Fundamentals and Principles of Solid-State Electrochemical Sensors for High Temperature Gas Detection
Abstract
:1. Introduction
2. Theory
2.1. Equilibrium Potentiometric Gas Sensors
2.2. Mixed Potential Gas Sensors
2.3. Amperometric Gas Sensors
2.4. Combined Gas Sensors (Amperometric-Potentiometric)
2.5. Impedance-Based Gas Sensors
3. Materials for Electrochemical Gas Sensors
3.1. Electrolytes
3.2. Electrodes
4. Solid Electrolyte-Based Electrochemical Gas Sensors (EGSs)
4.1. Detection of Combustible Gasses
4.1.1. Detection of COx (CO, CO2) and CnH2n+2 Hydrocarbons (HCs)
Mixed Potential Combustible Gas Sensors
Amperometric Combustible Gas Sensors
4.1.2. Hydrogen (H2) Sensors
Amperometric H2 Sensors
Combined (Amperometric-Potentiometric) H2 Sensors
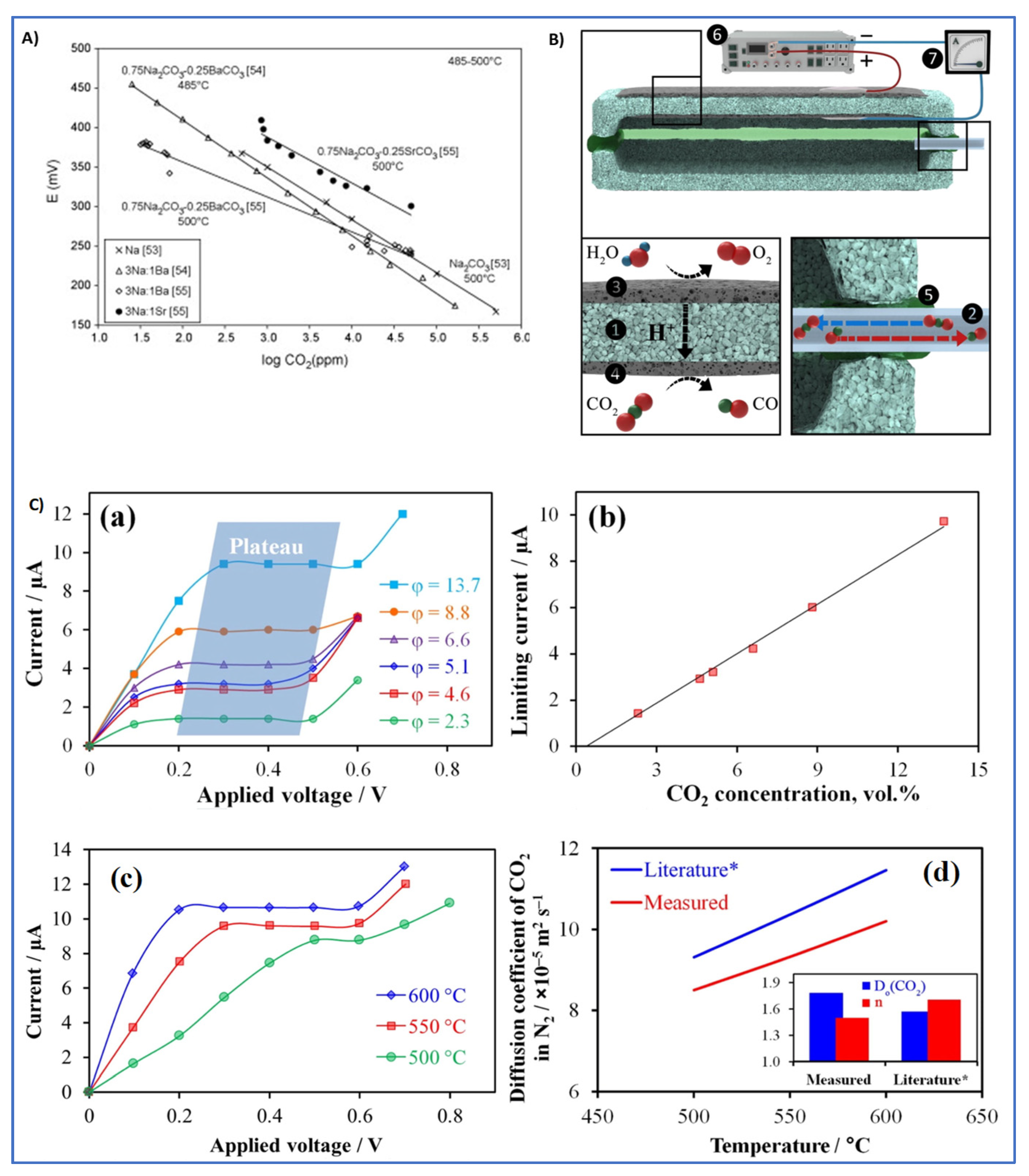
4.1.3. Carbon Dioxide (CO2) Sensors
4.2. Detection of Nitrogen Oxides (NOx) and Ammonia (NH3) Gas
4.2.1. Nitrogen Oxides Sensors
Amperometric NOx Sensors
Mixed Potential NOx Sensors
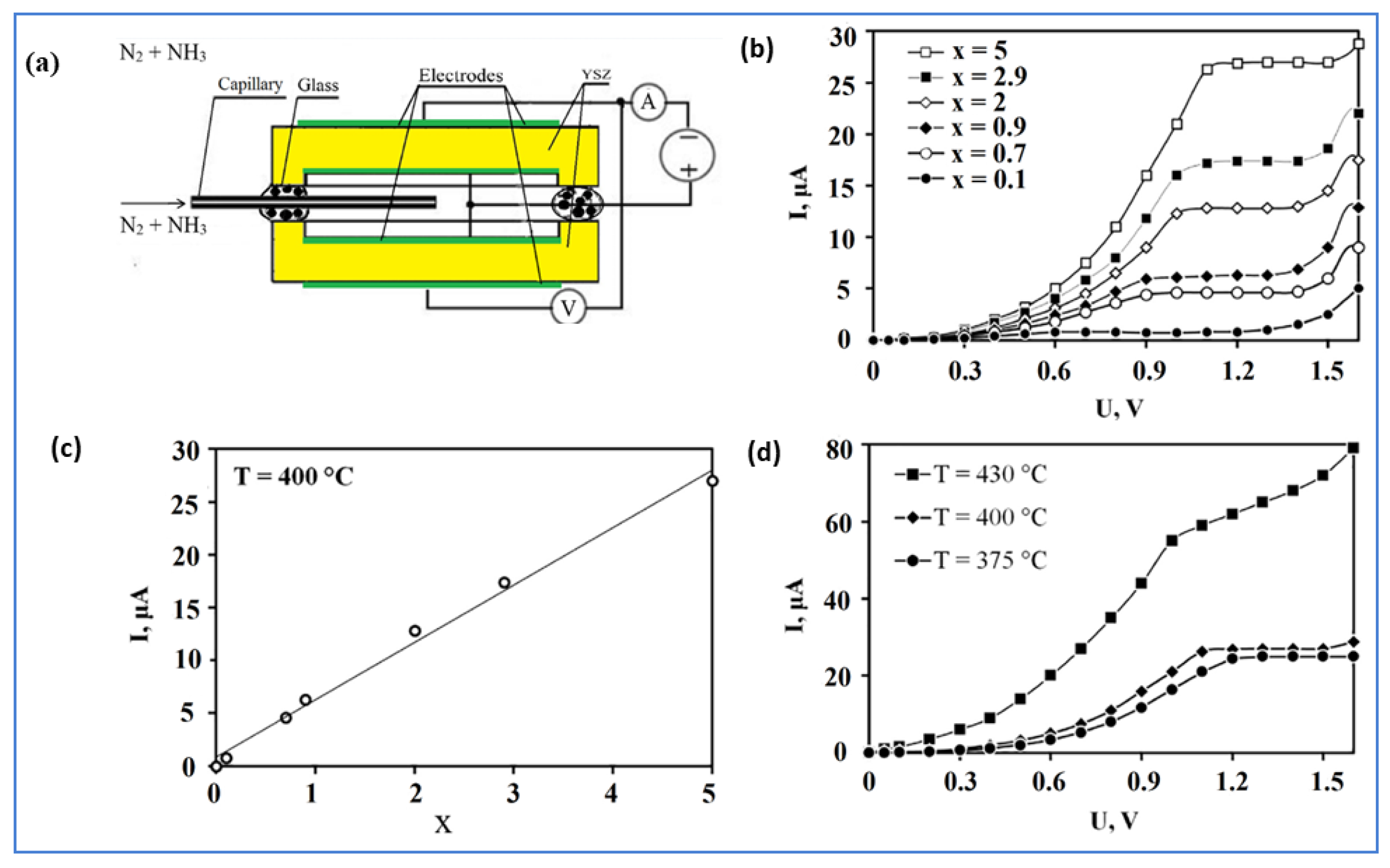
4.2.2. Ammonia (NH3) Sensors
Amperometric NH3 Sensors
4.3. Detection of Oxygen (O2) and Humidity (H2O Vapor)
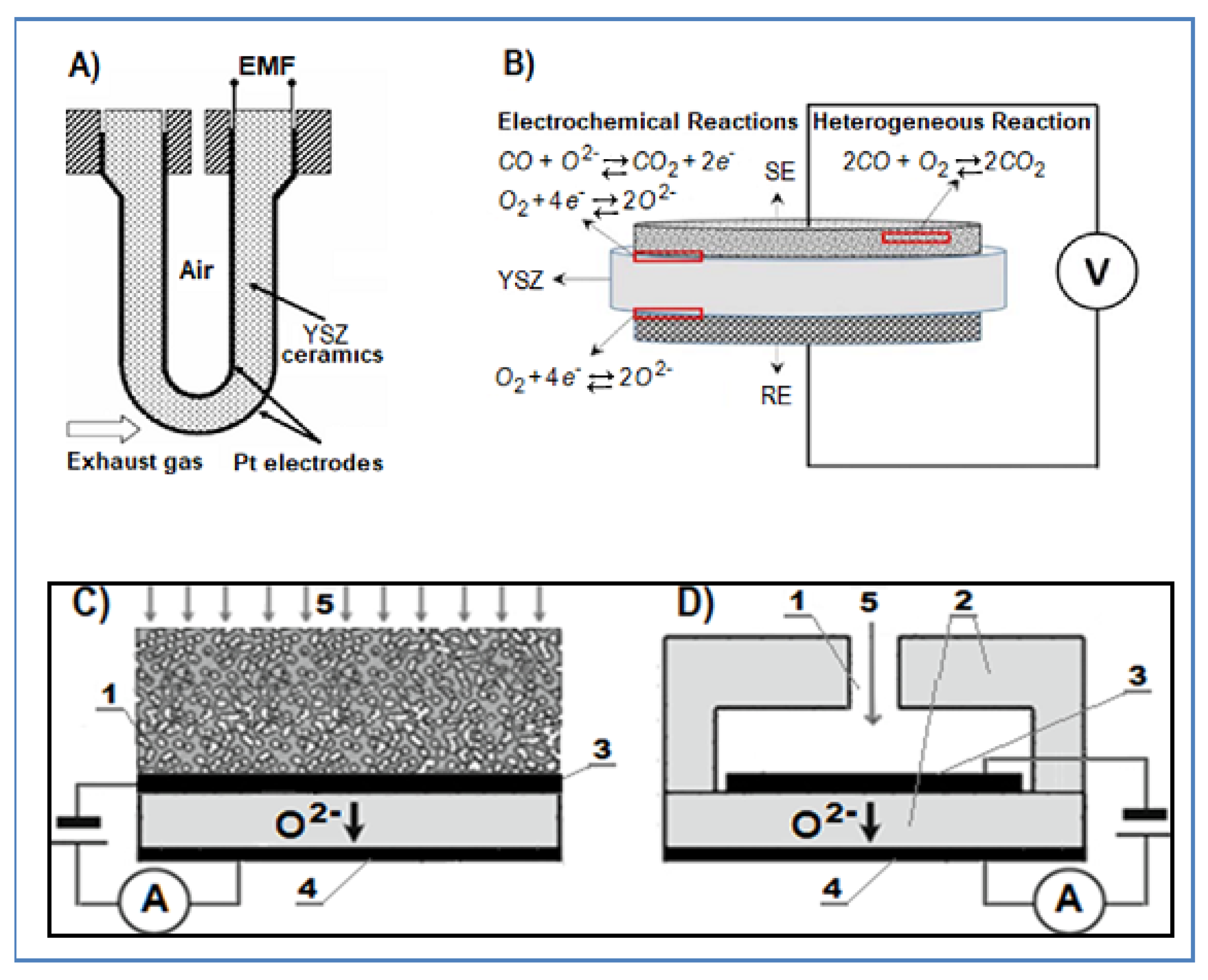
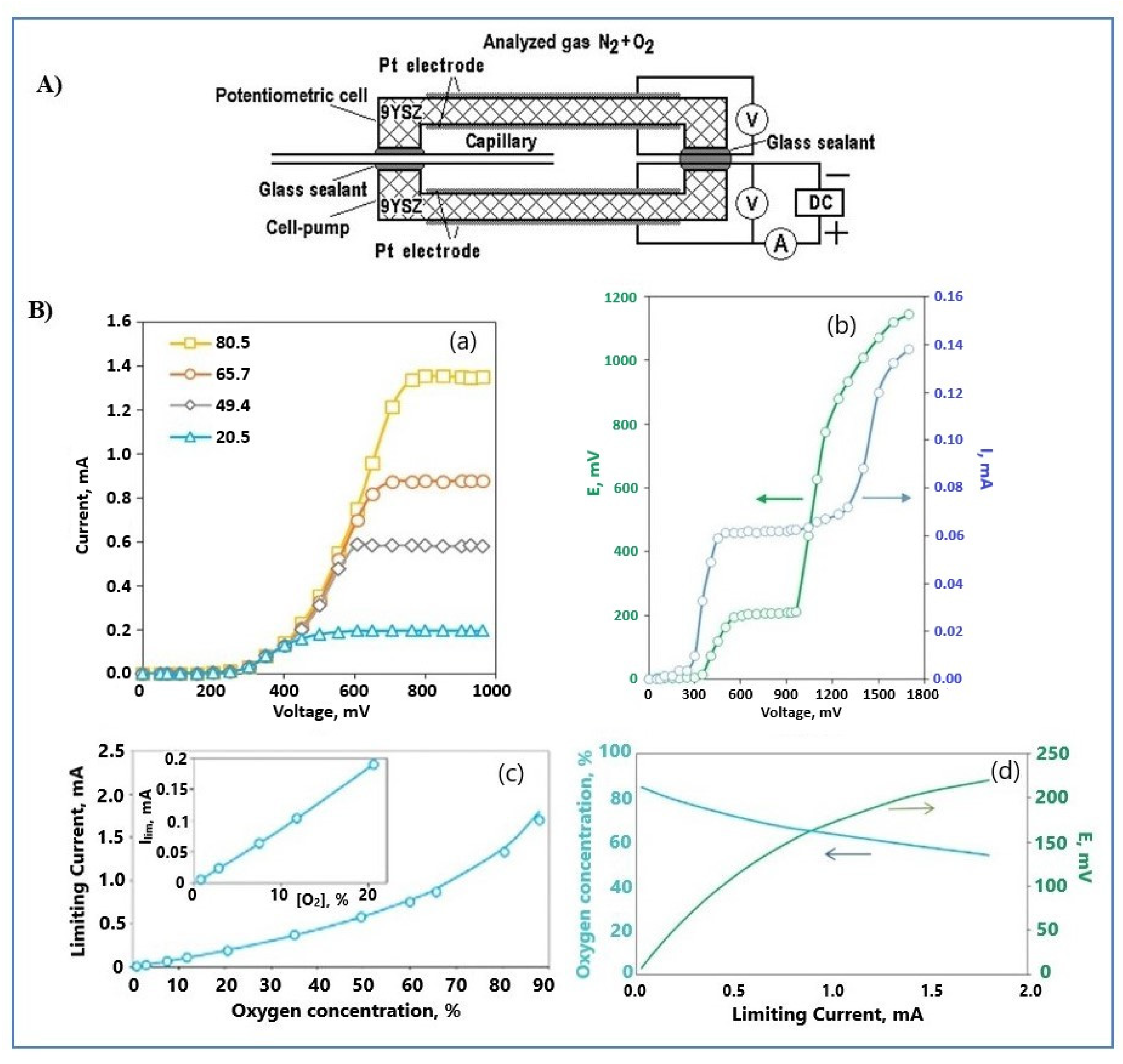
4.3.1. Oxygen Sensors
Potentiometric Oxygen Sensors
Amperometric Oxygen Sensors
Combined Oxygen Sensors
- First, the EMF value is lower compared to that of the calibration curve. This condition testifies that more than the expected oxygen reaches the electrode, either due to cracks in the electrolyte or the sealant. As a result, the measured oxygen concentration is higher than that in the analyzed gas.
- Second, the EMF value is higher compared to that of the calibration curve. This testifies that the characteristics of the metal capillary have changed. Corrosion or deposition of particles inside the capillary can change its inner diameter. As a result, the measured oxygen concentration is lower than that in the analyzed gas [69].
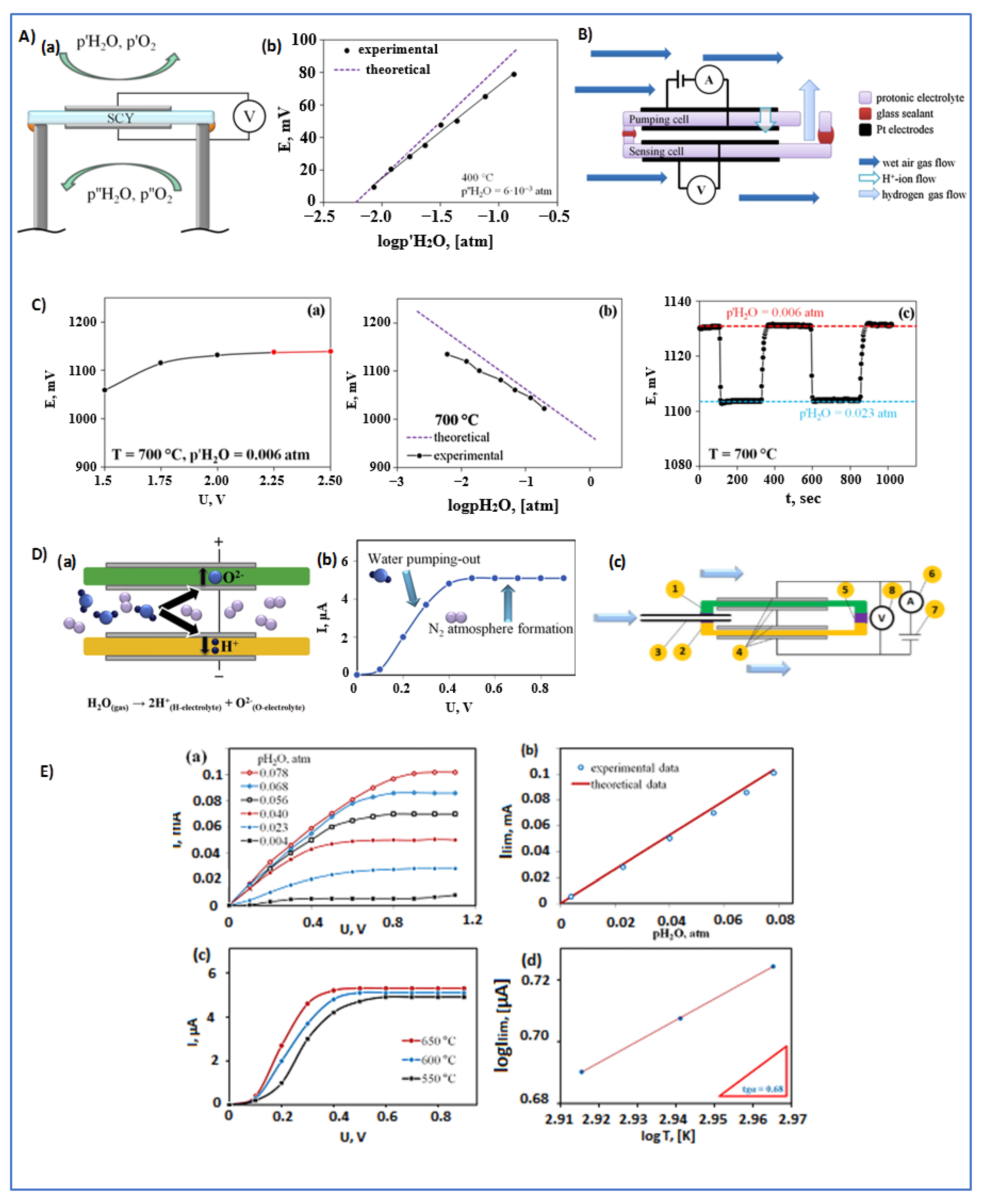
4.3.2. Humidity (H2O Steam) Sensors
5. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments

Conflicts of Interest
References
- Capone, S.; Forleo, A.; Francioso, L.; Rella, R.; Siciliano, P.; Spadavecchia, J.; Presicce, D.; Taurino, A. Solid state gas sensors: State of the art and future activities. J. Optoelectron. Adv. Mater. 2003, 5, 1335–1348. [Google Scholar] [CrossRef]
- Funke, K. Solid State Ionics: From Michael Faraday to green energy-the European dimension. Sci. Technol. Adv. Mater. 2013, 14, 043502. [Google Scholar] [CrossRef] [Green Version]
- Möbius, H.-H. On the history of solid electrolyte fuel cells. J. Solid State Electrochem. 1997, 1, 2–16. [Google Scholar] [CrossRef]
- Kiukkola, K.; Wagner, C. Measurements on Galvanic Cells Involving Solid Electrolytes. J. Electrochem. Soc. 1957, 104, 379. [Google Scholar] [CrossRef]
- Weissbart, J.; Ruka, R. Oxygen Gauge. Rev. Sci. Instrum. 1961, 32, 593–595. [Google Scholar] [CrossRef]
- West, A.R. Solid electrolytes. Ber. Bunsenges. Phys. Chem. 1989, 93, 1235–1241. [Google Scholar] [CrossRef]
- Liu, Y.; Parisi, J.; Sun, X.; Lei, Y. Solid-state gas sensors for high temperature applications—A review. J. Mater. Chem. A 2014, 2, 9919–9943. [Google Scholar] [CrossRef]
- Volkov, A.; Gorbova, E.; Vylkov, A.; Medvedev, D.; Demin, A.; Tsiakaras, P. Design and applications of potentiometric sensors based on proton-conducting ceramic materials. A brief review. Sens. Actuators B Chem. 2017, 244, 1004–1015. [Google Scholar] [CrossRef]
- Demin, A.; Gorbova, E.; Brouzgou, A.; Volkov, A.; Tsiakaras, P. Chapter 6—Sensors based on solid oxide electrolytes. In Solid Oxide-Based Electrochemical Devices; Lo Faro, M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 167–215. [Google Scholar]
- López-Gándara, C.; Ramos, F.M.; Cirera, A. YSZ-Based Oxygen Sensors and the Use of Nanomaterials: A Review from Classical Models to Current Trends. J. Sens. 2009, 2009, 1–15. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, X.; Yuan, L.; Yu, J. A review of high-temperature electrochemical sensors based on stabilized zirconia. Solid State Ion. 2015, 283, 91–102. [Google Scholar] [CrossRef]
- Liu, F.; Wang, J.; Jiang, L.; You, R.; Wang, Q.; Wang, C.; Lin, Z.; Yang, Z.; He, J.; Liu, A.; et al. Compact and planar type rapid response ppb-level SO2 sensor based on stabilized zirconia and SrMoO4 sensing electrode. Sens. Actuators B Chem. 2020, 307, 127655. [Google Scholar] [CrossRef]
- Khan, M.A.; Qazi, F.; Hussain, Z.; Idrees, M.U.; Soomro, S.; Soomro, S. Recent trends in electrochemical detection of NH3, H2S and NOx gases. Int. J. Electrochem. Sci. 2017, 12, 1711–1733. [Google Scholar] [CrossRef]
- Okamoto, H.; Obayashi, H.; Kudo, T. Carbon monoxide gas sensor made of stabilized zirconia. Solid State Ion. 1980, 1, 319–326. [Google Scholar] [CrossRef]
- Ramaiyan, K.P.; Mukundan, R. Editors’ Choice—Review—Recent Advances in Mixed Potential Sensors. J. Electrochem. Soc. 2020, 167, 037547. [Google Scholar] [CrossRef]
- Jasiński, P. Solid-state electrochemical gas sensors. Mater. Sci. Pol. 2006, 24, 269–278. [Google Scholar] [CrossRef]
- Morata, A.; Dezanneau, G.; Tarancon, A.; Peiro, F.; Morante, J.R. Novel design and preliminary results of YSZ electrolyte-based amperometric oxygen sensors. In Conference on Electron Devices, 2005 Spanish; IEEE: Piscataway, NJ, USA, 2005; pp. 585–588. [Google Scholar] [CrossRef]
- Kalyakin, A.; Lyagaeva, J.; Medvedev, D.; Volkov, A.; Demin, A.; Tsiakaras, P. Characterization of proton-conducting electrolyte based on La0. 9Sr0. 1YO3–δ and its application in a hydrogen amperometric sensor. Sens. Actuators B Chem. 2016, 225, 446–452. [Google Scholar] [CrossRef]
- Usui, T.; Asada, A.; Nakazawa, M.; Osanai, H. Gas Polarographic Oxygen Sensor Using an Oxygen/Zirconia Electrolyte. J. Electrochem. Soc. 1989, 136, 534–542. [Google Scholar] [CrossRef]
- Rheaume, J.M.; Pisano, A.P. A review of recent progress in sensing of gas concentration by impedance change. Ionics 2011, 17, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Nakatou, M.; Miura, N. Impedancemetric sensor based on YSZ and In2O3 for detection of low concentrations of water vapor at high temperature. Electrochem. Commun. 2004, 6, 995–998. [Google Scholar] [CrossRef]
- Wama, R.; Utiyama, M.; Plashnitsa, V.V.; Miura, N. Highly sensitive impedance-based propene sensor using stabilized zirconia and zinc oxide sensing-electrode. Electrochem. Commun. 2007, 9, 2774–2777. [Google Scholar] [CrossRef]
- Wu, N.; Chen, Z.; Xu, J.; Chyu, M.; Mao, S.X. Impedance-metric Pt/YSZ/Au–Ga2O3 sensor for CO detection at high temperature. Sens. Actuators B Chem. 2005, 110, 49–53. [Google Scholar] [CrossRef]
- Miura, N.; Nakatou, M.; Zhuiykov, S. Impedancemetric gas sensor based on zirconia solid electrolyte and oxide sensing electrode for detecting total NOx at high temperature. Sens. Actuators B Chem. 2003, 93, 221–228. [Google Scholar]
- Liu, T.; Zhang, X.; Wang, X.; Yu, J.; Li, L. A review of zirconia-based solid electrolytes. Ionics 2016, 22, 2249–2262. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Murashkina, A.A.; Maragou, V.I.; Demin, A.K.; Strekalovsky, V.N.; Tsiakaras, P.E. CeO2 based materials doped with lanthanides for applications in intermediate temperature electrochemical devices. Int. J. Hydrog. Energy 2011, 36, 6175–6183. [Google Scholar] [CrossRef]
- Inaba, H.; Tagawa, H. Ceria-based solid electrolytes. Solid State Ion. 1996, 83, 1–16. [Google Scholar] [CrossRef]
- Ishihara, T.; Matsuda, H.; Takita, Y. Doped LaGaO3 perovskite type oxide as a new oxide ionic conductor. J. Am. Chem. Soc. 1994, 116, 3801–3803. [Google Scholar] [CrossRef]
- Ishihara, T.; Matsuda, H.; bin Bustam, M.A.; Takita, Y. Oxide ion conductivity in doped Ga based perovskite type oxide. Solid State Ion. 1996, 86, 197–201. [Google Scholar] [CrossRef]
- Ishihara, T.; Honda, M.; Shibayama, T.; Minami, H.; Nishiguchi, H.; Takita, Y. Intermediate Temperature Solid Oxide Fuel Cells Using a New LaGaO3 Based Oxide Ion Conductor: I. Doped as a New Cathode Material. J. Electrochem. Soc. 1998, 145, 3177. [Google Scholar] [CrossRef]
- Skinner, S.J.; Kilner, J.A. Oxygen ion conductors. Mater. Today 2003, 6, 30–37. [Google Scholar] [CrossRef]
- Boivin, J.; Mairesse, G. Recent material developments in fast oxide ion conductors. Chem. Mater. 1998, 10, 2870–2888. [Google Scholar] [CrossRef]
- Iwahara, H.; Uchida, H.; Tanaka, S. High temperature-type proton conductive solid oxide fuel cells using various fuels. J. Appl. Electrochem. 1986, 16, 663–668. [Google Scholar] [CrossRef]
- Virkar, A.; Maiti, H. Oxygen ion conduction in pure and yttria-doped barium cerate. J. Power Sources 1985, 14, 295–303. [Google Scholar] [CrossRef]
- Mitsui, A.; Miyayama, M.; Yanagida, H. Evaluation of the activation energy for proton conduction in perovskite-type oxides. Solid State Ion. 1987, 22, 213–217. [Google Scholar] [CrossRef]
- Medvedev, D.; Brouzgou, A.; Demin, A.; Tsiakaras, P. Proton-Conducting Electrolytes for Solid Oxide Fuel Cell Applications. In Advances in Medium and High Temperature Solid Oxide Fuel Cell Technology; Springer: Cham, Switzerland, 2017; pp. 77–118. [Google Scholar]
- Medvedev, D.A.; Lyagaeva, J.G.; Gorbova, E.V.; Demin, A.K.; Tsiakaras, P. Advanced materials for SOFC application: Strategies for the development of highly conductive and stable solid oxide proton electrolytes. Prog. Mater. Sci. 2016, 75, 38–79. [Google Scholar] [CrossRef]
- Yajima, T.; Kazeoka, H.; Yogo, T.; Iwahara, H. Proton conduction in sintered oxides based on CaZrO3. Solid State Ion. 1991, 47, 271–275. [Google Scholar] [CrossRef]
- Liang, K.C.; Nowick, A.S. High-temperature protonic conduction in mixed perovskite ceramics. Solid State Ion. 1993, 61, 77–81. [Google Scholar] [CrossRef]
- Kochetova, N.; Animitsa, I.; Medvedev, D.; Demin, A.; Tsiakaras, P. Recent activity in the development of proton-conducting oxides for high-temperature applications. RSC Adv. 2016, 6, 73222–73268. [Google Scholar] [CrossRef]
- Zhuiykov, S. Electrochemistry of Zirconia Gas Sensors; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Yajima, T.; Koide, K.; Takai, H.; Fukatsu, N.; Iwahara, H. Application of hydrogen sensor using proton conductive ceramics as a solid electrolyte to aluminum casting industries. Solid State Ion. 1995, 79, 333–337. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, C.; Xia, J.; Zhou, G.; Jiang, D.; Wang, S.; Li, X.; Shen, Y.; Dai, M.; Wang, B.; et al. Mesoporous tungsten oxide electrodes for YSZ-based mixed potential sensors to detect NO2 in the sub ppm-range. Sens. Actuators B Chem. 2019, 284, 575–581. [Google Scholar] [CrossRef]
- Garzon, F.H.; Mukundan, R.; Brosha, E.L. Solid-state mixed potential gas sensors: Theory, experiments and challenges. Solid State Ion. 2000, 136–137, 633–638. [Google Scholar] [CrossRef]
- Brosha, E.L.; Mukundan, R.; Brown, D.R.; Garzon, F.H.; Visser, J. Development of ceramic mixed potential sensors for automotive applications. Solid State Ion. 2002, 148, 61–69. [Google Scholar] [CrossRef]
- Guillet, N.; Lalauze, R.; Pijolat, C. Oxygen and carbon monoxide role on the electrical response of a non-Nernstian potentiometric gas sensor; proposition of a model. Sens. Actuators B Chem. 2004, 98, 130–139. [Google Scholar] [CrossRef]
- Lu, G.; Miura, N.; Yamazoe, N. High-temperature hydrogen sensor based on stabilized zirconia and a metal oxide electrode. Sens. Actuators B Chem. 1996, 35, 130–135. [Google Scholar] [CrossRef]
- Di Bartolomeo, E.; Grilli, M.L. YSZ-based electrochemical sensors: From materials preparation to testing in the exhausts of an engine bench test. J. Eur. Ceram. Soc. 2005, 25, 2959–2964. [Google Scholar] [CrossRef]
- Zosel, J.; Westphal, D.; Jakobs, S.; Müller, R.; Guth, U. Au–oxide composites as HC-sensitive electrode material for mixed potential gas sensors. Solid State Ion. 2002, 152–153, 525–529. [Google Scholar] [CrossRef]
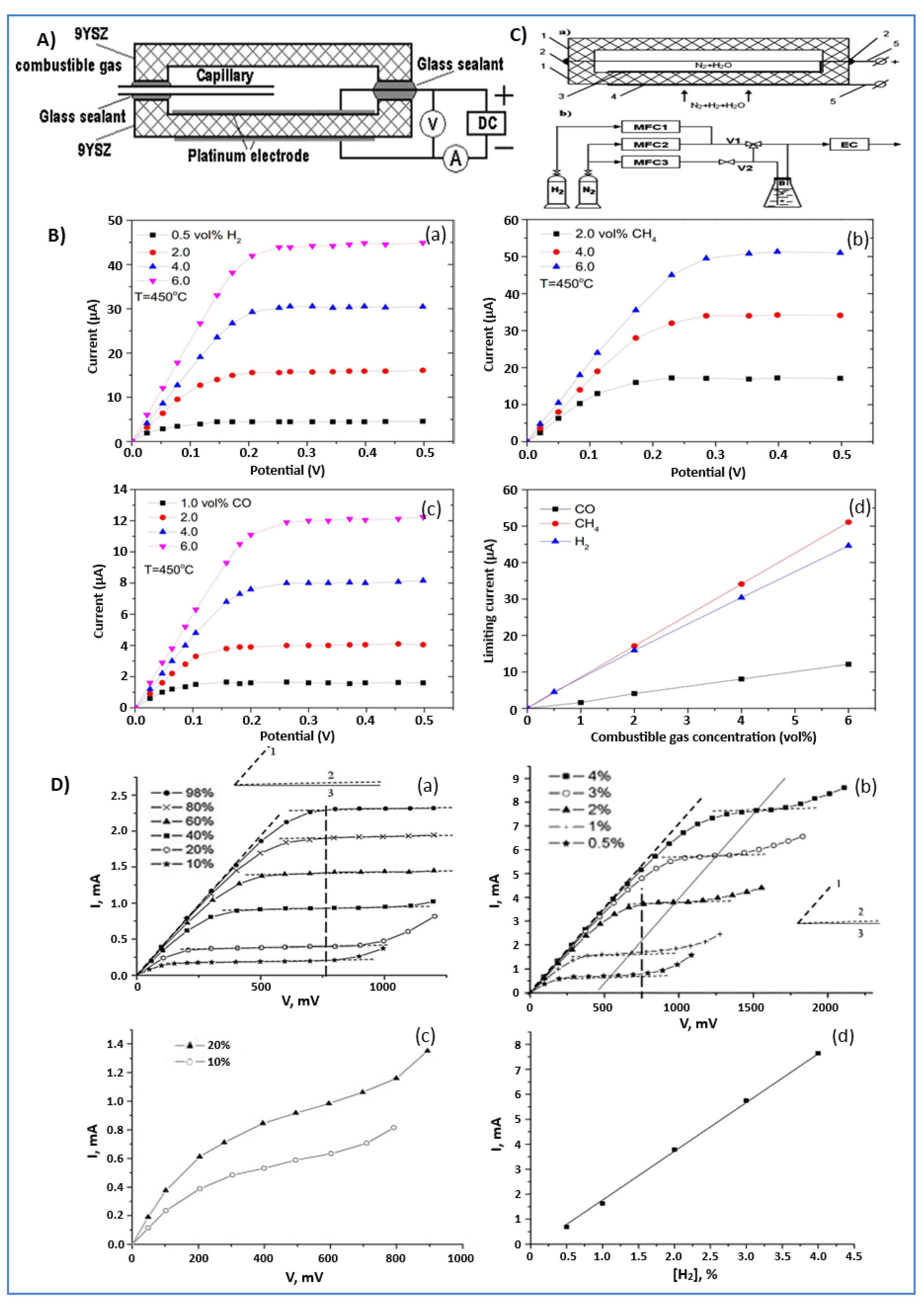
- Fadeyev, G.; Kalyakin, A.; Gorbova, E.; Brouzgou, A.; Demin, A.; Volkov, A.; Tsiakaras, P. A simple and low-cost amperometric sensor for measuring H2, CO, and CH4. Sens. Actuators B Chem. 2015, 221, 879–883. [Google Scholar] [CrossRef]
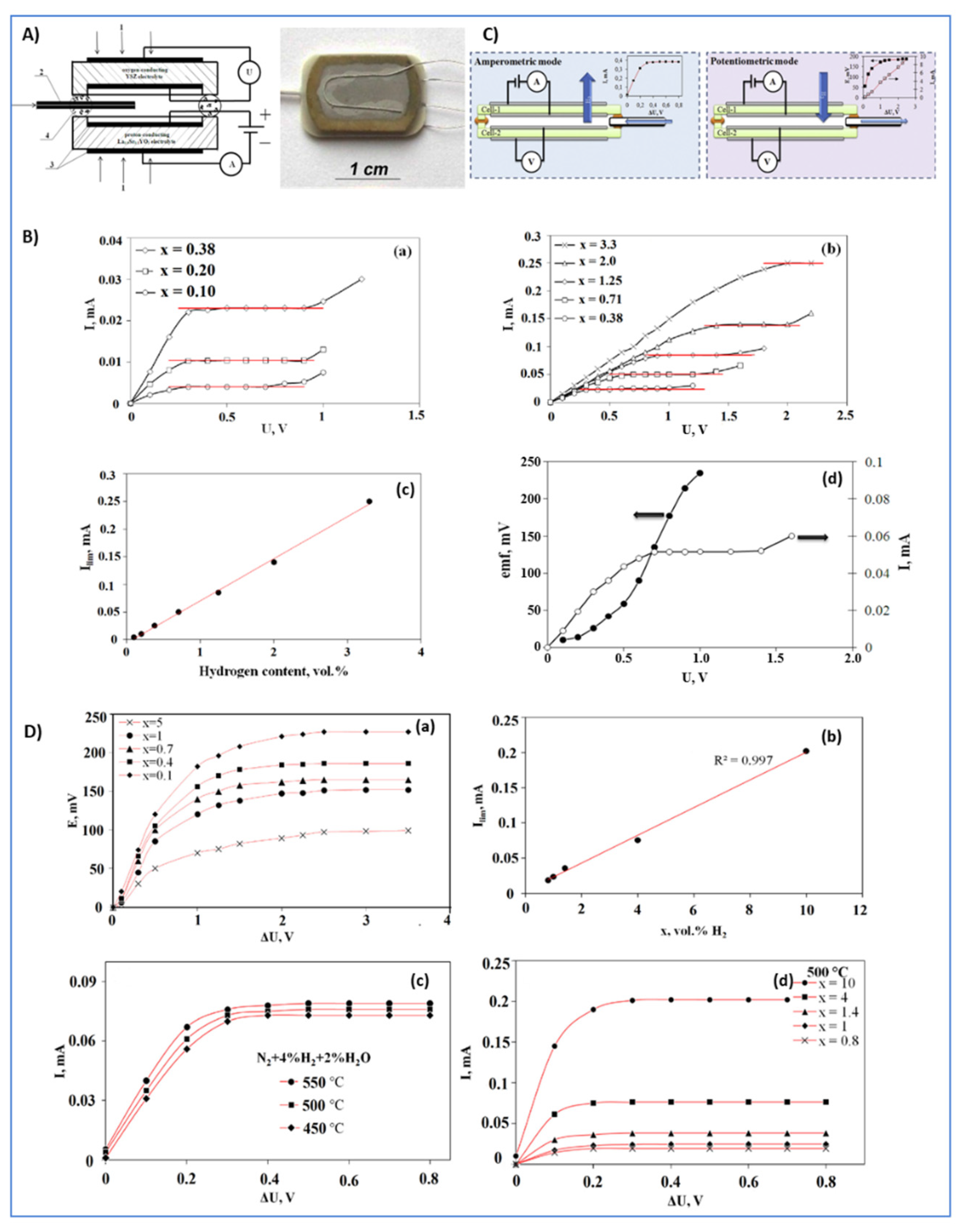
- Kalyakin, A.; Fadeyev, G.; Demin, A.; Gorbova, E.; Brouzgou, A.; Volkov, A.; Tsiakaras, P. Application of Solid oxide proton-conducting electrolytes for amperometric analysis of hydrogen in H2+N2+H2O gas mixtures. Electrochim. Acta 2014, 141, 120–125. [Google Scholar] [CrossRef]
- Kalyakin, A.; Volkov, A.; Lyagaeva, J.; Medvedev, D.; Demin, A.; Tsiakaras, P. Combined amperometric and potentiometric hydrogen sensors based on BaCe0.7Zr0.1Y0.2O3−δ proton-conducting ceramic. Sens. Actuators B Chem. 2016, 231, 175–182. [Google Scholar] [CrossRef]
- Fergus, J.W. A review of electrolyte and electrode materials for high temperature electrochemical CO2 and SO2 gas sensors. Sens. Actuators B Chem. 2008, 134, 1034–1041. [Google Scholar] [CrossRef]
- Kalyakin, A.S.; Medvedev, D.A.; Volkov, A.N. Electrochemical sensors based on proton-conducting electrolytes for determination of concentration and diffusion coefficient of CO2 in inert gases. Chem. Eng. Sci. 2021, 229, 116046. [Google Scholar] [CrossRef]
- Haagen-Smit, A.J.; Fox, M.M. Photochemical Ozone Formation with Hydrocarbons and Automobile Exhaust. Air Repair 1954, 4, 105–136. [Google Scholar] [CrossRef]
- Haagen-Smit, A.J. Photochemistry and Smog. J. Air Pollut. Control. Assoc. 1963, 13, 444–454. [Google Scholar] [CrossRef]
- Cooper, C.D.; Alley, F.C. Air Pollution Control: A Design Approach; Waveland Press: Long Grove, IL, USA, 2010. [Google Scholar]
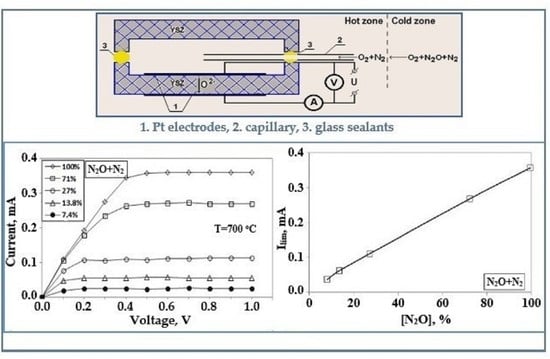
- Kalyakin, A.; Volkov, A.; Demin, A.; Gorbova, E.; Tsiakaras, P. Determination of nitrous oxide concentration using a solid-electrolyte amperometric sensor. Sens. Actuators B Chem. 2019, 297, 126750. [Google Scholar] [CrossRef]
- Miura, N.; Kurosawa, H.; Hasei, M.; Lu, G.; Yamazoe, N. Stabilized zirconia-based sensor using oxide electrode for detection of NOx in high-temperature combustion-exhausts. Solid State Ion. 1996, 86–88, 1069–1073. [Google Scholar] [CrossRef]
- Zhuiykov, S.; Nakano, T.; Kunimoto, A.; Yamazoe, N.; Miura, N. Potentiometric NOx sensor based on stabilized zirconia and NiCr2O4 sensing electrode operating at high temperatures. Electrochem. Commun. 2001, 3, 97–101. [Google Scholar] [CrossRef]
- Miura, N.; Akisada, K.; Wang, J.; Zhuiykov, S.; Ono, T. Mixed-potential-type NO x sensor based on YSZ and zinc oxide sensing electrode. Ionics 2004, 10, 1–9. [Google Scholar] [CrossRef]
- Di Bartolomeo, E.; Kaabbuathong, N.; Grilli, M.L.; Traversa, E. Planar electrochemical sensors based on tape-cast YSZ layers and oxide electrodes. Solid State Ion. 2004, 171, 173–181. [Google Scholar] [CrossRef]
- Wang, J.; Elumalai, P.; Terada, D.; Hasei, M.; Miura, N. Mixed-potential-type zirconia-based NOx sensor using Rh-loaded NiO sensing electrode operating at high temperatures. Solid State Ion. 2006, 177, 2305–2311. [Google Scholar] [CrossRef]
- Kalyakin, A.; Volkov, A.; Vylkov, A.; Gorbova, E.; Medvedev, D.; Demin, A.; Tsiakaras, P. An electrochemical method for the determination of concentration and diffusion coefficient of ammonia-nitrogen gas mixtures. J. Electroanal. Chem. 2018, 808, 133–136. [Google Scholar] [CrossRef]
- Poling, B.E.; Prausnitz, J.M.; O’connell, J.P. Properties of Gases and Liquids; McGraw-Hill Education: New York, NY, USA, 2001. [Google Scholar]
- Spirig, J.V.; Ramamoorthy, R.; Akbar, S.A.; Routbort, J.L.; Singh, D.; Dutta, P.K. High temperature zirconia oxygen sensor with sealed metal/metal oxide internal reference. Sens. Actuators B Chem. 2007, 124, 192–201. [Google Scholar] [CrossRef]
- Colominas, S.; Abella, J.; Victori, L. Characterisation of an oxygen sensor based on In/In2O3 reference electrode. J. Nucl. Mater. 2004, 335, 260–263. [Google Scholar] [CrossRef]
- Thangadurai, V.; Weppner, W. Ce0. 8Sm0. 2O1. 9: Characterization of electronic charge carriers and application in limiting current oxygen sensors. Electrochim. Acta 2004, 49, 1577–1585. [Google Scholar] [CrossRef]
- Kalyakin, A.; Demin, A.; Gorbova, E.; Volkov, A.; Tsiakaras, P. Combined amperometric-potentiometric oxygen sensor. Sens. Actuators B Chem. 2020, 313, 127999. [Google Scholar] [CrossRef]
- Iwahara, H.; Uchida, H.; Kondo, J. Galvanic cell-type humidity sensor using high temperature-type proton conductive solid electrolyte. J. Appl. Electrochem. 1983, 13, 365–370. [Google Scholar] [CrossRef]
- Katahira, K.; Matsumoto, H.; Iwahara, H.; Koide, K.; Iwamoto, T. A solid electrolyte steam sensor with an electrochemically supplied hydrogen standard using proton-conducting oxides. Sens. Actuators B Chem. 2000, 67, 189–193. [Google Scholar] [CrossRef]
- Medvedev, D.; Kalyakin, A.; Volkov, A.; Demin, A.; Tsiakaras, P. Electrochemical moisture analysis by combining oxygen- and proton-conducting ceramic electrolytes. Electrochem. Commun. 2017, 76, 55–58. [Google Scholar] [CrossRef]

| T, °C | Equation | Dexp·104m2/s | Dth·104m2/s | ΔD/Dth % | Byproduct |
|---|---|---|---|---|---|
| 400 | (35) | 1.11 | 1.12 | 0.9 | N2 |
| (36) | 0.83 | 26 | N2O | ||
| (37) | 0.67 | 40 | NO | ||
| 375 | (35) | 1.01 | 1.05 | 3.8 | N2 |
| (36) | 0.76 | 28 | N2O | ||
| (37) | 0.60 | 43 | NO |
| Type | Temperature | Target Gas | Electrolyte | Refs |
|---|---|---|---|---|
| amperometric | 450 | H2, CH4, CO | 9YSZ | [50] |
| amperometric | 700 | N2O | YSZ | [58] |
| amperometric | 375–430 | NH3 | 8YSZ | [64] |
| Amperometric/potentiometric | 400–700 | O2 | 9YSZ | [69] |
| Type | Temperature °C | Target Gas | Electrolyte | Refs |
|---|---|---|---|---|
| amperometric | 500–600 | H2 | La0.9Sr0.1YO3-δ | [18] |
| amperometric | 850 | H2 | La0.95Sr0.05YO3-CaZr0.9Sc0.1O3 | [51] |
| amperometric/potentiometric | 450–550 | H2 | BaCe0.9Zr0.1Y0.2O3-δ | [52] |
| amperometric | 650 | H2O | La0.9Sr0.1YO3-δ + YSZ | [72] |
| amperometric | 500–600 | CO2 | La0.9Sr0.1YO3-δ | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorbova, E.; Tzorbatzoglou, F.; Molochas, C.; Chloros, D.; Demin, A.; Tsiakaras, P. Fundamentals and Principles of Solid-State Electrochemical Sensors for High Temperature Gas Detection. Catalysts 2022, 12, 1. https://doi.org/10.3390/catal12010001
Gorbova E, Tzorbatzoglou F, Molochas C, Chloros D, Demin A, Tsiakaras P. Fundamentals and Principles of Solid-State Electrochemical Sensors for High Temperature Gas Detection. Catalysts. 2022; 12(1):1. https://doi.org/10.3390/catal12010001
Chicago/Turabian StyleGorbova, Elena, Fotini Tzorbatzoglou, Costas Molochas, Dimitris Chloros, Anatoly Demin, and Panagiotis Tsiakaras. 2022. "Fundamentals and Principles of Solid-State Electrochemical Sensors for High Temperature Gas Detection" Catalysts 12, no. 1: 1. https://doi.org/10.3390/catal12010001
APA StyleGorbova, E., Tzorbatzoglou, F., Molochas, C., Chloros, D., Demin, A., & Tsiakaras, P. (2022). Fundamentals and Principles of Solid-State Electrochemical Sensors for High Temperature Gas Detection. Catalysts, 12(1), 1. https://doi.org/10.3390/catal12010001