Island-Type Hybrid Catalysts Applied for Anion Exchange Membrane Water Electrolysis
Abstract
:1. Introduction
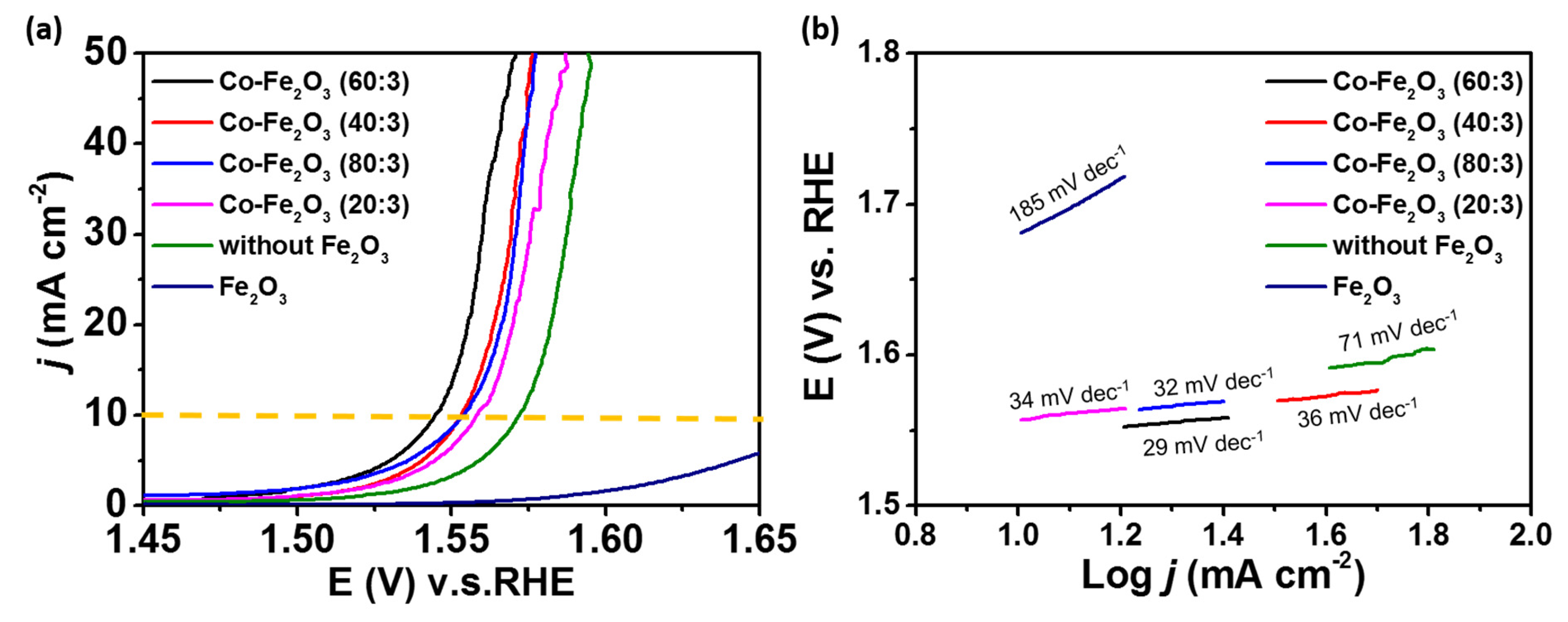
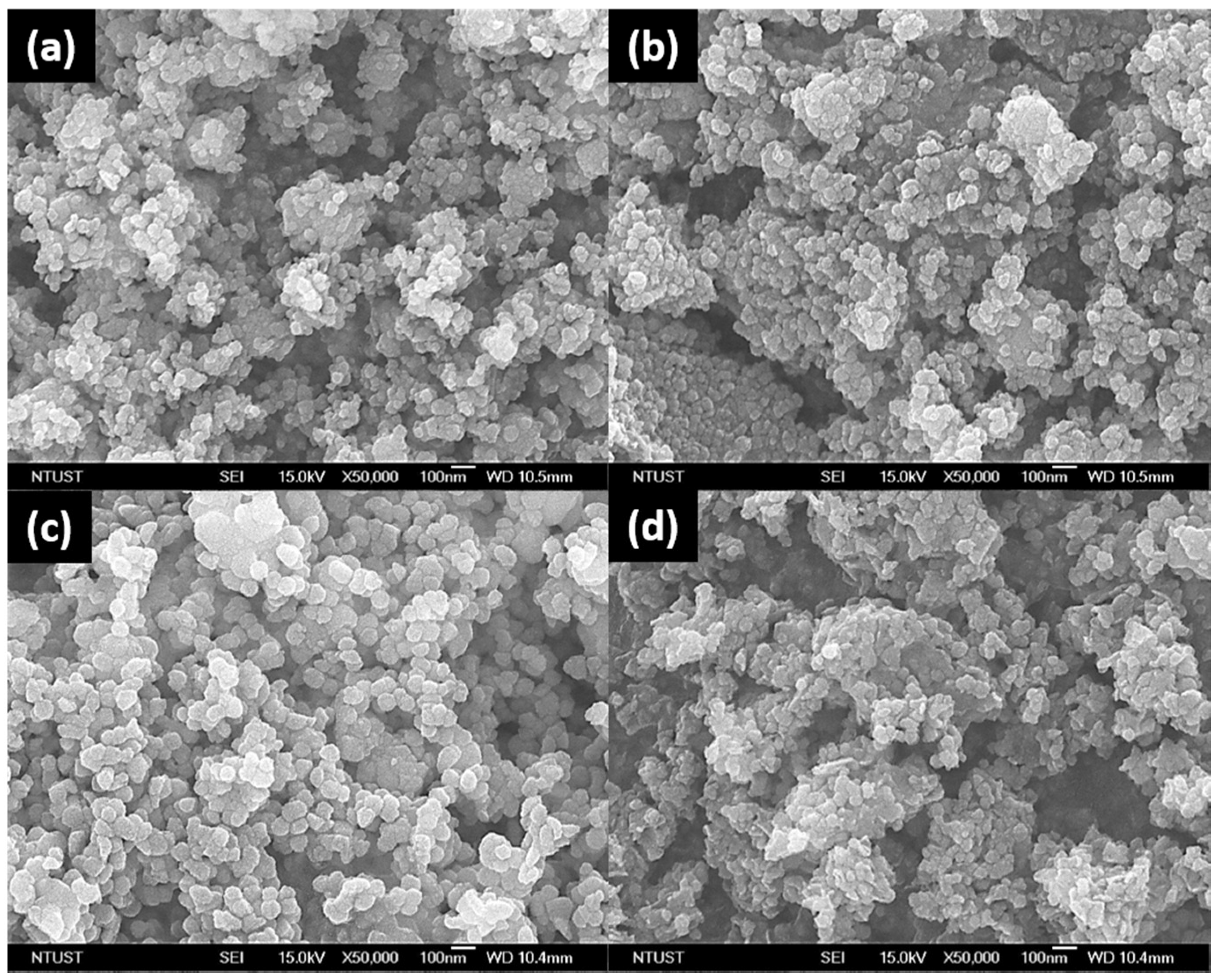
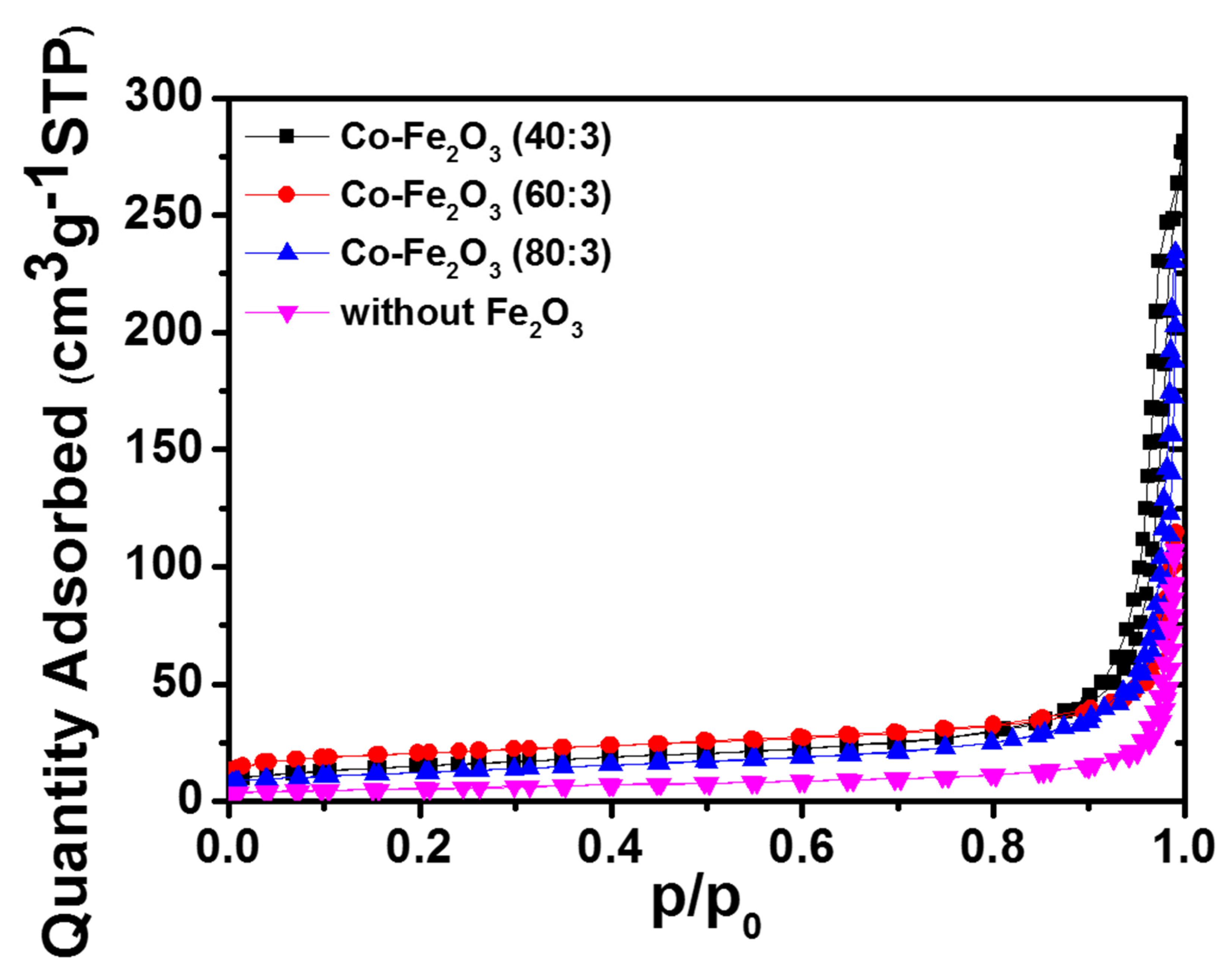
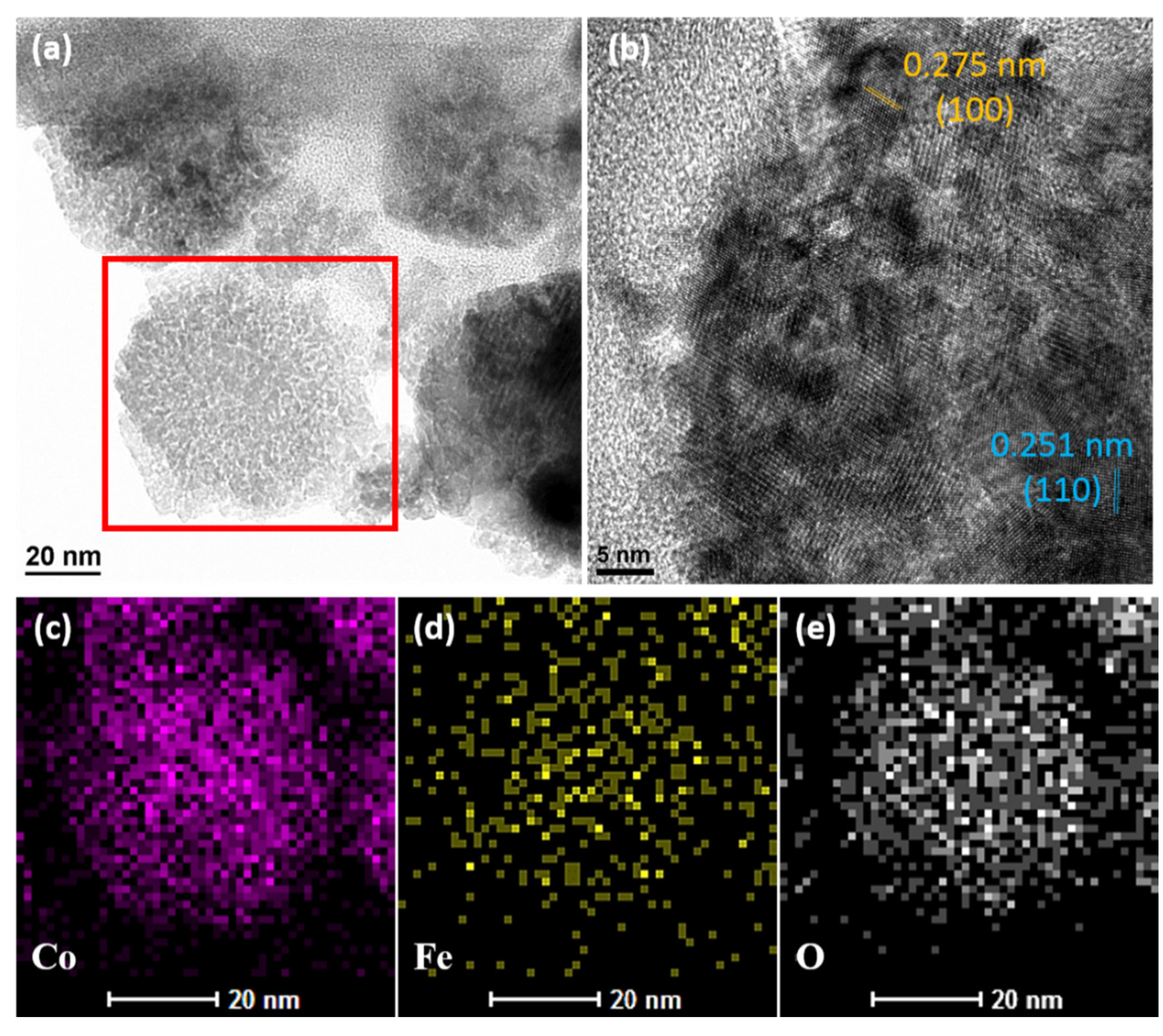
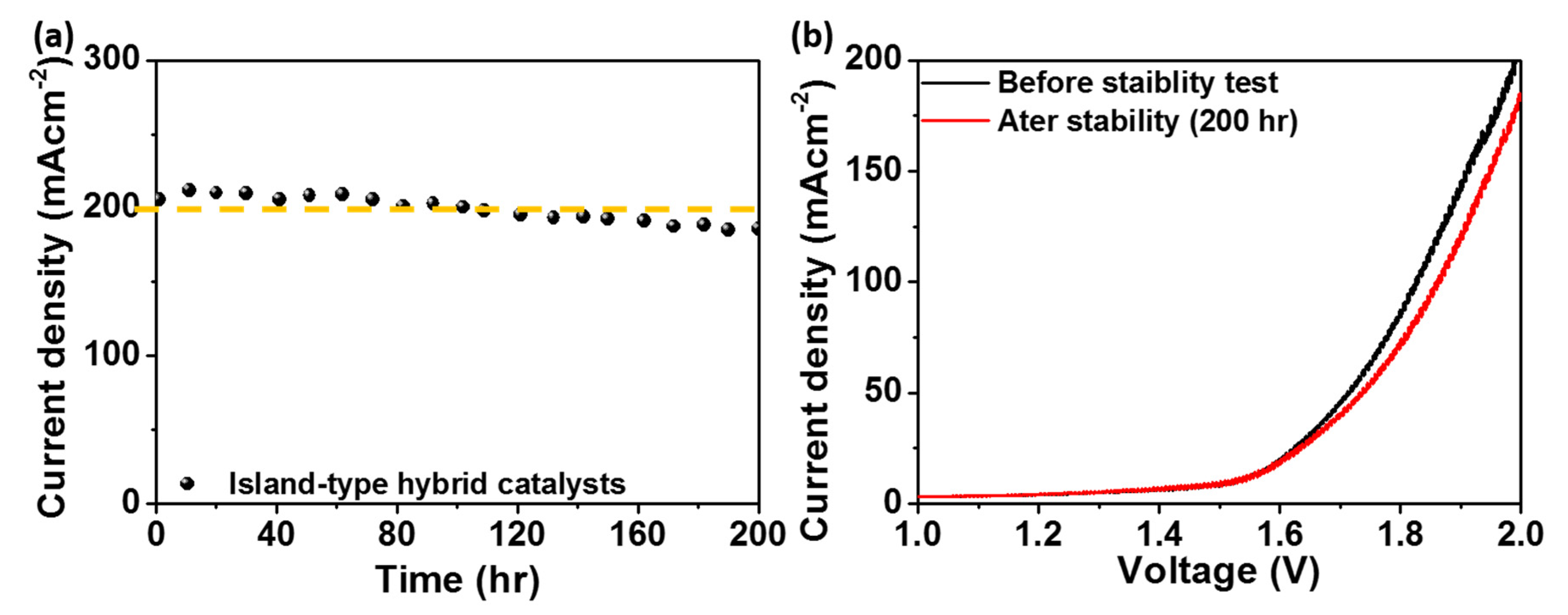
2. Results and Discussion
3. Experimental
3.1. Preparation of Fe2O3
3.2. Preparation of Island-Type Hybrid Catalysts
3.3. Electrochemical Measurement
3.4. Material Characterization
3.5. Full Water Electrolysis Test with Anion Exchange Membrane
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, Y.; Suntivich, J.; May, K.J.; Perry, E.E.; Shao-Horn, Y. Synthesis and Activities of Rutile IrO2 and RuO2 Nanoparticles for Oxygen Evolution in Acid and Alkaline Solutions. J. Phys. Chem. Lett. 2012, 3, 399–404. [Google Scholar] [CrossRef]
- Reier, T.; Oezaslan, M.; Strasser, P. Electrocatalytic Oxygen Evolution Reaction (OER) on Ru, Ir, and Pt Catalysts: A Comparative Study of Nanoparticles and Bulk Materials. ACS Catal. 2012, 2, 1765–1772. [Google Scholar] [CrossRef]
- Sardar, K.; Petrucco, E.; Hiley, C.I.; Sharman, J.D.B.; Wells, P.P.; Russell, A.E.; Kashtiban, R.J.; Sloan, J.; Walton, R.I. Water-splitting electrocatalysis in acid conditions using ruthenate-iridate pyrochlores. Angew. Chem. Int. Ed. Engl. 2014, 53, 10960–10964. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Hasin, P.; Wu, Y. NixCo3−xO4 Nanowire Arrays for Electrocatalytic Oxygen Evolution. Adv. Mater. 2010, 22, 1926–1929. [Google Scholar] [CrossRef] [PubMed]
- Dionigi, F.; Strasser, P. NiFe-Based (Oxy)hydroxide Catalysts for Oxygen Evolution Reaction in Non-Acidic Electrolytes. Adv. Energy Mater. 2016, 6, 1600621. [Google Scholar] [CrossRef]
- Osgood, H.; Devaguptapu, S.V.; Xu, H.; Cho, J.; Wu, G. Transition metal (Fe, Co, Ni, and Mn) oxides for oxygen reduction and evolution bifunctional catalysts in alkaline media. Nano Today 2016, 11, 601–625. [Google Scholar] [CrossRef]
- Castro, E.B.; Real, S.G.; Pinheiro Dick, L.F. Electrochemical characterization of porous nickel–cobalt oxide electrodes. Int. J. Hydrogen Energy 2004, 29, 255–261. [Google Scholar] [CrossRef]
- Chi, B.; Lin, H.; Li, J.; Wang, N.; Yang, J. Comparison of three preparation methods of NiCo2O4 electrodes. Int. J. Hydrogen Energy 2006, 31, 1210–1214. [Google Scholar] [CrossRef]
- Fan, G.; Li, F.; Evans, D.G.; Duan, X. Catalytic applications of layered double hydroxides: Recent advances and perspectives. Chem. Soc. Rev. 2014, 43, 7040–7066. [Google Scholar] [CrossRef]
- Cheng, F.; Shen, J.; Peng, B.; Pan, Y.; Tao, Z.; Chen, J. Rapid room-temperature synthesis of nanocrystalline spinels as oxygen reduction and evolution electrocatalysts. Nat. Chem. 2011, 3, 79–84. [Google Scholar] [CrossRef]
- Bragg, W.H. The Structure of Magnetite and the Spinels. Nature 1915, 95, 561. [Google Scholar] [CrossRef]
- Liu, X.-M.; Cui, X.; Dastafkan, K.; Wang, H.-F.; Tang, C.; Zhao, C.; Chen, A.; He, C.; Han, M.; Zhang, Q. Recent advances in spinel-type electrocatalysts for bifunctional oxygen reduction and oxygen evolution reactions. J. Energy Chem. 2021, 53, 290–302. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, T.; Chen, X.; Lu, Y.; Yang, W. Hierarchical hollow urchin-like NiCo2O4 nanomaterial as electrocatalyst for oxygen evolution reaction in alkaline medium. J. Power Source 2014, 268, 341–348. [Google Scholar] [CrossRef]
- Xia, H.; Huang, Z.; Lv, C.; Zhang, C. A Self-Supported Porous Hierarchical Core–Shell Nanostructure of Cobalt Oxide for Efficient Oxygen Evolution Reaction. ACS Catal. 2017, 7, 8205–8213. [Google Scholar] [CrossRef]
- Bandal, H.A.; Jadhav, A.R.; Tamboli, A.H.; Kim, H. Bimetallic iron cobalt oxide self-supported on Ni-Foam: An efficient bifunctional electrocatalyst for oxygen and hydrogen evolution reaction. Electrochim. Acta 2017, 249, 253–262. [Google Scholar] [CrossRef]
- Doyle, R.L.; Godwin, I.J.; Brandon, M.P.; Lyons, M.E.G. Redox and electrochemical water splitting catalytic properties of hydrated metal oxide modified electrodes. Phys. Chem. Chem. Phys. 2013, 15, 13737–13783. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef] [Green Version]
- Park, G.; Shin, C.-H.; Kang, J.; Lee, K.-S.; Zhang, C.; Lim, B.; Kim, C.; Yu, J.-S. Controllable synthesis of single-layer graphene over cobalt nanoparticles and insight into active sites for efficient oxygen evolution. J. Mater. Chem. A 2021, 9, 12060–12073. [Google Scholar] [CrossRef]
- Suen, N.-T.; Hung, S.-F.; Quan, Q.; Zhang, N.; Xu, Y.-J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, C.; Meng, X.; Lin, H.; Hu, C.; Long, X.; Yang, S. Hydrogen evolution electrocatalysis with binary-nonmetal transition metal compounds. J. Mater. Chem. A 2017, 5, 5995–6012. [Google Scholar] [CrossRef]
- Chen, K.; Rajendiran, R.; Lee, D.H.; Diao, J.; Li, O.L. Core-double shells heterostructure γ-Fe2O3@FeS2@C nanocubics with energy level matching double interfaces to boost the oxygen evolution reaction. J. Alloys Compd. 2021, 885, 160986. [Google Scholar] [CrossRef]
- Buchner, F.; Fuchs, S.; Behm, R.J. UHV preparation and electrochemical/-catalytic properties of well-defined Co- and Fe-containing unary and binary oxide model cathodes for the oxygen reduction and oxygen evolution reaction in Zn-air batteries. J. Electroanal. Chem. 2021, 896, 115497. [Google Scholar] [CrossRef]
- Ma, S.; Sun, L.; Cong, L.; Gao, X.; Yao, C.; Guo, X.; Tai, L.; Mei, P.; Zeng, Y.; Xie, H.; et al. Multiporous MnCo2O4 Microspheres as an Efficient Bifunctional Catalyst for Nonaqueous Li–O2 Batteries. J. Phys. Chem. C 2013, 117, 25890–25897. [Google Scholar] [CrossRef]
- Liu, L.; Yang, Y. Shape-controlled synthesis of MnCo complex oxide nanostructures via a polyol-based precursor route and their catalytic properties. Superlattices Microstruct. 2013, 54, 26–38. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, B.; Jiang, H.; Duan, X.; Zhu, Y.; Li, C. Boosting water oxidation electrocatalysts with surface engineered amorphous cobalt hydroxide nanoflakes. Nanoscale 2018, 10, 12991–12996. [Google Scholar] [CrossRef]
- Guzmán-Vargas, A.; Vazquez-Samperio, J.; Oliver-Tolentino, M.A.; Nava, N.; Castillo, N.; Macías-Hernández, M.J.; Reguera, E. Influence of cobalt on electrocatalytic water splitting in NiCoFe layered double hydroxides. J. Mater. Sci. 2018, 53, 4515–4526. [Google Scholar] [CrossRef]
- Thommes, M. Physical Adsorption Characterization of Nanoporous Materials. Chem. Ing. Tech. 2010, 82, 1059–1073. [Google Scholar] [CrossRef]
- Wei, X.; Li, Y.; Peng, H.; Gao, D.; Ou, Y.; Yang, Y.; Hu, J.; Zhang, Y.; Xiao, P. A novel functional material of Co3O4/Fe2O3 nanocubes derived from a MOF precursor for high-performance electrochemical energy storage and conversion application. Chem. Eng. J. 2019, 355, 336–340. [Google Scholar] [CrossRef]
- Samanta, A.; Das, S.; Jana, S. Doping of Ni in α-Fe2O3 Nanoclews To Boost Oxygen Evolution Electrocatalysis. ACS Sustain. Chem. Eng. 2019, 7, 12117–12124. [Google Scholar] [CrossRef]
- Zhu, S.; Huang, L.-A.; He, Z.; Wang, K.; Guo, J.; Pei, S.-E.; Shao, H.; Wang, J. Investigation of oxygen vacancies in Fe2O3/CoOx composite films for boosting electrocatalytic oxygen evolution performance stably. J. Electroanal. Chem. 2018, 827, 42–50. [Google Scholar] [CrossRef]
- Yang, X.; Chen, J.; Chen, Y.; Feng, P.; Lai, H.; Li, J.; Luo, X. Novel Co3O4 Nanoparticles/Nitrogen-Doped Carbon Composites with Extraordinary Catalytic Activity for Oxygen Evolution Reaction (OER). Nano-Micro Lett. 2017, 10, 15. [Google Scholar] [CrossRef] [Green Version]
- Pei, Z.; Xu, L.; Xu, W. Hierarchical honeycomb-like Co3O4 pores coating on CoMoO4 nanosheets as bifunctional efficient electrocatalysts for overall water splitting. Appl. Surf. Sci. 2018, 433, 256–263. [Google Scholar] [CrossRef]
- Li, D.; Park, E.J.; Zhu, W.; Shi, Q.; Zhou, Y.; Tian, H.; Lin, Y.; Serov, A.; Zulevi, B.; Baca, E.D.; et al. Highly quaternized polystyrene ionomers for high performance anion exchange membrane water electrolysers. Nat. Energy 2020, 5, 378–385. [Google Scholar] [CrossRef]
- Xiao, J.; Oliveira, A.M.; Wang, L.; Zhao, Y.; Wang, T.; Wang, J.; Setzler, B.P.; Yan, Y. Water-Fed Hydroxide Exchange Membrane Electrolyzer Enabled by a Fluoride-Incorporated Nickel–Iron Oxyhydroxide Oxygen Evolution Electrode. ACS Catal. 2021, 11, 264–270. [Google Scholar] [CrossRef]
- Zha, Q.; Li, M.; Liu, Z.; Ni, Y. Hierarchical Co,Fe-MOF-74/Co/Carbon Cloth Hybrid Electrode: Simple Construction and Enhanced Catalytic Performance in Full Water Splitting. ACS Sustain. Chem. Eng. 2020, 8, 12025–12035. [Google Scholar] [CrossRef]
- Li, M.; Liu, T.; Bo, X.; Zhou, M.; Guo, L. A novel flower-like architecture of FeCo@NC-functionalized ultra-thin carbon nanosheets as a highly efficient 3D bifunctional electrocatalyst for full water splitting. J. Mater. Chem. A 2017, 5, 5413–5425. [Google Scholar] [CrossRef]
- Shin, C.-H.; Wei, Y.; Park, G.; Kang, J.; Yu, J.-S. High performance binder-free Fe–Ni hydroxides on nickel foam prepared in piranha solution for the oxygen evolution reaction. Sustain. Energy Fuels 2020, 4, 6311–6320. [Google Scholar] [CrossRef]
- Jang, M.J.; Yang, J.; Lee, J.; Park, Y.S.; Jeong, J.; Park, S.M.; Jeong, J.-Y.; Yin, Y.; Seo, M.-H.; Choi, S.M.; et al. Superior performance and stability of anion exchange membrane water electrolysis: pH-controlled copper cobalt oxide nanoparticles for the oxygen evolution reaction. J. Mater. Chem. A 2020, 8, 4290–4299. [Google Scholar] [CrossRef]

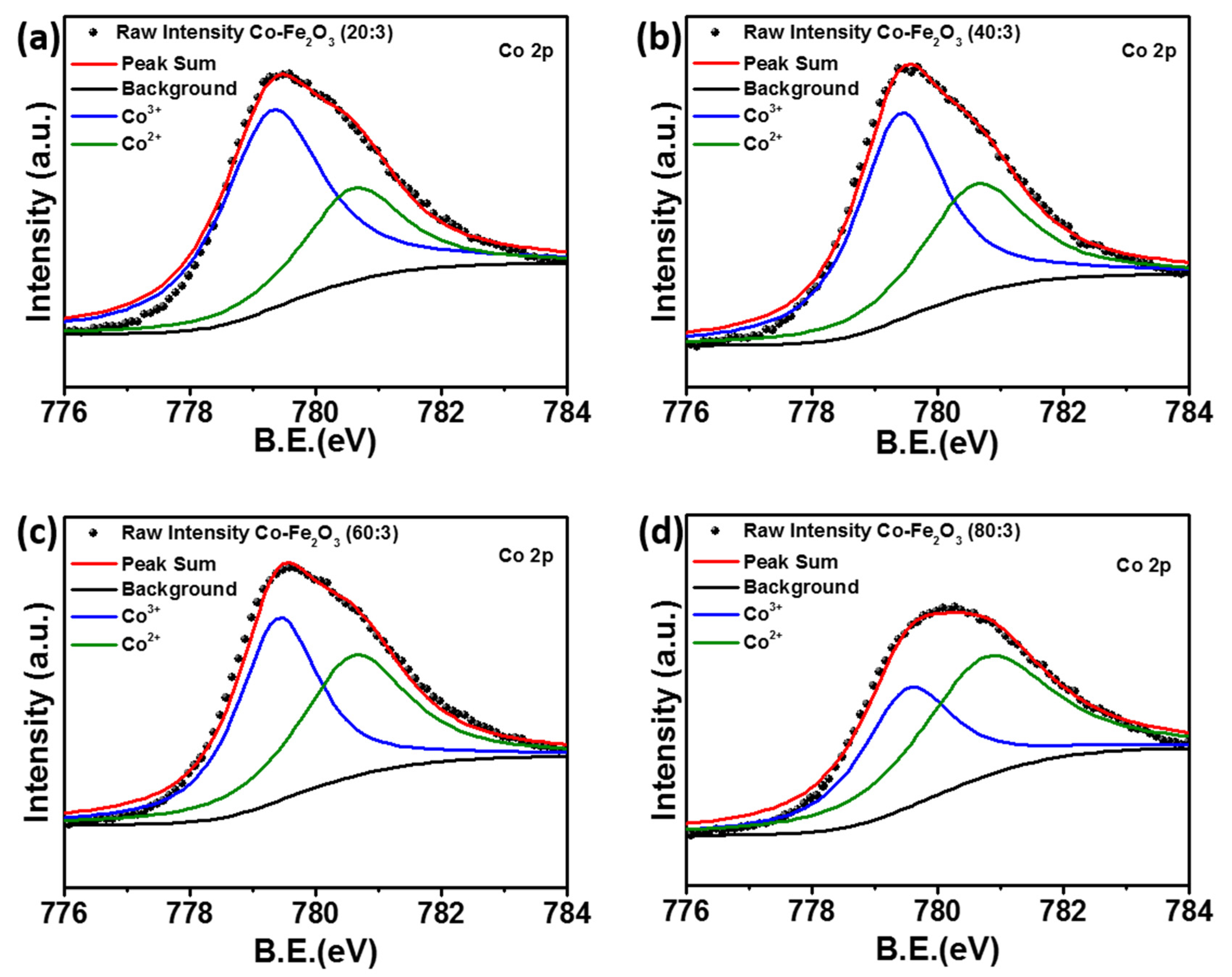
| Sample | Co3+ (%) | Co2+ (%) |
|---|---|---|
| Co-Fe2O3 (20:3) | 68.59 | 31.41 |
| Co-Fe2O3 (40:3) | 61.01 | 38.99 |
| Co-Fe2O3 (60:3) | 53.50 | 46.50 |
| Co-Fe2O3 (80:3) | 42.37 | 57.63 |
| Catalysts | OER Potential @10 mA/cm2 | Water Electrolysis Stability E@j (mA/cm2) | Operation Time (hour) | Temp. (°C) | Reference |
|---|---|---|---|---|---|
| Half-cell | |||||
| γ-Fe2O3@FeS2@C | 1.50 V | 1.50 V@10 | 10 | RT | [21] |
| Co3O4/Fe2O3 | 1.54 V | 1.54 V@10 | 20 | RT | [28] |
| Ni-Fe2O3 | 1.51 V | 1.51 V@10 | 10 | RT | [29] |
| Fe2O3/CoOx-Ar/H2 | 1.54 V | 1.55 V@10 | 16 | RT | [30] |
| M-Co3O4/NPC | 1.53 V | 1.53 V@10 | 10 | RT | [31] |
| Co3O4/CoMoO4/N | 1.50 V | 1.50 V@10 | 10 | RT | [32] |
| Full-cell | |||||
| Co-Fe2O3 (60:3) | 1.54 V | 2.0 V@200 | 200 | RT | This work |
| NiFe nanofoam | - | 2.1 V@200 | 160 | 60 °C | [33] |
| FexNiyOOH-20F | 1.48 V | 1.7 V@200 | 160 | 80 °C | [34] |
| Co-Fe-MOF74 | 1.49 V | 1.51 V@20 | 50 | RT | [35] |
| Fe0.5Co0.5@NC/NCNS-800 | 1.50 V | 1.50 V@10 | 30 | RT | [36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.-Y.; Chen, G.-C.; Liao, K.-W.; Wei, W.-H.; Huang, H.-C.; Wang, C.-H. Island-Type Hybrid Catalysts Applied for Anion Exchange Membrane Water Electrolysis. Catalysts 2022, 12, 102. https://doi.org/10.3390/catal12010102
Chen H-Y, Chen G-C, Liao K-W, Wei W-H, Huang H-C, Wang C-H. Island-Type Hybrid Catalysts Applied for Anion Exchange Membrane Water Electrolysis. Catalysts. 2022; 12(1):102. https://doi.org/10.3390/catal12010102
Chicago/Turabian StyleChen, Hsueh-Yu, Guan-Cheng Chen, Kuo-Wei Liao, Wen-Hui Wei, Hsin-Chih Huang, and Chen-Hao Wang. 2022. "Island-Type Hybrid Catalysts Applied for Anion Exchange Membrane Water Electrolysis" Catalysts 12, no. 1: 102. https://doi.org/10.3390/catal12010102
APA StyleChen, H.-Y., Chen, G.-C., Liao, K.-W., Wei, W.-H., Huang, H.-C., & Wang, C.-H. (2022). Island-Type Hybrid Catalysts Applied for Anion Exchange Membrane Water Electrolysis. Catalysts, 12(1), 102. https://doi.org/10.3390/catal12010102








