Degradation Kinetics and Mechanism of Polychloromethanes Reduction at Co-MoS2/Graphite Felt Electrode
Abstract
:1. Introduction
2. Results and Discussion
2.1. Material Characterization
2.2. Electrode Cyclic Voltammetric Analysis
2.3. Influence of Operational Parameters
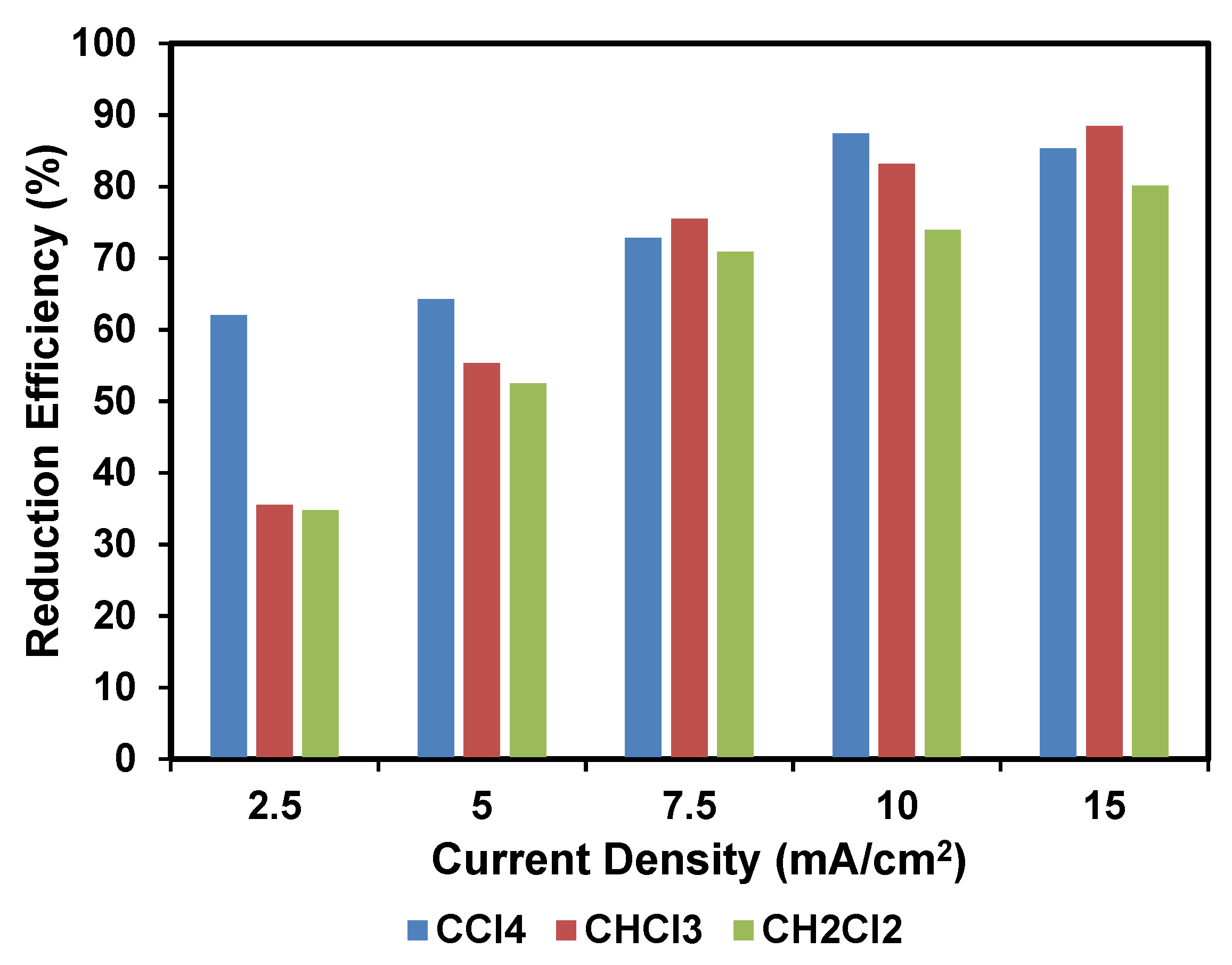
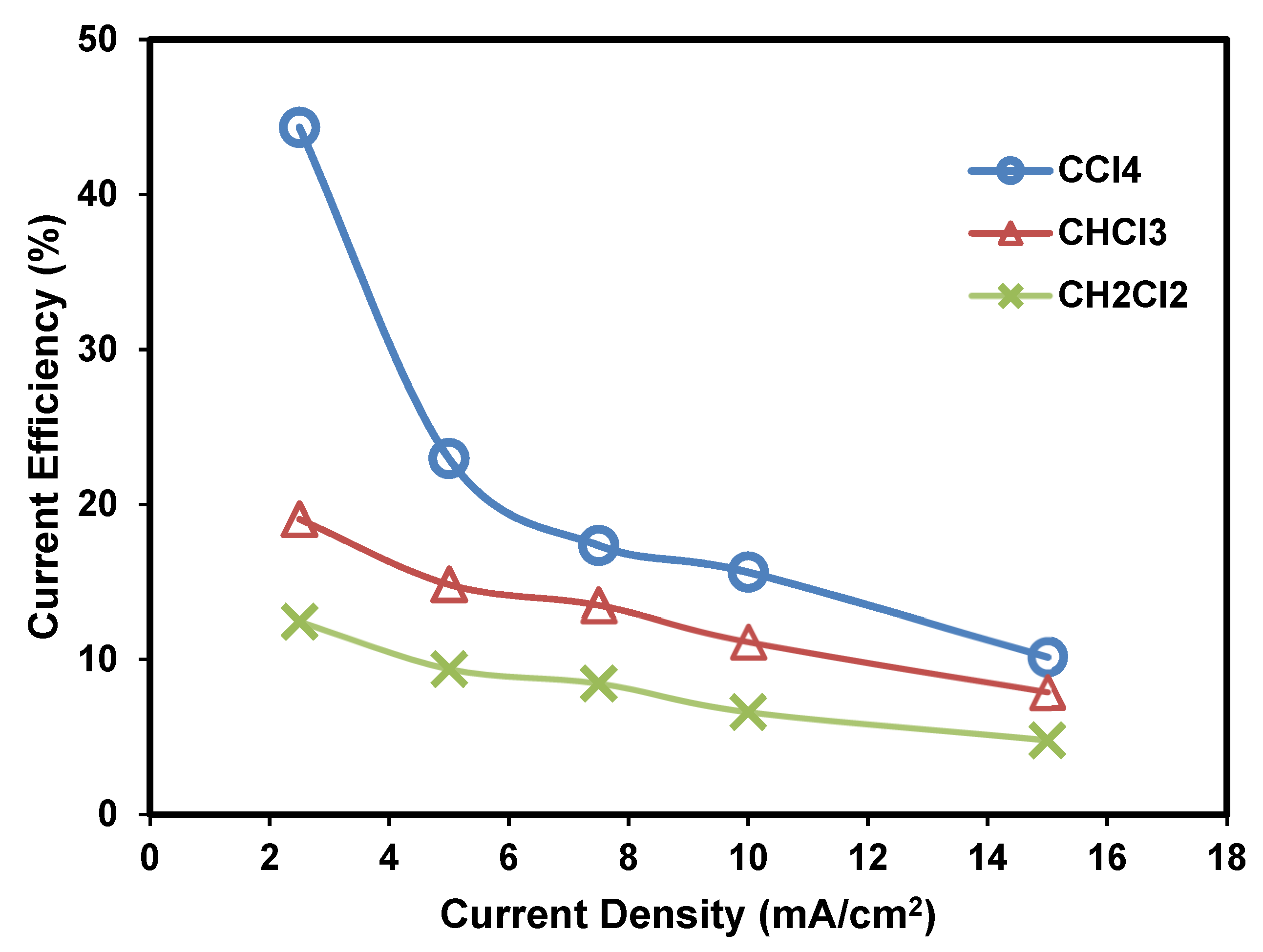
2.3.1. Effect of Applied Current Density
2.3.2. Effect of Solution pH
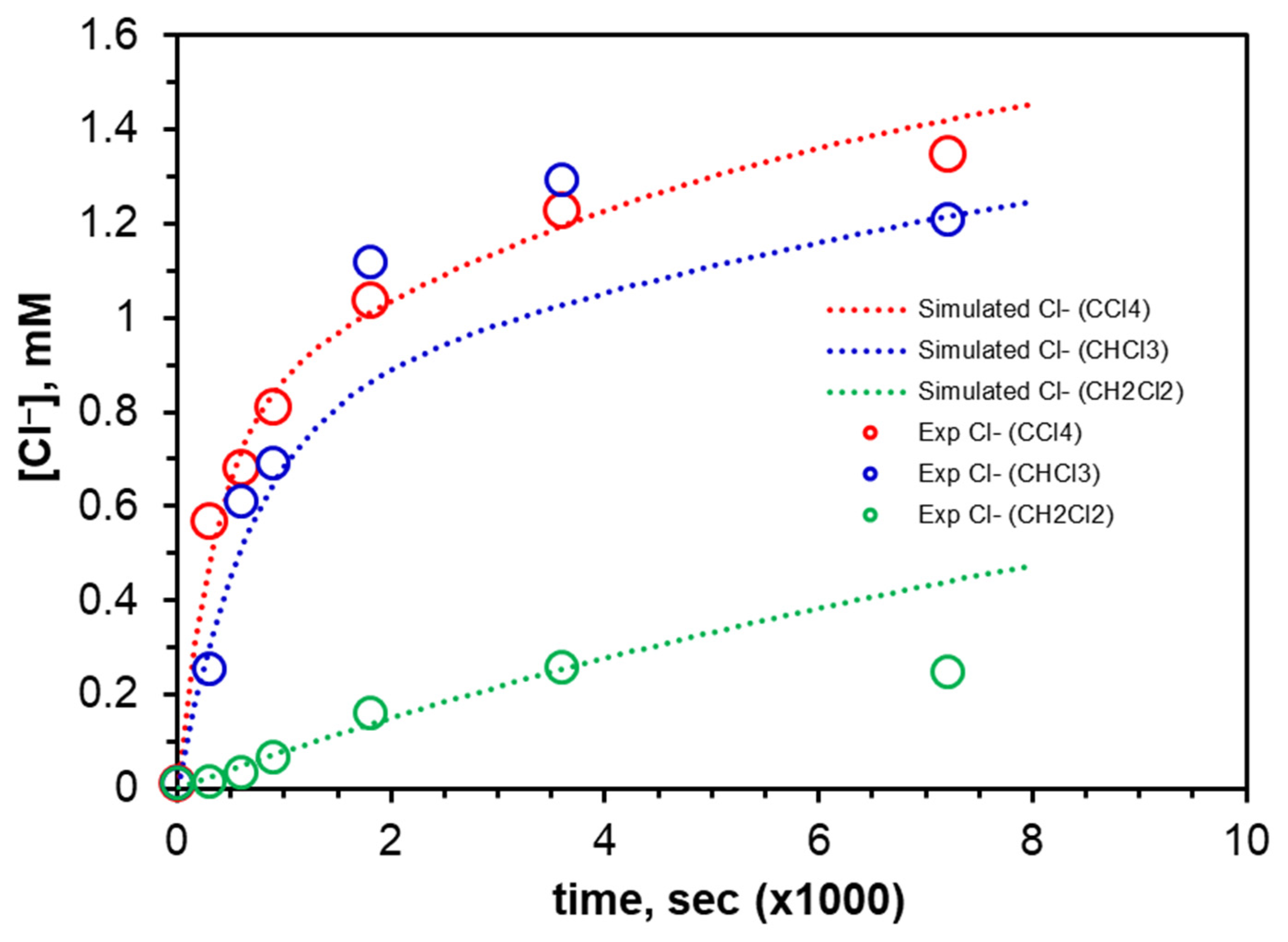
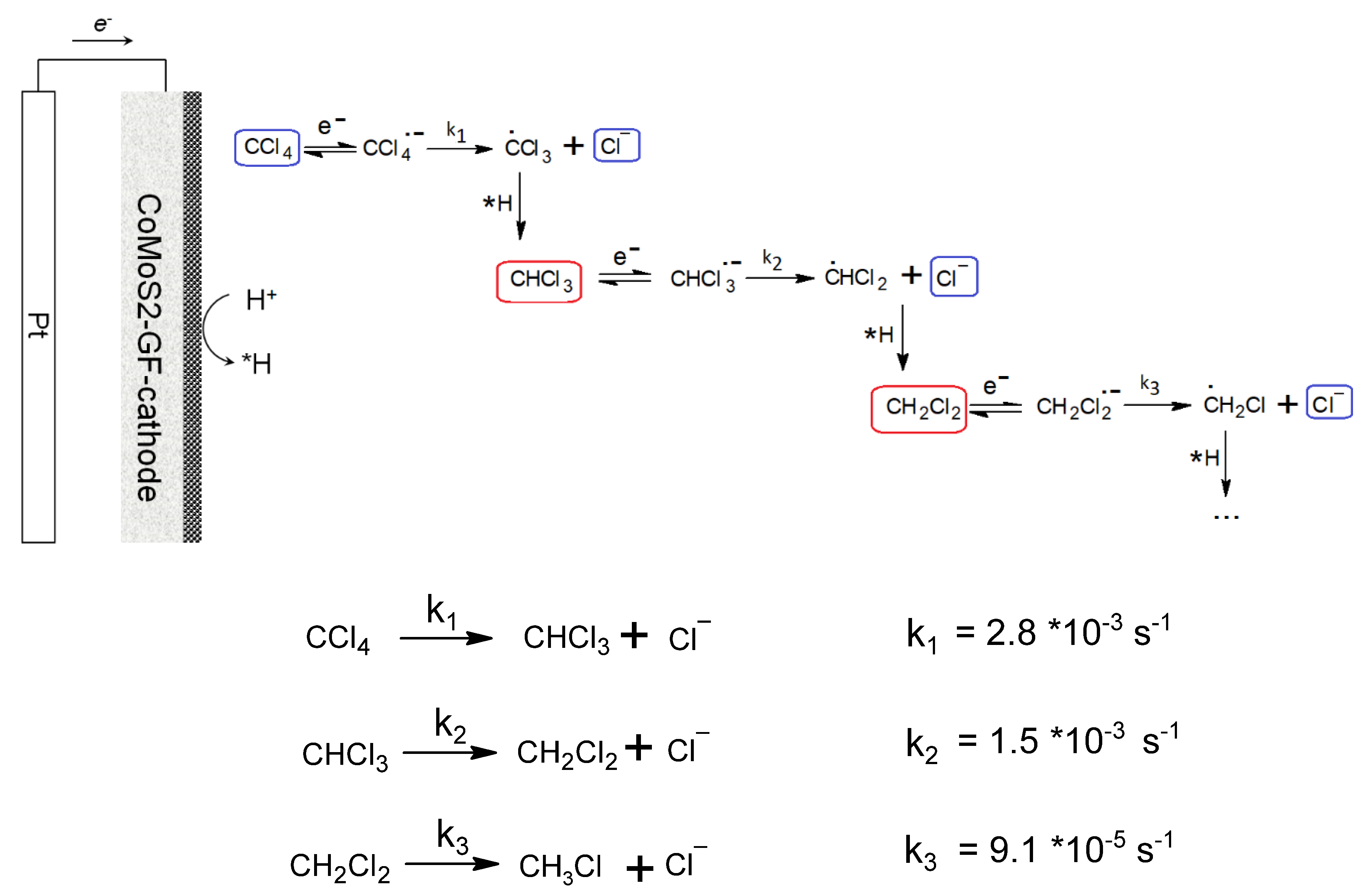
2.3.3. Kinetic Modeling and Mechanism of Degradation
3. Materials and Methods
3.1. Materials
3.2. Electrode Preparation and Characterization
3.3. Electrochemical Characterization
3.4. Electrolysis Experiments
3.5. Analytical Method
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lei, C.; Liang, F.; Li, J.; Chen, W.; Huang, B. Electrochemical reductive dechlorination of chlorinated volatile organic compounds (Cl-VOCs): Effects of molecular structure on the dehalogenation reactivity and mechanisms. Chem. Eng. J. 2019, 358, 1054–1064. [Google Scholar] [CrossRef]
- Yin, H.; Cao, X.; Lei, C.; Chen, W.; Huang, B. Insights into Electroreductive Dehalogenation Mechanisms of Chlorinated Environmental Pollutants. ChemElectroChem 2020, 1825–1837. [Google Scholar] [CrossRef]
- Huu, V.; Sakakibara, Y.; Komori, M.; Kishimoto, N.; Watanabe, T. Recent Developments in Electrochemical Technology for Water and Wastewater Treatments. J. Water Environ. Technol. 2016, 14, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Qi, X.; Shen, F.; Qiu, M.; Smith, R.L. Complete dechlorination of lindane over N-doped porous carbon supported Pd catalyst at room temperature and atmospheric pressure. Sci. Total Environ. 2020, 719, 137534. [Google Scholar] [CrossRef]
- Chang, M.B.; Fu, C.W.; Tsai, C.L. Effect of reducing agent on catalytic hydrodechlorination of aqueous-phase OCDD/F. Chemosphere 2018, 202, 322–329. [Google Scholar] [CrossRef]
- Nijenhuis, I.; Kuntze, K. Anaerobic microbial dehalogenation of organohalides—State of the art and remediation strategies. Curr. Opin. Biotechnol. 2016, 38, 33–38. [Google Scholar] [CrossRef]
- Hu, M.; Liu, Y.; Yao, Z.; Ma, L.; Wang, X. Catalytic reduction for water treatment. Front. Environ. Sci. Eng. 2018, 12, 1–18. [Google Scholar] [CrossRef]
- Xu, Y.H.; Cai, Q.Q.; Ma, H.X.; He, Y.; Zhang, H.; Ma, C.A. Electrochimica Acta Optimisation of electrocatalytic dechlorination of 2,4-dichlorophenoxyacetic acid on a roughened silver–palladium cathode. Electrochim. Acta 2013, 96, 90–96. [Google Scholar] [CrossRef]
- Shen, Y.; Tong, Y.; Xu, J.; Wang, S.; Wang, J.; Zeng, T.; He, Z.; Yang, W.; Song, S. Ni-based layered metal-organic frameworks with palladium for electrochemical dechlorination. Appl. Catal. B Environ. 2020, 264, 118505. [Google Scholar] [CrossRef]
- He, Z.; Lin, K.; Sun, J.; Wen, L.; Gao, C.; Chen, J.; Song, S.; Qian, Y.; Liub, W. Kinetics of electrochemical dechlorination of 2-chlorobiphenyl on apalladium-modified nickel foam cathode in a basic medium:From batch to continuous reactor operation. Electrochim. Acta 2013, 109, 502–511. [Google Scholar] [CrossRef]
- Shu, X.; Yang, Q.; Yao, F.; Zhong, Y.; Ren, W.; Chen, F.; Sun, J. Electrocatalytic hydrodechlorination of 4-chlorophenol on Pd supported multi-walled carbon nanotubes particle electrodes. Chem. Eng. J. 2019, 358, 903–911. [Google Scholar] [CrossRef]
- He, W.; Lou, Y.; Verlato, E.; Soutrel, I.; Floner, D.; Fourcade, F.; Amrane, A.; Geneste, F. Reductive dehalogenation of a chloroacetanilide herbicide in a flow electrochemical cell fitted with Ag-modified Ni foams. J. Chem. Technol. Biotechnol. 2018, 93, 1572–1578. [Google Scholar] [CrossRef]
- Durante, C.; Isse, A.A.; Sandonà, G.; Gennaro, A. Electrochemical hydrodehalogenation of polychloromethanes at silver and carbon electrodes. Appl. Catal. B Environ. 2009, 88, 479–489. [Google Scholar] [CrossRef]
- Park, J.E.; Yang, S.K.; Kim, J.H.; Park, M.J.; Lee, E.S. Electrocatalytic activity of Pd/Ir/Sn/Ta/TiO2 composite electrodes. Energies 2018, 11, 3356. [Google Scholar] [CrossRef] [Green Version]
- Fiori, G.; Rondinini, S.; Sello, G.; Vertova, A.; Cirja, M.; Conti, L. Electroreduction of volatile organic halides on activated silver cathodes. J. Appl. Electrochem. 2005, 35, 363–368. [Google Scholar] [CrossRef]
- He, Z.; Tong, Y.; Ni, S.; Ye, X.; Makwarimba, C.P.; Huang, X.; Zhang, S.; Song, S. Electrochemically reductive dechlorination of 3,6-dichloropicolinic acid on a palladium/nitrogen-doped carbon / nickel foam electrode. Electrochim. Acta 2018, 292, 685–696. [Google Scholar] [CrossRef]
- Dauda, M.; Basheer, C.; Al-Malack, M.H.; Siddiqui, M.N. Efficient Co-MoS2 electrocatalyst for cathodic degradation of halogenated disinfection by-products in water sample. Sep. Purif. Technol. 2021, 259, 118085. [Google Scholar] [CrossRef]
- Ye, J.; Chen, W.; Xu, S.; Yu, Z.; Hou, S. Synthesis of Co-doped MoS2/graphene hybrids as enhanced electrocatalysts for the hydrogen evolution reaction. RSC Adv. 2016, 6, 104925–104932. [Google Scholar] [CrossRef]
- Huang, L.Z.; Pedersen, S.U.; Bjerglund, E.T.; Lamagni, P.; Glasius, M.; Hansen, H.C.B.; Daasbjerg, K. Hierarchical MoS2 nanosheets on flexible carbon felt as an efficient flow-through electrode for dechlorination. Environ. Sci. Nano 2017, 4, 2286–2296. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, Y.; Huang, C.; Xiao, D.; Zhang, L.; Ma, S.; Qu, J.; Zheng, S.; Chu, P.K. Graphite felt incorporated with MoS2/rGO for electrochemical detoxification of high-arsenic fly ash. Chem. Eng. J. 2020, 382, 122763. [Google Scholar] [CrossRef]
- Escobedo, E.; Kim, J.; Oh, D.; Cho, K.; Chang, Y.S. Electrocatalytic dehalogenation of aqueous pollutants by dealloyed nanoporous Pd/Ti cathode. Catal. Today 2020, 361, 63–68. [Google Scholar] [CrossRef]
- Xiong, Q.; Zhang, X.; Wang, H.; Liu, G.; Wang, G.; Zhang, H.; Zhao, H. One-step synthesis of cobalt-doped MoS2 nanosheets as bifunctional electrocatalysts for overall water splitting under both acidic and alkaline conditions. Chem. Commun. 2018, 54, 3859–3862. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Ekspong, J.; Ashok, A.; Koroidov, S.; Gracia-Espino, E. Solid-state synthesis of few-layer cobalt-doped MoS2 with CoMoS phase on nitrogen-doped graphene driven by microwave irradiation for hydrogen electrocatalysis. RSC Adv. 2020, 10, 34323–34332. [Google Scholar] [CrossRef]
- Bose, R.; Jin, Z.; Shin, S.; Kim, S.; Lee, S.; Min, Y.S. Co-catalytic Effects of CoS2 on the Activity of the MoS2 Catalyst for Electrochemical Hydrogen Evolution. Langmuir 2017, 33, 5628–5635. [Google Scholar] [CrossRef]
- Chen, L.; Yang, W.; Liu, X.; Jia, J. Flower-like CoS2/MoS2 nanocomposite with enhanced electrocatalytic activity for hydrogen evolution reaction. Int. J. Hydrogen Energy 2017, 42, 12246–12253. [Google Scholar] [CrossRef]
- Hong, X.; Li, S.; Tang, X.; Sun, Z.; Li, F. Self-supporting porous CoS2/rGO sulfur host prepared by bottom-up assembly for lithium-sulfur batteries. J. Alloys Compd. 2018, 749, 586–593. [Google Scholar] [CrossRef]
- Sun, Z.; Ge, H.; Hu, X.; Peng, Y. Electrocatalytic dechlorination of chloroform in aqueous solution on palladium/titanium electrode. Chem. Eng. Technol. 2009, 32, 134–139. [Google Scholar] [CrossRef]
- Sun, Z.; Li, B.; Hu, X.; Shi, M.; Hou, Q.; Peng, Y. Electrochemical dechlorination of chloroform in neutral aqueous solution on palladium/foam-nickel and palladium/polymeric pyrrole film/foam-nickel electrodes. J. Environ. Sci. 2008, 20, 268–272. [Google Scholar] [CrossRef]
- Brzózka, A.; Jeleń, A.; Brudzisz, A.M.; Marzec, M.M.; Sulka, G.D. Electrocatalytic reduction of chloroform at nanostructured silver electrodes. Electrochim. Acta 2017, 225, 574–583. [Google Scholar] [CrossRef]
- Wang, G.; Feng, C.; Kang, C.; Zhang, B.; Chen, N.; Zhang, X. Electrochemical Degradation of Chloroform Using Ti/IrO2 Anode and Cu/Zn Cathode. J. Environ. Eng. 2016, 142, 04015066. [Google Scholar] [CrossRef] [Green Version]
- Cummins, D.R.; Martinez, U.; Sherehiy, A.; Kappera, R.; Martinez-Garcia, A.; Schulze, R.K.; Jasinski, J.; Zhang, J.; Gupta, R.K.; Lou, J.; et al. Efficient hydrogen evolution in transition metal dichalcogenides via a simple one-step hydrazine reaction. Nat. Commun. 2016, 7, 11857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.Y.; Lee, J.G.; Lee, S.H.; Seo, M.; Piao, L.; Bae, J.H.; Lim, S.Y.; Park, Y.J.; Chung, T.D. Hydrogen-atom-mediated electrochemistry. Nat. Commun. 2013, 4, 2766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razmjooei, F.; Liu, T.; Azevedo, D.A.; Hadjixenophontos, E.; Reissner, R.; Schiller, G.; Ansar, S.A.; Friedrich, K.A. Improving plasma sprayed Raney-type nickel–molybdenum electrodes towards high-performance hydrogen evolution in alkaline medium. Sci. Rep. 2020, 10, 10948. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dauda, M.; Al-Malack, M.H.; Basheer, C.; Siddiqui, M.N.; Jalilov, A. Degradation Kinetics and Mechanism of Polychloromethanes Reduction at Co-MoS2/Graphite Felt Electrode. Catalysts 2021, 11, 929. https://doi.org/10.3390/catal11080929
Dauda M, Al-Malack MH, Basheer C, Siddiqui MN, Jalilov A. Degradation Kinetics and Mechanism of Polychloromethanes Reduction at Co-MoS2/Graphite Felt Electrode. Catalysts. 2021; 11(8):929. https://doi.org/10.3390/catal11080929
Chicago/Turabian StyleDauda, Mohammed, Muhammad H. Al-Malack, Chanbasha Basheer, Mohammad Nahid Siddiqui, and Almaz Jalilov. 2021. "Degradation Kinetics and Mechanism of Polychloromethanes Reduction at Co-MoS2/Graphite Felt Electrode" Catalysts 11, no. 8: 929. https://doi.org/10.3390/catal11080929
APA StyleDauda, M., Al-Malack, M. H., Basheer, C., Siddiqui, M. N., & Jalilov, A. (2021). Degradation Kinetics and Mechanism of Polychloromethanes Reduction at Co-MoS2/Graphite Felt Electrode. Catalysts, 11(8), 929. https://doi.org/10.3390/catal11080929







