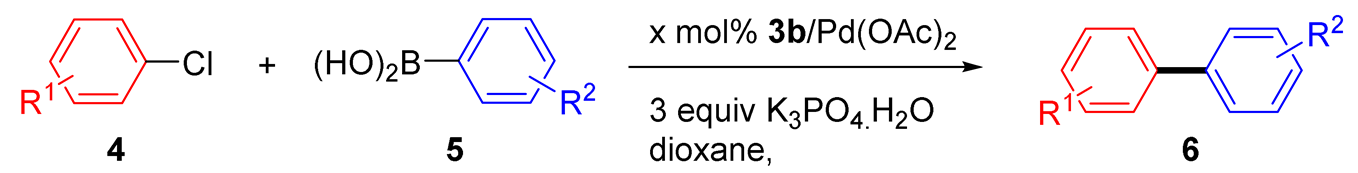Abstract
A new series of xylyl-linked bis-benzimidazolium salts were efficiently prepared using a simple preparation method from bis-benzimidazolium precursors featuring highly tunable linkers and wingtips. A highly efficient Suzuki–Miyaura cross-coupling reaction of aryl chlorides within the range of 0.5–2.0 mol% Pd-catalyst loading was observed. Also, di-ortho-substituted biaryl synthesis was achieved.
1. Introduction
The palladium-catalyzed Suzuki–Miyaura cross-coupling reaction (SMC), which involves the combination of an aryl halide with an arylboronic acid in the presence of a Pd catalyst, is one of the most practical protocols by which to achieve C–C bond formation [1,2,3,4,5]. Aryl chlorides, which are less expensive and more diverse than aryl bromides and iodides, are challenging compounds to use in SMC reactions due to their low C–Cl bond reactivity [6]. Numerous successful examples using a combination of phosphine ligands with Pd have been reported, however, the disadvantages of using such ligands are their difficult preparation, air-sensitivity, expense, and toxicity [7,8,9]. The development of Pd catalysts with phosphine-free ligands has thus received a great amount of attention.
Over the past three decades, the replacement of phosphine ligands with N-heterocyclic carbenes (NHCs) has been shown to be a good choice. Compared with bulky tertiary phosphines, these new NHC ligands exhibit strong σ-donor but poor π-acceptor properties [10]. Metal–NHC complexes exhibit extraordinary heat, air, and moisture stability due to the high dissociation energies of their metal–carbon bonds [11]. Bis-NHC binds to metals to form more stable complexes compared to monodentate NHC complexes, which has led to the development of such ligands attracting great attention in the literature. In addition, the diversity of bis-NHC compounds is also an advantage, as they have tunable linkers and wingtips. In 1998, a chelating bis-NHC ligand was synthesized by Herrmann and co-workers [12] and applied in the Pd-catalyzed SMC reaction of 4-chloroacetophenone and phenylboronic acid at 120 °C for 48 h, resulting in the desired product being formed with a yield of 60%. Numerous successful examples of SMC reactions between aryl chlorides and arylboronic acids employing Pd–bis-NHC complexes or in-situ-formed Pd(OAc)2/bis-NHC catalyst systems have been previously reported [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]. However, high catalyst loading and the variety of the substrates still present challenge.
The catalytic reaction rate is affected by the nature of the NHC ligand. To obtain efficient bis-NHC ligands, a series of new xylyl-linked bis-benzimidazolium salts was prepared by modifying the wingtips and linkers of a bis-NHC precursor. These salts have the following advantages: (1) our linker contains aryl group which can provide electronic effect, (2) our linker has an additional methylene group compared to currently available NHCs in which the aryl group is directly connected to the bis-benzimidazole moiety that offers more conformational flexibility, and (3) the wingtip delivers electronic and steric effects. Those advantages facilitate the oxidative addition and reductive elimination steps of the SMC reaction. We report herein their application in an in-situ-generated Pd(OAc)2/bis-NHC catalyst system for the SMC reaction of aryl chlorides with arylboronic acids (Figure 1).
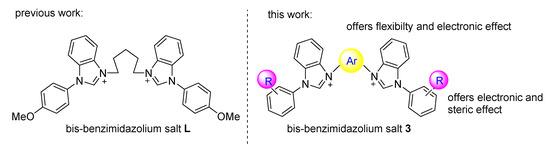
Figure 1.
The structures of the new xylyl-linked bis-benzimidazolium salts 3.
2. Results
2.1. Synthesis and Characterization of the Bis-Benzimidazolium Salts 3
According to Scheme 1, the bis-benzimidazolium salts 3 were prepared by the combination of two equivalents of the corresponding N-arylbenzimidazole 2 and one equivalent of the desired dibromides 1 in acetonitrile under reflux to produce the respective products with yields in the range of 50 to 89%. The newly prepared salts were found to be air- and moisture-stable both in the solid state and in solution, and were characterized by 1H and 13C nuclear magnetic resonance (NMR) spectroscopy.
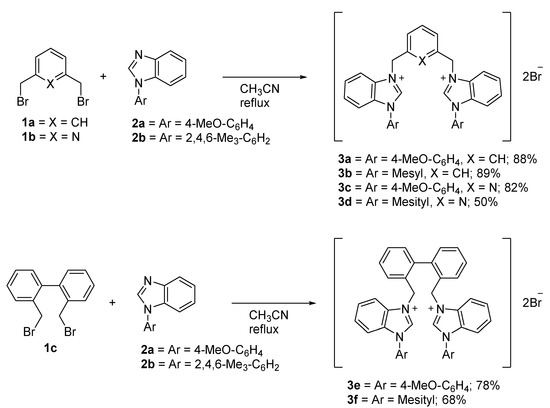
Scheme 1.
Overview of the synthesis of bis-benzimidazolium salts 3.
In the 1H NMR spectra of 3a–3d, the methylene protons could be observed as a singlet, while the 1H NMR spectra of 3e and 3f showed an AB spin system for the methylene protons. The benzimidazolium proton signals in the spectra of 3a–3f could be observed in a wide range of between δ 10.15 and 11.57 ppm. The 13C NMR spectra of 3a–3f showed the NCHN resonances between δ 142.4 and 161.0 ppm.
2.2. The Suzuki–Miyaura Cross-Coupling Reaction
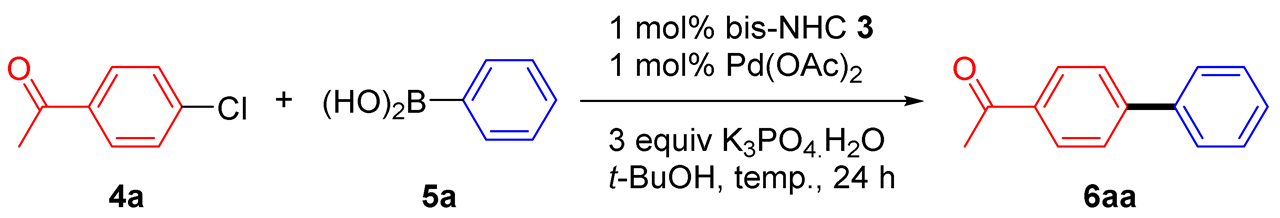
Continuing our previous studies on the application of in-situ-formed catalyst systems for the SMC reaction [28,29], 4-chloroacetophenone 4a and phenylboronic acid 5a were used as model starting materials (Table 1). Under classical conditions (K3PO4·H2O, Pd(OAc)2, t-BuOH, 30 °C, 24 h), the activity of the L/Pd(OAc)2 catalyst system was first compared with that of 3b/Pd(OAc)2 (Table 1, entries 1 and 2). It was found that the xylyl-bridged bis-benzimidazolium precursor 3b showed better activity in comparison to alkyl-linked bis-benzimidazolium salt L (Table 1, entries 1 and 2). Next, the activity of the 3/Pd(OAc)2 catalyst systems was compared. Upon increasing the reaction temperature to 80 °C, the gas chromatography (GC) yield of 6aa increased from 21% to 88% (Table 1, entries 2 and 3). The focus was shifted to investigate the influence that the xylyl-linked spacers and wingtips have on the benzimidazole ring. With the same wingtip on the benzimidazole ring (3b, 3d, and 3f), the bis-benzimidazolium precursor with a benzene ring as a xylyl-linked spacer showed better activity in comparison to when pyridine or biphenyl were incorporated in the xylyl-linked bridge bis-benzimidazolium salts (Table 1, entries 3, 6, and 8). The same results were also observed using bis-benzimidazolium salts 3a, 3c, and 3e (Table 1, entries 4, 5, and 7). The activity of the bis-benzimidazolium salts with a mesityl group on the benzimidazole ring as the wingtip (3d and 3f) was better than that when a methoxyphenyl group was incorporated as a wingtip (3c and 3e), regardless of whether pyridine or biphenyl was present as the xylyl-linked bridge in the bis-benzimidazolium salts (Table 1, entries 5–8). However, both 3a and 3b exhibited similar catalytic activity (Table 1, entries 3 and 4), an observation that shows that having a mesityl group on the benzimidazole ring leads to a sterically hindered effect, which is beneficial in the reductive elimination step of the catalytic cycle.

Table 1.
Screening the activity of the in-situ-formed bis-NHC/Pd catalyst 1.
The effects of different solvents, bases, and Pd loading amounts on the catalytic activity of this reaction are summarized in Table 2. Most of the solvents tested gave the products in good to excellent yields (87–94%, Table 2, entries 1–4) in this work, except for a mixed solvent of t-butanol (BuOH)/H2O, the use of which led to a poor product yield (55%, Table 2, entry 5). It was observed that 1,4-dioxane was the best choice of solvent for producing products with higher conversion rates (Table 2, entry 2). The replacement of K3PO4·H2O with KOtBu or KOAc led to lower GC yields of the respective products (Table 2, entries 6 and 9) and when K2CO3 or Cs2CO3 was used instead, excellent yields were observed (Table 2, entries 7 and 8). After screening Pd loading amount, the coupling reaction could be carried out in the presence of 0.5 mol% Pd (OAc)2/3b catalyst for 6 h in 98% yield (Table 2, entry 13).

Table 2.
Optimization of reaction conditions 1.
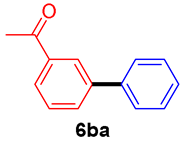
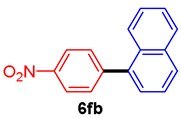
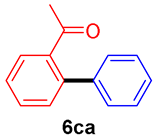
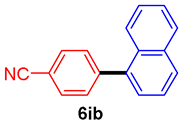
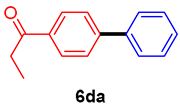
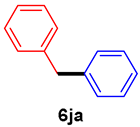
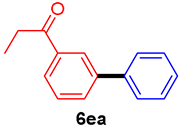
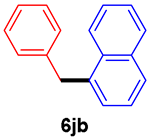
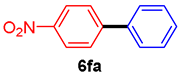
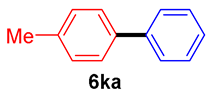
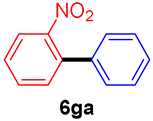
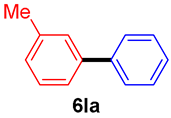
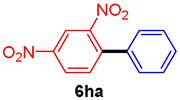
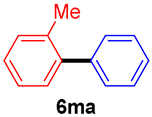
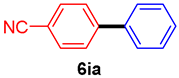
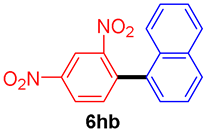
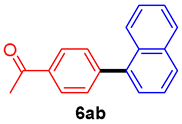
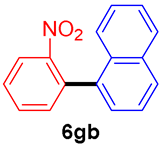
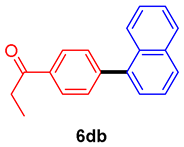
The scope of the SMC reaction was studied after screening the optimized reaction conditions (Table 3). The coupling reactions of aryl chlorides 4a, 4b, and 4c with electron-withdrawing groups proceeded in quantitative yields in line with an increase in the catalyst loading and temperature (6aa–6ca). Similarly, the SMC reaction was carried out at 80 °C in the presence of the catalyst with 1.0 mol% loading using m-chloropropiophenone and p-chloropropiophenone as starting materials and quantitative yields of the desired products 6da and 6ea were obtained. With other electron-withdrawing groups at the para position, such as in 4f and 4i, 96% and 90% isolated yields were obtained, respectively (6fa and 6ia), whereas, the coupling reactions of 1-chloro-2-nitrobenzene, 4g, and 1-chloro-2,4-dinitrobenzene, 4h, with phenylboronic acid showed good yields (81–89%) at 100 °C. Aryl chlorides (4a, 4d, 4e, 4f, and 4i) bearing electron-withdrawing groups at their m- or p-positions were coupled with 1-naphthylboronic acid at 100 °C in the presence of a catalyst with 1.5 mol% loading to give the respective products in yield of 90–99% (6ab–6ib). It was observed that the coupling reactions of benzyl chloride with phenylboronic acid or 1-naphthylboronic acid led to 95% and 99% yields of 6ja and 6jb, respectively, under the same reaction conditions. The aryl chlorides 4k and 4l with electron-donating groups gave the corresponding products (6ka and 6la) in poor yields. Interestingly, o-chlorotoluene was coupled with phenylboronic acid in the presence of a 1 mol% 3b/Pd(OAc)2 catalyst at 80 °C (6ma) to give the desired product in 95% yield. Finally, the di-ortho-substituted biaryl syntheses of o-nitroarenes (4n and 4g) with 1-naphthylboronic acid (5b) led to excellent yields of the respective products, with a 93% yield of 6hb and a 92% yield of 6gb observed.

Table 3.
In-situ-generated 3b/Pd(OAc)2 catalyst catalyzed Suzuki–Miyaura cross-coupling reactions 1.
3. Materials and Methods
3.1. General Methods
Unless otherwise stated, commercially available materials were received from Aldrich and Acros and used without further purification. Acetonitrile was distilled over calcium hydride prior to its use. Toluene, 1,4-dioxane, and t-BuOH were distillated over sodium prior to its use. Reactions were monitored using pre-coated silica gel 60 (F-254) plates. The products were purified by column chromatography (silica gel, 0.040–0.063 μm), eluting with n-hexane/ethyl acetate. 1H and 13C NMR spectra were recorded using an Agilent Mercury 400 spectrometer(Agilent Technologies Inc., Santa Clara, CA, USA), with the J-values given in Hz. Chemical shifts (δ) were referenced to CDCl3 (δ = 7.26 ppm) in the 1H NMR spectra and CDCl3 (δ = 77.0 ppm) in the 13C NMR spectra. Copies of 1H and 13C NMR spectra of all compounds are provided as Supplementary Materials. Melting points were determined using Thermo 1001D digital melting point apparatus and are uncorrected. GC-FID was recorded using a Shimadzu GC-2014 spectrometer (Shimadzu Co., Kyoto, Japan) equipped with a capillary column (SPB®®-5, 60 m × 0.25 mm × 0.25 μm). The conversion yields, GC yields, and ratios were determined using undecane as an internal standard. High-resolution mass spectra were recorded using a Finnigan/Thermo Quest MAT 95XL mass spectrometer (Finnigan MAT LCQ, San Jose, CA, USA) via either electron impact (EI) or electrospray ionization (ESI) methods. 1,3-Bis-(bromomethyl)benzene 1a [30], 2,6-bis-(bromomethyl)pyridine 1b [31], 2,2′-bis-(bromomethyl)benzene 1c [32], and 1-(4-methoxyphenyl)-1H-benzo[d]imidazole 2a [29] were synthesized according to modified literature procedures.
3.2. Experimental Procedures and Spectral Data
3.2.1. Synthesis of 1-(2,4,6-Trimethylphenyl)-1H-benzimidazole 2b
Procedure for 2-(2′,4′,6′-trimethylanilido)nitrobenzene (S1) [33]. A sealed tube was charged with 2-fluoronitrobenzene (0.15 mL, 1.43 mmol), 2,4,6-trimethylaniline (0.30 mL, 2.14 mmol), and anhydrous potassium fluoride (0.08 g, 1.43 mmol). The reaction mixture was heated at 180 °C for 48 h. After completion of the reaction (monitored by TLC), the mixture was cooled to room temperature and then quenched with water (15 mL). The aqueous phase was extracted with EtOAc (30 mL × 3). The combined organic layers were dried over MgSO4 and then filtered. The solvent was evaporated under reduced pressure to afford crude product which was purified by chromatography to afford S1 as an orange solid (0.35 g, 97%). 1H NMR (CDCl3, 400 MHz): δ 9.12 (s, 1H, NH), 8.22 (d, J = 7.6 Hz, 1H), 7.27 (t, J = 7.6 Hz, 1H), 7.00 (s, 2H), 6.68 (t, J = 7.6 Hz, 1H, 6.38 (d, J = 8.8 Hz, 1H), 2.33 (s, 3H), 2.15 (s, 6H); 13C NMR (CDCl3, 100 MHz): δ 144.7, 137.3, 136.3, 136.1, 132.6, 131.9, 129.4, 126.7, 116.2, 115.0, 21.0, 18.1.
Procedure for N-(2′,4′,6′-trimethylphenyl)-1,2-phenylenediamine (S2) [33]5. A 250 mL round-bottom flask was charged with 2-(2′,4′,6′-trimethylanilido)nitrobenzene S1 (2.18 g, 8.49 mmol) and ethanol (60 mL). The reaction mixture was heated at 60 °C. After the 2-(2′,4′,6′-trimethylanilido)nitrobenzene was dissolved completely, the solution was added ammonium chloride solution (0.125 g/1 mL H2O, 30 mL, 85.0 mmol), and iron (4.74 g, 85.0 mmol). The reaction mixture was refluxed for 1.5 h. After completion of the reaction (monitored by TLC), solvent was removed under reduced pressure, the residue was added with H2O (3 mL). The aqueous layer was extracted with EtOAc (3 mL × 3). The combined organic layers were dried over MgSO4 and then filtered. The solvent was evaporated under reduced pressure to afford S2 as a deep purple solid (1.59 g, 99%). 1H NMR (CDCl3, 400 MHz): δ 6.93 (s, 2H), 6.79 (d, J = 7.2 Hz, 1H), 6.74 (t, J = 7.2 Hz, 1H), 6.64 (t, J = 7.2 Hz, 1H), 6.23 (d, J = 7.2 Hz, 1H), 4.76 (s, 1H, NH), 3.63 (s, 2H, NH), 2.30 (s, 3H), 2.12 (s, 6H); 13C NMR (CDCl3, 100 MHz): δ 136.9, 135.6, 134.7, 133.9, 133.3, 129.3, 120.2, 120.0, 116.2, 114.9, 20.8, 18.0.
Procedure for 1-(2,4,6-trimethylphenyl)-1H-benzimidazole (2b) [34]6. To a solution of N-(2′,4′,6′-trimethylphenyl)-1,2-phenylenediamine S2 (0.40 g, 1.8 mmol) and sulfamic acid (19.0 mg, 0.20 mmol) in methanol (10 mL) was added triethyl orthoformate (1.2 mL, 6.9 mmol) at rt. After stirring overnight at rt (monitored by TLC), methanol was removed by rotary evaporator. H2O (3 mL) was added to the residue. The aqueous layer was extracted with EtOAc (3 mL × 3). The combined organic layers were dried over MgSO4 and then filtered. The solvent was evaporated under reduced pressure to afford crude product which was purified by chromatography to afford 2b as a deep purple solid (0.37 g, 90%). 1H NMR (CDCl3, 400 MHz): δ 7.89 (d, J = 8.0 Hz, 1H), 7.87 (s, 1H), 7.32 (t, J = 7.2 Hz, 1H), 7.26 (t, J = 7.2 Hz, 1H), 7.05–7.02 (m, 3H), 2.39 (s, 3H), 1.92 (s, 6H); 13C NMR (CDCl3, 100 MHz): δ 143.1, 142.8, 139.0, 136.0, 133.9, 130.8, 129.1, 123.2, 122.1, 120.1, 109.9, 20.9, 17.2.
3.2.2. General Procedures for the Synthesis of Bis-Benzimidazolium Salts 3
Procedure for bis-benzimidazolium salts 3a–d. A 10 mL round-bottom flask was charged with dibromide 1a or 1b (1.0 equiv), benzimidazole 2a or 2b (2.0 equiv to bromide), and MeCN (5.0 mL). After refluxing for 18 h, the precipitate was formed. The precipitate was filtered off, washed with EtOAc, and dried to afford pure product.
Procedure for 3,3′-(1,3-Phenylenebis(methylene))bis(1-(4-methoxyphenyl)-1H-benzo[d]imidazol-3-ium) dibromide (3a). 1,3-bis-(bromomethyl)benzene 1a (46.5 mg, 0.18 mmol) and 1-(4-methoxyphenyl)-1H-benzo[d]imidazole 2a (79.5 mg, 0.36 mmol) were used to afford 3a as white solid in 88% yield (0.10 g). Mp = 282–283 °C; 1H NMR (DMSO, 400 MHz): δ 10.36 (s, 2H), 7.97 (s, 1H), 7.94 (d, J = 8.0 Hz, 2H), 7.78–7.76 (m, 6H), 7.68–7.58 (m, 6H), 7.48 (t, J = 8.0 Hz, 1H), 7.26 (d, J = 8.8 Hz, 4H), 5.86 (s, 4H), 3.89 (s, 6H); 13C NMR (DMSO, 100 MHz): δ 158.6, 140.8, 132.4, 129.7, 128.7, 127.6, 127.0, 126.7, 125.4, 124.9, 124.8, 123.7, 113.4, 112.1, 111.7, 57.7, 53.8. HRMS-MALDI-TOF(m/z) [M-Br]+ Calculated for C36H32BrN4O2+: 631.1703, found: 631.1706.
Procedure for 3,3′-(1,3-Phenylenebis(methylene))bis(1-mesityl-1H-benzo[d]imidazol-3-ium) dibromide (3b). 1,3-bis-(bromomethyl)benzene 1a (0.10 g, 0.38 mmol) and 1-(2,4,6-trimethylphenyl)-1H-benzimidazole 2b (0.18 g, 0.77 mmol) were used to afford 3b as white solid in 89% yield (0.25 g). Mp = 210–213 °C; 1H NMR (CDCl3, 400 MHz): δ 11.47 (s, 2H), 8.51 (s, 1H), 8.23 (d, J = 8.4 Hz, 2H), 7.69 (d, J = 8.0 Hz, 2H), 7.67 (t, J = 8.4 Hz, 2H), 7.54 (t, J = 8.0 Hz, 2H), 7.33 (t, J = 8.0 Hz, 1H), 7.18 (d, J = 8.4 Hz, 2H), 7.06 (s, 4H), 6.26 (s, 4H), 2.37 (s, 6H), 1.99 (s, 12H); 13C NMR (CDCl3, 100 MHz): δ 142.4, 141.6, 135.0, 134.2, 131.5, 130.7, 130.3, 130.2, 130.1, 129.4, 128.2, 128.0, 127.8, 115.2, 112.9, 50.7, 21.1, 17.6. HRMS-MALDI-TOF(m/z) [M-Br]+ Calculated for C40H40BrN4+: 655.2431, found: 655.2435.
Procedure for 3,3′-(Pyridine-2,6-diylbis(methylene))bis(1-(4-methoxyphenyl)-1H-benzo[d]imidazol-3-ium) dibromide (3c). 2,6-bis-(bromomethyl)pyridine 1b (41.5 mg, 0.16 mmol) and 1-(4-methoxyphenyl)-1H-benzo[d]imidazole 2a (72.1 mg, 0.33 mmol) were used to afford 3c as white solid in 82% yield (0.095 g). Mp = 283–284 °C; 1H NMR (DMSO, 400 MHz): δ 10.15 (s, 2H), 8.06 (t, J = 7.6 Hz, 1H), 7.79 (t, J = 7.6 Hz, 4H), 7.68–7.55 (m, 8H), 7.47 (t, J = 7.6 Hz, 2H), 7.20 (d, J = 8.4 Hz, 4H), 5.92 (s, 4H), 3.89 (s, 6H); 13C NMR (DMSO, 100 MHz): δ 160.5, 152.9, 144.8, 142.8, 131.0, 130.97, 127.2, 126.8, 126.5, 125.4, 123.0, 115.3, 113.8, 113.4, 55.7, 50.8. HRMS-MALDI-TOF(m/z) [M-Br]+ Calculated for C35H31BrN5O2+: 632.1656, found: 632.1657.
Procedure for 3,3′-(Pyridine-2,6-diylbis(methylene))bis(1-mesityl-1H-benzo[d]imidazol-3-ium) dibromide (3d). 2,6-bis-(bromomethyl)pyridine 1b (24.7 mg, 0.09 mmol) and 1-(2,4,6-trimethylphenyl)-1H-benzimidazole 2b (44.4 mg, 0.18 mmol) were used to afford 3d as white solid in 50% yield (0.034 g). Mp = 204–205 °C; 1H NMR (CDCl3, 400 MHz): δ 11.57 (s, 2H), 7.97 (d, J = 8.0 Hz, 2H), 7.78–7.75 (m, 5H), 7.58 (t, J = 8.0 Hz, 2H), 7.23 (d, J = 8.0 Hz, 2H), 7.10 (s, 4H), 6.42 (s, 4H), 2.40 (s, 6H), 2.04 (s, 12H); 13C NMR (CDCl3, 400 MHz): δ 152.9, 143.1, 141.8, 139.5, 135.1, 131.5, 130.9, 130.2, 128.6, 128.1, 127.9, 123.5, 114.6, 113.0, 52.2, 21.2, 17.7; HRMS-MALDI-TOF(m/z) [M-Br]+ Calculated for C39H39BrN5+: 656.2383, found: 656.2377.
General Procedures for Preparation of bis-benzimidazolium salts 3e and 3f. A 10 mL round-bottom flask was charged with dibromide 1c (1.0 equiv), benzimidazole 2a or 2b (2.0 equiv to bromide), and MeCN (5.0 mL). After refluxing for 18 h, the precipitate was formed. The precipitate was filtered off, washed with EtOAc, and dried to afford pure product.
Procedure for 3,3′-([1,1′-Biphenyl]-2,2′-diylbis(methylene))bis(1-(4-methoxyphenyl)-1H-benzo[d]imidazol-3-ium) dibromide (3e). 2,2′-bis((bromomethyl)benzene 1c (46.0 mg, 0.14 mmol) and 1-(4-methoxyphenyl)-1H-benzo[d]imidazole 2a (62.2 mg, 0.29 mmol) were used to afford 3e as white solid in 78% yield (0.083 g). Mp = 200–201 °C; 1H NMR (CDCl3, 400 MHz): δ 10.52 (s, 2H), 7.68–7.53 (m, 12H), 7.49 (d, J = 7.6 Hz, 2H), 7.35 (t, J = 7.6 Hz, 2H), 7.26 (t, J = 7.6 Hz, 2H), 7.11 (d, J = 8.8 Hz, 4H), 6.88 (d, J = 7.6 Hz, 2H), 6.26 (d, J = 16.0 Hz, 2H), 5.91 (d, J = 16.0 Hz, 2H), 3.87 (s, 6H); 13C NMR (CDCl3, 100 MHz): δ 161.0, 142.0, 138.5, 131.2, 131.1, 131.0, 129.8, 129.7, 129.4, 129.3, 127.72, 127.65, 126.1, 125.1, 115.7, 114.2, 113.5, 55.8, 50.3. HRMS-MALDI-TOF(m/z) [M-Br]+ Calculated for C42H36BrN4O2+: 707.2016, found: 707.2017.
Procedure for 3,3′-([1,1′-Biphenyl]-2,2′-diylbis(methylene))bis(1-mesityl-1H-benzo[d]imidazol-3-ium) dibromide (3f). 2,2′-bis((bromomethyl)benzene 1c (36.5 mg, 0.11 mmol) and 1-(2,4,6-trimethylphenyl)-1H-benzimidazole 2b (54.3 mg, 0.24 mmol) were used to afford 3f as white solid in 68% yield (0.059 g). Mp = 297–298 °C; 1H NMR (CDCl3, 400 MHz): δ 11.00 (s, 2H), 7.89 (d, J = 7.6 Hz, 2H), 7.70 (t, J = 7.6 Hz, 2H), 7.61 (t, J = 7.6 Hz, 2H), 7.52 (d, J = 7.6 Hz, 2H), 7.46 (t, J = 7.6 Hz, 2H), 7.37 (t, J = 7.6 Hz, 2H), 7.25 (d, J = 7.6 Hz, 2H), 7.07 (s, 4H), 7.04 (d, J = 7.6 Hz, 2H), 6.55 (d, J = 16.8 Hz, 2H), 5.97 (d, J = 16.8 Hz, 2H), 2.38 (s, 6H), 2.05 (s, 6H), 2.01 (s, 6H); 13C NMR (CDCl3, 100 MHz): δ 143.4, 141.7, 138.0, 135.1, 135.0, 131.5, 131.4, 131.3, 130.6, 130.2, 129.44, 129.39, 128.3, 128.1, 126.7, 114.7, 113.0, 49.7, 21.2, 17.8, 17.7. HRMS-MALDI-TOF(m/z) [M-Br]+ Calculated for C46H44BrN4+: 731.2744, found: 731.2742.
3.2.3. General Procedures for Suzuki–Miyaura Cross-Coupling Reactions under N2
All manipulations were carried out under nitrogen using dried solvent. Pd(OAc)2 (1 mol%), salt 3b (1 mol%) and 1,4-dioxane (3 mL) were charged to the Schlenk tube at 80 °C for 1 h, followed by the addition of arylboronic acid 5 (1.50 mmol), aryl chloride 4 (1.00 mmol), and K3PO4·H2O (3.00 mmol) at the prescribed temperature for the prescribed time. After completion of the reaction, monitored by TLC, the reaction was quenched by water (3.0 mL). The aqueous layer was extracted with EtOAc (3.0 mL × 3). The organic layer was dried over anhydrous MgSO4 and then filtered. The solvent was evaporated under reduced pressure and the corresponding crude product of the Suzuki−Miyaura coupling reaction was purified by chromatography.
4-Acetylbiphenyl (6aa) [29]. 1H NMR (CDCl3, 400 MHz): δ 8.04 (d, J = 7.6 Hz, 2H), 7.69 (d, J = 7.6 Hz, 2H), 7.63 (d, J = 7.6 Hz, 2H), 7.47 (t, J = 7.6 Hz, 2H), 7.43–7.38 (m, 1H), 2.64 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ 197.7, 145.7, 139.8, 135.8, 128.92, 128.88, 128.2, 127.2, 127.2, 26.6.
3-Acetylbiphenyl (6ba) [35]. 1H NMR (CDCl3, 400 MHz): δ 8.19 (s, 1H), 7.94 (d, J = 7.6 Hz, 1H), 7.80 (d, J = 7.6 Hz, 1H), 7.63 (d, J = 7.2 Hz, 2H), 7.54 (t, J = 7.6 Hz, 1H), 7.47 (t, J = 7.2 Hz, 2H), 7.41–7.37 (m, 1H), 2.66 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ 197.8, 141.4, 139.9, 137.4, 131.5, 128.8, 128.7, 127.6, 127.0, 126.95, 126.7, 26.5.
2-Acetylbiphenyl (6ca) [36]. 1H NMR (CDCl3, 400 MHz): δ 7.57–7.50 (m, 2H), 7.44–7.39 (m, 5H), 7.36–7.34 (m, 2H), 2.01 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ 205.0, 140.8, 140.6, 140.5, 130.7, 130.2, 128.8, 128.6, 127.85, 127.82, 127.4, 30.4.
4′-Phenylpropiophenone (6da) [29]. 1H NMR (CDCl3, 400 MHz): δ 8.05 (d, J = 8.4 Hz, 2H), 7.68 (d, J = 8.4 Hz, 2H), 7.63 (d, J = 7.8 Hz, 2H), 7.47 (t, J = 7.8 Hz, 2H), 7.43–7.38 (m, 1H), 3.05 (q, J = 7.2 Hz, 2H), 1.26 (t, J = 7.2 Hz, 3H); 13C NMR (CDCl3,100 MHz): δ 200.5, 145.5, 139.9, 135.6, 128.9, 128.6, 128.2, 127.23, 127.19, 31.8, 8.3.
3′-Phenylpropiophenone (6ea) [29]. 1H NMR (CDCl3, 400 MHz): δ 8.20 (s, 1H), 7.95 (d, J = 7.6 Hz, 1H), 7.78 (d, J = 7.6 Hz, 1H), 7.63 (d, J = 7.6 Hz, 2H), 7.53 (t, J = 7.6 Hz, 1H), 7.48 (t, J = 7.6 Hz, 2H), 7.43–7.36 (m, 1H), 3.07 (q, J = 7.2 Hz, 2H), 1.27 (t, J = 7.2 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 200.7, 141.6, 140.2, 137.3, 131.5, 129.0, 128.9, 127.7, 127.1, 126.8, 126.6, 31.9, 8.2.
4-Nitrobiphenyl (6fa) [37]. 1H NMR (CDCl3, 400 MHz): δ 8.30 (d, J = 8.4 Hz, 2H), 7.74 (d, J = 8.4 Hz, 2H), 7.63 (d, J = 7.2 Hz, 2H), 7.55–7.41 (m, 3H); 13C NMR (CDCl3, 100 MHz): δ 147.6, 147.0, 138.7, 129.1, 128.9, 127.8, 127.3, 124.1.
2-Nitrobiphenyl (6ga) [37]. 1H NMR (CDCl3, 400 MHz): δ 7.86 (d, J = 7.6 Hz, 1H), 7.62 (t, J = 7.6 Hz, 1H), 7.51–7.39 (m, 5H), 7.34–7.32 (m, 2H); 13C NMR (CDCl3, 100 MHz): δ 149.2, 137.3, 136.3, 132.2, 131.9, 128.7, 128.2, 128.1, 127.9, 124.0.
2,4-Dinitrobiphenyl (6ha) [29]. 1H NMR (CDCl3, 400 MHz): δ 8.71 (d, J = 2.4 Hz, 1H), 8.47 (dd, J = 8.8, 2.4 Hz, 1H), 7.67 (d, J = 8.8 Hz, 1H), 7.50–7.47 (m, 3H), 7.36–7.33 (m, 2H); 13C NMR (CDCl3, 100 MHz): δ 148.8, 146.6, 142.1, 135.1, 133.2, 129.4, 128.9, 127.5, 126.4, 119.6.
4-Cyanobiphenyl (6ia) [38]. 1H NMR (CDCl3, 400 MHz): δ 7.73 (d, J = 8.4 Hz, 2H), 7.69 (d, J = 8.4 Hz, 2H), 7.59 (d, J = 7.6 Hz, 2H), 7.49 (t, J = 7.6 Hz, 2H), 7.46–7.40 (m, 1H); 13C NMR (CDCl3, 100 MHz): δ 145.2, 138.7, 132.2, 128.8, 128.4, 127.3, 126.9, 118.6, 110.5.
1-(4-(Naphthalen-1-yl)phenyl)ethan-1-one (6ab) [29]. 1H NMR (CDCl3, 400 MHz): δ 8.10 (d, J = 7.6 Hz, 2H), 7.94–7.84 (m, 3H), 7.61 (d, J = 7.6 Hz, 2H), 7.57–7.43 (m, 4H), 2.69 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ 197.9, 145.8, 139.0, 135.9, 133.7, 131.1, 130.3, 128.4, 128.35, 128.33, 126.9, 126.4, 126.0, 125.5, 125.3, 26.7.
1-(4-(Naphthalen-1-yl)phenyl)propan-1-one (6db) [29]. 1H NMR (CDCl3, 400 MHz): δ 8.10 (d, J = 8.0 Hz, 2H), 7.94–7.84 (m, 3H), 7.60 (d, J = 8.0 Hz, 2H), 7.57–7.42 (m, 4H), 3.09 (q, J = 7.2 Hz, 2H), 1.29 (t, J = 7.2 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 200.1, 145.2, 138.8, 135.5, 133.6, 130.9, 130.0, 128.2, 128.1, 127.8, 126.7, 126.2, 125.8, 125.3, 125.1, 31.6, 8.1.
1-(3-(Naphthalen-1-yl)phenyl)propan-1-one (6eb) [29]. 1H NMR (CDCl3, 400 MHz): δ 8.09 (s, 1H), 8.04 (d, J = 8.4 Hz, 1H), 7.94–7.89 (m, 2H), 7.81 (d, J = 8.0 Hz, 1H), 7.69 (d, J = 8.0 Hz, 1H), 7.61–7.50 (m, 3H), 7.44 (t, J = 8.4 Hz, 2H), 3.05 (q, J = 7.2 Hz, 2H), 1.25 (t, J = 7.2 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 200.1, 140.7, 138.8, 136.7, 134.0, 133.5, 131.1, 129.2, 128.2, 128.1, 127.8, 126.7, 126.5, 126.0, 125.6, 125.2, 125.0, 31.5, 7.9.
1-(4-Nitrophenyl)naphthalene (6fb) [37]. 1H NMR (CDCl3, 400 MHz): δ 8.37 (d, J = 8.4 Hz, 2H), 7.94 (dd, J = 8.4, 2.4 Hz, 2H), 7.78 (d, J = 8.4 Hz, 1H), 7.68 (d, J = 8.4 Hz, 2H), 7.59–7.41 (m, 4H); 13C NMR (CDCl3, 100 MHz): δ 147.6, 147.1, 137.7, 133.7, 130.9, 128.9, 128.5, 127.1, 126.7, 126.2, 125.3, 125.1, 123.6.
4-(1-Naphthyl)benzonitrile (6ib) [38]. 1H NMR (CDCl3, 400 MHz): δ 7.93 (d, J = 7.2 Hz, 2H), 7.80–7.76 (m, 3H), 7.62 (d, J = 8.4 Hz, 2H), 7.57–7.45 (m, 3H), 7.40 (d, J = 7.2 Hz, 1H); 13C NMR (CDCl3, 100 MHz): δ 145.2, 137.8, 133.5, 131.8, 130.6, 130.4, 128.5, 128.3, 126.8, 126.4, 125.9, 125.1, 124.9, 118.6, 110.8.
Diphenylmethane (6ja) [39]. 1H NMR (CDCl3, 400 MHz): δ 7.31–7.27 (m, 4H), 7.22–7.20 (m, 6H), 3.99 (s, 2H); 13C NMR (CDCl3, 100 MHz): δ 141.1, 128.9, 128.4, 126.0, 41.9.
1-Benzylnapthalene (6jb) [40]. 1H NMR (CDCl3, 400 MHz): δ 7.99 (d, J = 8.0 Hz, 1H), 7.86–7.84 (m, 1H), 7.76 (d, J = 8.0 Hz, 1H), 7.46–7.40 (m, 3H), 7.29–7.24 (m, 3H), 7.20–7.16 (m, 3H), 4.45 (s, 2H); 13C NMR (CDCl3, 100 MHz): δ 140.5, 136.5, 133.9, 132.1, 128.6, 128.58, 128.4, 127.2, 127.1, 125.9, 125.9, 125.5, 124.2, 38.9.
4-Methylbiphenyl (6ka) [37]. 1H NMR (CDCl3, 400 MHz): δ 7.58 (d, J = 7.2 Hz, 2H), 7.49 (d, J = 8.0 Hz, 2H), 7.42 (t, J = 7.2 Hz, 2H), 7.34–7.30 (m, 1H), 7.25 (d, J = 8.0 Hz, 2H), 2.40 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ 141.1, 138.3, 137.0, 129.5, 128.7, 127.0, 21.1.
3-Methylbiphenyl (6la) [41]. 1H NMR (CDCl3, 400 MHz): δ 7.60 (d, J = 7.2 Hz, 2H), 7.46–7.40 (m, 4H), 7.37–7.33 (m, 2H), 7.18 (d, J = 7.2 Hz, 1H), 2.44 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ 141.3, 141.2, 138.2, 128.6, 127.9, 127.87, 127.1, 124.2, 21.4.
2-Methylbiphenyl (6ma) [29]. 1H NMR (CDCl3, 400 MHz): δ 7.41 (t, J = 7.2 Hz, 2H), 7.35–7.31 (m, 3H), 7.26–7.24 (m, 4H,), 2.27 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ 141.9, 141.89, 135.3, 130.3, 129.8, 129.1, 128.0, 127.2, 126.7, 125.7, 20.4.
1-(2,4-Dinitrophenyl)naphthalene (6hb) [42]. 1H NMR (CDCl3, 400 MHz): δ 8.93 (s, 1H), 8.55 (d, J = 8.4 Hz, 1H), 7.96 (t, J = 9.2 Hz, 2H), 7.74 (d, J = 8.4 Hz, 1H), 7.57–7.52 (m, 2H), 7.46 (t, J = 7.6 Hz, 1H), 7.38–7.34 (m, 2H); 13C NMR (CDCl3, 100 MHz): δ 149.8, 147.4, 141.6, 134.5, 133.4, 133.2, 130.7, 129.7, 128.8, 127.2, 126.6, 126.5, 126.1, 125.2, 124.1, 119.9.
1-(2-Nitrophenyl)naphthalene (6gb) [43]. 1H NMR (CDCl3, 400 MHz): δ 8.08 (d, J = 8.4 Hz, 1H), 7.91 (d, J = 8.4 Hz, 2H), 7.71 (t, J = 7.2 Hz, 1H), 7.61 (t, J = 8.4 Hz, 1H), 7.52–7.39 (m, 5H), 7.35 (d, J = 7.2 Hz, 1H); 13C NMR (CDCl3, 100 MHz): δ 149.8, 135.5, 135.3, 133.4, 133.1, 132.5, 131.4, 128.6, 128.6, 128.5, 126.6, 126.0, 125.6, 125.2, 124.8, 124.23.
4. Conclusions
In summary, a series of new xylyl-linked bis-benzimidazolium salts 3a–3f were effectively synthesized via a simple synthetic route using cheap reactants. The changing of both the linkers and wingtips of the xylyl-linked bis-benzimidazolium salts 3a–3f led to different steric and electronic effects being observed. It was found in the Suzuki–Miyaura cross-coupling reactions of aryl chlorides that bis-benzimidazolium salts featuring benzene as a xylyl-linked spacer (3a and 3b) led to higher yields of the respective products being formed, whereas when pyridine (3c and 3d) and biphenyl (3e and 3f) were used as linkers lower yields were observed. Comparing the bis-NHC precursors 3a and 3b, the precursor with a sterically demanding wingtip (3b) led to the product being formed in a higher yield than when a precursor was used with an electronically demanding wingtip (3a). Various aryl chlorides, including carbonyl-, nitro-, and nitrile-functionalized compounds, were coupled with arylboronic acids to give the respective products in good to excellent yields at 80–100 °C using the in-situ-generated bis-NHC 3b/Pd catalyst system, with a loading in the range of 0.5–2.0 mol%. In addition, in di-ortho-substituted biaryl syntheses excellent yields were observed when 1-chloro-2,4-dinitrobenzene and 1-chloro-2-nitrobenzene were coupled with 1-naphthylboronic acid.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/catal11070817/s1, Figure S1: 1H NMR spectrum of 2-(2′,4′,6′-trimethylanilido)nitrobenzene S1 in CDCl3, Figure S2: 13C NMR spectrum of 2-(2′,4′,6′-trimethylanilido)nitrobenzene S1 in CDCl3, Figure S3: 1H NMR spectrum of N-(2′,4′,6′-trimethylphenyl)-1,2-phenylenediamine S1 in CDCl3, Figure S4: 13C NMR spectrum of N-(2′,4′,6′-trimethylphenyl)-1,2-phenylenediamine S2 in CDCl3, Figure S5: 1H NMR spectrum of 1-(2,4,6-trimethylphenyl)-1H-benzimidazole 2b in CDCl3, Figure S6: 13C NMR spectrum of 1-(2,4,6-trimethylphenyl)-1H-benzimidazole 2b in CDCl3, Figure S7: 1H NMR spectrum of (3,3′-(1,3-phenylenebis(methylene))bis(1-(4-methoxyphenyl)-1H-benzo[d]imidazol-3-ium)) dibromide (3a) in DMSO-d6, Figure S8: 13C NMR spectrum of (3,3′-(1,3-phenylenebis(methylene))bis(1-(4-methoxyphenyl)-1H-benzo[d]imidazol-3-ium)) dibromide (3a) in DMSO-d6, Figure S9: 1H NMR spectrum of (3,3′-(1,3-phenylenebis(methylene))bis(1-mesityl-1H-benzo[d]imidazol-3-ium)) dibromide (3b) in CDCl3, Figure S10: 13C NMR spectrum of (3,3′-(1,3-phenylenebis(methylene))bis(1-mesityl-1H-benzo[d]imidazol-3-ium)) dibromide (3b) in CDCl3, Figure S11: 1H NMR spectrum of (3,3′-(pyridine-2,6-diylbis(methylene))bis(1-(4-methoxyphenyl)-1H-benzo[d]imidazol-3-ium)) dibromide (3c) in DMSO-d6, Figure S12: 13C NMR spectrum of (3,3′-(pyridine-2,6-diylbis(methylene))bis(1-(4-methoxyphenyl)-1H-benzo[d]imidazol-3-ium)) dibromide (3c) in DMSO-d6, Figure S13: 1H NMR spectrum of (3,3′-(pyridine-2,6-diylbis(methylene))bis(1-mesityl-1H-benzo[d]imidazol-3-ium)) dibromide (3d) in CDCl3, Figure S14: 13C NMR spectrum of (3,3′-(pyridine-2,6-diylbis(methylene))bis(1-mesityl-1H-benzo[d]imidazol-3-ium)) dibromide (3d) in CDCl3, Figure S15: 1H NMR spectrum of 3,3′-([1,1′-biphenyl]-2,2′-diylbis(methylene))bis(1-(4-methoxyphenyl)-1H-benzo[d]imidazol-3-ium) dibromide (3e) in CDCl3, Figure S16: 13C NMR spectrum of 3,3′-([1,1′-biphenyl]-2,2′-diylbis(methylene))bis(1-(4-methoxyphenyl)-1H-benzo[d]imidazol-3-ium) dibromide (3e) in CDCl3, Figure S17: 1H NMR spectrum of 3,3′-([1,1′-biphenyl]-2,2′-diylbis(methylene))bis(1-mesityl-1H-benzo[d]imidazol-3-ium) dibromide (3f) in CDCl3, Figure S18: 13C NMR spectrum of 3,3′-([1,1′-biphenyl]-2,2′-diylbis(methylene))bis(1-mesityl-1H-benzo[d]imidazol-3-ium) dibromide (3f) in CDCl3, Figure S19: 1H NMR spectrum of 4-acetylbiphenyl (6aa) in CDCl3, Figure S20: 13C NMR spectrum of 4-acetylbiphenyl (6aa) in CDCl3, Figure S21: 1H NMR spectrum of 3-acetylbiphenyl (6ba) in CDCl3, Figure S22: 13C NMR spectrum of 3-acetylbiphenyl (6ba) in CDCl3, Figure S23: 1H NMR spectrum of 2-acetylbiphenyl (6ca) in CDCl3, Figure S24: 13C NMR spectrum of 2-acetylbiphenyl (6ca) in CDCl3, Figure S25: 1H NMR spectrum of 4′-phenylpropiophenone (6da) in CDCl3, Figure S26: 13C NMR spectrum of 4′-phenylpropiophenone (6da) in CDCl3, Figure S27: 1H NMR spectrum of 3′-phenylpropiophenone (6ea) in CDCl3, Figure S28: 13C NMR spectrum of 3′-phenylpropiophenone (6ea) in CDCl3, Figure S29: 1H NMR spectrum of 4-nitrobiphenyl (6fa) in CDCl3, Figure S30: 13C NMR spectrum of 4-nitrobiphenyl (6fa) in CDCl3, Figure S31: 1H NMR spectrum of 2-nitrobiphenyl (6ga) in CDCl3, Figure S32: 13C NMR spectrum of 2-nitrobiphenyl (6ga) in CDCl3, Figure S33: 1H NMR spectrum of 2,4-dinitrobiphenyl (6ha) in CDCl3, Figure S34: 13C NMR spectrum of 2,4-dinitrobiphenyl (6ha) in CDCl3, Figure S35: 1H NMR spectrum of 4-cyanobiphenyl (6ia) in CDCl3, Figure S36: 13C NMR spectrum of 4-cyanobiphenyl (6ia) in CDCl3, Figure S37: 1H NMR spectrum of diphenylmethane (6ja) in CDCl3, Figure S38: 13C NMR spectrum of diphenylmethane (6ja) in CDCl3, Figure S39: 1H NMR spectrum of 4-methylbiphenyl (6ka) in CDCl3, Figure S40: 13C NMR spectrum of 4-methylbiphenyl (6ka) in CDCl3, Figure S41: 1H NMR spectrum of 3-methylbiphenyl (6la) in CDCl3, Figure S42: 13C NMR spectrum of 3-methylbiphenyl (6la) in CDCl3, Figure S43: 1H NMR spectrum of 2-methylbiphenyl (6ma) in CDCl3, Figure S44: 13C NMR spectrum of 2-methylbiphenyl (6ma) in CDCl3, Figure S45: 1H NMR spectrum of 1-(4-(naphthalen-1-yl)phenyl)propan-1-one (6db) in CDCl3, Figure S46: 13C NMR spectrum of 1-(4-(naphthalen-1-yl)phenyl)propan-1-one (6db) in CDCl3, Figure S47: 1H NMR spectrum of 1-(4-nitrophenyl)naphthalene (6fb) in CDCl3, Figure S48: 13C NMR spectrum of 1-(4-nitrophenyl)naphthalene (6fb) in CDCl3, Figure S49: 1H NMR spectrum of 4-(1-naphthyl)benzonitrile (6ib) in CDCl3, Figure S50: 13C NMR spectrum of 4-(1-naphthyl)benzonitrile (6ib) in CDCl3, Figure S51: 1H NMR spectrum of 1-(3-(naphthalen-1-yl)phenyl)propan-1-one (6eb) in CDCl3, Figure S52: 13C NMR spectrum of 1-(3-(naphthalen-1-yl)phenyl)propan-1-one (6eb) in CDCl3, Figure S53: 1H NMR spectrum of 1-benzylnapthalene (6jb) in CDCl3, Figure S54: 13C NMR spectrum of 1-benzylnapthalene (6jb) in CDCl3, Figure S55: 1H NMR spectrum of 1-(2-nitrophenyl)naphthalene (6gb) in CDCl3, Figure S56: 13C NMR spectrum of 1-(2-nitrophenyl)naphthalene (6gb) in CDCl3, Figure S57: 1H NMR spectrum of 1-(2,4-dinitrophenyl)naphthalene (6hb) in CDCl3, Figure S58: 13C NMR spectrum of 1-(2,4-dinitrophenyl)naphthalene (6hb) in CDCl3.
Author Contributions
Conceptualization, D.-S.L.; methodology, T.W. and S.-J.H.; validation, T.W., T.-R.W., S.-J.H. and Y.-T.L.; formal analysis, T.W. and S.-J.H.; investigation, T.W., T.-R.W., S.-J.H. and Y.-T.L.; data curation, T.W., T.-R.W. and Y.-T.L.; writing—original draft preparation, D.-S.L.; writing—review and editing, T.-J.L.; supervision, D.-S.L.; project administration, D.-S.L.; funding acquisition, D.-S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Technology of the Republic of China, grant number 107WFA0510613.
Data Availability Statement
Data is contained within the article or Supplementary Materials.
Acknowledgments
We thank to National Center for High-performance Computing (NCHC) for providing computational and storage resources, the Instrument Center of National Chung Hsing University for help with measurements of the high-resolution mass spectrometer, and Dao-Wen Luo for help with the X-ray analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef] [Green Version]
- Stanforth, S.P. Catalytic Cross-Coupling Reactions in Biaryl Synthesis. Tetrahedron 1998, 54, 263–303. [Google Scholar] [CrossRef]
- Suzuki, A. Recent advances in the cross-coupling reactions of organoboron derivatives with organic electrophiles, 1995–1998. J. Organomet. Chem. 1999, 576, 147–168. [Google Scholar] [CrossRef]
- Mingji, D.; Liang, B.; Wang, C.; You, Z.; Xiang, J.; Dong, G.; Chen, J.; Yang, Z. A Novel Thiourea Ligand Applied in the Pd-Catalyzed Heck, Suzuki and Suzuki Carbonylative Reactions. Adv. Synth. Catal. 2004, 346, 1669–1673. [Google Scholar] [CrossRef]
- Suzuki, A.; Yamamoto, Y. Cross-coupling Reactions of Organoboranes: An Easy Method for C–C Bonding. Chem. Lett. 2011, 40, 894–901. [Google Scholar] [CrossRef]
- Grushin, V.V.; Alper, H. Transformations of Chloroarenes, Catalyzed by Transition-Metal Complexes. Chem. Rev. 1994, 94, 1047–1062. [Google Scholar] [CrossRef]
- Littke, A.F.; Fu, G.C. Palladium-Catalyzed Coupling Reactions of Aryl Chlorides. Angew. Chem. Int. Ed. 2002, 41, 4176–4211. [Google Scholar] [CrossRef]
- Corbet, J.-P.; Mignani, G. Selected Patented Cross-Coupling Reaction Technologies. Chem. Rev. 2006, 106, 2651–2710. [Google Scholar] [CrossRef]
- Wong, S.M.; Yuen, O.Y.; Choy, P.Y.; Kwong, F.Y. When Cross-Coupling Partners Meet Indolylphosphines. Coord. Chem. Rev. 2015, 293–294, 158–186. [Google Scholar] [CrossRef]
- Schwarz, J.B.; Böhm, V.P.W.; Gardiner, M.G.; Grosche, M.; Hermann, W.A.; Hieringer, W.; Raudaschl-Sieber, G. Polymer-Supported Carbene Complexes of Palladium: Well-Defined, Air-Stable, Recyclable Catalysts for the Heck Reaction. Chem. Eur. J. 2000, 6, 1773–1780. [Google Scholar] [CrossRef]
- Hermann, W.A.; Öfele, K.; Preysing, D.; Herdtweck, E. Metal complexes of acyclic diaminocarbenes: Links between N-heterocyclic carbene (NHC)- and Fischer-carbene complexes. J. Organomet. Chem. 2003, 684, 235–248. [Google Scholar] [CrossRef]
- Herrmann, W.A.; Reisinger, C.-P.; Spiegler, M. Chelating N-heterocyclic carbene ligands in palladium-catalyzed heck-type reactions. J. Organomet. Chem. 1998, 557, 93–96. [Google Scholar] [CrossRef]
- Zhang, C.; Trudell, M.L. Palladium-bisimidazol-2-ylidene complexes as catalysts for general and efficient Suzuki cross-coupling reactions of aryl chlorides with arylboronic acids. Tetrahedron Lett. 2000, 41, 595–598. [Google Scholar] [CrossRef]
- Özdemir, İ.; Çetinkaya, B.; Demir, S.; Gürbüz, N. Palladium-catalyzed Suzuki-Miyaura reaction using saturated N-heterocyclic ligands. Catal. Lett. 2004, 97, 37–40. [Google Scholar] [CrossRef]
- Özdemir, İ.; Gök, Y.; Gürbüz, N.; Çetinkaya, E.; Çetinkaya, B. Suzuki-Miyaura Reaction of Unactivated Aryl Chlorides Using Beznimidazol-2-Ylidene Ligands. Synth. Commun. 2004, 34, 4135–4144. [Google Scholar] [CrossRef]
- Özdemir, İ.; Gök, Y.; Gürbüz, N.; Çetinkaya, E.; Çetinkaya, B. Palladium-Catalyzed Suzuki Reaction Using 1,3-Dialkylbenzimidazol-2-ylidene Ligands in Aqueous Media. Heteroat. Chem. 2004, 15, 419–423. [Google Scholar] [CrossRef]
- Shi, M.; Qian, H.-X. A new dimeric bidentated NHC–Pd(II) complex from trans-cyclohexane-1,2-diamine for Suzuki reaction and Heck reaction. Tetrahedron 2005, 61, 4949–4955. [Google Scholar] [CrossRef]
- Xu, Q.; Duan, W.-L.; Lei, Z.-Y.; Zhu, Z.-B.; Shi, M. A novel cis-chelated Pd(II)–NHC complex for catalyzing Suzuki and Heck-type cross-coupling reactions. Tetrahedron 2005, 61, 11225–11229. [Google Scholar] [CrossRef]
- Demir, S.; Özdemir, İ.; Çetinkaya, B. Use of bis(benzimidazolium)–palladium system as a convenient catalyst for Heck and Suzuki coupling reactions of aryl bromides and chlorides. Appl. Organometal. Chem. 2006, 20, 254–259. [Google Scholar] [CrossRef]
- Özdemir, İ.; Demir, S.; Çetinkaya, B. Novel tetrahydropyrimidinium/palladium system as a convenient catalysts: Suzuki coupling reactions of aryl chlorides. Arkivoc 2007, 13, 71–78. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, T.; Shi, M. Cyclometalated cis-Chelated Bidentate N-Heterocyclic Carbene Palladium Complexes: Synthetic, Structural, and Catalytic Studies. Organometallics 2008, 27, 2668–2671. [Google Scholar] [CrossRef]
- Avery, K.B.; Devine, W.G.; Kormos, C.M.; Leadbeater, N.E. Use of a silicon carbide multi-well plate in conjunction with microwave heating for rapid ligand synthesis, formation of palladium complexes, and catalyst screening in a Suzuki coupling. Tetrahedron Lett. 2009, 50, 2851–2853. [Google Scholar] [CrossRef]
- Yilmaz, U.; Şireci, N.; Deniz, S.; Küçükbay, H. Synthesis and microwave-assisted catalytic activity of novel bis-benzimidazoles salts bearing furfuryl and thenyl moieties in Heck and Suzuki cross-coupling reactions. Appl. Organometal. Chem. 2010, 24, 414–420. [Google Scholar]
- Micksch, M.; Strassner, T. Palladium(II) Complexes with Chelating Biscarbene Ligands in the Catalytic Suzuki–Miyaura Cross-Coupling Reaction. Eur. J. Org. Chem. 2012, 5872–5880. [Google Scholar] [CrossRef]
- Li, Y.; Tang, J.; Gu, J.; Wang, Q.; Sun, P.; Zhang, D. Chiral 1,2-Cyclohexane-Bridged Bis-NHC Palladium Catalysts for Asymmetric Suzuki–Miyaura Coupling: Synthesis, Characterization, and Steric Effects on Enantiocontrol. Organometallics 2014, 33, 876–884. [Google Scholar] [CrossRef]
- Charbonneau, M.; Addoumieh, G.; Oguadinma, P.; Schmitzer, A.R. Support-Free Palladium–NHC Catalyst for High Recyclable Heterogeneous Suzuki–Miyaura Coupling in Neat Water. Organometallics 2014, 33, 6544–6549. [Google Scholar] [CrossRef]
- Thapa, R.; Kilyanek, S.M. Synthesis and structural characterization of 20-membered macrocyclic rings bearing trans-chelating bis(N-heterocyclic carbene) ligands and the catalytic activity of their palladium(II) complexes. Dalton Trans. 2019, 48, 12577–12590. [Google Scholar] [CrossRef]
- Lin, Y.-R.; Chiu, C.-C.; Chiu, H.-T.; Lee, D.-S.; Lu, T.-J. Bis-benzimidazolium-palladium system catalyzed Suzuki–Miyaura coupling reaction of aryl bromides under mild conditions. Appl. Organometal. Chem. 2017, 32, e3896. [Google Scholar] [CrossRef]
- Chiu, C.-C.; Chiu, H.-T.; Lee, D.-S.; Lu, T.-J. An efficient class of bis-NHC salts: Applications in Pd-catalyzed reactions under mild reaction conditions. RSC Adv. 2018, 8, 26407–26415. [Google Scholar] [CrossRef] [Green Version]
- Tran, V.M.; Nguyen, T.K.N.; Sorna, V.; Loganathan, D.; Kuberan, B. Synthesis and Assessment of Glycosaminoglycan Priming Activity of Cluster-xylosides for Potential Use as Proteoglycan Mimetics. ACS Chem. Biol. 2013, 8, 949–957. [Google Scholar] [CrossRef]
- Yu, K.-K.; Li, K.; Hou, J.-T.; Yu, X.-Q. Coumarin-TPA Derivative: A reaction-based ratiometric fluorescent probe for Cu(I). Tetrahedron Lett. 2013, 54, 5771–5774. [Google Scholar] [CrossRef]
- Cervantes-Reyes, A.; Rominger, F.; Rudolph, M.; Hashmi, A.S.K. Gold(I) Complexes Stabilized by Nine- and Ten-Membered N-Heterocyclic Carbene Ligands. Chem. Eur. J. 2019, 25, 11745–11757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Fridman, N.; Tamm, M.; Eisten, M.S. Addition of E–H (E = N, P, C, O, S) Bonds to Heterocumulenes Catalyzed by Benzimidazolin-2-iminato Actinide Complexes. Organometallics 2017, 36, 3896–3903. [Google Scholar] [CrossRef]
- Chan, A.; Scheidt, K.A. Highly Stereoselective Formal [3+3] Cycloaddition of Enals and Azomethine Imines Catalyzed by N-Heterocyclic Carbenes. J. Am. Che. Soc. 2007, 129, 5334–5335. [Google Scholar] [CrossRef] [PubMed]
- Glannerini, M.; Vila, C.; Hornillos, V.; Feringa, B.L. One-Pot, Fast and Modular Approach to Alkyl- and Aryl Ketones via Sequential 1,2-Addition,Pd-Catalyzed Cross-Coupling of Organolithium Reagents with Weinreb Amides. Chem. Commun. 2016, 52, 1206–1209. [Google Scholar] [CrossRef]
- Liu, Z.; Tan, H.; Wang, L.; Fu, T.; Xia, Y.; Zhang, Y.; Wang, J. Transition-Metal-Free Intramolecular Carbene Aromatic Substitution/Büchner Reaction: Synthesis of Fluorenes and [6,5,7]Benzo-fused Rings. Angew. Chem. Int. Ed. 2015, 54, 3056–3060. [Google Scholar] [CrossRef]
- Mao, P.; Yang, L.; Xiao, Y.; Yuan, J.; Liu, X.; Song, M. Suzuki cross-coupling catalyzed by palladium(II) complexes bearing 1-aryl-3,4,5,6-tetrahydropyrimidine ligands. J. Organomet. Chem. 2012, 705, 39–43. [Google Scholar] [CrossRef]
- Chen, L.; Lang, H.; Fang, L.; Yu, J.; Wang, L. Nickel-Catalyzed Desulfitative Suzuki–Miyaura Cross-Coupling of N,N-Disulfinylmethylamines and Arylboromic Acids. Eur. J. Org. Chem. 2014, 2014, 6385–6389. [Google Scholar] [CrossRef]
- Han, C.; Zhang, Z.; Xu, S.; Wang, K.; Chen, K.; Zhao, J. Palladium-Catalyzed Hiyama Coupling of Benzylic Ammonium Salts via C–N Bond Cleavage. J. Org. Chem. 2019, 84, 16308–16313. [Google Scholar] [CrossRef]
- Piontek, A.; Ochędzan-Siodłak, W.; Bisz, E.; Szostak, M. Nickel-Catalyzed C(sp2)-C(sp3) Kumada Cross-Coupling of Aryl Tosylates with Alkyl Grignard Reagents. Adv. Synth. Catal. 2019, 361, 2329–2335. [Google Scholar] [CrossRef]
- Liu, H.; Yin, B.; Gao, Z.; Li, Y.; Jiang, H. Transition-metal-free highly chemo- and regioselective arylation of unactivated arenes with aryl halides over recyclable heterogeneous catalysts. Chem. Commun. 2012, 48, 2033–2035. [Google Scholar] [CrossRef] [PubMed]
- Lücke, A.-L.; Wlechmann, S.; Freese, T.; Schmidt, A. Suzuki–Miyaura Cross-Coupling Reactions in Acetic Acid Employing Sydnone-Derived Catalyst Systems. Synlett 2017, 28, 1990–1993. [Google Scholar]
- Song, B.; Knauber, T.; Gooßen, L.J. Decarboxylative Cross-Coupling of Mesylates Catalyzed by Copper/Palladium Systems with Customized Imidazolyl Phosphine Ligands. Angew. Chem. Int. Ed. 2013, 52, 2954–2958. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).