Turbidity Changes during Carbamazepine Oxidation by Photo-Fenton
Abstract
:1. Introduction
2. Results
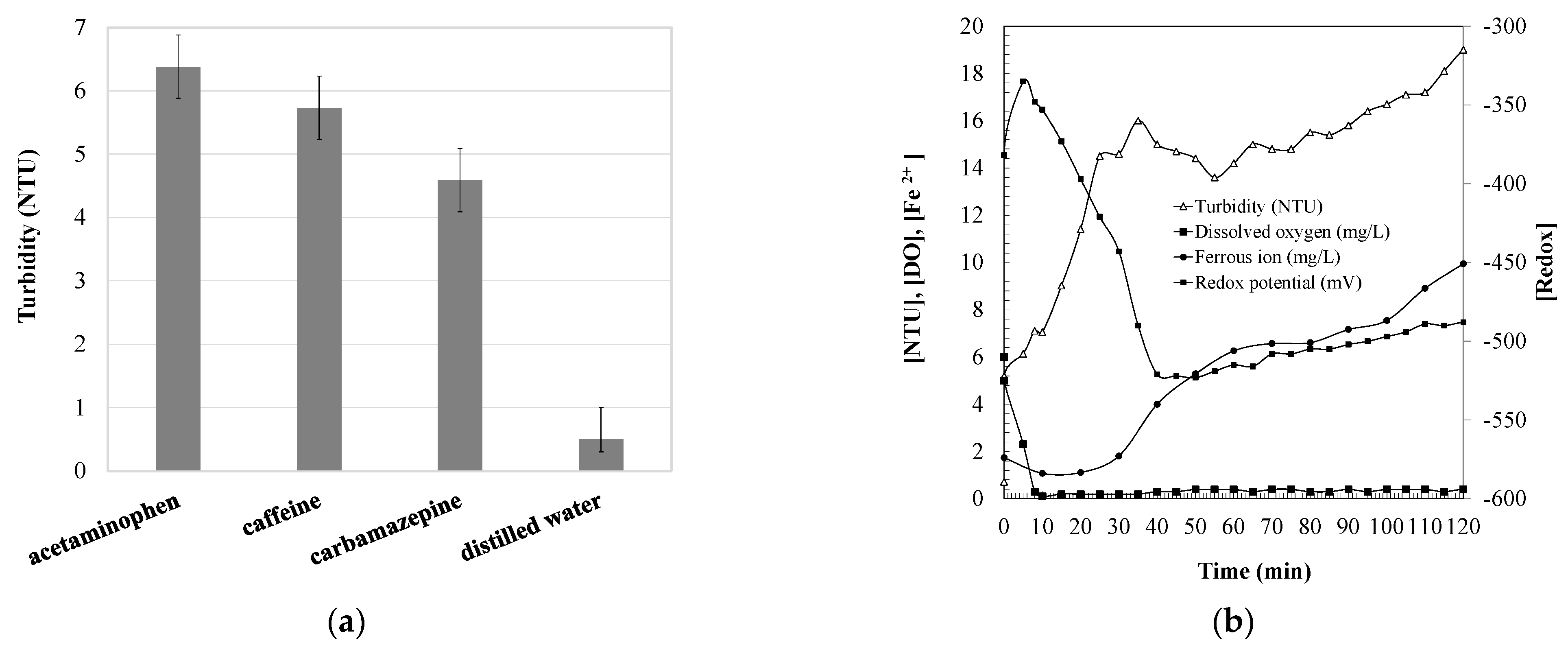
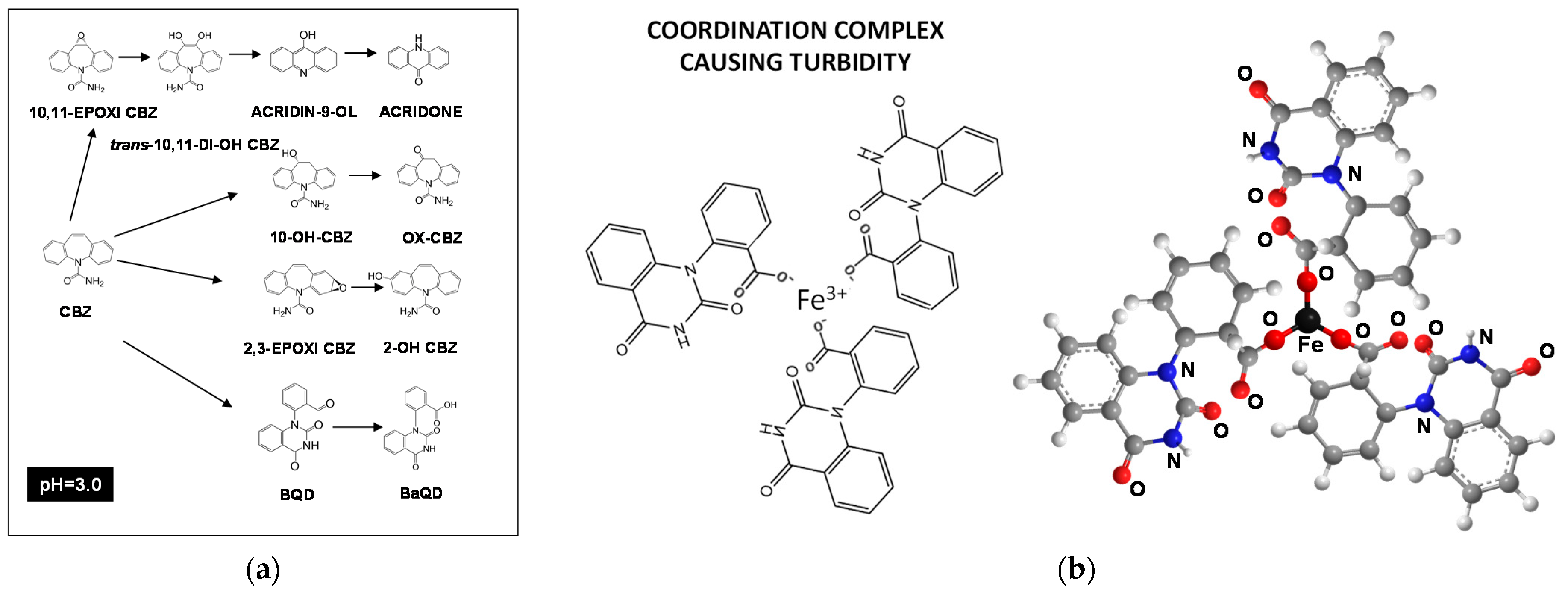
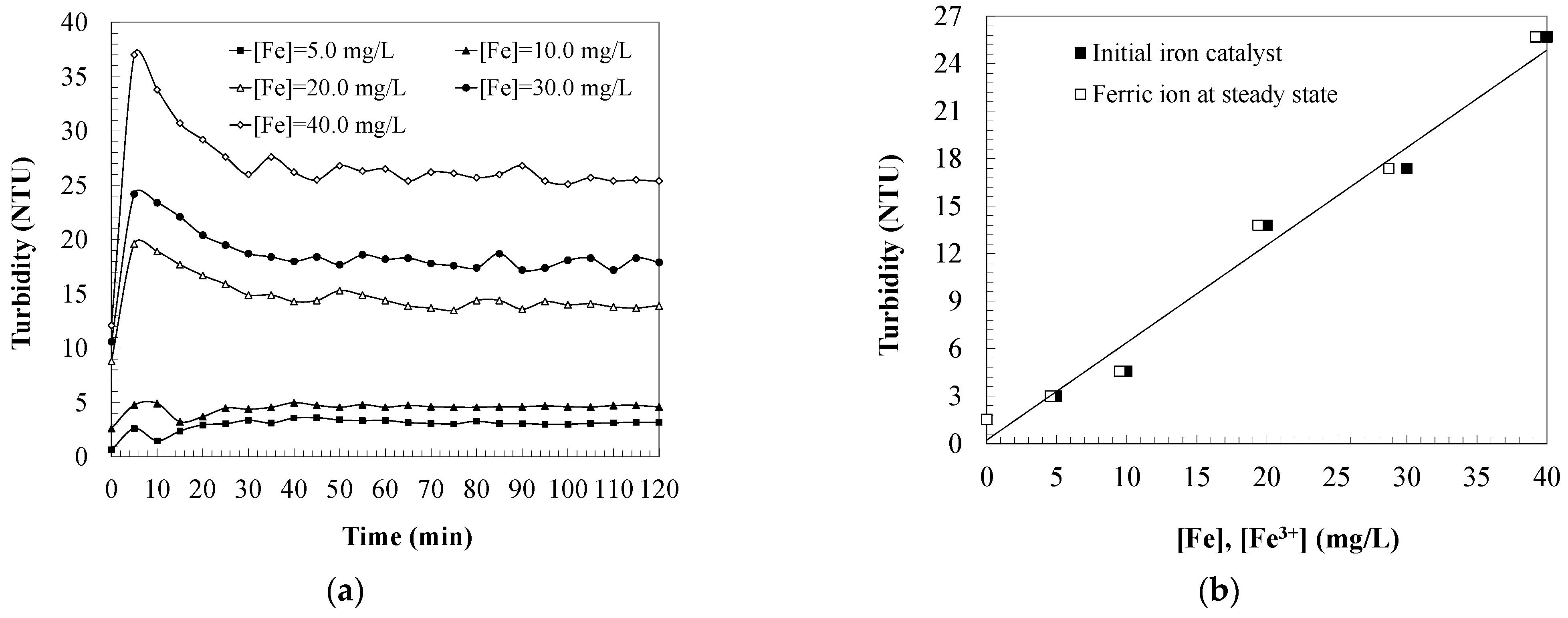
2.1. Turbidity Changes during Carbamazepine Oxidation
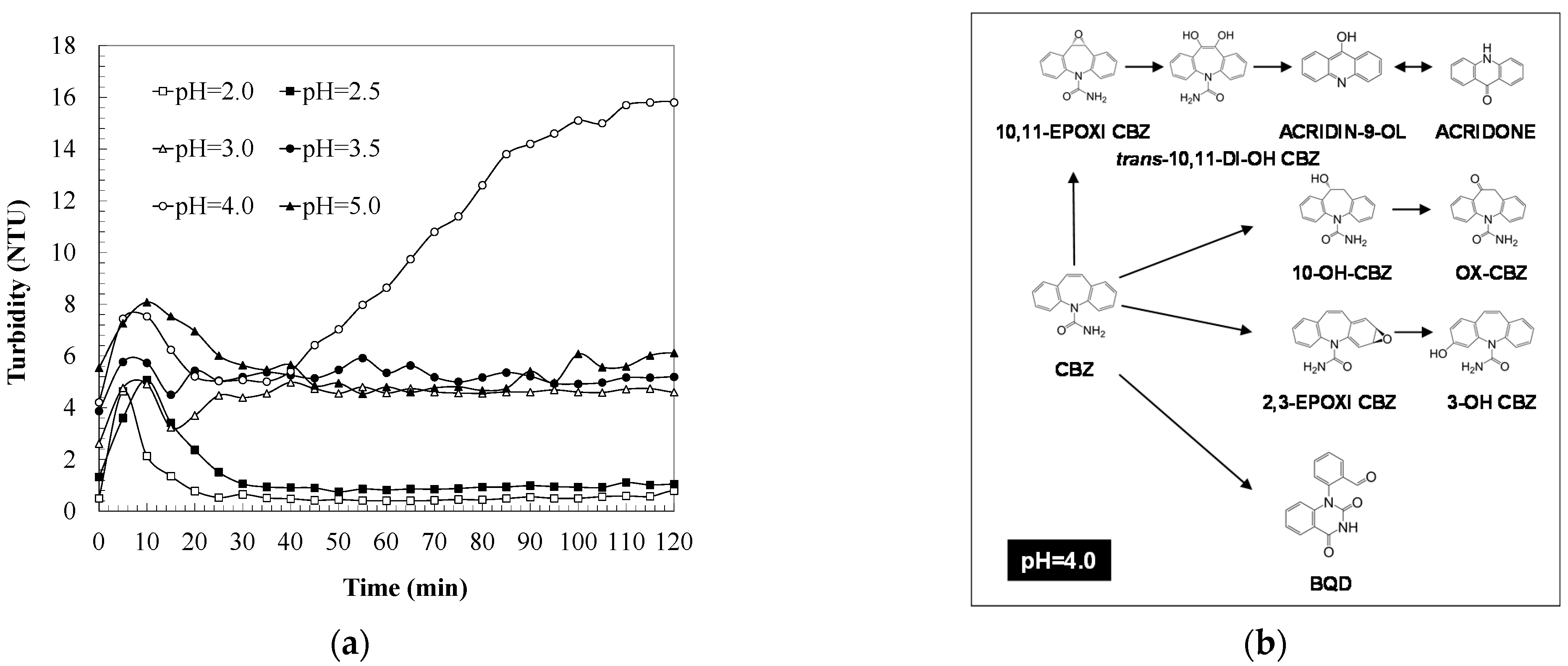
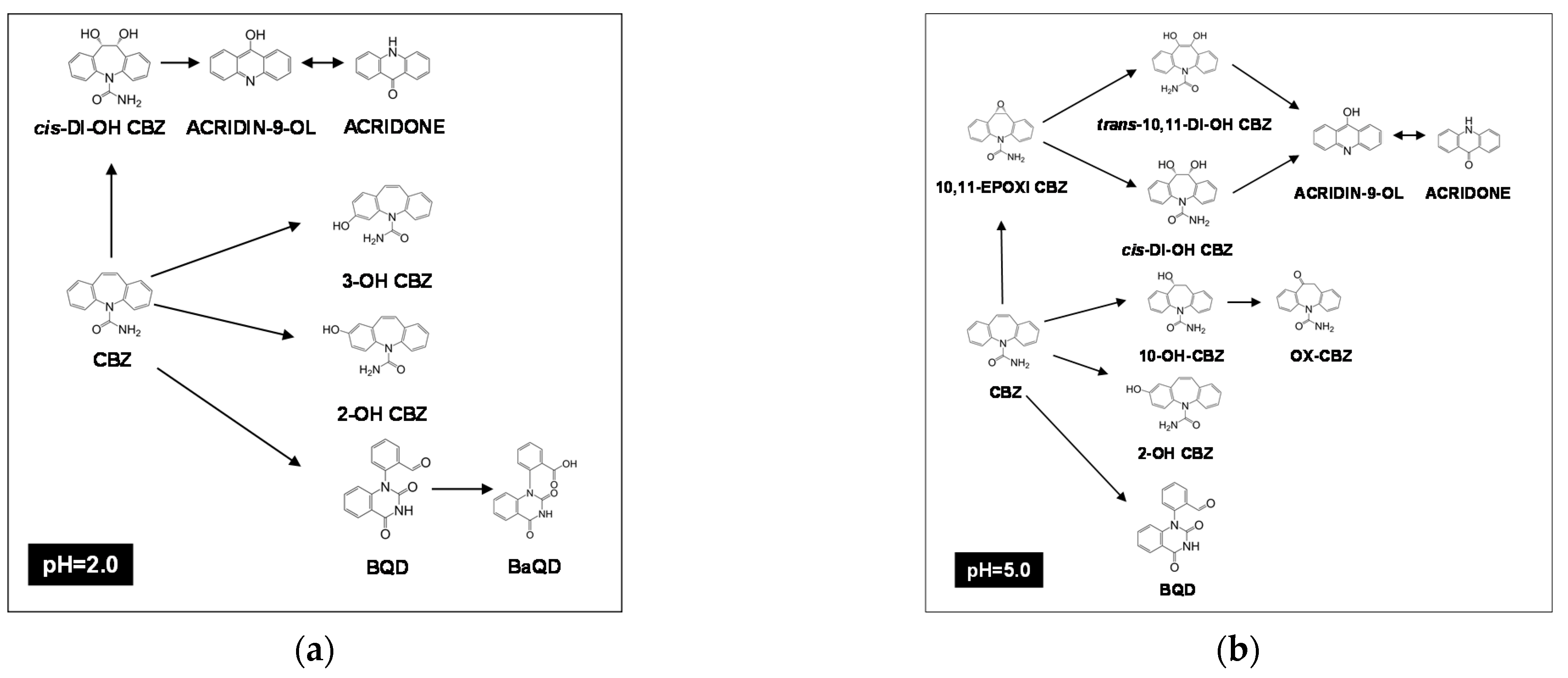
2.2. pH Effect
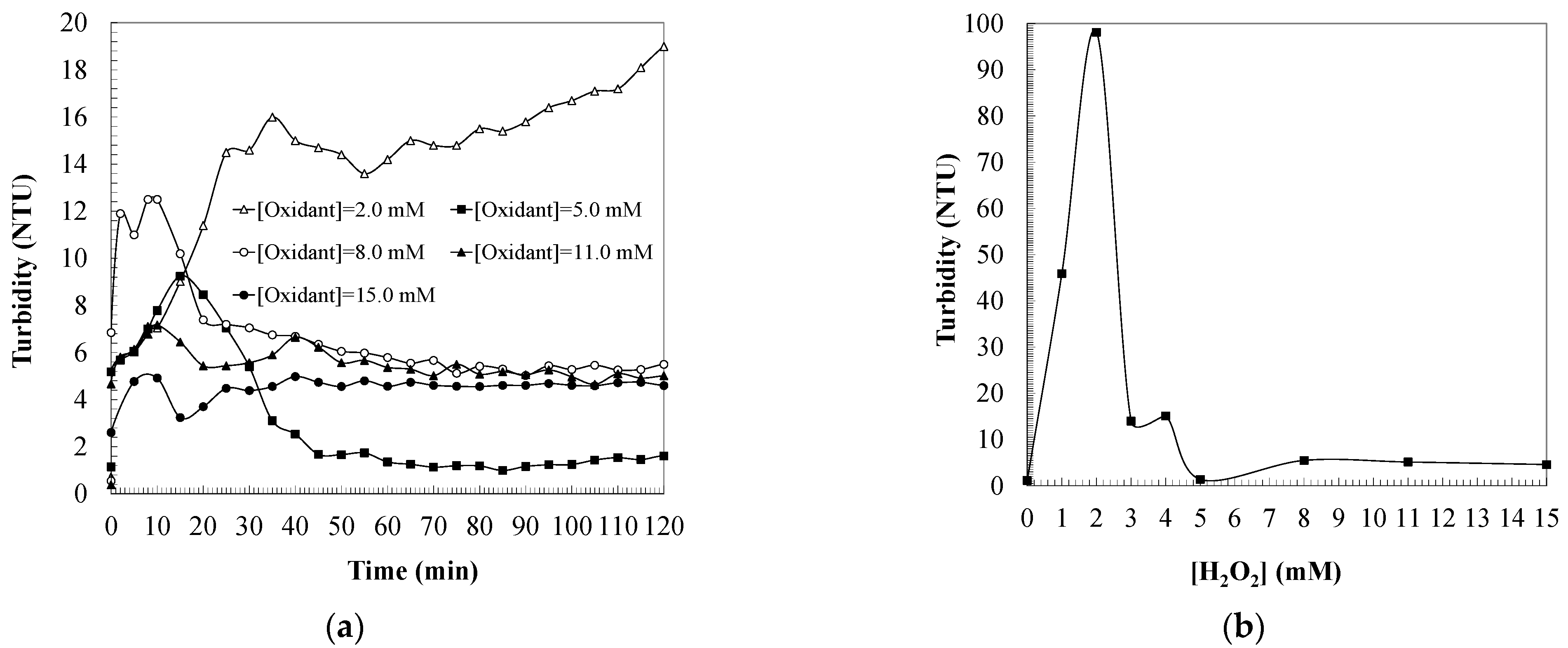
2.3. Effect of Iron Catalyst
3. Materials and Methods
3.1. Experimental Methods
3.2. Analytical Methods
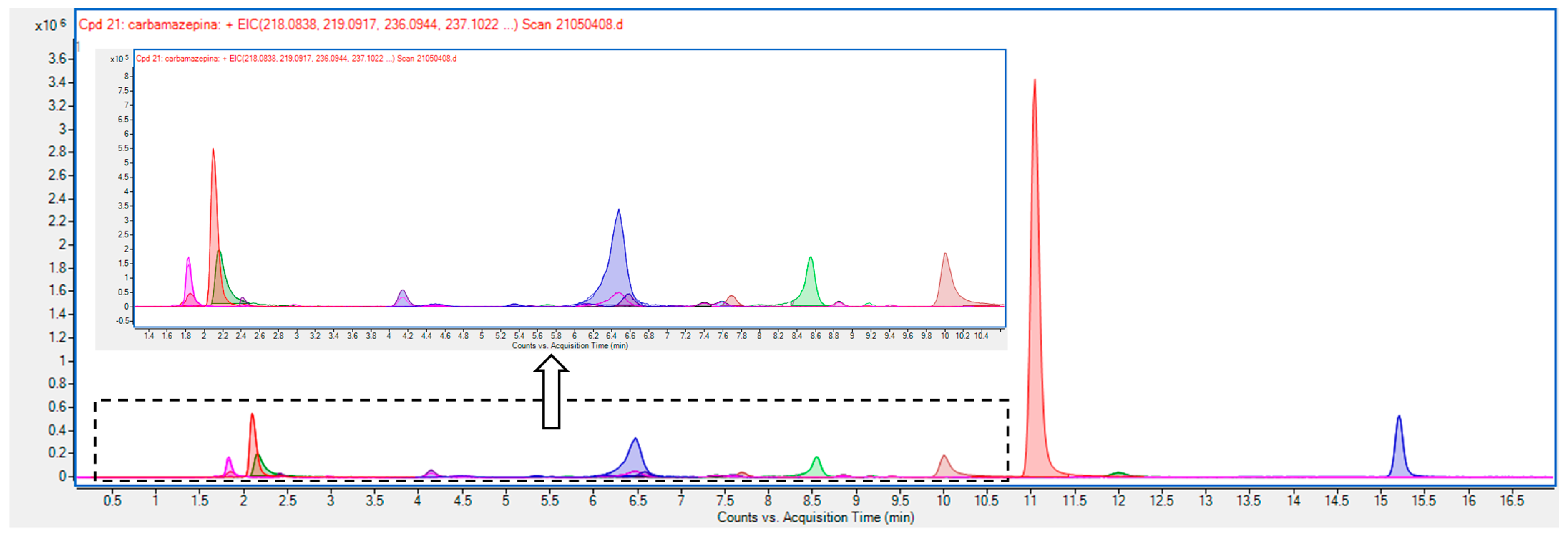
3.3. Liquid Chromatography-Mass Spectrometry to Elucidate the Intermediates of Carbamazepine Degradation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Label | Tr, min | m/z | Mass | Height | Name | Score | Diff (DB,ppm) | Ions |
|---|---|---|---|---|---|---|---|---|
| Comp 1 | 1.8 | 224.0718 | 223.0645 | 146,262 | - | - | - | 3 |
| Comp 2 | 1.8 | 163.0511 | 162.0438 | 41,946 | - | - | - | 2 |
| Comp 3 | 2.1 | 147.0557 | 146.0485 | 501,215 | - | - | - | 2 |
| Comp 4 | 2.2 | 180.0811 | 179.0739 | 172565 | Acridine | 99.09 | −2.02 | 2 |
| Comp 5 | 2.4 | 271.1054 | 270.0991 | 33,111 | CIS-d iOH -CBZ | 88.39 | 4.91 | 8 |
| Comp 6 | 4.1 | 271.1092 | 270.1023 | 58,811 | CIS-diOH-CBZ | 94.64 | −7.03 | 7 |
| Comp 7 | 4.5 | 267.0781 | 266.0708 | 8828 | BQD | 87.11 | −6.51 | 1 |
| Comp 8 | 5.4 | 267.0779 | 266.0706 | 8275 | BQD | 89.7 | −5.78 | 2 |
| Comp 9 | 5.8 | 255.1158 | 254.1093 | 8104 | - | - | - | |
| Comp 10 | 6.5 | 251.0828 | 250.0755 | 269,555 | T1251 | 92.29 | −5.1 | 5 |
| Comp 11 | 6.6 | 267.0778 | 266.0706 | 38,790 | BQD | 90.62 | −5.5 | 2 |
| Comp 12 | 7.3 | 224.0719 | 223.0639 | 1553 | - | - | - | 3 |
| Comp 13 | 7.4 | 253.0989 | 252.0919 | 12,234 | 2-OH-CBZ | 73.88 | −7.86 | 2 |
| Comp 14 | 7.6 | 253.0983 | 252.0911 | 16,164 | 2-OH-CBZ | 80.87 | −4.92 | 2 |
| Comp 15 | 7.7 | 283.0727 | 282.0654 | 32,599 | BaQD | 93.23 | −4.53 | 2 |
| Comp 16 | 8.5 | 267.0779 | 266.0706 | 147,319 | BQD | 90.13 | −5.65 | 3 |
| Comp 17 | 8.9 | 253.0994 | 252.0928 | 15,599 | 3-OH-CBZ | 77.71 | −11.53 | 3 |
| Comp 18 | 9.2 | 253.1034 | 252.096 | 3526 | - | - | - | 2 |
| Comp 19 | 9.9 | 196.077 | 195.0697 | 184,640 | Acridone or acridin-9-ol | 96.9 | −6.8 | 10 |
| Comp 20 | 11.2 | 259.0863 | 236.0971 | 3,433,925 | Carbamazepine | 94 | −9.09 | 7 |
| Comp 21 | 12.0 | 318.2826 | 317.2753 | 29,688 | - | - | - | 2 |
| Comp 22 | 15.2 | 226.0881 | 225.0808 | 402,491 | - | - | - | 5 |
| Label | Tr, min | m/z | Mass | Height | Name | Score | Diff (DB,ppm) | Ions |
|---|---|---|---|---|---|---|---|---|
| Comp 1 | 1.8 | 224.0716 | 223.0644 | 632,743 | - | - | - | 3 |
| Comp 2 | 2.1 | 147.0563 | 146.0491 | 436,509 | - | - | - | 3 |
| Comp 3 | 2.2 | 180.0818 | 179.0745 | 1,149,862 | Acridine | 92.86 | −5.76 | 3 |
| Comp 4 | 2.4 | 271.1095 | 270.1017 | 147,566 | TRANS-diOH-CBZ | 86.39 | −4.87 | 3 |
| Comp 5 | 3.5 | 271.109 | 270.102 | 12,320 | - | - | - | 2 |
| Comp 6 | 4.1 | 253.099 | 270.1023 | 169,843 | TRANS-diOH-CBZ | 93.57 | −6.99 | 3 |
| Comp 7 | 4.5 | 267.0774 | 266.0701 | 56,646 | BQD | 95.11 | −3.92 | 2 |
| Comp 8 | 5.3 | 267.0777 | 266.0704 | 45,947 | BQD | 91.98 | −5.07 | 2 |
| Comp 9 | 5.7 | 237.1033 | 254.1065 | 131,805 | 10-OH-CBZ | 83.69 | −4.02 | 2 |
| Comp 10 | 6.5 | 251.0834 | 250.0761 | 544,371 | T1251 | 84 | −7.51 | 5 |
| Comp 11 | 6.6 | 267.0776 | 266.0704 | 55,876 | BQD | 93.04 | −4.7 | 2 |
| Comp 12 | 7.1 | 269.0934 | 268.0861 | 141,392 | - | - | - | 4 |
| Comp 13 | 7.3 | 224.0718 | 223.0644 | 52,554 | - | - | - | 3 |
| Comp 14 | 7.4 | 253.0988 | 252.0916 | 256,182 | 2-OH-CBZ | 95.43 | −6.61 | 8 |
| Comp 15 | 7.6 | 253.0989 | 252.0916 | 357,248 | 2-OH-CBZ | 94.24 | −6.77 | 5 |
| Comp 16 | 7.7 | 283.0735 | 282.0662 | 9024 | BaQD | 82.12 | −7.58 | 2 |
| Comp 17 | 7.9 | 267.0784 | 266.0709 | 19,667 | BQD | 85.55 | −6.92 | 3 |
| Comp 18 | 8.2 | 253.0985 | 252.0914 | 17,230 | EP-CBZ | 73.45 | −6.07 | 2 |
| Comp 19 | 8.6 | 267.0795 | 266.0722 | 8216 | - | - | - | 2 |
| Comp 20 | 8.8 | 253.0994 | 252.0921 | 330,535 | 2-OH-CBZ | 90.52 | −8.92 | 4 |
| Comp 21 | 9.2 | 253.0988 | 252.0916 | 204,534 | Oxcarbamazepine | 94.32 | −6.72 | 5 |
| Comp 22 | 10.0 | 196.0772 | 195.0699 | 280,810 | Acridone or acridin-9-ol | 96.78 | −7.92 | 6 |
| Comp 23 | 11.2 | 237.1047 | 236.0974 | 10,672,680 | Carbamazepine | 88.67 | −10.08 | 15 |
| Comp 24 | 11.7 | 224.0733 | 223.066 | 276,891 | - | - | - | 5 |
| Comp 25 | 11.9 | 473.1992 | 472.1919 | 639,344 | - | - | - | 6 |
| Comp 26 | 15.2 | 226.0889 | 225.0816 | 1,183,745 | - | - | - | 5 |
| Label | Tr, min | m/z | Mass | Height | Name | Score | Diff (DB,ppm) | Ions |
|---|---|---|---|---|---|---|---|---|
| Comp 1 | 1.8 | 224.0722 | 223.0649 | 755,262 | - | - | - | 3 |
| Comp 2 | 2.1 | 147.0566 | 146.0493 | 372,649 | - | - | - | 3 |
| Comp 3 | 2.2 | 180.0822 | 179.0749 | 1,818,336 | Acridine | 87.37 | −7.78 | 3 |
| Comp 4 | 2.4 | 271.109 | 270.1018 | 67,258 | TRANS-diOH-CBZ | 80.63 | −5.01 | 2 |
| Comp 5 | 3.5 | 271.1091 | 270.1017 | 178,694 | TRANS-diOH-CBZ | 96.59 | −4.95 | 3 |
| Comp 6 | 4.2 | 253.0988 | 270.1021 | 236,040 | TRANS-diOH-CBZ | 94.47 | −6.47 | 3 |
| Comp 7 | 4.5 | 267.0778 | 266.0705 | 26,250 | BQD | 90.8 | −5.44 | 2 |
| Comp 8 | 5.3 | 267.078 | 266.0707 | 9285 | BQD | 88.48 | −6.13 | 1 |
| Comp 9 | 5.7 | 255.1141 | 254.1069 | 31,349 | 10-OH-CBZ | 80.45 | −5.52 | 3 |
| Comp 10 | 6.5 | 251.0825 | 250.0753 | 119,415 | T1251 | 94.04 | −4.46 | 3 |
| Comp 11 | 6.6 | 267.0779 | 266.0706 | 13,942 | BQD | 89.97 | −5.7 | 2 |
| Comp 12 | 7.1 | 269.0935 | 268.0861 | 166,675 | - | - | - | 5 |
| Comp 13 | 7.4 | 224.0716 | 223.0643 | 44,729 | - | - | - | 2 |
| Comp 14 | 7.3 | 253.0985 | 252.0912 | 385,655 | 3-OH CBZ | 96.47 | −5.18 | 5 |
| Comp 15 | 7.6 | 253.099 | 252.0917 | 514,816 | 3-OH CBZ | 93.53 | −7.24 | 8 |
| Comp 16 | 7.9 | 267.0781 | 266.0708 | 12,768 | BDQ | 87.89 | −6.29 | 2 |
| Comp 17 | 8.2 | 253.099 | 252.0918 | 70,044 | EP-CBZ | 93.04 | −7.69 | 3 |
| Comp 18 | 8.8 | 253.0996 | 252.0923 | 1,397,381 | 3-OH-CBZ | 89.34 | −9.65 | 11 |
| Comp 19 | 9.1 | 253.0989 | 252.0916 | 149,274 | Oxcarbamazepine | 78.9 | −6.89 | 4 |
| Comp 20 | 9.9 | 196.0767 | 195.0696 | 109,794 | Acridone or acridin-9-ol | 97.14 | −5.92 | 4 |
| Comp 21 | 11.2 | 237.105 | 236.0973 | 15,089,995 | Carbamazepine | 83.8 | −9.81 | 9 |
| Comp 22 | 11.7 | 224.0723 | 223.0653 | 95,064 | - | - | - | 2 |
| Comp 23 | 11.9 | 473.1994 | 472.192 | 688,405 | - | - | - | 8 |
| Comp 24 | 13.5 | 210.0931 | 209.0859 | 635,867 | - | - | - | 3 |
| Comp 25 | 15.2 | 226.0889 | 225.0817 | 1,793,645 | - | - | - | 8 |
| Label | Tr, min | m/z | Mass | Height | Name | Score | Diff (DB,ppm) | Ions |
|---|---|---|---|---|---|---|---|---|
| Comp 1 | 1.8 | 224.0718 | 223.0645 | 520,658 | - | - | - | 3 |
| Comp 2 | 2.1 | 147.0568 | 146.0496 | 588,299 | - | - | - | 3 |
| Comp 3 | 2.2 | 180.0822 | 179.0749 | 693,527 | Acridine | 87.18 | −7.84 | 3 |
| Comp 4 | 2.4 | 271.1092 | 270.1019 | 141,194 | TRANS-diOH-CBZ | 80.16 | −5.49 | 2 |
| Comp 5 | 3.5 | 271.1091 | 270.1019 | 432,132 | CIS-diOH-CBZ | 96.29 | −5.38 | 7 |
| Comp 6 | 4.1 | 271.1091 | 270.1018 | 409,566 | CIS-diOH-CBZ | 96.85 | −5.13 | 9 |
| Comp 7 | 4.5 | 267.0778 | 266.0705 | 114,157 | BQD | 91.47 | −5.23 | 2 |
| Comp 8 | 5.3 | 267.0779 | 266.0706 | 13,734 | BQD | 89.84 | −5.73 | 1 |
| Comp 9 | 5.7 | 255.114 | 254.1069 | 56,313 | 10-OH-CBZ | 96.03 | −5.69 | 10 |
| Comp 10 | 6.4 | 251.0827 | 250.0755 | 398,550 | T1251 | 92.37 | −5.07 | 6 |
| Comp 11 | 6.5 | 267.0782 | 266.0709 | 10,804 | BQD | 86.14 | −6.77 | 2 |
| Comp 12 | 7.1 | 269.0935 | 268.0862 | 148,854 | - | - | - | 8 |
| Comp 13 | 7.3 | 224.0721 | 223.0648 | 51,741 | - | - | - | 4 |
| Comp 14 | 7.3 | 253.0989 | 252.0916 | 473,737 | 2-OH-CBZ | 95.34 | −6.62 | 6 |
| Comp 15 | 7.5 | 253.0995 | 252.0922 | 802,934 | 2-OH-CBZ | 91.73 | −9.17 | 10 |
| Comp 16 | 7.9 | 267.0775 | 266.0701 | 34,402 | BQD | 95.57 | −3.73 | 4 |
| Comp 17 | 8.2 | 253.0985 | 252.0915 | 81,586 | EP-CBZ | 97.33 | −6.51 | 8 |
| Comp 18 | 8.8 | 253.0996 | 252.0924 | 1,722,757 | 2-OH-CBZ | 90.91 | −9.73 | 11 |
| Comp 19 | 9.1 | 253.0983 | 252.0911 | 37,573 | Oxcarbamazepine | 80.73 | -4.9 | 2 |
| Comp 20 | 10.0 | 196.0773 | 195.07 | 158,961 | Acridone or acridin-9-ol | 95.31 | −8.41 | 6 |
| Comp 21 | 11.2 | 237.1048 | 236.0973 | 11,711,134 | Carbamazepine | 89.16 | −9.9 | 15 |
| Comp 22 | 11.7 | 224.0731 | 223.0663 | 207,462 | - | - | - | 5 |
| Comp 23 | 12.0 | 473.1987 | 472.1915 | 239,021 | - | - | - | 7 |
| Comp 24 | 15.2 | 226.0886 | 225.0814 | 2,443,394 | - | - | - | 11 |
References
- Lapworth, D.J.; Baran, N.; Stuart, M.E.; Ward, R.S. Emerging Organic Contaminants in Groundwater: A Review of Sources, Fate and Occurrence. Environ. Pollut. 2012, 163, 287–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hai, F.I.; Yang, S.; Asif, M.B.; Sencadas, V.; Shawkat, S.; Sanderson-Smith, M.; Gorman, J.; Xu, Z.-Q.; Yamamoto, K. Carbamazepine as a Possible Anthropogenic Marker in Water: Occurrences, Toxicological Effects, Regulations and Removal by Wastewater Treatment Technologies. Water 2018, 10, 107. [Google Scholar] [CrossRef] [Green Version]
- Hughes, S.R.; Kay, P.; Brown, L.E. Global Synthesis and Critical Evaluation of Pharmaceutical Data Sets Collected from River Systems. Environ. Sci. Technol. 2013, 47, 661–677. [Google Scholar] [CrossRef]
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging Pollutants in the Environment: Present and Future Challenges in Biomonitoring, Ecological Risks and Bioremediation. New Biotechnol. 2015, 32, 147–156. [Google Scholar] [CrossRef]
- Brack, W.; Dulio, V.; Ågerstrand, M.; Allan, I.; Altenburger, R.; Brinkmann, M.; Bunke, D.; Burgess, R.M.; Cousins, I.; Escher, B.I.; et al. Towards the Review of the European Union Water Framework Directive: Recommendations for More Efficient Assessment and Management of Chemical Contamination in European Surface Water Resources. Sci. Total Environ. 2017, 576, 720–737. [Google Scholar] [CrossRef]
- Kumar, A.; Batley, G.E.; Nidumolu, B.; Hutchinson, T.H. Derivation of Water Quality Guidelines for Priority Pharmaceuticals. Environ. Toxicol. Chem. 2016, 35, 1815–1824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcelo, V.O.; Salette, R.; Jose, L.F.C.L.; Marcela, A.S. Analytical Features of Diclofenac Evaluation in Water as a Potential Marker of Anthropogenic Pollution. Curr. Pharm. Anal. 2016, 13, 39–47. [Google Scholar]
- Ferrer, I.; Thurman, E.M. Analysis of 100 Pharmaceuticals and Their Degradates in Water Samples by Liquid Chromatography/Quadrupole Time-of-Flight Mass Spectrometry. J. Chromatogr. A 2012, 1259, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Arye, G.; Dror, I.; Berkowitz, B. Fate and Transport of Carbamazepine in Soil Aquifer Treatment (SAT) Infiltration Basin Soils. Chemosphere 2011, 82, 244–252. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The Removal of Pharmaceuticals, Personal Care Products, Endocrine Disruptors and Illicit Drugs during Wastewater Treatment and Its Impact on the Quality of Receiving Waters. Water Res. 2009, 43, 363–380. [Google Scholar] [CrossRef]
- Chen, P.; Lin, J.-J.; Lu, C.-S.; Ong, C.-T.; Hsieh, P.F.; Yang, C.-C.; Tai, C.-T.; Wu, S.-L.; Lu, C.-H.; Hsu, Y.-C.; et al. Carbamazepine-Induced Toxic Effects and HLA-B*1502 Screening in Taiwan. N. Engl. J. Med. 2011, 364, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.A.; Mudgil, A.V.; Rosmarin, D.M. Toxic Epidermal Necrolysis. J. Am. Acad. Dermatol. 2007, 56, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Jentink, J.; Dolk, H.; Loane, M.A.; Morris, J.K.; Wellesley, D.; Garne, E.; de Jong-van den Berg, L.; EUROCAT Antiepileptic Study Working Group. Intrauterine Exposure to Carbamazepine and Specific Congenital Malformations: Systematic Review and Case-Control Study. BMJ 2010, 341, c6581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cummings, C.; Stewart, M.; Stevenson, M.; Morrow, J.; Nelson, J. Neurodevelopment of Children Exposed in Utero to Lamotrigine, Sodium Valproate and Carbamazepine. Arch. Dis. Child. 2011, 96, 643–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, D.E.; Brice-Bennett, S.; D’Souza, S.W. Antiepileptic Medication during Pregnancy: Does Fetal Genotype Affect Outcome? Pediatr. Res. 2007, 62, 120–127. [Google Scholar] [CrossRef]
- Hai, F.I.; Nghiem, L.D.; Khan, S.J.; Price, W.E.; Yamamoto, K. Wastewater Reuse: Removal of Emerging Trace Organic Contaminants. In Membrane Biological Reactors: Theory, Modeling, Design, Management and Applications to Wastewater Reuse; IWA Publishing: London, UK, 2014; ISBN 978-1-78040-065-5. [Google Scholar]
- Vieno, N.; Tuhkanen, T.; Kronberg, L. Elimination of Pharmaceuticals in Sewage Treatment Plants in Finland. Water Res. 2007, 41, 1001–1012. [Google Scholar] [CrossRef]
- Ying, G.-G.; Kookana, R.S.; Kolpin, D.W. Occurrence and Removal of Pharmaceutically Active Compounds in Sewage Treatment Plants with Different Technologies. J. Environ. Monit. 2009, 11, 1498–1505. [Google Scholar] [CrossRef]
- Tixier, C.; Singer, H.P.; Oellers, S.; Müller, S.R. Occurrence and Fate of Carbamazepine, Clofibric Acid, Diclofenac, Ibuprofen, Ketoprofen, and Naproxen in Surface Waters. Environ. Sci. Technol. 2003, 37, 1061–1068. [Google Scholar] [CrossRef]
- Wijekoon, K.C.; Hai, F.I.; Kang, J.; Price, W.E.; Guo, W.; Ngo, H.H.; Nghiem, L.D. The Fate of Pharmaceuticals, Steroid Hormones, Phytoestrogens, UV-Filters and Pesticides during MBR Treatment. Bioresour. Technol. 2013, 144, 247–254. [Google Scholar] [CrossRef]
- Guo, Y.C.; Krasner, S.W. Occurrence of Primidone, Carbamazepine, Caffeine, and Precursors for N-Nitrosodimethylamine in Drinking Water Sources Impacted by Wastewater1. JAWRA J. Am. Water Resour. Assoc. 2009, 45, 58–67. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A Review on the Occurrence of Micropollutants in the Aquatic Environment and Their Fate and Removal during Wastewater Treatment. Sci. Total Environ. 2014, 473, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Villota, N.; Lombraña, J.I.; Cruz-Alcalde, A.; Marcé, M.; Esplugas, S. Kinetic Study of Colored Species Formation during Paracetamol Removal from Water in a Semicontinuous Ozonation Contactor. Sci. Total Environ. 2019, 649, 1434–1442. [Google Scholar] [CrossRef]
- Mijangos, F.; Varona, F.; Villota, N. Changes in Solution Color During Phenol Oxidation by Fenton Reagent. Environ. Sci. Technol. 2006, 40, 5538–5543. [Google Scholar] [CrossRef] [PubMed]
- Villota, N.; Lomas, J.M.; Camarero, L.M. Study of the Paracetamol Degradation Pathway That Generates Color and Turbidity in Oxidized Wastewaters by Photo-Fenton Technology. J. Photochem. Photobiol. A Chem. 2016, 329, 113–119. [Google Scholar] [CrossRef]
- Villota, N.; Ferreiro, C.; Qulatein, H.A.; Lomas, J.M.; Camarero, L.M.; Lombraña, J.I. Colour Changes during the Carbamazepine Oxidation by Photo-Fenton. Catalysts 2021, 11, 386. [Google Scholar] [CrossRef]
- Sun, S.; Yao, H.; Fu, W.; Liu, F.; Wang, X.; Zhang, W. Enhanced Degradation of Carbamazepine in FeOCl Based Photo-Fenton Reaction. J. Environ. Chem. Eng. 2021, 9, 104501. [Google Scholar] [CrossRef]
- Yao, W.; Qu, Q.; von Gunten, U.; Chen, C.; Yu, G.; Wang, Y. Comparison of Methylisoborneol and Geosmin Abatement in Surface Water by Conventional Ozonation and an Electro-Peroxone Process. Water Res. 2017, 108, 373–382. [Google Scholar] [CrossRef]
- Oturan, M.A.; Aaron, J.-J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Chiron, S. Solar Photo-Fenton like Using Persulphate for Carbamazepine Removal from Domestic Wastewater. Water Res. 2014, 48, 229–236. [Google Scholar] [CrossRef]
- Expósito, A.J.; Monteagudo, J.M.; Durán, A.; San Martín, I.; González, L. Study of the Intensification of Solar Photo-Fenton Degradation of Carbamazepine with Ferrioxalate Complexes and Ultrasound. J. Hazard. Mater. 2018, 342, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhu, W.; Yang, D.; Wang, X.; Li, Y.; Gong, C.; Yan, J.; Zhai, J.; Gao, X.; Luo, Y. A Green Solar Photo-Fenton Process for the Degradation of Carbamazepine Using Natural Pyrite and Organic Acid with in-Situ Generated H2O2. Sci. Total Environ. 2021, 784, 147187. [Google Scholar] [CrossRef] [PubMed]
- Casierra-Martinez, H.A.; Madera-Parra, C.A.; Vargas-Ramírez, X.M.; Caselles-Osorio, A.; Torres-López, W.A. Diclofenac and Carbamazepine Removal from Domestic Wastewater Using a Constructed Wetland-Solar Photo-Fenton Coupled System. Ecol. Eng. 2020, 153, 105699. [Google Scholar] [CrossRef]
- BOE.Es—BOE-A-2003-3596 Royal Decree 140/2003, of February 7th, Establishing Sanitary Criteria for the Quality of Water for Human Consumption. Available online: https://www.boe.es/buscar/doc.php?id=BOE-A-2003-3596 (accessed on 24 June 2021).
- BOE.Es—BOE-A-2007-21092 Royal Decree 1620/2007, of December 7th, Establishing the Legal Regime for the Reuse of Purified Waters. Available online: https://www.boe.es/buscar/doc.php?id=BOE-A-2007-21092 (accessed on 24 June 2021).
- De Laurentiis, E.; Chiron, S.; Kouras-Hadef, S.; Richard, C.; Minella, M.; Maurino, V.; Minero, C.; Vione, D. Photochemical Fate of Carbamazepine in Surface Freshwaters: Laboratory Measures and Modeling. Environ. Sci. Technol. 2012, 46, 8164–8173. [Google Scholar] [CrossRef] [Green Version]
- Krakstrom, M.; Saeid, S.; Tolvanen, P.; Kumar, N.; Salmi, T.; Kronberg, L.; Eklund, P. Ozonation of Carbamazepine and Its Main Transformation Products: Product Determination and Reaction Mechanisms. Environ. Sci. Pollut. Res. 2020, 27, 23258–23269. [Google Scholar] [CrossRef] [PubMed]
- McDowell, D.C.; Huber, M.M.; Wagner, M.; von Gunten, U.; Ternes, T.A. Ozonation of Carbamazepine in Drinking Water: Identification and Kinetic Study of Major Oxidation Products. Environ. Sci. Technol. 2005, 39, 8014–8022. [Google Scholar] [CrossRef] [PubMed]
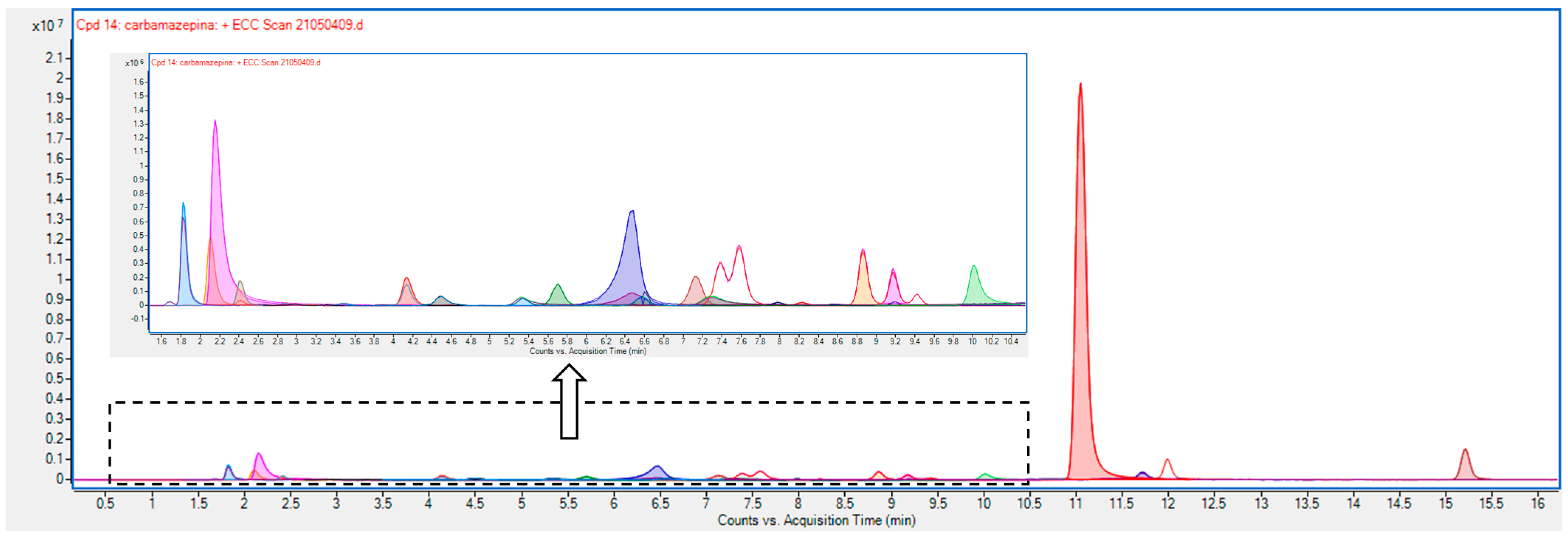


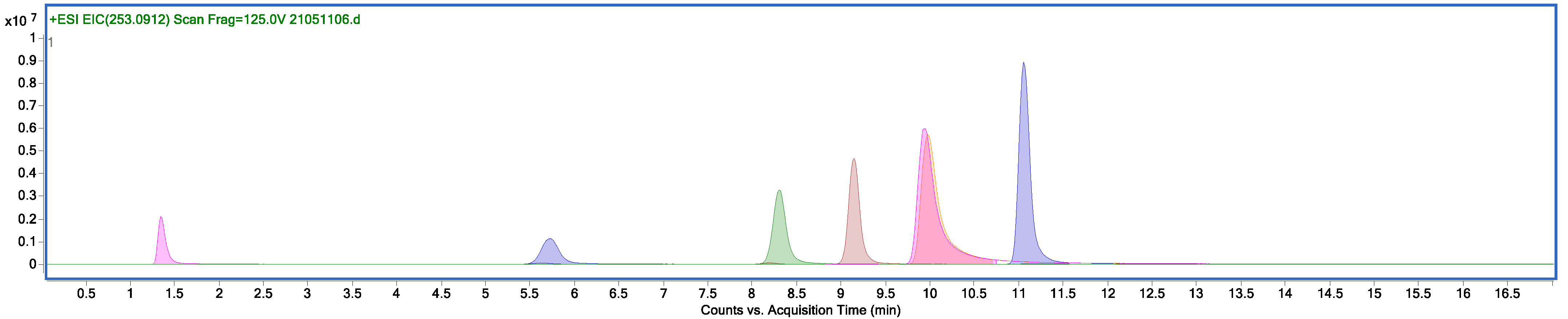
| Compound | Tr, min | m/z |
|---|---|---|
| 4-aminophenol | 1.3 | 110.0600 |
| Malonic acid | 1.8 | 105.0182 |
| 10-OH CBZ | 5.8 | 255.1128 |
| Epoxi-CBZ | 8.2 | 253.0972 |
| Oxo-CBZ | 9.1 | 253.0972 |
| 9-acridanone | 9.9 | 196.0757 |
| Acridin-9-ol | 9.9 | 196.0757 |
| CBZ | 11.2 | 237.1022 |
| Compound | pH = 2.0 | pH = 3.0 | pH = 4.0 | pH = 5.0 |
|---|---|---|---|---|
| CBZ | 1.8 | 10.8 | 34.4 | 17.0 |
| Oxo-CBZ | ˂LQL | 0.25 | 0.21 | 0.037 |
| Epoxi-CBZ | - | - | - | - |
| 10-OH CBZ | ˂LQL | 0.065 | 0.022 | 0.013 |
| 9-acridanone and acridin-9-ol | 0.013 | 0.026 | 0.004 | 0.010 |
| 4-aminophenol | n.d. | n.d. | n.d. | n.d. |
| Malonic acid | n.d. | n.d. | n.d. | n.d. |
| Compound | LOQ (ng/mL) | LOD (ng/mL) |
|---|---|---|
| CBZ | 100 | 30 |
| Oxo-CBZ | 2 | 0.6 |
| 10-OH CBZ | 2 | 0.6 |
| 9-acridanone | 1 | 0.3 |
| Acridin-9-ol | 1 | 0.3 |
| Compound | Range (µg/mL) | Regression Equation | R2 |
|---|---|---|---|
| CBZ | 0.1–50 | y = 3.580 x + 6.340 | 0.998 |
| Oxo-CBZ | 0.002–0.5 | y = 2.933 x + 5.480 | 0.998 |
| 10-OH CBZ | 0.002–0.5 | y = 2.008 x + 3.146 | 0.993 |
| 9-acridanone | 0.001–0.5 | y = 5.298 x + 9.636 | 0.998 |
| Acridin-9-ol | 0.001–0.5 | y = 5.034 x + 8.996 | 0.998 |
| Compound | Parameter | Amount Added (µg/mL) | Amount Recovered (µg/mL) | Degradation (%) |
|---|---|---|---|---|
| CBZ | Acidic degradation | 25.05 | 24.58 | 1.87 |
| Alkaline degradation | 25.05 | 24.29 | 3.04 | |
| Solar light | 25.05 | 24.87 | 0.71 | |
| OX-CBZ | Acidic degradation | 0.251 | 0.246 | 2.03 |
| Alkaline degradation | 0.251 | 0.241 | 3.87 | |
| Solar light | 0.251 | 0.249 | 0.98 | |
| 10-OH CBZ | Acidic degradation | 0.251 | 0.247 | 1.54 |
| Alkaline degradation | 0.251 | 0.240 | 4.20 | |
| Solar light | 0.251 | 0.249 | 0.85 | |
| Acridanone | Acidic degradation | 0.2505 | 0.2464 | 1.64 |
| Alkaline degradation | 0.2505 | 0.2412 | 3.72 | |
| Solar light | 0.2505 | 0.2480 | 0.99 | |
| Acridin 9-ol | Acidic degradation | 0.2505 | 0.2447 | 2.31 |
| Alkaline degradation | 0.2505 | 0.2418 | 3.47 | |
| Solar light | 0.2505 | 0.2479 | 1.02 |
| Compound | Range (µg/mL) | Recovery (Mean ± % RSD) |
|---|---|---|
| CBZ | 0.1–50 | 100.05 ± 0.023 |
| Oxo-CBZ | 0.002–0.5 | 100.24 ± 0.030 |
| 10-OH CBZ | 0.002–0.5 | 100.56 ± 0.011 |
| 9-acridanone | 0.001–0.5 | 100.08 ± 0.007 |
| Acridin-9-ol | 0.001–0.5 | 100.91 ± 0.024 |
| Compound | Concentration (µg/mL) | Standard Solution | Sample Solution | Mean | SD | % RSD | ||
|---|---|---|---|---|---|---|---|---|
| Intraday Precision | Interday Precision | Intraday Precision | Inter-Day Precision | |||||
| CBZ | 25.05 | 96.0190 | 96.9792 | 98.0188 | 98.9080 | 97.4812 | 98.7352 | 0.0128 |
| OX-CBZ | 0.251 | 6.2164 | 6.1542 | 6.0696 | 6.0908 | 6.1328 | 6.1988 | 0.0108 |
| 10-OH CBZ | 0.251 | 3.6500 | 3.7595 | 3.8475 | 3.7264 | 3.7459 | 3.8279 | 0.0118 |
| Acridanone | 0.2505 | 10.9633 | 10.6344 | 10.8027 | 10.7996 | 10.8000 | 10.934 | 0.0124 |
| Acridin 9-ol | 0.2505 | 10.2570 | 10.1544 | 10.0560 | 10.3688 | 10.2091 | 10.3431 | 0.0131 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villota, N.; Ferreiro, C.; Qulatein, H.A.; Lomas, J.M.; Lombraña, J.I. Turbidity Changes during Carbamazepine Oxidation by Photo-Fenton. Catalysts 2021, 11, 894. https://doi.org/10.3390/catal11080894
Villota N, Ferreiro C, Qulatein HA, Lomas JM, Lombraña JI. Turbidity Changes during Carbamazepine Oxidation by Photo-Fenton. Catalysts. 2021; 11(8):894. https://doi.org/10.3390/catal11080894
Chicago/Turabian StyleVillota, Natalia, Cristian Ferreiro, Hussein A. Qulatein, Jose M. Lomas, and Jose Ignacio Lombraña. 2021. "Turbidity Changes during Carbamazepine Oxidation by Photo-Fenton" Catalysts 11, no. 8: 894. https://doi.org/10.3390/catal11080894
APA StyleVillota, N., Ferreiro, C., Qulatein, H. A., Lomas, J. M., & Lombraña, J. I. (2021). Turbidity Changes during Carbamazepine Oxidation by Photo-Fenton. Catalysts, 11(8), 894. https://doi.org/10.3390/catal11080894









