Recent Progress of Metal Nanoparticle Catalysts for C–C Bond Forming Reactions
Abstract
:1. Introduction
2. Several Supports for Metal Nanoparticles
2.1. Inorganic Materials
2.2. Magnetically Recoverable Materials
2.3. Porous Materials
2.4. Organic–Inorganic Composites
2.5. Carbon Materials
2.6. Organic Polymers and Surfactants
2.7. Others
3. Conclusions and Perspective
Funding
Data Availability Statement
Conflicts of Interest
References
- Rampino, L.D.; Nord, F.F. Preparation of palladium and platinum synthetic high polymer catalysts and the relationship between particle size and rate of hydrogenation. J. Am. Chem. Soc. 1941, 63, 2745–2749. [Google Scholar] [CrossRef]
- Reetz, M.T.; Lohmer, G. Propylene carbonate stabilized nanostructured palladium clusters as catalysts in Heck reactions. Chem. Commun. 1996, 1921–1922. Available online: https://pubs.rsc.org/en/content/articlelanding/1996/CC/cc9960001921 (accessed on 10 August 2021). [CrossRef]
- Reetz, M.T.; Breinbauer, R.; Wanninger, K. Suzuki and Heck reactions catalyzed by preformed palladium clusters and palladiumnickel bimetallic clusters. Tetrahedron Lett. 1996, 37, 4499–4502. [Google Scholar] [CrossRef]
- Ayogu, J.I.; Onoabedje, E.A. Prospects and applications of palladium nanoparticles in the cross-coupling of (hetero)aryl halides and related analogues. ChemistryOpen 2021, 10, 430–450. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, M.; Kilic, H.; Nişanci, B.; Metin, Ö. Recent advances in the development of palladium nanocatalysts for sustainable organic transformations. Inorg. Chem. Front. 2021, 8, 499–545. [Google Scholar] [CrossRef]
- Söğütlü, I.; Mahmood, E.A.; Shendy, S.A.; Ebrahimiasl, S.; Vessally, E. Recent progress in application of nanocatalysts for carbonylative Suzuki cross-coupling reactions. RSC Adv. 2021, 11, 2112–2125. [Google Scholar] [CrossRef]
- Bhakta, S.; Ghosh, T. Nickel nanocatalysis: An efficient tool for Heck reaction. ChemCatChem 2021, 13, 828–835. [Google Scholar] [CrossRef]
- Wani, I.A.; Jain, S.K.; Khan, H.; Kalam, A.; Ahmad, T. Gold nanoparticles as efficient catalysts in organic transformations. Curr. Pharm. Biotechnol. 2021, 22, 724–732. [Google Scholar] [CrossRef]
- Ardakani, L.S.; Surendar, A.; Thangavelu, L.; Mandal, T. Silver nanoparticles (Ag NPs) as catalyst in chemical reactions. Synth. Commun. 2021, 51, 1516–1536. [Google Scholar]
- Jinfeng, F.; Yanmei, L.; Yan, H.; Moghadasi, Z. Synthesis of heterocycles catalyzed by metallic nanoparticles (NPs). Synth. Commun. 2021, 51. [Google Scholar] [CrossRef]
- Huang, W.; Jiang, J.; Mandal, T. Ferrite nanoparticles: Catalysis in multicomponent reactions (MCR). Synth. Commun. 2021, 51, 2397–2422. [Google Scholar] [CrossRef]
- Huang, J.; Lu, D.; Mandal, T. Catalytic application of copper iodide nanoparticles in organic synthesis. Synth. Commun. 2021, 51, 1923–1946. [Google Scholar]
- Alsalahi, W.; Trzeciak, A.M. Rhodium-catalyzed hydroformylation under green conditions: Aqueous/organic biphasic, “on water”, solventless and Rh nanoparticle based systems. Cood. Chem. Rev. 2021, 430, 213732. [Google Scholar] [CrossRef]
- Gholinejad, M.; Khosravi, F.; Afrasi, M.; Sansano, J.M.; Nájera, C. Applications of bimetallic PdCu catalysts. Catal. Sci. Technol. 2021, 11, 2652–2702. [Google Scholar] [CrossRef]
- Recent advances in metal-nanoparticle-catalyzed coupling reactions assisted by microwave irradiation. Synthesis 2021, 53, 3513–3521. [CrossRef]
- Din Reshi, N.U.; Samuelson, A.G. Recent advances in soluble ruthenium(0) nanocatalysts and their reactivity. Appl. Catal. A Gen. 2020, 598, 117561. [Google Scholar] [CrossRef]
- Sankar, M.; He, Q.; Engel, R.V.; Sainna, M.A.; Jogsdail, A.J.; Roldan, A.; Willock, D.J.; Agarwal, N.; Kiely, C.J.; Hutchings, G.J. Role of the support in gold-containing nanoparticles as heterogeneous catalysts. Chem. Rev. 2020, 120, 3890–3938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponti, A.; Molteni, G. Nanoparticle-catalyzed 1,3-dipolar cycloadditions. Eur. J. Org. Chem. 2020, 6173–6191. [Google Scholar] [CrossRef]
- Dhameliya, T.M.; Donga, H.A.; Vaghela, P.V.; Panchal, B.G.; Sureja, D.K.; Bodiwala, K.B.; Chhabria, M.T. A decennary update on applications of metal nanoparticles (MNPs) in the synthesis of nitrogen- and oxygen-containing heterocyclic scaffolds. RSC Adv. 2020, 10, 32740–32820. [Google Scholar] [CrossRef]
- Van Vaerenbergh, B.; Lauwaert, J.; Vermeir, P.; Thybaut, J.W.; De Clercq, J. Towards high-performance heterogeneous palladium nanoparticle catalysts for sustainable liquid-phase reactions. React. Chem. Eng. 2020, 5, 1556–1618. [Google Scholar] [CrossRef]
- Van Vaerenbergh, B.; Lauwaert, J.; Vermeir, P.; De Clercq, J.; Thybaut, J.W. Chapter one–Synthesis and support interaction effects on the palladium nanoparticle catalyst characteristics. Adv. Catal. 2019, 65, 1–120. [Google Scholar]
- Trzeciak, A.M.; Augustyniak, A.W. The role of palladium nanoparticles in catalytic C-C cross-coupling reactions. Cood. Chem. Rev. 2019, 384, 1–20. [Google Scholar] [CrossRef]
- Ohtaka, A. Transition-metal nanoparticles catalyzed carbon-carbon coupling reactions in water. Curr. Org. Chem. 2019, 23, 689–703. [Google Scholar] [CrossRef]
- Monfared, A.; Mohammadi, R.; Ahmadi, S.; Nikpassand, M.; Hosseinian, A. Recent advances in the application of nano-catalysts for Hiyama cross-coupling reactions. RSC Adv. 2019, 9, 3185–3202. [Google Scholar] [CrossRef] [Green Version]
- Roy, D.; Uozumi, Y. Recent advances in palladium-catalyzed cross-coupling reactions at ppm to ppb molar catalyst loading. Adv. Synth. Catal. 2018, 360, 602–625. [Google Scholar] [CrossRef]
- Liu, L.; Corma, A. Metal catalysts for heterogeneous catalysis: From single atoms to nanoclusters and nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef] [Green Version]
- Mpungose, P.P.; Vundla, Z.P.; Maguire, G.E.M.; Friedrich, H.B. The current status of heterogeneous palladium catalysed Heck and Suzuki cross-coupling reactions. Molecules 2018, 23, 1676. [Google Scholar] [CrossRef] [Green Version]
- Biffis, A.; Centomo, P.; Del Zotto, A.; Zecca, M. Pd metal catalysts for cross-couplings and related reactions in the 21st century: A critical review. Chem. Rev. 2018, 118, 2249–2295. [Google Scholar] [CrossRef]
- Ojha, N.K.; Zyryanov, G.V.; Majee, A.; Charushin, V.N.; Chupakhin, O.N.; Santra, S. Copper nanoparticles as inexpensive and efficient catalyst: A valuable contribution in organic synthesis. Coord. Chem. Rev. 2017, 353, 1–57. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Atarod, M.; Alizadeh, M.; Hatamifard, A.; Sajadi, S.M. Recent advances in the application of heterogeneous nanocatalysts for Sonogashira coupling reactions. Curr. Org. Chem. 2017, 21, 708–749. [Google Scholar] [CrossRef]
- Díaz-Sánchez, M.; Díaz-García, D.; Prashar, S.; Gómez-Ruiz, S. Palladium nanoparticles supported on silica, alumina or titania: Greener alternatives for Suzuki-Miyaura and other C-C coupling reactions. Environ. Chem. Lett. 2019, 17, 1585–1602. [Google Scholar] [CrossRef]
- Veerakumar, P.; Thanasekaran, P.; Lu, K.-L.; Liu, S.-B.; Rajagopal, S. Functionalized silica matrices and palladium: A versatile heterogeneous catalyst for Suzuki, Heck, and Sonogashira reactions. ACS Sustainable Chem. Eng. 2017, 5, 6357–6376. [Google Scholar] [CrossRef]
- Del Zotto, A.; Zuccaccia, D. Metallic palladium, PdO, and palladium supported on metal oxides for the Suzuki-Miyaura cross-coupling reaction: A unified view of the process of formation of the catalytically active species in solution. Catal. Sci. Technol. 2017, 7, 3934–3951. [Google Scholar] [CrossRef]
- Utsunomiya, M.; Kondo, R.; Oshima, T.; Safumi, M.; Suzuki, T.; Obora, Y. Cross β-arylmethylation of alcohols catalyzed by recyclable Ti-Pd alloys not requiring pre-activation. Chem. Commun. 2021, 57, 5139–5142. [Google Scholar] [CrossRef]
- Handa, S.; Jin, B.; Bora, P.P.; Wang, Y.; Zhang, X.; Gallou, F.; Reilly, J.; Lipshutz, B.H. Sonogashira coupling catalyzed by Fe nanoparticles containing ppm levels of reusable Pd, under mild aqueous micellar conditions. ACS Catal. 2019, 9, 2423–2431. [Google Scholar] [CrossRef]
- Elazab, H.A.; Radwan, M.A.; El-Idreesy, T.T. Facile microwave-assisted synthetic approach to palladium nanoparticles supported on copper oxide as an efficient catalyst for Heck and Sonogashira cross-coupling reactions. Int. J. Nanosci. 2019, 18, 1850032. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.; Gao, P.; Xiao, Y.; Chen, J.; Wang, W. Pd-Co nanoparticles supported on calcined Mg-Fe hydrotalcites for the Suzuki-Miyaura reaction in water with high turnover numbers. Catalysts 2019, 9, 1061. [Google Scholar] [CrossRef] [Green Version]
- Yamada, M.; Shio, Y.; Akiyama, T.; Honma, T.; Ohki, Y.; Takahashi, M.; Murai, K.; Arisawa, M. Ligand-free Suzuki-Miyaura coupling reaction of an aryl chloride using a continuous irradiation type microwave and a palladium nanoparticle catalyst: Effect of a co-existing solid. Green Chem. 2019, 21, 4541–4549. [Google Scholar] [CrossRef]
- Taniguchi, T.; Saito, N.; Doi, R.; Kimoto, A.; Hoshiya, N.; Fujiki, K.; Shuto, S.; Fujioka, H.; Arisawa, M.; Sato, Y. Nickel nanoparticle-catalyzed carboxylation of unsaturated hydrocarbon with CO2 using sulfur-modified Au-supported nickel material. Chem. Lett. 2019, 48, 1406–1409. [Google Scholar] [CrossRef]
- Mahanta, A.; Raul, P.K.; Saikia, S.; Bora, U.; Thakur, A.J. Methanol aided synthesis of PdNPs decorated on montmorillonite K 10 and its implication in Suzuki Miyaura type cross coupling reaction under base free condition. Appl. Organomet. Chem. 2018, 32, e4192. [Google Scholar] [CrossRef]
- Elazab, H.A.; Sadek, M.A.; El-Idreesy, T.T. Microwave-assisted synthesis of palladium nanoparticles supported on copper oxide in aqueous medium as an efficient catalyst for Suzuki cross-coupling reaction. Adsorpt. Sci. Technol. 2018, 36, 1352–1365. [Google Scholar] [CrossRef]
- Yu, D.; Jie, B.; Wang, J.; Li, C. Design a fabrication of PdO/CexOy composite catalysts with coaxial nanotuber and studies of their synergistic performance in Suzuki-Miyaura reactions. J. Catal. 2018, 365, 195–203. [Google Scholar] [CrossRef]
- Miura, H.; Nagao, M.; Hosokawa, S.; Shishido, T.; Inoue, M.; Wada, K. Generation of active ruthenium catalysts for hydroarylation of C-C multiple bonds from isolated Ru(IV)=O species supported on CeO2. Bull. Chem. Soc. Jpn 2018, 91, 1397–1401. [Google Scholar] [CrossRef]
- Ketike, T.; Velpula, V.R.K.; Madduluri, V.R.; Kamaraju, S.R.R.; Burri, D.R. Carbonylative Suzuki-Miyaura cross-coupling over Pd NPs/Rice-Husk carbon-silica solid catalyst: Effect of 1,4-dioxane solvent. ChemistrySelect 2018, 3, 7164–7169. [Google Scholar] [CrossRef]
- Del Zotto, A.; Colussi, S.; Trovarelli, A. Pd/REOs catalysts applied to the Suzuki-Miyaura coupling. A comparison of their catalytic performance and reusability. Inorg. Chim. Acta 2018, 470, 275–283. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, X.; Tang, L.; Min, D.; Shi, T.; Zhang, W. Pd-ZnO nanowire arrays as recyclable catalyst for 4-nitrophenol reduction and Suzuki coupling reactions. RSC Adv. 2017, 7, 7964–7972. [Google Scholar] [CrossRef] [Green Version]
- Shabbir, S.; Lee, S.; Lim, M.; Lee, H.; Ko, H.; Lee, Y.; Rhee, H. Pd nanoparticles on reverse phase silica gel as recyclable catalyst for Suzuki-Miyaura cross coupling reaction and hydrogenation in water. J. Organomet. Chem. 2017, 846, 296–304. [Google Scholar] [CrossRef]
- Raza, F.; Yim, D.; Park, J.H.; Kim, H.-I.; Jeon, S.-J.; Kim, J.-H. Structuring Pd nanoparticles on 2H-WS2 nanosheets induced excellent photocatalytic activity for cross-coupling reactions under visible light. J. Am. Chem. Soc. 2017, 139, 14767–14774. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.M.S.; Balbín, A.; Erami, R.S.; Prashar, S.; Fajardo, M.; Gómez-Ruiz, S. Synthesis and study of the catalytic applications in C-C coupling reactions of hybrid nanosystems based on alumina and palladium nanoparticles. Inorg. Chim. Acta 2017, 455, 645–652. [Google Scholar] [CrossRef]
- Pitre, S.P.; Scaiano, J.C.; Yoon, T.P. Photocatalytic indole Diels-Alder cycloadditions mediated by heterogeneous platinum-modified titanium dioxide. ACS Catal. 2017, 7, 6440–6444. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.H.; Kang, E.; Park, H.; Han, T.; Lee, C.-H.; Lim, D.-K. Pd-nanodot decorated MoS2 nanosheets as a highly efficient photocatalyst for the visible-light-induced Suzuki-Miyaura coupling reaction. J. Mater. Chem. A 2017, 5, 24965–24971. [Google Scholar] [CrossRef]
- Hamdi, J.; Blanco, A.A.; Diehl, B.; Wiley, J.B.; Trudell, M.L. Room-temperature aqueous Suzuki-Miyaura cross-coupling reactions catalyzed via a recyclable palladium@halloysite nanocomposite. Org. Lett. 2019, 21, 3471–3475. [Google Scholar] [CrossRef]
- Van Vaerenbergh, B.; De Vlieger, K.; Claeys, K.; Vanhoutte, G.; De Clercq, J.; Vermeir, P.; Verberckmoes, A. The effect on the hydrotalcite structure and nanoparticle size on the catalytic performance of supported palladium nanoparticle catalysts in Suzuki cross-coupling. Appl. Catal. A Gen. 2018, 550, 236–244. [Google Scholar] [CrossRef]
- Gholinejad, M.; Bahrami, M.; Nájera, C. A fluorescence active catalyst support comprising carbon quantum dots and magnesium oxide doping for stabilization of palladium nanoparticles: Application as a recoverable catalyst for Suzuki reaction in water. Mol. Catal. 2017, 433, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Miura, H.; Tanaka, Y.; Nakahara, K.; Shishido, T. Reductive cycloisomerization of diynes by supported palladium catalysts and subsequent [4+2] cycloaddition for one-pot synthesis of cyclohexenes. ChemCatChem 2020, 12, 455–458. [Google Scholar] [CrossRef]
- Miura, H.; Tanaka, Y.; Nakahara, K.; Hachiya, Y.; Endo, K.; Shishido, T. Concerted catalysis by adjacent palladium and gold in alloy nanoparticles for the versatile and practical [2+2+2] cycloaddition of alkynes. Angew. Chem. Int. Ed. 2018, 57, 6136–6140. [Google Scholar] [CrossRef]
- Miura, H.; Shishido, T. Concerted catalysis of Pd and Au on alloy nanoparticles for efficient heterogeneous molecular transformation. Chem. Lett. 2021, 50, 346–352. [Google Scholar] [CrossRef]
- An, J.; Wang, Y.; Zhang, Z.; Zhang, J.; Gocyla, M.; Dunin-Borkowski, R.E.; Wang, F. Linear-regioselective hydromethoxycarbonylation of styrene using Ru-clusters/CeO2 catalyst. Chin. J. Catal. 2020, 41, 963–969. [Google Scholar] [CrossRef]
- An, J.; Wang, Y.; Lu, J.; Zhang, J.; Zhang, Z.; Xu, S.; Liu, X.; Zhang, T.; Gocyla, M.; Heggen, M.; et al. Acid-promoter-free ethylene methoxycarbonylation over Ru-Clusters/ceria: The catalysis of interfacial Lewis acid-base pair. J. Am. Chem. Soc. 2018, 140, 4172–4181. [Google Scholar] [CrossRef]
- An, J.; Wang, Y.; Zhang, Z.; Zhao, Z.; Zhang, J.; Wang, F. The synthesis of quinazolinones from olefins, CO and amines over a heterogeneous Ru-clusters/ceria catalyst. Angew. Chem. Int. Ed. 2018, 57, 12308–12312. [Google Scholar] [CrossRef]
- Diacon, A.; Rusen, E.; Mocanu, A.; Nistor, L.C. Supported Cu0 nanoparticles catalyst for controlled radical polymerization reaction and block-copolymer synthesis. Sci. Rep. 2017, 7, 10345. [Google Scholar] [CrossRef] [Green Version]
- Alexander, M.; Kalaivani, R.A.; Sriraman, V.; Senthilkumar, U. Electro polymerization of o-phenylenediamine using palladium nanoparticles coated fabricated TiO2 nanotubes modified glassy carbon electrode. Asian J. Org. Chem. 2019, 31, 2229–2232. [Google Scholar] [CrossRef]
- Koohgard, M.; Hosseini-Sarvari, M. Enhancement of Suzuki-Miyaura coupling reaction by photocatalytic palladium nanoparticles anchored to TiO2 under visible light irradiation. Catal. Commun. 2018, 111, 10–15. [Google Scholar] [CrossRef] [Green Version]
- Hosseini-Sarvari, M.; Bazyar, Z. Visible light driven photocatalytic cross-coupling reactions on nano Pd/ZnO photocatalyst at room-temperature. ChemistrySelect 2018, 3, 1898–1907. [Google Scholar] [CrossRef]
- Elhage, A.; Lanterna, A.E.; Scaiano, J.C. Light-induced Sonogashira C-C coupling under mild conditions using supported palladium nanoparticles. ACS Sustainable Chem. Eng. 2018, 6, 1717–1722. [Google Scholar] [CrossRef]
- Marina, N.; Lanterna, A.E.; Scaiano, J.C. Expanding the color space in the two-color heterogeneous photocatalysis of Ullmann C-C coupling reactions. ACS Catal. 2018, 8, 7593–7597. [Google Scholar] [CrossRef]
- Wang, B.; Guo, X.; Jin, G.; Guo, X. Visible-light-enhanced photocatalytic Sonogashira reaction over silicon carbide supported Pd nanoparticles. Catal. Commun. 2017, 98, 81–84. [Google Scholar] [CrossRef]
- Tyagi, A.; Yamamoto, A.; Yoshida, H. Photocatalytic Ullmann coupling of aryl halides by a novel blended catalyst consisting of a TiO2 photocatalyst and an Al2O3 supported Pd-Au bimetallic catalyst. Catal. Sci. Technol. 2018, 8, 6196–6203. [Google Scholar] [CrossRef] [Green Version]
- Tyagi, A.; Yamamoto, A.; Yamamoto, M.; Yoshida, T.; Yoshida, H. Direct cross-coupling between alkenes and tetrahydrofuran with platinum-loaded titanium oxide photocatalyst. Catal. Sci. Technol. 2018, 8, 2546–2556. [Google Scholar] [CrossRef]
- Tyagi, A.; Yamamoto, A.; Kato, T.; Yoshida, H. Bifunctional property of Pt nanoparticles deposited on TiO2 for the photocatalytic sp3C-sp3C cross-coupling reactions between THF and alkanes. Catal. Sci. Technol. 2017, 7, 2616–2623. [Google Scholar] [CrossRef]
- Tyagi, A.; Yamamoto, A.; Yoshida, H. Novel blended catalysts consisting of a TiO2 photocatalyst and an Al2O3 supported Pd-Au bimetallic catalyst for direct dehydrogenative cross-coupling between arenes and tetrahydrofuran. RSC Adv. 2018, 8, 24021–24028. [Google Scholar] [CrossRef] [Green Version]
- Naniwa, S.; Hishitani, S.; Yamamoto, A.; Yoshida, H. Ligand-to-metal charge transfer of a pyridine surface complex on TiO2 for selective dehydrogenative cross-coupling with benzene. Phys. Chem. Chem. Phys. 2021, 23, 11366–11373. [Google Scholar] [CrossRef] [PubMed]
- Rohani, S.; Ziarati, A.; Ziarani, G.M.; Badiei, A.; Burgi, T. Engineering of highly active Au/Pd supported on hydrogenated urchin-like yolk@shell TiO2 for visible light photocatalytic Suzuki coupling. Catal. Sci. Technol. 2019, 9, 3820–3827. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, I.N.; Roy, S.; Devatha, G.; Rao, A.; Pillai, P.P. InP/ZnS quantum dots as efficient visible-light photocatalysts for redox and carbon-carbon coupling reactions. Chem. Mater. 2019, 31, 2258–2262. [Google Scholar] [CrossRef]
- Li, Y.; Ren, P.; Zhang, D.; Qiao, W.; Wang, D.; Yang, X.; Wen, X.; Rummeli, M.H.; Niemantsverdriet, H.; Lewis, J.P.; et al. Rationally designed metal cocatalyst for selective photosynthesis of bibenzyls via dehalogenative C-C homocoupling. ACS Catal. 2021, 11, 4338–4348. [Google Scholar] [CrossRef]
- Sharma, A.S.; Sharma, V.S.; Kaur, H. Graphitic carbon nitride decorated with Cu2O nanoparticles for the visible light activated synthesis of ynones, aminoindolizines, and pyrrolo[1,2-a]quinoline. ACS Appl. Nano Mater. 2020, 3, 1191–1202. [Google Scholar] [CrossRef]
- Ma, J.; Tan, X.; Zhang, Q.; Wang, Y.; Zhang, J.; Wang, L. Exploring the size effect of Pt nanoparticles on the photocatalytic nonoxidative coupling of methane. ACS Catal. 2021, 11, 3352–3360. [Google Scholar] [CrossRef]
- Tyagi, A.; Matsumoto, T.; Yamamoto, A.; Kato, T.; Yoshida, H. Metal cocatalyst directing photocatalytic acetonylation of toluene via dehydrogenative cross-coupling with acetone. Catal. Lett. 2020, 150, 31–38. [Google Scholar] [CrossRef]
- Huang, W.; Cheng, Q.; Ma, D. Recent reports on magnetic nanoparticles supported metallic catalysts: Synthesis of heterocycles. Synth. Commun. 2021, 51, 1321–1339. [Google Scholar] [CrossRef]
- Kazemi, M. Based on CuFe2O4 MNPs: Magnetically recoverable nanocatalysts in coupling reactions. Synth. Commun. 2020, 50, 2114–2131. [Google Scholar] [CrossRef]
- Kazemi, M. Based on magnetic nanoparticles: Gold reusable nanomagnetic catalysts in organic synthesis. Synth. Commun. 2020, 50, 2079–2094. [Google Scholar] [CrossRef]
- Kazemi, M. Based on MFe2O4 (M = Co, Cu, and Ni): Magnetically recoverable nanocatalysts in synthesis of heterocyclic structural scaffolds. Synth. Commun. 2020, 50, 1899–1935. [Google Scholar] [CrossRef]
- Kazemi, M.; Mohammadi, M. Magnetically recoverable catalysts: Catalysis in synthesis of polyhydroquinolines. Appl. Organomet. Chem. 2019, 34, e5400. [Google Scholar] [CrossRef]
- Kazemi, M.; Ghobadi, M.; Mirzaie, A. Cobalt ferrite nanoparticles (CoFe2O4 MNPs) as catalyst and support: Magnetically recoverable nanocatalysts in organic synthesis. Nanotechnol. Rev. 2018, 7, 43–68. [Google Scholar] [CrossRef]
- Sydnes, M.O. The use of palladium on magnetic support as catalyst for Suzuki-Miyaura cross-coupling reactions. Catalysts 2017, 7, 35. [Google Scholar] [CrossRef] [Green Version]
- Mulahmetovic, E.; Hargaden, G.C. Recent advances in the development of magnetic catalysts for the Suzuki reaction. Rev. J. Chem. 2017, 7, 373–398. [Google Scholar] [CrossRef]
- Sobhani, S.; Zarei, H.; Sansano, J.M. A new nanomagnetic Pd-Co bimetallic alloy as catalyst in the Mizoroki-Heck and Buchwald-Hartwig amination reactions in aqueous media. Sci. Rep. 2021, 11, 17025. [Google Scholar] [CrossRef]
- Veisi, H.; Zohrabi, A.; Kamangar, S.A.; Karmakar, B.; Saremi, S.G.; Varmira, K.; Hamelian, M. Green synthesis of Pd/Fe3O4 nanoparticles using Chamomile extract as highly active and recyclable catalyst for Suzuki coupling reaction. J. Organomet. Chem. 2021, 951, 122005. [Google Scholar] [CrossRef]
- Veisi, H.; Joshani, Z.; Karmakar, B.; Tamoradi, T.; Heravi, M.M.; Gholami, J. Ultrasound assisted synthesis of Pd NPs decorated chitosan-starch functionalized Fe3O4 nanocomposite catalyst towards Suzuki-Miyaura coupling and reduction of 4-nitrophenol. Int. J. Biol. Macromol. 2021, 172, 104–113. [Google Scholar] [CrossRef]
- Baloutaki, B.A.; Sayahi, M.H.; Nikpassand, M.; Kefayati, H. Palladium supported terpyridine modified magnetic nanoparticles as an efficient catalyst for carbon-carbon bond formation. J. Organomet. Chem. 2021, 935, 121682. [Google Scholar] [CrossRef]
- Nasseri, M.A.; Kazemnejadi, M.; Allahresani, A.; HussainZadeh, M. NiFe2O4@SiO2@ZrO2/SO42-/Cu/Co nanoparticles: A novel, efficient, magnetically recyclable and bimetallic catalyst for Pd-free Suzuki, Heck and C-N cross-coupling reactions in aqueous media. New J. Chem. 2021, 45, 7741–7757. [Google Scholar]
- Aghbash, K.O.; Alamgholiloo, H.; Pesyan, N.N.; Khaksar, S.; Rostamnia, S. Gold nanoparticle stabilized dithiocarbamate functionalized magnetite carbon as promise clean nanocatalyst for A3-coupling organic transformation. Mol. Catal. 2021, 499, 111252. [Google Scholar] [CrossRef]
- Dabiri, M.; Nikbakht, R.; Movahed, S.K. Palladium nanoparticle supported on core-shell FeOx@nitrogen-doped carbon cubes and their photocatalytic activities in selective oxidation of alcohols and Ullmann homocoupling in one reaction system. Mater. Chem. Phys. 2021, 258, 123908. [Google Scholar] [CrossRef]
- Sharma, A.K.; Joshi, H.; Singh, A.K. Catalysis with magnetically retrievable and recyclable nanoparticles layered with Pd(0) for C-C/C-O coupling in water. RSC Adv. 2020, 10, 6452–6459. [Google Scholar] [CrossRef] [Green Version]
- Solyman, S.M.; Darwish, M.S.A.; Yoon, J. Catalytic activity of hybrid iron oxide silver nanoparticles in methyl methacrylate polymerization. Catalysts 2020, 10, 422. [Google Scholar] [CrossRef]
- Heydari, F.; Mobinikhaledi, A.; Zolfigol, M.A. Synthesis of a novel Pd supported polymeric magnetic nanoparticles with urea-pyridine bridge: Application as an efficient catalyst for the C-C and C-N bond formation. J. Porous Mater. 2020, 27, 395–411. [Google Scholar] [CrossRef]
- Jahanshahi, R.; Khazaee, A.; Sobhani, S.; Sansano, J.M. g-C3N4/γ-Fe2O3/TiO2/Pd: A new magnetically separable photocatalyst for visible-light-driven fluoride-free Hiyama and Suzuki-Miyaura cross-coupling reactions at room temperature. New J. Chem. 2020, 44, 11513–11526. [Google Scholar] [CrossRef]
- Veisi, H.; Ozturk, T.; Karmakar, B.; Tamoradi, T.; Hemmati, S. In situ decorated Pd NPs on chitosan-encapsulated Fe3O4/SiO2-NH2 as magnetic catalyst in Suzuki-Miyaura coupling and 4-nitrophenol reduction. Carbohyd. Polym. 2020, 235, 115966. [Google Scholar] [CrossRef] [PubMed]
- Gholami, A.; Mokhtary, M.; Nikpassand, M. Glycolic acid-supported cobalt ferrite-catalyzed one-pot synthesis of pyrimido[4,5-b]quinoline and indenopyrido[2,3-d]pyrimidine derivative. Appl. Organomet. Chem. 2020, e6007. [Google Scholar] [CrossRef]
- Kaldareh, M.F.; Mokhtary, M.; Nikpassand, M. Nicotinic acid-supported cobalt ferrite-catalyzed one-pot synthesis of substituted chromeno[3,4-b]quinolines. Appl. Organomet. Chem. 2020, 34, e5469. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/aoc.5469 (accessed on 10 August 2021).
- Nikpassand, M.; Hsseinnezhad, E. Green synthesis of 4,6-bisarylpyrimidin-2(1H)-ones and azo-linked 4-arylpyrimidin-2(1H)-ones using NiFe2O4@SiO2nPr@glucose amine as a mild nano catalyst. Arab. J. Chem. 2020, 13, 8995–9004. [Google Scholar] [CrossRef]
- Nikpassand, M.; Kasmaei, S.A. Tannic acid-functionalized silica coated Fe3O4 nanoparticles as a novel and magnetically separable catalyst for green synthesis of aryl naptho[1,3]oxazine-2-thiones. J. Inorg. Organomet. P. 2020, 30, 4926. [Google Scholar]
- Aghazadeh, B.; Nikpassand, M. “2-Amino glucose” as a substrate for synthesis of magnetically recoverable nanocatalyst NiFe3O4@SiO2@amino glucose for the green synthesis of novel bis(1,2-dihydro-4-hydroxy-2-oxoquinolin-3-yl)methanes. Carbohyd. Res. 2019, 483, 107755. [Google Scholar] [CrossRef]
- Elazab, H.A.; Gadalla, M.A.; Sadek, M.A.; El-Idreesy, T.T. Hydrothermal synthesis of graphene supported Pd/Fe3O4 nanoparticles as efficient magnetic catalysts for Suzuki cross-coupling. Biointerface Res. Appl. Chem. 2019, 9, 3906–3911. [Google Scholar]
- De Cattelle, A.; Billen, A.; O’Rourke, G.; Brullot, W.; Verbiest, T.; Koeckelberghs, G. Ligand-free, recyclable palladium-functionalized magnetite nanoparticles as a catalyst in the Suzuki-, Sonogashira, and Stille reaction. J. Organomet. Chem. 2019, 904, 121005. [Google Scholar] [CrossRef]
- Tamoradi, T.; Veisi, H.; Karmakar, B. Pd nanoparticle fabricated tetrahydroharman-3-carboxylic acid analog immobilized CoFe2O4 catalyzed fast and expedient C-C cross and C-S coupling. ChemistrySelect 2019, 4, 10953–10959. [Google Scholar] [CrossRef]
- Veisi, H.; Mohammadi, L.; Hemmati, S.; Tamoradi, T.; Mohammadi, P. In situ immobilized silver nanoparticles on Rubia tinctorum extract-coated ultrasmall iron oxide nanoparticles: An efficient nanocatalyst with magnetic recyclability for synthesis of propargylamines by A3 coupling reaction. ACS Omega 2019, 4, 13991–14003. [Google Scholar] [CrossRef] [Green Version]
- Veisi, H.; Hemmati, S.; Safarimehr, P. In situ immobilized palladium nanoparticles on surface of poly-methyldopa coated-magnetic nanoparticles (Fe3O4@PMDA/Pd): A magnetically recyclable nanocatalyst for cyanation of aryl halides with K4[Fe(CN)6]. J. Catal. 2018, 365, 204–212. [Google Scholar] [CrossRef]
- Veisi, H.; Pirhayati, M.; Kakanejadifard, A.; Mohammadi, P.; Abdi, M.R.; Gholami, J.; Hemmati, S. In situ green synthesis of Pd nanoparticles on tannic acid-modified magnetite nanoparticles as a green reductant and stabilizer agent: Its application as a recyclable nanocatalyst (Fe3O4@TA/Pd) for reduction of 4-nitrophenol and Suzuki reactions. ChemistrySelect 2018, 3, 1820–1826. [Google Scholar] [CrossRef]
- Tanhaei, M.; Mahjoub, A.; Nejat, R. Three-dimensional graphene-magnetic palladium nanohybrid: A highly efficient and reusable catalyst for promoting organic reactions. Catal. Lett. 2018, 148, 1549–1561. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Jiang, S.; Zhang, H. Facile synthesis of magnetic recyclable palladium-gold alloy nanoclusters catalysts PdAur/Fe3O4@LDH and its catalytic applications in Heck reaction. J. Organomet. Chem. 2018, 878, 84–95. [Google Scholar] [CrossRef]
- Dadras, A.; Naimi-Jamal, M.R.; Moghaddam, F.M.; Ayati, S.E. Suzuki-Miyaura coupling reaction in water in the presence of robust palladium immobilized on modified magnetic Fe3O4 nanoparticles as a recoverable catalyst. Appl. Organomet. Chem. 2018, 32, e3993. [Google Scholar] [CrossRef]
- Veisi, H.; Pirhayati, M.; Kakanejadifard, A. Immobilization of palladium nanoparticles on ionic liquid-triethylammonium chloride functionalized magnetic nanoparticles: As a magnetically separable, stable and recyclable catalyst for Suzuki-Miyaura cross-coupling reactions. Tetrahedron Lett. 2017, 58, 4269–4276. [Google Scholar] [CrossRef]
- Rostamizadeh, S.; Estiri, H.; Azad, M. Ullmann homocoupling of aryl iodides catalyzed by gold nanoparticles stabilized on magnetic mesoporous silica. J. Iran. Chem. Soc. 2017, 14, 1005–1010. [Google Scholar] [CrossRef]
- Sarvi, I.; Gholizadeh, M.; Izadyar, M. Highly dispersed palladium nanoparticle-loaded magnetic catalyst (FeS@Ep-AG-Pd) for Suzuki reaction in water. Catal. Lett. 2017, 147, 1162–1171. [Google Scholar] [CrossRef]
- Khazaei, A.; Khazaei, M.; Nasrollahzadeh, M. Nano-Fe3O4@SiO2 supported Pd(0) as a magnetically recoverable nanocatalyst for Suzuki coupling reaction in the presence of waste eggshell as low-cost natural base. Tetrahedron 2017, 73, 5624–5633. [Google Scholar] [CrossRef]
- Rezaei, S.J.T.; Shamseddin, A.; Ramazani, A.; Malekzadeh, A.M.; Asiabi, P.A. Palladium nanoparticles immobilized on amphiphilic and hyperbranched polymer-functionalized magnetic nanoparticles: An efficient semi-heterogeneous catalyst for Heck reaction. Appl. Organomet. Chem. 2017, 31, e3707. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Rezaei, F.; Khohrsandi, Z. Pd/Cu-free Heck and Sonogashira cross-coupling reaction by Co nanoparticles immobilized on magnetic chitosan as reusable catalyst. Green Chem. 2017, 19, 1353–1361. [Google Scholar] [CrossRef]
- Nasseri, M.A.; Rezazadeh, Z.; Kazemnejadi, M.; Allahresani, A. A Co-Cu bimetallic magnetic nanocatalyst with synergistic and bifunctional performance for the base-free Suzuki, Sonogashira, and C-N cross-coupling reactions in water. Dalton Trans. 2020, 49, 10645–10660. [Google Scholar] [CrossRef]
- Nasseri, M.A.; Rezazadeh, Z.; Kazemnejadi, M.; Allahresani, A. Magnetic Cu-Schiff base complex with an ionic tail as a recyclable bifunctional catalyst for base/Pd-free Sonogashira coupling reaction. J. Iran. Chem. Soc. 2019, 16, 2693–2705. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Tavangar-Rizi, Z. Palladium nanoparticles immobilized on magnetic methionine-functionalized chitosan: A versatile catalyst for Suzuki and copper-free Sonogashira reactions of aryl halides at room temperature in water as only solvent. Appl. Organomet. Chem. 2017, 31, e3701. [Google Scholar] [CrossRef]
- Lamei, K.; Eshghi, H.; Bakavoli, M.; Rostamnia, S. Highly dispersed copper/ppm palladium nanoparticles as novel magnetically recoverable catalyst for Suzuki reaction under aqueous conditions at room temperature. Appl. Organomet. Chem. 2017, 31, e3743. [Google Scholar] [CrossRef]
- Wang, L.L.; Zhu, L.P.; Bing, N.C.; Wang, L.J. Facile green synthesis of Pd/N-doped carbon nanotubes catalysts and their application in Heck reaction and oxidation of benzyl alcohol. J. Phys. Chem. Solids 2017, 107, 125–130. [Google Scholar] [CrossRef]
- Movahed, S.K.; Dabiri, M.; Bazgir, A. Palladium nanoparticle decorated high nitrogen-doped graphene with high catalytic activity for Suzuki-Miyaura and Ullmann-type coupling reactions in aqueous media. Appl. Catal. A. Gen. 2014, 488, 265–274. [Google Scholar] [CrossRef]
- Zheng, K.; Shen, C.; Qiao, J.; Tong, J.; Jin, J.; Zhang, P. Novel magnetically-recyclable, nitrogen-doped Fe3O4@Pd NPs for Suzuki-Miyaura coupling and their application in the synthesis of Crizotinib. Catalysts 2018, 8, 443. [Google Scholar] [CrossRef] [Green Version]
- Hajipour, A.R.; Abolfathi, P. Nickel embedded on triazole-modified magnetic nanoparticles: A novel and sustainable heterogeneous catalyst for Hiyama reaction in fluoride-free condition. Catal. Commun. 2018, 103, 92–95. [Google Scholar] [CrossRef]
- Moghadam, H.H.; Sobhani, S.; Sansano, J.M. New nanomagnetic heterogeneous cobalt catalyst for the synthesis of aryl nitriles and biaryls. ACS Omega 2020, 5, 18619–18627. [Google Scholar] [CrossRef] [PubMed]
- Cattelle, A.D.; Billen, A.; Brullot, W.; Verbiest, T.; Koeckelberghs, G. Synthesis of poly(phenylene ethynylene) using an easily recyclable Pd-functionalized magnetite nanoparticle catalyst. Macromolecules 2020, 53, 1998–2005. [Google Scholar] [CrossRef]
- Yazdani, H.; Pardis, S.; Loni, M.; Bazgir, A. Gold nanoparticles as a Lewis acid catalyst in 1,3-dipolar cycloaddition reaction. Catal. Commun. 2020, 134, 105844. [Google Scholar] [CrossRef]
- Kaur, H.; Kumar, M.; Bhalla, V. Photocatalytic ensemble HP-T@Au-Fe3O4: Synergistic and balanced operation in Kumada and Heck coupling reactions. Green Chem. 2020, 22, 8036–8045. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, M.; Walia, P.K.; Kumar, M.; Bhalla, V. Encapsulating Au-Fe3O4 nanodots into AIE active supramolecular assemblies: Ambient visible light harvesting “Dip-Strip” photocatalyst for C-C/C-N bond formation reactions. Chem. Asian J. 2019, 14, 809–813. [Google Scholar] [CrossRef] [Green Version]
- Kaur, H.; Pramanik, S.; Kumar, M.; Bhalla, V. Polythiophene-encapsulated bimetallic Au-Fe3O4 nano-hybrid materials: A potential tandem photocatalytic system for nondirected C(sp2)-H activation for the synthesis of quinoline carboxylates. ACS Catal. 2017, 7, 2007–2021. [Google Scholar] [CrossRef]
- Azadi, G.; Kazemi, F.; Firouzeh, E. Visible-light-driven photocatalytic Suzuki-Miyaura coupling reaction using novel retrievable magnetic photocatalyst. ChemistrySelect 2021, 6, 630–639. [Google Scholar] [CrossRef]
- Churipard, S.R.; Kanakikodi, K.S.; Maradur, S.P. Metal nanoparticles supported on mesoporous polymers: Realizing the synergetic effect to achieve superior catalytic performance. In Advanced Heterogeneous Catalysts Volume 1: Applications at the Nano-Scale; Chapter 16; American Chemical Society: Washington, DC, USA, 2020; pp. 483–511. Available online: https://pubs.acs.org/doi/abs/10.1021/bk-2020-1359.ch016 (accessed on 10 August 2021).
- Tao, R.; Ma, X.; Wei, X.; Jin, Y.; Qiu, L.; Zhang, W. Porous organic polymer material supported palladium nanoparticles. J. Mater. Chem. A 2020, 8, 17360–17391. [Google Scholar] [CrossRef]
- Hong, K.; Sajjadi, M.; Suh, J.M.; Zhang, K.; Nasrollahzadeh, M.; Jang, H.W.; Varma, R.S.; Shokouhimehr, M. Palladium nanoparticles on assorted nanostructured supports: Applications for Suzuki, Heck, and Sonogashira cross-coupling reactions. ACS Appl. Nano Mater. 2020, 3, 2070–2103. [Google Scholar] [CrossRef]
- Dabiri, M.; Fazli, H.; Salarinejad, N.; Movahed, S.K. Pd nanoparticles supported on cubic shaped ZIF-based materials and their catalytic activities in organic reactions. Mater. Res. Bull. 2021, 133, 111015. [Google Scholar] [CrossRef]
- Cho, H.J.; Kim, D.; Xu, B. Selective control in tandem catalytic furfural upgrading on zeolite-encapsulated Pt nanoparticles through site and solvent engineering. ACS Catal. 2020, 10, 4770–4779. [Google Scholar] [CrossRef]
- Cho, H.J.; Kim, D.; Li, S.; Su, D.; Ma, D.; Xu, B. Molecular-level proximity of metal and acid sites in zeolite-encapsulated Pt nanoparticles for selective multistep tandem catalysis. ACS Catal. 2020, 10, 3340–3348. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Khorsandi, Z. Pd/Cu-free Heck and Sonogashira coupling reactions applying cobalt nanoparticles supported on multifunctional porous organic hybrid. Appl. Organomet. Chem. 2020, 34, e5398. [Google Scholar] [CrossRef]
- Kalay, E.; Cetin, S.; Kolemen, S.; Metin, Ö. A facile synthesis of mesoporous graphitic carbon nitride supported palladium nanoparticles as highly effective and reusable catalysts for the Stille coupling reactions under mild conditions. New J. Chem. 2020, 44, 6714–6723. [Google Scholar] [CrossRef]
- Dastjerdi, F.H.; Ghorbani-Vaghei, R.; Alavinia, S. Copper iodide nanoparticles immobilized porous polysulfonamide: An effective nanocatalyst for synthesis of imidazo[1,2-a]pyridines. Catal. Lett. 2020, 150, 3514–3522. [Google Scholar] [CrossRef]
- Guo, B.; Li, H.-X.; Zha, C.-H.; Young, D.J.; Li, H.-Y.; Lang, J.-P. Visible-light-enhanced Suzuki-Miyaura reactions of aryl chlorides in water with Pd NPs supported on a conjugated nanoporous polycarbazole. ChemSusChem 2019, 12, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Ju, P.; Wu, S.; Su, Q.; Li, X.; Liu, Z.; Li, G.; Wu, Q. Salen-porphyrin-based conjugated microporous polymer supported Pd nanoparticles: Highly efficient heterogeneous catalysts for aqueous C-C coupling reactions. J. Mater. Chem. A 2019, 7, 2660–2666. [Google Scholar] [CrossRef]
- Cho, H.J.; Kim, D.; Li, J.; Su, D.; Xu, B. Zeolite-encapsulated Pt nanoparticles for tandem catalysis. J. Am. Chem. Soc. 2018, 140, 13514–13520. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wu, L.; Han, J.; Xu, X.; He, C.; Wang, P.; Wei, Q.; Yang, W. Facile synthesis of palladium nanoparticles on hierarchical hollow silica spheres and its catalytic properties in Suzuki-reaction. Roy. Soc. Open Sci. 2018, 5, 180545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.; Wang, X.; Li, T.; Shen, R.; Hao, S.-J.; Li, Y.; Wang, Q.; Li, Z.; Gu, Z.-G. Porphyrin-based porous polyimide polymer/Pd nanoparticle composites as efficient catalysts for Suzuki-Miyaura coupling reactions. Polym. Chem. 2018, 9, 1430–1438. [Google Scholar] [CrossRef]
- Mondal, P.; Khatun, R.; Bhanja, P.; Bhaumik, A.; Das, D.; Islam, S.M. Palladium nanoparticles embedded on mesoporous TiO2 material (Pd@MTiO2) as an efficient heterogeneous catalyst for Suzuki-coupling reactions in water medium. J. Colloid Interf. Sci. 2017, 508, 378–386. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, C.; Wu, Q.; Guo, B.; Liu, W.; Li, G.; Su, Q.; Mu, Y. Palladium nanoparticles supported on carbazole functionalized mesoporous organic polymer: Synthesis and their application as efficient catalysts for Suzuki-Miyaura cross coupling reaction. Polym. Chem. 2017, 8, 1488–1494. [Google Scholar] [CrossRef]
- Wang, K.; Yang, L.; Zhao, W.; Cao, L.; Sun, Z.; Zhang, F. A facile synthesis of copper nanoparticles supported on ordered mesoporous polymer as an efficient and stable catalyst for solvent-free Sonogashira coupling reactions. Green Chem. 2017, 19, 1949–1957. [Google Scholar] [CrossRef]
- Puthiaraj, P.; Ahn, W.-S. Ullmann coupling of aryl chlorides in water catalyzed by palladium nanoparticles supported on amine-grafted porous aromatic polymer. Mol. Catal. 2017, 437, 73–79. [Google Scholar] [CrossRef]
- Ke, W.; Cui, T.; Yu, Q.; Wang, M.; Lv, L.; Wang, H.; Jiang, Z.; Li, X. Mesoporous H-ZSM-5 nanocrystals with programmable number of acid sites as “solid ligands” to activate Pd nanoparticles for C-C coupling reactions. Nano Res. 2018, 11, 874–881. [Google Scholar] [CrossRef]
- Lu, S.; Hu, Y.; Wan, S.; McCaffrey, R.; Jin, Y.; Gu, H.; Zhang, W. Synthesis of ultrafine and highly dispersed metal nanoparticles confined in a thioether-containing covalent organic framework and their catalytic applications. J. Am. Chem. Soc. 2017, 139, 17082–17088. [Google Scholar] [CrossRef]
- Akiyama, T.; Wada, Y.; Yamada, M.; Shio, Y.; Honma, T.; Shimoda, S.; Tsuruta, K.; Tamenori, Y.; Haneoka, H.; Suzuki, T.; et al. Self-assembled multilayer iron(0) nanoparticles catalyst for ligand-free carbon-carbon/carbon-nitrogen bond-forming reactions. Org. Lett. 2020, 22, 7244–7249. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Wada, Y.; Jenkinson, K.; Honma, T.; Tsuruta, K.; Tamenori, Y.; Haneoka, H.; Takehara, T.; Suzuki, T.; Murai, K.; et al. Reusable immobilized iron(II) nanoparticle precatalysts for ligand-free Kumada coupling. ACS Appl. Nano Mater. 2018, 1, 6950–6958. [Google Scholar] [CrossRef]
- Akiyama, T.; Taniguchi, T.; Saito, N.; Doi, R.; Honma, T.; Tamenori, Y.; Ohki, Y.; Takahashi, N.; Fujioka, H.; Sato, Y.; et al. Ligand-free Suzuki-Miyaura coupling using ruthenium(0) nanoparticles and a continuously irradiating microwave system. Green Chem. 2017, 19, 3357–3369. [Google Scholar] [CrossRef]
- Hoshiya, N.; Fujiki, K.; Taniguchi, T.; Honma, T.; Tamenori, Y.; Xiao, M.; Saito, N.; Yokoyama, M.; Ishii, A.; Fujioka, H.; et al. Self-assembled multilayer-stabilized nickel nanoparticle catalyst for ligand-free cross-coupling reactions: In situ metal nanoparticle and nanospace simultaneous organization. Adv. Synth. Catal. 2016, 358, 2449–2459. [Google Scholar] [CrossRef]
- Arisawa, M.; Al-Amin, M.; Honma, T.; Tamenori, Y.; Arai, S.; Hoshiya, N.; Sato, T.; Yokoyama, M.; Ishii, A.; Takeguchi, M.; et al. Formation of self-assembled multilayer stable palladium nanoparticles for ligand-free coupling reactions. RSC Adv. 2015, 5, 676–683. [Google Scholar] [CrossRef]
- Norouzi, N.; Das, M.K.; Richard, A.J.; Ibrahim, A.A.; El-Kaderi, H.M.; El-Shall, M.S. Heterogeneous catalysis by ultra-small bimetallic nanoparticles surpassing homogeneous catalysis for caron-carbon bond forming reactions. Nanoscale 2020, 12, 19191–19202. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.; Yun, J.; Ludwig, L.; Jang, S.G.; Bae, J.Y.; Byun, H.; Kim, J.-H. Comparative catalytic properties of supported and encapsulated gold nanoparticles in homocoupling reactions. Front. Chem. 2020, 8, 834. [Google Scholar] [CrossRef] [PubMed]
- Dewan, A.; Sarmah, M.; Bharali, P.; Thakur, A.J.; Boruah, P.K.; Das, M.R.; Bora, U. Pd nanoparticles-loaded honeycomb-structured bio-nanocellulose as a heterogeneous catalyst for heteroaryl cross-coupling reaction. ACS Sustain. Chem. Eng. 2021, 9, 954–966. [Google Scholar] [CrossRef]
- Zhuang, Q.; Gao, R.; Shi, M.; Lin, X.; Xie, A.; Dong, W. Confining palladium nanoparticles in microporous tetrastyrene polymer enables efficient size-selective heterogeneous catalysis. ACS Appl. Nano Mater. 2021, 4, 3869–3876. [Google Scholar] [CrossRef]
- Cho, H.J.; Kim, D.; Xu, B. Pore size engineering enabled selectivity control in tandem catalytic upgrading of cyclopentanone on zeolite-encapsulated Pt nanoparticles. ACS Catal. 2020, 10, 8850–8859. [Google Scholar] [CrossRef]
- Sun, P.; Lin, S.; Guo, H.; Su, J.; Shi, L. A highly dispersed copper nanoparticles catalyst with a large number of weak acid centers for efficiently synthesizing the high value-added 3-methylindole by aniline and biomass-derived glycerin. Catal. Lett. 2021, 151, 463–477. [Google Scholar] [CrossRef]
- Díaz-Sánchez, M.; Gómez, J.; Prashar, S.; Horáček, M.; Lamač, M.; Urbán, B.; Pinkas, J.; Gómez-Ruiz, S. Multifunctional catalysts based on palladium nanoparticles supported on functionalized halloysites: Application in catalytic C-C coupling, selective oxidation and dehalogenation reactions. Appl. Clay Sci. 2021, 214, 106272. [Google Scholar] [CrossRef]
- Luo, S.; Zeng, Z.; Zeng, G.; Liu, Z.; Xiao, R.; Chen, M.; Tang, L.; Tang, W.; Lai, C.; Cheng, M.; et al. Metal organic frameworks as robust host of palladium nanoparticles in heterogeneous catalysis: Synthesis, application, and prospect. ACS Appl. Mater. Interfaces 2019, 11, 32579–32598. [Google Scholar] [CrossRef]
- Xiang, W.; Zhang, Y.; Lin, H.; Liu, C.-J. Nanoparticle/metal-organic framework composites for catalytic applications: Current status and perspective. Molecules 2017, 22, 2103. [Google Scholar] [CrossRef] [Green Version]
- Ferlin, F.; Sciosci, D.; Valentini, F.; Menzio, J.; Cravotto, G.; Martina, K.; Vaccaro, L. si-Gly-CD-PdNPs as a hybrid heterogeneous catalyst for environmentally friendly continuous flow Sonogashira cross-coupling. Green Chem. 2021, 23, 7210–7218. [Google Scholar] [CrossRef]
- Gorji, S.; Ghorbani-Vaghei, R. Ag nanoparticles stabilized on basalt fibers as a novel, stable, and reusable catalyst for Suzuki-Miyaura coupling reactions. Appl. Organomet. Chem. 2021, 35, e6018. [Google Scholar] [CrossRef]
- Alamgholiloo, H.; Pesyan, N.N.; Rostamnia, S. A novel strategy for stabilization of sub-nanometric Pd colloids on kryptofix functionalized MCM-41: Nanoengineered material for Stille coupling transformation. Sci. Rep. 2021, 11, 18417. [Google Scholar] [CrossRef]
- Li, J.; Song, Y.; Wang, Y.; Zhang, H. Ultrafine PdCu nanoclusters by ultrasonic-assisted reduction on the LDHs/rGO hybrid with significantly enhanced Heck reactivity. ACS Appl. Mater. Interfaces 2020, 12, 50365–50376. [Google Scholar] [CrossRef]
- Anastasiou, I.; Van Velthoven, N.; Tomarelli, E.; Lombi, A.; Lanari, D.; Liu, P.; Bals, S.; De Vos, D.E.; Vaccaro, L. C2-H Arylation of Indoles Catalyzed by Palladium-Containing Metal-Organic-Framework in γ-Valerolactone. ChemSusChem 2020, 13, 2786–2791. [Google Scholar] [CrossRef]
- Rohani, S.; Ziarani, G.M.; Ziarati, A.; Badiei, A. Designer 3D CoAl-layered double hydroxide@N,S doped graphene hollow architecture decorated with Pd nanoparticles for Sonogashira couplings. Appl. Surf. Sci. 2019, 496, 143599. [Google Scholar] [CrossRef]
- Tamoradi, T.; Ghadermazi, M.; Ghorbani-Choghamarani, A. SBA-15@adenine-Pd: A novel and green heterogeneous nanocatalyst in Suzuki and Stille reactions and synthesis of sulfides. J. Porous Mater. 2019, 26, 121–131. [Google Scholar] [CrossRef]
- Elazab, H.A.; El-Idreesy, T. T Polyvinylpyrrolidone-reduced graphene oxide–Pd nanoparticles as an efficient nanocomposite for catalysis applications in cross-coupling reactions. Bull. Chem. React. Eng. Catal. 2019, 14, 490–501. [Google Scholar] [CrossRef] [Green Version]
- Hajipour, A.R.; Khorsandi, Z.; Abeshtian, Z. Pd/Cu-free Heck and Sonogashira reactions using cobalt immobilized on in situ magnetic cross-linked chitosan fibers: A highly efficient and reusable catalyst. Inorg. Chem. Commun. 2019, 107, 107470. [Google Scholar] [CrossRef]
- Hemmati, S.; Mehrazin, L.; Pirhayati, M.; Veisi, H. Immobilization of palladium nanoparticles on Metformin-functionalized graphene oxides as a heterogeneous and recyclable nanocatalyst for Suzuki coupling reactions and reduction of 4-nitrophenol. Polyhedron 2019, 158, 414–422. [Google Scholar] [CrossRef]
- Veisi, H.; Nikseresht, A.; Ahmadi, N.; Khosravi, K.; Saeidifar, F. Suzuki-Miyaura reaction by heterogeneously supported Pd nanoparticles on thio-modified multi walled carbon nanotubes as efficient nanocatalyst. Polyhedron 2019, 162, 240–244. [Google Scholar] [CrossRef]
- Veisi, H.; Kamangar, S.A.; Mohammadi, P.; Hemmati, S. Palladium nanoparticles-decorated triethanolammonium chloride ionic liquid-modified TiO2 nanoparticles (TiO2/IL-Pd): A highly active and recoverable catalyst for Suzuki-Miyaura cross-coupling reaction in aqueous medium. Appl. Organomet. Chem. 2019, 33, e4909. [Google Scholar] [CrossRef]
- Li, X.; Zhang, B.; Van Zeeland, R.; Tang, L.; Pei, Y.; Qi, Z.; Goh, T.W.; Stanley, L.M.; Huang, W. Unveiling the effects of linker substitution in Suzuki coupling with palladium nanoparticles in metal-organic frameworks. Catal. Lett. 2018, 148, 940–945. [Google Scholar] [CrossRef] [Green Version]
- Dabiri, M.; Alavioon, S.I.; Movahed, S.K. Palladium supported on mesoporous silica/graphene nanohybrid as a highly efficient and reusable heterogeneous catalyst for C-H functionalization. ChemistrySelect 2018, 3, 3487–3494. [Google Scholar] [CrossRef]
- Amini, M.M.; Mohammadkhani, A.; Bazgir, A. Dicarboxylic acid-functionalized MCM-41 with embedded palladium nanoparticles as an efficient heterogeneous catalyst for C-C coupling reactions. ChemistrySelect 2018, 3, 1439–1444. [Google Scholar] [CrossRef]
- Azaroon, M.; Kiasat, A.R. An efficient and new protocol for the Heck reaction using palladium nanoparticle-engineered dibenzo-18-crown-6-ether/MCM-41 nanocomposite in water. Appl. Organomet. Chem. 2018, 32, e4271. [Google Scholar] [CrossRef]
- Ghasemi, S.; Karim, S. Mizoroki-Heck cross-coupling reaction of haloarenes mediated by a well-controlled modified polyacrylamide brush grafted silica/Pd nanoparticle system. Bull. Chem. Soc. Jpn. 2017, 90, 485–490. [Google Scholar] [CrossRef]
- Veisi, H.; Azadbakht, R.; Saeidifar, F.; Abdi, M.R. Schiff base-functionalized multi walled carbon nano tubes to immobilization of palladium nanoparticles as heterogeneous and recyclable nanocatalyst for Suzuki reaction in aqueous media under mild conditions. Catal. Lett. 2017, 147, 976–986. [Google Scholar] [CrossRef]
- Veisi, H.; Mirzaee, N. Ligand-free Mizoroki-Heck reaction using reusable modified graphene oxide-supported Pd(0) nanoparticles. Appl. Organomet. Chem. 2017, 31, e4067. [Google Scholar]
- Nikoorazm, M.; Ghorbani-Choghamarani, A.; Ghobadi, M.; Massahi, S. Pd-SBT@MCM-41: As an efficient, stable and recyclable organometallic catalyst for C-C coupling reactions and synthesis of 5-substituted tetrazoles. Appl. Organomet. Chem. 2017, 31, e3848. [Google Scholar] [CrossRef]
- de Borros, S.D.; Duarte, J.P.P.; Rocha, L.D.S.; Ramos, V.S.; Navarro, M.I.R.; Archanjo, B.S.; Medeiros, M.E.; De Campos, J.B.; De Costa, M.E.H.M.; Lachter, E.R.; et al. Cyclodextrin-stabilized palladium nanoparticles on ceria: Investigation of support interactions and pivotal promotion in the Suzuki-Miyaura reaction. ChemistrySelect 2020, 5, 7227–7235. [Google Scholar] [CrossRef]
- Doustkhah, E.; Rostamnia, S.; Imura, M.; Ide, Y.; Mohammadi, S.; Hyland, C.J.T.; You, J.; Tsunoji, N.; Zeynizadeh, B.; Yamauchi, Y. Thiourea bridged periodic mesoporous organosilica with ultra-small Pd nanoparticles for coupling reactions. RSC Adv. 2017, 7, 56306–56310. [Google Scholar] [CrossRef] [Green Version]
- Mohan, A.; Rout, L.; Thomas, A.M.; Peter, J.; Nagappan, S.; Parambadath, S.; Ha, C.-S. Palladium nanoparticles-anchored dual-responsive SBA-15-PNIPAM/PMAA nanoreactor: A novel heterogeneous catalyst for a green Suzuki-Miyaura cross-coupling reaction. RSC Adv. 2020, 10, 28193–28204. [Google Scholar] [CrossRef]
- Ghorbani-Vaghei, R.; Sarmast, N.; Rahmatpour, F. Immobilization of palladium nanoparticles as a recyclable heterogeneous catalyst for the Suzuki-Miyaura coupling reaction. Comptes Rendus Chim. 2018, 21, 644–651. [Google Scholar] [CrossRef]
- Patel, A.; Patel, A. Stabilized palladium nanoparticles: Synthesis, multi-spectroscopic characterization and application for Suzuki-Miyaura reaction. Catal. Lett. 2018, 148, 3534–3547. [Google Scholar] [CrossRef]
- Veisi, H.; Mirzaei, A.; Mohammadi, P. Palladium nanoparticles decorated into a biguanidine modified-KIT-5 mesoporous structure: A recoverable nanocatalyst for ultrasound-assisted Suzuki-Miyaura cross-coupling. RSC Adv. 2019, 9, 41581–51890. [Google Scholar] [CrossRef] [Green Version]
- Kozell, V.; Giannoni, T.; Nocchetti, M.; Vivani, R.; Piermatti, O.; Vaccaro, L. Immobilized palladium nanoparticles on zirconium carboxy-aminophosphonates nanosheets as an efficient recoverable heterogeneous catalyst for Suzuki-Miyaura and Heck coupling. Catalysts 2017, 7, 186. [Google Scholar] [CrossRef] [Green Version]
- Massaro, M.; Colletti, C.G.; Buscemi, G.; Cataldo, S.; Guernelli, S.; Lazzara, G.; Liotta, L.F.; Parisi, F.; Pettignano, A.; Riela, S. Palladium nanoparticles immobilized on halloysite nanotubes covered by multilayer network for catalytic applications. New J. Chem. 2018, 42, 13938–13947. [Google Scholar] [CrossRef]
- Ghasemi, S.; Karim, S. Organic/inorganic hybrid composed of modified polyacrylamide grafted silica supported Pd nanoparticles using RAFT polymerization process: Controlled synthesis, characterization and catalytic activity. Mater. Chem. Phys. 2018, 205, 347–358. [Google Scholar] [CrossRef]
- Wang, W.; Chen, S.; Cal, E.G.; Moro, M.M.; Moya, S.; Coy, E.; Wang, C.; Hamon, J.-R.; Astruc, D. ZIF-8-based vs. ZIF-8-derived Au and Pd nanoparticles as efficient catalysts for the Ullmann homocoupling reaction. Inorg. Chem. Front. 2020, 7, 3945–3952. [Google Scholar] [CrossRef]
- Yasukawa, T.; Zhu, Z.; Yamashita, Y.; Kobayashi, S. Carbonylative Suzuki-Miyaura coupling reactions of aryl iodides with readily available polymer-immobilized palladium nanoparticles. Synlett 2021, 32, 502–504. [Google Scholar] [CrossRef]
- Miyamura, H.; Yasukawa, T.; Zhu, Z.; Kobayashi, S. Asymmetric 1,4-addition of arylboronic acids to β,γ-unsaturated α-ketoesters using heterogeneous chiral metal nanoparticle systems. Adv. Synth. Catal. 2020, 362, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Sadeghzadeh, S.M.; Zhiani, R.; Emrani, S. Pd/APTPOSS@KCC-1 as a new and efficient support catalyst for C-H activation. RSC Adv. 2017, 7, 24885–24894. [Google Scholar] [CrossRef] [Green Version]
- Şen, B.; Akdere, E.H.; Şavk, A.; Gültekin, E.; Parali, Ö.; Göksu, H.; Şen, F. A novel thiocarbamide functionalized graphene oxide supported bimetallic monodisperse Rh-Pd nanoparticles (RhPd/TC@GO NPs) for Knoevenagel condensation of aryl aldehydes together with malononitrile. Appl. Catal. B Environ. 2018, 225, 148–153. [Google Scholar] [CrossRef]
- Subudhi, S.; Mansingh, S.; Tripathy, S.P.; Mohanty, A.; Mohapatra, P.; Rath, D.; Parida, K. The fabrication of Au/Pd plasmonic alloys on UiO-66-NH2: An efficient visible light-induced photocatalyst towards the Suzuki Miyaura coupling reaction under ambient conditions. Catal. Sci. Technol. 2019, 9, 6585–6597. [Google Scholar] [CrossRef]
- Sahoo, M.; Mansingh, S.; Subudhi, S.; Mohapatra, P.; Parida, K. A plasmonic AuPd bimetallic nanoalloy decorated over a GO/LDH hybrid nanocomposite via a green synthesis route for robust Suzuki coupling reactions: A paradigm shift towards a sustainable future. Catal. Sci. Technol. 2019, 9, 4678–4692. [Google Scholar] [CrossRef]
- Luo, Y.; Peng, Y.; Liu, W.; Chen, F.; Wang, B. Soluble inorganic nanoparticle constructed porous coordination frameworks as homogenized heterogeneous photocatalysts for Suzuki coupling reaction under near-infrared light. Chem. Eur. J. 2017, 23, 8879–8885. [Google Scholar] [CrossRef]
- Hoseinim, C.; Seyede, M.; Adadi, S.; Heravi, M.M. Application of bimetallic and trimetallic nanoparticles supported on graphene as novel heterogeneous catalysts in the reduction of nitroarenes, homo-coupling, Suzuki-Miyaura and Sonogashira reactions. Curr. Org. Chem. 2020, 24, 2216–2234. [Google Scholar] [CrossRef]
- Zhao, M.; Wu, Y.; Cao, J.-P. Carbon-based material-supported palladium nanocatalysis in coupling reactions: Discussion on their stability and heterogeneity. Appl. Organomet. Chem. 2020, 34, e5539. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Issaabadi, Z.; Tohidi, M.M.; Sajadi, S.M. Recent progress in application of graphene supported metal nanoparticles in C-C and C-X coupling reactions. Chem. Rec. 2018, 18, 165–229. [Google Scholar] [CrossRef]
- Labulo, A.H.; Martincigh, B.S.; Omondi, B.; Nyamori, V.O. Advances in carbon nanotubes as efficacious supports for palladium-catalyzed carbon-carbon cross-coupling reactions. J. Mater. Sci. 2017, 52, 9225–9248. [Google Scholar] [CrossRef]
- Anastasiou, I.; Ferlin, F.; Viteritti, O.; Santoro, S.; Vaccaro, L. Pd/C-catalyzed aerobic oxidative C-H alkenylation of arenes in γ-valerolactone (GVL). Mol. Catal. 2021, 513, 111787. [Google Scholar] [CrossRef]
- Campana, F.; Massaccesi, B.M.; Santoro, S.; Piermatti, O.; Vaccaro, L. Polarclean/water as a safe and recoverable medium for selective C2-arylation of indoles catalyzed by Pd/C. ACS Sustain. Chem. Eng. 2020, 8, 16441–16450. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Boruah, P.K.; Das, M.R.; Bora, U. Direct C-H bond activation: Palladium-on-carbon as a reusable heterogeneous catalyst for C-2 arylation of indoles with arylboronic acid. New J. Chem. 2020, 44, 7675–7682. [Google Scholar] [CrossRef]
- Shabestari, M.E.; Martín, O.; Díaz-García, D.; Gómez-Ruiz, S.; Gonzalez, V.J.; Baselga, J. Facile and rapid decoration of graphene oxide with copper double salt, oxides and metallic copper as catalysts in oxidation and coupling reactions. Carbon 2020, 161, 7–16. [Google Scholar] [CrossRef]
- Sultana, S.; Mech, S.D.; Hussain, F.L.; Pahari, P.; Borah, G.; Gogoi, P.K. Green synthesis of graphene oxide (GO)-anchored Pd/Cu bimetallic nanoparticles using Ocimum sanctum as bio-reductant: An efficient heterogeneous catalyst for the Sonogashira cross-coupling reaction. RSC Adv. 2020, 10, 23108–23120. [Google Scholar] [CrossRef]
- Pang, Q.; Fan, X. Facile synthesis for anchoring highly efficient superfine Pd nanoparticles on carbon: Boosting catalytic C-C coupling. ChemistrySelect 2020, 5, 7959–7966. [Google Scholar] [CrossRef]
- Saptal, V.B.; Saptal, M.V.; Mane, R.S.; Sasaki, T.; Bhanage, B.M. Amine-functionalized graphene oxide-stabilized Pd nanoparticles (Pd@APGO): A novel and efficient catalyst for the Suzuki and carbonylative Suzuki-Miyaura coupling reactions. ACS Omega 2019, 4, 643–649. [Google Scholar] [CrossRef]
- Golestanzadeh, M.; Naeimi, H. Palladium decorated on a new dendric complex with nitrogen ligation grafted to graphene oxide: Fabrication, characterization, and catalytic application. RSC Adv. 2019, 9, 27560–27573. [Google Scholar] [CrossRef] [Green Version]
- Kumari, S.; Mittal, A.; Kumar, A.; Sharma, S.K.; Parmanand; Krishna. Palladium nanoparticles immobilized on Schiff base-functionalized graphene-oxide: Application in carbon-carbon cross-coupling reactions. ChemistrySelect 2019, 4, 10828–10837. Available online: https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/slct.201902242 (accessed on 10 September 2021).
- Mohazzab, B.F.; Jaleh, B.; Issaabadi, Z.; Nasrollahzadeh, M.; Varma, R.S. Stainless steel mesh-GO/Pd NPs: Catalytic applications of Suzuki-Miyaura and Stille coupling reactions in eco-friendly media. Green Chem. 2019, 21, 3319–3327. [Google Scholar] [CrossRef]
- Lu, W.; Sun, W.; Tan, X.; Gao, L.; Zheng, G. Stabilized Cu/Cu2O nanoparticles on rGO as an efficient heterogeneous catalyst for Glaser homo-coupling. Catal. Commun. 2019, 125, 98–102. [Google Scholar] [CrossRef]
- Beigbaghlou, S.S.; Kalbasi, R.J.; Habibi, A. Ni-Pd nanoparticles embedded in N-doped hierarchical porous carbon derived from Luffa sponge: Preparation, characterization and its catalytic activity. ChemistrySelect 2019, 4, 12875–12885. [Google Scholar] [CrossRef]
- Trzeciak, A.M.; Wojcik, P.; Lisiecki, R.; Gerasymchuk, Y.; Strek, W.; Legendziewicz, J. Palladium nanoparticles supported on graphene oxide as catalysts for the synthesis of diarylketones. Catalysts 2019, 9, 319. [Google Scholar] [CrossRef] [Green Version]
- Veisi, H.; Tamoradi, T.; Karmakar, B.; Mohammadi, P.; Hemmati, S. In situ biogenic synthesis of Pd nanoparticles over reduced graphene oxide by using a plant extract (Thymbra spicata) and its catalytic evaluation towards cyanation of aryl halides. Mater. Sci. Eng. C 2019, 104, 109919. [Google Scholar] [CrossRef]
- Shi, X.; Cai, C. Imidazolium-based ionic liquid functionalized reduced graphene oxide supported palladium as a reusable catalyst for Suzuki-Miyaura reactions. New J. Chem. 2018, 42, 2364–2367. [Google Scholar] [CrossRef]
- Poly, S.S.; Hakim Siddiki, S.M.A.; Touchy, A.S.; Ting, K.W.; Toyao, T.; Maeno, Z.; Kanda, Y.; Shimizu, K. Acceptorless dehydrogenative synthesis of pyrimidines from alcohols and amidines catalyzed by supported platinum nanoparticles. ACS Catal. 2018, 8, 11330–11341. [Google Scholar] [CrossRef]
- Elazab, H.A.; Moussa, S.; Siamaki, A.R.; Gupton, B.F.; El-Shall, M.S. The effect of graphene on catalytic performance of palladium nanoparticles decorated with Fe3O4, Co3O4, and Ni(OH)2: Potential efficient catalysts used for Suzuki cross-coupling. Catal. Lett. 2017, 147, 1510–1522. [Google Scholar] [CrossRef]
- Sarvestani, M.; Azadi, R. Palladium nanoparticles deposited on a graphene-benzimidazole support as an efficient and recyclable catalyst for aqueous-phase Suzuki-Miyaura coupling reaction. Appl. Organomet. Chem. 2017, 31, e3667. [Google Scholar] [CrossRef]
- Hoseini, S.J.; Agahi, B.H.; Fard, Z.S.; Fath, R.H.; Bahrami, M. Modification of palladium-copper thin film by reduced graphene oxide or platinum as catalyst for Suzuki-Miyaura reactions. Appl. Organomet. Chem. 2017, 31, e3607. [Google Scholar] [CrossRef]
- Kwon, T.H.; Cho, K.Y.; Baek, J.-Y.; Yoon, H.G.; Kim, B.M. Recyclable palladium-graphene nanocomposite catalysts containing ionic polymers: Efficient Suzuki coupling reactions. RSC Adv. 2017, 7, 11684–11690. [Google Scholar] [CrossRef] [Green Version]
- Lamei, K.; Eshghi, H.; Bakavoli, M.; Rounaghi, S.A.; Esmaeili, E. Carbon coated copper nanostructures as a green and ligand free nanocatalyst for Suzuki cross-coupling reaction. Catal. Commun. 2017, 92, 40–45. [Google Scholar] [CrossRef]
- Erami, R.S.; Díaz-García, D.; Prashar, S.; Rodríguez-Diéguez, A.; Fajardo, M.; Amirnasr, M.; Gómez-Ruiz, S. Suzuki-Miyaura C-C coupling reactions catalyzed by supported Pd nanoparticles for the preparation of fluorinated biphenyl derivatives. Catalysts 2017, 7, 76. [Google Scholar] [CrossRef] [Green Version]
- Mahanta, A.; Hussain, N.; Das, M.R.; Thakur, A.J.; Bora, U. Palladium nanoparticles decorated on reduced graphene oxide: An efficient catalyst for ligand- and copper-free Sonogashira reaction at room temperature. Appl. Organomet. Chem. 2017, 31, e3679. [Google Scholar] [CrossRef]
- Nan, L.; Yalan, C.; Jixiang, L.; Dujuan, O.; Wenhui, D.; Rouhi, J.; Mustapha, M. Carbonylative Suzuki-Miyaura cross-coupling by immobilized Ni@Pd NPs supported on carbon nanotubes. RSC Adv. 2020, 10, 27923–27931. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Khorsandi, Z.; Farrokhpour, H. In situ synthesis of carbon nanotube-encapsulated cobalt nanoparticles by a novel and simple chemical treatment process: Efficient and green catalysts for the Heck reaction. New J. Chem. 2019, 43, 8215–8219. [Google Scholar] [CrossRef]
- Gopiraman, M.; Saravanamoorthy, S.; Ullah, S.; Ilangovan, A.; Kim, I.S.; Chung, I.M. Reducing-agent-free facile preparation of Rh-nanoparticles uniformly anchored on onion-like fullerene for catalytic applications. RSC Adv. 2020, 10, 2545–2559. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.-Y.; Su, H.; Zhai, G.-Y.; Zhang, S.-N.; Sun, L.-H.; Chen, J.-S.; Li, X.-H. Designed electron-deficient gold nanoparticles for a room-temperature Csp3-Csp3 coupling reaction. Chem. Commun. 2021, 57, 741–744. [Google Scholar] [CrossRef]
- Wang, C.; Salmon, L.; Ciganda, R.; Yate, L.; Moya, S.; Ruiz, J.; Astruc, D. An efficient parts-per-million α-Fi2O3 nanocluster/graphene oxide catalyst for Suzuki-Miyaura coupling reactions and 4-nitrophenol reduction in aqueous solution. Chem. Commun. 2017, 53, 644–646. [Google Scholar] [CrossRef]
- Tran, T.P.N.; Thakur, A.; Trinh, D.X.; Dao, A.T.N.; Taniike, T. Design of Pd@graphene oxide framework nanocatalyst with improved activity and recyclability in Suzuki-Miyaura cross-coupling reaction. Appl. Catal. A Gem. 2018, 549, 60–67. [Google Scholar] [CrossRef]
- Zhang, Q.; Mao, Z.; Wang, K.; Phan, N.T.S.; Zhang, F. Microwave-assisted aqueous carbon-carbon cross-coupling reactions of aryl chlorides catalyzed by reduced graphene oxide supported palladium nanoparticles. Green Chem. 2020, 22, 3239–3247. [Google Scholar] [CrossRef]
- Fatahi, P.; Hoseini, S.J. Formation of PdNiZn thin film at oil-water interface: XPS study and application as Suzuki-Miyaura catalyst. Appl. Organomet. Chem. 2018, 32, e4187. [Google Scholar] [CrossRef]
- Murugan, K.; Nainamalai, D.; Kanagaraj, P.; Nagappan, S.G.; Palaniswamy, S. Green-synthesized nickel nanoparticles on reduced graphene oxide as an active and selective catalyst for Suzuki and Glaser-Hay coupling reactions. Appl. Organomet. Chem. 2020, e5778. [Google Scholar] [CrossRef]
- Blanco, M.; Mosconi, D.; Tubaro, C.; Biffis, A.; Badocco, D.; Pastore, P.; Otyepka, M.; Bakandritsos, A.; Liu, Z.; Ren, W.; et al. Palladium nanoparticles supported on graphene acid: A stable and eco-friendly bifunctional C-C homo- and cross-coupling catalyst. Green Chem. 2019, 21, 5238–5247. [Google Scholar] [CrossRef]
- Dabiri, M.; Kashi, S.R.B.; Lehi, N.F.; Bashiribod, S. Synthesis of gold nanoparticles decorated on sulfonated three-dimensional graphene nanocomposite and application as a highly efficient and recyclable heterogeneous catalyst for Ullmann homocoupling of aryl iodides and reduction of p-nitrophenol. Appl. Organomet. Chem. 2018, 32, e4189. [Google Scholar] [CrossRef]
- Dabiri, M.; Vajargahy, M.P. PdCo bimetallic nanoparticles supported on three-dimensional graphene as a highly active catalyst for Sonogashira cross-coupling reaction. Appl. Organomet. Chem. 2017, 31, e3594. [Google Scholar] [CrossRef]
- Siddiki, S.M.A.H.; Touchy, A.S.; Jamil, M.A.R.; Toyao, T.; Shimizu, K. C-methylation of alcohols, ketones, and indoles with methanol using heterogeneous platinum catalysts. ACS Catal. 2018, 8, 3091–3103. [Google Scholar] [CrossRef]
- Kramer, S.; Hejjo, F.; Rasmussen, K.H.; Kegnæs, S. Silylative pinacol coupling catalyzed by nitrogen-doped carbon-encapsulated nickel/cobalt nanoparticles: Evidence for a silyl radical pathway. ACS Catal. 2018, 8, 754–759. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Ma, L.; Wang, J.; Zhang, Y.; Jiang, R. Graphitic carbon nitride (g-C3N4) supported Pd species: An efficient heterogeneous photocatalyst surpassing homogeneous thermal heating systems for Suzuki coupling. ChemPlusChem 2019, 84, 1164–1168. [Google Scholar] [CrossRef]
- Movahed, S.K.; Miraghaee, S.; Dabiri, M. AuPd alloy nanoparticles decorated graphitic carbon nitride as an excellent photocatalyst for the visible-light-enhanced Suzuki-Miyaura cross-coupling reaction. J. Alloy. Compd. 2020, 819, 152994. [Google Scholar] [CrossRef]
- Kang, E.; Shin, H.H.; Lim, D.-K. Interface-controlled Pd nanodot-Au nanoparticle colloids for efficient visible-light-induced photocatalytic Suzuki-Miyaura coupling reaction. Catalysts 2018, 8, 463. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Hong, X.M.; Collard, D.M.; El-Sayed, M.A. Suzuki cross-coupling reactions catalyzed by palladium nanoparticles in aqueous solution. Org. Lett. 2000, 2, 2385–2388. [Google Scholar] [CrossRef]
- Zhao, M.; Sun, L.; Crooks, R.M. Preparation of Cu nanoclusters within dendrimer templates. J. Am. Chem. Soc. 1998, 120, 4877–4878. [Google Scholar] [CrossRef]
- Balogh, L.; Tomalia, D.A. Poly(amidoamine) dendrimer-templated nanocompsites. 1. Synthesis of zerovalent copper nanoclusters. J. Am. Chem. Soc. 1998, 120, 7355–7356. [Google Scholar] [CrossRef]
- Esumi, K.; Suzuki, A.; Aihara, N.; Usui, K.; Torigoe, K. Preparation of gold colloids with UV irradiation using dendrimers as stabilizer. Langmuir 1998, 14, 3157–3159. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Favier, I.; Pia, D.; Gómez, M. Palladium nanoparticles in polyols: Synthesis, catalytic couplings, and hydrogenations. Chem. Rev. 2020, 120, 1146–1183. [Google Scholar] [CrossRef]
- Wolfson, A.; Levy-Ontman, O. Development and application of palladium nanoparticles on renewable polysaccharides as catalysts for the Suzuki cross-coupling of halobenzenes and phenylboronic acids. Mol. Catal. 2020, 493, 111048. [Google Scholar] [CrossRef]
- Yasukawa, T.; Miyamura, H.; Kobayashi, S. Chiral rhodium nanoparticle-catalyzed asymmetric arylation reactions. Acc. Chem. Res. 2020, 53, 2950–2963. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Ghorbannezhad, F.; Issaabadi, Z.; Sajadi, S.M. Recent developments in the biosynthesis of Cu-based recyclable nanocatalysts using plant extracts and their application in the chemical reactions. Chem. Rec. 2019, 19, 601–643. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Yek, S.M.-G.; Motahharifar, N.; Gorab, M.G. Recent development in the plant-mediated green synthesis of Ag-based nanoparticles for environmental and catalytic applications. Chem. Rec. 2019, 19, 2436–2479. [Google Scholar] [CrossRef]
- Ferlin, F.; Valentini, F.; Sciosci, D.; Calamante, M.; Petricci, E.; Vaccaro, L. Biomass waste-derived Pd-PiNe catalyst for the continuous-flow copper-free Sonogashira reaction in a CPME-water azeotropic mixture. ACS Sustain. Chem. Eng. 2021, 9, 12196–12204. [Google Scholar] [CrossRef]
- Valentini, F.; Ferlin, F.; Lilli, S.; Marrocchi, A.; Ping, L.; Gu, Y.; Vaccaro, L. Valorisation of urban waste to access low-cost heterogeneous palladium catalysts for cross-coupling reactions in biomass-derived γ-valerolactone. Green Chem. 2021, 23, 5887–5895. [Google Scholar] [CrossRef]
- Valentini, F.; Ferlin, F.; Tomarelli, E.; Mahmoudi, H.; Bagherzadeh, M.; Calamante, M.; Vaccaro, L. A waste-minimized approach to Cassar-Heck reaction based on POLITAG-Pd0 heterogeneous catalyst and recoverable acetonitrile azeotrope. ChemSusChem 2021, 14, 3359–3366. [Google Scholar] [CrossRef]
- Dos Santos, R.V.; Vitoi, V.H.M.; Casta, M.V.; da Silva, L.C.L.L.F.; Archanjo, B.S.; Achete, C.A.; Silva, R.S.F.; Aguiar, L.C.S.; Malta, L.F.B.; Senra, J.D. Thermoresponsive starch hydrogel stabilized Pd nanoparticles: Soft catalyst for the preparation of (±)-α-methylbiphenylalanine in water aiming at bioorthogonal chemistries. Catal. Lett. 2021, 151, 844–852. [Google Scholar] [CrossRef]
- Úbeda, M.Á.; Amorós, P.; Sánhez-Royo, J.F.; Haskouri, J.E.; Marcos, M.D.; Pérez-Pla, F. Precatalyst or dosing-device? The [Pd2{μ-(C6H4)PPh2}2{μ-O2C(C6H5)}2] complex anchored on a carboxypolystyrene polymer as an effective supplier of palladium catalytically active nanoparticles for the Suzuki-Miyaura reaction. J. Catal. 2020, 381, 26–37. [Google Scholar] [CrossRef]
- Patel, S.B.; Vasava, D.V. The study on encapsulation of copper nanoparticles in modified poly-styrene resin matrix and its catalytic evaluation in microwave-assisted Sonogashira coupling. ChemistrySelect 2020, 5, 7040–7048. [Google Scholar] [CrossRef]
- Tirumala, R.T.A.; Dadgar, A.P.; Mohammadparast, F.; Ramakrishnan, S.B.; Mou, T.; Wang, B.; Andiappan, M. Homogeneous versus heterogeneous catalysis in Cu2O-nanoparticle-catalyzed C-C coupling reactions. Green Chem. 2019, 21, 5284–5290. [Google Scholar] [CrossRef]
- Chopra, R.; Kumar, M.; Neelam Bhalla, V. Visible light promoted PANI@Au:CuO catalyzed sequential amination, azidation and annulation for the preparation of 2-arylbenzimidazoles. Green Chem. 2019, 21, 3666–3674. [Google Scholar] [CrossRef]
- Ferlin, F.; Yetra, S.R.; Warratz, S.; Vaccaro, L.; Ackermann, L. Reusable Pd@PEG catalyst for aerobic dehydrogenative C-H/C-H arylations of 1,2,3-triazoles. Chem. Eur. J. 2019, 25, 11427–11431. [Google Scholar] [CrossRef]
- Valentini, F.; Mahmoudi, H.; Bivona, L.A.; Piermatti, O.; Bagherzadeh, M.; Fusaro, L.; Aprile, C.; Marrocchi, A.; Vaccaro, L. Polymer-supported bis-1,2,4-triazolium ionic tag framework for an efficient Pd(0) catalytic system in biomass derived γ-valerolactone. ACS Sustain. Chem. Eng. 2019, 7, 6939–6946. [Google Scholar] [CrossRef]
- Zhang, W.; Yao, Z.-J.; Deng, W. Palladium nanoparticles supported on β-cyclodextrin functionalized poly(amidoamine)s and their application in Suzuki-Miyaura cross-coupling reactions. J. Braz. Chem. Soc. 2019, 30, 1667–1677. [Google Scholar] [CrossRef]
- Sarmah, M.; Neog, A.B.; Boruah, P.K.; Das, M.R.; Bharali, P.; Bora, U. Effect of substrates on catalytic activity of biogenic palladium nanoparticles in C-C cross-coupling reactions. ACS Omega 2019, 4, 3329–3340. [Google Scholar] [CrossRef] [Green Version]
- Ghasemi, S.; Harandi, Z.A. PdNPs@thermo-responsive block copolymers composed of PNIPAM and poly(ionic liquid) via RAFT polymerization. Polym. Bull. 2019, 76, 2819–2834. [Google Scholar] [CrossRef]
- Veisi, H.; Dadres, N.; Mohammadi, P.; Hemmati, S. Green synthesis of silver nanoparticles based on oil-water interface method with essential oil of orange peel and its application as nanocatalyst for A3 coupling. Matel. Sci. Eng. C 2019, 105, 110031. [Google Scholar] [CrossRef] [PubMed]
- Viesi, H. Efficient cyanation of aryl halides with K4[Fe(CN)6] catalyzed by encapsulated palladium nanoparticles in biguanidine-chitosan matrix as core-shell recyclable heterogeneous nanocatalyst. Polyhedron 2019, 159, 212–216. [Google Scholar] [CrossRef]
- Singh, G.; Kumar, M.; Bhalla, V. Ultrafine hybrid Cu2O-Fe2O3 nanoparticles stabilized by hexaphenylbenzene-based supramolecular assembles: A photocatalytic system for the Ullmann-Goldberg coupling reaction. Green Chem. 2018, 20, 5346–5357. [Google Scholar] [CrossRef]
- Kaur, N.; Kaur, G.; Bhalla, A.; Dhau, J.S.; Chaudhary, G.R. Metallosurfactant based Pd-Ni alloy nanoparticles as a proficient catalyst in the Mizoroki Heck coupling reaction. Green Chem. 2018, 20, 1506–1514. [Google Scholar] [CrossRef]
- Alsalahi, W.; Tylus, W.; Trzeciak, A.M. Green synthesis of rhodium nanoparticles, catalytically active in benzene hydrogenation and 1-hexene hydroformylation. ChemCatChem 2018, 10, 2051–2058. [Google Scholar] [CrossRef]
- Lu, Z.; Jasinski, J.B.; Handa, S.; Hammond, G.B. Recyclable-cellulose-palladium nanoparticles for clean cross-coupling chemistry. Org. Biomol. Chem. 2018, 16, 2748–2752. [Google Scholar] [CrossRef]
- Jebali, Z.; Granados, A.; Nabili, A.; Boufi, S.; do Rego, A.M.B.; Majboub, H.; Vallribera, A. Cationic cellulose nanofibrils as a green support of palladium nanoparticles: Catalyst evaluation in Suzuki reactions. Cellulose 2018, 25, 6963–6975. [Google Scholar] [CrossRef]
- Dewan, A.; Sarmah, M.; Thakur, A.J.; Bharali, P.; Bora, U. Greener biogenic approach for the synthesis of palladium nanoparticles using papaya peel: An eco-friendly catalyst for C-C coupling reaction. ACS Omega 2018, 3, 5327–5335. [Google Scholar] [CrossRef]
- Chatterjee, S.; Bhattacharya, S.K. Size-dependent catalytic activity and fate of palladium nanoparticles in Suzuki-Miyaura coupling reactions. ACS Omega 2018, 3, 12905–12913. [Google Scholar] [CrossRef] [Green Version]
- Kandathil, V.; Dateer, R.B.; Sasidhar, B.S.; Patil, S.A.; Patil, S.A. Green synthesis of palladium nanoparticles: Applications in aryl halide cyanation and Hiyama cross-coupling reaction under ligand free conditions. Catal. Lett. 2018, 148, 1562–1578. [Google Scholar] [CrossRef]
- Gaikwad, D.S.; Undale, K.A.; Patil, D.B.; Pore, D.M.; Kamble, A.A. Triton X-100 stabilized Pd nanoparticles and their catalytic application in one-pot sequential Heck and Hiyama coupling in water. Res. Chem. Intermed. 2018, 44, 265–275. [Google Scholar] [CrossRef]
- Kuchkina, N.V.; Rajadurai, M.; Pal, M.; Basaveni, S.; Sorokina, S.A.; Krasnova, I.Y.; Serkova, E.S.; Shifrina, Z.B. Catalysts cased on hyperbranched pyridylphenylene polymers and palladium nanoparticles for the Suzuki-Miyaura cross-coupling reaction. Russ. Chem. Bull. Int. Ed. 2018, 67, 1035–1040. [Google Scholar] [CrossRef]
- Veisi, H.; Farokhi, M.; Hamelian, M.; Hemmati, S. Green synthesis of Au nanoparticles using an aqueous extract of Stachys lavandulifolia and their catalytic performance for alkyne/aldehyde/amine A3 coupling reactions. RSC Adv. 2018, 8, 38186–38195. [Google Scholar] [CrossRef] [Green Version]
- Veisi, H.; Mirshokraie, S.A.; Ahmadian, H. Synthesis of biaryls using palladium nanoparticles immobilized on metformine-functionalized polystyrene resin as a reusable and efficient nanocatalyst. Int. J. Biol. Macromol. 2018, 108, 419–425. [Google Scholar] [CrossRef]
- Veisi, H.; Adib, M.; Karimi-Nami, R.; Yasaei, Z.; Tajik, M.; Mosavat, T.S.; Hemmati, S. Suzuki-Miyaura coupling catalyzed by palladium nanoparticles biosynthesized using Glycyrrhiza glabra as reducing and stabilizing agent. Appl. Organomet. Chem. 2018, 32, e4138. [Google Scholar] [CrossRef]
- Ghasemi, S.; Karim, S. Controlled synthesis of modified polyacrylamide grafted nano-sized supported Pd nanoparticles via RAFT polymerization through “grafting to” approach: Application to the Heck reaction. Colloid Polym. Sci. 2018, 296, 1323. [Google Scholar] [CrossRef]
- Ghasemi, S.; Harandi, Z.A. Thermo-responsive poly(N-isopropylacrylamide)-block-poly(ionic liquid) of pyridinium sulfonate immobilized Pd nanoparticles in C-C coupling reactions. RSC Adv. 2018, 8, 14570–14578. [Google Scholar] [CrossRef] [Green Version]
- Baran, T.; Baran, N.Y.; Menteş, A. Sustainable chitosan/starch composite material for stabilization of palladium nanoparticles: Synthesis, characterization and investigation of catalytic behaviour of Pd@chitosan/starch nanocomposite in Suzuki-Miyaura reaction. Appl. Organomet. Chem. 2017, 31, e4075. [Google Scholar] [CrossRef]
- Dewan, A.; Sarmah, M.; Bora, U.; Thakur, A.J. In situ generation of palladium nanoparticles using agro waste and their use as catalyst for copper-, amine- and ligand-free Sonogashira reaction. Appl. Organomet. Chem. 2017, 31, e3646. [Google Scholar] [CrossRef]
- Veisi, H.; Rostami, A.; Shirinbayan, M. Greener approach for synthesis of monodispersed palladium nanoparticles using aqueous extract of green tea and their catalytic activity for the Suzuki-Miyaura coupling reaction and the reduction of nitroarenes. Appl. Organomet. Chem. 2017, 31, e3609. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, X.; Chen, X.; Wei, Y. N-Methylimidazole functionalized carboxymethylcellulose-supported Pd catalyst and its applications in Suzuki cross-coupling reaction. Carbohyd. Polym. 2017, 160, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Kumara, K.S.J.; Krishnamurthy, G.; Swamy, B.E.K.; Kumar, N.S.; Kumar, M. Catalytic performance study of nano-cobalt: A catalyst for complement to the Heck coupling reaction. J. Porous Mater. 2017, 24, 1095–1103. [Google Scholar] [CrossRef]
- Tarnowicz, S.; Alsalahi, W.; Mieczynska, E.; Trzeciak, A.M. Heck arylation of allyl alcohol catalyzed by Pd(0) nanoparticles. Tetrahedron 2017, 73, 5605–5612. [Google Scholar] [CrossRef]
- Huang, T.; Sheng, G.; Manchanda, P.; Emwas, A.H.; Lai, Z.; Nunes, S.P.; Peinemann, K.-V. Cyclodextrin polymer networks decorated with subnanometer metal nanoparticles for high-performance low-temperature catalysis. Sci. Adv. 2019, 5, eaax6976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doherty, S.; Knight, J.G.; Backhouse, T.; Abood, E.; Al-shaikh, H.; Clemmet, A.R.; Ellison, J.R.; Bourne, R.A.; Chamberlain, T.W.; Stones, R.; et al. Heteroatom donor-decorated polymer-immobilized ionic liquid stabilized palladium nanoparticles: Efficient catalysts for room-temperature Suzuki-Miyaura cross-coupling in aqueous media. Adv. Synth. Catal. 2018, 360, 3716–3731. [Google Scholar] [CrossRef]
- Kunfi, A.; May, Z.; Németh, P.; London, G. Polydopamine supported palladium nanoparticles: Highly efficient catalysts in Suzuki cross-coupling and tandem Suzuki cross-coupling/nitroarene reductions under green conditions. J. Catal. 2018, 361, 84–93. [Google Scholar] [CrossRef]
- Borah, R.K.; Mahanta, A.; Dutta, A.; Bora, U.; Thakur, A.J. A green synthesis of palladium nanoparticles by Sapindus mukorossi seed extract and use in efficient room temperature Suzuki-Miyaura cross-coupling reaction. Appl. Organomet. Chem. 2017, 31, e3784. [Google Scholar] [CrossRef]
- Mäsing, F.; Nüsse, H.; Klingauf, J.; Studer, A. Visible-light-enabled preparation of palladium nanoparticles and application as catalysts for Suzuki-Miyaura coupling. Org. Lett. 2018, 20, 752–755. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, M.; Zhang, L.; Liu, Y.; Han, J. Poly(o-aminothiophenol)-stabilized Pd nanoparticles as efficient heterogeneous catalysts for Suzuki cross-coupling reactions. RSC Adv. 2017, 7, 47104–47110. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.O.; You, J.M.; Jang, H.-S.; Choi, S.K.; Jung, B.Y.; Kang, O.; Kim, J.W.; Lee, Y.-S. Eumelanin as a support for efficient palladium nanoparticle catalyst for Suzuki coupling reaction of aryl chlorides in water. Tetrahedron Lett. 2017, 58, 2149–2152. [Google Scholar] [CrossRef]
- Yan, X.; Luo, Y.; Liu, W.; Liang, L.; Gan, Y.; Chen, Z.; Xu, Z.; Wan, H.; Tang, D.; Shi, H.; et al. Strategy used to synthesize high activity and low Pd catalyst for Suzuki coupling reaction: An experimental and theoretical investigation. Phys. Chem. Chem. Phys. 2020, 22, 6222–6230. [Google Scholar] [CrossRef]
- Wan, J.; Fan, B.; Thang, S.H. Sonochemical preparation of polymer-metal nanocomposites with catalytic and plasmonic properties. Nanoscale Adv. 2021, 3, 3306–3315. [Google Scholar] [CrossRef]
- Llevot, A.; Monney, B.; Sehlinger, A.; Behrens, S.; Meier, M.A.R. Highly efficient Tsuji-Trost allylation in water catalyzed by Pd-nanoparticles. Chem. Commun. 2017, 53, 5175–5178. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Yu, J. A polysalen based on polyacrylamide stabilized palladium nanoparticle catalyst for efficient carbonylative Sonogashira reaction in aqueous media. RSC Adv. 2017, 7, 31850–31857. [Google Scholar] [CrossRef] [Green Version]
- Piras, C.C.; Slavik, P.; Smith, D.K. Self-assembling supramolecular hybrid hydrogel beads. Angew. Chem. Int. Ed. 2020, 59, 853–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slavik, P.; Smith, D.K. Hybrid hydrogels loaded with palladium nanoparticles-catalysts for environmentally-friendly Sonogashira and Heck cross-coupling reactions. Tetrahedron 2020, 76, 131344. [Google Scholar] [CrossRef]
- Slavik, P.; Kurka, D.W.; Smith, D.K. Palladium-scavenging self-assembled hybrid hydrogels-reusable highly-active green catalysts for Suzuki-Miyaura cross-coupling reactions. Chem. Sci. 2018, 9, 8673–8681. [Google Scholar] [CrossRef] [Green Version]
- Ohtaka, A.; Kawase, M.; Usami, A.; Fukui, S.; Yamashita, M.; Yamaguchi, K.; Sakon, A.; Shiraki, T.; Ishida, T.; Nagata, S.; et al. Mechanistic study on allylic arylation in water with linear polystyrene-stabilized Pd and PdO nanoparticles. ACS Omega 2019, 4, 15764–15770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohtaka, A.; Kawase, M.; Aihara, S.; Miyamoto, Y.; Terada, A.; Nakamura, K.; Hamasaka, G.; Uozumi, Y.; Shinagawa, T.; Shimomura, O.; et al. Poly(tetrafluoroethylene)-stabilized metal nanoparticles: Preparation and evaluation of catalytic activity for Suzuki, Heck, and arene hydrogenation in water. ACS Omega 2018, 3, 10066–10073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohtaka, A.; Fukui, S.; Sakon, A.; Hamasaka, G.; Uozumi, Y.; Shinagawa, T.; Shimomura, O.; Nomura, R. Linea polystyrene-stabilized Rh(III) nanoparticles for oxidative coupling of arylboronic acids with alkenes in water. J. Organomet. Chem. 2018, 873, 1–7. [Google Scholar] [CrossRef]
- Ohtaka, A.; Sakon, A.; Yasui, A.; Kawaguchi, T.; Hamasaka, G.; Uozumi, Y.; Shinagawa, T.; Shimomura, O.; Nomura, R. Catalytic specificity of linear polystyrene-stabilized Pd nanoparticles during Ullmann coupling reaction in water and the associated mechanism. J. Organomet. Chem. 2018, 854, 87–93. [Google Scholar] [CrossRef]
- Sakon, A.; Ii, R.; Hamasaka, G.; Uozumi, Y.; Shinagawa, T.; Shimomura, O.; Nomura, R.; Ohtaka, A. Detailed mechanism for Hiyama coupling reaction in water catalyzed by linear polystyrene-stabilized PdO nanoparticles. Organometallics 2017, 36, 1618–1622. [Google Scholar] [CrossRef]
- Da Costa, A.P.; Nunes, D.R.; Tharaud, M.; Oble, J.; Poli, G.; Rieger, J. Pd(0)-nanoparticles embedded in core-shell nanogels as recoverable catalysts for the Mizoroki-Heck reaction. ChemCatChem 2017, 9, 2167–2175. [Google Scholar] [CrossRef] [Green Version]
- Pang, H.; Hu, Y.; Yu, J.; Gallou, F.; Lipshutz, B.H. Water-sculpting of a heterogeneous nanoparticle precatalyst for Mizoroki-Heck couplings under aqueous micellar catalysis conditions. J. Am. Chem. Soc. 2021, 143, 3373–3382. [Google Scholar] [CrossRef] [PubMed]
- Ansari, T.N.; Sharma, S.; Hazra, S.; Jasinski, J.B.; Wilson, A.J.; Hicks, F.; Leahy, D.K.; Handa, S. Shielding effect of nanomicelles: Stable and catalytically active oxidizable Pd(0) nanoparticle catalyst compatible for cross-couplings of water-sensitive acid chlorides in water. JACS Au 2021, 1, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Ansari, T.N.; Jasinski, J.B.; Leahy, D.K.; Handa, S. Metal-micelle cooperativity: Phosphine ligand-free ultrasmall palladium(II) nanoparticles for oxidative Mizoroki-Heck-type couplings in water at room temperature. JACS Au 2021, 1, 308–315. [Google Scholar] [CrossRef]
- Bihani, M.; Ansari, T.N.; Finck, L.; Bora, P.P.; Jasinski, J.B.; Pavuluri, B.; Leahy, D.K.; Handa, S. Scalable α-arylation of nitriles in aqueous micelles using ultrasmall Pd nanoparticles: Surprising formation of carbanion in water. ACS Catal. 2020, 10, 6816–6821. [Google Scholar] [CrossRef]
- Petkova, D.; Borlinghaus, N.; Shama, S.; Kaschel, J.; Lindner, T.; Klee, J.; Jolit, A.; Haller, V.; Heitz, S.; Britze, K.; et al. Hydrophobic pockets of HPMC enable extremely short reaction times in water. ACS Sustain. Chem. Eng. 2020, 8, 12612–12617. [Google Scholar] [CrossRef]
- Lee, N.; Moghadam, F.A.; Braga, F.C.; Lippincott, D.J.; Zhu, B.; Gallou, F.; Lipshutz, B.H. Sustainable palladium-catalyzed Tsuji-Trost reactions enabled by aqueous micellar catalysis. Org. Lett. 2020, 22, 4949–4954. [Google Scholar] [CrossRef]
- Takale, B.S.; Thakore, R.R.; Irvine, N.M.; Schuitman, A.D.; Li, X.; Lipshutz, B.H. Sustainable and cost-effective Suzuki-Miyaura coupling toward the key biaryl subunits of Arylex and Rinskor active. Org. Lett. 2020, 22, 4823–4827. [Google Scholar] [CrossRef]
- Pang, H.; Wang, Y.; Gallou, F.; Lipshutz, B.H. Fe-catalyzed reductive couplings of terminal (hetero)aryl alkenes and alkyl halides under aqueous micellar conditions. J. Am. Chem. Soc. 2019, 141, 17117–17124. [Google Scholar] [CrossRef] [PubMed]
- Duong, U.T.; Gade, A.B.; Plummer, S.; Gallou, F.; Handa, S. Reactivity of carbenes in aqueous nanomicelles containing palladium nanoparticles. ACS Catal. 2019, 9, 10963–10970. [Google Scholar] [CrossRef]
- Bihani, M.; Bora, P.P.; Nachtegaal, M.; Jasinski, J.B.; Plummer, S.; Gallou, F.; Handa, S. Microballs containing Ni(0)Pd(0) NPs for highly selective micellar catalysis in water. ACS Catal. 2019, 9, 7520–7526. [Google Scholar] [CrossRef]
- Lee, N.R.; Linstadt, R.T.H.; Gloisten, D.J.; Gallou, F.; Lipshutz, B.H. B-alkyl sp3-sp2 Suzuki-Miyaura couplings under mild aqueous micellar conditions. Org. Lett. 2018, 20, 2902–2905. [Google Scholar] [CrossRef] [PubMed]
- Handa, S.; Smith, J.D.; Zhang, Y.; Takale, B.S.; Gallou, F.; Lipshutz, B.H. Sustainable HandaPhos-ppm Palladium technology for copper-free Sonogashira couplings in water under mild conditions. Org. Lett. 2018, 20, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Isley, N.A.; Wang, Y.; Gallou, F.; Handa, S.; Aue, D.H.; Lipshutz, B.H. A micellar catalysis strategy for Suzuki-Miyaura cross-couplings of 2-pyridyl MIDA boronates: No copper, in water, very mild conditions. ACS Catal. 2017, 7, 8331–8337. [Google Scholar] [CrossRef]
- Brals, J.; Smith, J.D.; Ibrahim, F.; Gallou, F.; Handa, S. Micelle-enabled palladium catalysis for convenient sp2-sp3 coupling of nitroalkanes with aryl bromides in water under mild conditions. ACS Catal. 2017, 7, 7245–7250. [Google Scholar] [CrossRef]
- Cen, J.; Wu, Y.; Li, J.; Huang, L.; Wu, W.; Zhu, Z.; Yang, S.; Jiang, H. Switchable reactivity between vinyl azides and terminal alkyne by nano copper catalysis. Org. Lett. 2019, 21, 2090–2094. [Google Scholar] [CrossRef]
- Nandi, D.; Perla, V.K.; Ghosh, S.K.; Arderne, C.; Mallick, K. Copper-azide nanoparticle: A ‘catalyst-cum-reagent’ for the designing of 5-alkynyl 1,4-disubstituted triazoles. Sci. Rep. 2020, 10, 16720. [Google Scholar] [CrossRef]
- Wang, X.; Wu, C.; Sun, Y.; Yao, X. Copper nanoparticles catalyzed economical synthesis of 3-substituted isocoumarins from 2-chlorobenzoic acids/amides and 1,3-diketones. Tetrahedron Lett. 2017, 58, 3164–3167. [Google Scholar] [CrossRef]
- Shadmehr, A.; Abdolmohammadi, S. An expedient synthesis of [1]benzopyrano[b]pyridine-3-carbonitriles catalyzed by NiCr2O4 NPs. Polycycl. Aromat. Comp. 2020, 1739083. Available online: https://www.tandfonline.com/doi/full/10.1080/10406638.2020.1739083 (accessed on 10 August 2021).
- Mart, M.; Trzeciak, A.M. The synthesis of β-enaminones using trialkylamines and a Pd/DNA catalyst. Mol. Catal. 2021, 502, 111365. [Google Scholar] [CrossRef]
- Mart, M.; Trzeciak, A.M. Solvent switchable Pd/DNA catalyst in carbonylative Sonogashira coupling. Mol. Catal. 2020, 494, 111124. [Google Scholar] [CrossRef]
- Mart, M.; Tylus, W.; Trzeciak, A.M. Pd/DNA as a highly active and recyclable catalyst for aminocarbonylation and hydroxycarbonylation in water: The effect of Mo(CO)6 on the reaction course. Mol. Catal. 2019, 462, 28–36. [Google Scholar] [CrossRef]
- Mart, M.; Tylus, W.; Trzeciak, A.M. Pd/DNA as highly active and recyclable catalyst of Suzuki-Miyaura coupling. Catalysts 2018, 8, 552. [Google Scholar] [CrossRef] [Green Version]
- Alsalahi, W.; Trzeciak, A.M. Rh/DNA nanoparticles, synthesis, characterization and catalytic activity in “on water” asymmetric hydroformylation reaction. ChemistrySelect 2018, 3, 1727–1736. [Google Scholar] [CrossRef]
- Kataria, M.; Deol, H.; Singh, G.; Kumar, M.; Bhalla, V. Visible-light-mediated dehydrogenative cross-coupling between terminal alkynes and aldehydes by employing supramolecular polymeric ensemble of PBI derivative. New J. Chem. 2018, 42, 822–826. [Google Scholar] [CrossRef]
- Kataria, M.; Kumar, M.; Singh, Z.; Bhalla, V. Gold nanoparticles immobilized polymeric PBI derivative: Productive, portable, and photocatalytic system for Heck coupling. ACS Sustain. Chem. Eng. 2018, 6, 8223–8229. [Google Scholar] [CrossRef]
- Jeffery, T. Heck-type reactions in water. Tetrahedron Lett. 1994, 35, 3051–3054. [Google Scholar] [CrossRef]
- Nagata, T.; Obora, Y. N,N-Dimethylformamide-protected single-sized metal nanoparticles and their use as catalysts for organic transformations. ACS Omega 2020, 5, 98–103. [Google Scholar] [CrossRef] [Green Version]
- Prechtl, M.H.G.; Scholten, J.D.; Dupont, J. Carbon-carbon cross coupling reactions in ionic liquids catalyzed by palladium metal nanoparticles. Molecules 2010, 15, 3441–3461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gálvez-Martínez, E.; Aguilar-Granda, A.; Rodríguez-Molina, B.; Haropérez, G.; Kozina, A. Catalytic evaluation of citrate-stabilized palladium nanoparticles in the Sonogashira reaction for the synthesis of 1,4-bis[(trimethylsilyl)ethynyl] benzene. Catal. Commun. 2021, 153, 106269. [Google Scholar] [CrossRef]
- Ishida, J.; Nakatsuji, M.; Nagata, T.; Kawasaki, H.; Suzuki, T.; Obora, Y. Synthesis and characterization of N,N-dimethylformamide-protected palladium nanoparticles and their use in the Suzuki-Miyaura cross-coupling reaction. ACS Omega 2020, 5, 9598–9604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olekszyszen, D.N.; Albuquerque, B.L.; de Silva, O.D.; Tripodi, G.L.; de Oliveira, D.C.; Domingos, J.B. Core-shell PdCu bimetallic colloidal nanoparticles in Sonogashira cross-coupling reaction: Mechanistic insights into the catalyst mode of action. Nanocale 2020, 12, 1171–1179. [Google Scholar] [CrossRef]
- Fernández, E.; Rivero-Crespo, M.A.; Domínguez, I.; Rubio-Marués, P.; Oliver-Meseguer, J.; Liu, L.; Cabrero-Antonino, M.; Gavara, R.; Hernández-Garido, J.C.; Boronat, M.; et al. Base-controlled Heck, Suzuki, and Sonogashira reactions catalyzed by ligand-free platinum or palladium single atom and subnanometer clusters. J. Am. Chem. Soc. 2019, 141, 1928–1940. [Google Scholar] [CrossRef]
- Sarhid, I.; Abdellah, I.; Martini, C.; Huc, V.; Dragoe, D.; Beaunier, P.; Lampre, I.; Remita, H. Plasmonic catalysis for the Suzuki-Miyaura cross-coupling reaction using palladium nanoflowers. New J. Chem. 2019, 43, 4349–4355. [Google Scholar] [CrossRef]
- Wirwis, A.; Trzeciak, A.M. Ligand-free palladium-catalyzed tandem pathways for the synthesis of 4,4-diarylbutanones and 4,4-diaryl-3-butenones under microwave conditions. Appl. Organomet. Chem. 2019, 33, e4870. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/aoc.4870 (accessed on 10 August 2021). [CrossRef]
- Molteni, G.; Ferretti, A.M.; Mondini, S.; Ponti, A. Nitrilimine cycloadditions catalyzed by iron oxide nanoparticles. J. Nanopart. Res. 2018, 20, 79. [Google Scholar] [CrossRef]
- Sable, V.; Maindan, K.; Bhilare, S.; Chrysochos, N.; Schulzke, C.; Kapdi, A.R. An active palladium colloidal catalyst for the selective oxidative heterocoupling of (hetero)aryl boronic acids. Chem. Asian J. 2018, 13, 2489–2498. [Google Scholar] [CrossRef]
- Jeanne-Julien, L.; Astier, E.; Lai-Kuen, R.; Genta-Jouve, G.; Roulland, E. Palladium nanoparticle-catalyzed stereoretentive cross-coupling of alkenyl sulfides with Grignard reagents. Org. Lett. 2018, 20, 1430–1434. [Google Scholar] [CrossRef]
- Vargas, D.R.; Cook, S.P. Palladium nanoparticles: Chemoselective control for reductive Heck with aryl triflates and 2,3-dihydrofuran. Tetrahedron 2018, 74, 3314–3317. [Google Scholar] [CrossRef]
- Wirwis, A.; Feder-Kubis, J.; Trzeciak, A.M. Two efficient pathways for the synthesis of aryl ketones catalyzed by phosphorus-free palladium catalysts. Mol. Catal. 2018, 445, 61–72. [Google Scholar] [CrossRef]
- Onishi, K.; Oikawa, K.; Yano, H.; Suzuki, T.; Obora, Y. N,N-Dimethylformamide-stabilized palladium nanoclusters as a catalyst for Larock indole synthesis. RSC Adv. 2018, 8, 11324–11329. [Google Scholar] [CrossRef] [Green Version]
- Reina, A.; Serrano-Maldonado, A.; Teuma, E.; Martin, E.; Gómez, M. Palladium nanocatalysts in glycerol: Tuning the reactivity by effect of the stabilizer. Catal. Commun. 2018, 104, 22–27. [Google Scholar] [CrossRef]
- Arvelos, M.S.; Silva, A.C.; de Souza, A.L.F.; Achete, C.A.; Vasconcelos, T.L.; Robertis, E.; Archanjo, B.S.; Aguiar, L.C.S.; Malta, L.F.B.; Senra, J.D. Revealing Pd nanoparticles formation from PEG-mediated decomposition of organometallic precursor and their application as catalyst for the synthesis of n-extended carbazoles. ChemistrySelect 2018, 3, 9725–9730. [Google Scholar] [CrossRef]
- Feiz, A.; Loni, M.; Naderi, S.; Bazgir, A. The β-cyclodextrin decorated with palladium nanoparticles without pretreatment: An efficient heterogenous catalyst for biaryls synthesis. Appl. Organomet. Chem. 2018, 32, e4608. [Google Scholar] [CrossRef]
- Scattolin, T.; Canovese, L.; Visentin, F.; Paganelli, S.; Canton, P.; Demitri, N. Synthesis of novel allyl palladium complexes bearing purine based NHC and a water soluble phosphine and their catalytic activity in the Suzuki-Miyaura coupling in water. Appl. Organomet. Chem. 2018, 32, e4034. [Google Scholar] [CrossRef]
- Oka, H.; Kitai, K.; Suzuki, T.; Obora, Y. N,N-Dimethylformamide-stabilized copper nanoparticles as a catalyst precursor for Sonogashira-Hagihara cross coupling. RSC Adv. 2017, 7, 22869–22874. [Google Scholar] [CrossRef] [Green Version]
- Zong, Y.; Wang, J.; An, P.; Yue, G.; Pan, Y.; Wang, X. Self-assembled Pd nanoparticle-containing ionic liquid: Efficient and reusable catalyst for the Heck reaction in water. Appl. Organomet. Chem. 2017, 31, e3762. [Google Scholar] [CrossRef]
- Asada, S.; Nito, A.; Miyagi, Y.; Ishida, J.; Obora, Y.; Sanda, F. Sonogashira-Hagihara and Mizoroki-Heck coupling polymerizations catalyzed by Pd nanoclusters. Macromolecules 2017, 50, 4083–4087. [Google Scholar] [CrossRef]
- Ge, J.; Jiang, J.; Yuan, C.; Zhang, C.; Liu, M. Palladium nanoparticles stabilized by phosphine ligand for aqueous phase room temperature Suzuki-Miyaura coupling. Tetrahedron Lett. 2017, 58, 1142–1145. [Google Scholar] [CrossRef]
- Sarmah, M.; Mondal, M.; Gohain, S.B.; Bora, U. Gallic acid-derived palladium(0) nanoparticles as in situ-formed catalyst for Sonogashira cross-coupling reaction in ethanol under open air. Catal. Commun. 2017, 90, 31–34. [Google Scholar] [CrossRef]
- Mondal, M.; Begum, T.; Gogoi, P.K.; Bora, U. Gallic acid derived palladium(0) nanoparticles: An in situ formed “green and recyclable” catalyst for Suzuki-Miyaura coupling in water. ChemistrySelect 2016, 1, 4645–4651. [Google Scholar] [CrossRef]
- Arora, A.; Oswal, P.; Rao, G.K.; Kumar, S.; Singh, A.K.; Kumar, A. Catalytically active nanosized Pd9Te4 (telluropalladinite) and PdTe (kotulskite) alloys: First precursor-architecture controlled synthesis using palladium complexes of organotellurium compounds as single source precursors. RSC Adv. 2021, 11, 7214–7224. [Google Scholar] [CrossRef]
- Oswal, P.; Arora, A.; Kaushal, J.; Rao, G.K.; Kumar, S.; Singh, A.K.; Kumar, A. Ultra-small palladium nano-particles synthesized using bulky S/Se and N donor ligands as a stabilizer: Application as catalysts for Suzuki-Miyaura coupling. RSC Adv. 2019, 9, 22313–22319. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Arora, A.; Oswal, P.; Rao, G.K.; Kaushal, J.; Kumar, S.; Kumar, S.; Singh, M.P.; Singh, A.K.; Kumar, A. Bidentate organochalcogen ligands (N, E.; E = S/Se) as stabilizers for recyclable palladium nanoparticles and their application in Suzuki-Miyaura coupling reactions. Polyhedron 2019, 171, 120–127. [Google Scholar] [CrossRef]
- Dachwitz, S.; Duwe, D.H.; Wang, Y.H.; Gruß, H.; Hannappel, Y.; Hellweg, T.; Sewald, N. Suzuki-Miyaura cross-coupling of bromotryptophan derivatives at ambient temperature. Chem. Eur. J. 2020, 26, 16357–16364. [Google Scholar] [CrossRef]
- Song, J.; Feng, X.; Yamamoto, Y.; Almansour, A.I.; Arumugam, N.; Kumar, R.S.; Bao, M. Carboxylative coupling of chloromethyl(hetero)arenes with allytrimethoxysilane catalyzed by palladium nanoparticles. Asian J. Org. Chem. 2017, 6, 177–183. [Google Scholar] [CrossRef]
- Feng, X.; Sun, A.; Zhang, S.; Yu, X.; Bao, M. Palladium-catalyzed carboxylative coupling of benzyl chlorides with allyltributylstannane: Remarkable effect of palladium nanoparticles. Org. Lett. 2013, 15, 108–111. [Google Scholar] [CrossRef]
- Raheem, A.A.; Thangasamy, P.; Sathish, M.; Praveen, C. Supercritical water assisted preparation of recyclable gold nanoparticles and their catalytic utility in cross-coupling reactions under sustainable conditions. Nanoscale Adv. 2019, 1, 3177–3191. [Google Scholar] [CrossRef] [Green Version]
- Kiani, M.; Bagherzadeh, M.; Meghdadi, S.; Fadaei-Tirani, F.; Babaie, M.; Schenk-Joß, K. Promising new catalytic properties of a Co(II)-carboxamide complex and its derived Co3O4 nanoparticles for the Mizoroki-Heck and the epoxidation reactions. Appl. Organomet. Chem. 2020, 34, e5911. [Google Scholar] [CrossRef]
- Tanaka, K.; Fujiki, K. Bis[N,N’-(2-indalolyl)]-1,5-diazacyclooctane as unique metal ligand: Self-assemble of palladium nanoparticles and catalytic reactivity on C-C bond formation. Synthesis 2018, 50, 1097–1104. [Google Scholar] [CrossRef]
- Xia, Y.-T.; Wu, J.-J.; Zhang, C.-Y.; Mao, M.; Ji, Y.-G.; Wu, L. Cascade alkynylation and highly selective hydrogenation catalyzed by binaphthyl-palladium nanoparticles accessing phosphinyl (Z)-[3]dendralenes. Org. Lett. 2019, 21, 6383–6387. [Google Scholar] [CrossRef] [PubMed]
- Parveen, N.; Sekar, G. Palladium nanoparticle-catalyzed stereoselective domino synthesis of all-carbon tetrasubstituted olefin containing oxindoles via carbopalladation/C-H activation. J. Org. Chem. 2020, 85, 10514–10524. [Google Scholar] [CrossRef] [PubMed]
- Parveen, N.; Sekar, G. Palladium nanoparticle-catalyzed stereoselective domino synthesis of 3-allylidene-2(3H)-oxindoles and 3-allylidene-2(3H)-benzofuranones. J. Org. Chem. 2020, 85, 4682–4694. [Google Scholar] [CrossRef] [PubMed]
- Parveen, N.; Sekar, G. Palladium nanoparticles-catalyzed synthesis of indanone derivatives via intramolecular reductive Heck reaction. Adv. Synth. Catal. 2019, 361, 4581–4595. [Google Scholar] [CrossRef]
- Parveen, N.; Saha, R.; Sekar, G. Stable and reusable palladium nanoparticles-catalyzed conjugate addition of aryl iodides to enones: Route to reductive Heck products. Adv. Synth. Catal. 2017, 359, 3741–3751. [Google Scholar] [CrossRef]
- Kobayashi, M.; Yamaguchi, H.; Suzuki, T.; Obora, Y. Cross β-alkylation of primary alcohols catalyzed by DMF-stabilized iridium nanoparticles. Org. Biomol. Chem. 2021, 19, 1950–1954. [Google Scholar] [CrossRef]
- Oikawa, K.; Itoh, S.; Yano, H.; Kawasaki, H.; Obora, Y. Preparation and use of DMF-stabilized iridium nanoclusters as methylation catalysts using methanol as the C1 source. Chem. Commun. 2017, 53, 1080–1083. [Google Scholar] [CrossRef]
- Wang, J.; Feng, X.; Song, J.; Gao, Z.; Wang, W.-H.; Bao, M. The ultrasmall palladium nanoparticles catalyzed telomerization of CO2 with 1,3-butadiene at room temperature: Selective synthesis of δ-lactone. ChemistrySelect 2020, 5, 9404–9408. [Google Scholar] [CrossRef]
- Selvaraj, K.; Daoud, A.; Alarifi, S.; Idhayadhulla, A. Tel-Cu-NPs catalyst: Synthesis of naphtho[2,3-g]phthalazine derivatives as potential inhibiters of tyrosinase enzymes and their investigation in kinetic, molecular docking, and cytotoxicity studies. Catalysts 2020, 10, 1442. [Google Scholar] [CrossRef]
- Mišurović, J.; Mojović, M.; Marjanović, B.; Vulić, P.; Ćirić-Marjanović, G. Magnetite nanoparticles-catalyzed synthesis of conductive poly(p-aminodiphenylamine). Synth. Met. 2020, 269, 116577. [Google Scholar] [CrossRef]
- Mišurović, J.; Mojović, M.; Marjanović, B.; Vulić, P.; Ćirić-Marjanović, G. Magnetite nanoparticles-catalyzed synthesis of conductive polyaniline. Synth. Met. 2019, 257, 116174. [Google Scholar] [CrossRef]
- Yuan, M.; Xu, L.; Cui, X.; Lv, J.; Zhang, P.; Tang, H. Facile synthesis of ultrahigh molecular weight poly(methyl methacrylate) by organic halides in the presence of palladium nanoparticles. Polymers 2020, 12, 2747. [Google Scholar] [CrossRef]
- Rosa-Pardo, I.; Casadevall, C.; Schmidt, L.; Claros, M.; Galian, R.E.; Lloret-Fillol, J.; Pérez-Prieto, J. The synergy between the CsPbBr3 nanoparticle surface and the organic ligand becomes manifest in a demanding carbon-carbon coupling reaction. Chem. Commun. 2020, 56, 5026–5029. [Google Scholar] [CrossRef]
















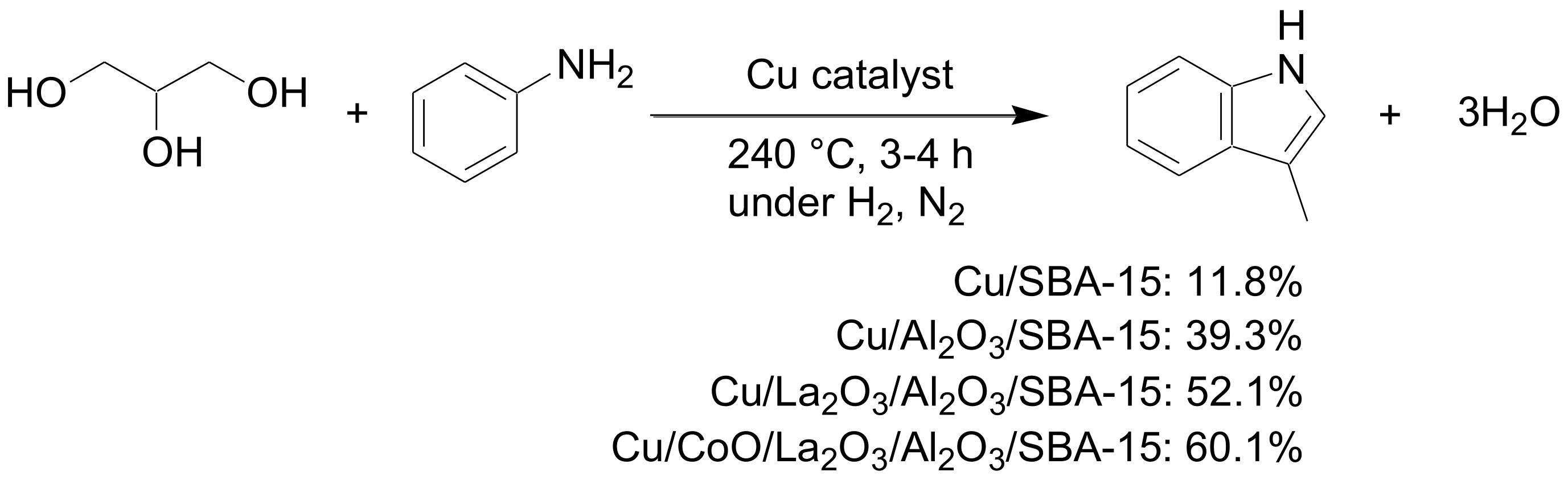






















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohtaka, A. Recent Progress of Metal Nanoparticle Catalysts for C–C Bond Forming Reactions. Catalysts 2021, 11, 1266. https://doi.org/10.3390/catal11111266
Ohtaka A. Recent Progress of Metal Nanoparticle Catalysts for C–C Bond Forming Reactions. Catalysts. 2021; 11(11):1266. https://doi.org/10.3390/catal11111266
Chicago/Turabian StyleOhtaka, Atsushi. 2021. "Recent Progress of Metal Nanoparticle Catalysts for C–C Bond Forming Reactions" Catalysts 11, no. 11: 1266. https://doi.org/10.3390/catal11111266
APA StyleOhtaka, A. (2021). Recent Progress of Metal Nanoparticle Catalysts for C–C Bond Forming Reactions. Catalysts, 11(11), 1266. https://doi.org/10.3390/catal11111266





