Degradation Efficiency and Kinetics Analysis of an Advanced Oxidation Process Utilizing Ozone, Hydrogen Peroxide and Persulfate to Degrade the Dye Rhodamine B
Abstract
1. Introduction
2. Results and Discussion
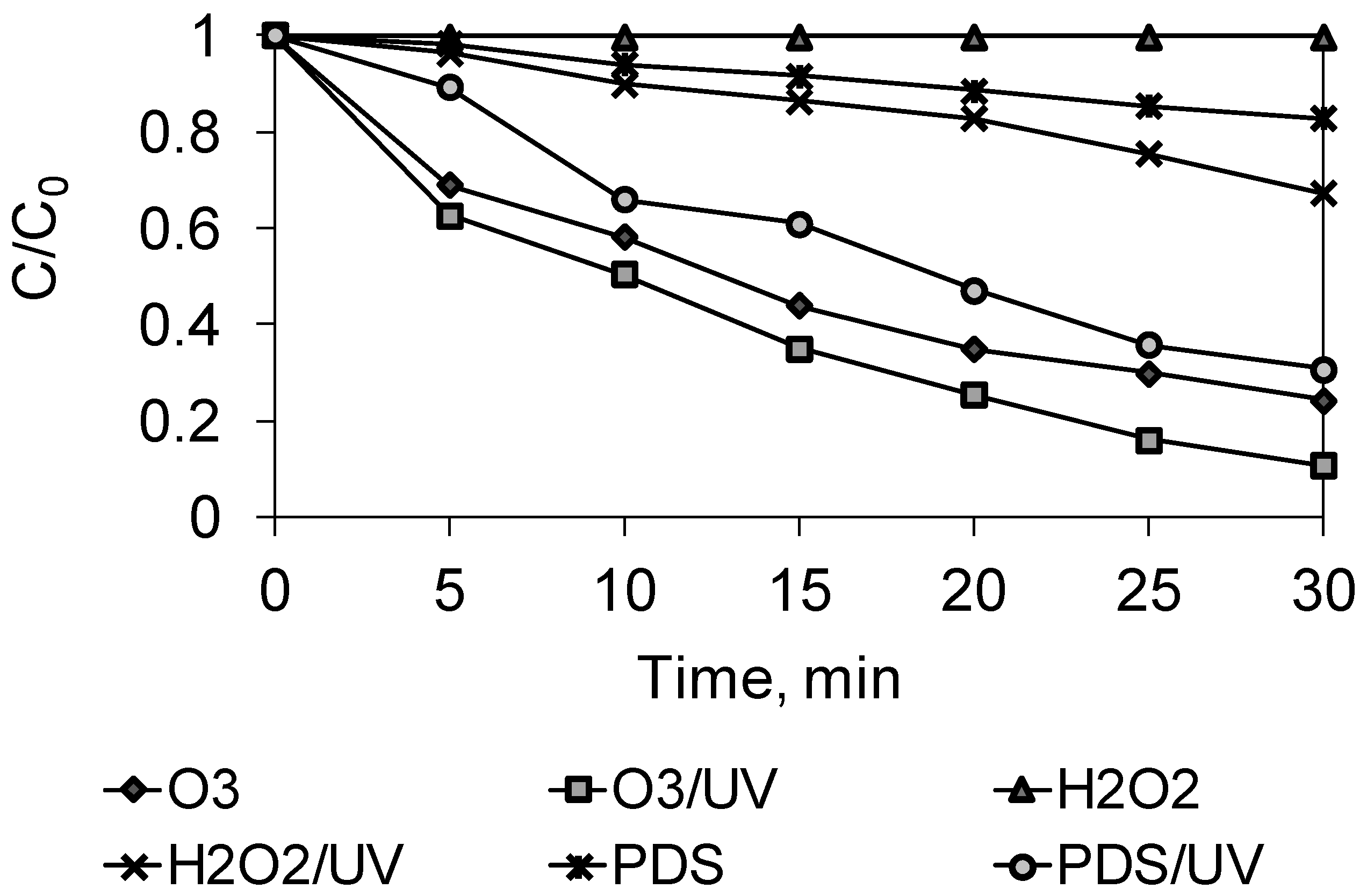
2.1. Advanced Oxidation Study
2.2. Kinetics Studies
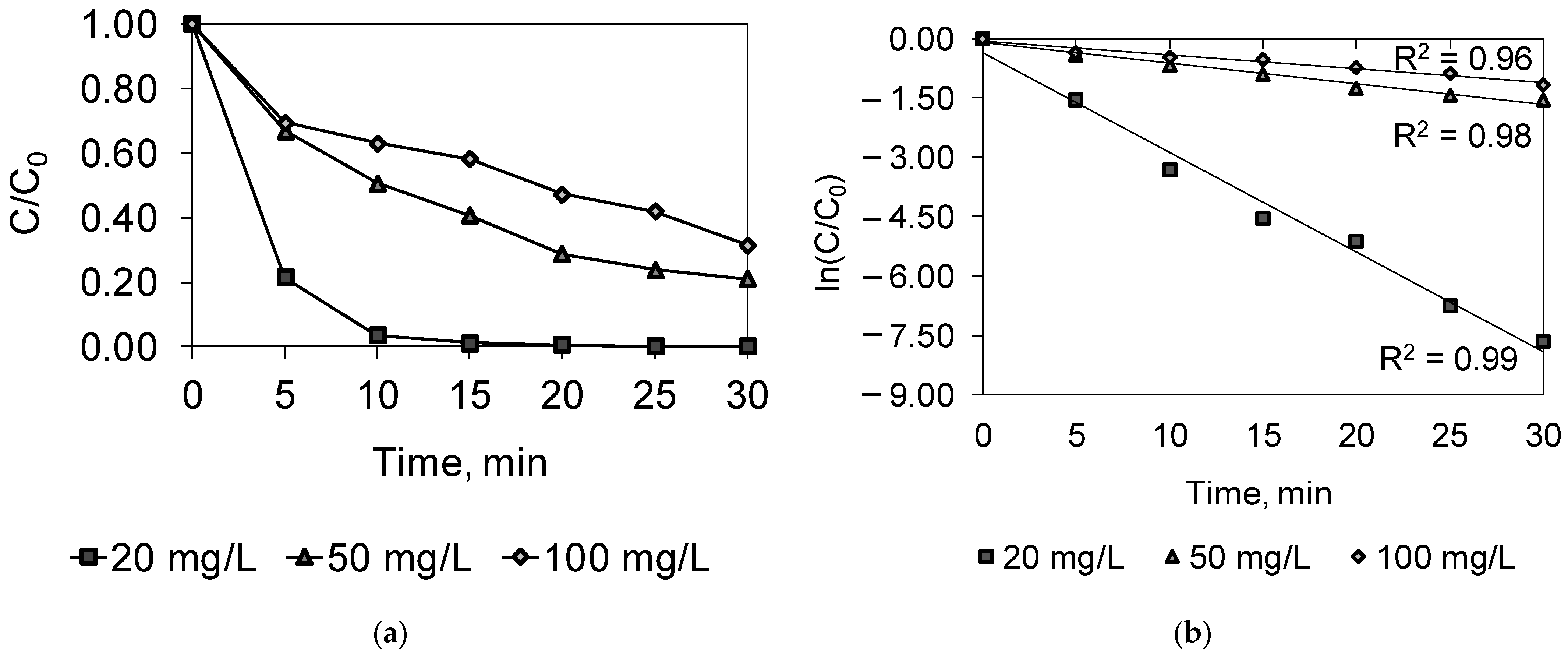
2.3. Combined PDS/O3/UV
3. Comparison with Other AOPs
4. Materials and Methods
4.1. Materials
4.2. Advanced Oxidation Experiments
4.3. Instruments and Analytical Methods
4.4. Kinetics Studies
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Syafiuddin, A.; Fulazzaky, M.A. Decolorization kinetics and mass transfer mechanisms of Remazol brilliant blue R dye mediated by different fungi. Biotechnol. Rep. 2020, 29, e00573. [Google Scholar] [CrossRef]
- Wang, C.; Yuan, Z.; Wang, A.; Qu, J.; Fang, Z.; Wen, Y. Ultraviolet light enhanced sodium persulfate oxida-tion of cellulose to facilitate the preparation of cellulose nanofibers. Cellulose 2020, 27, 2041–2051. [Google Scholar] [CrossRef]
- Zawadzki, P.; Kudlek, E.; Dudziak, M. Titanium (IV) oxide modified with activated carbon and ultrasounds for caffeine photodegradation: Adsorption isotherm and kinetics study. J. Ecol. Eng. 2020, 21, 137–145. [Google Scholar] [CrossRef]
- Jain, R.; Mathur, M.; Sikarwar, S.; Mittal, D.A. Removal of the hazardous dye rhodamine B through photocatalytic and adsorption treatments. J. Environ. Manag. 2007, 85, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Yahia, I.S.; Rammah, Y.S.; Khaled, K.F. Fabrication of an electrochemical cell based on Rhodamine B dye for low power applications. J. Mater. Environ. Sci. 2013, 4, 442–447. [Google Scholar]
- Garrido-Cardenas, J.A.; Esteban-García, B.; Agüera, A.; Sánchez-Pérez, J.A.; Manzano-Agugliaro, F. Wastewater treatment by advanced oxidation process and their worldwide research trends. Int. J. Environ. Res. Public Health 2019, 17, 170. [Google Scholar] [CrossRef]
- Tariqa, M.; Muhammada, M.; Khana, J.; Raziqa, A.; KashifUddinbc, M.; Niazd, A.; Ahmedef, S.; Rahimg, A. Removal of Rhodamine B dye from aqueous solutions using photo-fenton processes and novel Ni-Cu@MWCNTs photocatalyst. J. Mol. Liq. 2020, 312, 113399. [Google Scholar] [CrossRef]
- Dhahir, S.A.; Al-saade, K.A.; Al-Jobouri, I.S. Degradation studies of rhodamine b in the presence of UV/H2O2/Fe2+. Int. J. Tech. Res. Appl. 2014, 2, 123–127. [Google Scholar]
- Devi, P.; Das, U.; Dalai, A.K. In-situ chemical oxidation: Principle and applications of peroxide and persulfate treatments in wastewater systems. Sci. Total Environ. 2016, 571, 643–657. [Google Scholar] [CrossRef]
- Ike, I.; Linden, K.G.; Orbell, J.D.; Duke, M. Critical review of the science and sustainability of persulphate advanced oxidation processes. Chem. Eng. J. 2018, 338, 651–669. [Google Scholar] [CrossRef]
- Matzek, L.W.; Carter, K.E. Activated persulfate for organic chemical degradation: A review. Chemosphere 2016, 151, 178–188. [Google Scholar] [CrossRef]
- Wacławek, S.; Lutze, H.; Grübel, K.; Padil, V.V.T.; Černík, M.; Dionysiou, D. Chemistry of persulfates in water and wastewater treatment: A review. Chem. Eng. J. 2017, 330, 44–62. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Giannakis, S.; Lin, K.-Y.A.; Ghanbari, F. A review of the recent advances on the treatment of industrial wastewaters by sulfate radical-based advanced oxidation processes (SR-AOPs). Chem. Eng. J. 2020, 406, 127083. [Google Scholar] [CrossRef]
- Oh, W.-D.; Dong, Z.; Lim, T.-T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, M.; Dionysiou, D.D. What is the role of light in persulfate-based advanced oxidation for water treatment? Water Res. 2020, 189, 116627. [Google Scholar] [CrossRef]
- Zawadzki, P. Comparative studies of Rhodamine B decolorization in the combined process Na2S2O8/visible light/ultrasound. Desalination Water Treat. 2021, 213, 269–278. [Google Scholar] [CrossRef]
- Heidarpour, H.; Padervand, M.; Soltanieh, M.; Vossoughi, M. Enhanced decolorization of rhodamine B solution through simultaneous photocatalysis and persulfate activation over Fe/C3N4 photocatalyst. Chem. Eng. Res. Des. 2019, 153, 709–720. [Google Scholar] [CrossRef]
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2018, 16, 1193–1226. [Google Scholar] [CrossRef]
- Mathon, B.; Coquery, M.; Miege, C.; Penru, Y.; Choubert, J.-M. Removal efficiencies and kinetic rate constants of xenobiotics by ozonation in tertiary treatment. Water Sci. Technol. 2017, 75, 2737–2746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Duan, L.; Zhang, D. Decolorization of methyl orange by ozonation in combination with ultrasonic irradiation. J. Hazard. Mater. 2006, 138, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.; Baulch, D.L.; Cox, R.A.; Hampson, R.F.; Kerr, J.A.; Rossi, M.J.; Troe, J. Evaluated kinetic and photochemical data for atmospheric chemistry: Supplement VI. IUPAC subcommittee on gas kinetic data evaluation for atmospheric chemistry. J. Phys. Chem. Ref. Data 1997, 26, 1329. [Google Scholar] [CrossRef]
- Faryadi, M.; Rahimi, M.; Akbari, M. Process modeling and optimization of Rhodamine B dye ozonation in a novel microreactor equipped with high frequency ultrasound wave. Korean J. Chem. Eng. 2015, 33, 922–933. [Google Scholar] [CrossRef]
- Sierra, B.J.; Cabrera, A.M.; Arias, A.N.H.; Eguiluz, R.P.; Callejas, R.L.; Méndez, B.G.R.; Alvarado, R.V. Methylene blue degradation assessment by advance oxidation methods. J. Appl. Res. Technol. 2019, 17. [Google Scholar] [CrossRef][Green Version]
- Ding, X.; Gutierrez, L.; Croue, J.-P.; Li, M.; Wang, L.; Wang, Y. Hydroxyl and sulfate radical-based oxidation of RhB dye in UV/H2O2 and UV/persulfate systems: Kinetics, mechanisms, and comparison. Chemosphere 2020, 253, 126655. [Google Scholar] [CrossRef] [PubMed]
- Smaali, A.; Berkani, M.; Merouane, F.; Le, V.T.; Vasseghian, Y.; Rahim, N.; Kouachi, M. Photocatalytic persulfate oxidation for diclofenac removal from aqueous solutions: Modeling, optimization and biotoxicity test assessment. Chemosphere 2020, 266, 129158. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, J.; Jiang, D.; Gao, Y.; Chen, Y.; Wang, W.; Cao, B.; Tao, Y.; Wang, L.; Zhang, Y. Removal of atrazine by biochar-supported zero-valent iron catalyzed persulfate oxidation: Reactivity, radical production and transformation pathway. Environ. Res. 2020, 184, 109260. [Google Scholar] [CrossRef]
- Usman, M.; Alam Cheema, S.; Farooq, M. Heterogeneous fenton and persulfate oxidation for treatment of landfill leachate: A review supplement. J. Clean. Prod. 2020, 256, 120448. [Google Scholar] [CrossRef]
- Ahmadi, S.; Igwegbe, C.; Rahdar, S. The application of thermally activated persulfate for degradation of acid blue 92 in aqueous solution. Int. J. Ind. Chem. 2019, 10, 249–260. [Google Scholar] [CrossRef]
- Piotr, Z. Decolorisation of methylene blue with sodium persulfate activated with visible light in the presence of glucose and sucrose. Water Air Soil Pollut. 2019, 230, 313. [Google Scholar] [CrossRef]
- Liu, C.S.; Higgins, C.P.; Wang, F.; Shih, K. Effect of temperature on oxidative transformation of perfluorooctanoic acid (PFOA) by persulfate activation in water. Sep. Purif. Technol. 2012, 91, 46–51. [Google Scholar] [CrossRef]
- Tsitonaki, A.; Petri, B.; Crimi, M.; Mosbæk, H.; Siegrist, R.L.; Bjerg, P.L. In situ chemical oxidation of contaminated soil and groundwater using persulfate: A review. Crit. Rev. Environ. Sci. Technol. 2010, 40, 55–91. [Google Scholar] [CrossRef]
- Perkowski, J.; Ledakowicz, S. Decomposition of anthraquinone dye in the aqueous solution by ozone, hydrogen peroxide or UV radiation. Fibres Text. East. Eur. 2002, 10, 72–77. [Google Scholar]
- Simonenko, E.; Gomonov, A.; Rolle, N.; Molodkina, L. Modeling of H2O2 and UV oxidation of organic pollutants at wastewater post-treatment. Procedia Eng. 2015, 117, 337–344. [Google Scholar] [CrossRef][Green Version]
- Hamedi, F.H.; Rauf, M.; Ashraf, S. Degradation studies of Rhodamine B in the presence of UV/H2O2. Desalination 2009, 239, 159–166. [Google Scholar] [CrossRef]
- Haddad, M.E.; Regti, A.; Laamari, M.R.; Mamouni, R.; Saffaj, N. Use of fenton reagent as advanced oxidative process for removing textile dyes from aqueous solutions. J. Mater. Environ. Sci. 2014, 5, 667–674. [Google Scholar]
- Aljuboury, D.A.D.; Palaniandy, P. Kinetic study of inorganic carbon (IC) removal and COD removal from refinery wastewater by solar photo-fenton. Glob. NEST J. 2017, 19, 641–649. [Google Scholar]
- Zawadzki, P.; Kudlek, E.; Dudziak, M. Kinetics of the photocatalytic decomposition of bisphenol a on modified photocatalysts. J. Ecol. Eng. 2018, 19, 260–268. [Google Scholar] [CrossRef]
- Covinich, L.G.; Felissia, F.; Massa, P.; Fenoglio, R.; Area, M.C. Kinetic modeling of a heterogeneous fenton-type oxidative treatment of complex industrial effluent. Int. J. Ind. Chem. 2018, 9, 215–229. [Google Scholar] [CrossRef]
- Idrissi, M.; Miyah, Y.; Benjelloun, Y.; Chaouch, M. Degradation of crystal violet by heterogeneous fenton-like reaction using Fe/Clay catalyst with H2O2. J. Mater. Environ. Sci. 2016, 7, 50–58. [Google Scholar]
- Martínez, J.M.L.; Denis, M.F.L.; Piehl, L.L.; de Celis, E.R.; Buldain, G.Y.; Orto, V.C.D. Studies on the activation of hydrogen peroxide for color removal in the presence of a new Cu(II)-polyampholyte heterogeneous catalyst. Appl. Catal. B Environ. 2008, 82, 273–283. [Google Scholar] [CrossRef]
- Ziembowicz, S.; Kida, M.; Koszelnik, P. Sonochemical formation of hydrogen peroxide. Proceedings 2017, 2, 188. [Google Scholar] [CrossRef]
- Mandal, T.; Maity, S.; Dasgupta, D.; Datta, S. Advanced oxidation process and biotreatment: Their roles in combined industrial wastewater treatment. Desalination 2010, 250, 87–94. [Google Scholar] [CrossRef]
- Ku, Y.; Tu, Y.-H.; Ma, C.-M. Effect of hydrogen peroxide on the decomposition of monochlorophenols by sonolysis in aqueous solution. Water Res. 2005, 39, 1093–1098. [Google Scholar] [CrossRef]
- Thangavadivel, K.; Megharaj, M.; Mudhoo, A.; Naidu, R. Degradation of organic pollutants using ultra-sound. In Handbook on Application of Ultrasound: Sonochemistry for Sustainability; Sharma, S.K., Mudhoo, A., Eds.; Taylor and Francis Group: Abingdon-on-Thames, UK, 2011. [Google Scholar]
- Daneshvar, N.; Behnajady, M.A.; Mohammadi, M.K.A.; Dorraji, M.S.S. UV/H2O2 treatment of Rhodamine B in aqueous solution: Influence of operational parameters and kinetic modeling. Desalination 2008, 230, 16–26. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, H.; Zhong, X.; Hou, L. Ultrasound enhanced heterogeneous activation of peroxymonosulfate by a bimetallic Fe–Co/SBA-15 catalyst for the degradation of orange II in water. J. Hazard. Mater. 2015, 283, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Ahmed, N.; Vione, D.; Rivoira, L.; Carena, L.; Castiglioni, M.; Bruzzoniti, M. A review on the degradation of pollutants by fenton-like systems based on zero-valent iron and persulfate: Effects of reduction potentials, pH, and anions occurring in waste waters. Molecules 2021, 26, 4584. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.R. An Introduction to Chemical Kinetics; John Wiley and Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Rokesh, K.; Mohan, S.C.; Karuppuchamy, S.; Jothivenkatachalam, K. Photo-assisted advanced oxidation processes for Rhodamine B degradation using ZnO-Ag nanocomposite materials. J. Environ. Chem. Eng. 2018, 6, 3610–3620. [Google Scholar] [CrossRef]
- Jinisha, R.; Gandhimathi, R.; Ramesh, S.; Nidheesh, P.; Velmathi, S. Removal of rhodamine B dye from aqueous solution by electro-fenton process using iron-doped mesoporous silica as a heterogeneous catalyst. Chemosphere 2018, 200, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhang, Y.; Zhou, M.; Liang, L.; Li, K. Oxidation of Rhodamine B by persulfate activated with porous carbon aerogel through a non-radical mechanism. J. Hazard. Mater. 2018, 358, 53–61. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Thomas, M.; Natarajan, K.; Bajaj, H.C.; Tayade, R.J. Study on UV-LED/TiO2 process for degradation of Rhodamine B dye. Chem. Eng. J. 2011, 169, 126–134. [Google Scholar] [CrossRef]
- Cuiping, B.; Xianfeng, X.; Wenqi, G.; Dexin, F.; Mo, X.; Zhongxue, G.; Nian, X. Removal of rhodamine B by ozone-based advanced oxidation process. Desalination 2011, 278, 84–90. [Google Scholar] [CrossRef]
- Maharana, D.; Niu, J.; Gao, D.; Xu, Z.; Shi, J. Electrochemical degradation of rhodamine B over Ti/SnO2-sb electrode. Water Environ. Res. 2015, 87, 304–311. [Google Scholar] [CrossRef]
- Hou, M.-F.; Liao, L.; Zhang, W.-D.; Tang, X.; Wan, H.-F.; Yin, G.-C. Degradation of rhodamine B by Fe(0)-based Fenton process with H2O2. Chemosphere 2011, 83, 1279–1283. [Google Scholar] [CrossRef]
- Hauser-Davis, R.A.; Monteiro, F.; Chavez da Rocha, R.C.; Lemos, L.; Cardoso, M.D.; Siciliano, S. Titanium as a contaminant of emerging concern in the aquatic environment and the current knowledge gap regarding sea-bird contamination. Ornithologia 2020, 11, 7–15. [Google Scholar]
- Asztemborska, M.; Jakubiak, M.; Stęborowski, R.; Chajduk, E.; Bystrzejewska-Piotrowska, G. Titanium dioxide nanoparticle circulation in an aquatic ecosystem. Water Air Soil Pollut. 2018, 229, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Borghesi, F.; Migani, F.; Andreotti, A.; Baccetti, N.; Bianchi, N.; Birke, M.; Dinelli, E. Metals and trace elements in feathers: A geochemical approach to avoid misinterpretation of analytical responses. Sci. Total Environ. 2016, 544, 476–494. [Google Scholar] [CrossRef] [PubMed]
- Sodium Persulfate—The Dossier. Available online: https://www.santos.com/wp-content/uploads/2021/04/Sodium-persulfate-March-2021.pdf (accessed on 12 August 2021).
- Yasar, A.; Ahmad, N.; Chaudhry, M.N.; Rehman, M.S.U.; Khan, A.A.A. Ozone for color and COD removal of raw and anaerobically biotreated combined industrial wastewater. Pol. J. Environ. Stud. 2007, 16, 289–294. [Google Scholar]
- Collivignarelli, M.C.; Pedrazzani, R.; Sorlini, S.; Abbà, A.; Bertanza, G. H2O2 based oxidation processes for the treatment of real high strength aqueous wastes. Sustainability 2017, 9, 244. [Google Scholar] [CrossRef]
- Asenjo, N.G.; Santamaría, R.; Blanco, C.; Granda, M.; Álvarez, P.; Menéndez, R. Correct use of the Langmuir-Hinshelwood equation for proving the absence of a synergy effect in the photocatalytic degradation of phenol on a suspended mixture of titania and activated carbon. Carbon 2013, 55, 62–69. [Google Scholar] [CrossRef]
- Armenise, S.; Garcia-Bordeje, E.; Valverde, J.L.; Romeo, E.; Monzon, A. A Langmuir–Hinshelwood approach to the kinetic modelling of catalytic ammonia decomposition in an integral reactor. Phys. Chem. Chem. Phys. 2013, 15, 12104. [Google Scholar] [CrossRef] [PubMed]
- Marcinkowski, D.; Wałęsa-Chorab, M.; Patroniak, V.; Kubicki, M.; Kądziołka, G.; Michalkiewicz, B. A new polymeric complex of silver(i) with a hybrid pyrazine-bipyridine ligand-synthesis, crystal structure and its photocatalytic activity. New J. Chem. 2013, 38, 604–610. [Google Scholar] [CrossRef]


| Chemical Structure | Physicochemical Properties | |
|---|---|---|
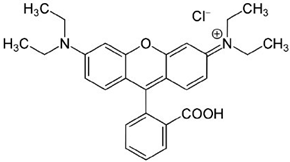 | Chemical formula | C28H31CIN2O3 |
| Molecular weight [g/mol] | 479.00 | |
| CAS number | 81-88-9 | |
| Water solubility at 20 °C [g/L] | 15.0 | |
| Dissociation constant (pKa) [–] | 3.7 | |
| logKOW [–] | 1.9–2.0 | |
| λmax in water [nm] | 554.0 | |
| Model | Parameter | Process | |||||
|---|---|---|---|---|---|---|---|
| H2O2 | H2O2/UV | PDS | PDS/UV | O3 | O3/UV | ||
| pseudo-first-order | k, 1/min | 0.00 | 0.0065 | 0.0126 | 0.0408 | 0.0459 | 0.0726 |
| R2 | 0.62 | 0.99 | 0.96 | 0.99 | 0.99 | 0.99 | |
| pseudo-second-order | k, 1/min | 0.00 | 0.0070 | 0.0150 | 0.0768 | 0.1022 | 0.2625 |
| R2 | 0.61 | 0.99 | 0.94 | 0.95 | 0.98 | 0.87 | |
| Parameter | Dye Concentration, mg/L | ||
|---|---|---|---|
| 20 | 50 | 100 | |
| k, 1/min | 0.2513 | 0.0523 | 0.0341 |
| R2 | 0.99 | 0.98 | 0.96 |
| t/2 | 2.8 | 13.3 | 20.3 |
| Half-Life, s | Time Span for Near-Completion | Rate Classification |
|---|---|---|
| 10−15–10−12 | ps or less | ultra fast rate |
| 10−12–10−6 | µs or less | very fast rate |
| 10−6–1 | seconds | fast rate |
| 1–103 | minutes or hours | moderate rate |
| 106–103 | weeks | slow rate |
| >106 | weeks or years | very slow rate |
| AOP | Conditions | Removal Efficiency, % | References |
|---|---|---|---|
| PDS/O3/UV | C0[RhB] = 100 mg/L pH = 6.0 time = 30 min O3 flow = 10 mL/min T = 295.0 K Lamp = 10 W H2O2 dose = 40 mg/L PDS dose = 20 mM | 60.0 | This study |
| PDS/Vis/US | C0[RhB] = 10 mg/L pH = 6.0 time = 60 min T = 295.0 K US = 40 kHz, 60 W PDS dose = 20 mM Lamp = 10 W | 85.0 | [17] |
| Peroxide assisted photocatalytic degradation in the presence of ZnO | C0[RhB] = 5 mg/L time = 90 min Catalyst dose = 500 mg/L lamp (300 W) | 60.0 | [51] |
| Electro-Fenton | C0[RhB] = 10 mg/L pH = 2.0 time = 180 min Electrode dose = 15 mg/L Voltage = 8 V | 97.7 | [52] |
| Carbon aerogel/persulfate | C0[RhB] = 10 mg/L pH = 7.0 time = 60 min Persulfate dose = 1.0 mM Carbon aerogel dose = 100 mg/L | 80.0 | [53] |
| UV-LED/TiO2 | C0[RhB] = 49 mg/L time = 180 min pH = 3.05 TiO2 = 1.6 g/L Lamp = LED (5) luminous intensity = 350 mcd radiant flux = 10–12 mW at 20 mA | 51.0 | [54] |
| O3/UV | C0[RhB] = 100 mg/L pH = 3.0 time = 15 min ozone flow = 30 mL/min Lamp = 16 W | 99.8 | [55] |
| Ti/SnO2-Sb electrode | C0[RhB] = 50 mg/L pH = 3.0 time = 20 min at a constant current density of 20 mA/cm2 with a 10 mmol/L Na2SO4 supporting electrolyte solution | 97.8 | [56] |
| Fe(0)-based Fenton process with H2O2 | C0[RhB] = 49 mg/L Time = 30 min pH = 4.0 Fe(0) = 1.0 g/L H2O2 = 2.0 mM | 100.0 | [57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zawadzki, P.; Deska, M. Degradation Efficiency and Kinetics Analysis of an Advanced Oxidation Process Utilizing Ozone, Hydrogen Peroxide and Persulfate to Degrade the Dye Rhodamine B. Catalysts 2021, 11, 974. https://doi.org/10.3390/catal11080974
Zawadzki P, Deska M. Degradation Efficiency and Kinetics Analysis of an Advanced Oxidation Process Utilizing Ozone, Hydrogen Peroxide and Persulfate to Degrade the Dye Rhodamine B. Catalysts. 2021; 11(8):974. https://doi.org/10.3390/catal11080974
Chicago/Turabian StyleZawadzki, Piotr, and Małgorzata Deska. 2021. "Degradation Efficiency and Kinetics Analysis of an Advanced Oxidation Process Utilizing Ozone, Hydrogen Peroxide and Persulfate to Degrade the Dye Rhodamine B" Catalysts 11, no. 8: 974. https://doi.org/10.3390/catal11080974
APA StyleZawadzki, P., & Deska, M. (2021). Degradation Efficiency and Kinetics Analysis of an Advanced Oxidation Process Utilizing Ozone, Hydrogen Peroxide and Persulfate to Degrade the Dye Rhodamine B. Catalysts, 11(8), 974. https://doi.org/10.3390/catal11080974






