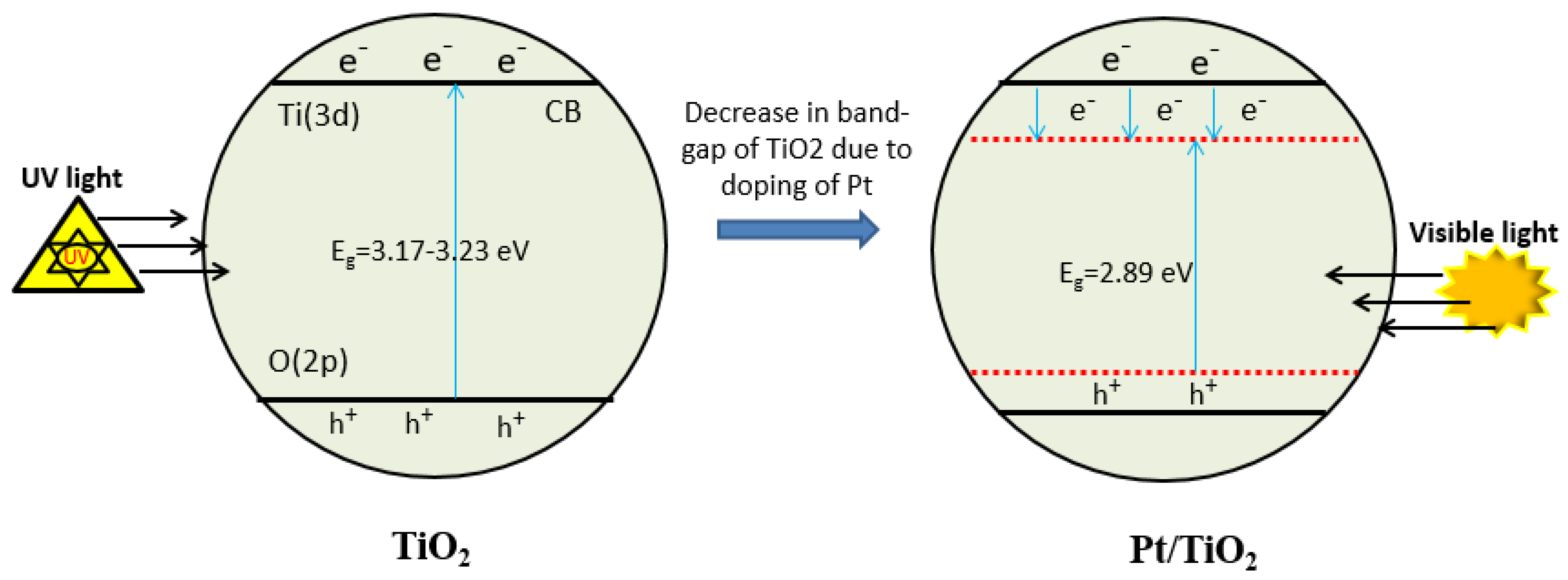
2.2. Mechanism of Doping of Noble Metal Pt on the Surface of TiO2
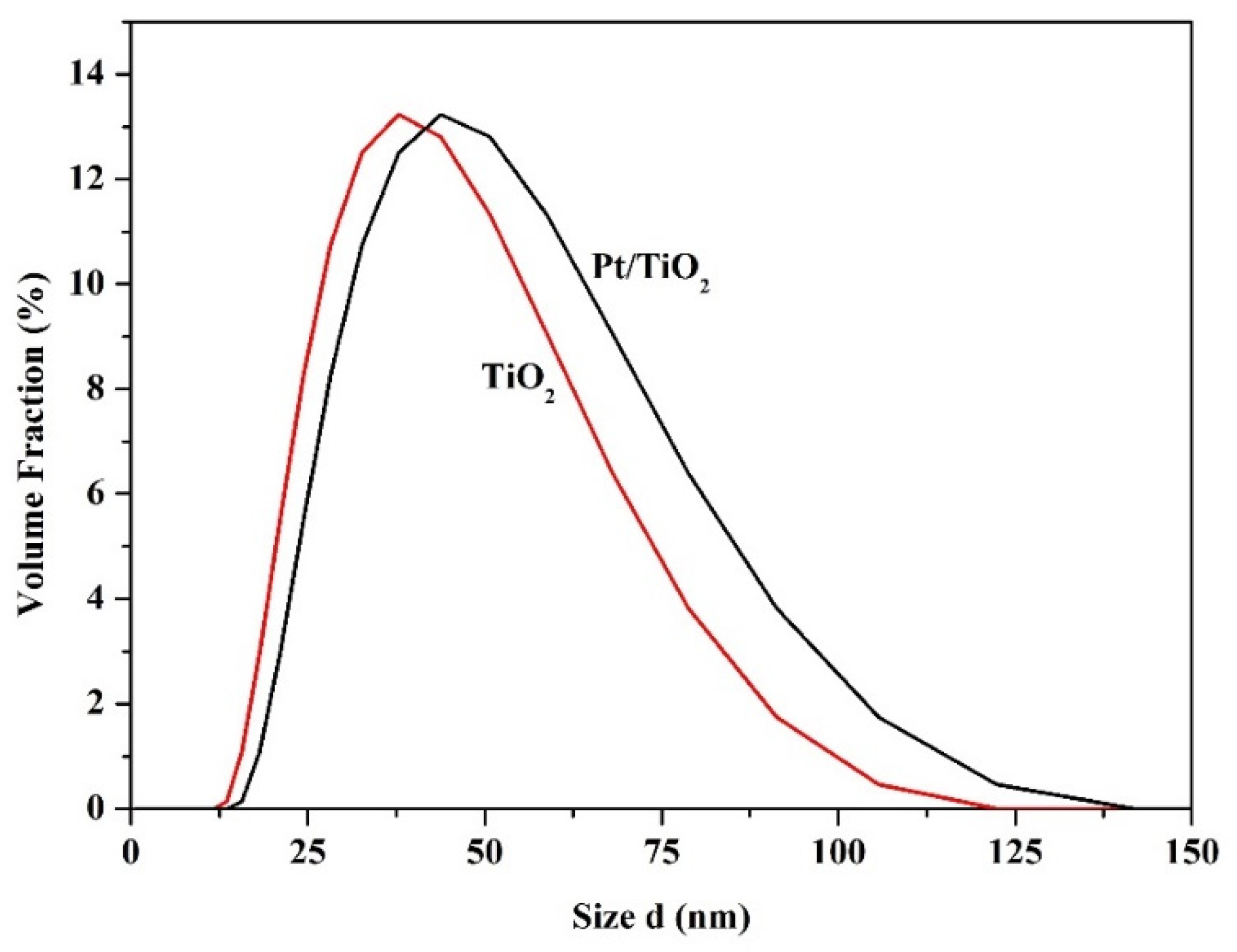
2.3. Particle Size Analysis of TiO2 Support and TiO2 Supported Pt Photocatalyst
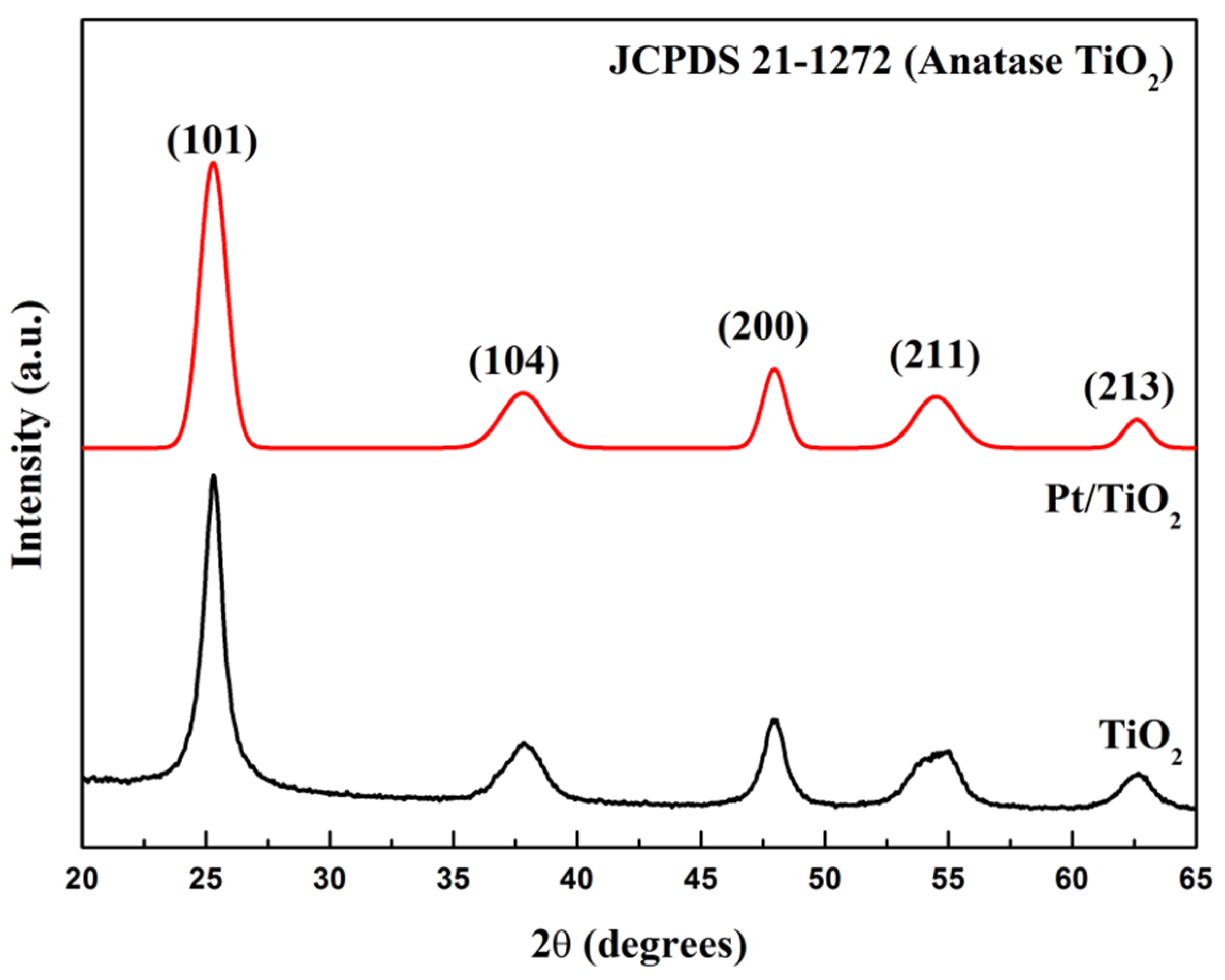
2.4. XRD and BET Analysis of TiO2 Support and TiO2 Supported Pt Photocatalyst
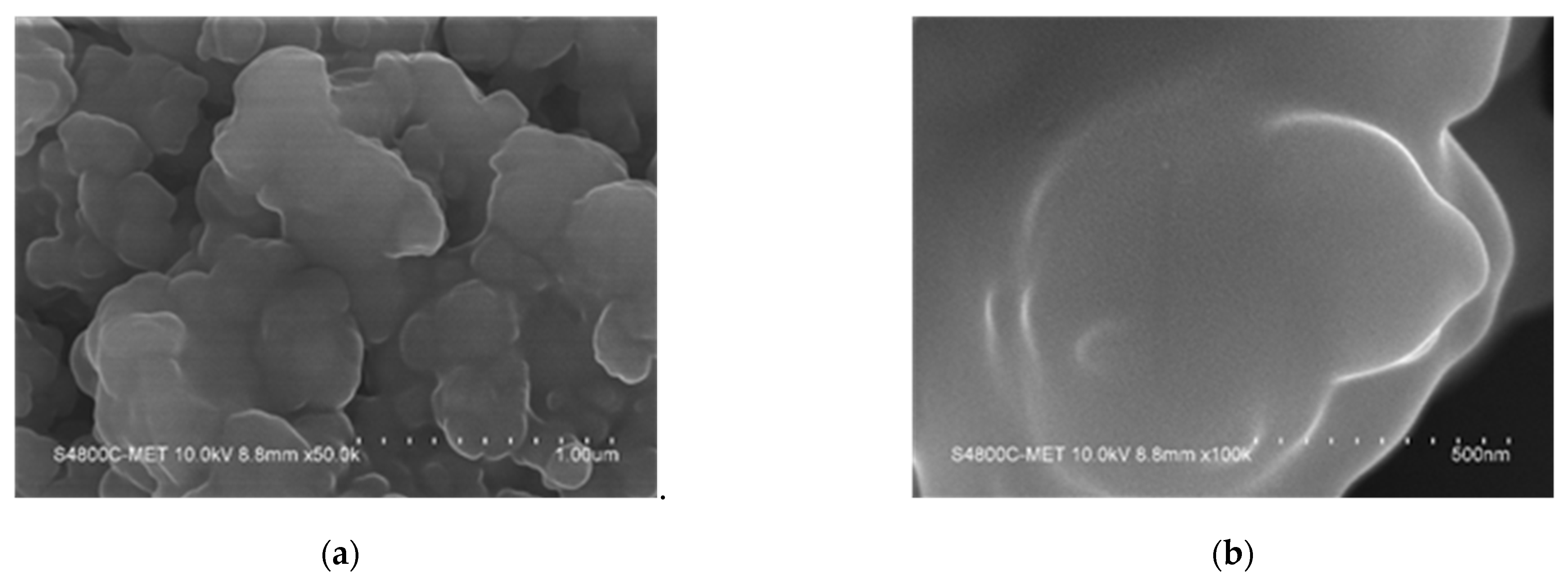
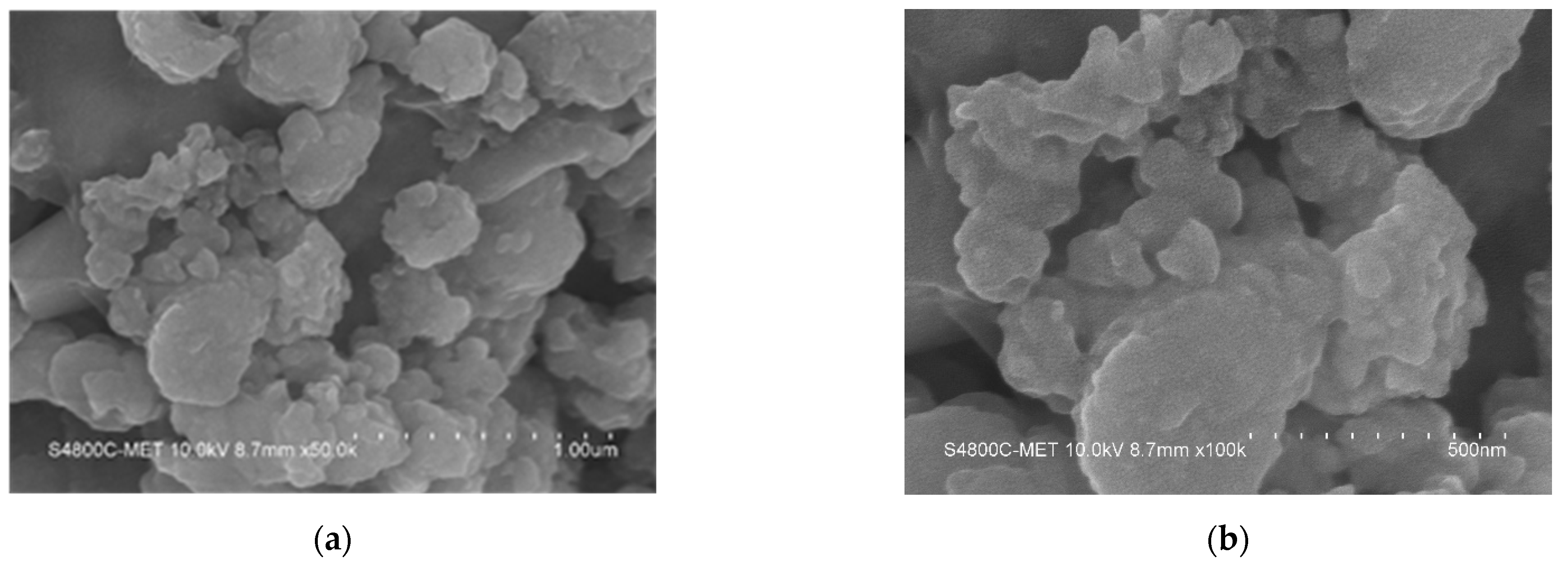
4.4.1. FE-SEM Analysis of TiO2 and TiO2 Supported Pt Catalyst
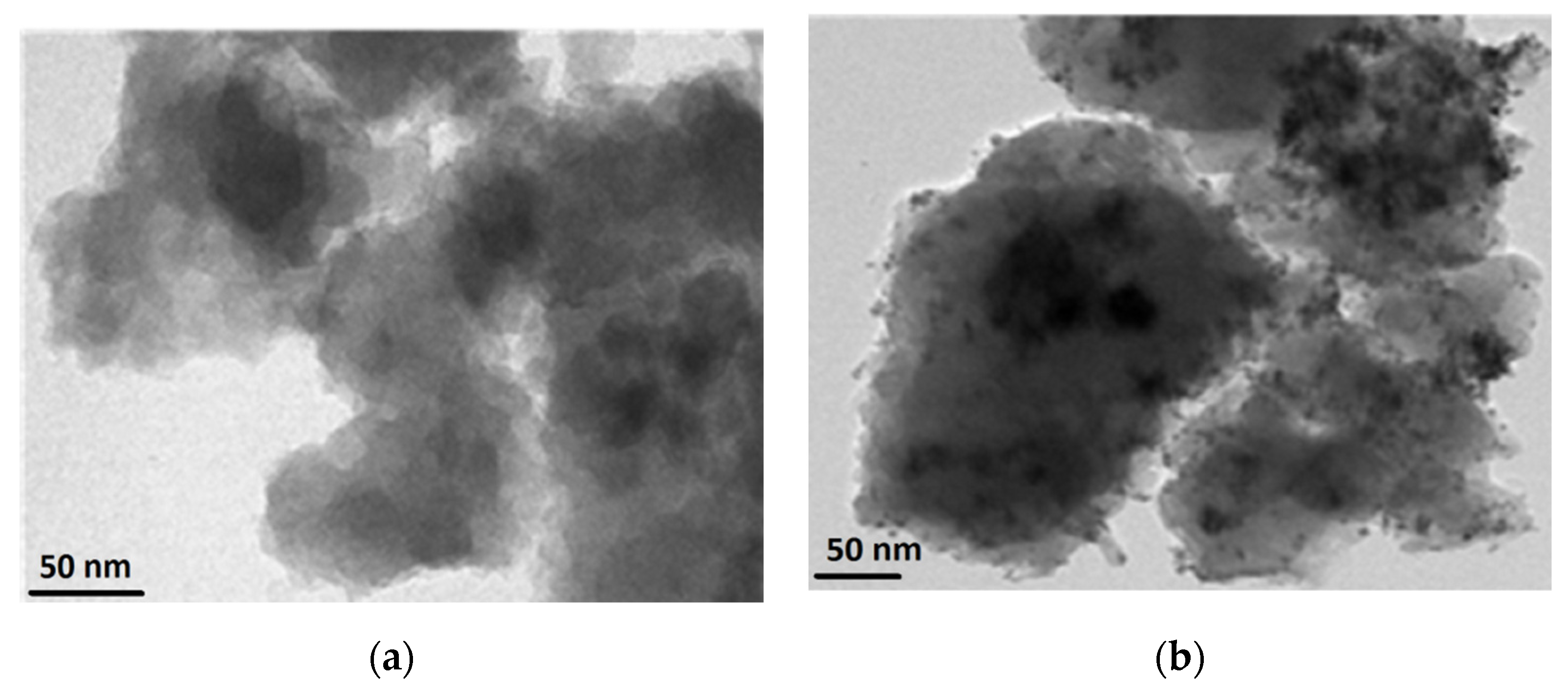
4.4.2. TEM Analysis of TiO2 and Pt/TiO2
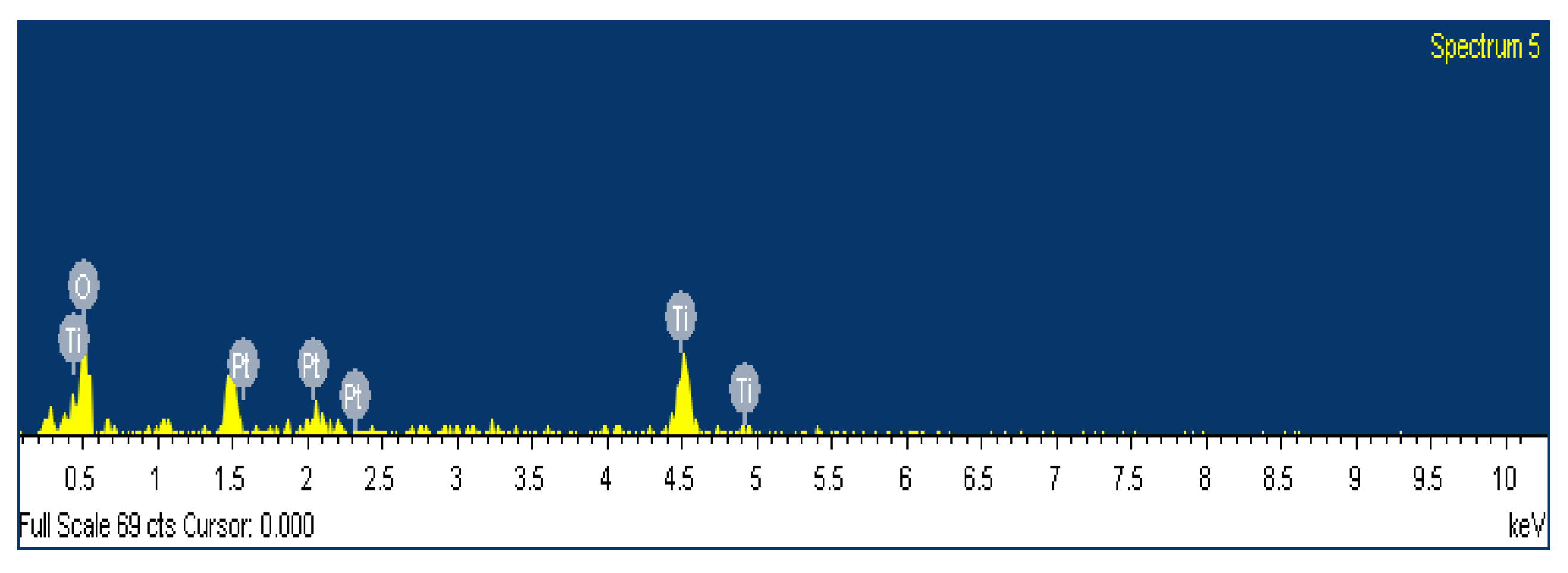
2.5. Energy Dispersive X-Ray Analysis (EDX) (Pt/TiO2)
3. Materials and Methods
3.1. Materials
3.2. Synthesis of TiO2 as the Support: Ultrasound Approach
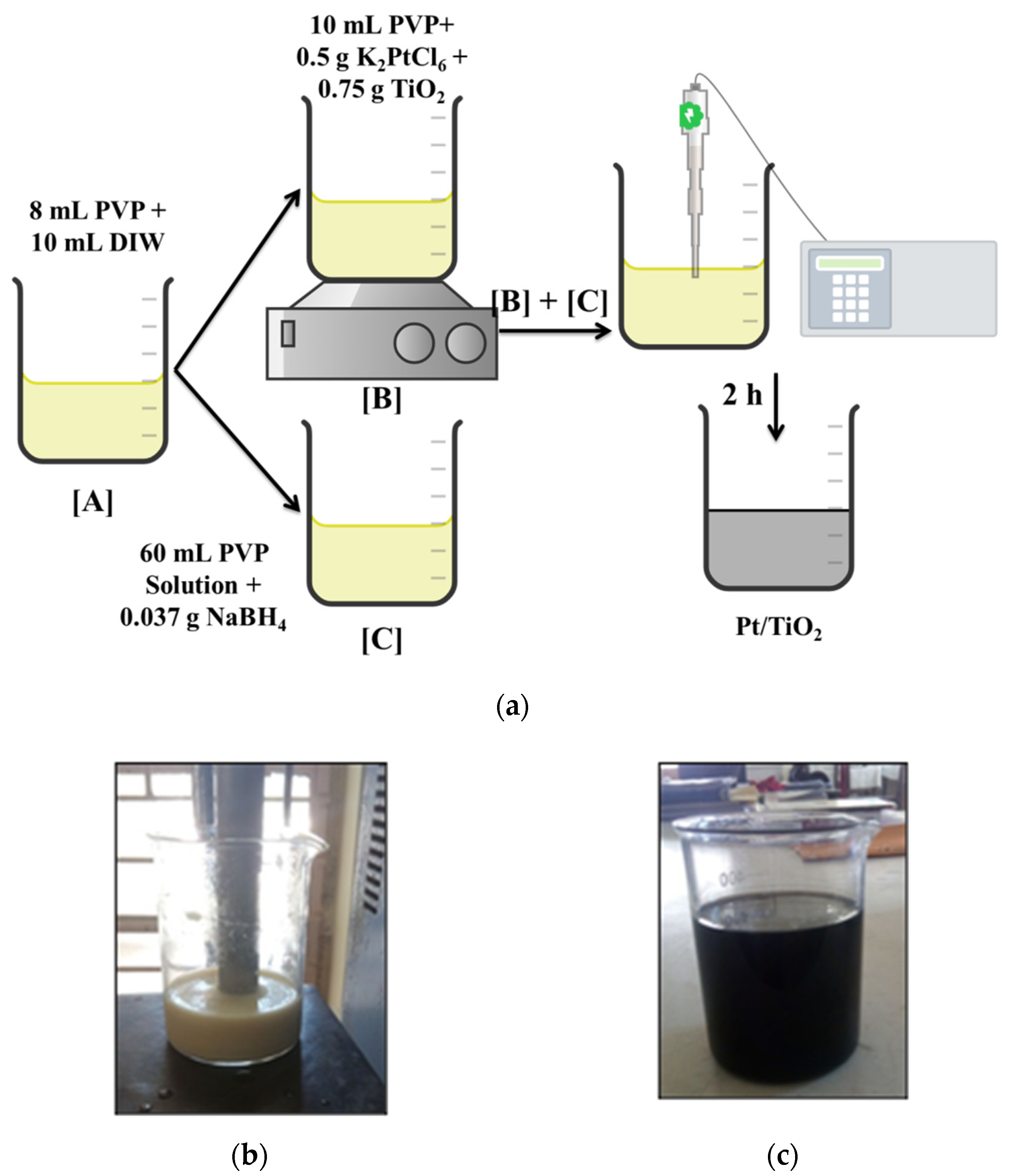
3.3. Synthesis of TiO2 Supported Pt Catalyst
4. Characterisation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Liu, B.; Zhang, P.; Morikawa, T.; Yamanaka, K.I.; Sato, T. Photocatalytic oxidation of NOx under visible led light irradiation over nitrogen-doped titania particles with iron or platinum loading. J. Phys. Chem. C 2008, 112, 12425–12431. [Google Scholar] [CrossRef]
- Sakthivel, S.; Neppolian, B.; Arabindoo, B.; Palanichamy, M.; Murugesan, V. TiO2 catalysed photodegradation of leather dye, Acid Green 16. J. Sci. Ind. Res. 2000, 59, 556–562. [Google Scholar]
- Zaleska, A. Doped-TiO₂: A Review. Recent Pat. Eng. 2008, 2, 157–164. [Google Scholar] [CrossRef]
- Landge, V.K.; Sonawane, S.H.; Chaudhari, R.V.; Babu, G.U.B. Selective Oxidation of Glycerol: A Biomass-Derived Feedstock Using the Pt-Cu Janus Catalyst for Value-Added Products. Ind. Eng. Chem. Res. 2020, 60, 185–195. [Google Scholar] [CrossRef]
- Lü, X.; Mou, X.; Wu, J.; Zhang, D.; Zhang, L.; Huang, F.; Xu, F.; Huang, S. Improved-Performance Dye-Sensitized solar cells using Nb-Doped TiO2 electrodes: Efficient electron Injection and transfer. Adv. Funct. Mater. 2010, 20, 509–515. [Google Scholar] [CrossRef]
- Mohamed, N.M.; Bashiri, R.; Chong, F.K.; Sufian, S.; Kakooei, S. Photoelectrochemical behaviour of bimetallic Cu-Ni and monometallic Cu, Ni doped TiO2 for hydrogen production. Int. J. Hydrog. Energy 2015, 40, 14031–14038. [Google Scholar] [CrossRef]
- Chen, L.; He, S.; He, B.Y.; Wang, T.J.; Su, C.L.; Zhang, C.; Jin, Y. Synthesis of iron-doped titanium oxide nano adsorbent and its adsorption characteristics for fluoride in drinking water. Ind. Eng. Chem. Res. 2012, 51, 13150–13156. [Google Scholar] [CrossRef]
- Cheng, T.; Gao, H.; Liu, G.; Pu, Z.; Wang, S.; Yi, Z.; Wu, X.; Yang, H. Preparation of Core-Shell Heterojunction Photocatalysts by Coating CdS Nanoparticles onto Bi4Ti3O12 Hierarchical Microspheres and Their Photocatalytic Removal of Organic Pollutants and Cr(VI) Ions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127918. [Google Scholar] [CrossRef]
- Khalid, N.R.; Majid, A.; Tahir, M.B.; Niaz, N.A.; Khalid, S. Carbonaceous-TiO2 nanomaterials for photocatalytic degradation of pollutants: A review. Ceram. Int. 2017, 43, 14552–14571. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, C.; Doña-Rodríguez, J.M.; González-Díaz, O.; Seck, I.; Zerbani, D.; Portillo, D.; Perez-Peña, J. Synthesis of highly photoactive TiO2 and Pt/TiO2 nanocatalysts for substrate-specific photocatalytic applications. Appl. Catal. B Environ. 2012, 125, 383–389. [Google Scholar] [CrossRef]
- Sadanandam, G.; Lalitha, K.; Kumari, V.D.; Shankar, M.V.; Subrahmanyam, M. Cobalt doped TiO2: A stable and efficient photocatalyst for continuous hydrogen production from glycerol: Water mixtures under solar light irradiation. Int. J. Hydrog. Energy 2013, 38, 9655–9664. [Google Scholar] [CrossRef]
- Bagheri, S.; Muhd Julkapli, N.; Bee Abd Hamid, S. Titanium dioxide as a catalyst support in heterogeneous catalysis. Sci. World J. 2014, 2014, 1–21. [Google Scholar] [CrossRef]
- Rao, T.N.; Babji, P.; Ahmad, N.; Khan, R.A.; Hassan, I.; Shahzad, S.A.; Husain, F.M. Green synthesis and structural classification of Acacia nilotica mediated-silver doped titanium oxide (Ag/TiO2) spherical nanoparticles: Assessment of its antimicrobial and anticancer activity. Saudi J. Biol. Sci. 2019, 26, 1385–1391. [Google Scholar] [CrossRef]
- Ould-Chikh, S.; Proux, O.; Afanasiev, P.; Khrouz, L.; Hedhili, M.N.; Anjum, D.H.; Harb, M.; Geantet, C.; Basset, J.M.; Puzenat, E. Photocatalysis with chromium-doped TiO2: Bulk and surface doping. ChemSusChem 2014, 7, 1361–1371. [Google Scholar] [CrossRef]
- Wang, S.; Bai, L.N.; Sun, H.M.; Jiang, Q.; Lian, J.S. Structure and photocatalytic property of Mo-doped TiO2 nanoparticles. Powder Technol. 2013, 244, 9–15. [Google Scholar] [CrossRef]
- Wu, J.C.S.; Chen, C.H. A visible-light response vanadium-doped titania nanocatalyst by sol-gel method. J. Photochem. Photobiol. A Chem. 2004, 163, 509–515. [Google Scholar] [CrossRef]
- Gupta, K.; Singh, R.P.; Pandey, A.; Pandey, A. Photocatalytic antibacterial performance of TiO2 and Ag-doped TiO2 against S. aureus. P. aeruginosa and E. coli. Beilstein J. Nanotechnol. 2013, 4, 345–351. [Google Scholar] [CrossRef]
- Singh, S.A.; Madras, G. Sonochemical synthesis of Pt, Ru doped TiO2 for methane reforming. Appl. Catal. A Gen. 2016, 518, 102–114. [Google Scholar] [CrossRef]
- Ihnatiuk, D.; Tossi, C.; Tittonen, I.; Linnik, O. Effect of synthesis conditions of nitrogen and platinum co-doped titania films on the photocatalytic performance under simulated solar light. Catalysts 2020, 10, 1074. [Google Scholar] [CrossRef]
- Grabowska, E.; Remita, H.; Zaleska, A. Photocatalytic activity of TiO2 loaded with metal clusters. Physicochem. Probl. Miner. Process. 2010, 45, 29–38. [Google Scholar]
- Li, Y.; Li, M.; Xu, P.; Tang, S.; Liu, C. Efficient Photocatalytic Degradation of Acid Orange 7 over N-Doped Ordered Mesoporous Titania on Carbon Fibers under Visible-Light Irradiation Based on Three Synergistic Effects. Appl. Catal. A Gen. 2016, 524, 163–172. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Baeissa, E.S. Mordenite encapsulated with Pt-TiO2: Characterisation and applications for photocatalytic degradation of direct blue dye. J. Alloys Compd. 2013, 558, 68–72. [Google Scholar] [CrossRef]
- Xiong, S.; Yin, Z.; Zhou, Y.; Peng, X.; Yan, W.; Liu, Z.; Zhang, X. The Dual-Frequency (20/40 KHz) Ultrasound Assisted Photocatalysis with the Active Carbon Fiber-Loaded Fe3+-TiO2 as Photocatalyst for Degradation of Organic Dye. Bull. Korean Chem. Soc. 2013, 34, 3039–3045. [Google Scholar] [CrossRef][Green Version]
- Devipriya, S.P.; Yesodharan, S.; Yesodharan, E.P. Solar photocatalytic removal of chemical and bacterial pollutants from water using Pt/TiO2-coated ceramic tiles. Int. J. Photoenergy 2012, 2012, 1–8. [Google Scholar] [CrossRef][Green Version]
- Kim, S.; Hwang, S.J.; Choi, W. Visible light active platinum-ion-doped TiO2 photocatalyst. J. Phys. Chem. B 2005, 109, 24260–24267. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Li, M.; Li, Y.; Chen, W. Enhancement of the Catalytic Activity of Ordered Mesoporous TiO2 by Using Carbon Fiber Support and Appropriate Evaluation of Synergy between Surface Adsorption and Photocatalysis by Langmuir-Hinshelwood (L-H) Integration Equation. RSC Adv. 2015, 5, 105227–105238. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, C.; García-Domínguez, Á.E.; Guerrero-Robles, F.; Saavedra-Díaz, R.O.; Torres-Torres, G.; Felipe, C.; Ojeda-López, R.; Silahua-Pavón, A.; Cervantes-Uribe, A. Synthesis of supported metal nanoparticles (Au/TiO2) by the suspension impregnation method. J. Compos. Sci. 2020, 4, 89. [Google Scholar] [CrossRef]
- Abbas, M.M.; Rasheed, M. Solid State Reaction Synthesis and Characterization of Cu doped TiO2 Nanomaterials. J. Phys. Conf. Ser. 2021, 1795, 1–9. [Google Scholar] [CrossRef]
- Tang, N.; Li, Y.; Chen, F.; Han, Z. In Situ Fabrication of a Direct Z-Scheme Photocatalyst by Immobilising CdS Quantum Dots in the Channels of Graphene-Hybridized and Supported Mesoporous Titanium Nanocrystals for High Photocatalytic Performance under Visible Light. RSC Adv. 2018, 8, 42233–42245. [Google Scholar] [CrossRef]
- Fan, C.; Chen, C.; Wang, J.; Fu, X.; Ren, Z.; Qian, G.; Wang, Z. Black Hydroxylated Titanium Dioxide Prepared via Ultrasonication with Enhanced Photocatalytic Activity. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef]
- Teoh, W.Y.; Mädler, L.; Beydoun, D.; Pratsinis, S.E.; Amal, R. Direct (one-step) synthesis of TiO2 and Pt/TiO2 nanoparticles for photocatalytic mineralisation of sucrose. Chem. Eng. Sci. 2005, 60, 5852–5861. [Google Scholar] [CrossRef]
- Shanmugam, S.; Gedanken, A. Synthesis and electrochemical oxygen reduction of platinum nanoparticles supported on mesoporous TiO2. J. Phys. Chem. C 2009, 113, 18707–18712. [Google Scholar] [CrossRef]
- Neppolian, B.; Bruno, A.; Bianchi, C.L.; Ashokkumar, M. Graphene oxide based Pt-TiO2 photocatalyst: Ultrasound assisted synthesis, characterisation and catalytic efficiency. Ultrason. Sonochem. 2012, 19, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Bedolla-Valdez, Z.I.; Verde-Gómez, Y.; Valenzuela-Muñiz, A.M.; Gochi-Ponce, Y.; Oropeza-Guzmán, M.T.; Berhault, G.; Alonso-Núñez, G. Sonochemical synthesis and characterization of Pt/CNT, Pt/TiO2, and Pt/CNT/TiO2 electrocatalysts for methanol electro-oxidation. Electrochim. Acta 2015, 186, 76–84. [Google Scholar] [CrossRef]
- Abdulrazzak, F.H.; Hussein, F.H.; Alkaim, A.F.; Ivanova, I.; Emeline, A.V.; Bahnemann, D.W. Sonochemical/hydration-dehydration synthesis of Pt-TiO2 NPs/decorated carbon nanotubes with enhanced photocatalytic hydrogen production activity. Photochem. Photobiol. Sci. 2016, 15, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Driessen, M.D.; Grassian, V.H. Photooxidation of Trichloroethylene on Pt/TiO2. J. Phys. Chem. B. 1997, 102, 1418–1423. [Google Scholar] [CrossRef]
- Zhu, Z.; Wu, R.J. The Degradation of Formaldehyde Using a Pt@TiO2 Nanoparticles in Presence of Visible Light Irradiation at Room Temperature. J. Taiwan Inst. Chem. Eng. 2015, 50, 276–281. [Google Scholar] [CrossRef]
- Pinedo-Escobar, J.A.; Fan, J.; Moctezuma, E.; Gomez-Solís, C.; Martinez, C.J.C.; Gracia-Espino, E. Nanoparticulate double-heterojunction photocatalysts comprising TiO2 (Anatase)/WO3/TiO2 (Rutile)with enhanced photocatalytic activity toward the degradation of methyl orange under near-ultraviolet and visible light. ACS Omega 2021, 6, 11840–11848. [Google Scholar] [CrossRef]
- Zhao, Z.J.; Hwang, S.H.; Jeon, S.; Hwang, B.; Jung, J.Y.; Lee, J.; Park, S.H.; Jeong, J.H. Three-Dimensional Plasmonic Ag/TiO2 Nanocomposite Architectures on Flexible Substrates for Visible-Light Photocatalytic Activity. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Kim, H.; Choi, Y.; Kanuka, N.; Kinoshita, H.; Nishiyama, T.; Usami, T. Preparation of Pt-loaded TiO2 nanofibers by electrospinning and their application for WGS reactions. Appl. Catal. A Gen. 2009, 352, 265–270. [Google Scholar] [CrossRef]
- Boccuzzi, F.; Chiorino, A.; Manzoli, M.; Andreeva, D.; Tabakova, T.; Ilieva, L.; Iadakiev, V. Gold, silver and copper catalysts supported on TiO2 for pure hydrogen production. Catal. Today 2002, 75, 169–175. [Google Scholar] [CrossRef]
- Balachandramohan, J.; Sivasankar, T.; Sivakumar, M. Facile sonochemical synthesis of Ag2O-guar gum nanocomposite as a visible light photocatalyst for the organic transformation reactions. J. Hazard. Mater. 2020, 385, 121621. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, M.; Towata, A.; Yasui, K.; Tuziuti, T.; Kozuka, T.; Tsujimoto, M.; Zhong, Z.; Iida, Y. Fabrication of nanosized Pt on rutile TiO2 using a standing wave sonochemical reactor (SWSR)—Observation of an enhanced catalytic oxidation of CO. Ultrason. Sonochem. 2010, 17, 213–218. [Google Scholar] [CrossRef]
- Ahmed, L.M.; Ivanova, I.; Hussein, F.H.; Bahnemann, D.W. Role of Platinum Deposited on TiO2 in Photocatalytic Methanol Oxidation and Dehydrogenation Reactions. Int. J. Photoenergy 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Abdennouri, M.; Elhalil, A.; Farnane, M.; Tounsadi, H.; Mahjoubi, F.Z.; Elmoubarki, R.; Sadiq, M.; Khamar, L.; Galadi, A.; Baâlala, M.; et al. Photocatalytic degradation of 2,4-D and 2,4-DP herbicides on Pt/TiO2 nanoparticles. J. Saudi Chem. Soc. 2015, 19, 485–493. [Google Scholar] [CrossRef]
- Potdar, S.B.; Praveen, B.V.S.; Sonawane, S.H. Sonochemical approach for synthesis of zinc oxide-poly methyl methacrylate hybrid nanoparticles and its application in corrosion inhibition. Ultrason. Sonochem. 2020, 68, 1–7. [Google Scholar] [CrossRef]
- Hong, G.B.; Ma, C.M. Photocatalytic degradation of indoor air pollutants by Pt-TiO2. J. Nanomater. 2012, 2012, 88–92. [Google Scholar] [CrossRef]
- Bethi, B.; Sonawane, S.H.; Rohit, G.S.; Holkar, C.R.; Pinjari, D.V.; Bhanvase, B.A.; Pandit, A.B. Investigation of TiO2 photocatalyst performance for decolorisation in the presence of hydrodynamic cavitation as hybrid AOP. Ultrason. Sonochem. 2016, 28, 150–160. [Google Scholar] [CrossRef]
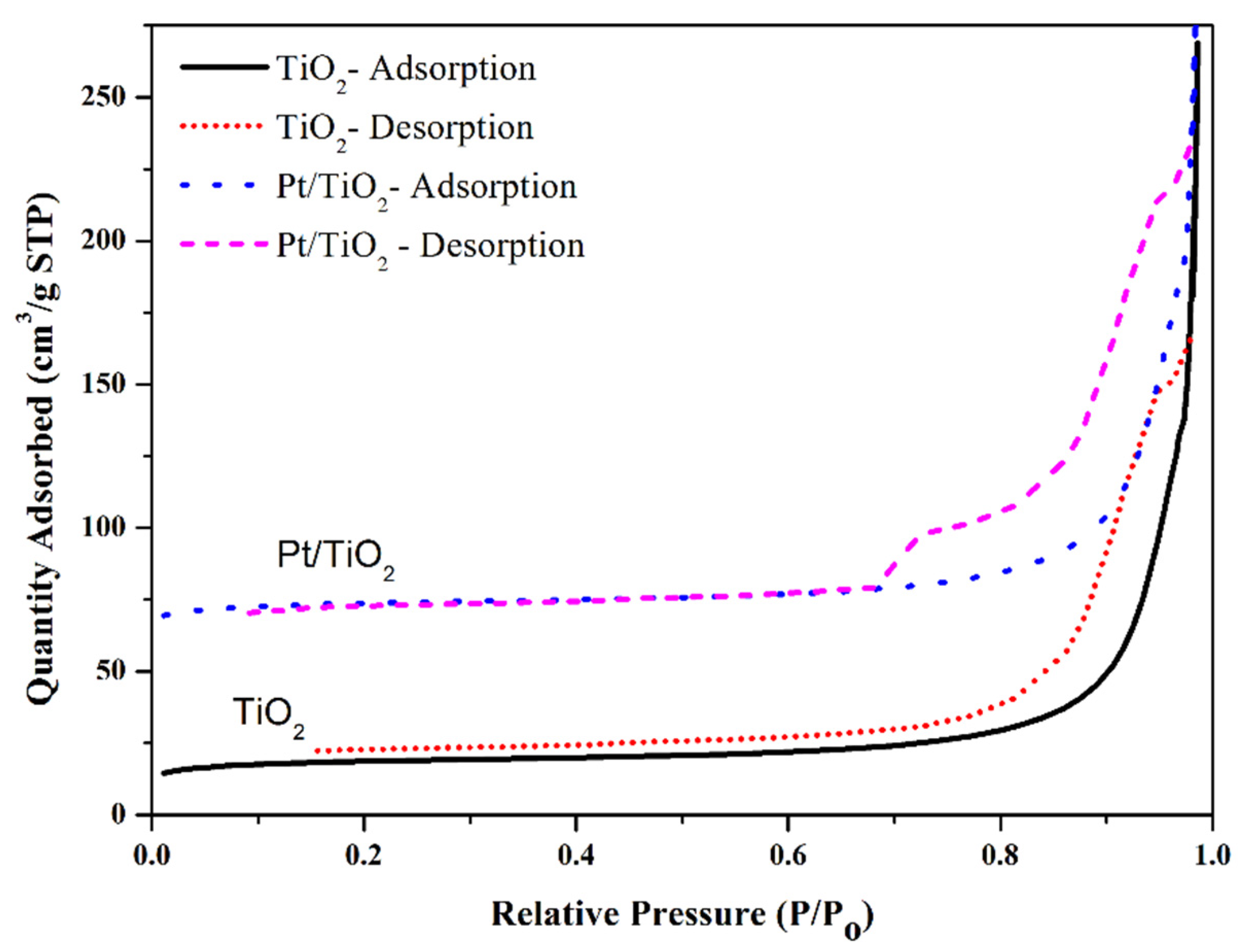
| Activated Carbon | Surface Area (m2/g) | Pore Diameter (nm) | Pore Volume (cm3/g) | Particle Size (TEM) (nm) | Crystallite Size Using Scherrer Equation (nm) |
|---|---|---|---|---|---|
| TiO2 | 71 | 13.6 | 0.31 | 11 | 10.132 |
| Pt/TiO2 | 129 | 17.2 | 0.45 | 15 | 13.43 |
| Elements | Wt% | Atomic % |
|---|---|---|
| O | 73.52 | 90.21 |
| Ti | 23.03 | 9.44 |
| Pt | 2.45 | 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potdar, S.B.; Huang, C.-M.; Praveen, B.; Manickam, S.; Sonawane, S.H. Highly Photoactive Titanium Dioxide Supported Platinum Catalyst: Synthesis Using Cleaner Ultrasound Approach. Catalysts 2022, 12, 78. https://doi.org/10.3390/catal12010078
Potdar SB, Huang C-M, Praveen B, Manickam S, Sonawane SH. Highly Photoactive Titanium Dioxide Supported Platinum Catalyst: Synthesis Using Cleaner Ultrasound Approach. Catalysts. 2022; 12(1):78. https://doi.org/10.3390/catal12010078
Chicago/Turabian StylePotdar, Shital B., Chao-Ming Huang, BVS Praveen, Sivakumar Manickam, and Shirish H. Sonawane. 2022. "Highly Photoactive Titanium Dioxide Supported Platinum Catalyst: Synthesis Using Cleaner Ultrasound Approach" Catalysts 12, no. 1: 78. https://doi.org/10.3390/catal12010078
APA StylePotdar, S. B., Huang, C.-M., Praveen, B., Manickam, S., & Sonawane, S. H. (2022). Highly Photoactive Titanium Dioxide Supported Platinum Catalyst: Synthesis Using Cleaner Ultrasound Approach. Catalysts, 12(1), 78. https://doi.org/10.3390/catal12010078








