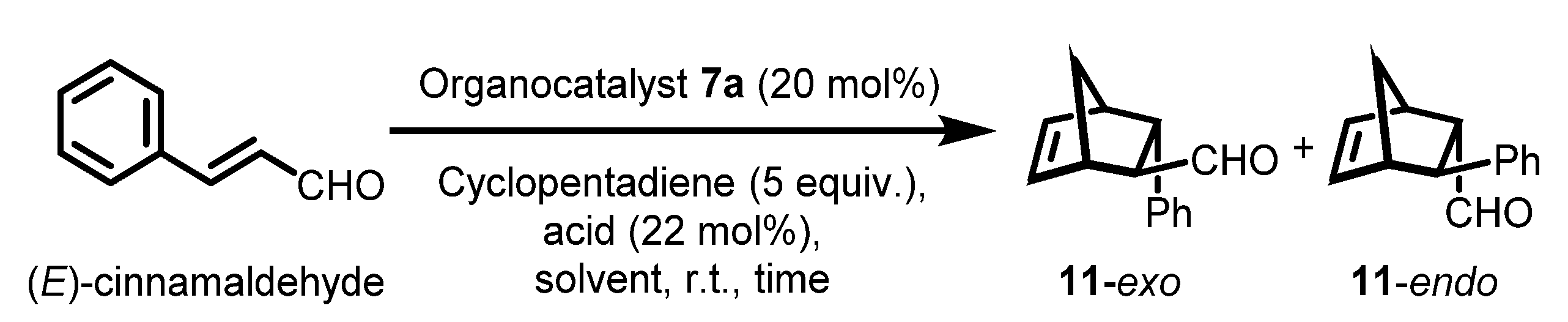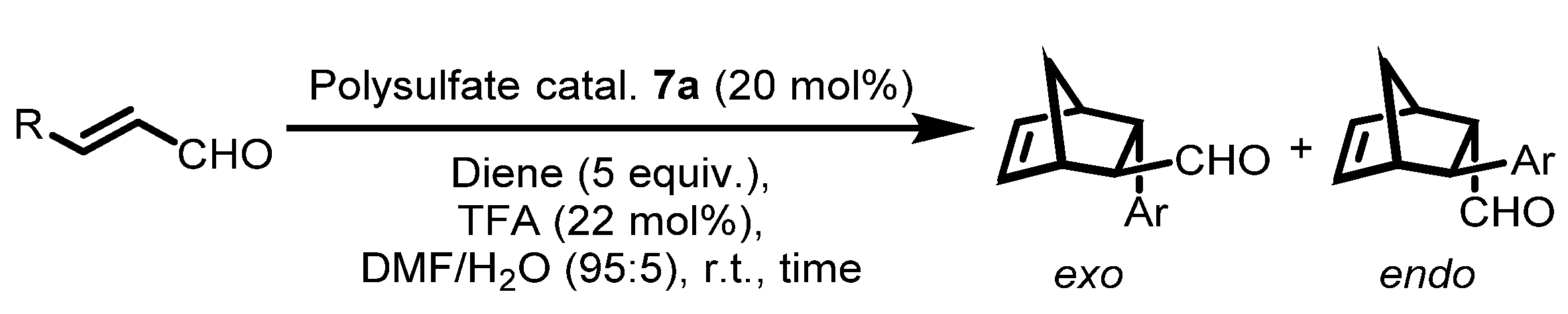Abstract
Novel polymeric MacMillan catalysts were prepared from modified chiral imidazolidin-4-one monomers via sulfur(VI) fluoride exchange chemistry. The resulting polysulfates containing chiral imidazolidin-4-one units could be employed as polymeric organocatalysts for the asymmetric Diels–Alder reaction. With the use of these polysulfate catalysts, sufficient catalytic activity and enantioselectivity were obtained, which were similar to those obtained by monomeric catalysts in a homogeneous catalytic reaction. In addition, the polysulfate catalysts could be recovered and reused five times without a considerable loss of activity and selectivity.
1. Introduction
In the past decades, polymer-bound chiral organocatalysts have attracted attention because of their distinctive benefits, such as catalyst reusability and convenient product purification [1,2,3,4,5,6]. Numerous types of polymer-bound chiral organocatalysts have been designed and synthesized, and they can be classified according to synthetic strategies for the structure of polymer products, that is, main-chain (backbone) and side-chain functionalized polymeric catalysts. Both types of polymeric chiral catalysts have been synthesized and applied to asymmetric catalysis [7].
Among efficient organocatalysts, chiral imidazolidin-4-one (1a), the first-generation MacMillan catalyst, has proven to be a powerful versatile catalyst, operating via the lowest unoccupied molecular orbital (LUMO)-lowering iminium activation [8,9,10,11,12,13,14,15]. Nonetheless, its utilization in asymmetric reactions has some deficiencies such as low turnover frequency and laborious separation. Polymeric immobilization of chiral organocatalysts provides strategic solutions to these drawbacks and is practically applicable for industrial use and flow chemistry [16,17,18,19]. There have been many approaches for immobilizing MacMillan imidazolidinone catalysts onto polymers in order to retain selectivity and maintain reusability [20]. Some examples of these include chiral imidazolidin-4-one linked to JandaJel™ [21], poly (ethylene glycol) [22], polymeric imidazolidin-4-one salt [23,24,25,26,27], methacrylate resin [28], poly (methylhydrosiloxane) [29], ionic liquid support [30,31], fluorous tag [32], grafted polystyrene via a Cu(I)-catalyzed click reaction [33], polyacrylate copolymer [34], organic and inorganic silica networks [35,36], multivalent polyglycerol [37], porous organic polymers [38], sulfated chitin [39], and core-corona polymer microspheres [40]. Despite these efforts, polymer-bound MacMillan catalysts commonly show lower yields and enantioselectivities than their homogeneous counterparts. Moreover, reusability issues are still present pending better solutions. This is why the development of efficient and reusable polymer-bound MacMillan catalysts has been continuously attempted. To overcome the drawbacks associated with heterogeneous catalysis, there has been great efforts to utilize soluble polymer-immobilized catalysts [41,42,43,44,45,46,47].
In 2014, Sharpless et al. developed a unique metal-free click reaction based on sulfur(VI) fluoride exchange (SuFEx) between an aryl fluorosulfate and an aryl silyl ether [48,49,50,51,52]. Through the click reaction catalyzed by an organic superbase, namely fluoride or bifluoride salt, the newly generated SVI–O bonds produce stable linkages between two terminal moieties with comprehensive orthogonality and high efficiency. Like many other click reactions for the production of functional materials, SuFEx click chemistry has been successfully recognized as a reliable method for polymer synthesis [53,54,55,56,57], surface modification [58,59,60,61,62], and post-synthetic modification of macromolecules [63,64,65,66,67]. Linear polysulfate products, prepared by copolymerization in N-methyl-2-pyrrolidone (NMP) and N,N-dimethylformamide (DMF) as optimal solvents, show a high molecular weight and excellent solubility [49,53,54].
Inspired by the synthetic versatility and good solubility of the linear polysulfates, we envisioned that the solubility of polymeric organocatalysts in NMP or DMF can facilitate homogeneous catalysis, and the catalysts can be recovered by heterogeneous recycling, as is the case with soluble polymer catalysts [68,69,70,71,72]. In this work, we applied SuFEx click chemistry to the synthesis of polysulfate-bound MacMillan catalysts and examined both their catalytic activity and selectivity in the asymmetric Diels–Alder (DA) reaction. Furthermore, soluble polymer-bound organocatalysts can be recovered by separation through precipitation. These polymeric organocatalysts were employed effectively in homogeneous reactions and heterogeneous recycling.
2. Results and Discussion
2.1. Design of Monomeric and Polymeric MacMillan Catalysts
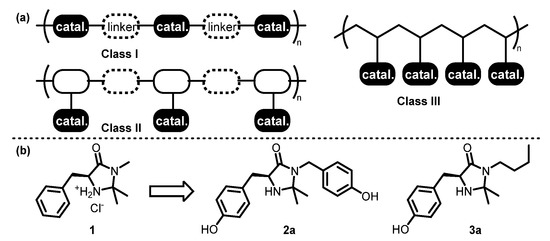
Three different classes of polymeric organocatalysts were designed. Each class is used to provide a specific polymer structure, such as self-supported main-chain polymers (Class I), side-chain functionalized polymers via copolymerization (Class II), and polymers obtained via post-polymerization modification (PPM) (Class III), as shown in Scheme 1. The synthetic design of the phenolic monomers of chiral imidazolidin-4-one is illustrated in Scheme 1b. To synthesize the bisphenol-type and monophenol-type chiral imidazolidin-4-one 2a and 3a, respectively, the phenyl and N-methyl groups of the original MacMillan catalyst 1 were modified to accommodate aryl silyl ether or aryl fluorosulfate, the two clickable functionalities for SuFEx chemistry. All SuFEx copolymerization reactions were catalyzed by tetrabutylammonium bifluoride (TBAHF2), which was found to be superior to 1,8-diazabicyclo[5. 4.0]undec-7-ene (DBU) in terms of catalytic activity and functional group tolerance [53,54]. The bifluoride salt-mediated polycondensation required low catalyst loading (2.0 mol%) and proceeded almost quantitatively with no side reactions on chiral imidazolidin-4-one units.
Scheme 1.
(a) Three classes of polymeric MacMillan catalysts based on SuFEx-click polymerization. (b) Core structure modification.
2.2. Synthesis of Class I Polymeric MacMillan Catalysts
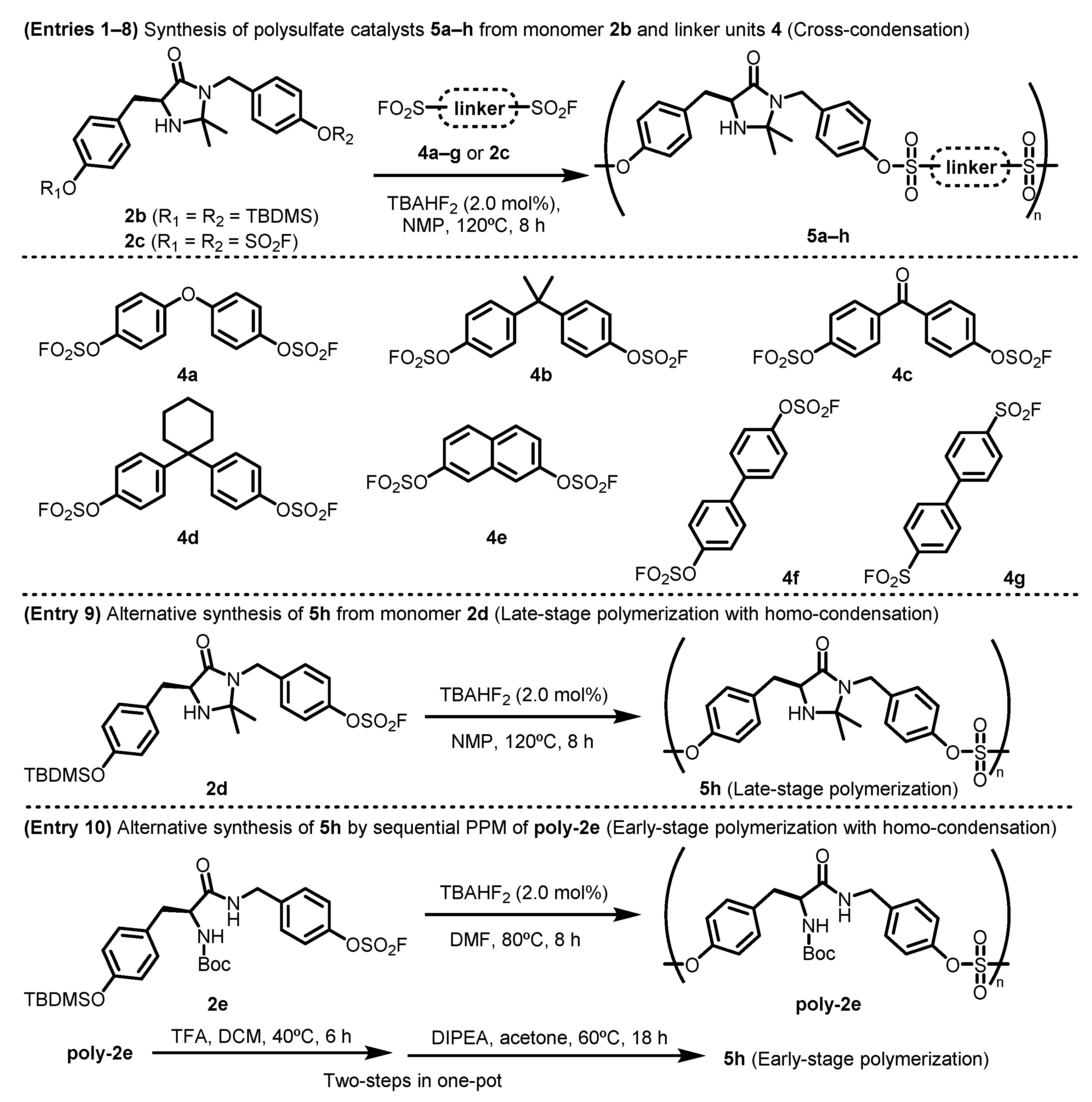
For the construction of Class I polymeric catalysts, a chiral imidazolidin-4-one derivative functionalized with aryl bis (silyl ether) 2b was obtained from commercially available N-(tert-butoxycarbonyl)-l-tyrosine (N-Boc-l-tyrosine). In addition, several types of aryl bis (fluorosulfate) linkers 4a–g were prepared (Table 1). The synthetic details of aryl silyl ether-type (2b) and aryl bis (fluorosulfate)-type monomers (4a–g) are described in the Supplementary Materials. The SuFEx-click polymerization of aryl bis (silyl ether) 2b with aryl bis (fluorosulfate) monomer 4a–g afforded the corresponding chiral imidazolidin-4-one polysulfates 5a–f in good yields, with the MacMillan catalyst monomer embedded as a repeating unit in the backbone (main-chain) of the synthetic polymers. The presence of both the MacMillan catalyst and the linker unit was confirmed by the NMR spectra of the polysulfate products. The molecular weight and polydispersity index (PDI) of the polymer products were measured by gel permeation chromatography (GPC) in tetrahydrofuran (THF) or DMF. The chiral polysulfates 5a–f were synthesized in high yields (91–97%), with moderate number-average molecular weights (Mn over 9 kDa) and good molecular weight distribution (PDI below 2), and exhibited good solubility in NMP and DMF (Table 1, entries 1–6). Only a trace amount of 5g was obtained from bis (sulfonyl fluorides) monomer 4g, presumably due to the formation of methanol-soluble low-molecular-weight products (entry 7).

Table 1.
Synthesis of Class I polymeric MacMillan catalysts a.
To obtain a high-load polymeric organocatalyst 5h, which has no linker units embedded on the polymer backbone, we prepared three additional monomers, namely an aryl bis (fluorosulfate) monomer bearing chiral imidazolidin-4-one 2c, 2d with two functional groups in a single molecule, and its N-Boc-tyrosine precursor 2e. Product 5h was obtained via three distinct methods: cross-condensation and both late-stage and early-stage polymerization (entries 8–10). First, monomer 2b underwent cross-condensation with 2c under the same reaction conditions, giving 5h a lower yield and molecular weight than the polymer products bearing linker units 5a–f. To obtain a higher molecular-weight polymer 5h, we devised two alternative approaches for homo-condensation polymerization. One method involved late-stage polymerization in the final step using MacMillan catalyst monomer 2d, while the other involved early-stage polymerization. N-Boc-tyrosine monomer 2e underwent polymerization first, followed by two-step PPM and Boc removal from the resulting product, and N,N-ketal formation proceeded sequentially. The polymer product 5h obtained from monomer 2d possessing two functionalities by late-stage polymerization was readily soluble in NMP and DMF, reaching a higher molecular weight (Mn: 17.7 kDa) (entry 9) compared to that of 5h prepared by other synthetic approaches. We assumed that the low-molecular-weight products by early-stage polymerization were formed because of the low reaction temperature, which was needed to avoid decomposition of the N-Boc group of monomer 2e (entry 10).
2.3. Synthesis of Class II Polymeric MacMillan Catalysts
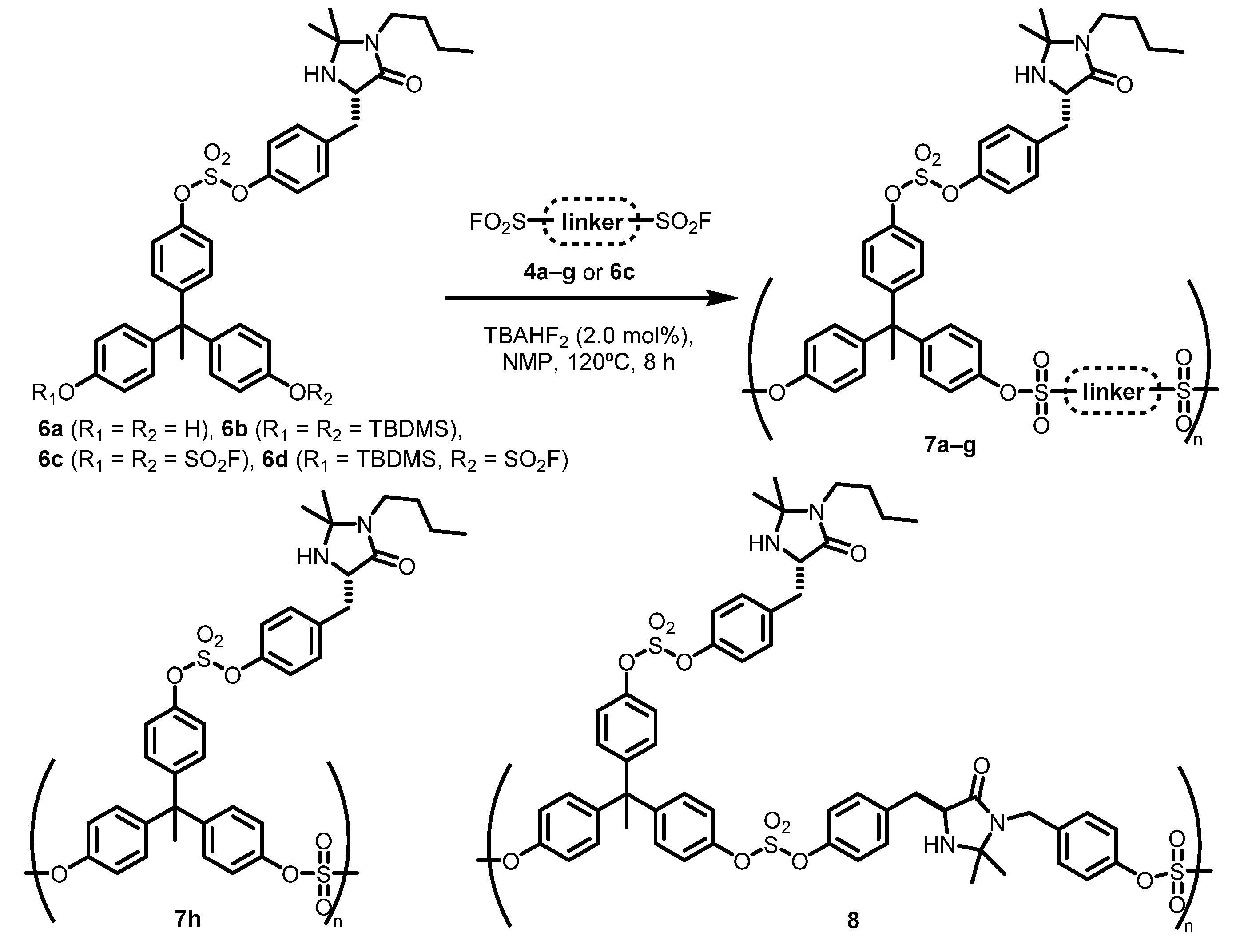
Next, for the construction of side-chain functionalized polymeric organocatalysts, we adopted a drastically different approach. The construction of two new types (Class II and III) of polymers were devised. Of these two types, the former (Class II) is equipped with a bisphenol-type polysulfate backbone produced by copolymerization of organocatalyst-attached monomers, while the latter (Class III) is composed of a polystyrene backbone functionalized by PPM. From commercially available 1,1,1-tris (4-hydroxyphenyl)ethane, we obtained bisphenol-type monomers 6a–d attached to a MacMillan catalyst unit. The synthetic details of aryl bis (silyl ether) 6b, aryl bis (fluorosulfate) 6c, and bifunctional monomer 6d are described in the Supplementary Materials. Under the same copolymerization conditions as used in the construction of the self-supported polymer 5, the SuFEx-click polymerization between aryl bis (silyl ether) monomer 6b and linker unit monomer 4a–g gave bisphenol-type polysulfates 7a–g in high yields (88–98%). The structures of 7a–g were confirmed by NMR spectra of the products and they showed lower molecular weights (Mn over 4.4 kDa) and broader molecular weight distribution than those of polymer 5 (PDI over 1.8), although they exhibited decent solubility in NMP and DMF, as expected (Table 2, entries 1–7).

Table 2.
Synthesis of Class II polymeric MacMillan catalysts a.
To obtain a high-load polymeric organocatalyst 7h with no linker units on the main-chain, we prepared two additional monomers bearing chiral imidazolidin-4-one, aryl bis (fluorosulfate) 6c, and bifunctional monomer 6d. We achieved the synthesis of the anticipated polymer product from the cross-condensation of monomers 6b and 6c with acceptable properties (Table 2, entry 8). However, from homo-condensation polymerization of monomer 6d, no polymer products precipitated with the addition of MeOH, despite the complete disappearance of monomer 6d. This unexpected result was different from that of the self-supported polymer 5h, where the highest Mn was achieved from the reaction of the monomer of two functionalities of 2c (entry 9). Additionally, we synthesized copolymer 8, combining two species, namely self-supported MacMillan catalyst unit 2b and side-chain functionalized bisphenol-type monomer 6c, in an alternating sequence. The cross-condensation of monomers 2b and 6c produced the anticipated copolymer 8, which revealed very poor solubility in common organic solvents including DMF, NMP, chloroform, and THF probably due to strong intramolecular hydrogen-bonding, which inevitably increases the rigidity of the polymer backbone (entry 10) [73].
2.4. Synthesis of Class III Polymeric MacMillan Catalysts
As the SuFEx-click reactions have been efficiently employed in the post-synthetic modification of macromolecules [58,59,60,61,62,63,64,65,66,67], we implemented the PPM method for the functionalization of the phenolic polymers. Due to the high selectivity and conversion of phenolic hydroxy groups to aryl fluorosulfates from the reaction with sulfuryl fluoride gas, we envisioned that commercially available plastic powder, poly(4-hydroxystyrene) 9a, could be utilized for the straightforward SuFEx-mediated construction of organocatalysts. Polymer 9a has been extensively exploited as an organic transistor and photoresist material in the device industry [74,75]. For the construction of Class III polymers based on the PPM technique, we devised two synthetic pathways starting from poly (4-hydroxystyrene): first, the combination of silyl ether-modified polymer 9b with fluorosulfate-derivatized monomer 3c (Table 3, entry 1), and second, the reaction of fluorosulfate-modified polymer 9c with silyl ether-derivatized monomer 3b (entry 2). The synthetic details for the preparation of modified polymers 9b–c and their clickable monomers 3b–c are described in the Supplementary Materials. The SuFEx-click reaction of polymer 9 with its compatible monomer 3 was catalyzed with 5.0 mol% of TBAHF2 in NMP. The resulting polymer 10, verified by NMR, indicated the complete conversion of the clickable fluorosulfate or silyl ether groups in the polymer intermediates 9b–c. Both two-step post-polymerization modification pathways for SuFEx-mediated covalent attachment of the MacMillan catalyst unit 3 proceeded quantitatively to produce polymer 10 with high efficiency. Polymer 10a generated from entry 1 exhibited a higher molecular weight than 10b from entry 2. Polymers 10a and 10b exhibited low solubility in DMF and NMP.

Table 3.
Synthesis of Class III polymeric MacMillan catalysts a.
2.5. Optimization of Reaction Conditions for the Asymmetric Diels–Alder Reaction
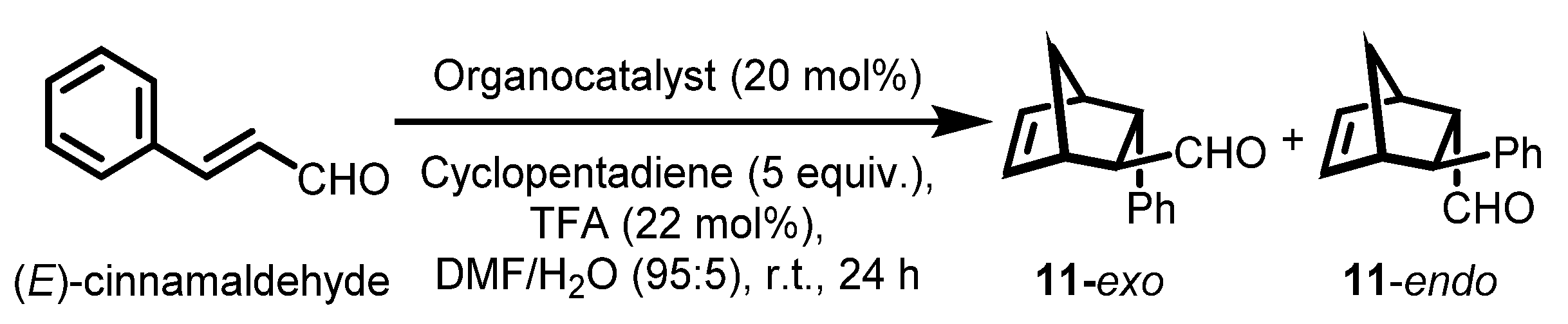
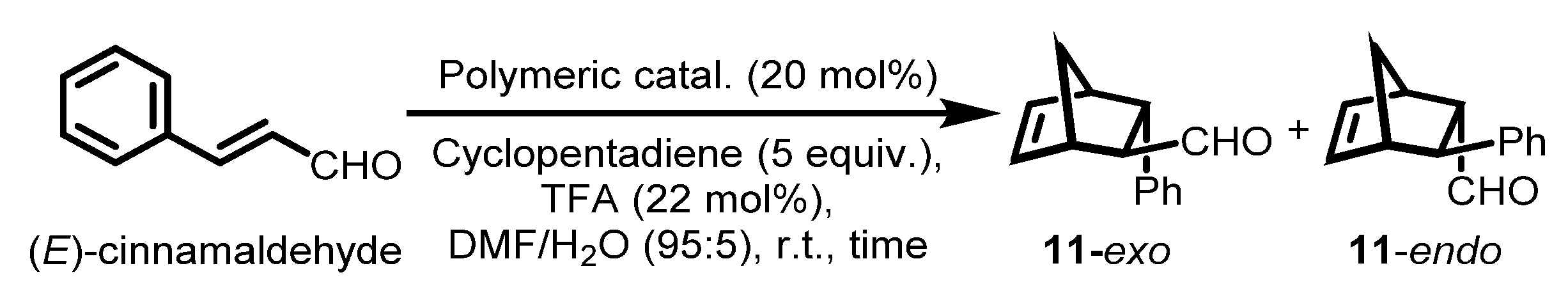
With the various polymeric chiral imidazolidin-4-ones in hand, we evaluated the catalytic activities by means of an asymmetric DA reaction between (E)-cinnamaldehyde and cyclopentadiene. To optimize the reaction conditions, polymeric catalyst 7a was chosen because of its simple structure and scalable preparation, and several common solvents and acid co-catalysts were screened (Table 4). Both monomeric and polymeric MacMillan catalysts showed improved catalytic performance in organic solvent-H2O mixed media [26,76]. First, we examined various solvents such as H2O, MeOH-H2O (95:5), toluene-H2O (95:5), dichloromethane (DCM)-H2O (95:5), acetonitrile (ACN)-H2O (95:5), THF-H2O (95:5), and DMF-H2O (95:5). Except for H2O and MeOH-H2O (95:5 (v/v)), all the reaction conditions we examined could dissolve polysulfate catalyst 7a for homogeneous catalysis. When the catalyst was not completely soluble in the reaction media, no or low yields and moderate enantioselectivities were observed (Table 4, entries 1 and 2, respectively). In contrast to heterogeneous catalysis, homogeneous reactions with solvents such as DCM, ACN, THF, and DMF led to improved yields and good enantioselectivities (92–93% ee) for both endo and exo DA products. There was almost no diastereoselectivity between the endo and exo DA products, as was the case for many other asymmetric DA reactions employing recoverable MacMillan catalysts [7,20]. When 10 mol% of polysulfate catalyst 7a was used instead of 20 mol%, the yield dropped even when the reaction time was prolonged (entry 13). When the reaction was performed at 0 °C, the enantioselectivity increased to 94% ee, while the reaction took a much longer time (48 h) to give an acceptable yield (80%) (entry 14). When various acid co-catalysts such as trifluoroacetic acid (TFA), p-toluenesulfonic acid (p-TsOH), HCl, HClO4, methanesulfonic acid (MsOH), and HBF4 were examined, the yields and both the diastereo and enantioselectivities of the asymmetric reactions remain almost similar (entries 8–12). Thus, we chose DMF:H2O (95:5 (v/v)) with 20 mol% of the polymeric MacMillan catalyst and 22 mol% of the TFA co-catalyst at room temperature as the optimized DA reaction conditions (entry 7).

Table 4.
Optimization of reaction conditions for the asymmetric Diels–Alder reaction a.
2.6. Asymmetric Diels–Alder Reaction with Class I–III Organocatalysts
With the optimized homogeneous reaction conditions, polymeric MacMillan catalysts 5, 7, and 10 were investigated in asymmetric DA reactions. With the first two classes of polymeric MacMillan catalysts 5a–f and 7a–g, interestingly, their catalytic activities and enantioselectivities were comparably satisfactory, despite differences in their backbone structures. DA reactions using 5a–f and 7a–g all proceeded in good yields (86–93%) and enantioselectivities of the desired product 11 (88–93% ee for exo and endo DA products) (Table 5, entries 4–9 and 13–19, respectively). Comparing the yields and enantioselectivities of the DA product 11 with those of monomeric catalysts 2a, 3a, and 6a as references (entries 1–3), we conclude that the catalytic effectiveness of the polysulfate MacMillan catalysts was comparable to that of the monomeric organocatalysts, except for the slightly lower enantioselectivities. However, the enantioselectivities of the DA reactions employing high-load polysulfate organocatalysts with no linker units embedded, that is, 5h and 7h, were lower than those of catalysts with linker units such as 5a–f and 7a–g (84–86% ee for exo and 82–88% ee for endo, entries 10–12 and 20). In the case of the reaction with polymeric catalyst 10 obtained by the SuFEx-mediated PPM method with compact immobilization density on a polyphenolic support, the catalytic activity was lower, giving 64% and 69% yields, and product enantioselectivities dropped to 61% and 66% ee for exo and 67% and 69% ee for endo (entries 21 and 22). This is presumably due to the sterically crowded catalytic sites and the partial insolubility of the catalysts. It was observed that the catalytic performance was not directly correlated with the molecular weight (Mn) but was more strongly affected by the microenvironment of the catalytic site on the polymer. These findings are in accordance with a previous report by Gutmann et al. [36,77].

Table 5.
Asymmetric Diels–Alder reaction with monomeric 2a, 3a, and 6a, and polymeric organocatalysts Class I–III a.
2.7. Substrate Scope of Asymmetric Diels–Alder Reaction with Chiral Polysulfate Organocatalyst 7a
Next, to explore the versatility of the polysulfate MacMillan catalyst, we extended the scope of the asymmetric DA reaction between several α,β-unsaturated aldehyde substrates such as dienophiles, cyclopentadiene, and diphenyisobenzofuran. Under the same reaction conditions employing polymeric MacMillan catalyst 7a, these aldehyde substrates were converted to the respective products in good yields (76–97%) and enantioselectivities (52–95% ee for exo and 82–95% ee for endo) (Table 6). In the case of 1,3-diphenylisobenzofuran as a diene, high exo-selectivity was observed with 85% ee for the exo-product.

Table 6.
Substrate scope of the asymmetric Diels–Alder reaction with chiral polysulfate organocatalyst 7a a.
2.8. Comparisons of Soluble Polymeric MacMillan Catalysts for the Asymmetric Diels–Alder Reaction
We compared the results of soluble polymeric MacMillan catalysts 7a with those of other reported soluble and reusable MacMillan catalysts for asymmetric DA reactions (Table 7). The results indicate that polymeric MacMillan catalysts generally show lower turnover numbers (TON) than that of the monomer catalyst. Notably, the enatio-selectivities observed from the reactions employing catalyst 7a were among the highest of those obtained from the reactions using polymeric organocatalysts (entry 5). In addition, reactions using polymeric catalyst 7a showed acceptable TON when compared with those of other reported polymeric catalysts.

Table 7.
Comparisons of various soluble polymeric MacMillan catalysts for asymmetric Diels–Alder reaction between (E)-cinnamaldehyde and cyclopentadiene.
2.9. Recycling Test of Polysulfate Organocatalysts in Asymmetric Diels–Alder Reaction
Confirming that homogeneous catalytic reactions using polymeric organocatalysts were efficacious, we further studied the recyclability of the catalysts. The copolymer catalysts 5b and 7a were selected for the repetitive asymmetric DA reaction, providing product 11. After each reaction cycle in DMF:H2O, the used catalyst was recovered by simple precipitation with the addition of diethyl ether, followed by decantation of the supernatant containing the aldehyde product, and washing and drying of the precipitated polymer. After drying, the recovered polymeric organocatalyst was used directly in the next catalytic cycle. The visual overview of the recycling procedures is depicted in the Supplementary Materials.
As shown in Table 8, the recovered polymers 5b and 7a could be reused for the iterative reactions. In the case of the reactions with catalyst 5b, the yields and enantioselectivities of the recycle reaction were maintained up to the fourth reuse of the catalyst and then decreased to a noticeable degree at the fifth (Table 8, entries 1–5). In the case of the reactions employing catalyst 7a, the yields and enantioselectivities were maintained until the sixth recycle (entries 6–11). No obvious structural change or loss of the MacMillan catalyst unit in the polymer was detected by Fourier-transform infrared spectroscopy (FT-IR) and 13C NMR after the fourth and fifth cycles. Presumably, the results could be triggered not only by catalyst loss during the recovery process but also by partial and gradual hydrolytic degradation of the N,N-ketal of the imidazolidin-4-one moiety, which may induce a gradual decline in the catalytic activity and enantioselectivity.

Table 8.
Recycle use of polysulfate organocatalysts 5b and 7a in the asymmetric Diels–Alder reaction a.
3. Materials and Methods
3.1. General Remarks
All solvents and reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA), Alfa Aesar (Tewksbury, MA, USA), Tokyo Chemical Industry Co. (Tokyo, Japan), and Samchun Chemicals (Seoul, Korea), and were used without further purification unless otherwise noted. Sulfuryl fluoride gas (purity 99%) was purchased from SynQuest Laboratories (Alachua, FL, USA) and used as received. Tetrabutylammonium bifluoride (TBAHF2) was prepared in a large scale according to the method described in the literature [78]. Reactions were monitored by thin layer chromatography (TLC), which was performed using silica gel 60 F254 coated on an aluminum sheet (E. Merck, Art. 5554). Chromatograms were visualized using an UV lamp and/or stained with 2% ninhydrin/ethanol solution and KMnO4 solution. Column chromatography was performed on silica gel (Merck. 7734 or 9385 Kiesel gel 60) and the eluent is mentioned in each procedure. The 1H, 13C, and 19F NMR spectra were measured with the Agilent 400-MR DD2 Magnetic Resonance System (1H, 400 MHz; 13C, 100 MHz; 19F, 376 MHz) (Santa Clara, CA, USA). Chemical shifts were measured as part per million (δ values) using tetramethylsilane as an internal standard at the probe temperature in CDCl3, DMF-d7, and acetone-d6. Coupling constants are provided in Hz with the following spectral pattern designations: s, singlet; d, doublet; t, triplet; q, quartet; quint, quintet; m, multiplet; and br, broad. Gas chromatography–mass spectrometry (GC–MS) analyses were performed using the Agilent Technologies 7820A series with an HP-5 capillary column and an Agilent 5977E network mass-selective detector (MSD). High-performance liquid chromatography (HPLC) analysis was carried out on a Hewlett-Packard 1100 system, composed of an autosampler, quaternary pump, photodiode array detector (DAD), and Agilent ChemStation software. UV detection was monitored at 254 nm using a Chiralcel OJ-H or Chiralpak AD-H column (25 cm). Liquid chromatography–mass spectrometry (LC–MS) was performed on the Agilent 6100 Series LC/MSD with LC (Agilent 1260 infinity) and Agilent 6120 quadrupole LC/MS (electrospray ionization, ESI). The separation was carried out on a C18 Shiseido Capcell pak column (5 µm, 4.6 mm i.d. × 25 cm) with 0.1% trifluoroacetic acid in water (A) and acetonitrile (B) as the mobile phase at a flow rate of 1 mL/min at ambient temperature. High-resolution mass spectrometry (HRMS) data were obtained from the Organic Chemistry Research Center at Sogang University with a Bruker compact™ spectrometer. Gel permeation chromatography (GPC) for polymer molecular weight analysis was carried out with a Thermo Dionex HPLC, Ultimate 3000 RI System (quaternary pump, refractive index detector, and UV detector) and Shodex GPC KF-806L or KD-806M column eluted with tetrahydrofuran or N,N-dimethylformamide (GPC grade, Honeywell Burdick & Jackson, Charlotte, NC, USA). Samples were diluted in 0.10–0.20 wt% with THF or DMF and filtered with a 0.20 μm polytetrafluoroethylene (PTFE) membrane filter before injection into the GPC. Optical rotation was obtained at 589 nm using a JASCO P-1030 automatic polarimeter. Fourier transform infrared (FT-IR) spectra were recorded on a PerkinElmer Spectrum Two spectrophotometer (Waltham, MA, USA) for solid and liquid samples, at νmax cm−1.
3.2. Synthesis of Class I Polymeric MacMillan Catalysts
Bis (tert-butyldimethylsilyl ether) 2b (0.30 mmol) and bis (fluorosulfate) or bis (fluorosulfonate) 4a–4h or 2c (0.30 mmol) was added to a dried vial with stirring bar. The tube was purged with Ar gas and then charged with anhydrous NMP (0.60 mL) and TBAHF2 (2.0 mol%). The mixture was heated to 120 °C with stirring for 8 h under an inert atmosphere. The solution was added slowly by pipette into a flask containing MeOH (25 mL) with vigorous stirring to precipitate the desired polysulfates 5a–5f and 5h. The precipitate was then isolated by filtration, washed with MeOH (20 mL), and dried under high vacuum at 50 °C to obtain a yellow to yellowish-brown powder.
3.3. Synthesis of Class II Polymeric MacMillan Catalysts
Bis (tert-butyldimethylsilyl ether) 6b or 2b (0.20 mmol) and bis (fluorosulfate) or bis (fluorosulfonate) 4a–4g, 6c (0.20 mmol) was added to a dried vial with stirring bar. The tube was purged with argon gas and then charged with anhydrous NMP (0.40 mL) and TBAHF2 (2.0 mol%). The mixture was heated to 120 °C with stirring for 8 h under an inert atmosphere. The solution was added slowly by pipette into a flask containing methanol (30 mL) with vigorous stirring to precipitate the desired polysulfonate 7a–7h and 8. The precipitate was then isolated by filtration, washed with methanol (20 mL), and dried under high vacuum at 50 °C, to obtain a yellow to yellowish-brown powder.
3.4. Synthesis of Class III Polymeric MacMillan Catalysts
TBAHF2 (5.0 mol%) was added to a stirred solution of modified polymer 9b or 9c (1.5 mmol), aryl fluorosulfate 3c, or aryl silyl ether organocatalyst monomer 3b (3.0 mmol) in NMP (4.0 mL). The reaction mixture was stirred at 120 °C for 12 h. The residue was precipitated in MeOH to give the desired product of 10a or 10b as a yellowish powder.
3.5. General Procedure for the Asymmetric Diels–Alder Reaction and Recycling
Trifluoroacetic acid (8.4 μL, 0.11 mmol, 22 mol%) was added to a stirred solution of the polymer catalyst (0.10 mmol, 20 mol%) in DMF:H2O (95:5 (v/v), 1.0 mL) and the mixture was stirred for 10 min at room temperature. Cinnamaldehydes (0.50 mmol) and cyclopentadiene (0.21 mL, 2.50 mmol) were added sequentially. The mixture was vigorously stirred at room temperature and monitored by TLC. When the reaction was complete, diethyl ether (5.0 mL) was added to the stirred mixture to precipitate the polymer catalyst and the organic solution was separated by decantation. After evaporation of the decanted solution using a vacuum pump, the residue was purified by flash column chromatography (hexane/ethyl acetate as an eluent) to give the aldehyde product. The precipitated polymer was washed with diethyl ether (5.0 mL), dried under high vacuum at 40 °C, and subsequently reused. The ratio of exo and endo isomers was determined by 1H NMR spectroscopy. The products were reduced to the corresponding alcohols with sodium borohydride and enantiomeric excess was determined by HPLC using a Daicel CHIRALCEL® OJ-H, CHIRALPAK® AD-H, CHIRALPAK® IA, or CHIRALCEL® OD-H column.
4. Conclusions
In conclusion, by employing robust and versatile SuFEx-mediated polymerization, we developed three novel types of polymeric MacMillan catalysts with main or side-chains that are functionalized via linear copolymerization or PPM. These polymeric catalysts could be effectively applied to the asymmetric DA reaction under homogeneous reaction conditions. Out of the three class polymeric catalysts, Class I and II exhibited similar catalytic activities and enantioselectivities, while Class II catalysts performed comparatively more satisfactorily for their better precipitation capability, which enabled them to be recycled. As expected, the polymeric organocatalysts could be reused at least five times by heterogeneous separation without losing their structural integrity as a chiral organocatalyst. We point out that the SuFEx-mediated copolymerization strategy can be a powerful bottom-up technique for immobilizing various chiral secondary amine organocatalysts.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/catal11091044/s1, Figure S1: Process diagram for the asymmetric Diels–Alder reaction and solid-liquid biphasic separation, in which an excess of the poor solvent (5.0 mL of diethyl ether) is added to precipitate the soluble polysulfate-bound MacMillan catalyst; Figure S2: (a) FT-IR spectra of polymer catalyst 5b as the fresh catalyst (below) and the recovered polymer catalyst (above) after fifth-time catalytic reactions, and (b) FT-IR spectra of polymer catalyst 7a as the fresh catalyst (below) and the recovered polymer catalyst (above) after sixth-time catalytic reactions; Figure S3: 13C NMR spectra of polymer catalyst 5b as the fresh catalyst (below) and the recovered polymer catalyst (above) after fifth-time catalytic reactions; and Figure S4: 13C NMR spectra of polymer catalyst 7a as the fresh catalyst (below) and the recovered polymer catalyst (above) after sixth-time catalytic reactions.
Author Contributions
Conceptualization, W.-S.L.; methodology, W.-S.L. and L.L.; formal analysis, W.-S.L.; investigation, W.-S.L.; data curation, W.-S.L.; writing—original draft preparation, W.-S.L.; writing—review and editing, B.M.K.; visualization, W.-S.L.; supervision, B.M.K.; project administration, B.M.K.; funding acquisition, B.M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Nano Material Development Program for an NRF grant funded by MEST (NRF-2012M3A7B4049644).
Data Availability Statement
All the data presented in this study are available in the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cozzi, F. Immobilization of Organic Catalysts: When, Why, and How. Adv. Synth. Catal. 2006, 348, 1367–1390. [Google Scholar] [CrossRef]
- Benaglia, M. Recoverable and recyclable chiral organic catalysts. New J. Chem. 2006, 30, 1525–1533. [Google Scholar] [CrossRef]
- Gruttadauria, M.; Giacalone, F.; Noto, R. Supported proline and proline-derivatives as recyclable organocatalysts. Chem. Soc. Rev. 2008, 37, 1666–1688. [Google Scholar] [CrossRef]
- Kristensen, T.E.; Hansen, T. Polymer-Supported Chiral Organocatalysts: Synthetic Strategies for the Road Towards Affordable Polymeric Immobilization. Eur. J. Org. Chem. 2010, 3179–3204. [Google Scholar] [CrossRef]
- Itsuno, S.; Parveza, M.M.; Haraguchi, N. Polymeric chiral organocatalysts. Polym. Chem. 2011, 2, 1942–1949. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, S.; Cheng, J.-P. Non-covalent immobilization of asymmetric organocatalysts. Catal. Sci. Technol. 2011, 1, 507–516. [Google Scholar] [CrossRef]
- Itsuno, S.; Hassan, M.M. Polymer-immobilized chiral catalysts. RSC Adv. 2014, 4, 52023–52043. [Google Scholar] [CrossRef]
- Ahrendt, K.A.; Borths, C.J.; MacMillan, D.W.C. New Strategies for Organic Catalysis: The First Highly Enantioselective Organocatalytic Diels−Alder Reaction. J. Am. Chem. Soc. 2000, 122, 4243–4244. [Google Scholar] [CrossRef]
- Jen, W.S.; Wiener, J.J.M.; MacMillan, D.W.C. New Strategies for Organic Catalysis: The First Enantioselective Organocatalytic 1,3-Dipolar Cycloaddition. J. Am. Chem. Soc. 2000, 122, 9874–9875. [Google Scholar] [CrossRef]
- Pares, N.A.; MacMillan, D.W.C. New Strategies in Organic Catalysis: The First Enantioselective Organocatalytic Friedel−Crafts Alkylation. J. Am. Chem. Soc. 2001, 123, 4370–4371. [Google Scholar] [CrossRef]
- Austin, J.F.; MacMillan, D.W.C. Enantioselective Organocatalytic Indole Alkylations. Design of a New and Highly Effective Chiral Amine for Iminium Catalysis. J. Am. Chem. Soc. 2002, 124, 1172–1173. [Google Scholar] [CrossRef]
- Brochu, M.P.; Brown, S.P.; MacMillan, D.W.C. Direct and Enantioselective Organocatalytic α-Chlorination of Aldehydes. J. Am. Chem. Soc. 2004, 126, 4108–4109. [Google Scholar] [CrossRef]
- Beeson, T.D.; MacMillan, D.W.C. Enantioselective Organocatalytic α-Fluorination of Aldehydes. J. Am. Chem. Soc. 2005, 127, 8826–8828. [Google Scholar] [CrossRef]
- Fonseca, M.T.H.; List, B. Catalytic Asymmetric Intramolecular Michael Reaction of Aldehydes. Angew. Chem. Int. Ed. 2004, 43, 3958–3960. [Google Scholar] [CrossRef]
- Lee, S.; MacMillan, D.W.C. Enantioselective organocatalytic epoxidation using hypervalent iodine reagents. Tetrahedron 2006, 62, 11413–11424. [Google Scholar] [CrossRef]
- Bartók, M. Advances in Immobilized Organocatalysts for the Heterogeneous Asymmetric Direct Aldol Reactions. Catal. Rev. Sci. Eng. 2015, 57, 192–255. [Google Scholar] [CrossRef]
- Sater, M.A.E.; Jaber, N.; Schulz, E. Chiral Salen Complexes for Asymmetric Heterogeneous Catalysis: Recent Examples for Recycling and Cooperativity. ChemCatChem 2019, 11, 3662–3687. [Google Scholar] [CrossRef]
- Franconetti, A.; De Gonzalo, G. Recent Developments on Supported Hydrogen-bond Organocatalysts. ChemCatChem 2018, 10, 5554–5572. [Google Scholar] [CrossRef]
- De Oliveira, P.H.R.; Santos, B.M.D.S.; Leão, R.A.C.; Miranda, L.S.M.; San Gil, R.A.S.; De Souza, R.O.M.A.; Finelli, F.G. From Immobilization to Catalyst Use: A Complete Continuous-Flow Approach Towards the Use of Immobilized Organocatalysts. ChemCatChem 2019, 11, 5553–5561. [Google Scholar] [CrossRef]
- Deepa; Singh, S. Recent Development of Recoverable MacMillan Catalyst in Asymmetric Organic Transformations. Adv. Synth. Catal. 2021, 363, 629–656. [Google Scholar] [CrossRef]
- Selkälä, S.A.; Tois, J.; Pihko, P.M.; Koskinen, A.M.P. Asymmetric Organocatalytic Diels–Alder Reactions on Solid Support. Adv. Synth. Catal. 2002, 344, 941–945. [Google Scholar] [CrossRef]
- Benaglia, M.; Celentano, G.; Cinquini, M.; Puglisi, A.; Cozzi, F. Poly(ethylene glycol)-Supported Chiral Imidazolidin-4-one: An Efficient Organic Catalyst for the Enantioselective Diels–Alder Cycloaddition. Adv. Synth. Catal. 2002, 344, 149–152. [Google Scholar] [CrossRef]
- Haraguchi, N.; Takemura, Y.; Itsuno, S. Novel polymer-supported organocatalyst via ion exchange reaction: Facile immobilization of chiral imidazolidin-4-one and its application to Diels–Alder reaction. Tetrahedron Lett. 2010, 51, 1205–1208. [Google Scholar] [CrossRef]
- Haraguchi, N.; Kiyono, H.; Takemura, Y.; Itsuno, S. Design of main-chain polymers of chiral imidazolidinone for asymmetric organocatalysis application. Chem. Commun. 2012, 48, 4011–4013. [Google Scholar] [CrossRef]
- Itsuno, S.; Oonami, T.; Takenaka, N.; Haraguchi, N. Synthesis of Chiral Polyethers Containing Imidazolidinone Repeating Units and Application as Catalyst in Asymmetric Diels–Alder Reaction. Adv. Synth. Catal. 2015, 357, 3995–4002. [Google Scholar] [CrossRef]
- Haraguchi, N.; Nguyen, T.L.; Itsuno, S. Polyesters Containing Chiral Imidazolidinone Salts in Polymer Main Chain: Heterogeneous Organocatalysts for the Asymmetric Diels–Alder Reaction. ChemCatChem 2017, 9, 3786–3794. [Google Scholar] [CrossRef]
- Haraguchi, N.; Takenaka, N.; Najwa, A.; Takahara, Y.; Mun, M.K.; Itsuno, S. Synthesis of Main-Chain Ionic Polymers of Chiral Imidazolidinone Organocatalysts and Their Application to Asymmetric Diels–Alder Reactions. Adv. Synth. Catal. 2018, 360, 112–123. [Google Scholar] [CrossRef]
- Kristensen, T.E.; Vestli, K.; Jakobsen, M.G.; Hansen, F.K.; Hansen, T. A General Approach for Preparation of Polymer-Supported Chiral Organocatalysts via Acrylic Copolymerization. J. Org. Chem. 2010, 75, 1620–1629. [Google Scholar] [CrossRef]
- Guizzetti, S.; Benaglia, M.; Siegel, J.S. Poly(methylhydrosiloxane)-supported chiral imidazolinones: New versatile, highly efficient and recyclable organocatalysts for stereoselective Diels–Alder cycloaddition reactions. Chem. Commun. 2012, 48, 3188–3190. [Google Scholar] [CrossRef]
- Shen, Z.-L.; Cheong, H.-L.; Lai, Y.-C.; Loo, W.-Y.; Loh, T.-P. Application of recyclable ionic liquid-supported imidazolidinonecatalyst in enantioselective Diels–Alder reactions. Green Chem. 2012, 14, 2626–2627. [Google Scholar] [CrossRef]
- Chauhan, M.S.; Kumar, P.; Singh, S. Synthesis of MacMillan catalyst modified with ionic liquid as a recoverable catalyst for asymmetric Diels–Alder reaction. RSC Adv. 2015, 5, 52636–52641. [Google Scholar] [CrossRef]
- Chu, Q.; Zhang, W.; Curran, D.P. A recyclable fluorous organocatalyst for Diels–Alder reactions. Tetrahedron Lett. 2006, 47, 9287–9290. [Google Scholar] [CrossRef][Green Version]
- Riente, P.; Yadav, J.; Pericàs, M.A. A Click Strategy for the Immobilization of MacMillan Organocatalysts onto Polymers and Magnetic Nanoparticles. Org. Lett. 2012, 14, 3668–3671. [Google Scholar] [CrossRef]
- Moore, B.L.; Lu, A.; Longbottom, D.A.; O’Reilly, R.K. Immobilization of MacMillan catalyst via controlled radical polymerization: Catalytic activity and reuse. Polym. Chem. 2013, 4, 2304–2312. [Google Scholar] [CrossRef]
- Wang, C.A.; Zhang, Y.; Shi, J.Y.; Wang, W. A Self-Supported Polymeric MacMillan Catalyst for Homogeneous Organocatalysis and Heterogeneous Recycling. Chem. Asian J. 2013, 8, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, A.; Benaglia, M.; Annunziata, R.; Chiroli, V.; Porta, R.; Gervasini, A. Chiral Hybrid Inorganic–Organic Materials: Synthesis, Characterization, and Application in Stereoselective Organocatalytic Cycloadditions. J. Org. Chem. 2013, 78, 11326–11334. [Google Scholar] [CrossRef]
- Pecchioli, T.; Muthyala, M.K.; Haag, R.; Christmann, M. Multivalent polyglycerol supported imidazolidin-4-one organocatalysts for enantioselective Friedel–Crafts alkylations. Beilstein J. Org. Chem. 2015, 11, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-A.; Li, Y.-W.; Han, Y.-F.; Zhang, J.-P.; Wu, R.-T.; He, G.-F. The “bottom-up” construction of chiral porous organic polymers for heterogeneous asymmetric organocatalysis: MacMillan catalyst built-in nanoporous organic frameworks. Polym. Chem. 2017, 8, 5561–5569. [Google Scholar] [CrossRef]
- Watanabe, M.; Sakai, T.; Oka, M.; Makinose, Y.; Miyazaki, H.; Iida, H. Non-Covalently Immobilized Chiral Imidazolidinone on Sulfated-Chitin: Reusable Heterogeneous Organocatalysts for Asymmetric Diels–Alder Reaction. Adv. Synth. Catal. 2020, 362, 255–260. [Google Scholar] [CrossRef]
- Ullah, M.W.; Haraguchi, N. Ionic, Core-Corona Polymer Microsphere-Immobilized MacMillan Catalyst for Asymmetric Diels–Alder Reaction. Catalysts 2019, 9, 960. [Google Scholar] [CrossRef]
- Gravert, D.J.; Janda, K.D. Organic Synthesis on Soluble Polymer Supports: Liquid-Phase Methodologies. Chem. Rev. 1997, 97, 489–510. [Google Scholar] [CrossRef]
- Toy, P.H.; Janda, K.D. Soluble Polymer-Supported Organic Synthesis. Acc. Chem. Res. 2000, 33, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, T.J.; Reed, N.N.; Janda, K.D. Soluble Polymers as Scaffolds for Recoverable Catalysts and Reagents. Chem. Rev. 2002, 102, 3325–3344. [Google Scholar] [CrossRef] [PubMed]
- Bergbreiter, D.E. Using Soluble Polymers To Recover Catalysts and Ligands. Chem. Rev. 2002, 102, 3345–3384. [Google Scholar] [CrossRef] [PubMed]
- Bergbreiter, D.E.; Tian, J.; Hongfa, C. Using Soluble Polymer Supports To Facilitate Homogeneous Catalysis. Chem. Rev. 2009, 109, 530–582. [Google Scholar] [CrossRef]
- Bergbreiter, D.E. Soluble Polymers as Tools in Catalysis. ACS Macro Lett. 2014, 3, 260–265. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Bergbreiter, D.E. Soluble polymer-supported organocatalysts. Pure Appl. Chem. 2013, 85, 493–509. [Google Scholar] [CrossRef]
- Dong, J.; Krasnova, L.; Finn, M.G.; Sharpless, K.B. Sulfur(VI) Fluoride Exchange (SuFEx): Another Good Reaction for Click Chemistry. Angew. Chem. Int. Ed. 2014, 53, 9430–9448. [Google Scholar] [CrossRef]
- Dong, J.; Sharpless, K.B.; Kwisnek, L.; Oakdale, J.S.; Fokin, V.V. SuFEx-Based Synthesis of Polysulfates. Angew. Chem. Int. Ed. 2014, 53, 9466–9470. [Google Scholar] [CrossRef]
- Li, S.; Wu, P.; Moses, J.E.; Sharpless, K.B. Multidimensional SuFEx Click Chemistry: Sequential Sulfur(VI) Fluoride Exchange Connections of Diverse Modules Launched From An SOF4 Hub. Angew. Chem. Int. Ed. 2017, 56, 2903–2908. [Google Scholar] [CrossRef]
- Guo, T.; Meng, G.; Zhan, X.; Yang, Q.; Ma, T.; Xu, L.; Sharpless, K.B.; Dong, J. A New Portal to SuFEx Click Chemistry: A Stable Fluorosulfuryl Imidazolium Salt Emerging as an “F−SO2+” Donor of Unprecedented Reactivity, Selectivity, and Scope. Angew. Chem. Int. Ed. 2018, 57, 2605–2610. [Google Scholar] [CrossRef]
- Gao, B.; Li, S.; Wu, P.; Moses, J.E.; Sharpless, K.B. SuFEx Chemistry of Thionyl Tetrafluoride (SOF4) with Organolithium Nucleophiles: Synthesis of Sulfonimidoyl Fluorides, Sulfoximines, Sulfonimidamides, and Sulfonimidates. Angew. Chem. Int. Ed. 2018, 57, 1939–1943. [Google Scholar] [CrossRef]
- Gao, B.; Zhang, L.; Zheng, Q.; Zhou, F.; Klivansky, L.M.; Lu, J.; Liu, Y.; Dong, J.; Wu, P.; Sharpless, K.B. Bifluoride-catalysed sulfur(VI) fluoride exchange reaction for the synthesis of polysulfates and polysulfonates. Nat. Chem. 2017, 9, 1083–1088. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, F.; Ren, G.; Zheng, Q.; Chen, H.; Gao, B.; Klivansky, L.; Liu, Y.; Wu, B.; Xu, Q.; et al. SuFEx-Based Polysulfonate Formation from Ethenesulfonyl Fluoride–Amine Adducts. Angew. Chem. Int. Ed. 2017, 56, 11203–11208. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jin, H.-S.; Chen, X.-M.; Lin, B.-P.; Yang, H. A sulfur(vi) fluoride exchange click chemistry approach towards main chain liquid crystal polymers bearing sulfate ester groups. Polym. Chem. 2019, 10, 3657–3664. [Google Scholar] [CrossRef]
- Cao, Z.; Zhou, F.; Gu, P.-Y.; Chen, D.; He, J.; Cappiello, J.R.; Wu, P.; Xu, Q.; Lu, J. Preparation of aryl polysulfonates via a highly efficient SuFEx click reaction, their controllable degradation and functionalized behavior. Polym. Chem. 2020, 11, 3120–3124. [Google Scholar] [CrossRef]
- Kulow, R.W.; Wu, J.W.; Kim, C.; Michaudel, Q. Synthesis of unsymmetrical sulfamides and polysulfamides via SuFEx click chemistry. Chem. Sci. 2020, 11, 7807–7812. [Google Scholar] [CrossRef] [PubMed]
- Yatvin, J.; Brooks, K.; Locklin, J. SuFEx on the Surface: A Flexible Platform for Postpolymerization Modification of Polymer Brushes. Angew. Chem. Int. Ed. 2015, 54, 13370–13373. [Google Scholar] [CrossRef] [PubMed]
- Durie, K.; Yatvin, J.; McNitt, C.D.; Reese, R.A.; Jung, C.; Popik, V.V.; Locklin, J. Multifunctional Surface Manipulation Using Orthogonal Click Chemistry. Langmuir 2016, 32, 6600–6605. [Google Scholar] [CrossRef] [PubMed]
- Gahtory, D.; Sen, R.; Pujari, S.; Li, S.; Zheng, Q.; Moses, J.E.; Sharpless, K.B.; Zuilhof, H. Quantitative and Orthogonal Formation and Reactivity of SuFEx Platforms. Chem. Eur. J. 2018, 24, 10550–10556. [Google Scholar] [CrossRef]
- Randall, J.D.; Eyckens, D.J.; Stojcevski, F.; Francis, P.S.; Doeven, E.H.; Barlow, A.J.; Barrow, A.S.; Arnold, C.L.; Moses, J.E.; Henderson, L.C. Modification of Carbon Fibre Surfaces by Sulfur-Fluoride Exchange Click Chemistry. ChemPhysChem 2018, 19, 3176–3181. [Google Scholar] [CrossRef]
- Liu, W.; Dong, Y.; Zhang, S.; Wu, Z.; Chen, H. A rapid one-step surface functionalization of polyvinyl chloride by combining click sulfur(vi)-fluoride exchange with benzophenone photochemistry. Chem. Commun. 2019, 55, 858–861. [Google Scholar] [CrossRef] [PubMed]
- Durie, K.; Razavi, M.J.; Wang, X.; Locklin, J. Nanoscale Surface Creasing Induced by Post-polymerization Modification. ACS Nano 2015, 9, 10961–10969. [Google Scholar] [CrossRef]
- Li, S.; Beringer, L.T.; Chen, S.; Averick, S. Combination of AGET ATRP and SuFEx for post-polymerization chain-end modifications. Polymer 2015, 78, 37–41. [Google Scholar] [CrossRef]
- Oakdale, J.S.; Kwisnek, L.; Fokin, V.V. Selective and Orthogonal Post-Polymerization Modification using Sulfur(VI) Fluoride Exchange (SuFEx) and Copper-Catalyzed Azide–Alkyne Cycloaddition (CuAAC) Reactions. Macromolecules 2016, 49, 4473–4479. [Google Scholar] [CrossRef]
- Wang, P.; Dong, Y.; Lu, X.; Wu, Z.; Chen, H. Combining Click Sulfur(VI)-Fluoride Exchange with Photoiniferters: A Facile, Fast, and Efficient Strategy for Postpolymerization Modification. Macromol. Rapid Commun. 2018, 39, 1700523. [Google Scholar] [CrossRef]
- Park, S.; Song, H.; Ko, N.; Kim, C.; Kim, K.; Lee, E. SuFEx in Metal–Organic Frameworks: Versatile Postsynthetic Modification Tool. ACS Appl. Mater. Interfaces 2018, 10, 33785–33789. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-y.; Liu, X.-y.; Zhao, G. Synthesis of Dendrimer-Supported Prolinols and Their Application in Enantioselective Reduction of Ketones. Synlett 2006, 1150–1154. [Google Scholar] [CrossRef]
- Zeitler, K.; Mager, I. An Efficient and Versatile Approach for the Immobilization of Carbene Precursors via Copper-Catalyzed [3+2]-Cycloaddition and their Catalytic Application. Adv. Synth. Catal. 2007, 349, 1851–1857. [Google Scholar] [CrossRef]
- Chung, C.W.Y.; Toy, P.H. Multipolymer Reaction System for Selective Aerobic Alcohol Oxidation: Simultaneous Use of Multiple Different Polymer-Supported Ligands. J. Comb. Chem. 2007, 9, 115–120. [Google Scholar] [CrossRef]
- Zhao, Y.-H.; Zheng, C.-W.; Zhao, G.; Cao, W.-G. Highly enantioselective tandem cyclopropanation/Wittig reaction of α,β-unsaturated aldehydes with arsonium ylides catalyzed by recyclable dendritic catalyst. Tetrahedron Asymmetry 2008, 19, 701–708. [Google Scholar] [CrossRef]
- Zhao, W.-X.; Liu, N.; Li, G.-W.; Chen, D.-L.; Zhang, A.-A.; Wang, M.-C.; Liu, L. Synthesis of dendrimer-supported ferrocenylmethyl aziridino alcohol ligands and their application in asymmetric catalysis. Green Chem. 2015, 17, 2924–2930. [Google Scholar] [CrossRef]
- Zhu, C.; Mu, A.U.; Wang, C.; Ji, X.; Fang, L. Synthesis and Solution Processing of a Rigid Polymer Enabled by Active Manipulation of Intramolecular Hydrogen Bonds. ACS Macro Lett. 2018, 7, 801–806. [Google Scholar] [CrossRef]
- Barclay, G.G.; Hawker, C.J.; Ito, H.; Orellana, A.; Malenfant, P.R.L.; Sinta, R.F. The “Living” Free Radical Synthesis of Poly(4-hydroxystyrene): Physical Properties and Dissolution Behavior. Macromolecules 1998, 31, 1024–1031. [Google Scholar] [CrossRef]
- Cho, H.; Kim, J.; Patil, P.; Kim, J.Y.; Kim, T.-H. Synthesis of succinylated poly(4-hydroxystyrene) and its application for negative-tone photoresist. J. Appl. Polym. Sci. 2007, 103, 3560–3566. [Google Scholar] [CrossRef]
- Brazier, J.B.; Jones, K.M.; Platts, J.A.; Tomkinson, N.C.O. On the Roles of Protic Solvents in Imidazolidinone-Catalyzed Transformations. Angew. Chem. Int. Ed. 2011, 50, 1613–1616. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Gall, T.; Lee, J.-W.; Opwis, K.; List, B.; Gutmann, J.S. Textile Catalysts—An unconventional approach towards heterogeneous catalysis. ChemCatChem 2016, 8, 1428–1436. [Google Scholar] [CrossRef]
- Landini, D.; Molinari, H.; Pensa, M.; Rampoldi, A. Convenient Procedures for the Preparation of Lipophilic Quaternary Onium Fluorides, Hydrogendifluorides and Dihydrogentrifluorides via Ion Exchange in Two-Phase Systems. Synthesis 1988, 953–955. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).