A Comparative Study of the Effect of Graphene Oxide, Graphitic Carbon Nitride, and Their Composite on the Photocatalytic Activity of Cu3SnS4
Abstract
:1. Introduction
2. Results
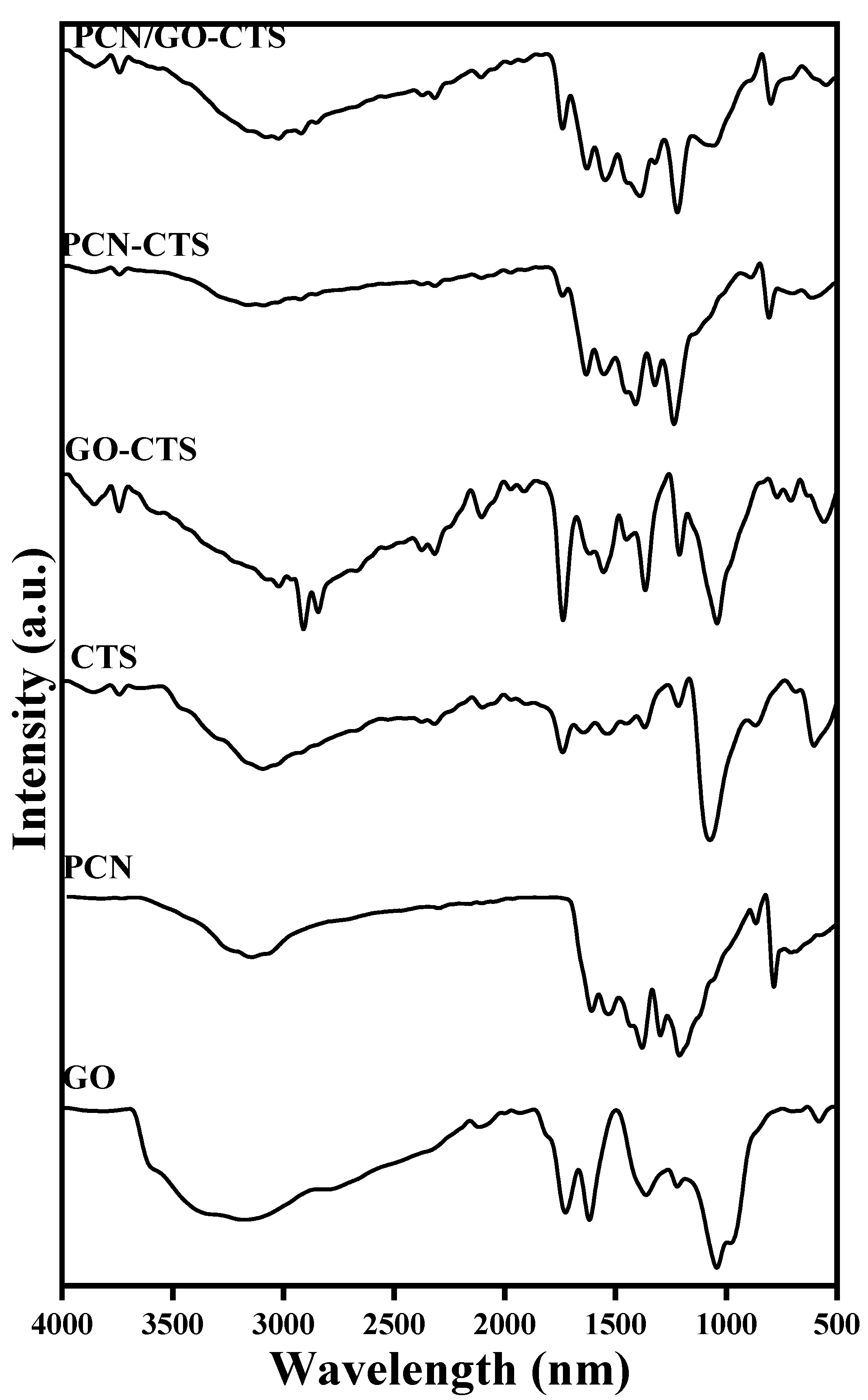
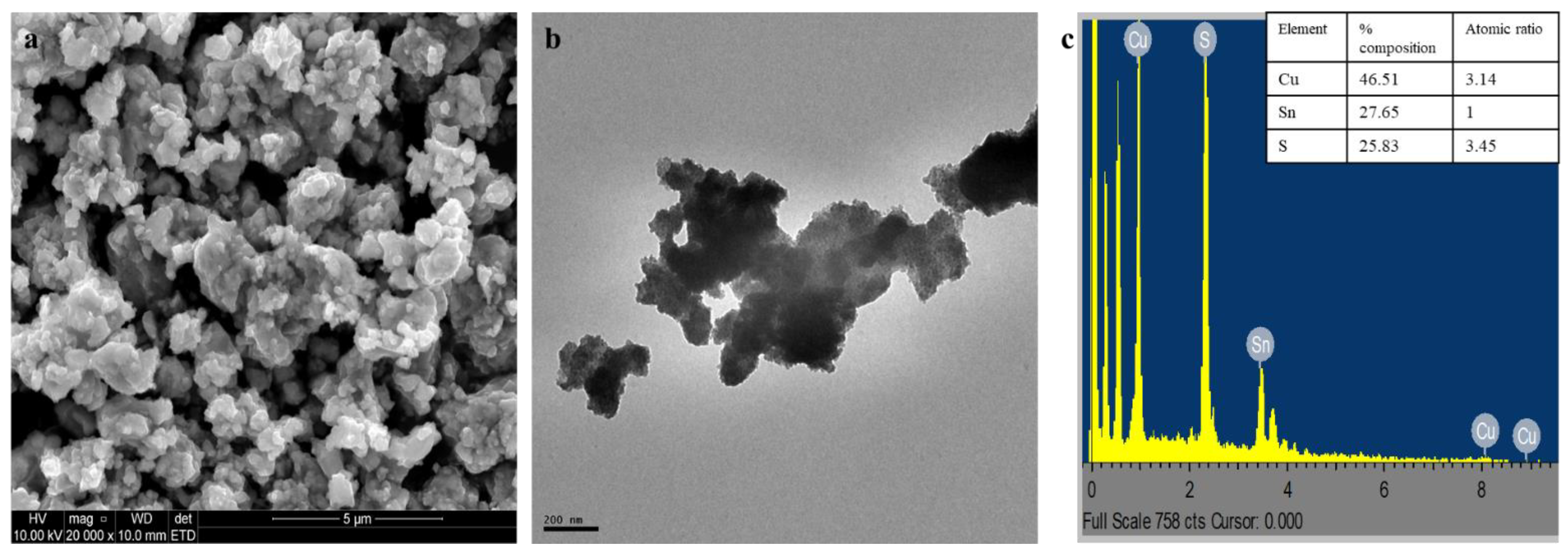
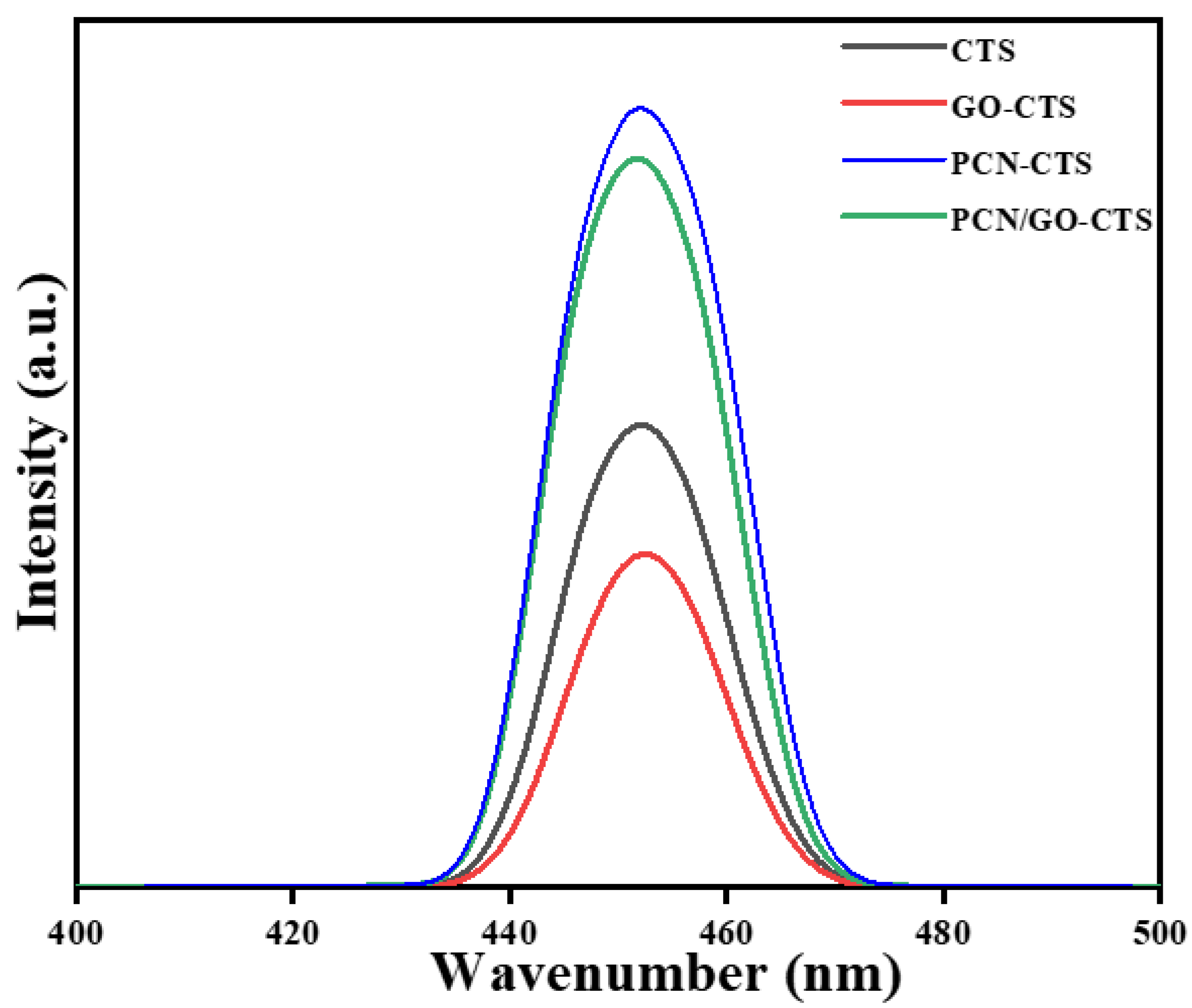
2.1. Characterization of GO, PCN, GO-CTS, PCN-CTS, and GO/PCN-CTS
2.2. Evaluation of Photocatalytic Activity
2.3. Proposed Mechanism for the Degradation Process
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Protonated Graphitic Carbon Nitride
3.3. Synthesis of Graphene Oxide (GO)
3.4. Synthesis of Graphene Oxide/PCN Composites
3.5. Synthesis of Cu3SnS4
3.6. Characterization of Prepared Nanoparticles
3.7. Preparation of GO-CTS, PCN-CTS, and GO/PCN-CTS Composites
3.8. Evaluation of Photocatalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olatunde, O.C.; Onwudiwe, D.C. Graphene-Based Composites as Catalysts for the Degradation of Pharmaceuticals. Int. J. Environ. Res. Public Health 2021, 18, 1529. [Google Scholar] [CrossRef] [PubMed]
- Su, D.S.; Zhang, J.; Frank, B.; Thomas, A.; Wang, X.; Paraknowitsch, J.; Schlogl, R. Metal-free heterogeneous catalysis for sustainable chemistry. ChemSusChem 2010, 3, 169–180. [Google Scholar] [CrossRef]
- Julkapli, N.M.; Bagheri, S. Graphene supported heterogeneous catalysts: An overview. Int. J. Hydrog. Energy 2015, 40, 948–979. [Google Scholar] [CrossRef]
- Heske, J.; Walczak, R.; Epping, J.D.; Youk, S.; Sahoo, S.K.; Antonietti, M.; Kühne, T.D.; Oschatz, M. When water becomes an integral part of carbon—Combining theory and experiment to understand the zeolite-like water adsorption properties of porous C2N materials. J. Mater. Chem. A 2021, 9, 22563–22572. [Google Scholar] [CrossRef]
- Cheng, Y.; Fan, Y.; Pei, Y.; Qiao, M. Graphene-supported metal/metal oxide nanohybrids: Synthesis and applications in heterogeneous catalysis. Catal. Sci. Technol. 2015, 5, 3903–3916. [Google Scholar] [CrossRef]
- Radsar, T.; Khalesi, H.; Ghods, V. Graphene properties and applications in nanoelectronic. Opt. Quantum Electron. 2021, 53, 178. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Soldano, C.; Mahmood, A.; Dujardin, E. Production, properties and potential of graphene. Carbon 2010, 48, 2127–2150. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Jiao, G.; Zhao, W.; Jin, P.; Li, X. Magnetic Fe3O4–graphene composites as targeted drug nanocarriers for pH-activated release. Nanoscale 2013, 5, 1143–1152. [Google Scholar] [CrossRef]
- Pramanik, N.; Ranganathan, S.; Rao, S.; Suneet, K.; Jain, S.; Rangarajan, A.; Jhunjhunwala, S. A Composite of Hyaluronic Acid-Modified Graphene Oxide and Iron Oxide Nanoparticles for Targeted Drug Delivery and Magnetothermal Therapy. ACS Omega 2019, 4, 9284–9293. [Google Scholar] [CrossRef] [Green Version]
- Vinothkannan, M.; Kim, A.R.; Gnana Kumar, G.; Yoo, D.J. Sulfonated graphene oxide/Nafion composite membranes for high temperature and low humidity proton exchange membrane fuel cells. RSC Adv. 2018, 8, 7494–7508. [Google Scholar] [CrossRef] [Green Version]
- Xia, Z.; Wang, S.; Jiang, L.; Sun, H.; Qi, F.; Jin, J.; Sun, G. Rational design of a highly efficient Pt/graphene–Nafion® composite fuel cell electrode architecture. J. Mater. Chem. A 2015, 3, 1641–1648. [Google Scholar] [CrossRef]
- Dey, R.S. Development of Biosensors from Polymer Graphene Composites. In Graphene-Based Polymer Nanocomposites in Electronics; Sadasivuni, K.K., Ponnamma, D., Kim, J., Thomas, S., Eds.; Springer: Cham, Switzerland, 2015; pp. 277–305. [Google Scholar]
- Zhang, W.; Jia, B.; Furumai, H. Fabrication of graphene film composite electrochemical biosensor as a pre-screening algal toxin detection tool in the event of water contamination. Sci. Rep. 2018, 8, 10686. [Google Scholar] [CrossRef]
- Lu, Z.; Kong, Z.; Jing, L.; Wang, T.; Liu, X.; Fu, A.; Guo, P.; Guo, Y.-G.; Li, H. Porous SnO2/Graphene Composites as Anode Materials for Lithium-Ion Batteries: Morphology Control and Performance Improvement. Energy Fuels 2020, 34, 13126–13136. [Google Scholar] [CrossRef]
- Cong, R.; Choi, J.-Y.; Song, J.-B.; Jo, M.; Lee, H.; Lee, C.-S. Characteristics and electrochemical performances of silicon/carbon nanofiber/graphene composite films as anode materials for binder-free lithium-ion batteries. Sci. Rep. 2021, 11, 1283. [Google Scholar] [CrossRef] [PubMed]
- Tabish, T.A.; Memon, F.A.; Gomez, D.E.; Horsell, D.W.; Zhang, S. A facile synthesis of porous graphene for efficient water and wastewater treatment. Sci. Rep. 2018, 8, 1817. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, M.R.; Vasudevan, S.; Shibayama, A.; Yamada, M. Graphene and Graphene-Based Composites: A Rising Star in Water Purification—A Comprehensive Overview. ChemistrySelect 2016, 1, 4358–4385. [Google Scholar] [CrossRef] [Green Version]
- Sachdeva, H. Recent advances in the catalytic applications of GO/rGO for green organic synthesis. Green Process. Synth. 2020, 9, 515–537. [Google Scholar] [CrossRef]
- Kong, X.-K.; Chen, C.-L.; Chen, Q.-W. Doped graphene for metal-free catalysis. Chem. Soc. Rev. 2014, 43, 2841–2857. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of graphene: Covalent and non-covalent approaches, derivatives and applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef]
- Zhang, H. Molecularly imprinted nanoparticles for biomedical applications. Adv. Mater. 2020, 32, 1806328. [Google Scholar] [CrossRef]
- Wang, X.; Blechert, S.; Antonietti, M. Polymeric Graphitic Carbon Nitride for Heterogeneous Photocatalysis. ACS Catal. 2012, 2, 1596–1606. [Google Scholar] [CrossRef]
- Idris, M.B.; Devaraj, S. Few-layered mesoporous graphitic carbon nitride: A graphene analogue with high capacitance properties. New J. Chem. 2019, 43, 11626–11635. [Google Scholar] [CrossRef]
- Suter, T.; Brázdová, V.; McColl, K.; Miller, T.S.; Nagashima, H.; Salvadori, E.; Sella, A.; Howard, C.A.; Kay, C.W.M.; Corà, F.; et al. Synthesis, Structure and Electronic Properties of Graphitic Carbon Nitride Films. J. Phys. Chem. C 2018, 122, 25183–25194. [Google Scholar] [CrossRef] [Green Version]
- Shcherban, N.D.; Mäki-Arvela, P.; Aho, A.; Sergiienko, S.A.; Yaremov, P.S.; Eränen, K.; Murzin, D.Y. Melamine-derived graphitic carbon nitride as a new effective metal-free catalyst for Knoevenagel condensation of benzaldehyde with ethylcyanoacetate. Catal. Sci. Technol. 2018, 8, 2928–2937. [Google Scholar] [CrossRef]
- Sreekanth, T.V.M.; Basivi, P.K.; Nagajyothi, P.C.; Dillip, G.R.; Shim, J.; Ko, T.J.; Yoo, K. Determination of surface properties and Gutmann’s Lewis acidity-basicity parameters of thiourea and melamine polymerized graphitic carbon nitride sheets by inverse gas chromatography. J. Chromatogr. A 2018, 1580, 134–141. [Google Scholar] [CrossRef]
- Jin, X.; Balasubramanian, V.V.; Selvan, S.T.; Sawant, D.P.; Chari, M.A.; Lu, G.Q.; Vinu, A. Highly Ordered Mesoporous Carbon Nitride Nanoparticles with High Nitrogen Content: A Metal-Free Basic Catalyst. Angew. Chem. Int. Ed. 2009, 48, 7884–7887. [Google Scholar] [CrossRef] [PubMed]
- Humayun, M.; Ullah, H.; Usman, M.; Habibi-Yangjeh, A.; Tahir, A.A.; Wang, C.; Luo, W. Perovskite-type lanthanum ferrite based photocatalysts: Preparation, properties, and applications. J. Energy Chem. 2022, 66, 314–338. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Liu, H.; Liu, C.; Wan, Y.; Long, Y.; Cai, Z. Recent Advances and Applications of Semiconductor Photocatalytic Technology. Appl. Sci. 2019, 9, 2489. [Google Scholar] [CrossRef] [Green Version]
- Daghrir, R.; Drogui, P. Tetracycline antibiotics in the environment: A review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Khalili, D. Graphene oxide: A promising carbocatalyst for the regioselective thiocyanation of aromatic amines, phenols, anisols and enolizable ketones by hydrogen peroxide/KSCN in water. New J. Chem. 2016, 40, 2547–2553. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Aslam, S.; Mustafa, F.; Arshad, U. Synergistic antibacterial activity of surfactant free Ag–GO nanocomposites. Sci. Rep. 2021, 11, 196. [Google Scholar] [CrossRef] [PubMed]
- Ben-Refael, A.; Benisti, I.; Paz, Y. Transient photoinduced phenomena in graphitic carbon nitride as measured at nanoseconds resolution by step-scan FTIR. Catal. Today 2020, 340, 97–105. [Google Scholar] [CrossRef]
- Zhu, G.X.; Lu, T.L.; Han, L.; Zhan, Y.Z. Graphitic carbon nitride (g-C3N4) as an efficient metal-free Fenton-like catalyst for degrading organic pollutants: The overlooked non-photocatalytic activity. Water Sci. Technol. 2020, 81, 518–528. [Google Scholar] [CrossRef]
- Jayabharathi, J.; Sundari, G.A.; Thanikachalam, V.; Jeeva, P.; Panimozhi, S. A dodecanethiol-functionalized Ag nanoparticle-modified ITO anode for efficient performance of organic light-emitting devices. RSC Adv. 2017, 7, 38923–38934. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.; Xia, P.; Li, Y.; Ho, W.; Yu, J. Fabrication and photocatalytic activity enhanced mechanism of direct Z-scheme g-C3N 4/Ag2 WO4 photocatalyst. Appl. Surf. Sci. 2017, 391, 175–183. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, L.; Deng, H. Ag modified g-C3N4 composites with enhanced visible-light photocatalytic activity for diclofenac degradation. J. Mol. Catal. A Chem. 2016, 423, 270–276. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Ruan, Q.; Tang, J. Ru and RuOx decorated carbon nitride for efficient ammonia photosynthesis. Nanoscale 2020, 12, 12329–12335. [Google Scholar] [CrossRef]
- Sridharan, K.; Jang, E.; Park, J.H.; Kim, J.-H.; Lee, J.-H.; Park, T.J. Silver Quantum Cluster (Ag9)-Grafted Graphitic Carbon Nitride Nanosheets for Photocatalytic Hydrogen Generation and Dye Degradation. Chem. Eur. J. 2015, 21, 9126–9132. [Google Scholar] [CrossRef]
- Fina, F.; Callear, S.; Carins, G.; Irvine, J. Structural Investigation of Graphitic Carbon Nitride via XRD and Neutron Diffraction. Chem. Mater. 2015, 27, 2612–2618. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Fujitsuka, M.; Majima, T. The role of nitrogen defects in graphitic carbon nitride for visible-light-driven hydrogen evolution. Phys. Chem. Chem. Phys. 2019, 21, 2318–2324. [Google Scholar] [CrossRef]
- Arranz-Mascarós, P.; Godino-Salido, M.L.; López-Garzón, R.; García-Gallarín, C.; Chamorro-Mena, I.; López-Garzón, F.J.; Fernández-García, E.; Gutiérrez-Valero, M.D. Non-covalent Functionalization of Graphene to Tune Its Band Gap and Stabilize Metal Nanoparticles on Its Surface. ACS Omega 2020, 5, 18849–18861. [Google Scholar] [CrossRef]
- Huang, J.; Fu, K.; Deng, X.; Yao, N.; Wei, M. Fabrication of TiO2 Nanosheet Aarrays/Graphene/Cu2O Composite Structure for Enhanced Photocatalytic Activities. Nanoscale Res. Lett. 2017, 12, 310. [Google Scholar] [CrossRef]
- Yeh, T.-F.; Cihlář, J.; Chang, C.-Y.; Cheng, C.; Teng, H. Roles of graphene oxide in photocatalytic water splitting. Mater. Today 2013, 16, 78–84. [Google Scholar] [CrossRef]
- Yu, X.; Ng, S.-F.; Putri, L.K.; Tan, L.-L.; Mohamed, A.R.; Ong, W.-J. Point-Defect Engineering: Leveraging Imperfections in Graphitic Carbon Nitride (g-C3N4) Photocatalysts toward Artificial Photosynthesis. Small 2021, 17, 2006851. [Google Scholar] [CrossRef]
- Liqiang, J.; Yichun, Q.; Baiqi, W.; Shudan, L.; Baojiang, J.; Libin, Y.; Wei, F.; Honggang, F.; Jiazhong, S. Review of photoluminescence performance of nano-sized semiconductor materials and its relationships with photocatalytic activity. Sol. Energy Mater. Sol. Cells 2006, 90, 1773–1787. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, M.; Gong, Y.; Li, Q.; Yang, M.; Yang, Q.; Zhang, Z. Three-dimensional bandgap-tuned Ag2S quantum dots/reduced graphene oxide composites with enhanced adsorption and photocatalysis under visible light. Catal. Sci. Technol. 2018, 8, 5225–5235. [Google Scholar] [CrossRef]
- Abdullah, E.A. Band edge positions as a key parameter to a systematic design of heterogeneous photocatalyst. Eur. J. Chem. 2019, 10, 82–94. [Google Scholar] [CrossRef]
- Ansari, S.A.; Cho, M.H. Highly Visible Light Responsive, Narrow Band gap TiO2 Nanoparticles Modified by Elemental Red Phosphorus for Photocatalysis and Photoelectrochemical Applications. Sci. Rep. 2016, 6, 25405. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; He, T.; Wang, Y.; Ning, G.; Xu, Z.; Chen, X.; Hu, X.; Wu, Y.; Zhao, Y. Synergistic adsorption-photocatalytic degradation effect and norfloxacin mechanism of ZnO/ZnS@BC under UV-light irradiation. Sci. Rep. 2020, 10, 11903. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.A.; Isborn, C.M.; Prezhdo, O.V. Excited states and optical absorption of small semiconducting clusters: Dopants, defects and charging. Chem. Sci. 2011, 2, 400–406. [Google Scholar] [CrossRef]
- Yang, F.; Chu, X.; Sun, J.; Zhang, Y.; Li, Z.; Liu, H.; Bai, L.; Qu, Y.; Jing, L. Efficient singlet oxygen generation by excitonic energy transfer on ultrathin g-C3N4 for selective photocatalytic oxidation of methyl-phenyl-sulfide with O2. Chin. Chem. Lett. 2020, 31, 2784–2788. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, S.; Chen, S.; Li, D.; Zhang, X.; Shao, W.; Sun, X.; Xie, J.; Zhao, Z.; Zhang, Q.; et al. Enhanced Singlet Oxygen Generation in Oxidized Graphitic Carbon Nitride for Organic Synthesis. Adv. Mater. 2016, 28, 6940–6945. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.-J.; Tana, L.-L.; Chaia, S.-P.; Yonga, S.-T.; Mohamed, A.R. Surface charge modification via protonation of graphitic carbon nitride (g-C3N4) for electrostatic self-assembly construction of 2D/2D reduced graphene oxide (rGO)/g-C3N4 nanostructures toward enhanced photocatalytic reduction of carbon dioxide to methane. Nano Energy 2015, 13, 757–770. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhang, W.; Bai, J.; Lu, G.; Zhang, L.; Wu, G. Construction of a 2D/2D g-C3N4/rGO hybrid heterojunction catalyst with outstanding charge separation ability and nitrogen photofixation performance via a surface protonation process. RSC Adv. 2016, 6, 25695–25702. [Google Scholar] [CrossRef]
- Onwudiwe, D.C.; Olatunde, O.C.; Mathur, S. Structural studies and morphological properties of antimony sulphide nanorods obtained by solvothermal synthesis. Phys. B Condens. Matter 2021, 605, 412691. [Google Scholar] [CrossRef]



| CP (%) | EEO (KW/h) | T0.9 (min) | |
|---|---|---|---|
| CTS | - | 22.1 | 158.9 |
| PCN−CTS | 6.1 | 12.4 | 89.6 |
| GO−CTS | 15.9 | 7.2 | 52.0 |
| PCN/GO−CTS | 22.4 | 2.1 | 14.9 |
| CTS | PCN-CTS | GO-CTS | PCN/GO-CTS | |
|---|---|---|---|---|
| ECB (eV) | −0.075 | 0.02 | 0.035 | 0.17 |
| EVB (eV) | 1.65 | 1.48 | 1.48 | 1.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olatunde, O.C.; Onwudiwe, D.C. A Comparative Study of the Effect of Graphene Oxide, Graphitic Carbon Nitride, and Their Composite on the Photocatalytic Activity of Cu3SnS4. Catalysts 2022, 12, 14. https://doi.org/10.3390/catal12010014
Olatunde OC, Onwudiwe DC. A Comparative Study of the Effect of Graphene Oxide, Graphitic Carbon Nitride, and Their Composite on the Photocatalytic Activity of Cu3SnS4. Catalysts. 2022; 12(1):14. https://doi.org/10.3390/catal12010014
Chicago/Turabian StyleOlatunde, Olalekan C., and Damian C. Onwudiwe. 2022. "A Comparative Study of the Effect of Graphene Oxide, Graphitic Carbon Nitride, and Their Composite on the Photocatalytic Activity of Cu3SnS4" Catalysts 12, no. 1: 14. https://doi.org/10.3390/catal12010014
APA StyleOlatunde, O. C., & Onwudiwe, D. C. (2022). A Comparative Study of the Effect of Graphene Oxide, Graphitic Carbon Nitride, and Their Composite on the Photocatalytic Activity of Cu3SnS4. Catalysts, 12(1), 14. https://doi.org/10.3390/catal12010014







