Dynamic Pyrolysis Characteristics, Kinetics and Products Analysis of Waste Tire Catalytic Pyrolysis with Ni/Fe-ZSM-5 Catalysts Using TG-IR-GC/MS
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Catalyst
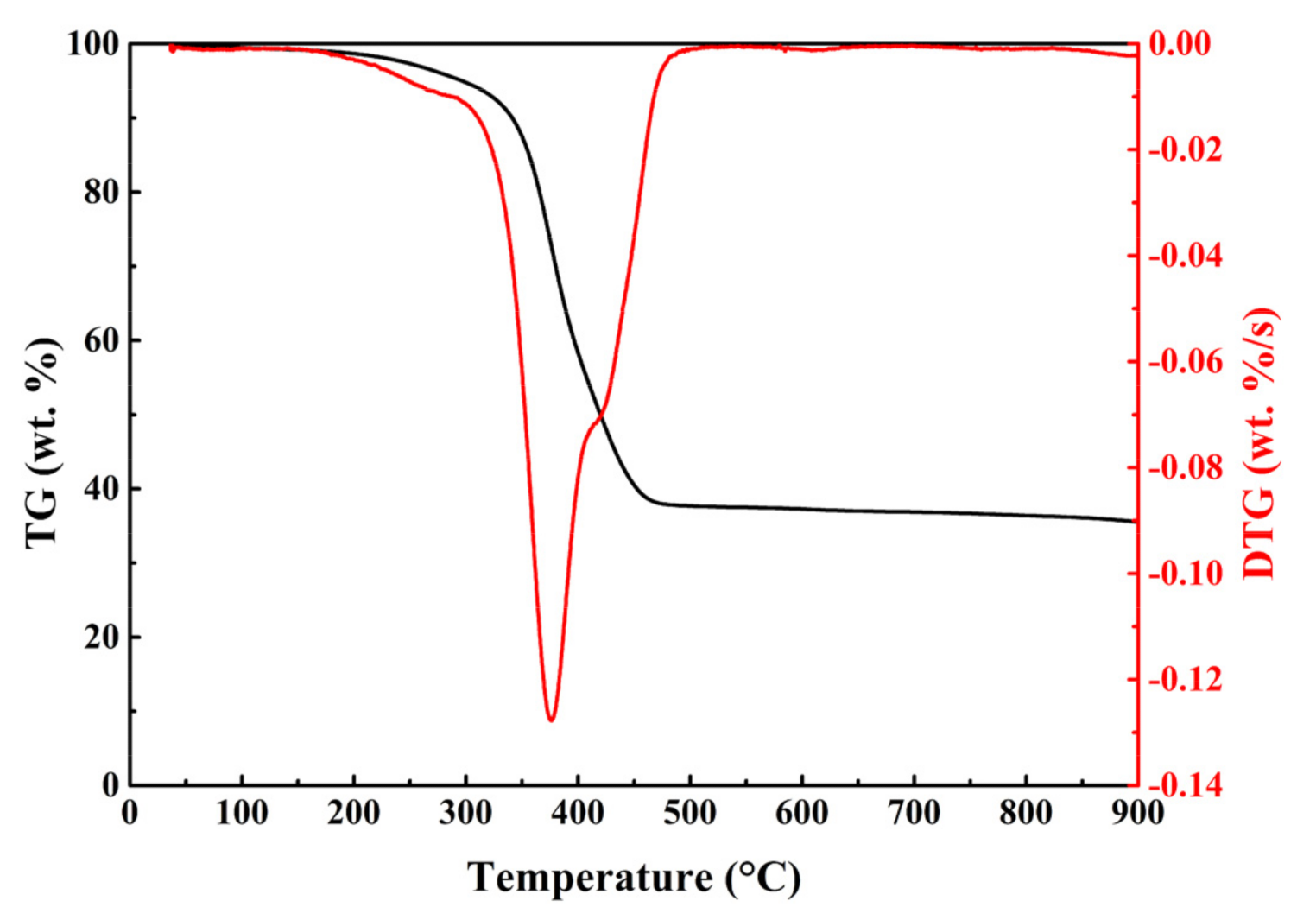
2.2. Thermal Characteristic Analysis
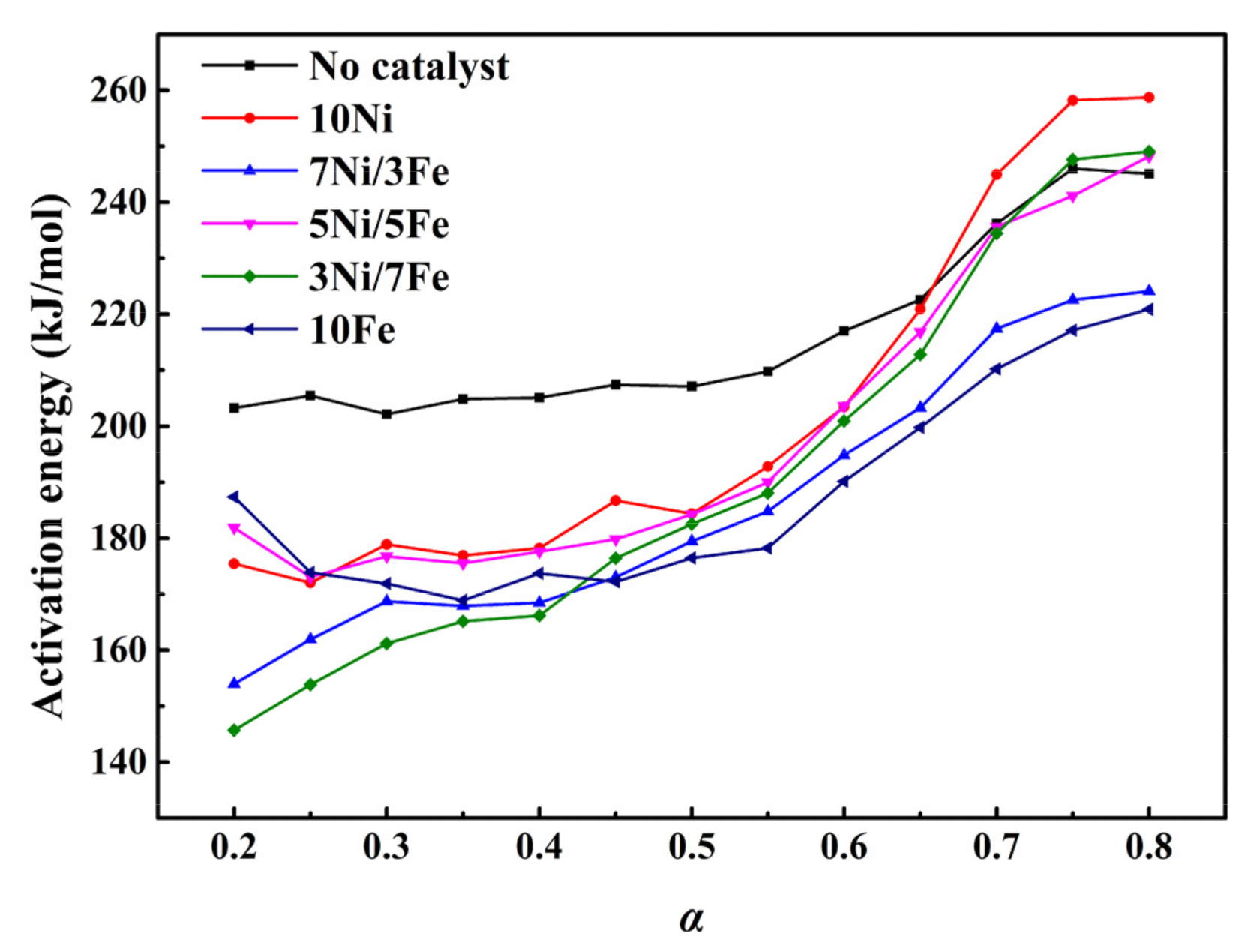
2.3. Estimation of Activation Energy
2.4. Determination of Reaction Model
2.5. Product Analysis
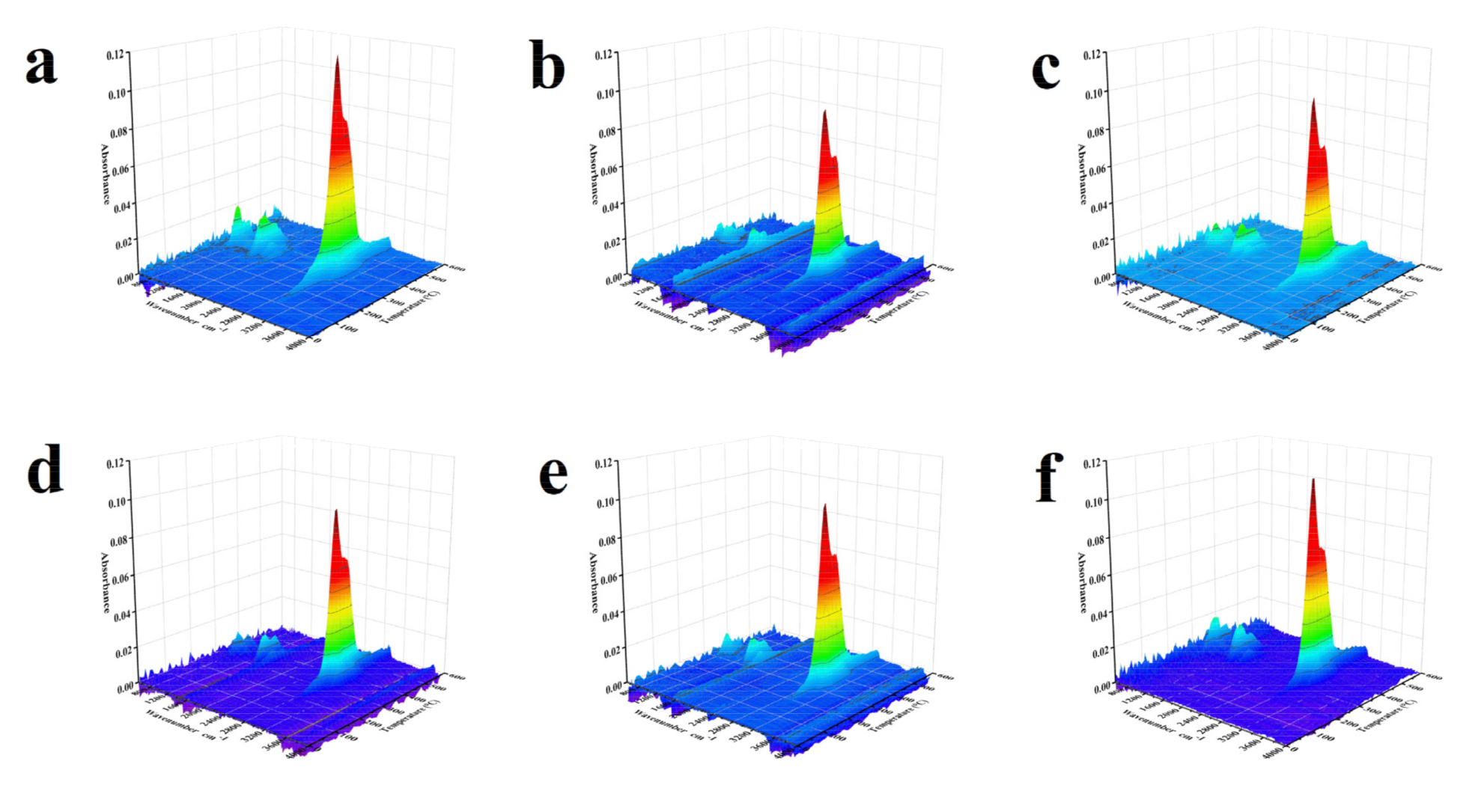
2.5.1. TG-FTIR
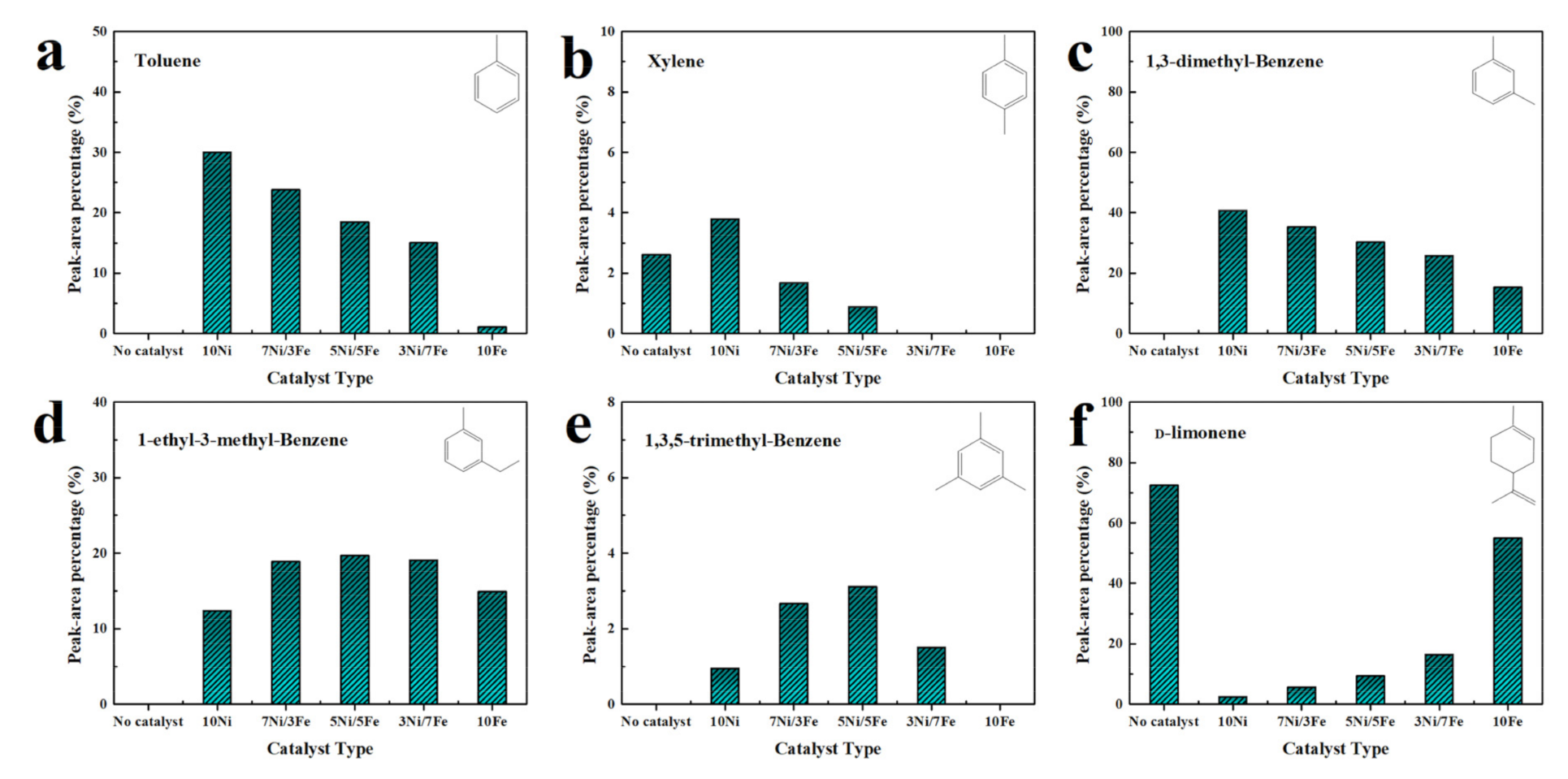
2.5.2. TG-GCMS
3. Materials and Methods
3.1. Materials and Catalysts
3.2. TG-IR-GC/MS Experiments
3.3. Kinetic Analysis Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pakdel, H.; Roy, C.; Aubin, H.; Jean, G.; Coulombe, S. Formation of dl-limonene in used tire vacuum pyrolysis oils. Environ. Sci. Technol. 1991, 25, 1646–1649. [Google Scholar] [CrossRef]
- Williams, P.T. Pyrolysis of waste tyres: A review. Waste Manag. 2013, 33, 1714–1728. [Google Scholar] [CrossRef] [Green Version]
- Williams, P.T.; Brindle, A.J. Aromatic chemicals from the catalytic pyrolysis of scrap tyres. J. Anal. Appl. Pyrolysis 2003, 67, 143–164. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, J.; Wang, X.; Liu, P.; Li, J.; Liu, G.; Wang, K.; Li, M.; Zhong, Z.; Xu, J.; et al. Catalytic conversion of rubber wastes to produce aromatic hydrocarbons over USY zeolites: Effect of SiO2/Al2O3 mole ratio. Energy Convers. Manag. 2019, 197, 111857. [Google Scholar] [CrossRef]
- Zhao, M.; Church, T.L.; Harris, A.T. SBA-15 supported Ni-Co bimetallic catalysts for enhanced hydrogen production during cellulose decomposition. Appl. Catal. B Environ. 2011, 101, 522–530. [Google Scholar] [CrossRef]
- Yu, L.; Farinmade, A.; Ajumobi, O.; Su, Y.; John, V.T.; Valla, J.A. MCM-41/ZSM-5 composite particles for the catalytic fast pyrolysis of biomass. Appl. Catal. A Gen. 2020, 602, 117727. [Google Scholar] [CrossRef]
- Ding, K.; Zhong, Z.; Zhang, B.; Wang, J.; Min, A.; Ruan, R. Catalytic pyrolysis of waste tire to produce valuable aromatichydrocarbons: An analytical Py-GC/MS study. J. Anal. Appl. Pyrolysis 2016, 122, 55–63. [Google Scholar] [CrossRef]
- Ji, G.; Xu, X.; Yang, H.; Zhao, X.; He, X.; Zhao, M. Enhanced Hydrogen Production from Sawdust Decomposition Using Hybrid-Functional Ni-CaO-Ca2SiO4 Materials. Env. Sci. Technol. 2017, 51, 11484–11492. [Google Scholar] [CrossRef] [PubMed]
- Kantarelis, E.; Yang, W.; Blasiak, W. Effect of zeolite to binder ratio on product yields and composition during catalytic steam pyrolysis of biomass over transition metal modified HZSM5. Fuel 2014, 122, 119–125. [Google Scholar] [CrossRef]
- Hong, Y.; Hensley, A.; McEwen, J.-S.; Wang, Y. Perspective on Catalytic Hydrodeoxygenation of Biomass Pyrolysis Oils: Essential Roles of Fe-Based Catalysts. Catal. Lett. 2016, 146, 1621–1633. [Google Scholar] [CrossRef]
- Widayatno, W.B.; Guan, G.; Rizkiana, J.; Yang, J.; Hao, X.; Tsutsumi, A.; Abudula, A. Upgrading of bio-oil from biomass pyrolysis over Cu-modified β-zeolite catalyst with high selectivity and stability. Appl. Catal. B Environ. 2016, 186, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Yu, J.; Chen, T.; Liu, X.; Sun, L. Study on catalytic pyrolysis mechanism of natural rubber (NR) over Zn-modified ZSM5 catalysts. J. Energy Inst. 2021, 94, 210–221. [Google Scholar] [CrossRef]
- Botas, J.A.; Serrano, D.P.; García, A.; de Vicente, J.; Ramos, R. Catalytic conversion of rapeseed oil into raw chemicals and fuels over Ni- and Mo-modified nanocrystalline ZSM-5 zeolite. Catal. Today 2012, 195, 59–70. [Google Scholar] [CrossRef]
- Kantarelis, E.; Yang, W.; Blasiak, W. Effects of Silica-Supported Nickel and Vanadium on Liquid Products of Catalytic Steam Pyrolysis of Biomass. Energy Fuels 2014, 28, 591–599. [Google Scholar] [CrossRef]
- Namchot, W.; Jitkarnka, S. Upgrading of waste tyre-derived oil from waste tyre pyrolysis over Ni catalyst supported on HZSM-5 zeolite. Chem. Eng. Trans. 2015, 45, 775–780. [Google Scholar] [CrossRef]
- Muenpol, S.; Jitkarnka, S. Effects of Fe supported on zeolites on structures of hydrocarbon compounds and petrochemicals in waste tire-derived pyrolysis oils. J. Anal. Appl. Pyrolysis 2016, 117, 147–156. [Google Scholar] [CrossRef]
- Wu, C.; Nahil, M.A.; Miskolczi, N.; Huang, J.; Williams, P.T. Production and application of carbon nanotubes, as a co-product of hydrogen from the pyrolysis-catalytic reforming of waste plastic. Process. Saf. Environ. Prot. 2016, 103, 107–114. [Google Scholar] [CrossRef]
- Popovska, N.; Danova, K.; Jipa, I.; Zenneck, U. Catalytic growth of carbon nanotubes on zeolite supported iron, ruthenium and iron/ruthenium nanoparticles by chemical vapor deposition in a fluidized bed reactor. Powder Technol. 2011, 207, 17–25. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, P.; Shao, Q.; Takahashi, F.; Yoshikawa, K. In situ catalytic conversion of tar using rice husk char/ash supported nickel–iron catalysts for biomass pyrolytic gasification combined with the mixing-simulation in fluidized-bed gasifier. Appl. Energy 2015, 160, 808–819. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Z.; Zhu, Y.-A.; Liu, Z.; Sui, Z.; Zhu, K.; Zhou, X. Dry reforming of methane on Ni-Fe-MgO catalysts: Influence of Fe on carbon-resistant property and kinetics. Appl. Catal. B Environ. 2020, 264, 118497. [Google Scholar] [CrossRef]
- Vyazovkina, S.; Burnhamb, A.K.; Criadoc, J.M.; Pérez-Maquedac, L.A.; Popescud, C.; Sbirrazzuolie, N. ICTAC Kinetics Committee recommendations for performing kineticcomputations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Pérez-Maqueda, L.A.; Ortega, A.; Criado, J.M. The use of master plots for discriminating the kinetic model of solid state reactions from a single constant-rate thermal analysis (CRTA) experiment. Thermochim. Acta 1996, 277, 165–173. [Google Scholar] [CrossRef]
- Chai, M.; Liu, R.; He, Y. Effects of SiO2/Al2O3 ratio and Fe loading rate of Fe-modified ZSM-5 on selection of aromatics and kinetics of corn stalk catalytic pyrolysis. Fuel Process. Technol. 2020, 206, 106458. [Google Scholar] [CrossRef]
- Al-Dughaither, A.S.; de Lasa, H. HZSM-5 Zeolites with Different SiO2/Al2O3 Ratios. Characterization and NH3 Desorption Kinetics. Ind. Eng. Chem. Res. 2014, 53, 15303–15316. [Google Scholar] [CrossRef]
- Ma, C.; Kamo, T. Enhanced debromination by Fe particles during the catalytic pyrolysis of non-metallic fractions of printed circuit boards over ZSM-5 and Ni/SiO2-Al2O3 catalyst. J. Anal. Appl. Pyrolysis 2019, 138, 170–177. [Google Scholar] [CrossRef]
- Chen, H.S.; Polk, D.E. Mechanical properties of Ni-Fe based alloy glasses. J. Non-Cryst. Solids 1974, 15, 174–178. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, X.; Chen, L.; Zhao, B.; Yang, S.; Xie, X. Comparision of catalytic fast pyrolysis of biomass to aromatic hydrocarbons over ZSM-5 and Fe/ZSM-5 catalysts. J. Anal. Appl. Pyrolysis 2016, 121, 342–346. [Google Scholar] [CrossRef]
- Leung, C.L.W. Kinetic Modeling of Scrap Tire Pyrolysis. Energy Fuels 1999, 13, 421–427. [Google Scholar] [CrossRef]
- Seidelt, S.; Müller-Hagedorn, M.; Bockhorn, H. Description of tire pyrolysis by thermal degradation behaviour of main components. J. Anal. Appl. Pyrolysis 2006, 75, 11–18. [Google Scholar] [CrossRef]
- Irmak Aslan, D.; Parthasarathy, P.; Goldfarb, J.L.; Ceylan, S. Pyrolysis reaction models of waste tires: Application of master-plots method for energy conversion via devolatilization. Waste Manag. 2017, 68, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Zhan, J.-H.; Lai, D.; Li, M.; Liu, X.; Xu, G. Primary understanding of non-isothermal pyrolysis behavior for oil shale kerogen using reactive molecular dynamics simulation. Int. J. Hydrogen Energy 2016, 41, 12093–12100. [Google Scholar] [CrossRef]
- Starink, M.J. The Determination of Activation Energy from Linear Heating Rate Experiments: A Comparison of the Accuracy of Isoconversion Methods. Thermochim. Acta 2003, 404, 163–176. [Google Scholar] [CrossRef] [Green Version]
- Qu, B.; Li, A.; Qu, Y.; Wang, T.; Zhang, Y.; Wang, X.; Gao, Y.; Fu, W.; Ji, G. Kinetic analysis of waste tire pyrolysis with metal oxide and zeolitic catalysts. J. Anal. Appl. Pyrolysis 2020, 152, 104949. [Google Scholar] [CrossRef]
- Osorio-Vargas, P.; Lick, I.D.; Sobrevía, F.; Correa-Muriel, D.; Menares, T.; Manrique, R.; Casella, M.L.; Arteaga-Pérez, L.E. Thermal Behavior, Reaction Pathways and Kinetic Implications of Using a Ni/SiO2 Catalyst for Waste Tire Pyrolysis. Waste Biomass Valorization 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Alsurakji, I.H.; El-Qanni, A.; El-Hamouz, A.M.; Warad, I.; Odeh, Y. Thermogravimetric Kinetics Study of Scrap Tires Pyrolysis Using Silica Embedded With NiO and/or MgO Nanocatalysts. J. Energy Resour. Technol. 2021, 143, 092302. [Google Scholar] [CrossRef]
- Kordoghli, S.; Paraschiv, M.; Kuncser, R.; Tazerout, M.; Zagrouba, F. Catalysts’ influence on thermochemical decomposition of waste tires. Environ. Prog. Sustain. Energy 2017, 36, 1560–1567. [Google Scholar] [CrossRef]
- Yang, H.; Ji, G.; Clough, P.T.; Xu, X.; Zhao, M. Kinetics of catalytic biomass pyrolysis using Ni-based functional materials. Fuel Process. Technol. 2019, 195, 106145. [Google Scholar] [CrossRef]
- Chen, J.; Mu, L.; Jiang, B.; Yin, H.; Song, X.; Li, A. TG/DSC-FTIR and Py-GC investigation on pyrolysis characteristics of petrochemical wastewater sludge. Bioresour. Technol. 2015, 192, 1–10. [Google Scholar] [CrossRef]
- Fong, M.J.B.; Loy, A.C.M.; Chin, B.L.F.; Lam, M.K.; Yusup, S.; Jawad, Z.A. Catalytic pyrolysis of Chlorella vulgaris: Kinetic and thermodynamic analysis. Bioresour. Technol. 2019, 289, 121689. [Google Scholar] [CrossRef]
- Chong, C.T.; Mong, G.R.; Ng, J.-H.; Chong, W.W.F.; Ani, F.N.; Lam, S.S.; Ong, H.C. Pyrolysis characteristics and kinetic studies of horse manure using thermogravimetric analysis. Energy Convers. Manag. 2019, 180, 1260–1267. [Google Scholar] [CrossRef]
- Tian, B.; Qiao, Y.; Bai, L.; Feng, W.; Jiang, Y.; Tian, Y. Pyrolysis behavior and kinetics of the trapped small molecular phase in a lignite. Energy Convers. Manag. 2017, 140, 109–120. [Google Scholar] [CrossRef]
- Xu, F.; Wang, B.; Yang, D.; Ming, X.; Jiang, Y.; Hao, J.; Qiao, Y.; Tian, Y. TG-FTIR and Py-GC/MS study on pyrolysis mechanism and products distribution of waste bicycle tire. Energy Convers. Manag. 2018, 175, 288–297. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, X.; Shen, J.; Zhang, H. Pyrolysis of superfine pulverized coal. Part 1. Mechanisms of methane formation. Energy Convers. Manag. 2014, 87, 1027–1038. [Google Scholar] [CrossRef]
- Hao, J.; Feng, W.; Qiao, Y.; Tian, Y.; Zhang, J.; Che, Y. Thermal cracking behaviors and products distribution of oil sand bitumen by TG-FTIR and Py-GC/TOF-MS. Energy Convers. Manag. 2017, 151, 227–239. [Google Scholar] [CrossRef]
- Zeng, M.; Yuan, W.; Wang, Y.; Zhou, W.; Zhang, L.; Qi, F.; Li, Y. Experimental and kinetic modeling study of pyrolysis and oxidation of n-decane. Combust. Flame 2014, 161, 1701–1715. [Google Scholar] [CrossRef]
- Danon, B.; van der Gryp, P.; Schwarz, C.E.; Görgens, J.F. A review of dipentene (dl-limonene) production from waste tire pyrolysis. J. Anal. Appl. Pyrolysis 2015, 112, 1–13. [Google Scholar] [CrossRef]
- Pakdel, H.; Pantea, D.M.; Roy, C. Production of dl-limonene by vacuum pyrolysis of used tires. J. Anal. Appl. Pyrolysis 2001, 57, 91–107. [Google Scholar] [CrossRef]
- Busca, G. Chapter 9—Metal Catalysts for Hydrogenations and Dehydrogenations. In Heterogeneous Catalytic Materials; Busca, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 297–343. [Google Scholar]
- Zhao, M.; Raheem, A.; Memon, Z.M.; Vuppaladadiyam, A.K.; Ji, G. Iso-conversional kinetics of low-lipid micro-algae gasification by air. J. Clean. Prod. 2019, 207, 618–629. [Google Scholar] [CrossRef]
- Murray, P.; White, J. Kinetic of the thermal dehydration of clays. Part IV. Interpretation of the differential thermal analysis of the clay minerals. Trans. Brot Ceram. Soc. 1955, 54, 204–238. [Google Scholar]
- Qu, Y.; Li, A.; Wang, D.; Zhang, L.; Ji, G. Kinetic study of the effect of in-situ mineral solids on pyrolysis process of oil sludge. Chem. Eng. J. 2019, 374, 338–346. [Google Scholar] [CrossRef]





| Sample | SBET a (m2/g) | Smic b (m2/g) | Sext c (m2/g) | Vtotal d (cm3/g) | Vmic b (cm3/g) | Da (nm) |
|---|---|---|---|---|---|---|
| ZSM-5 | 324 | 131 | 193 | 0.348 | 0.082 | 4.30 |
| 10Ni | 282 | 136 | 146 | 0.265 | 0.082 | 4.32 |
| 7Ni/3Fe | 262 | 138 | 124 | 0.254 | 0.084 | 4.35 |
| 5Ni/5Fe | 241 | 120 | 121 | 0.245 | 0.071 | 4.36 |
| 3Ni/7Fe | 220 | 104 | 116 | 0.236 | 0.062 | 4.42 |
| 10Fe | 180 | 86 | 94 | 0.203 | 0.050 | 4.52 |
| Sample | Ts a (°C) | Tmax (°C) | DTGmax (wt.%/s) | Te b (°C) | △T c (°C) | Residue (wt.%) |
|---|---|---|---|---|---|---|
| No catalyst | 316.11 | 376.20 | −0.1277 | 444.15 | 128.04 | 37.16 |
| 10Ni | 265.11 | 373.99 | −0.1372 | 442.04 | 176.93 | 32.24 |
| 7Ni/3Fe | 214.70 | 374.20 | −0.1296 | 441.50 | 226.80 | 32.69 |
| 5Ni/5Fe | 202.76 | 373.77 | −0.1279 | 443.51 | 240.75 | 33.94 |
| 3Ni/7Fe | 235.97 | 374.16 | −0.1270 | 445.86 | 209.89 | 32.86 |
| 10Fe | 294.81 | 373.53 | −0.1366 | 444.79 | 149.98 | 32.47 |
| α | No Catalyst | 10Ni | 7Ni/3Fe | 5Ni/5Fe | 3Ni/7Fe | 10Fe |
|---|---|---|---|---|---|---|
| E (kJ/mol) | E (kJ/mol) | E (kJ/mol) | E (kJ/mol) | E (kJ/mol) | E (kJ/mol) | |
| 0.20 | 203.25 | 175.42 | 153.97 | 181.88 | 145.69 | 187.34 |
| 0.25 | 205.48 | 172.03 | 161.90 | 173.05 | 153.84 | 173.89 |
| 0.30 | 202.15 | 178.88 | 168.73 | 176.75 | 161.16 | 171.87 |
| 0.35 | 204.85 | 176.91 | 167.90 | 175.52 | 165.14 | 168.89 |
| 0.40 | 205.09 | 178.22 | 168.46 | 177.60 | 166.16 | 173.70 |
| 0.45 | 207.42 | 186.72 | 173.02 | 179.80 | 176.42 | 172.18 |
| 0.50 | 207.12 | 184.37 | 179.45 | 184.24 | 182.54 | 176.45 |
| 0.55 | 209.76 | 192.80 | 184.79 | 189.99 | 188.03 | 178.23 |
| 0.60 | 217.01 | 203.46 | 194.82 | 203.60 | 200.93 | 190.16 |
| 0.65 | 222.60 | 220.89 | 203.28 | 216.85 | 212.81 | 199.77 |
| 0.70 | 236.1 | 244.95 | 217.43 | 235.60 | 234.42 | 210.22 |
| 0.75 | 246.029 | 258.18 | 222.55 | 241.16 | 247.63 | 217.14 |
| 0.80 | 245.09 | 258.71 | 224.09 | 248.20 | 249.05 | 220.86 |
| Ave | 216.31 | 202.43 | 186.19 | 198.79 | 191.06 | 187.75 |
| Sample | Method | E (kJ/mol) | Reference |
|---|---|---|---|
| WT | KAS | 168.4 | [34] |
| WT + Ni/SiO2 | 111.0 | ||
| WT | OFW | 142.0 | [35] |
| WT + SBNs1 | 96.0 | ||
| WT + SBNs2 | 146.0 | ||
| WT + SBNs3 | 103.0 | ||
| WT | Friedman | 246.9 | [36] |
| WT + CaCO3 | 128.3 | ||
| WT + Al2O3 | 190.2 | ||
| WT + Zeolite | 448.3 | ||
| WT + MgO | 121.8 |
| Sample | β (°C/min) | E (kJ/mol) | A (s−1) | f(α) | R2 |
|---|---|---|---|---|---|
| No catalyst | 10 | 216.31 | 4.63 × 1014 | (1 − α)3.45 | 0.997899 |
| 20 | 9.26 × 1014 | 0.998599 | |||
| 30 | 1.39 × 1015 | 0.999673 | |||
| 10Ni | 10 | 202.43 | 3.81 × 1013 | (1 − α)3.02 | 0.998722 |
| 20 | 7.62 × 1013 | 0.999672 | |||
| 30 | 1.14 × 1014 | 0.999412 | |||
| 7Ni/3Fe | 10 | 186.19 | 1.69 × 1012 | (1 − α)2.85 | 0.998865 |
| 20 | 3.38 × 1012 | 0.999399 | |||
| 30 | 5.07 × 1012 | 0.999431 | |||
| 5Ni/5Fe | 10 | 198.79 | 1.93 × 1012 | (1 − α)2.95 | 0.998385 |
| 20 | 3.85 × 1012 | 0.999537 | |||
| 30 | 5.78 × 1012 | 0.999649 | |||
| 3Ni/7Fe | 10 | 191.06 | 4.30 × 1012 | (1 − α)2.92 | 0.998623 |
| 20 | 8.60 × 1012 | 0.999419 | |||
| 30 | 1.29 × 1013 | 0.999817 | |||
| 10Fe | 10 | 187.75 | 2.37 × 1012 | (1 − α)2.91 | 0.999864 |
| 20 | 4.74 × 1012 | 0.999436 | |||
| 30 | 7.11 × 1012 | 0.999361 |
| Wavenumber (cm−1) | Group/Product | Vibration |
|---|---|---|
| 950 | C2H4 | / |
| 1459 | –C–H | Bending |
| 1520 | –C=C– (Aromatic) | Stretching |
| 1542 | ||
| 1650 | –C=C– | Stretching |
| 2887 | –C–H | Stretching |
| 2932 | ||
| 2970 | ||
| 3014 | CH4 | / |
| 3080 | –C–H (Aromatic) | Stretching |
| Proximate Analysis (wt.%) | |
|---|---|
| Moisture | 0.43 ± 0.05 |
| Volatile matter | 63.35 ± 0.13 |
| Fixed carbon | 28.54 ± 0.14 |
| Ash | 7.68 ± 0.01 |
| Ultimate analysis (wt.%) | |
| C | 80.87 ± 0.09 |
| H | 7.69 ± 0.02 |
| O a | 1.00 ± 0.06 |
| N | 1.05 ± 0.02 |
| S | 1.71 ± 0.07 |
| Reaction Name | f(α) | g(α) |
|---|---|---|
| Power law (P2/3) | 2/3α−1/2 | α3/2 |
| Power law (P2) | 2α1/2 | α1/2 |
| Power law (P3) | 3α2/3 | α1/3 |
| Power law (P4) | 4α3/4 | α1/4 |
| Avrami-Erofeev (A2) | 2 (1 − α) [−ln (1 − α)]1/2 | [−ln (1 − α)]1/2 |
| Avrami-Erofeev (A3) | 3 (1 − α) [−ln (1 − α)]2/3 | [−ln (1 − α)]2/3 |
| Avrami-Erofeev (A4) | 4 (1 − α) [−ln (1 − α)]3/4 | [−ln (1 − α)]3/4 |
| 1-D diffusion (D1) | 1/(2α) | α2 |
| 2-D diffusion (D2) | −[1/ln (1 − α)] | (1 − α) ln (1 − α) + α |
| Contracting cylinder (R2) | 2 (1 − α)1/2 | 1 − (1 − α)1/2 |
| Contracting sphere (R3) | 3 (1 − α)2/3 | 1 − (1 − α)1/3 |
| Zero order (F0) | 1 | α |
| First order (F1) | 1 − α | −ln (1 − α) |
| Second order (F2) | (1 − α)2 | [1/(1 − α)] − 1 |
| Third order (F3) | (1 − α)3 | (1/2) [1/(1 − α)−2 − 1] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, B.; Zhang, Y.; Wang, T.; Li, A.; Wu, Z.; Ji, G. Dynamic Pyrolysis Characteristics, Kinetics and Products Analysis of Waste Tire Catalytic Pyrolysis with Ni/Fe-ZSM-5 Catalysts Using TG-IR-GC/MS. Catalysts 2021, 11, 1031. https://doi.org/10.3390/catal11091031
Qu B, Zhang Y, Wang T, Li A, Wu Z, Ji G. Dynamic Pyrolysis Characteristics, Kinetics and Products Analysis of Waste Tire Catalytic Pyrolysis with Ni/Fe-ZSM-5 Catalysts Using TG-IR-GC/MS. Catalysts. 2021; 11(9):1031. https://doi.org/10.3390/catal11091031
Chicago/Turabian StyleQu, Boyu, Yulin Zhang, Tian Wang, Aimin Li, Zhiqiang Wu, and Guozhao Ji. 2021. "Dynamic Pyrolysis Characteristics, Kinetics and Products Analysis of Waste Tire Catalytic Pyrolysis with Ni/Fe-ZSM-5 Catalysts Using TG-IR-GC/MS" Catalysts 11, no. 9: 1031. https://doi.org/10.3390/catal11091031
APA StyleQu, B., Zhang, Y., Wang, T., Li, A., Wu, Z., & Ji, G. (2021). Dynamic Pyrolysis Characteristics, Kinetics and Products Analysis of Waste Tire Catalytic Pyrolysis with Ni/Fe-ZSM-5 Catalysts Using TG-IR-GC/MS. Catalysts, 11(9), 1031. https://doi.org/10.3390/catal11091031







