Synthetic Routes to Arylsulfonyl Fluorides
Abstract
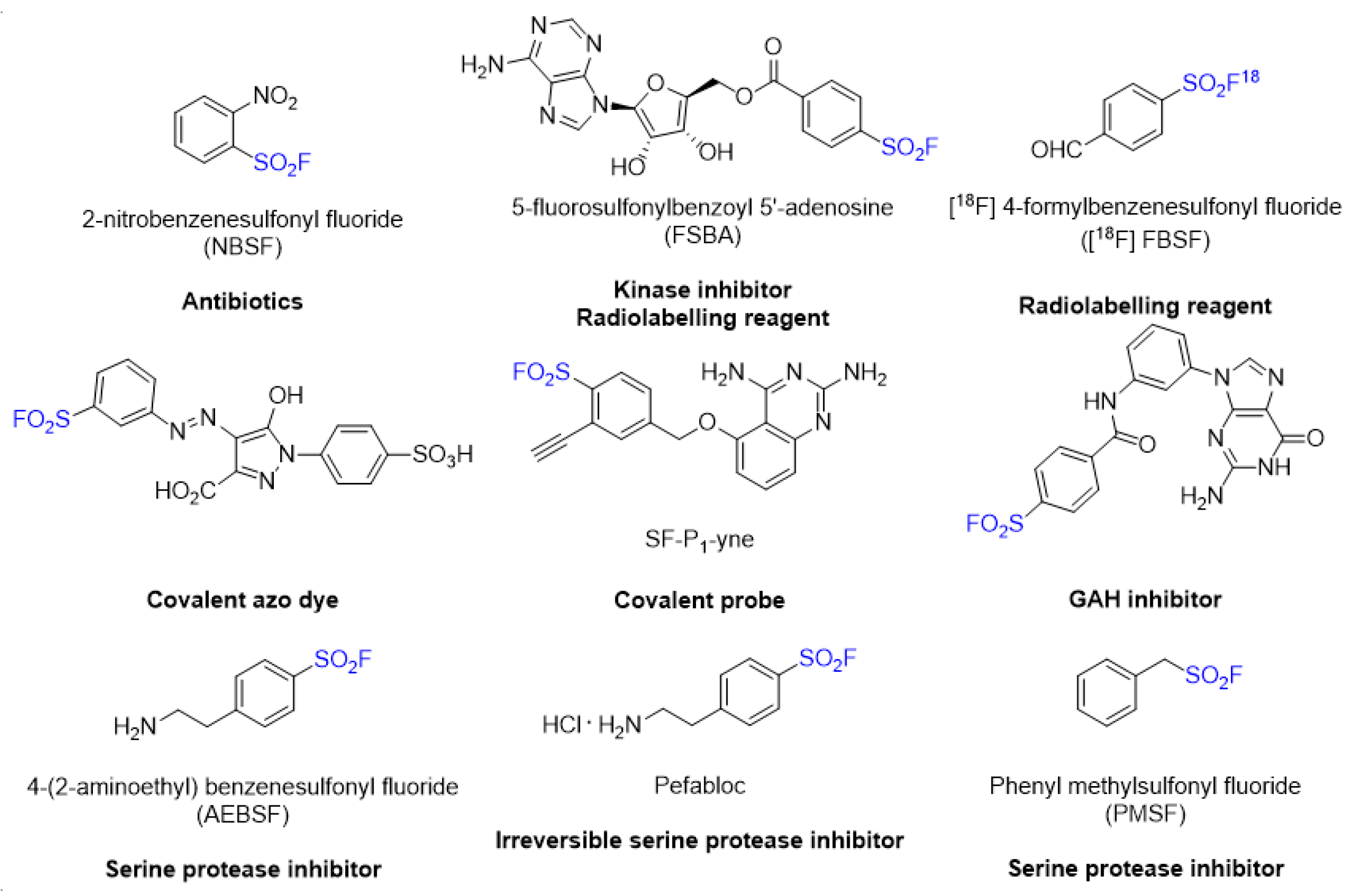
:1. Introduction
2. Direct Arylsulfonyl Fluoride Synthesis Reactions
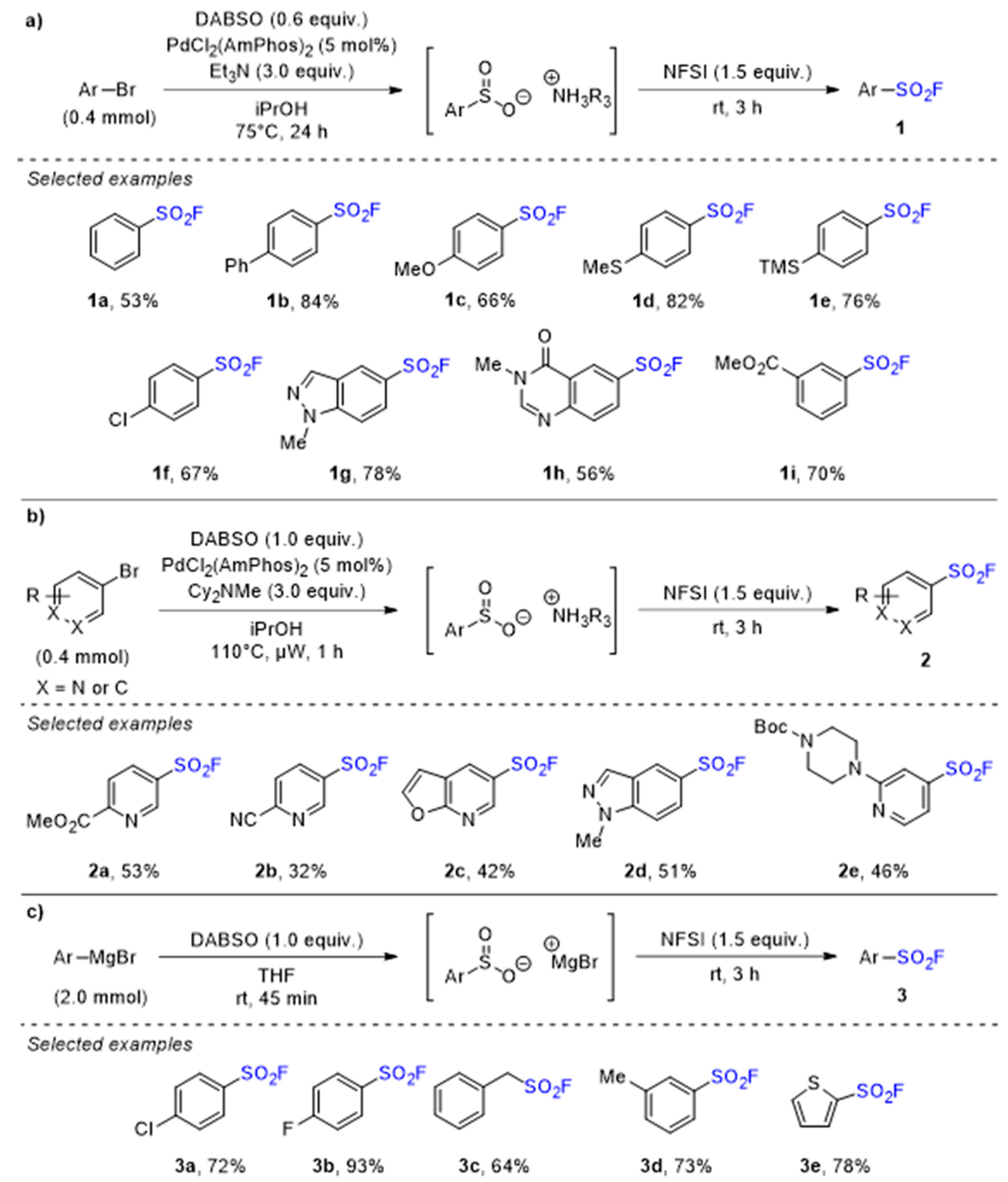
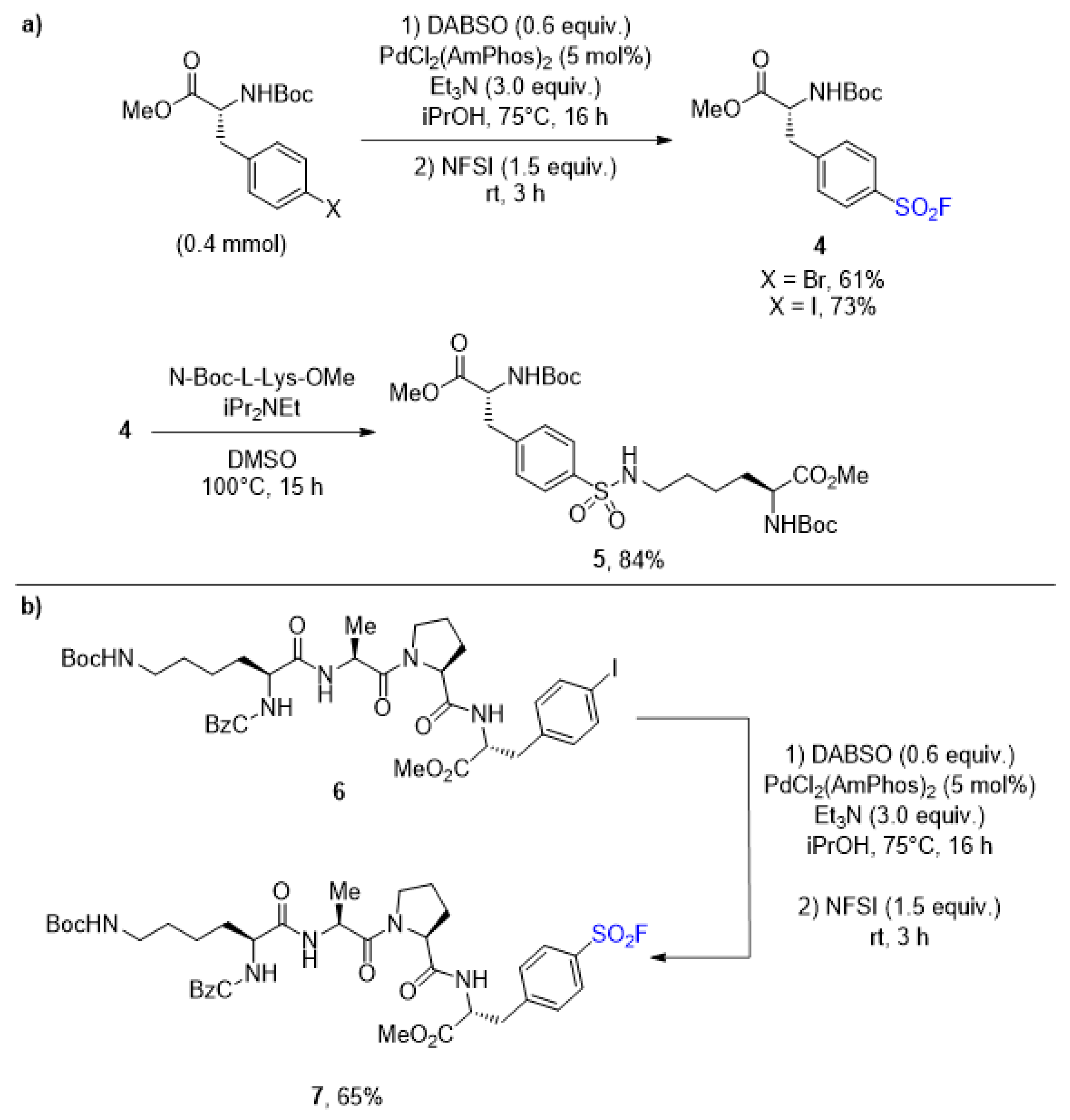
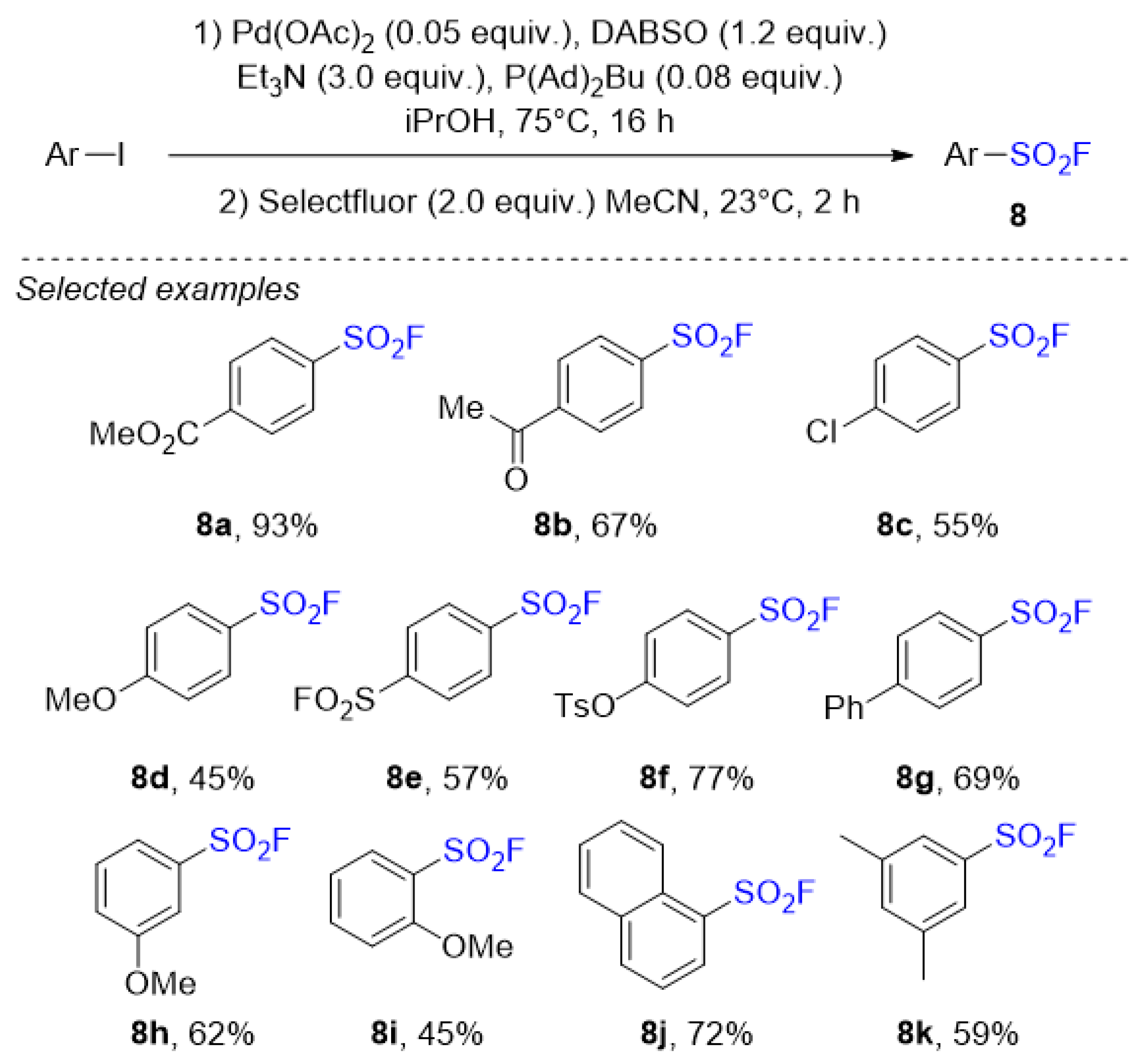
2.1. Sulfonyl Fluorides Synthesis from Aryl Halides
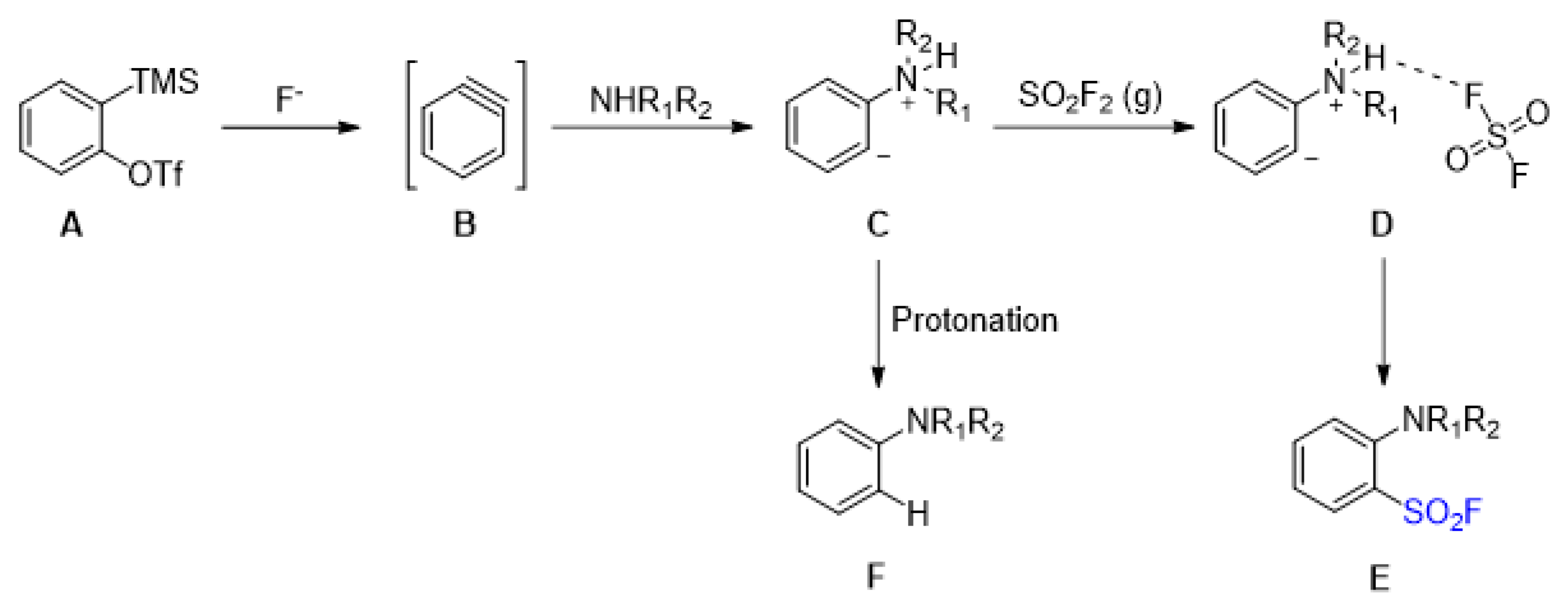
2.2. Sulfonyl Fluorides Synthesis from Arynes
2.3. Sulfonyl Fluorides Synthesis from Grignard Reagents
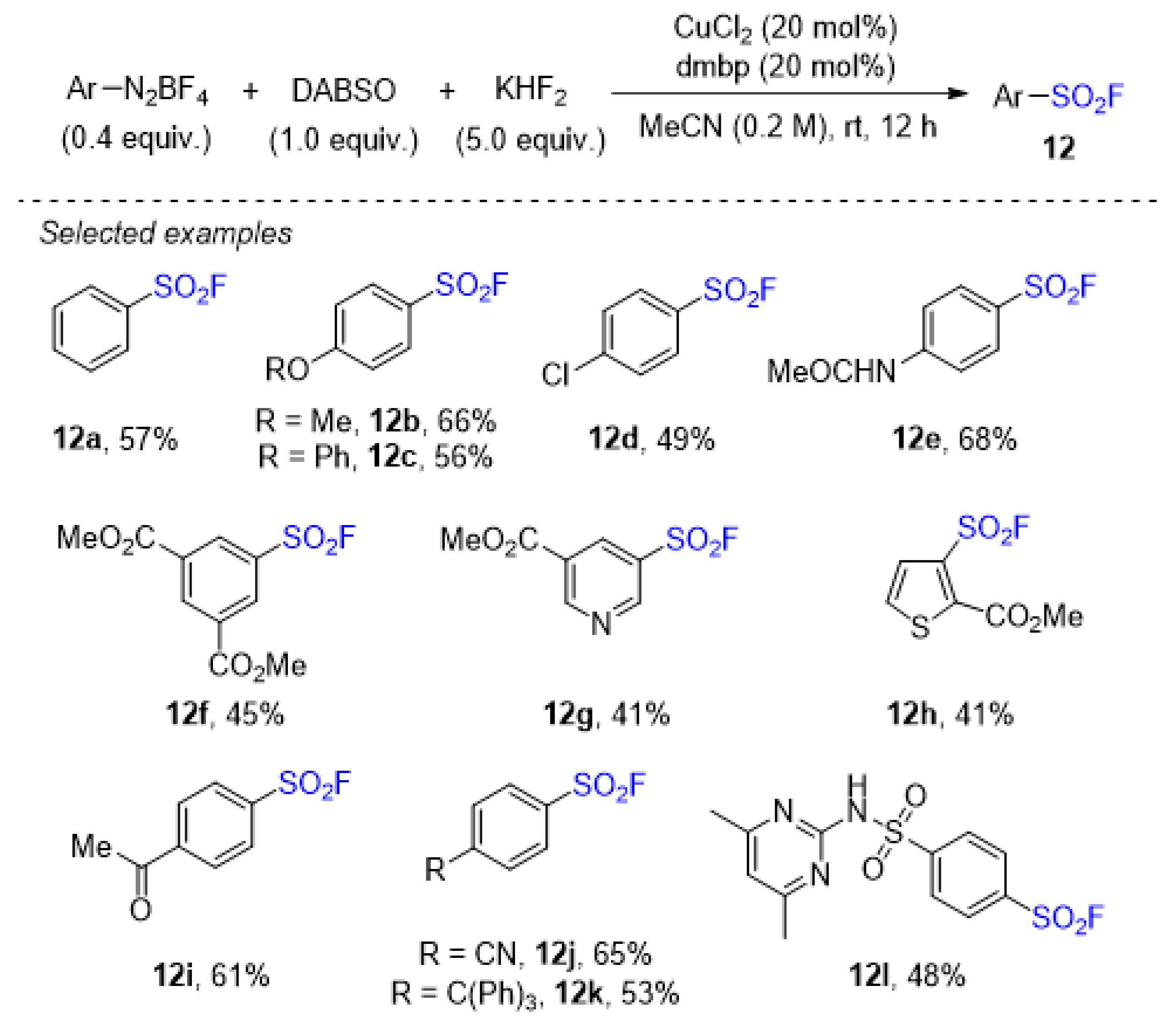
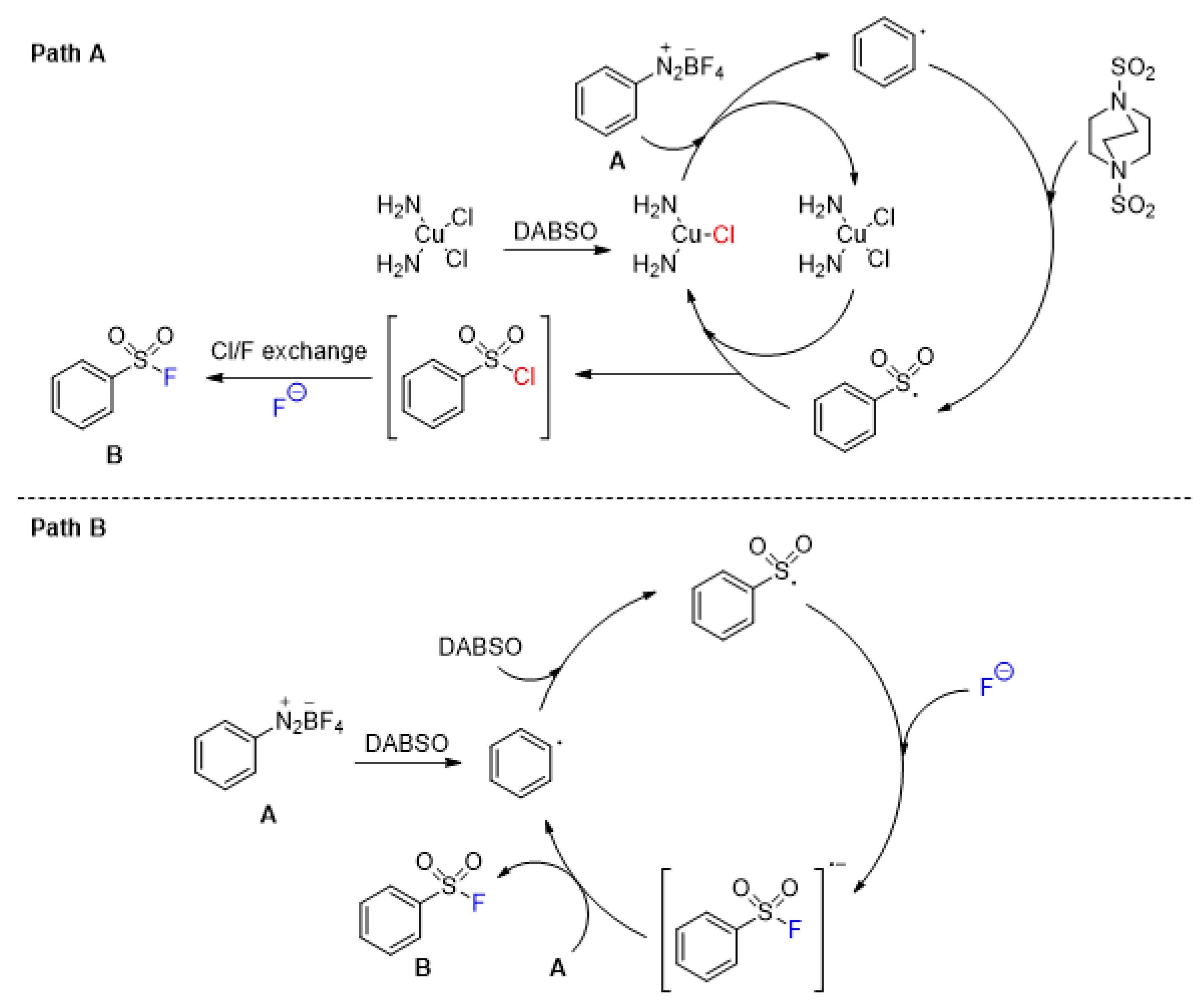
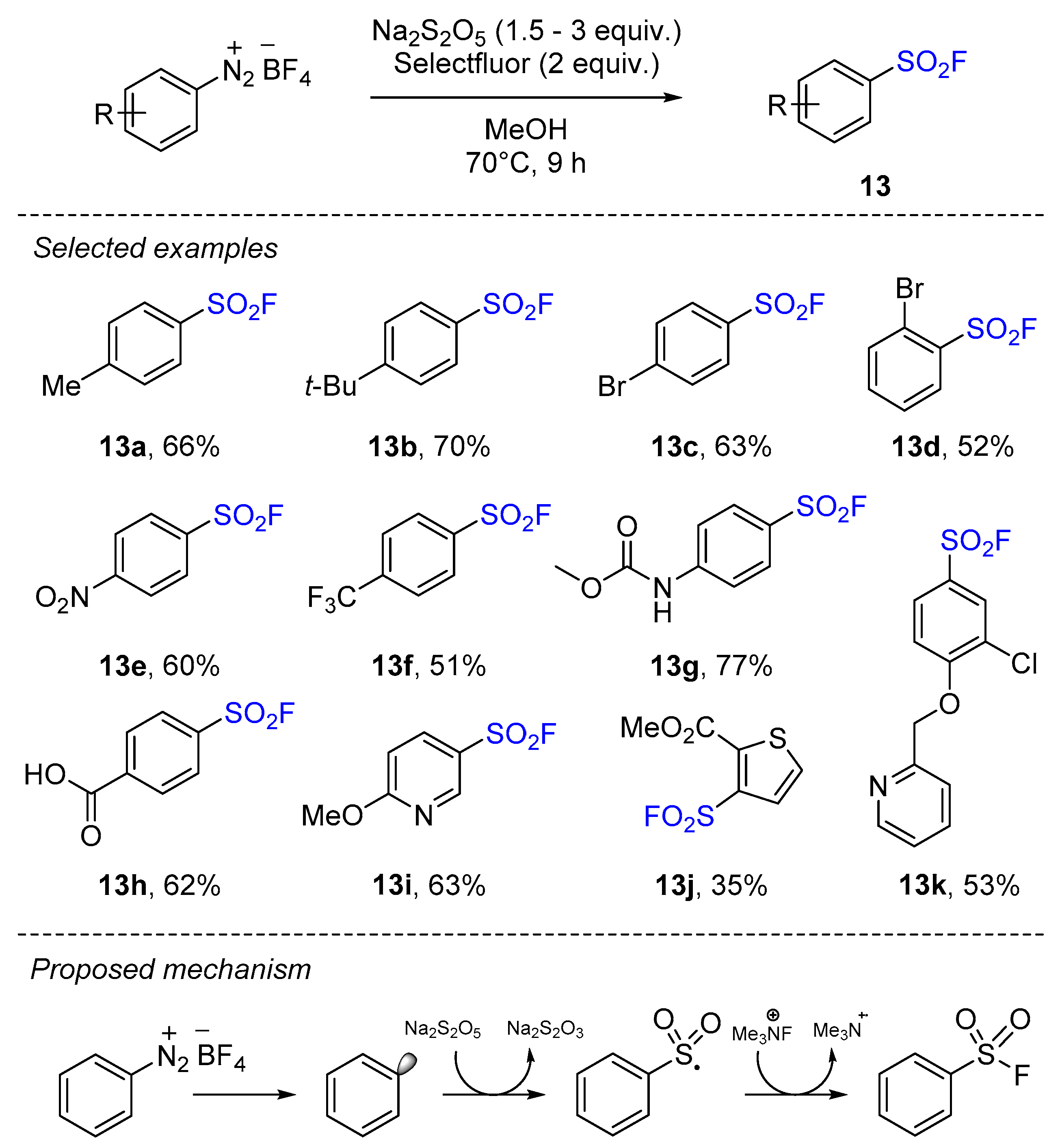
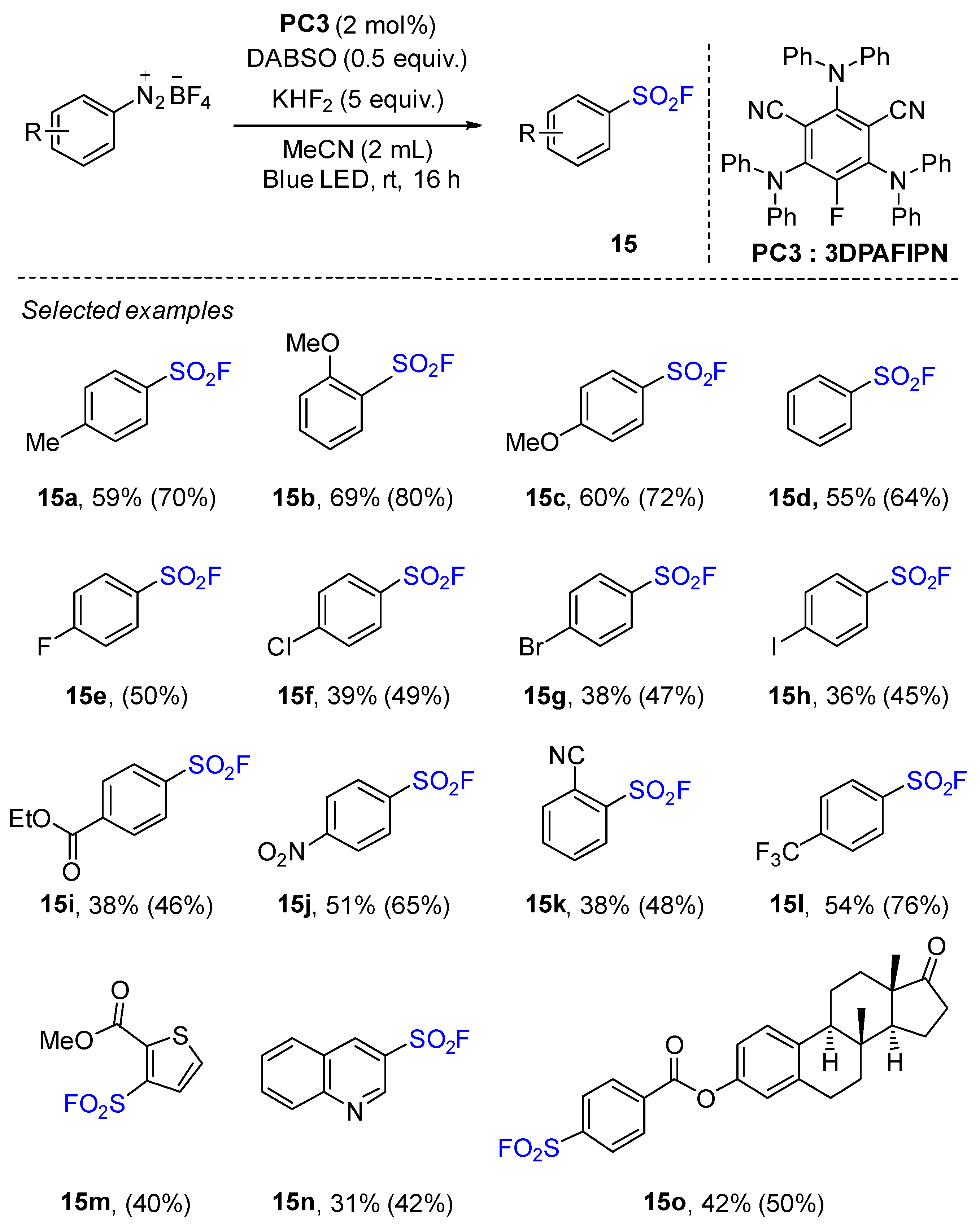
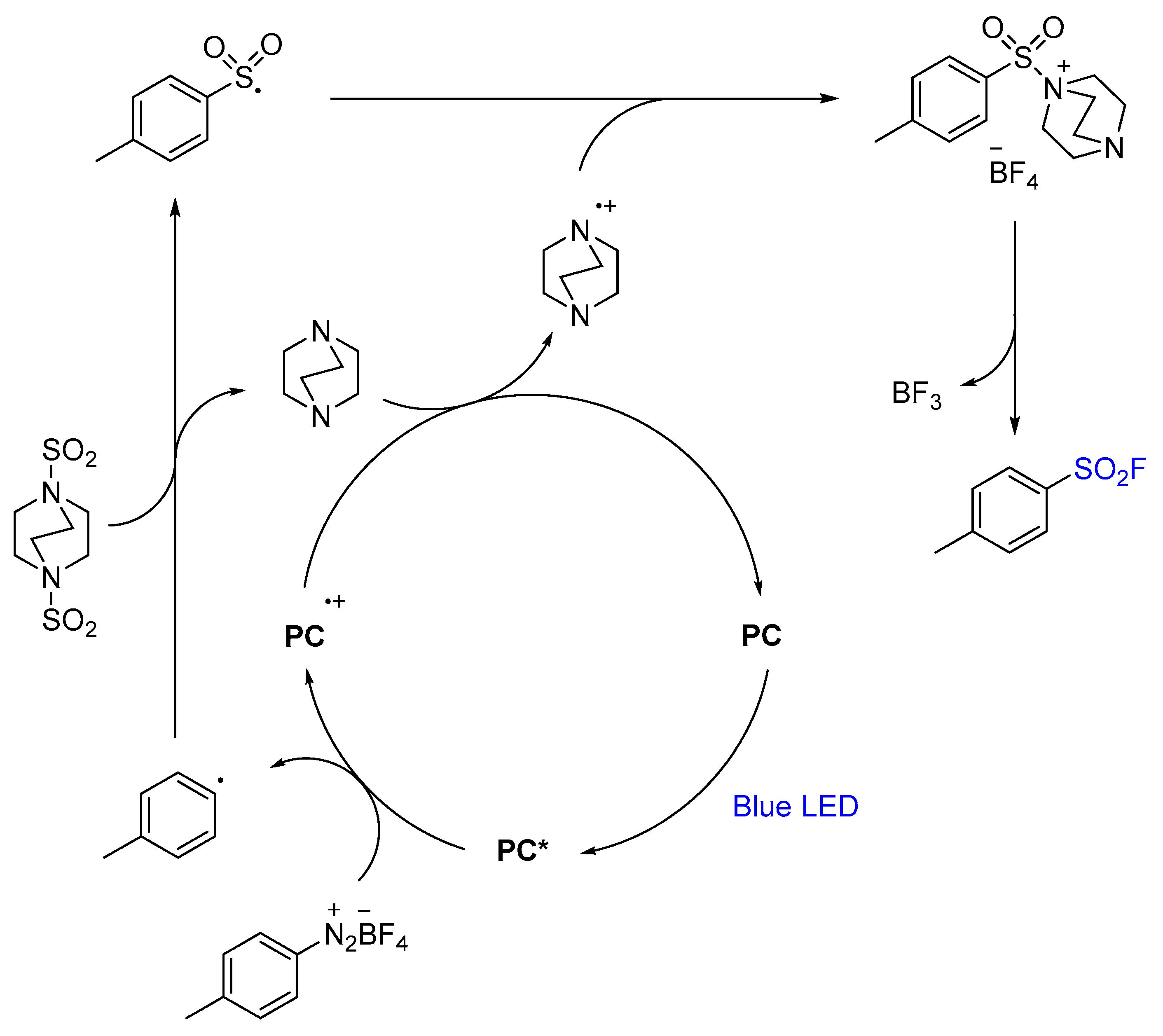
2.4. Sulfonyl Fluorides Synthesis from Aryldiazonium Salts
3. Indirect Arylesulfonyl Fluoride Synthesis Reactions
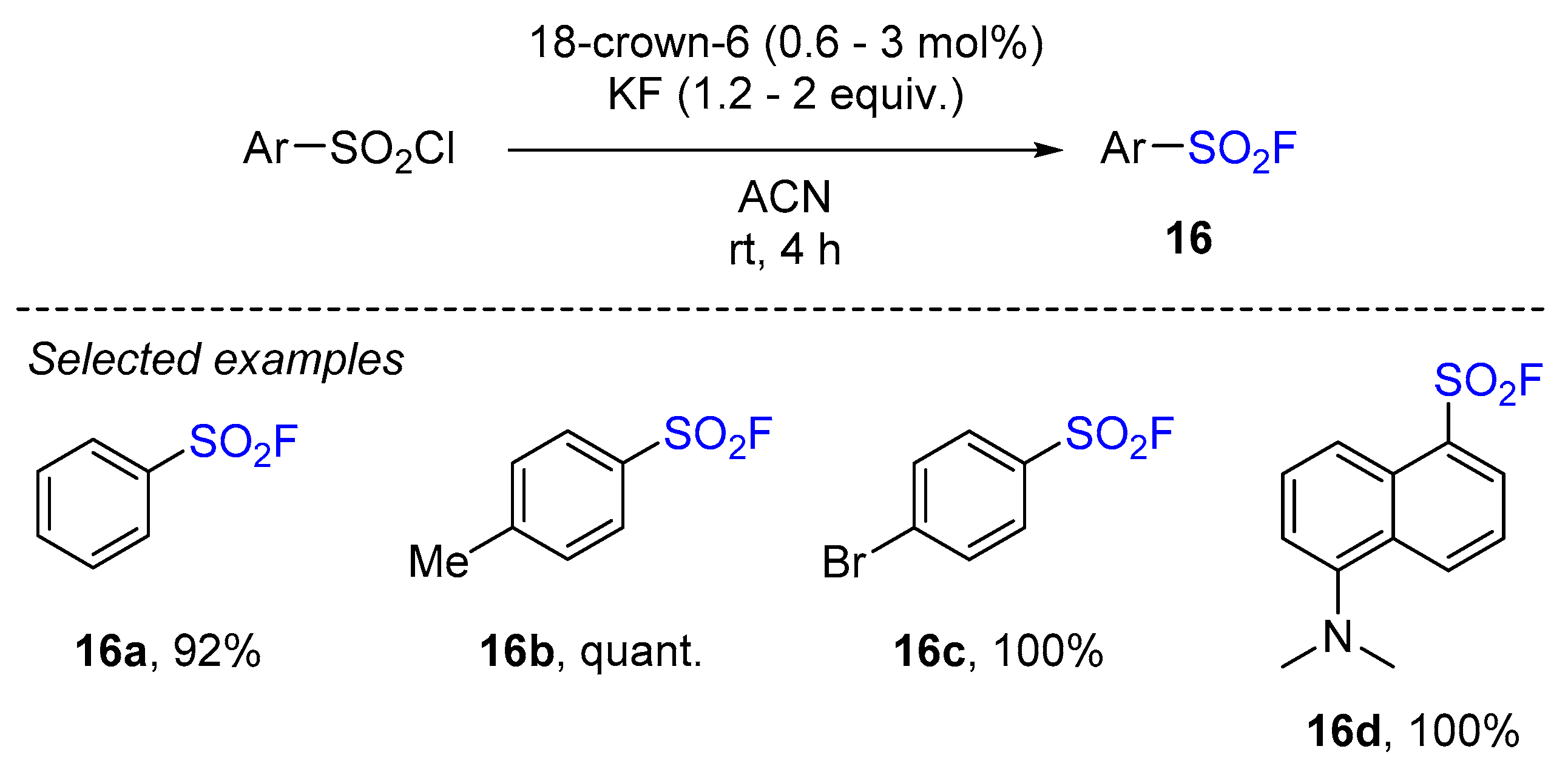
3.1. Sulfonyl Fluorides Synthesis from Arylsulfonyl Chlorides
3.2. Sulfonyl Fluorides Synthesis from Sulfonyl Hydrazides and Sodium Arylsulfinates
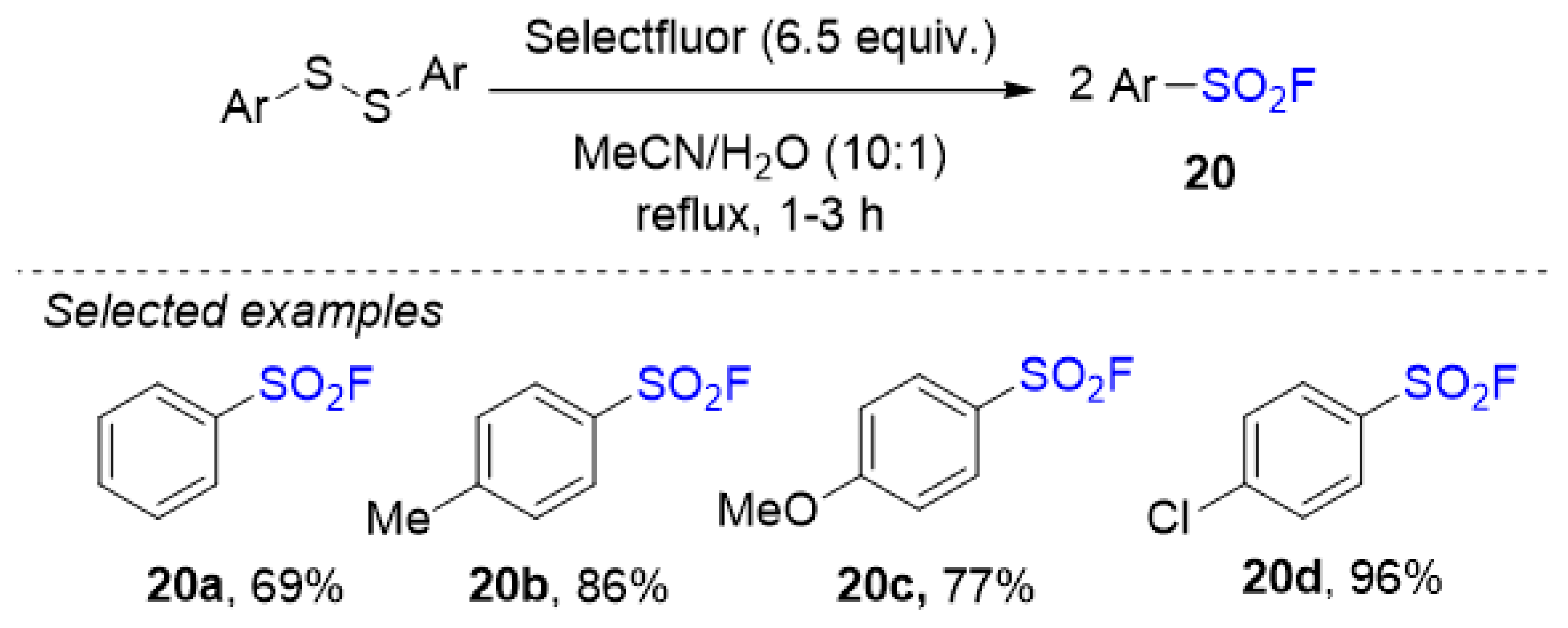
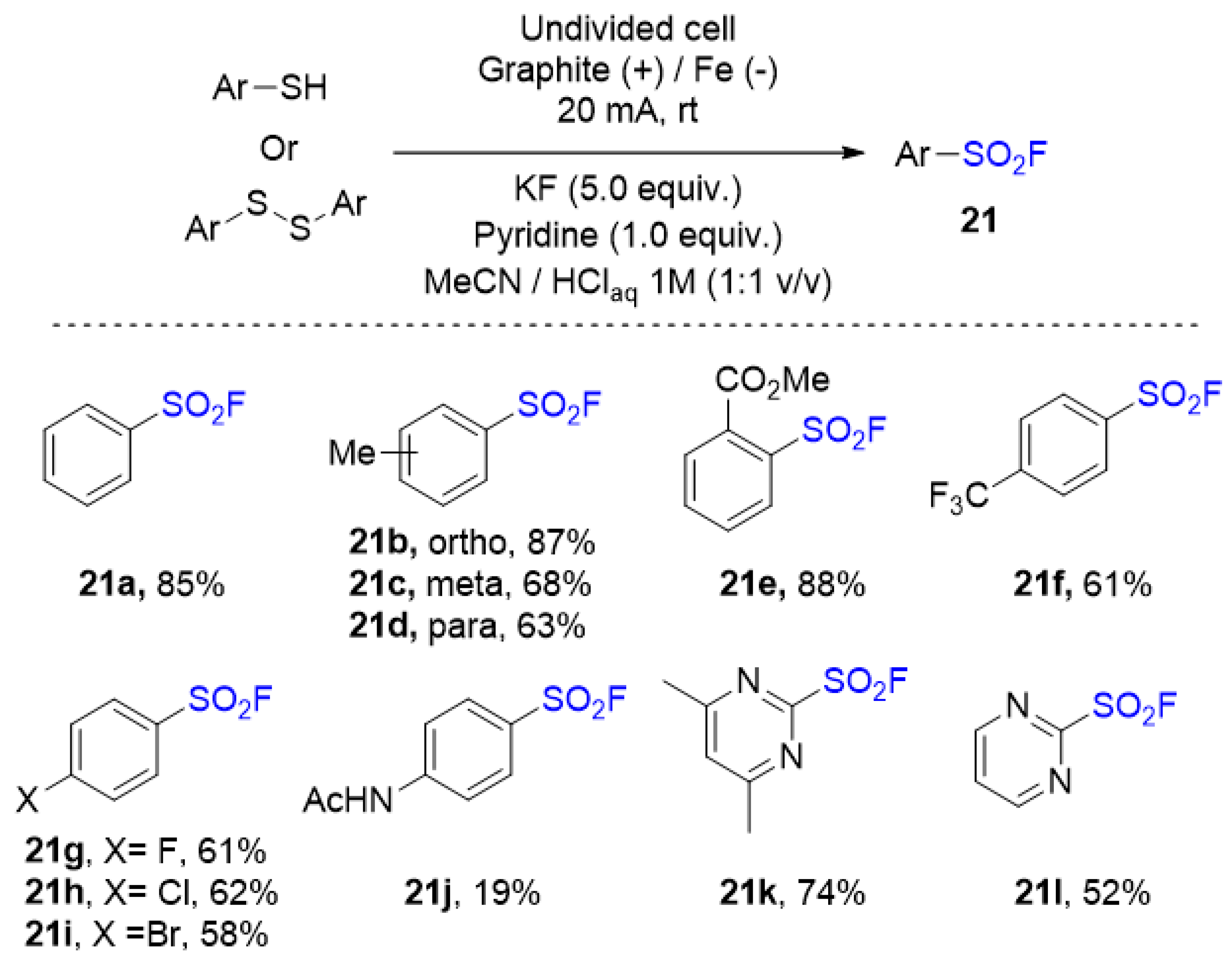
3.3. Sulfonyl Fluorides Synthesis from Thiols and Disulfides
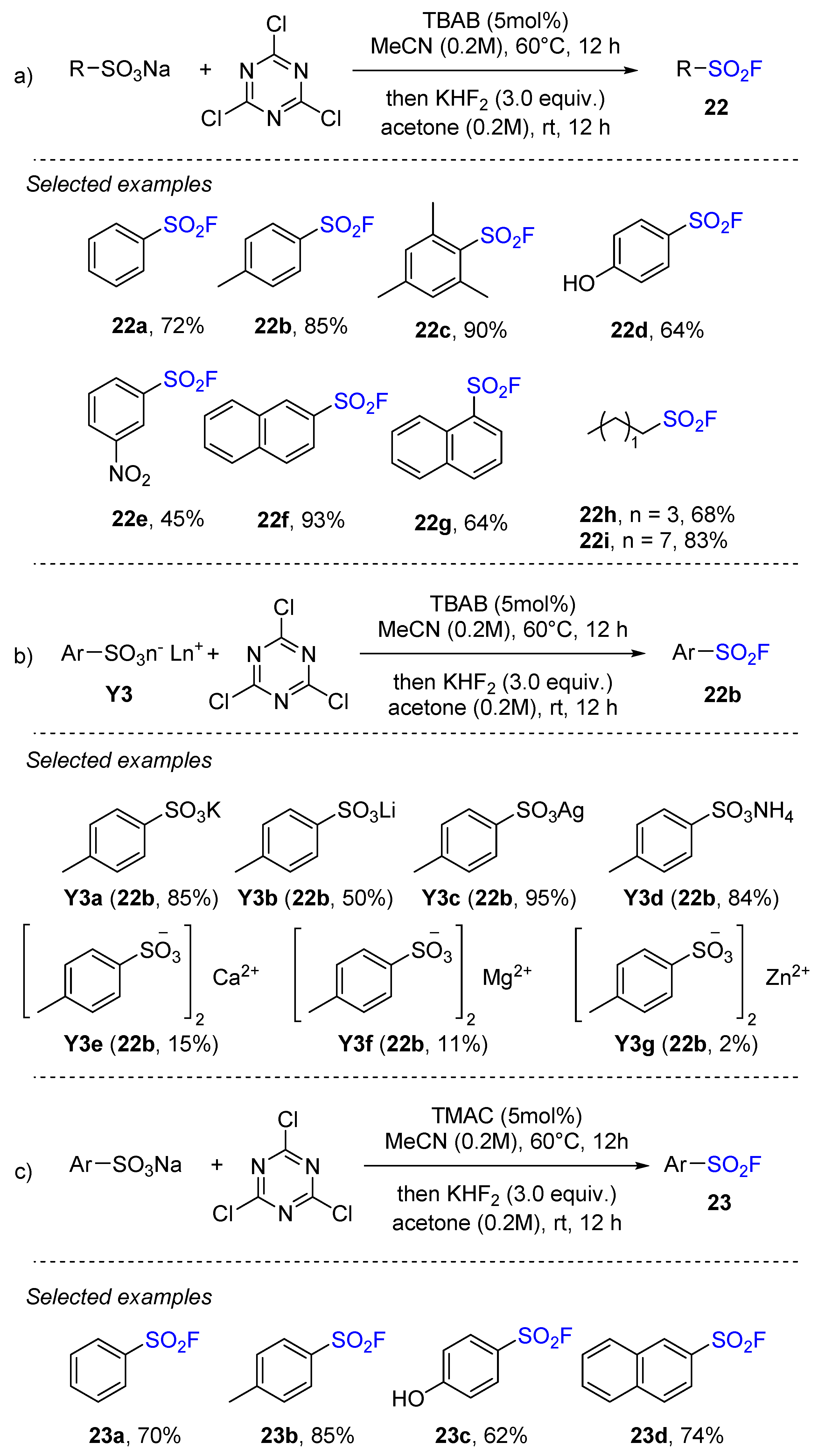
3.4. Sulfonyl Fluorides Synthesis from Sulfonates and Sulfonic Acids
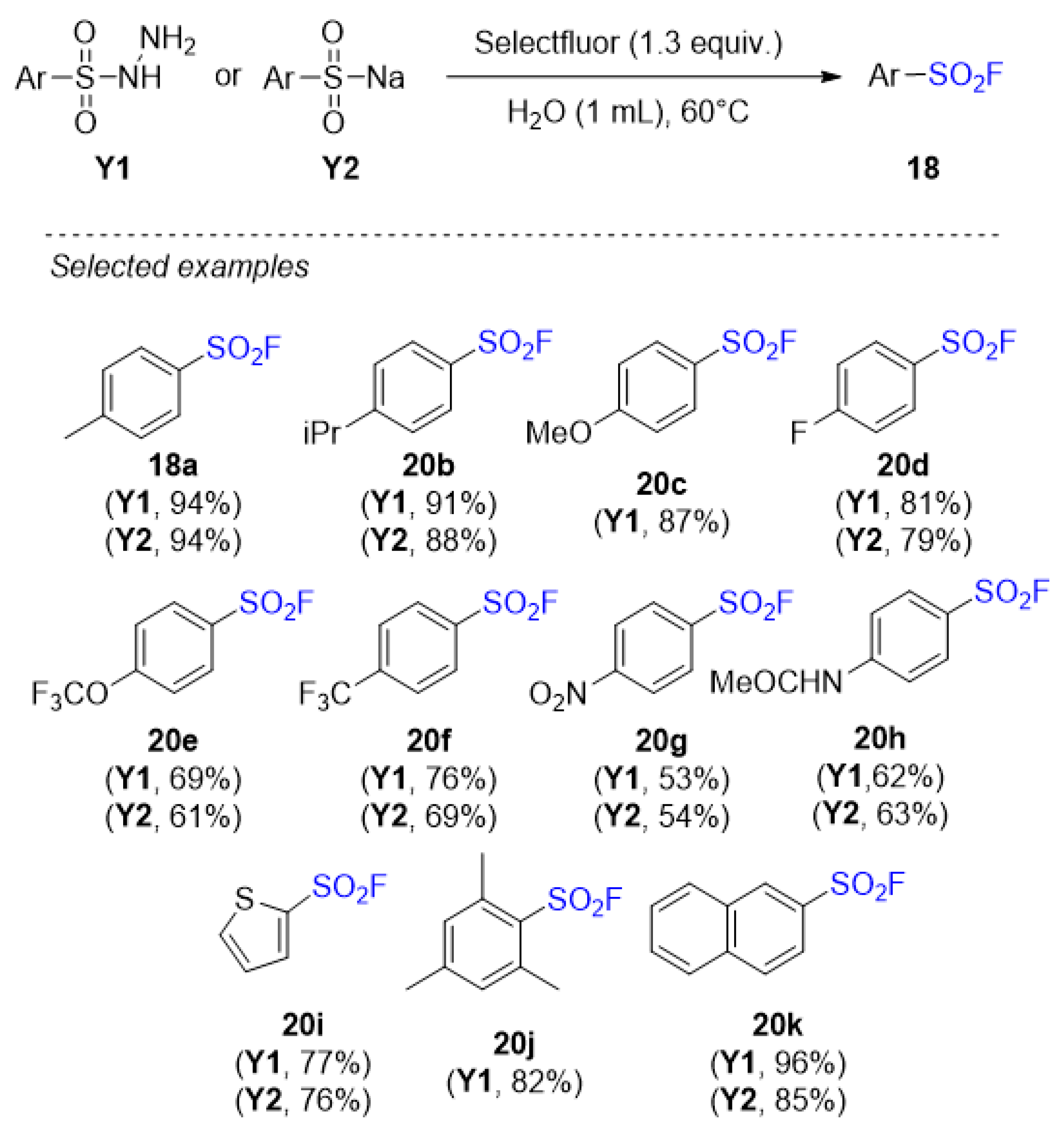
3.5. Sulfonyl Fluorides Synthesis from Sulfonamides
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shannon, D.A.; Gu, C.; McLaughlin, C.J.; Kaiser, M.; van der Hoorn, R.A.L.; Weerapana, E. Sulfonyl fluoride analogues as activity-based probes for serine proteases. ChemBioChem 2012, 13, 2327–2330. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, A.; Jones, L.H. Sulfonyl fluorides as privileged warheads in chemical biology. Chem. Sci. 2015, 6, 2650–2659. [Google Scholar] [CrossRef] [Green Version]
- Barrow, A.S.; Smedley, C.J.; Zheng, Q.; Li, S.; Dong, J.; Moses, J.E. The growing applications of SuFEx click chemistry. Chem. Soc. Rev. 2019, 48, 4731–4758. [Google Scholar] [CrossRef] [PubMed]
- Fattah, T.A.; Saeed, A.; Albericio, F. Recent advances towards sulfur (VI) fluoride exchange (SuFEx) click chemistry. J. Fluor. Chem. 2018, 213, 87–112. [Google Scholar] [CrossRef]
- Chinthakindi, P.K.; Arvidsson, P.I. Sulfonyl Fluorides (SFs): More Than Click Reagents? Eur. J. Org. Chem. 2018, 2018, 3648–3666. [Google Scholar] [CrossRef]
- Lee, C.; Cook, A.J.; Elisabeth, J.E.; Friede, N.C.; Sammis, G.M.; Ball, N.D. The Emerging Applications of Sulfur(VI) Fluorides in Catalysis. ACS Catal. 2021, 11, 6578–6589. [Google Scholar] [CrossRef]
- Grimster, N.P.; Connelly, S.; Baranczak, A.; Krasnova, L.B.; Powers, E.T.; Wilson, I.A.; Kelly, J.W.; Dong, J.; Sharpless, K.B. Aromatic Sulfonyl Fluorides Covalently Kinetically Stabilize Transthyretin to Prevent Amyloidogenesis while Affording a Fluorescent Conjugate. J. Am. Chem. Soc. 2013, 135, 5656–5668. [Google Scholar] [CrossRef] [Green Version]
- Hett, E.C.; Xu, H.; Geoghegan, K.F.; Gopalsamy, A.; Kyne, R.E.; Menard, C.A.; Narayanan, A.; Parikh, M.D.; Liu, S.; Roberts, L.; et al. Rational targeting of active-site tyrosine residues using sulfonyl fluoride probes. ACS Chem. Biol. 2015, 10, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Shannon, D.A.; Colby, T.; Wang, Z.; Shabab, M.; Kumari, S.; Villamor, J.G.; McLaughlin, C.J.; Weerapana, E.; Kaiser, M.; et al. Chemical proteomics with sulfonyl fluoride probes reveals selective labeling of functional tyrosines in glutathione transferases. Chem. Biol. 2013, 20, 541–548. [Google Scholar] [CrossRef] [Green Version]
- Kruse, C.H.; Holden, K.G.; Pritchard, M.L.; Feild, J.A.; Rieman, D.J.; Greig, R.G.; Poste, G. Synthesis and Evaluation of Multisubstrate Inhibitors of an Oncogene-Encoded Tyrosine-Specific Protein Kinase. J. Med. Chem. 1988, 31, 1762–1767. [Google Scholar] [CrossRef]
- Brouwer, A.J.; Alvarez, N.H.; Ciaffoni, A.; van de Langemheen, H.; Liskamp, R.M.J. Proteasome inhibition by new dual warhead containing peptido vinyl sulfonyl fluorides. Bioorg. Med. Chem. 2016, 24, 3429–3435. [Google Scholar] [CrossRef] [Green Version]
- Inkster, J.A.H.; Liu, K.; Ait-Mohand, S.; Schaffer, P.; Guérin, B.; Ruth, T.J.; Storr, T. Sulfonyl Fluoride-Based Prosthetic Compounds as Potential 18F Labelling Agents. Chem. Eur. J. 2012, 18, 11079–11087. [Google Scholar] [CrossRef]
- Matesic, L.; Wyatt, N.A.; Fraser, B.H.; Roberts, M.P.; Pham, T.Q.; Greguric, I. Ascertaining the suitability of aryl sulfonyl fluorides for [18F]radiochemistry applications: A systematic investigation using microfluidics. J. Org. Chem. 2013, 78, 11262–11270. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Zhou, F.; Jiang, J.; Chen, H.; Wang, L.; Chen, D.; Xu, Q.; Lu, J. Highly efficient polymerization via sulfur(vi)-fluoride exchange (SuFEx): Novel polysulfates bearing a pyrazoline–naphthylamide conjugated moiety and their electrical memory performance. Polym. Chem. 2018, 9, 1040–1044. [Google Scholar] [CrossRef]
- Yang, C.; Flynn, J.P.; Niu, J. Facile Synthesis of Sequence-Regulated Synthetic Polymers Using Orthogonal SuFEx and CuAAC Click Reactions. Angew. Chem. Int. Ed. 2018, 57, 16194–16199. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, F.; Ren, G.; Zheng, Q.; Chen, H.; Gao, B.; Klivansky, L.; Liu, Y.; Wu, B.; Xu, Q.; et al. SuFEx-Based Polysulfonate Formation from Ethenesulfonyl Fluoride-Amine Adducts. Angew. Chem. Int. Ed. 2017, 56, 11203–11208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, J.; Krasnova, L.; Finn, M.G.; Sharpless, K.B. Sulfur(VI) Fluoride Exchange (SuFEx): Another Good Reaction for Click Chemistry. Angew. Chem. Int. Ed. 2014, 53, 9430–9448. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.L.; Zheng, Q.; Bare, G.A.L.; Wu, P.; Sharpless, K.B. A Heck-Matsuda Process for the Synthesis of β-Arylethenesulfonyl Fluorides: Selectively Addressable Bis-electrophiles for SuFEx Click Chemistry. Angew. Chem. Int. Ed. 2016, 55, 14155–14158. [Google Scholar] [CrossRef] [Green Version]
- Yatvin, J.; Brooks, K.; Locklin, J. SuFEx Click: New Materials from SOxF and Silyl Ethers. J. Chem. Eur. J. 2016, 22, 16348–16354. [Google Scholar] [CrossRef]
- Dondoni, A.; Marra, A. SuFEx: A metal-free click ligation for multivalent biomolecules. Org. Biomol. Chem. 2017, 15, 1549–1553. [Google Scholar] [CrossRef]
- Nielsen, M.K.; Ugaz, C.R.; Li, W.; Doyle, A.G. PyFluor: A Low-Cost, Stable, and Selective Deoxyfluorination Reagent. J. Am. Chem. Soc. 2015, 137, 9571–9574. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, M.K.; Ahneman, D.T.; Riera, O.; Doyle, A.G. Deoxyfluorination with Sulfonyl Fluorides: Navigating Reaction Space with Machine Learning. J. Am. Chem. Soc. 2018, 140, 5004–5008. [Google Scholar] [CrossRef]
- Kice, J.L.; Lunney, E.A. Catalysis of the hydrolysis of aryl sulfonyl fluorides by acetate ion and trimethylamine. J. Org. Chem. 1975, 40, 2125–2127. [Google Scholar] [CrossRef]
- Mukherjee, H.; Debreczeni, J.; Breed, J.; Tentarelli, S.; Aquila, B.; Dowling, J.E.; Whitty, A.; Grimster, N.P. A study of the reactivity of S(VI)–F containing warheads with nucleophilic amino-acid side chains under physiological conditions. Org. Biomol. Chem. 2017, 15, 9685–9695. [Google Scholar] [CrossRef] [PubMed]
- Ciuffarin, E.; Senatore, L.; Isola, M. Nucleophilic substitution at four-co-ordinate sulphur. Mobility of the leaving group. J. Chem. Soc. Perkin Trans. 1972, 2, 468–471. [Google Scholar] [CrossRef]
- Baker, R.B.; Lourens, G.J. Irreversible Enzyme Inhibitors. CV.12 Differential Irreversible Inhibition of Vertebrate Dihydrofolic Reductases by Derivatives of 4,6-Diamino-l,2-dihydro-2,2-dimethyl-l-phenyl-s-triazines Substituted with a Terminal Sulfonyl Fluoride. J. Med. Chem. 1967, 10, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, M.P. Recent progress in the use of sulfonyl radicals in organic synthesis. A review. Org. Prep. Proced. Int. 1994, 26, 257–290. [Google Scholar] [CrossRef]
- Chinthakindi, P.K.; Kruger, H.G.; Govender, T.; Naicker, T.; Arvidsson, P.I. On-Water Synthesis of Biaryl Sulfonyl Fluorides. J. Org. Chem. 2016, 81, 2618–2623. [Google Scholar] [CrossRef]
- Davis, A.T.; Curto, J.M.; Bagley, S.W.; Willis, M.C. One-pot palladium-catalyzed synthesis of sulfonyl fluorides from aryl bromides. Chem. Sci. 2017, 8, 1233–1237. [Google Scholar] [CrossRef] [Green Version]
- Tribby, A.L.; Rodriguez, I.; Shariffudin, S.; Ball, N.D. Pd-Catalyzed Conversion of Aryl Iodides to Sulfonyl Fluorides Using SO2 Surrogate DABSO and Selectfluor. J. Org. Chem. 2017, 82, 2294–2299. [Google Scholar] [CrossRef]
- Kwon, J.; Kim, B.M. Synthesis of Arenesulfonyl Fluorides via Sulfuryl Fluoride Incorporation from Arynes. Org. Lett. 2019, 21, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Ball, N.D.; Sammis, G.M. One-pot fluorosulfurylation of Grignard reagents using sulfuryl fluoride. Chem. Commun. 2019, 55, 14753. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, D.; Guo, Y.; Xiao, J.; Chen, Q.; Liu, C. Arenesulfonyl Fluoride Synthesis via Copper-Catalyzed Fluorosulfonylation of Arenediazonium Salts. Org. Lett. 2020, 22, 2281–2286. [Google Scholar] [CrossRef]
- Zhong, T.; Pang, M.; Chen, Z.; Zhang, B.; Weng, J.; Lu, G. Copper-free Sandmeyer-type Reaction for the Synthesis of Sulfonyl Fluorides. Org. Lett. 2020, 22, 3072–3078. [Google Scholar] [CrossRef] [PubMed]
- Louvel, D.; Chelagha, A.; Rouillon, J.; Payard, P.-A.; Khrouz, L.; Monnereau, C.; Tlili, A. Metal-Free Visible-Light Synthesis of Arylsulfonyl Fluorides: Scope and Mechanism. Chem. Eur. J. 2021, 27, 8704–8708. [Google Scholar] [CrossRef] [PubMed]
- Louvel, D.; Ghiazza, C.; Debrauwer, V.; Khrouz, L.; Monnereau, C.; Tlili, A. Forging C-SeCF3 Bonds with Trifluoromethyl Tolueneselenosulfonate under Visible-Light. Chem. Rec. 2021, 21, 417–426. [Google Scholar] [CrossRef]
- Ghiazza, C.; Debrauwer, V.; Monnereau, C.; Khrouz, L.; Médebielle, M.; Billard, T.; Tlili, A. Visible-Light-Mediated Metal-Free Synthesis of Trifluoromethylselenolated Arenes. Angew. Chem. Int. Ed. 2018, 57, 11781–11785. [Google Scholar] [CrossRef]
- Ghiazza, C.; Monnereau, C.; Khrouz, L.; Médebielle, M.; Billard, T.; Tlili, A. New Avenues in Radical Trifluoromethylselenylation with Trifluoromethyl Tolueneselenosulfonate. Synlett 2019, 30, 777–782. [Google Scholar] [CrossRef]
- Ghiazza, C.; Khrouz, L.; Monnereau, C.; Billard, T.; Tlili, A. Visible-light promoted fluoroalkylselenolation: Toward the reactivity of unsaturated compounds. Chem. Commun. 2018, 54, 9909–9912. [Google Scholar] [CrossRef]
- Ghiazza, C.; Khrouz, L.; Billard, T.; Monnereau, C.; Tlili, A. Fluoroalkylselenolation of Alkyl Silanes/Trifluoroborates under Metal-Free Visible-Light Photoredox Catalysis. Eur. J. Org. Chem. 2020, 1559–1566. [Google Scholar] [CrossRef] [Green Version]
- Bianchi, T.A.; Cate, L.A. Phase Transfer Catalysis. Preparation of Aliphatic and Aromatic Sulfonyl Fluorides. J. Org. Chem. 1977, 42, 2031–2032. [Google Scholar] [CrossRef]
- Talko, A.; Barbasiewicz, M. Nucleophilic Fluorination with Aqueous Bifluoride Solution: Effect of the Phase-Transfer Catalyst. ACS Sustain. Chem. Eng. 2018, 6, 6693–6701. [Google Scholar] [CrossRef]
- Tang, L.; Yang, Y.; Wen, L.; Yang, X.; Wang, Z. Catalyst-free radical fluorination of sulfonyl hydrazides in water. Green Chem. 2016, 18, 1224–1228. [Google Scholar] [CrossRef]
- Wright, S.W.; Hallstrom, K.N. A Convenient Preparation of Heteroaryl Sulfonamides and Sulfonyl Fluorides from Heteroaryl Thiols. J. Org. Chem. 2006, 71, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Kirihara, M.; Naito, S.; Nishimura, Y.; Ishikuza, Y.; Iwai, T.; Takeuchi, H.; Ogata, T.; Hanai, H.; Kinoshita, Y.; Kishida, M.; et al. Oxidation of disulfides with electrophilic halogenating reagents: Concise methods for preparation of thiosulfonates and sulfonyl halides. Tetrahedron 2014, 70, 2464–2471. [Google Scholar] [CrossRef]
- Laudadio, G.; Bartolomeu, A.d.A.; Verwijlen, L.M.H.M.; Cao, Y.; de Oliveira, K.T.; Noël, T. Sulfonyl Fluoride Synthesis through Electrochemical Oxidative Coupling of Thiols and Potassium Fluoride. J. Am. Chem. Soc. 2019, 141, 11832–11836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldvogel, S.R.; Lips, S.; Selt, M.; Riehl, B.; Kampf, C.J. Electrochemical Arylation Reaction. Chem. Rev. 2018, 118, 6706–6765. [Google Scholar] [CrossRef]
- Yan, M.; Kawamata, Y.; Baran, P.S. Synthetic Organic Electrochemistry: Calling All Engineers. Angew. Chem. Int. Ed. 2018, 57, 4149–4155. [Google Scholar] [CrossRef]
- Wiebe, A.; Gieshoff, T.; Möhle, S.; Rodrigo, E.; Zirbes, M.; Waldvogel, S.R. Electrifying Organic Synthesis. Angew. Chem. Int. Ed. 2018, 57, 5594–5619. [Google Scholar] [CrossRef]
- Sauermann, N.; Meyer, T.H.; Ackermann, L. Electrochemical Cobalt-Catalyzed C−H Activation. Chem. Eur. J. 2018, 24, 16209–16217. [Google Scholar] [CrossRef]
- Yan, M.; Kawamata, Y.; Baran, P.S. Synthetic Organic Electrochemical Methods Since 2000: On the Verge of a Renaissance. Chem. Rev. 2017, 117, 13230–13319. [Google Scholar] [CrossRef]
- Pitts, C.R.; Bornemann, D.; Liebing, P.; Santschi, N.; Togni, A. Making the SF5 Group More Accessible: A Gas-Reagent-Free Approach to Aryl Tetrafluoro-λ6-sulfanyl Chlorides. Angew. Chem. Int. Ed. 2019, 58, 1950–1954. [Google Scholar] [CrossRef] [PubMed]
- Umemoto, T.; Garrick, L.M.; Saito, N. Discovery of practical production processes for arylsulfur pentafluorides and their higher homologues, bis- and tris(sulfur pentafluorides): Beginning of a new era of “super-trifluoromethyl” arene chemistry and its industry. J. Org. Chem. 2012, 8, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Umemoto, T.; Singh, R.P.; Xu, Y.; Saito, N. Discovery of 4-tert-Butyl-2,6-dimethylphenylsulfur Trifluoride as a Deoxofluorinating Agent with High Thermal Stability as Well as Unusual Resistance to Aqueous Hydrolysis, and Its Diverse Fluorination Capabilities Including Deoxofluoro-Arylsulfinylation with High Stereoselectivity. J. Am. Chem. Soc. 2010, 132, 18199–18205. [Google Scholar] [PubMed]
- Jiang, Y.; Alharbi, N.S.; Sun, B.; Qin, H. Facile one-pot synthesis of sulfonyl fluorides from sulfonates or sulfonic acids. RSC Adv. 2019, 9, 13863–13867. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Palau, M.; Cornella, J. Synthesis of Sulfonyl Fluorides from Sulfonamides. J. Eur. J. Org. Chem. 2020, 2020, 2497–2500. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chelagha, A.; Louvel, D.; Taponard, A.; Berthelon, R.; Tlili, A. Synthetic Routes to Arylsulfonyl Fluorides. Catalysts 2021, 11, 830. https://doi.org/10.3390/catal11070830
Chelagha A, Louvel D, Taponard A, Berthelon R, Tlili A. Synthetic Routes to Arylsulfonyl Fluorides. Catalysts. 2021; 11(7):830. https://doi.org/10.3390/catal11070830
Chicago/Turabian StyleChelagha, Aida, Dan Louvel, Alexis Taponard, Rodolphe Berthelon, and Anis Tlili. 2021. "Synthetic Routes to Arylsulfonyl Fluorides" Catalysts 11, no. 7: 830. https://doi.org/10.3390/catal11070830
APA StyleChelagha, A., Louvel, D., Taponard, A., Berthelon, R., & Tlili, A. (2021). Synthetic Routes to Arylsulfonyl Fluorides. Catalysts, 11(7), 830. https://doi.org/10.3390/catal11070830





