Potential Valorization of Organic Waste Streams to Valuable Organic Acids through Microbial Conversion: A South African Case Study
Abstract
1. Introduction
2. The Carboxylate Platform
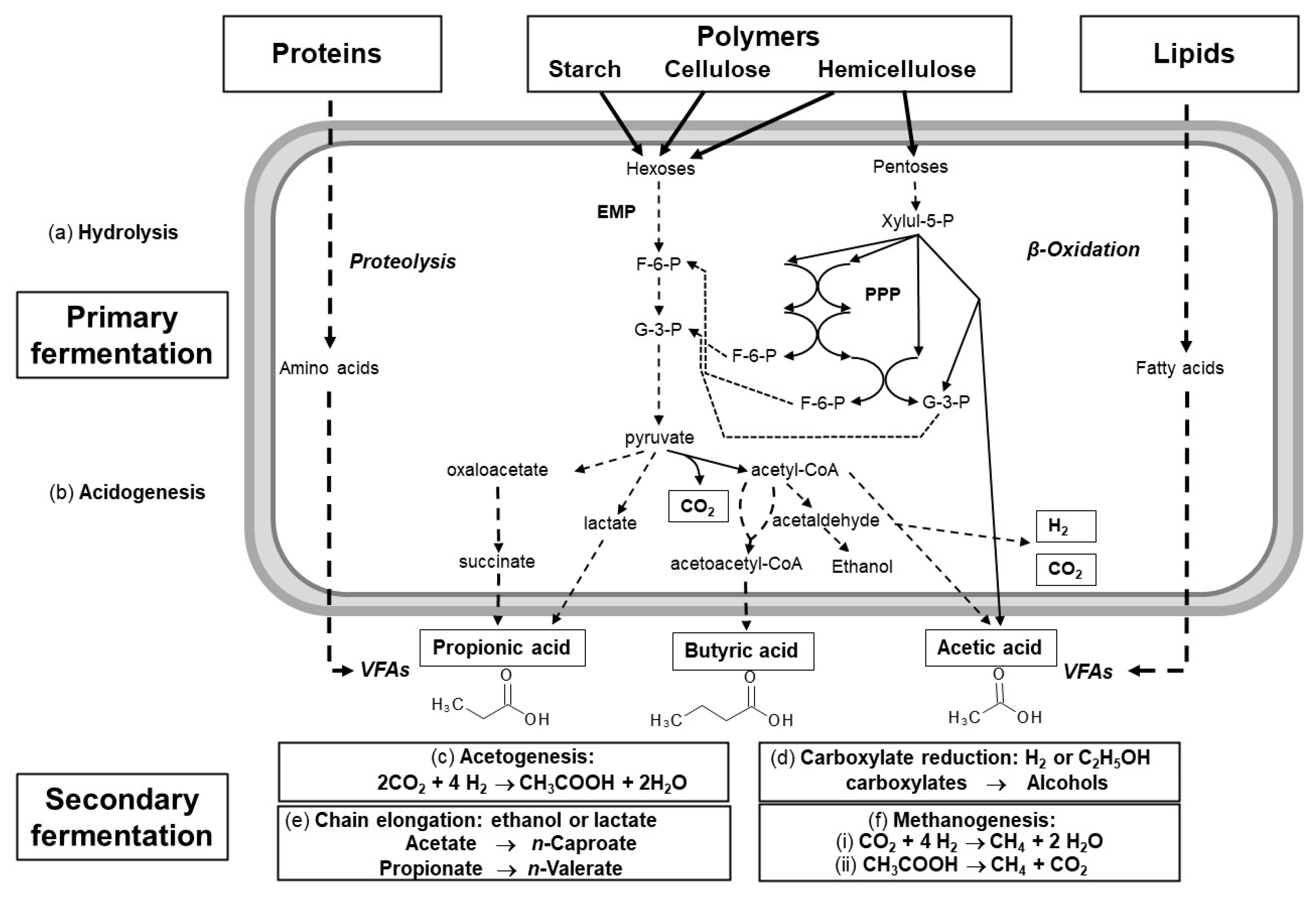
2.1. VFA Production
2.2. Types of Wastes Suitable for VFA Production
2.3. Pretreatment Techniques
2.4. Fermentation Parameters Influencing VFA Production
2.4.1. Temperature
2.4.2. pH
2.4.3. Retention Time
2.4.4. Organic Loading Rate
2.4.5. Inoculum Concentration
2.5. Microbial Communities Adapted for VFA Production
2.6. The Rumen-Modeled Carboxylate Platform
2.7. Characteristics of Rumen That Favor VFA Production
2.8. VFAs Conversion Route Suitable for Rumen Fermentation towards Hydrocarbon Fuel
2.9. Main Challenges Associated with a Rumen Carboxylate Platform and Incentives
2.9.1. Increasing the Cost Differential between Input Biomass and Product
2.9.2. Towards Implementing a Cost-Effective Carboxylate Platform Industry
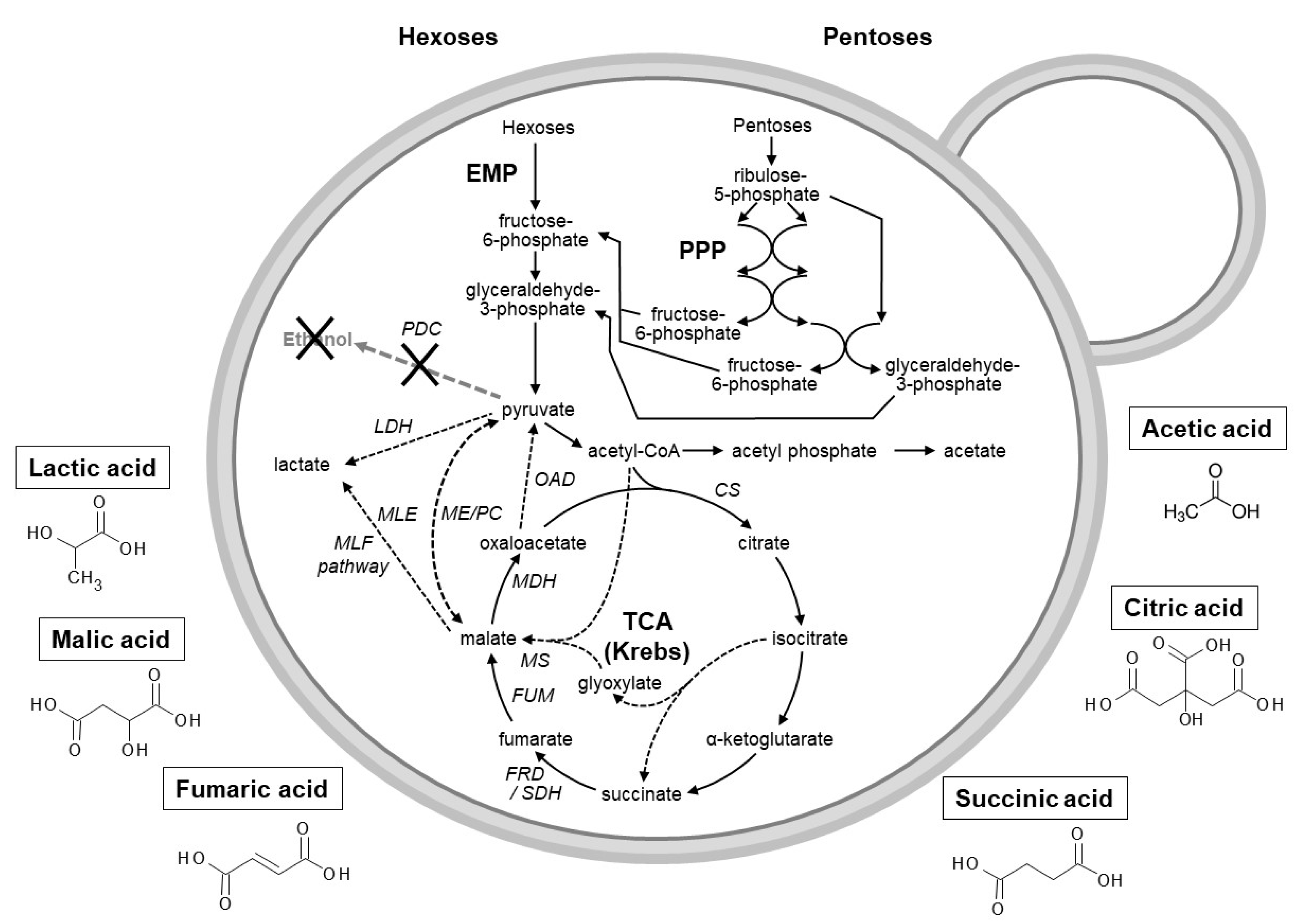
3. Production of Organic Acids
3.1. Use of Microbial Hosts to Produce Industrial Important Organic Acids
3.1.1. Fumaric Acid
3.1.2. Succinic Acid
3.1.3. Citric Acid
3.1.4. Lactic Acid
3.1.5. Acetic Acid
3.2. Natural versus Genetically Modified Yeasts
3.3. Challenges and Incentives
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kohler, M. CO2 emissions, energy consumption, income and foreign trade: A South African perspective. Energy Policy 2013, 63, 1042–1050. [Google Scholar] [CrossRef]
- Fiorentino, G.; Ripa, M.; Ulgiati, S. Chemicals from biomass: Technological versus environmental feasibility. A review. Biofuels Bioprod. Biorefining 2017, 11, 195–214. [Google Scholar] [CrossRef]
- Hugo, W. Bioenergy Atlas of South Africa—Synopsis Report; South African Environmental Observation Network: Pretoria, South Africa, 2016. [Google Scholar]
- Van den Berg, E.C.; Kotze, I.; Beukes, H. Detection, quantification and monitoring of Prosopis in the Northern Cape Province of South Africa using remote sensing and GIS. S. Afr. J. Geomat. 2013, 2, 68–81. [Google Scholar]
- Richardson, D.M.; Van Wilgen, B.W. Invasive alien plants in South Africa: How well do we understand the ecological impacts? S. Afr. J. Sci. 2004, 100, 45–52. [Google Scholar]
- Ndhlovu, T.; Milton, S.J.; Esler, K.J. Impact of Prosopis (mesquite) invasion and clearing on vegetation species composition and diversity in semi-arid Nama-Karoo rangeland, South Africa. Afr. J. Range Forage Sci. 2016, 33, 101–110. [Google Scholar] [CrossRef]
- Pancholy, R. Effective utilisation of Prosopis juliflora pods by ensiling with desert grass Lasiurus sindicus. Bioresour. Technol. 1999, 69, 1999–2001. [Google Scholar] [CrossRef]
- Shackleton, R.T.; Le Maitre, D.C.; van Wilgen, B.W.; Richardson, D.M. Towards a national strategy to optimise the man-agement of a widespread invasive tree (Prosopis species; mesquite) in South Africa. Ecosyst. Serv. 2017, 27, 242–252. [Google Scholar] [CrossRef]
- Chen, D.M.C.; Bodirsky, B.L.; Krueger, T.; Mishra, A.; Popp, A. The world’s growing municipal solid waste: Trends and impacts. Environ. Res. Lett. 2020, 15, 074021. [Google Scholar] [CrossRef]
- Averda. South African Waste Management Sector Can Still Improve. Available online: https://www.averda.com/rsa/news/south-african-waste-management-sector-can-still-improve (accessed on 23 June 2021).
- Gibril, M.E.; Lekha, P.; Andrew, J.; Sithole, B.; Tesfaye, T.; Ramjugernath, D. Beneficiation of pulp and paper mill sludge: Production and characterisation of functionalised crystalline nanocellulose. Clean Technol. Environ. Policy 2018, 20, 1835–1845. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extrac-tion, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef]
- FOASTAT FOASTAT. Production—Crops. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 22 June 2021).
- Bordiga, M.; Travaglia, F.; Locatelli, M. Valorisation of grape pomace: An approach that is increasingly reaching its maturity–A review. Int. J. Food Sci. Technol. 2019, 54, 933–942. [Google Scholar] [CrossRef]
- Sirohi, R.; Tarafdar, A.; Singh, S.; Negi, T.; Gaur, V.K.; Gnansounou, E.; Bharathiraja, B. Green processing and biotechnological potential of grape pomace: Current trends and opportunities for sustainable biorefinery. Bioresour. Technol. 2020, 314, 123771. [Google Scholar] [CrossRef]
- Sousa, E.C.; Uchôa-Thomaz, A.M.A.; Carioca, J.O.B.; de Morais, S.M.; de Lima, A.; Martins, C.G.; Alexandrino, C.D.; Ferreira, P.A.T.; Rodrigues, A.L.M.; Rodrigues, S.P.; et al. Chemical composition and bioactive compounds of grape pomace (Vitis vinifera L.), Benitaka variety, grown in the semiarid region of Northeast Brazil. Food Sci. Technol. 2014, 34, 135–142. [Google Scholar] [CrossRef]
- Tseng, A.; Zhao, Y. Wine grape pomace as antioxidant dietary fibre for enhancing nutritional value and improving storability of yogurt and salad dressing. Food Chem. 2013, 138, 356–365. [Google Scholar] [CrossRef]
- Antonic, B.; Jancikova, S.; Dordevic, D.; Tremlova, B. Apple pomace as food fortification ingredient: A systematic review and meta-analysis. J. Food Sci. 2020, 85, 2977–2985. [Google Scholar] [CrossRef]
- Tikhonova, A.N.; Ageyeva, N.M.; Biryukova, S.A.; Globa, E.V.; Abakumova, A.A. Effect of grape variety, place of growth, and processing technology on the physical and chemical indicators of grape pomace. Food Process. Tech. Technol. 2020, 58, 493–502. [Google Scholar] [CrossRef]
- Ben-Iwo, J.; Manovic, V.; Longhurst, P. Biomass resources and biofuels potential for the production of transportation fuels in Nigeria. Renew. Sustain. Energy Rev. 2016, 63, 172–192. [Google Scholar] [CrossRef]
- Sims, R.E.H.; Mabee, W.; Saddler, J.N.; Taylor, M. An overview of second-generation biofuel technologies. Bioresour. Technol. 2010, 101, 1570–1580. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Calderon, O.; Arantes, V. A review on commercial-scale high-value products that can be produced alongside cel-lulosic ethanol. Biotechnol. Biofuels 2019, 12, 240. [Google Scholar] [CrossRef]
- Weimer, P.J.; Russell, J.B.; Muck, R.E. Lessons from the cow: What the ruminant animal can teach us about consolidated bioprocessing of cellulosic biomass. Bioresour. Technol. 2009, 100, 5323–5331. [Google Scholar] [CrossRef] [PubMed]
- Weimer, P.J.; Kohn, R.A. Impacts of ruminal microorganisms on the production of fuels: How can we intercede from the outside? Appl. Microbiol. Biotechnol. 2016, 100, 3389–3398. [Google Scholar] [CrossRef]
- IEA. Technology Roadmap Biofuels for Transport; The International Energy Agency: Paris, France, 2011; Available online: http://www.iea.org/publications/freepublications/publication/biofuels_roadmap_web.pdf (accessed on 21 June 2021).
- Peng, W.; Pivato, A. Sustainable management of digestate from the organic fraction of municipal solid waste and food waste under the concepts of back to earth alternatives and circular economy. Waste Biomass Valorization 2019, 10, 465–481. [Google Scholar] [CrossRef]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A review of the production and applications of waste-derived volatile fatty acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Steinbusch, K.J.J. Liquid Biofuel Production from Volatile Fatty Acids. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2010. [Google Scholar]
- Cherubini, F.; Jungmeier, G.; Mandl, M.; Philips, C. IEA Bioenergy Task 42 on biorefineries: Co-production of fuels, chemicals, power and materials from biomass. In Proceedings of the IEA Task 42 Biorefineries Workshop, Dublin, Ireland, 25 March 2009. [Google Scholar]
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Khan, N.; Le Roes-Hill, M.; Welz, P.J.; Grandin, K.A.; Kudanga, T.; Van Dyk, J.S.; Ohlhoff, C.; Van Zyl, W.H.; Pletschke, B.I. Fruit waste streams in South Africa and their potential role in developing a bio-economy. S. Afr. J. Sci. 2015, 111, 1–11. [Google Scholar] [CrossRef][Green Version]
- Werpy, T.; Petersen, G. Top Value-Added Chemicals from Biomass Volume 1—Results of Screening for Potential Candidates from Sugars and Synthesis Gas (No. DOE/GO-102004-1992); National Renewable Energy Laboratory: Golden, CO, USA, 2004. [Google Scholar]
- De Jong, E.; Higson, A.; Walsh, P.; Wellisch, M. Bio-Based Chemicals: Value Added Products from Biorefineries. In IEA Bioenergy: Task-42 Biorefinery; Avantium, Biomass Research and Wageningen University and Research Centre: Wageningen, The Netherlands, 2021. [Google Scholar]
- Made-in-China Connecting Buyers with Chinese Suppliers. Available online: https://www.made-in-china.com/productdirectory (accessed on 26 July 2021).
- Markets and Markets. Available online: https://www.marketsandmarkets.com/ (accessed on 23 June 2021).
- ImarcGgroup Imarc Group. Available online: https://www.imarcgroup.com/ (accessed on 23 June 2021).
- Grand View Research. Available online: https://www.grandviewresearch.com/ (accessed on 23 June 2021).
- Global Market Insights. Available online: https://www.gminsights.com/ (accessed on 23 June 2021).
- Expert Market Research. Available online: https://www.expertmarketresearch.com/ (accessed on 23 June 2021).
- 360 Research Reports. Available online: https://www.360researchreports.com/ (accessed on 23 June 2021).
- Wang, K.; Yin, J.; Shen, D.; Li, N. Anaerobic digestion of food waste for volatile fatty acids (VFAs) production with different types of inoculum: Effect of pH. Bioresour. Technol. 2014, 161, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Holm-Nielsen, J.B.; Al Seadi, T.; Oleskowicz-Popiel, P. The future of anaerobic digestion and biogas utilization. Bioresour. Technol. 2009, 100, 5478–5484. [Google Scholar] [CrossRef] [PubMed]
- Coma, M.; Martinez-Hernandez, E.; Abeln, F.; Raikova, S.; Donnelly, J.; Arnot, T.C.; Allen, M.J.; Hong, D.D.; Chuck, C.J. Organic waste as a sustainable feedstock for platform chemicals. Faraday Discuss. 2017, 202, 175–195. [Google Scholar] [CrossRef]
- Chang, H.N. Biofuels production from volatile fatty acid platform what are VFAs? In Proceedings of the Bioenergy II: Fuels and Chemicals from Renewable Resources, Rio De Janeiro, Brazil, 8–13 March 2009. [Google Scholar]
- Granda, C.B.; Holtzapple, M.T.; Luce, G.; Searcy, K.; Mamrosh, D.L. Carboxylate platform: The MixAlco process part 2: Process economics. Appl. Biochem. Biotechnol. 2009, 156, 107–124. [Google Scholar] [CrossRef]
- Wu, Q.; Bao, X.; Guo, W.; Wang, B.; Li, Y.; Luo, H.; Wang, H.; Ren, N. Medium-chain carboxylic acids production from waste biomass: Current advances and perspectives. Biotechnol. Adv. 2019, 37, 599–615. [Google Scholar] [CrossRef]
- Li, L.; Peng, X.; Wang, X.; Wu, D. Anaerobic digestion of food waste: A review focusing on process stability. Bioresour. Technol. 2018, 248, 20–28. [Google Scholar] [CrossRef]
- Padella, M.; O’Connell, A.; Prussi, M. What is still limiting the deployment of cellulosic ethanol? Analysis of the current status of the sector. Appl. Sci. 2019, 9, 4523. [Google Scholar] [CrossRef]
- Agler, M.T.; Wrenn, B.A.; Zinder, S.H.; Angenent, L.T. Waste to bioproduct conversion with undefined mixed cultures: The carboxylate platform. Trends Biotechnol. 2011, 29, 70–78. [Google Scholar] [CrossRef] [PubMed]
- De Groof, V.; Coma, M.; Arnot, T.; Leak, D.J.; Lanham, A.B. Medium-chain carboxylic acids from complex organic feedstocks by mixed culture fermentation. Molecules 2019, 24, 1–32. [Google Scholar] [CrossRef]
- Dong, L.; Zhenhong, Y.; Yongming, S.; Xiaoying, K.; Yu, Z. Hydrogen production characteristics of the organic fraction of municipal solid wastes by anaerobic mixed culture fermentation. Int. J. Hydrog. Energy 2009, 34, 812–820. [Google Scholar] [CrossRef]
- Yin, J.; Liu, J.; Chen, T.; Long, Y.; Shen, D. Influence of melanoidins on acidogenic fermentation of food waste to produce volatility fatty acids. Bioresour. Technol. 2019, 284, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, H.; Feng, K.; Liu, J. Oriented fermentation of food waste towards high-value products: A review. Energies 2020, 13, 5638. [Google Scholar] [CrossRef]
- Holtzapple, M.T.; Lonkar, S.; Granda, C.B. Producing biofuels via the carboxylate platform. Chem. Eng. Prog. 2015, 111, 41–44. [Google Scholar]
- Zhou, M.; Zhou, J.; Tan, M.; Du, J.; Yan, B.; Wong, J.W.C.; Zhang, Y. Enhanced carboxylic acids production by decreasing hydrogen partial pressure during acidogenic fermentation of glucose. Bioresour. Technol. 2017, 245, 44–51. [Google Scholar] [CrossRef]
- Broudiscou, L.P.; Offner, A.; Sauvant, D. Effects of inoculum source, pH, redox potential and headspace di-hydrogen on rumen in vitro fermentation yields. Animal 2014, 8, 931–937. [Google Scholar] [CrossRef]
- Bentsen, N.S.; Felby, C.; Thorsen, B.J. Agricultural residue production and potentials for energy and materials services. Prog. Energy Combust. Sci. 2014, 40, 59–73. [Google Scholar] [CrossRef]
- Ho, D.P.; Ngo, H.H.; Guo, W. A mini review on renewable sources for biofuel. Bioresour. Technol. 2014, 169, 742–749. [Google Scholar] [CrossRef]
- Dahiya, S.; Kumar, A.N.; Shanthi Sravan, J.; Chatterjee, S.; Sarkar, O.; Mohan, S.V. Food waste biorefinery: Sustainable strategy for circular bioeconomy. Bioresour. Technol. 2018, 248, 2–12. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y.; Ge, X.; Yang, L.; Li, Y. Anaerobic digestion of food waste—Challenges and opportunities. Bioresour. Technol. 2018, 247, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, P.C. Ash content of forages. In Focus on Forage; University of Wisconsin-Madison: Madison, WI, USA, 2005; Volume 7, pp. 7–8. [Google Scholar]
- Clapp, C.E.; Stark, S.A.; Clay, D.E.; Larson, W.E. Sewage sludge organic matter and soil properties. In The Role of Organic Matter in Modern Agriculture; Springer Science & Business Media: Berlin, Germany, 1986; pp. 209–210. ISBN 902473360X. [Google Scholar]
- Amin, F.R.; Khalid, H.; Zhang, H.; Rahman, S.; Zhang, R.; Liu, G.; Chen, C. Pretreatment methods of lignocellulosic biomass for anaerobic digestion. AMB Express 2017, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.S.A.; Inoue, H.; Endo, T.; Yano, S.; Bon, E.P.S. Milling pretreatment of sugarcane bagasse and straw for enzy-matic hydrolysis and ethanol fermentation. Bioresour. Technol. 2010, 101, 7402–7409. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.K.; Xu, C.; Qin, W. Biological pretreatment of lignocellulosic biomass for biofuels and bioproducts: An overview. Waste Biomass Valorization 2019, 10, 235–251. [Google Scholar] [CrossRef]
- Lazuka, A.; Auer, L.; O’Donohue, M.; Hernandez-Raquet, G. Anaerobic lignocellulolytic microbial consortium derived from termite gut: Enrichment, lignocellulose degradation and community dynamics. Biotechnol. Biofuels 2018, 11, 284. [Google Scholar] [CrossRef]
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second-generation biofuels: A comprehensive review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Rughoonundun, H.; Mohee, R.; Holtzapple, M.T. Influence of carbon-to-nitrogen ratio on the mixed-acid fermentation of wastewater sludge and pretreated bagasse. Bioresour. Technol. 2012, 112, 91–97. [Google Scholar] [CrossRef]
- Agler, M.T.; Werner, J.J.; Iten, L.B.; Dekker, A.; Cotta, M.A.; Dien, B.S.; Angenent, L.T. Shaping reactor microbiomes to produce the fuel precursor n-butyrate from pretreated cellulosic hydrolysates. Environ. Sci. Technol. 2012, 46, 10229–10238. [Google Scholar] [CrossRef]
- Nachiappan, B.; Fu, Z.; Holtzapple, M.T. Ammonium carboxylate production from sugarcane trash using long-term air-lime pretreatment followed by mixed-culture fermentation. Bioresour. Technol. 2011, 102, 4210–4217. [Google Scholar] [CrossRef]
- Li, X.L.; Peng, Y.Z.; Ren, N.Q.; Li, B.K.; Chai, T.Z.; Zhang, L. Effect of temperature on short-chain fatty acids (SCFAs) ac-cumulation and microbiological transformation in sludge alkaline fermentation with Ca(OH)2 adjustment. Water Res. 2014, 61, 34–45. [Google Scholar] [CrossRef]
- Castilla-Archilla, J.; Papirio, S.; Lens, P.N.L. Two step process for volatile fatty acid production from brewery spent grain: Hydrolysis and direct acidogenic fermentation using anaerobic granular sludge. Process Biochem. 2021, 100, 272–283. [Google Scholar] [CrossRef]
- Domingos, J.M.B.; Martinez, G.A.; Scoma, A.; Fraraccio, S.; Kerckhof, F.M.; Boon, N.; Reis, M.A.M.; Fava, F.; Bertin, L. Effect of operational parameters in the continuous anaerobic fermentation of cheese whey on titers, yields, productivities, and microbial community structures. ACS Sustain. Chem. Eng. 2017, 5, 1400–1407. [Google Scholar] [CrossRef]
- Woo, H.C.; Kim, Y.H. Eco-efficient recovery of bio-based volatile C2-6 fatty acids. Biotechnol. Biofuels 2019, 12, 92. [Google Scholar] [CrossRef]
- Naseeruddin, S.; Yadav, K.S.; Sateesh, L.; Manikyam, A.; Desai, S.; Rao, L.V. Selection of the best chemical pretreatment for lignocellulosic substrate Prosopis juliflora. Bioresour. Technol. 2013, 136, 542–549. [Google Scholar] [CrossRef]
- Shrestha, S.; Fonoll, X.; Khanal, S.K.; Raskin, L. Biological strategies for enhanced hydrolysis of lignocellulosic biomass during anaerobic digestion: Current status and future perspectives. Bioresour. Technol. 2017, 245, 1245–1257. [Google Scholar] [CrossRef]
- Zhang, Y.; McKechnie, J.; MacLean, H.L.; Spatari, S. Environmental life cycle assessment of lignocellulose-to-bioalcohol production. In Bioalcohol Production: Biochemical Conversion of Lignocellulosic Biomass; Waldron, K., Ed.; Woodhead Publishing: Oxford, UK, 2010; pp. 365–390. ISBN 9781845695101. [Google Scholar]
- Jiang, J.; Zhang, Y.; Li, K.; Wang, Q.; Gong, C.; Li, M. Volatile fatty acids production from food waste: Effects of pH, temperature, and organic loading rate. Bioresour. Technol. 2013, 143, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, E.; Chwialkowska, J.; Stodolny, M.; Oleskowicz-Popiel, P. Effect of pH and retention time on volatile fatty acids production during mixed culture fermentation. Bioresour. Technol. 2015, 190, 274–280. [Google Scholar] [CrossRef]
- Jankowska, E.; Duber, A.; Chwialkowska, J.; Stodolny, M.; Oleskowicz-Popiel, P. Conversion of organic waste into volatile fatty acids—The influence of process operating parameters. Chem. Eng. J. 2018, 345, 395–403. [Google Scholar] [CrossRef]
- Zhou, M.; Yan, B.; Wong, J.W.C.; Zhang, Y. Enhanced volatile fatty acids production from anaerobic fermentation of food waste: A mini-review focusing on acidogenic metabolic pathways. Bioresour. Technol. 2018, 248, 68–78. [Google Scholar] [CrossRef]
- Cope, J.L.; Hammett, A.J.M.; Kolomiets, E.A.; Forrest, A.K.; Golub, K.W.; Hollister, E.B.; DeWitt, T.J.; Gentry, T.J.; Holtzapple, M.T.; Wilkinson, H.H. Evaluating the performance of carboxylate platform fermentations across diverse inocula originating as sediments from extreme environments. Bioresour. Technol. 2014, 155, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.O.; Denman, S.E.; Mackie, R.I.; Morrison, M.; Rae, A.L.; Attwood, G.T.; McSweeney, C.S. Opportunities to im-prove fiber degradation in the rumen: Microbiology, ecology, and genomics. FEMS Microbiol. Rev. 2003, 27, 663–693. [Google Scholar] [CrossRef]
- Mouriño, F.; Akkarawongsa, R.; Weimer, P.J. Initial pH as a determinant of cellulose digestion rate by mixed ruminal microorganisms in vitro. J. Dairy Sci. 2001, 84, 848–859. [Google Scholar] [CrossRef]
- Hu, Z.H.; Wang, G.; Yu, H.Q. Anaerobic degradation of cellulose by rumen microorganisms at various pH values. Biochem. Eng. J. 2004, 21, 59–62. [Google Scholar] [CrossRef]
- Khan, M.A.; Ngo, H.H.; Guo, W.; Liu, Y.; Nghiem, L.D.; Chang, S.W.; Nguyen, D.D.; Zhang, S.; Luo, G.; Jia, H. Optimization of hydraulic retention time and organic loading rate for volatile fatty acid production from low strength wastewater in an anaerobic membrane bioreactor. Bioresour. Technol. 2019, 271, 100–108. [Google Scholar] [CrossRef]
- Abdl-Rahman, M.A. In vitro manipulation of rumen fermentation efficiency by fumaric acid—Bentonite coupled addition as an alternative to antibiotics. J. Agric. Sci. 2010, 2, 174–180. [Google Scholar] [CrossRef]
- Nerdahl, M.A.; Weimer, P.J. Redox mediators modify end product distribution in biomass fermentations by mixed ruminal microbes in vitro. AMB Express 2015, 5, 1–8. [Google Scholar] [CrossRef]
- Pagliano, G.; Ventorino, V.; Panico, A.; Pepe, O. Integrated systems for biopolymers and bioenergy production from organic waste and by-products: A review of microbial processes. Biotechnol. Biofuels 2017, 10, 1–24. [Google Scholar] [CrossRef]
- Arslan, D.; Steinbusch, K.J.J.; Diels, L.; Hamelers, H.V.M.; Strik, D.P.B.T.B.; Buisman, C.J.N.; De Wever, H. Selective short-chain carboxylates production: A review of control mechanisms to direct mixed culture fermentations. Crit. Rev. Environ. Sci. Technol. 2016, 46, 592–634. [Google Scholar] [CrossRef]
- Hollister, E.B.; Forrest, A.K.; Wilkinson, H.H.; Ebbole, D.J.; Malfatti, S.A.; Tringe, S.G.; Holtzapple, M.T.; Gentry, T.J. Structure and dynamics of the microbial communities underlying the carboxylate platform for biofuel production. Appl. Microbiol. Biotechnol. 2010, 88, 389–399. [Google Scholar] [CrossRef]
- Lambrecht, J.; Cichocki, N.; Schattenberg, F.; Kleinsteuber, S.; Harms, H.; Müller, S.; Sträuber, H. Key sub-community dy-namics of medium-chain carboxylate production. Microb. Cell Fact. 2019, 18, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zapletalová, M.; Kašparovská, J.; Křížová, L.; Kašparovský, T.; Šerý, O.; Lochman, J. Bacterial community dynamics in a rumen fluid bioreactor during in-vitro cultivation. J. Biotechnol. 2016, 234, 43–49. [Google Scholar] [CrossRef]
- Cavalcante, W.d.A.; Leitão, R.C.; Gehring, T.A.; Angenent, L.T.; Santaella, S.T. Anaerobic fermentation for n-caproic acid production: A review. Process Biochem. 2017, 54, 106–119. [Google Scholar] [CrossRef]
- Knol, W.; Van Der Most, M.M.; De Waart, J. Biogas production by anaerobic digestion of fruit and vegetable waste. A pre-liminary study. J. Sci. Food Agric. 1978, 29, 822–830. [Google Scholar] [CrossRef]
- Spirito, C.M.; Richter, H.; Rabaey, K.; Stams, A.J.M.; Angenent, L.T. Chain elongation in anaerobic reactor microbiomes to recover resources from waste. Curr. Opin. Biotechnol. 2014, 27, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Yarimtepe, C.C.; Oz, N.A.; Ince, O. Volatile fatty acid production dynamics during the acidification of pretreated olive mill wastewater. Bioresour. Technol. 2017, 241, 936–944. [Google Scholar] [CrossRef]
- Gijzen, H.J.; Lubberding, H.J.; Verhagen, F.J.; Zwart, K.B.; Vogels, G.D. Application of rumen microorganisms for an en-hanced anaerobic degradation of solid organic waste materials. Biol. Wastes 1987, 22, 81–95. [Google Scholar] [CrossRef]
- Hungate, R.E.I. Microbial ecology of the rumen. Bacteriol. Rev. 1960, 24, 353–364. [Google Scholar] [CrossRef]
- Wang, Y.; McAllister, T.A. Rumen microbes, enzymes and feed digestion-A review. Asian Australas. J. Anim. Sci. 2002, 15, 1659–1676. [Google Scholar] [CrossRef]
- Dhaese, C. Chain elongation by mixed microbial community: Key parameters for high-value products generation. Master’s Thesis, Faculty of Bioscience Engineering, University Gent, Gent, Belgium, 2015. [Google Scholar]
- Mohammed, R.; Brink, G.E.; Stevenson, D.M.; Anthony, P.; Beauchemin, K.A.; Suen, G.; Weimer, P.J.; Henderson, G. Bac-terial communities in the rumen of Holstein heifers differ when fed orchardgrass as pasture vs. hay. Front. Energy Res. 2014, 5, 689. [Google Scholar] [CrossRef]
- Weimer, P.J. Redundancy, resilience, and host specificity of the ruminal microbiota: Implications for engineering improved ruminal fermentations. Front. Microbiol. 2015, 6, 296. [Google Scholar] [CrossRef]
- Shrivastava, B.; Jain, K.K.; Kumar, R.; Prusty, S.; Kumar, S.; Chakraborty, S.; Chaudhary, H.S.; Puniya, M.; Kuhad, R.C. Rumen Microbiology: From Evolution to Revolution; Puniya, A.K., Singh, R., Eds.; Springer: New Delhi, India, 2015; ISBN 9788132224013. [Google Scholar]
- Ali Shah, F.; Mahmood, Q.; Maroof Shah, M.; Pervez, A.; Ahmad Asad, S. Microbial ecology of anaerobic digesters: The key players of anaerobiosis. Sci. World J. 2014, 2014, 183752. [Google Scholar] [CrossRef]
- Lin, M.; Dai, X.; Weimer, P.J. Shifts in fermentation end products and bacterial community composition in long-term, se-quentially transferred in vitro ruminal enrichment cultures fed switchgrass with and without ethanol as a co-substrate. Bioresour. Technol. 2019, 285, 121324. [Google Scholar] [CrossRef]
- Weimer, P.J.; French, A.D.; Calamari, T.A. Differential fermentation of cellulose allomorphs by ruminal cellulolytic bacteria. Appl. Environ. Microbiol. 1991, 57, 3101–3106. [Google Scholar] [CrossRef]
- Dai, X.; Weimer, P.J.; Dill-McFarland, K.A.; Brandao, V.L.N.; Suen, G.; Faciola, A.P. Camelina seed supplementation at two dietary fat levels change ruminal bacterial community composition in a dual-flow continuous culture system. Front. Microbiol. 2017, 8, 2147. [Google Scholar] [CrossRef]
- Christopherson, M.R.; Suen, G. Nature’s bioreactor: The rumen as a model for biofuel production. Biofuels 2013, 4, 511–521. [Google Scholar] [CrossRef]
- Njokweni, S.G.; Weimer, P.J.; Botes, M.; van Zyl, W.H. Effects of preservation of rumen inoculum on volatile fatty acids production and the community dynamics during batch fermentation of fruit pomace. Bioresour. Technol. 2021, 321, 124518. [Google Scholar] [CrossRef]
- Russell, J.B. The importance of pH in the regulation of ruminal acetate to propionate ratio and methane production in vitro. J. Dairy Sci. 1998, 81, 3222–3230. [Google Scholar] [CrossRef]
- Seshadri, R.; Leahy, S.C.; Attwood, G.T.; Teh, K.H.; Lambie, S.C.; Cookson, A.L.; Eloe-Fadrosh, E.A.; Pavlopoulos, G.A.; Hadjithomas, M.; Varghese, N.J.; et al. Cultivation and sequencing of rumen microbiome members from the Hungate1000 Collection. Nat. Biotechnol. 2018, 36, 359–367. [Google Scholar] [CrossRef]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analysis (Apparatus, Reagents, Procedures, and Some Applications); United States Department of Agriculture: Washington, DC, USA, 1970. [Google Scholar]
- Trujillo, A.I.; Marichal, M.d.J.; Carriquiry, M. Comparison of dry matter and neutral detergent fibre degradation of fibrous feedstuffs as determined with in situ and in vitro gravimetric procedures. Anim. Feed Sci. Technol. 2010, 161, 49–57. [Google Scholar] [CrossRef]
- Njokweni, S.G.; Weimer, P.J.; Botes, M.; Cruywagen, C.W.; van Zyl, W.H. Extraruminal fermentation of citrus, grape and apple pomaces: Assessing the potential to serve as feedstock for production of volatile fatty acids. Waste Biomass Valorization 2021, 12, 3671–3681. [Google Scholar] [CrossRef]
- Leiva, E.; Hall, M.B.; Van Horn, H.H. Performance of dairy cattle fed citrus pulp or corn products as sources of neutral detergent-soluble carbohydrates. J. Dairy Sci. 2000, 83, 2866–2875. [Google Scholar] [CrossRef]
- Tayengwa, T.; Mapiye, C. Citrus and winery wastes: Promising dietary supplements for sustainable ruminant animal nu-trition, health, production, and meat quality. Sustainability 2018, 10, 3718. [Google Scholar] [CrossRef]
- Cherney, D.J.R.; Cherney, J.R.; Davidson, A.H. Characterization of legume and grass residues following in vitro and in sacco ruminal digestion. In Proceedings of the XVIII International Grassland Congress; International Grassland Congress: Winnipeg, MB, Canada; Saskatoon, SK, Canada, 1997; p. 401. [Google Scholar]
- Njokweni, S.G.; Weimer, P.J.; Warburg, L.; Botes, M.; van Zyl, W.H. Valorisation of the invasive species, Prosopis juliflora, using the carboxylate platform to produce volatile fatty acids. Bioresour. Technol. 2019, 288, 121602. [Google Scholar] [CrossRef]
- Weimer, P.J. Why don’t ruminal bacteria digest cellulose faster? J. Dairy Sci. 1996, 79, 1496–1502. [Google Scholar] [CrossRef]
- Russell, J.B.; Muck, R.E.; Weimer, P.J. Quantitative analysis of cellulose degradation and growth of cellulolytic bacteria in the rumen. FEMS Microbiol. Ecol. 2009, 67, 183–197. [Google Scholar] [CrossRef]
- Weimer, P.J. End product yields from the extraruminal fermentation of various polysaccharide, protein and nucleic acid components of biofuels feedstocks. Bioresour. Technol. 2011, 102, 3254–3259. [Google Scholar] [CrossRef]
- Weimer, P.J. Ruminal fermentations to produce liquid and gaseous fuels. In Rumen Microbiology: From Evolution to Revolution; Puniya, A.K., Sing, R., Kamra, D.N., Eds.; Springer: New Delhi, India, 2015; pp. 265–280. ISBN 978-81-322-2400-6. [Google Scholar]
- Pham, V.; Holtzapple, M.; El-halwagi, M.M. Lignocellulose-to-hydrocarbons process using a carboxylate platform. In Inte-Grated Biorefineries, Design, Analysis and Optimization; Taylor & Francis: Boca Raton, FL, USA, 2012; p. 874. ISBN 978-104398-0347-9. [Google Scholar]
- Freguia, S.; Teh, E.H.; Boon, N.; Leung, K.M.; Keller, J.; Rabaey, K. Microbial fuel cells operating on mixed fatty acids. Bio Resour. Technol. 2010, 101, 1233–1238. [Google Scholar] [CrossRef]
- Weimer, P.J.; Nerdahl, M.; Brandl, D.J. Production of medium-chain volatile fatty acids by mixed ruminal microorganisms is enhanced by ethanol in co-culture with Clostridium kluyveri. Bioresour. Technol. 2015, 175, 97–101. [Google Scholar] [CrossRef]
- Kuhry, A.B.; Weimer, P.J. Biological/Electrolytic Conversion of Biomass to Hydrocarbons. U.S. Patent US8518680B2, 30 May 2017. [Google Scholar]
- Russell, J.B. The bacteriocins of ruminal bacteria and their potential as an alternative to antibiotics. J. Mol. Microbiol. Biotechnol. 2002, 4, 347–355. [Google Scholar]
- Sauer, M.; Porro, D.; Mattanovich, D.; Branduardi, P. Microbial production of organic acids: Expanding the markets. Trends Biotechnol. 2008, 26, 100–108. [Google Scholar] [CrossRef]
- Wang, X.; Gong, C.S.; Tsao, G.T. Production of L-malic acid via biocatalysis employing wild-type and respiratory-deficient yeasts. Appl. Biochem. Biotechnol. 1998, 70, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Saayman, M.; Viljoen-Bloom, M. The biochemistry of malic acid metabolism by wine yeasts—A review. S. Afr. J. Enol. Vitic. 2006, 27, 113–122. [Google Scholar] [CrossRef]
- Theron, M.M.; Lues, J.F.R. Organic Acids and Food Preservation; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2011; p. 1763. ISBN 9780429191763. [Google Scholar]
- Souci, S.W.; Fachmann, W.; Kraut, H. Food Composition and Nutrition Tables 1981/82; Wissenschaftliiche Verlagsgesellschaft mbH: Stuttgart, Germany, 2000; Volume 6. [Google Scholar]
- Sánchez-Mata, M.C.; Loera, R.D.C.; Morales, P.; Fernández-Ruiz, V.; Cámara, M.; Marqués, C.D.; Pardo-de-Santayana, M.; Tardío, J. Wild vegetables of the Mediterranean area as valuable sources of bioactive compounds. Genet. Resour. Crop. Evol. 2012, 59, 431–443. [Google Scholar] [CrossRef]
- Guo, F.; Wu, M.; Dai, Z.; Zhang, S.; Zhang, W.; Dong, W.; Zhou, J.; Jiang, M.; Xin, F. Current advances on biological production of fumaric acid. Biochem. Eng. J. 2020, 153, 107397. [Google Scholar] [CrossRef]
- Koutinas, A.A.; Vlysidis, A.; Pleissner, D.; Kopsahelis, N.; Lopez Garcia, I.; Kookos, I.K.; Papanikolaou, S.; Kwan, T.H.; Lin, C.S.K. Valorization of industrial waste and by-product streams via fermentation for the production of chemicals and biopolymers. Chem. Soc. Rev. 2014, 43, 2587. [Google Scholar] [CrossRef] [PubMed]
- Lohbeck, K.; Haferkorn, H.; Fuhrmann, W.; Fedtke, N. Maleic and fumaric acids. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000. [Google Scholar]
- Roa Engel, C.A.; Straathof, A.J.J.; Zijlmans, T.W.; van Gulik, W.; van der Wielen, L.A.M. Fumaric acid production by fer-mentation. Appl. Microbiol. Biotechnol. 2008, 78, 379–389. [Google Scholar] [CrossRef]
- Foster, J.W.; Waksman, S.A. The production of fumaric acid by molds belonging to the genus Rhizopus. J. Am. Chem. Soc. 1939, 61, 127–135. [Google Scholar] [CrossRef]
- Gangl, I.C.; Weigand, W.A.; Keller, F.A. Economic comparison of calcium fumarate and sodium fumarate production by Rhizopus arrhizus. Appl. Biochem. Biotechnol. 1990, 24–25, 663–677. [Google Scholar] [CrossRef]
- Kenealy, W.; Zaady, E.; du Preez, J.C.; Stieglitz, B.; Goldberg, I. Biochemical aspects of fumaric acid accumulation by Rhi-zopus arrhizus. Appl. Environ. Microbiol. 1986, 52, 128–133. [Google Scholar] [CrossRef]
- Romano, A.H.; Bright, M.M.; Scott, W.E. Mechanism of fumaric acid accumulation in Rhizopus nigricans. J. Bacteriol. 1967, 93, 600–604. [Google Scholar] [CrossRef]
- Rhodes, R.A.; Lagoda, A.A.; Misenheimer, T.J.; Smith, M.L.; Anderson, R.F.; Jackson, R.W. Production of fumaric acid in 20-lter fermentors. Appl. Environ. Microbiol. 1962, 10, 9–15. [Google Scholar] [CrossRef]
- Riscaldati, E.; Moresi, M.; Federici, F.; Petruccioli, M. Direct ammonium fumarate production by Rhizopus arrhizus under phosphorous limitation. Biotechnol. Lett. 2000, 22, 1043–1047. [Google Scholar] [CrossRef]
- Xu, Q.; He, S.; Jiang, L.; Li, S.; Wen, J.; Guan, R.; Huang, H. Extractive fermentation for fumaric acid production by Rhizopus oryzae. Sep. Sci. Technol. 2017, 52, 1512–1520. [Google Scholar] [CrossRef]
- Zhou, Y.; Du, J.; Tsao, G.T. Mycelial pellet formation by Rhizopus oryzae ATCC 20344. Appl. Biochem. Biotechnol. 2000, 84, 779–789. [Google Scholar] [CrossRef]
- Cao, N.; Du, J.; Gong, C.S.; Tsao, G.T. Simultaneous production and recovery of fumaric acid from immobilized Rhizopus oryzae with a rotary biofilm contactor and an adsorption column. Appl. Environ. Microbiol. 1996, 62, 2926–2931. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, H.U.; Chae, T.U.; Cho, J.S.; Kim, J.W.; Shin, J.H.; Kim, D.I.; Ko, Y.-S.; Jang, W.D.; Jang, Y.-S. A comprehensive metabolic map for production of bio-based chemicals. Nat. Catal. 2019, 2, 18–33. [Google Scholar] [CrossRef]
- Wang, G.; Huang, D.; Li, Y.; Wen, J.; Jia, X. A metabolic-based approach to improve xylose utilization for fumaric acid production from acid pretreated wheat bran by Rhizopus oryzae. Bioresour. Technol. 2015, 180, 119–127. [Google Scholar] [CrossRef]
- Liao, W.; Liu, Y.; Frear, C.; Chen, S. A new approach of pellet formation of a filamentous fungus—Rhizopus oryzae. Bioresour. Technol. 2007, 98, 3415–3423. [Google Scholar] [CrossRef]
- Huang, L.; Wei, P.; Zang, R.; Xu, Z.; Cen, P. High-throughput screening of high-yield colonies of Rhizopus oryzae for en-hanced production of fumaric acid. Ann. Microbiol. 2010, 60, 287–292. [Google Scholar] [CrossRef]
- Mondala, A.H. Direct fungal fermentation of lignocellulosic biomass into itaconic, fumaric, and malic acids: Current and future prospects. J. Ind. Microbiol. Biotechnol. 2015, 42, 487–506. [Google Scholar] [CrossRef]
- Tamakawa, H.; Ikushima, S.; Yoshida, S. Efficient production of l-lactic acid from xylose by a recombinant Candida utilis strain. J. Biosci. Bioeng. 2012, 113, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Chen, A.; Zhang, B.; Kong, P.; Liu, C.; Zhao, J. Rumen degradability and post-ruminal digestion of dry matter, nitrogen and amino acids of three protein supplements. Asian Australas. J. Anim. Sci. 2015, 28, 485–493. [Google Scholar] [CrossRef][Green Version]
- Yan, D.; Wang, C.; Zhou, J.; Liu, Y.; Yang, M.; Xing, J. Construction of reductive pathway in Saccharomyces cerevisiae for effective succinic acid fermentation at low pH value. Bioresour. Technol. 2014, 156, 232–239. [Google Scholar] [CrossRef]
- Ahn, J.H.; Jang, Y.S.; Lee, S.Y. Production of succinic acid by metabolically engineered microorganisms. Curr. Opin. Biotechnol. 2016, 42, 54–66. [Google Scholar] [CrossRef]
- Cok, B.; Tsiropoulos, I.; Roes, A.L.; Patel, M.K. Succinic acid production derived from carbohydrates: An energy and greenhouse gas assessment of a platform chemical toward a bio-based economy. Biofuels Bioprod. Biorefining 2014, 8, 16–29. [Google Scholar] [CrossRef]
- Kidwell, H. Bio-succinic Acid to Go Commercial. Available online: http://www.biopharma-reporter.com/Downstream-Processing/Bio-succinic-acid-to-go-commercial (accessed on 23 June 2021).
- Jansen, M.L.A.; van Gulik, W.M. Towards large scale fermentative production of succinic acid. Curr. Opin. Biotechnol. 2014, 30, 190–197. [Google Scholar] [CrossRef]
- Beauprez, J.J.; De Mey, M.; Soetaert, W.K. Microbial succinic acid production: Natural versus metabolic engineered producers. Process. Biochem. 2010, 45, 1103–1114. [Google Scholar] [CrossRef]
- Dai, Z.; Guo, F.; Zhang, S.; Zhang, W.; Yang, Q.; Dong, W.; Jiang, M.; Ma, J.; Xin, F. Bio-based succinic acid: An overview of strain development, substrate utilization, and downstream purification. Biofuels Bioprod. Biorefining 2020, 14, 965–985. [Google Scholar] [CrossRef]
- Finley, K.R.; Huryta, J.M.; Mastel, B.M.; McMullin, T.W.; Poynter, G.M.; Rush, B.J.; Watts, K.T.; Fosmer, A.M.; McIntosh, V.L.; Brady, K.M. Compositions and Methods for Succinate Production. U.S. Patent US20130302866A1, 14 November 2013. [Google Scholar]
- Raab, A.M.; Gebhardt, G.; Bolotina, N.; Weuster-Botz, D.; Lang, C. Metabolic engineering of Saccharomyces cerevisiae for the biotechnological production of succinic acid. Metab. Eng. 2010, 12, 518–525. [Google Scholar] [CrossRef]
- Rush, B.J.; Fosmer, A.M. Methods for Succinate Production. C. krusei Mutant Strain was Developed by Bioamber for the Enhanced Production of SA under Low pH Condition. U.S. Patent US20140363862A1, 11 December 2014. [Google Scholar]
- Yuzbashev, T.V.; Yuzbasheva, E.Y.; Sobolevskaya, T.I.; Laptev, I.A.; Vybornaya, T.V.; Larina, A.S.; Matsui, K.; Fukui, K.; Sineoky, S.P. Production of succinic acid at low pH by a recombinant strain of the aerobic yeast Yarrowia lipolytica. Biotechnol. Bioeng. 2010, 107, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, X.F.; Gao, S.; Wang, H.M.; Lin, C.S.K. High efficiency succinic acid production from glycerol via in situ fibrous bed bioreactor with an engineered Yarrowia lipolytica. Bioresour. Technol. 2017, 225, 9–16. [Google Scholar] [CrossRef]
- Cui, Z.; Gao, C.; Li, J.; Hou, J.; Lin, C.S.K.; Qi, Q. Engineering of unconventional yeast Yarrowia lipolytica for efficient suc-cinic acid production from glycerol at low pH. Metab. Eng. 2017, 42, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Castillo Martinez, F.A.; Balciunas, E.M.; Salgado, J.M.; Domínguez González, J.M.; Converti, A.; Oliveira, R.P.d.S. Lactic acid properties, applications and production: A review. Trends Food Sci. Technol. 2013, 30, 70–83. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, P.; Liu, L. Modular optimization of multi-gene pathways for fumarate production. Metab. Eng. 2016, 33, 76–85. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Tong, T.; Liu, L. Spatial modulation and cofactor engineering of key pathway enzymes for fumarate production in Candida glabrata. Biotechnol. Bioeng. 2019, 116, 622–630. [Google Scholar] [CrossRef]
- Chen, X.; Dong, X.; Wang, Y.; Zhao, Z.; Liu, L. Mitochondrial engineering of the TCA cycle for fumarate production. Metab. Eng. 2015, 31, 62–73. [Google Scholar] [CrossRef]
- Chen, X.; Wu, J.; Song, W.; Zhang, L.; Wang, H.; Liu, L. Fumaric acid production by Torulopsis glabrata: Engineering the urea cycle and the purine nucleotide cycle. Biotechnol. Bioeng. 2015, 112, 156–167. [Google Scholar] [CrossRef]
- Xu, G.; Chen, X.; Liu, L.; Jiang, L. Fumaric acid production in Saccharomyces cerevisiae by simultaneous use of oxidative and reductive routes. Bioresour. Technol. 2013, 148, 91–96. [Google Scholar] [CrossRef]
- Wei, L.; Liu, J.; Qi, H.; Wen, J. Engineering Scheffersomyces stipitis for fumaric acid production from xylose. Bioresour. Technol. 2015, 187, 246–254. [Google Scholar] [CrossRef]
- Xu, G.; Liu, L.; Chen, J. Reconstruction of cytosolic fumaric acid biosynthetic pathways in Saccharomyces cerevisiae. Microb. Cell Fact. 2012, 11, 1–10. [Google Scholar] [CrossRef]
- Rywińska, A.; Rymowicz, W.; Marcinkiewicz, M. Valorization of raw glycerol for citric acid production by Yarrowia lipolytica yeast. Electron. J. Biotechnol. 2010, 13, 1–9. [Google Scholar] [CrossRef]
- Good, D.W.; Droniuk, R.; Lawford, G.R.; Fein, J.E. Isolation and characterization of a Saccharomycopsis lipolytica mutant showing increased production of citric acid from canola oil. Can. J. Microbiol. 1985, 31, 436–440. [Google Scholar] [CrossRef]
- Morgunov, I.; Kamzolova, S.; Lunina, J. Citric acid production by Yarrowia lipolytica yeast on different renewable raw materials. Fermentation 2018, 4, 36. [Google Scholar] [CrossRef]
- Rymowicz, W.; Rywińska, A.; Żarowska, B.; Juszczyk, P. Citric acid production from raw glycerol by acetate mutants of Yarrowia lipolytica. Chem. Pap. 2006, 60, 391–394. [Google Scholar] [CrossRef]
- Ishida, N.; Saitoh, S.; Ohnishi, T.; Tokuhiro, K. Metabolic Engineering of Saccharomyces Cerevisiae for Efficient Production of Pure L-(+)-Lactic Acid; McMillan, J.D., Adney, W.S., Mielenz, J.R., Klasson, K.T., Eds.; Humana Press: Totowa, NJ, USA, 2006. [Google Scholar]
- Saitoh, S.; Ishida, N.; Onishi, T.; Tokuhiro, K.; Nagamori, E.; Kitamoto, K.; Takahashi, H. Genetically engineered wine yeast produces a high concentration of L-lactic acid of extremely high optical purity. Appl. Environ. Microbiol. 2005, 71, 2789–2792. [Google Scholar] [CrossRef] [PubMed]
- Ikushima, S.; Fujii, T.; Kobayashi, O.; Yoshida, S.; Yoshida, A. Genetic engineering of Candida utilis yeast for efficient production of L-lactic acid. Biosci. Biotechnol. Biochem. 2009, 73, 1818–1824. [Google Scholar] [CrossRef]
- Pavlovich, S.S.; Mikhajlovic, V.M.; Vladimirovich, J.T.; Aleksandrovich, R.J.; Isaakovna, R.E.; Georgievna, T.N.; Aleksandrovna, V.M.; Mikhajlovna, A.A.; Georgievich, D.V. Method for Microbiological Synthesis of Lactic Acid and Recombinant Strain of Yeast Schizosaccharomyces pombe for Its Realization. R.U. Patent RU000002268304, 20 January 2006. [Google Scholar]
- Kong, X.; Zhang, B.; Hua, Y.; Zhu, Y.; Li, W.; Wang, D.; Hong, J. Efficient L-lactic acid production from corncob residue using metabolically engineered thermo-tolerant yeast. Bioresour. Technol. 2019, 273, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Hause, B.; Rajgarhia, V.; Suominen, P. Methods and Materials for the Production of L-Lactic Acid in Yeast. U.S. Patent 7,534,597, 19 May 2009. [Google Scholar]
- Osawa, F.; Fujii, T.; Nishida, T.; Tada, N.; Ohnishi, T.; Kobayashi, O.; Komeda, T.; Yoshida, S. Efficient production of L-lactic acid by Crabtree-negative yeast Candida boidinii. Yeast 2009, 26, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Sawant, O.; Mahale, S.; Ramchandran, V.; Nagaraj, G.; Bankar, A. Fungal citric acid production using waste materials: A mini-review. J. Microbiol. Biotechnol. Food Sci. 2018, 8, 821–828. [Google Scholar] [CrossRef]
- Gupta, G.K.; De, S.; Franco, A.; Balu, A.; Luque, R. Sustainable biomaterials: Current trends, challenges and applications. Molecules 2015, 21, 48. [Google Scholar] [CrossRef]
- Yin, X.; Shin, H.-D.; Li, J.; Du, G.; Liu, L.; Chen, J. Comparative genomics and transcriptome analysis of Aspergillus niger and metabolic engineering for citrate production. Sci. Rep. 2017, 7, 41040. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, X.; Sun, W.; Xi, X.; Zhao, N.; Huang, Z.; Ying, Z.; Liu, L.; Liu, D.; Niu, H.; et al. Continuous citric acid production in repeated-fed batch fermentation by Aspergillus niger immobilized on a new porous foam. J. Biotechnol. 2018, 276–277, 1–9. [Google Scholar] [CrossRef]
- Papagianni, M. Advances in citric acid fermentation by Aspergillus niger: Biochemical aspects, membrane transport and modeling. Biotechnol. Adv. 2007, 25, 244–263. [Google Scholar] [CrossRef]
- Kappoor, K.K.; Chudhary, K.; Tauro, P.; Reed, G. Citric acid. In Prescott and Dunn’s Industrial Microbiology; MacMillan Publishers Ltd.: London, UK, 1983; pp. 709–747. [Google Scholar]
- Abbas, N.; Safdar, W.; Ali, S.; Choudhry, S.; Ilahi, S. Citric acid production from Aspergillus niger using banana peel. Int. J. Sci. Eng. Res. 2016, 7, 1580–1583. [Google Scholar]
- Alnassar, M.; Tayfour, A.; Afif, R. The study of lactose effect on citric acid production by Aspergillus niger PLA30 in cheese whey. Int. J. Chem. Tech. Res. 2016, 9, 318–322. [Google Scholar]
- Grewal, H.S.; Kalra, K.L. Fungal production of citric acid. Biotechnol. Adv. 1995, 13, 209–234. [Google Scholar] [CrossRef]
- Ikeno, Y.; Masuda, M.; Tanno, K.; Oomori, I.; Takahashi, N. Citric acid production from various raw materials by yeasts. J. Ferment. Technol. 1975, 53, 752–756. [Google Scholar]
- Käppeli, O.; Muller, M.; Fiechter, A. Chemical and structural alterations at the cell surface of Candida tropicalis, induced by hydrocarbon substrate. J. Bacteriol. 1978, 133, 952–958. [Google Scholar] [CrossRef]
- Crolla, A.; Kennedy, K.J. Optimization of citric acid production from Candida lipolytica Y-1095 using n-paraffin. J. Biotechnol. 2001, 89, 27–40. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 2013, 31, 877–902. [Google Scholar] [CrossRef]
- Sauer, M.; Porro, D.; Mattanovich, D.; Branduardi, P. 16 Years research on lactic acid production with yeast—Ready for the market? Biotechnol. Genet. Eng. Rev. 2010, 27, 229–256. [Google Scholar] [CrossRef]
- Porro, D.; Brambilla, L.; Ranzi, B.M.; Martegani, E.; Alberghina, L. Development of metabolically engineered Saccharomyces cerevisiae cells for the production of lactic acid. Biotechnol. Prog. 1995, 11, 294–298. [Google Scholar] [CrossRef]
- Litchfield, J.H. Microbiological production of lactic acid. Adv. Appl. Microbiol. 1996, 42, 45–95. [Google Scholar] [CrossRef]
- John, R.P.; Nampoothiri, K.M.; Pandey, A. Fermentative production of lactic acid from biomass: An overview on process developments and future perspectives. Appl. Microbiol. Biotechnol. 2007, 74, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.L.; Zhang, G.C.; Oh, E.J.; Subramaniam, V.; Adiputra, A.; Subramaniam, V.; Skory, C.D.; Jang, J.Y.; Yu, B.J.; Park, I.; et al. Lactic acid production from cellobiose and xylose by engineered Saccharomyces cerevisiae. Biotechnol. Bioeng. 2016, 113, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, J.J.; Murphy, C.; Mota, F.M.; Pauli, T. Impurity and cost considerations for nutrient supplementation of whey permeate fermentations to produce lactic acid for biodegradable plastics. Int. Dairy J. 2003, 13, 575–580. [Google Scholar] [CrossRef]
- Abbott, D.A.; Zelle, R.M.; Pronk, J.T.; van Maris, A.J.A. Metabolic engineering of Saccharomyces cerevisiae for production of carboxylic acids: Current status and challenges. FEMS Yeast Res. 2009, 9, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Banat, F.; Taher, H. A review on the lactic acid fermentation from low-cost renewable materials: Recent developments and challenges. Environ. Technol. Innov. 2020, 20, 101138. [Google Scholar] [CrossRef]
- Su, J.; Wang, T.; Wang, Y.; Li, Y.Y.; Li, H. The use of lactic acid-producing, malic acid-producing, or malic acid-degrading yeast strains for acidity adjustment in the wine industry. Appl. Microbiol. Biotechnol. 2014, 98, 2395–2413. [Google Scholar] [CrossRef]
- Schümann, C.; Michlmayr, H.; del Hierro, A.M. Malolactic enzyme from Oenococcus oeni: Heterologous expression in Escherichia coli and biochemical characterization. Bioengineered 2013, 4, 147–152. [Google Scholar] [CrossRef]
- Schümann, C.; Michlmayr, H.; Eder, R.; Del Hierro, A.M.; Kulbe, K.D.; Mathiesen, G.; Nguyen, T.-H. Heterologous expression of Oenococcus oeni malolactic enzyme in Lactobacillus plantarum for improved malolactic fermentation. AMB Express 2012, 2, 19. [Google Scholar] [CrossRef]
- Ragsdale, S.W.; Pierce, E. Acetogenesis and the Wood–Ljungdahl pathway of CO2 fixation. Biochim. Biophys. Acta Proteins Proteomics 2008, 1784, 1873–1898. [Google Scholar] [CrossRef] [PubMed]
- Vidra, A.; Németh, Á. Bio-produced acetic acid: A review. Period. Polytech. Chem. Eng. 2018, 63, 245–256. [Google Scholar] [CrossRef]
- Ljungdhal, L.G. The autotrophic pathway of acetate synthesis in acetogenic bacteria. Annu. Rev. Microbiol. 1986, 40, 415–450. [Google Scholar] [CrossRef]
- Karekar, S.C.; Srinivas, K.; Ahring, B.K. Kinetic study on heterotrophic growth of Acetobacterium woodii on lignocellulosic substrates for acetic acid production. Fermentation 2019, 5, 17. [Google Scholar] [CrossRef]
- Steyn, A.; Viljoen-Bloom, M.; van Zyl, W.H. Valorization of apple and grape wastes with malic acid-degrading yeasts. Folia Microbiol. 2021, 66, 341–354. [Google Scholar] [CrossRef]
- Parmar, I.; Rupasinghe, H.P.V. Bio-conversion of apple pomace into ethanol and acetic acid: Enzymatic hydrolysis and fermentation. Bioresour. Technol. 2013, 130, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Vashisht, A.; Thakur, K.; Kauldhar, B.S.; Kumar, V.; Yadav, S.K. Waste valorization: Identification of an ethanol tolerant bacterium Acetobacter pasteurianus SKYAA25 for acetic acid production from apple pomace. Sci. Total Environ. 2019, 690, 956–964. [Google Scholar] [CrossRef]
- Wang, Q.-M.; Liu, W.-Q.; Liti, G.; Wang, S.-A.; Bai, F.-Y. Surprisingly diverged populations of Saccharomyces cerevisiae in natural environments remote from human activity. Mol. Ecol. 2012, 21, 5404–5417. [Google Scholar] [CrossRef] [PubMed]
- Liti, G.; Carter, D.M.; Moses, A.M.; Warringer, J.; Parts, L.; James, S.A.; Davey, R.P.; Roberts, I.N.; Burt, A.; Koufopanou, V.; et al. Population genomics of domestic and wild yeasts. Nature 2009, 458, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Steensels, J.; Snoek, T.; Meersman, E.; Nicolino, M.P.; Voordeckers, K.; Verstrepen, K.J. Improving industrial yeast strains: Exploiting natural and artificial diversity. FEMS Microbiol. Rev. 2014, 38, 947–995. [Google Scholar] [CrossRef]
- Panda, S.K.; Mishra, S.S.; Kayitesi, E.; Ray, R.C. Microbial-processing of fruit and vegetable wastes for production of vital enzymes and organic acids: Biotechnology and scopes. Environ. Res. 2016, 146, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Zelle, R.M.; de Hulster, E.; van Winden, W.A.; de Waard, P.; Dijkema, C.; Winkler, A.A.; Geertman, J.A.; van Dijken, J.P.; Pronk, J.T.; van Maris, A.J.A. Malic acid production by Saccharomyces cerevisiae: Engineering of pyruvate carboxylation, oxaloacetate reduction, and malate export. Appl. Environ. Microbiol. 2008, 74, 2766–2777. [Google Scholar] [CrossRef] [PubMed]
- Hermann, B.G.; Blok, K.; Patel, M.K. Producing bio-based bulk chemicals using industrial biotechnology saves energy and combats climate change. Environ. Sci. Technol. 2007, 41, 7915–7921. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Sanders, J.P.M. Toward concise metrics for the production of chemicals from renewable biomass. Catal. Today 2015, 239, 3–6. [Google Scholar] [CrossRef]
- Ilica, R.A.; Kloetzer, L.; Galaction, A.I.; Caşcaval, D. Fumaric acid: Production and separation. Biotechnol. Lett. 2019, 41, 47–57. [Google Scholar] [CrossRef]
- Pines, O.; Even-Ram, S.; Elnathan, N.; Battat, E.; Aharonov, O.; Gibson, D.; Goldberg, I. The cytosolic pathway of L-malic acid synthesis in Saccharomyces cerevisiae: The role of fumarase. Appl. Microbiol. Biotechnol. 1996, 46, 393–399. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Kaur, S.; Brar, S.K. Perspective of apple processing wastes as low-cost substrates for bioproduction of high value products: A review. Renew. Sustain. Energy Rev. 2013, 27, 789–805. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Brar, S.K.; Verma, M.; Tyagi, R.D. Recent advances in citric acid bio-production and recovery. Food Bioprocess. Technol. 2011, 4, 505–529. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Oberoi, H.S.; Kaur, S.; Bansal, S.; Brar, S.K. Value-addition of agricultural wastes for augmented cellulase and xylanase production through solid-state tray fermentation employing mixed-culture of fungi. Ind. Crops Prod. 2011, 34, 1160–1167. [Google Scholar] [CrossRef]
- Nizami, A.S.; Rehan, M.; Waqas, M.; Naqvi, M.; Ouda, O.K.M.; Shahzad, K.; Miandad, R.; Khan, M.Z.; Syamsiro, M.; Ismail, I.M.I.; et al. Waste biorefineries: Enabling circular economies in developing countries. Bioresour. Technol. 2017, 241, 1101–1117. [Google Scholar] [CrossRef]
- Li, Z.; Liu, N.; Cao, Y.; Jin, C.; Li, F.; Cai, C.; Yao, J. Effects of fumaric acid supplementation on methane production and rumen fermentation in goats fed diets varying in forage and concentrate particle size. J. Anim. Sci. Biotechnol. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Swart, R.M.; Le Roux, F.; Naude, A.; De Jongh, N.W.; Nicol, W. Fumarate production with Rhizopus oryzae: Utilising the Crabtree effect to minimise ethanol by-product formation. Biotechnol. Biofuels 2020, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, S.; Wasewar, K.L.; Ak, N.; Uslu, H. Reactive extraction as an intensifying approach for the recovery of organic acids from aqueous solution: A comprehensive review on experimental and theoretical studies. J. Chem. Eng. Data 2021, 66, 1557–1573. [Google Scholar] [CrossRef]
| Fruit Crop | World (MMT) | South Africa (MMT) |
|---|---|---|
| Citrus | 108.04 | 2.58 |
| Grapes | 77.14 | 1.99 |
| Apples | 87.24 | 0.89 |
| Bananas | 11.68 | 0.41 |
| Pears | 23.92 | 0.41 |
| Peaches and nectarines | 25.74 | 0.14 |
| Pineapples | 28.18 | 0.11 |
| Mangoes and guavas | 55.85 | 0.11 |
| Watermelons and melons | 12.79 | 0.09 |
| Plums | 12.60 | 0.06 |
| Total of all fruits | 742.83 | 7.06 |
| Organic Acid | Global Market Size and CAGR * | Reference |
|---|---|---|
| Citric acid | USD 3.6 billion by 2020; CAGR of 5.5% | [35] |
| Fumaric acid | USD 660.9 million by 2020; CAGR of 6.1% | [36] |
| Succinic acid | USD 198.5 million in 2020; CAGR of 9.2% | [37] |
| Lactic acid | USD 2.7 billion in 2020, CAGR of 8.0% | [37] |
| Butyric acid | USD 175 million in 2020; CAGR of 13.2% | [38] |
| Propionic acid | USD 1.53 billion in 2020; CAGR of 2.7% | [37] |
| Acetic acid | USD 9.3 billion in 2020; CAGR of 5.2% | [37] |
| Valeric acid | USD 15.06 billion in 2020; CAGR of 5.3%. | [39] |
| Caproic acid | USD 38 million in 2020; CAGR of 2.9% | [40] |
| Feedstock | Pretreatment | Inoculum | Temp. (°C) | Peak VFAs (g/L) | Fermentation Period (Days) | Initial pH | Reference |
|---|---|---|---|---|---|---|---|
| Agricultural wastes | |||||||
| Bagasse (~19% lignin) | Ca(OH)2 at 50 °C for 8 weeks | Adapted marine wastewater | 55 | 5.63 | 40 | 7 | [68] |
| Corn Fiber (~13% lignin) | Dilute H2SO4 at 160 °C for 20 min | Reactor Microbes | 55 | 11.1 | 419 | 5.5 | [69] |
| Wheat Straw (~18% lignin) | Autoclaved at 120 °C for 20 min | Termite gut (N. ephratae) | 35 | 6.54 (190 mCmol) | 11 | 6.15 | [66] |
| Sugarcane trash and 20% chicken manure | Air-lime pretreatment at 50 °C for 4–8 weeks | Marine wastewater | 55 | 29.9 | 20 | 7 | [70] |
| Municipal and Industrial wastes | |||||||
| Mixed Sludge | - | Adapted marine wastewater | 55 | 10.67 | 36 | 7 | [68] |
| Waste activated sludge | - | Reactor microbes | 15–55 | 0.9–1.77 | 48 | 10 | [71] |
| Brewery wastes (spent grain) (~16% lignin) | H2SO4 at 121 °C for 20 min | Anaerobic granular sludge | 37 | 10 | 3 | 7 | [72] |
| Kitchen Waste (~14% lignin) | Liquid stream treatment | Reactor microbes | 35 | 36 | 32 | 6 | [73] |
| Other sources | |||||||
| Microalgae (Brown alginate neutralized with CaCO2, filtered) | 3% H2SO4 at 120 °C for 250 min | Municipal wastewater microbes | 35 | 9.8 | 15.5 | 7 | [74] |
| Parameter | Optimal VFA Production Conditions |
|---|---|
| Temperature | 20–40 °C |
| pH | 5–11 |
| Retention time | 0–20 days |
| Organic loading rate | 5–11 gTS/L x d |
| Inoculum concentration | 15–25% v/v |
| Feedstock | Period (h) | NDFd % | Reference | |
|---|---|---|---|---|
| Brewers’ grains | - | 96 | 37 | [114] |
| Alfalfa hay | - | 96 | 45 | [114] |
| Citrus Pulp | - | 48 | 76 | [115,116] |
| Apple pomace | - | 96 | 75 | [115] |
| Grape pomace | - | 96 | 55 | [115,117] |
| Ryegrass | - | 96 | 79 | [114] |
| Oat | - | 96 | 80 | [114] |
| White clover | - | 96 | 77 | [114] |
| Reed cannary grass | - | 48 | 58 | [118] |
| Prosopis juliflora | Leaves | 96 | 36 | [119] |
| Stems | 96 | 31 | [119] | |
| Branches | 96 | 20 | [119] |
| Microbial Strain | Genetic Modifications | Substrate; Culturing Method | Titer (g/L) | Yield (g/g) | Reference |
|---|---|---|---|---|---|
| Fumaric acid | |||||
| S. cerevisiae TGFA091-16 | Expression of RoMDH-SDH1, RoPYC-KGD2-SUCLG2 and SFC1-SpMAE1; Deletion of thi2, fum1, ura3, leu2, trp1 and his3 | Glucose; shake flasks | 33.1 | 0.33 | [169] |
| C. glabrata T.G-4G-S(1:1:2)-P(M)-F(H) | Expression of ADB1-RoPYC-AsPCK-SpMAE1 and ADB2-RoMDH-ScFDH1-ADB3-RoFUM; Deletion of ura3 and arg8; Scaffold (1:1:2) | Glucose; batch fermentation | 21.6 | 0.22 | [170] |
| C. glabrata KS(H)-S(M)–A-2 S | Expression of kgd2, SUCLG2, sdh1, Spmae1, sfc1 and ASL; Deletion of ura3 and arg8 | Glucose; shake flasks | 15.8 | 0.15 | [171] |
| T. glabrata SpMAE1 | Expression of ASL, ADSL and Spmae1; Deletion of ura3 and arg8 | Glucose; shake flasks | 8.8 | 0.15 | [172] |
| S. cerevisiae FMME004-6 | Expression of Ropyc, Romdh and Rofum1; Deletion of thi2 and fum1 | Glucose; shake flasks | 5.6 | 0.11 | [173] |
| S. stipitis PSYPMFfS | Expression of Ymae1; Deletion of ura3, leu2, Psfum1 and Psfum2 | Xylose; shake flasks | 4.7 | 0.10 | [174] |
| S. cerevisiae FMME-001 | Expression of Romdh, Rofum1 and pyc2 | Glucose; shake flasks | 3.2 | 0.05 | [175] |
| Succinic acid | |||||
| S. cerevisiae PMCFfg | Expression of pyc2, mdh3, fumC, frdS1; deletion of his3, fum1, gpd1, pdc1, pdc5 and pdc6 | Glucose; batch fermentation | 13.0 | 0.13 | [155] |
| S. cerevisiae AH22ura3 | Deletion of sdh1, sdh2, idh1 and idp1 | Glucose; anaerobic batch fermentation | 3.6 | 0.07 | [163] |
| P. kudriavzevii 13 723 | Expression of pyc1, fum1, mae, mdh and frd1; deletion of ura and pdc | Glucose | 48.2 | 0.45 | [164] |
| Y. lipolytica Y-3314 | Deletion of sdh1, sdh2 and suc2 | Glycerol; aerobic batch fermentation | 45.4 | 0.36 | [165] |
| P. kudriavzevii 13 171 | Expression of pyc1, fum1, mdh and frd1; deletion of cyb2a | Glucose | 23.0 | n/a | [162] |
| Y. lipolytica PGC01003 | Deletion of sdh5 | Glycerol; fed-batch fermentation | 198.2 | n/a | [166] |
| Y. lipolytica Y-3314 | Expression of pck, scs2; deletion of ach | Glycerol; fed-batch fermentation | 110.7 | 0.53 | [167] |
| Citric acid | |||||
| Y. lipolytica Wratislavia 1.31 | Acetate-negative mutant was obtained after wild strain Y. lipolytica A-101 was exposed to UV irradiation | Crude glycerol (86% wt/wt); fed-batch fermentation | 155.20 | 0.55 | [176] |
| S. lipolytica NTG9 | A citrate nonutilizing strain (NTG9) was obtained after mutagenesis of S. lipolytica ATCC 20228 with nitrosoguanidine | Canola oil; NBS MultiGen fermentor | 152.30 | 113.4 | [177] |
| Y. lipolytica NG40/UV5 | Mutagenesis with UV irradiation and N-methyl-NT-nitro-N-nitrosoguanidine | Rapeseed oil; 10-L ANKUM-2M fermenter | 140.0 | 1.50 | [178] |
| Y. lipolytica 1.31 | Acetate-negative mutant was obtained after mutagenesis | Glycerol; stirred tank bioreactor | 124.5 | 0.62 | [179] |
| Lactic acid | |||||
| S. cerevisiae strain | Bos Taurus L-LDH Integrated (6 copies) | Cane juice-based media; microaerobic batch fermentation | 122 | n/a | [180,181] |
| C. utilis Cupdc1 Δ 4-LDH2 | Bos Taurus L-LDH (optimized) Integrated (2 copies) | Glucose, shake flasks | 103.3 | 0.95 | [182] |
| S. pombe VKPM Y-3127 | S. pombe VKPM Y-285 transformed with R. oryzae IdhA gene | Glucose | 80–100 | n/a | [183] |
| K. marxianus YKX071 | YKX056, pKX055, PfLDH, ΔKmDLD1, BmLDH, ScJEN1, KmPFK | Corncob residue; fed batch fermentation | 103 | n/a | [184] |
| K. marxianus CD607 | L. helveticus L-LDH Integrated into PDC1 locus | Glucose; shake flasks | 94–99 | 0.9–0.98 | [185] |
| C. boidinii KY2199 | Disruption of the PDC1 gene with bovine L-lactate dehydrogenase-encoding gene | Glucose; aerobic batch fermentation | 85.9 | 1.01 | [186] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Njokweni, S.G.; Steyn, A.; Botes, M.; Viljoen-Bloom, M.; van Zyl, W.H. Potential Valorization of Organic Waste Streams to Valuable Organic Acids through Microbial Conversion: A South African Case Study. Catalysts 2021, 11, 964. https://doi.org/10.3390/catal11080964
Njokweni SG, Steyn A, Botes M, Viljoen-Bloom M, van Zyl WH. Potential Valorization of Organic Waste Streams to Valuable Organic Acids through Microbial Conversion: A South African Case Study. Catalysts. 2021; 11(8):964. https://doi.org/10.3390/catal11080964
Chicago/Turabian StyleNjokweni, Sesethu Gift, Annica Steyn, Marelize Botes, Marinda Viljoen-Bloom, and Willem Heber van Zyl. 2021. "Potential Valorization of Organic Waste Streams to Valuable Organic Acids through Microbial Conversion: A South African Case Study" Catalysts 11, no. 8: 964. https://doi.org/10.3390/catal11080964
APA StyleNjokweni, S. G., Steyn, A., Botes, M., Viljoen-Bloom, M., & van Zyl, W. H. (2021). Potential Valorization of Organic Waste Streams to Valuable Organic Acids through Microbial Conversion: A South African Case Study. Catalysts, 11(8), 964. https://doi.org/10.3390/catal11080964






