Abstract
Rice is the second most extensively consumed food ingredient, and its by-products in the paddy field include rice husk and straw. Rice husk ash, resulting from rice husk burning, is considered an environment menace, inducing negative effects on the area in which it is disposed of. In this study, rice husk was applied as a silicate source to obtain mesoporous silica material. Characterization techniques confirmed the well-ordered mesophase and resemblance of mesoporous silica resulting from rice husk ash with one obtained from conventional silica sources. The mesoporous silica material was further used as catalyst support. The resulting catalysts were used for rhodamine 110 oxidation, proving high potential for oxidizing hazardous organic compounds, such as dyes from water, resulting in environmentally harmless products.
1. Introduction
Nowadays, disappearance of raw resources and excess waste constitute major issues worldwide. The only solution to overcome these issues is reutilization of these wastes [1].
The commercial exploitation of industrial and agricultural wastes is increasing, which is observed mainly in agribusiness. This can be considered a potential income and also an option for reducing waste production and environmental effects [2,3].
Moreover, increasing demands from the ceramic industry has led to the production of various raw materials.
Rice (Oryza sativa), from the Gramineae family, is considered to be in second position in terms of being the most extensively eaten food ingredient. It typically contains 20–25% rice husk (RH), which is discarded as waste from the rice mill industry. Rice paddy production during the period of 2018–2019 from major consuming countries is presented in Figure 1 [4]. As can be observed, China and India are the major producers, accounting for around 50 wt% of the total rice production.
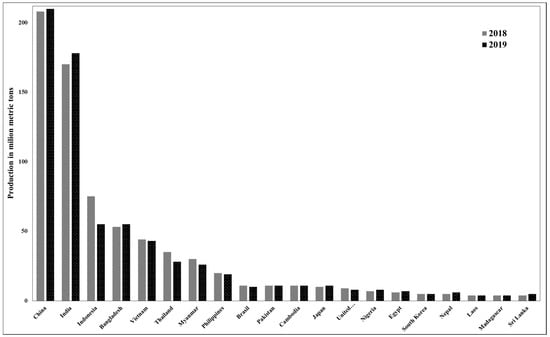
Figure 1.
Global paddy rice output in 2018 and 2019 (million metric tons) [4].
Rice husk (RH) is mainly constituted by organic compounds (70–80%) (including cellulose or lignin) and mineralogical components, among them being silica, alkalis, and trace elements (20–30%) [5]. Important quantities of SiO2 have been determined in husk, varying from 8.7% to 22% [6]. SiO2 is present as an amorphous state that is hydrated, comparable to that from other compounds in the biosphere [7,8].
Due to unsuitable disposal and burning techniques in developing countries, where rice husk is used as fuel, rice husk ash (RHA) is considered a major environmental pollutant, although with amorphous silica content.
The resulting RHA can be used in various industries (chemicals, electronics, ceramics, and construction) because of its high silica concentration [1,9]. In this respect, many studies have targeted this research area, with the use of RHA being considered sustainable on a “triple bottom line” basis (environmental, economic, and social) [9,10]. Additionally, RHA has been considered raw material for obtaining advanced materials valuable in various fields [11,12]. Numerous investigations have described the application of silica from RH to obtain advanced non-oxide ceramics, silicon, and last but not least, nano-silica [1]. Additionally, it has been used for the synthesis of zeolite materials, mesoporous or hierarchical carbon, non-oxide ceramics, and silica aerogels (SAs) [13].
Overall, RHA is more appropriate than RH, with its efficiency as potential raw material varying with the calcination temperature [14]. Additionally, the silica obtained from RHA can have structural modification as a consequence of the obtained parameters (time, temperature, etc.). As a consequence, at an interval of 550–800 °C, amorphous silica is obtained, with crystalline silica resulting at temperatures higher than 800 °C [15,16,17].
Therefore, using RHA for extracting silica not only provides a valuable product but also helps in mitigating the milestone of disposal, eventually having a positive impact on the environment by reducing pollution. Moreover, since rice husk is organic and a waste product, its use as raw material has an almost negligible cost.
Figure 2 presents the most common methods for extracting silica from RH and the methods used for removing impurities at the beginning and end of thermal treatment. It is concluded that a chemical procedure involving acid leach, followed by annealing, can be considered among the easiest and most efficient procedures for obtaining powder nano-silica particles [15,16,17].
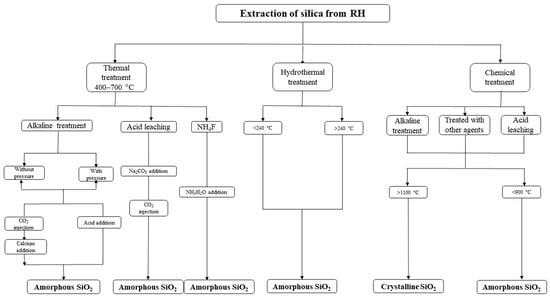
Figure 2.
Various processes for silica obtained from RH.
Additionally, rice husk can be treated with mineral acid and subsequent calcination, resulting in high-purity silica [18,19]. This silica precursor can be considered a potential candidate for obtaining porous materials such as MCM-41 and zeolites X and Y [19,20]. MCM-41-type mesoporous silica has an ordered porous network, having one-dimensional and hexagonal mesopores (pore sizes of 2–4 nm). MCM-41 has gained increasing attention as it is able to act as both an adsorbent and support for catalysts [19,21].
The neutral framework of MCM-41 materials restrains the application as catalysts. Hence, by introducing metallic ions, active sites can be created, increasing the activity. Among transitional metals, Ni and Fe have increased redox characteristics. For example, nickel catalysts have high catalytic activity in various reactions, including alkane or alkene oxidation [22], CO2 methanation [23], and syngas obtainment [24].
The textile and dye industry generates effluents rich in various dyes and pigments. Colored effluents result also from several other industries, such as pharmaceutical, cosmetic, food, and liquid crystal manufacturing [25]. Most of these dyes are environmentally hazardous, many of them having carcinogenic effects [26]. Their negative effects include inhibition of sunlight penetration into water streams, affecting the natural disinfection and reducing photosynthesis of aquatic plants [27]. These dyes can be harmful even as traces, therefore making it mandatory to destroy them before they reach the environment [28]. Various methods have been investigated to oxidize dyes from wastewater, including oxidation with H2O2 [29,30], photocatalysis [31], catalytic ozonation [32,33], and ultrasonic irradiation [34].
The aim of this study was to obtain MCM-41 support and efficient catalysts with high specific surface area. The sodium silicate solution obtained from RHA was used as silica source through an easy temperature-controlled procedure. The synthesis proved to be accessible, cost-efficient, and green. The catalytic activity of the mesoporous materials was tested in rhodamine 110 oxidation using H2O2 as oxidant agent. Although many studies have presented wet peroxidative removal of dyes on Fe-containing zeolites or mesoporous silica [35,36,37], no study has reported the use of Fe and Ni-like catalysts using mesoporous silica obtained from rice husk for heterogeneous Fenton-like degradation dyes.
2. Materials and Methods
2.1. Mesoporous Silica Synthesis
RHA resulted from heat treatment of rice husk (5 h, 600 °C) in an air oven (Nabertherm GmbH, Lilienthal, Germany).
Figure 3 displays a step-by-step diagram for obtaining mesoporous MCM-41. Rice husks were rinsed and then treated at reflux with HCl (Sigma-Aldrich, Steinheim, Germany) in order to remove residual metals, followed by thermal decomposition of the organic matter to produce rice husk ash (RHA) as a dirty white powder.
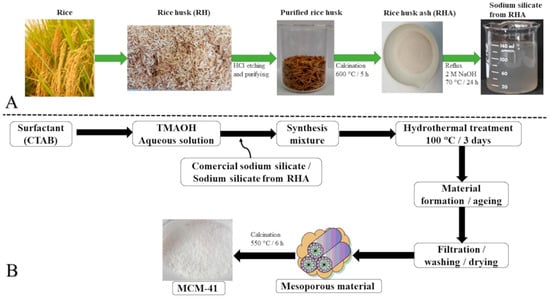
Figure 3.
(A) Flow chart showing RHA obtainment from rice. (B) Comprehensive scheme of the MCM-41 synthesis from commercial sodium silicate or RHA-extracted sodium silicate.
Sodium silicate solution was extracted by refluxing for 24 h (70 °C) rice husk ash in aqueous solution of 2 M NaOH (Sigma-Aldrich, Steinheim, Germany) (1 g RHA: 50 mL NaOH). The surfactant–silica mixture resulted from adding, under stirring at ambient temperature, 7.6 g of the obtained silicate solution to 6 g of hexadecyltrimethylammonium bromide (CTAB) (Sigma-Aldrich, Steinheim, Germany) dissolved in 60 mL of ultrapure water (Purelab Flex 3-Elga, Wycombe, UK). Finally, the pH was adjusted to 10.5, and the mixture was further stirred for another 12 h. The mixture was subjected to hydrothermal treatment (100 °C, 72 h) in an acid digestion vessel (PARR Instrument Company, Moline, IL, USA). After this period, the solid was filtrated under vacuum, rinsed with ultrapure water, dried and calcined (550 °C, 6 h), and it was denoted as MCM-41r. Additionally, a typical MCM-41 was synthesized using CTAB, TMAOH (tetramethylammonium hydroxide), and sodium silicate (Sigma-Aldrich, Steinheim, Germany), in conformity with a previously developed procedure [38,39]. Briefly, 6 g of CTAB was dissolved in 60 mL of ultrapure H2O, under stirring at ambient temperature, for 2 h. Then, 7.6 g of commercial sodium silicate was added, with stirring continuing for another 2 h. After this, 43 g of TMAOH was dropwise incorporated under stirring for another 30 min, finally, with the pH corrected to 10.5. The mixture was stirred for 24 h and then transferred to an acid digestion vessel for the hydrothermal treatment (100 °C, 72 h). Finally, the solid was filtrated, rinsed with ultrapure water, dried, and calcined (550 °C, 6 h), and it was further denoted as MCM-41c.
2.2. Catalysts Synthesis
The support, MCM-41r (0.6 g), was impregnated with aqueous solution of nickel acetate (3.0 mL solution at a concentration of 1.34% Ni) (Sigma-Aldrich, Steinheim, Germany) [38]. After impregnation, the precursor was dried for 24 h in air and then calcined (550 °C, 6 h).
The iron catalyst was obtained by treating 0.75 g of MCM-41r with 12.5 mL of 1 M NaCl, to which a 50 mL solution of 20 mM FeCl3 (Sigma-Aldrich, Steinheim, Germany) was added [40]. The mixture volume was then adjusted to 500 mL with ultrapure water under stirring. Then, the pH was measured and corrected to be around 3, with the mixture left under agitation for 24 h, at ambient temperature. After this interval, the pH was checked again, and it was allowed to settle in a separation funnel for 24 h. The mixture was centrifuged, the supernatant was removed, and the brick-colored precipitate was dried at 100 °C for 5 h.
2.3. Material Characterization
The initial RHA chemical composition was obtained by treating it with microwave digestion using a Mars 6 extraction system (CEM Corporation, Matthews, NC, USA). The digested fluid was then analyzed using a Hach DR 3900 spectrophotometer (Hach, Loveland, CO, USA).
The gas chromatographic method coupled with the combustion method [41] was used for qualitative and quantitative determinations of the carbon, nitrogen, and hydrogen concentrations. The oxygen concentration was determined by pyrolysis coupled with the gas chromatographic method. The equipment was a Flash EA 2000 (Thermo Scientific, Loughborough, UK).
The quantitative determination of Ni and Fe in the samples was performed by flame atomic absorption spectrophotometry, according to standard analysis methods. A novAA 300 atomic absorption spectrophotometer (Analytik Jena, Jena, Germany) was used, with air/acetylene flow at 0.9–1.1 L/min.
The specific surface areas and pore distribution were assessed by BET (Brunauer–Emmett–Teller) and BJH (Barrett–Joyner–Halenda) procedures [42]. The isotherms, BET area, and BJH pore distribution were obtained with a Quantachrome Autosorb IQ porosity equipment (Quantachrome Instruments, Boynton Beach, FL, USA). Samples were degassed at 423 K beforehand. N2 adsorption–desorption experiments were achieved at 77 K.
The mesoporous materials were structurally investigated by scanning electron microscopy using FESEM VP Scanning Electron Microscope (Carl Zeiss, Oberkochen, Germany) at resolutions of 0.8 and 2.5 nm, VP mode, at 30 kV.
For TEM analysis, both mesoporous silica support and catalysts were crushed in an agate mortar, dispersed in ethyl alcohol, and deposited on a microgrid. A TEM model Hitachi HD2700 scanning-transmission electron microscope (Oxford Instruments, Abingdon, Oxfordshire, UK) was used: cold field emission gun, 200 kV, secondary and transmitted electron detectors, and two windowless X-ray detectors.
Functional groups from mesoporous materials were highlighted using FTIR analysis (Cary 630 ATR–FTIR spectrophotometer, Agilent Technologies, Inc., Santa Clara, CA, USA).
The materials’ thermal stability was tested by thermogravimetric analysis with a SDT Q600 equipment (TA Instruments, New Castle, DE, USA). Mass modification throughout the thermal analysis was evaluated at an interval of 303–1273 K, under 99.999% vol purity N2, with a flow rate of 100 mL/min and a ramp of 283 K/min.
Raman measurements were performed using an Alpha 300RAS Atomic Force Microscope (WITec GmbH, Ulm, Germany) equipped with an inverted microscope connected to a 75 mWAr laser and a grating spectrometer with an ultrafast CCD camera for spectrum acquisition at every 5 ms with a resolution higher than 3 cm−1 [43,44].
2.4. Catalytic Testing
Oxidation reactions were carried out in 20 mL of glass recipients at natural light, at ambient temperature and pressure, using rhodamine 110 (Sigma-Aldrich, Steinheim, Germany) as substrate, acetonitrile as solvent (Sigma-Aldrich, Steinheim, Germany), and hydrogen peroxide as oxidant agent (Sigma-Aldrich, Steinheim, Germany). The decolorization experiments were monitored by UV–VIS spectrophotometry (Specord 200 Plus, Analytik Jena, Jena, Germany). COD (chemical oxygen demand) reduction was detected by dichromate method with the LCI 400 cuvette test (Hach Lange GmbH, Dusseldorf, Germany) and a Hach DR 3900 spectrophotometer (Hach, Loveland, CO, USA).
The COD reduction was evaluated using the equation:
where COD0 represents COD value at the initial moment t = 0, and CODt represents COD at t moment.
3. Results and Discussion
The obtaining approach relied on the hydrothermal treatment of the porous silica resulting from the rice husk, which was not only the matrix but also the raw material for further processing.
Table 1 summarizes the elemental analysis results for RHA, MCM-41r, MCM-41c, and the Ni and Fe catalysts, denoted as Ni-MCM-41r and Fe-MCM-41r.

Table 1.
Elemental composition of RHA and MCM-41.
Table 1 reveals that the silicon content is relatively high in RHA, highlighting its suitability for obtaining silica materials. By introducing a surfactant in the hydrothermal treatment, a mesoporous material (MCM-41r) was obtained. A similar composition to MCM-41c (obtained when commercial sodium silicate was used) was confirmed by morphostructural characterization. EDS analysis confirmed the Si percent spectrophotometrically determined.
One of the main characterization issues for mesoporous silica is identifying the pore network based on physical adsorption–desorption analysis. Type IV isotherms were identified (Figure 4), in accordance with the IUPAC definition, which were associated with the presence of mesopores [45].
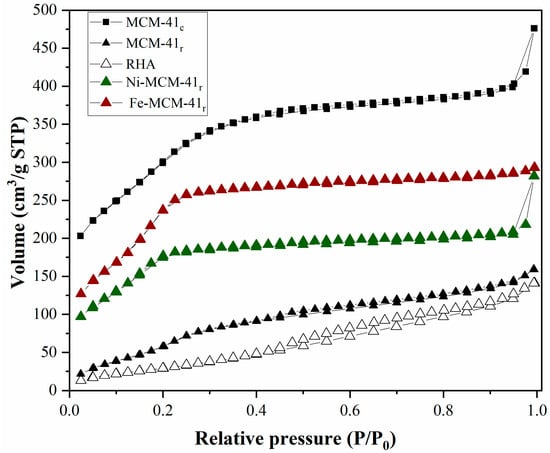
Figure 4.
Adsorption–desorption isotherms and pore distribution for MCM-41 resulting from classical synthesis (MCM-41c) and RHA (MCM-41r).
The initial segment of the adsorption isotherm (P/P0 < 0.2) was attributed to the monolayer and multilayer occurrences, similar to that given by a nonporous material. The second segment, at higher P/P0, exhibited an upward deviation correlated to the continuous filling of mesopores by capillary condensation. The obtained MCM-41 contained no macropores since the isotherm reached a plateau at a high P/P0. On reducing P/P0, desorption took place, resulting in a hysteresis loop, with its form related to the shape of the pores [46].
The BET specific surface area and the mesopore volume (786 m2/g, 0.990 cm3/g) of the mesoporous silica obtained from RHA were slightly lower than those of MCM-41c (791 m2/g, 0.991 cm3/g) (Figure 4 and Table 2).

Table 2.
Textural parameters of RHA and silica materials.
The next phase was the determination of the pore size distribution. In this respect, the BJH procedure was used to calculate the mesopore distribution (from the desorption curve) in the capillary condensation segment. The medium pore diameter slightly increased from 3.7 nm for MCM-41 from classical synthesis to 3.92 nm for MCM-41 from RHA (Figure 4 and Table 2). When introducing metals, the specific surface area decreased to 528 m2/g for Ni-MCM-41r and 175 m2/g for Fe-MCM-41r, but the medium pore diameter remained constant for the first catalyst and decreased to 2.93 for the second one. It can also be observed that the mesopore volume drastically decreased when metals were introduced in the silica matrix by up to 0.161 cm3/g for Ni-MCM-41r and 0.062 cm3/g for Fe-MCM-41r. The Fe-MCM-41r isotherm change can be explained by the obtaining method, dynamic wet impregnation, which also led to a decrease in specific surface area, mesopore diameter, and volume.
Field emission scanning electron microscopy revealed the morphology of the samples (Figure 5). Rice husk contained longitudinal fibers, interspaced within the lignin and glucose network (Figure 5a). This arrangement is similar to a composite structure, with fibers regularly located within the framework. Rice husk fibers are formed by silica, cellulose, and lignin, forming a compact matrix [47]. In RHA, SEM revealed many residual channels and pores, indicating that it is a highly porous material with an interesting internal surface area (Figure 5b). Rice husk was destroyed during thermal treatment, resulting in a porous structure. The MCM-41 samples (Figure 5c,d) displayed a similar morphology with spherical and partially elongated particles of around 0.2 μm dimensions. EDS confirmed the high silica contents for both MCM-41 samples.
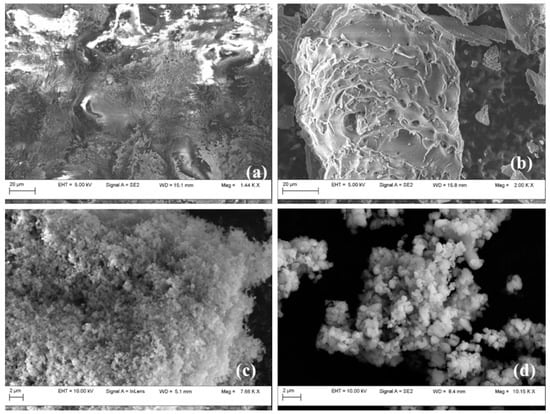
Figure 5.
SEM images for RH (a), RHA (b), MCM-41r (c), and MCM-41c (d).
After the metal introduction, SEM images confirmed that the ordered morphology was maintained (Figure 6).
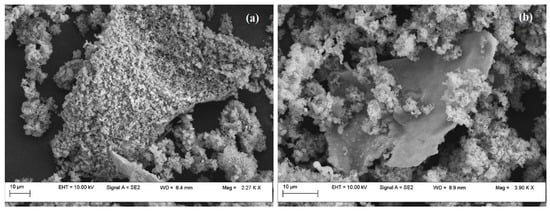
Figure 6.
SEM images for Fe-MCM-41r (a) and Ni-MCM-41r (b).
TEM images for the selected samples are shown in Figure S1 (Supplementary Material). The unit cell parameters and pore wall thicknesses derived from the TEM data are listed in Table S1. The obtained unit cell parameters were similar to those estimated from N2 adsorption/desorption analysis. Figure S1a indicates that the MCM-41r pores are almost hexagonal, in accordance with the literature [48]. The hexagonal arrangement was kept after Ni and Fe introduction, the pore diameters remaining constant in the Ni-MCM-41r catalyst and decreasing in the Fe-MCM-41r catalyst. Figure S2 represents the EDS layered image for MCM-41r, confirming the elemental composition and distribution of elements. Figures S3 and S4 illustrate the EDS mapping of Ni-MCM-41r and Fe-MCM-41r, where both Ni and Fe were successfully and homogenously impregnated on MCM-41r. EDS mapping confirmed that all Ni ions attached to the silica surface, while part of the Fe ions dispersed into the silica matrix, inducing modification to the textural and chemical properties.
Figure 7 represents the FTIR spectra for MCM-41c and MCM-41r and for the obtained catalysts.
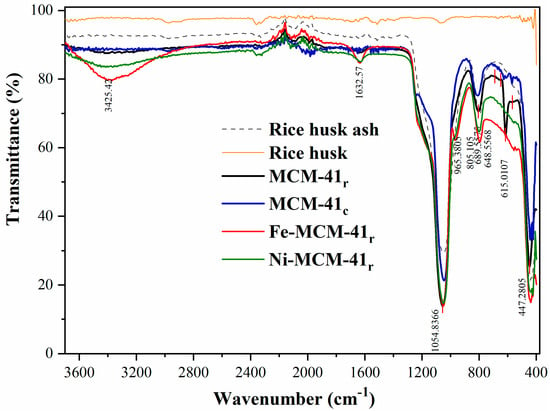
Figure 7.
FTIR spectra of RHA, MCM-41r, MCM-41c, Fe-MCM-41r, and Ni-MCM-41r.
The two FTIR spectra of the results for mesoporous silica (Figure 7) were similar. The surfactant was completely removed throughout calcination, with the bands from around 1466 cm−1 corresponding to dC-H vibrations not observed [49,50,51]. The bandwidth from about 3400 cm−1 was attributed to the surface hydroxyl groups’ stretching mode and to the physical surface-adsorbed water molecules. The peak at about 1632 cm−1 corresponded to the deformation mode for the surface hydroxyl groups. The two bands from about 1054 and 805 cm−1 were assigned to stretch vibrations for symmetrical and asymmetrical Si–O groups. The peaks from about 615–648 cm−1 and 447 cm−1 were attributed to the stretching and deformation vibrations for Si–O groups from the silica surface [50].
When Ni was introduced, the FTIR spectra suffered a modification: the bands from 3400 and 1632 cm−1 (specific for surface silanols and the surface-adsorbed water) widened, suggesting that once the metal was impregnated, the quantity of the hydroxyl groups and absorbed water molecules increased, suggesting the formation of Ni hydrate on the silica surface [52]. A new shoulder occurred at 980 cm−1 after nickel impregnation, which was attributed to Si–O–Ni vibration, concluding that Ni species was successfully impregnated on MCM-41r. Ni content was confirmed also by the disappearance of the peak from 615 cm−1, confirming the metal positioning at the silica surface.
The introduction of Fe in silica support also produced a modification of the FTIR spectra: the band from 965 cm−1 was assigned to Si–O–Fe vibration within Fe-silicate, a potential evidence of the isomorphous replacement of Si with Fe [53]. Fe introduction was confirmed also by the disappearance of the peak from 615 cm−1, confirming the metal positioning at the silica surface.
Figure 8 represents thermogravimetric curves for both MCM-41c and MCM-41r, revealing two intervals of mass loss, the first generated by the loss of the water from the silica surface and the second associated with the mesoporous framework disruption.
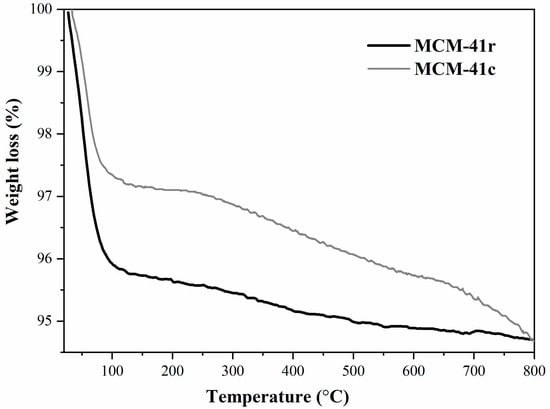
Figure 8.
TG analysis of MCM-41r and MCM-41c.
The total mass lost was approximately 5.2% for MCM-41c and 5.3% for MCM-41r. The difference between the two curves is due to the slightly increased content in residual carbon for MCM-41r compared with that for MCM-41c, which seems to influence the allure, which also explains the little variation in mass loss.
The Raman spectra for the mesoporous silica are illustrated in Figure 9, exhibiting four weak Raman bands at 458, 626, 995, and 1090 cm−1. The bands from 458 and 626 cm−1 were assigned to the Si–O–Si bond asymmetrical and symmetrical stretch vibration, and the band from 995 cm−1 was associated with the vibration mode of the siloxane. The 1090 cm−1 band was attributed to the Si–O–Si bond correlated to frame defects [54,55]. The low intensities from MCM-41r were harmonized to the silica specific surface area and pore diameter reduction.
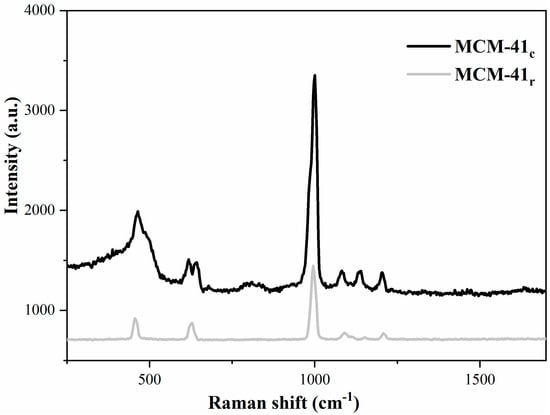
Figure 9.
Raman spectra of MCM-41r and MCM-41c.
The Raman and FTIR results confirmed that the samples were MCM-41-type mesoporous silica.
In order to assess the materials’ catalytic activity, rhodamine oxidation was carried out with hydrogen peroxide as oxidant and acetonitrile as solvent.
Figure 10 presents the results of rhodamine oxidation in the presence of Ni-MCM-41r and Fe-MCM-41r as catalysts.
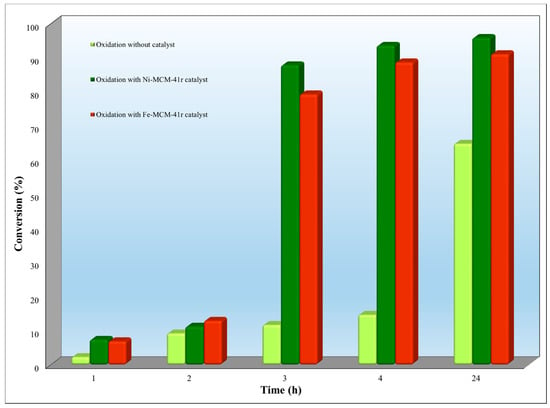
Figure 10.
Ni-MCM-41r and Fe-MCM-41r catalytic activity on rhodamine oxidation (5 mg/L dye, 50 mg catalyst, 5 mL H2O2, 9.2 mL acetonitrile as solvent).
When Ni-MCM-41r was used as a catalyst, a salt in the conversion was observed from 11.30% after 2 h up to 87.95% after 3 h (Figure 10). The maximum conversion of rhodamine (95.98%) was obtained after 24 h. After this period, no changes were registered in the conversion degree.
When Fe-MCM-41r was used as a catalyst, the conversion increased from 12.90% after 2 h up to 79.30% after 3 h (Figure 10). The maximum conversion of rhodamine (91.10%) was obtained after 24 h. After this period, no changes were registered in the conversion degree.
In the absence of the catalysts, the maximum rhodamine conversion was 64.79% after 24 h.
Observing the trends of the conversion, it can be concluded that Ni-MCM-41r presented a higher catalytic activity than Fe-MCM-41r.
After rhodamine oxidation, COD measurements were carried out, the COD load of the reaction mixtures decreasing by 83.11% when Ni-MCM-41r was used and by 80.51% when Fe-MCM-41r was used. The high COD reduction indicated that rhodamine was oxidized to simple organic compounds and also considerably mineralized. For comparison, the literature mentioned a COD removal of around 90% when rhodamine 110 was oxidized in the presence of modified TiO2 catalysts [56]. The high conversion induced by the Ni-based catalyst compared with the Fe-based catalyst can be explained by the obtaining methods: while the Ni catalyst was prepared by static wet impregnation, the Fe catalyst was prepared by dynamic impregnation. Due to this, as SEM showed, all Ni ions attached to the silica surface, while part of the Fe ions dispersed into the silica matrix, thus reducing the specific area and, as a consequence, the potential catalytic activity. The reduction of the COD (chemical oxygen demand) value when the Fe catalyst was used is explained by its lower catalytic activity, meaning that a lower-quantity organic dye was oxidized, with the results confirmed by the measurements of the dye concentrations during the reaction.
Because H2O2 manifests an excellent potential to remove rhodamine over mesoporous silica catalysts, various amounts of H2O2 were tested in order to improve the oxidation parameters. Figure 11 reveals the degradation varying with the volume of H2O2 (1–5 mL).
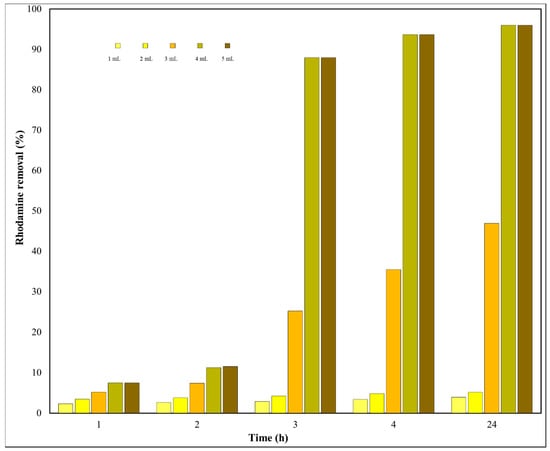
Figure 11.
H2O2 effect on the rhodamine removal (5 mg/L dye, 50 mg Ni-MCM-41r as catalyst, 9.2 mL acetonitrile as solvent).
The removal level of rhodamine changed from 3.96% to 95.98% after 24 h. The catalytic activity tended to reach a plateau, at about 4%, at a low initial volume of H2O2. Without a doubt, the rhodamine removal is correlated with additional HO. radicals resulting from hydrogen peroxide segregation as the H2O2 volume grows [57]. An elevated H2O2 volume resulted in a sharp increase in rhodamine removal by up to approximately 96% after 24 h. It can also be observed that, at 4 and 5 mL, the removal efficiency registered almost the same values, which indicated that the maximum removal was reached.
The catalyst dose can also severely influence the removal of organic dyes. In this respect, the effect of catalyst quantity was examined in the range of 0–60 mg (Figure 12).
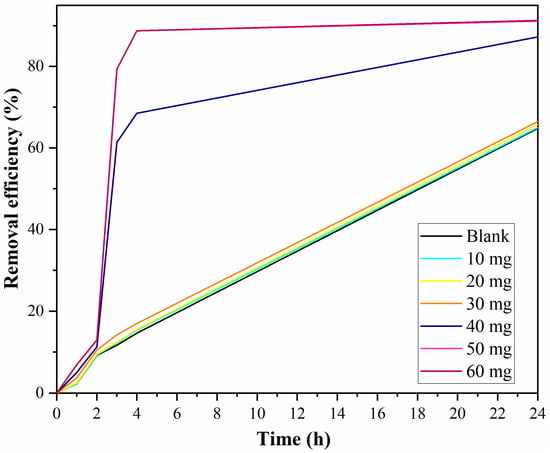
Figure 12.
Dye concentration effect on the removal (50 mg catalyst, 5 mL H2O2, 9.2 mL acetonitrile as solvent).
The removal of rhodamine varied proportionally with the catalyst dose, indicating that the catalytic activity is correlated with the number of accessible active sites at the catalyst surface. The highest efficiency was obtained with the 50 mg Fe-MCM-41r catalyst, and thereafter, increasing the catalyst quantity had no other notable effect on rhodamine degradation. It can be observed that the efficiency removal when 50 mg catalysts were used overlapped with the values from the 60 mg catalyst.
The effect of dye concentration was studied by changing the concentration of rhodamine in the range of 0–7 mg/L, while the other parameters remained constant. A concentration increase above 5 mg/L led to the decrease in degradation rate (Figure 13).
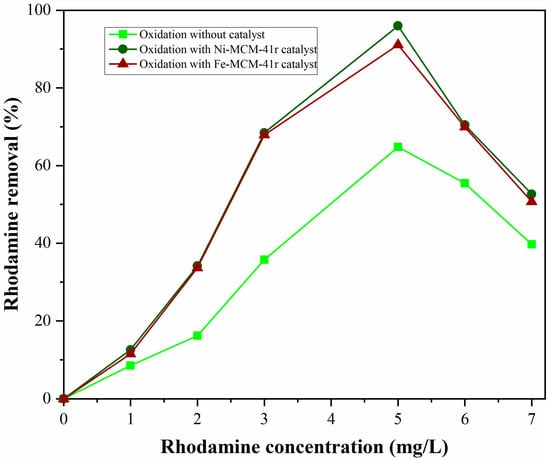
Figure 13.
Catalyst dosage effect on removal efficiency (5 mg/L dye, Fe-MCM-41r as catalyst, 5 mL H2O2, 9.2 mL acetonitrile as solvent).
This effect might be attributed to two factors: increasing the rhodamine adsorption onto the catalysts or to the rhodamine acting as an inner filter, preventing the passage of light, leading to the decrease of the photoelectrons and photohole number, resulting in the degradation rate.
After their use, the catalysts were extracted by centrifugation, and they were rinsed with ultrapure water to separate the residual dye linked to the catalyst. Then, the catalysts were soaked in 100 mL ultrapure water, and the mixture was stirred for 4 h. After this, the mixtures were centrifuged, and the solids were dried in an air oven at approximately 378 K. The recovered catalysts were reused for the same reaction. It was observed that even after five cycles, the efficiency of the catalysts diminished by less than 5.0%.
The products of rhodamine 110 decomposition were analyzed by FTIR measurements (Figure 14).
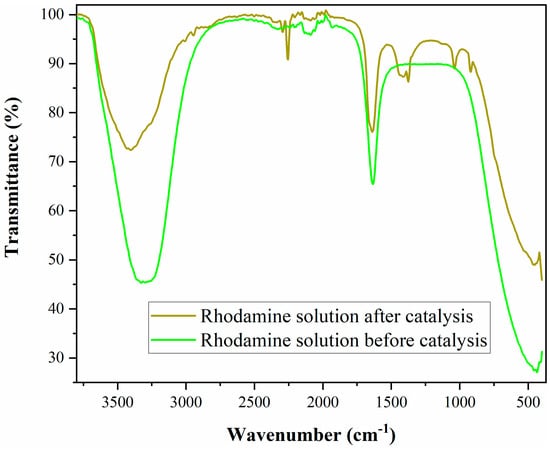
Figure 14.
FTIR spectra of rhodamine solution before and after catalytic oxidation.
FTIR spectroscopy was used to identify the presence or absence of some bonds before and after the oxidation. It was observed that the band found for rhodamine before catalysis at 3306 cm−1 (assigned to the presence of secondary amine) decreased in intensity after the catalytic reaction, indicating its partial destruction in rhodamine. This band overlapped with the new band at 3429 cm−1, indicating the formation of –OH. Still-existing absorption bands of conjugated carbonyl at 1640 cm−1, as well as the new band at 3432 cm−1 (–OH), indicated the formation of carboxyl groups after catalysis [58].
After oxidation, a band appeared at 924 cm−1 attributed to N–H stretching vibrations. The appearance of new bands at 1375, 1415, and 2255 cm−1 demonstrated the total disruption of aromatic rings. Similar results were found for rhodamine B degradation using Coelastrella sp. green microalgae [59]. The FTIR results showed that dye molecules were transformed into small-molecular-weight organics and inorganics, which were less harmful, and finally completely converted to CO2, water, and some inorganic anions.
The rhodamine solution, after catalyst usage, was subjected to SAA analysis, the results indicating a concentration of Ni and Fe under the method quantification limits: under 50 µg/L for Ni ions and under 70 µg/L for Fe ions. These results suggest that the catalysts are very stable during the oxidation reaction.
The literature mentioned that the Fe catalysts are more active than the Ni catalysts [60], but under photocatalytic conditions, where it is well known that Fe ions together with hydrogen peroxide act as Fenton reagent. The present study revealed that the Ni catalyst proved to be more efficient than the Fe catalyst. Up to now, Ni-MCM-41 has not been applied as a catalyst for rhodamine oxidation, being used for methane partial oxidation [61] or for carbon and oxygenate reduction in the copyrolysis process of polypropylene and cellulose [62]. Additionally, Ni-MCM-41 was applied as adsorbent for dye removal [52]. Comparing the adsorption with the oxidation, it can be observed that Ni-MCM-41 was more efficient when catalysis was involved due to the transformation of the dye into less harmful compounds.
From an economic point of view, the MCM-41-type mesoporous silica obtained from rice husk proved to be cheaper than the mesoporous silica synthesis from silicates available on the market. For example, in order to obtain sodium silicate from sodium carbonate and quartz sand, a temperature of 1573 K and high energy demand are necessary [63,64]. The costs increase if mesoporous silicates are obtained from organic silicon sources [65]. By using rice husk as raw material for silicate solution, the cost of obtaining MCM-41, at the laboratory scale, including reagents, instruments, energy, and labor costs, was estimated to be about 1400 euro/kg [66]. In comparison, the cost of a commercial MCM-41-type mesoporous silica can reach approximately 26,000 euro/kg.
4. Conclusions
This investigation showed that rice husk not only is an agricultural waste by-product but also can be assessed as raw material for extracting amorphous silica.
Rice husk, as a low-cost silica source, has been successfully applied to synthesize high-purity MCM-41-type silica material. Chemical and structural investigations have shown that rice-husk-derived MCM-41 material has similar chemistry and morphology to those obtained from commercial silicate. The obtained catalysts are inexpensive, and they can be effortlessly synthesized and do not demand hard initial treatments. The obtained catalysts prove high activity for the removal of hazardous organics (dyes from water), resulting in environmentally harmless products. The catalytic oxidation results show that Ni-MCM-41r is more efficient than Fe-MCM-41r. The increased catalytic activity of the first one can be attributed to the obtaining method, namely, static wet impregnation, compared with the Fe catalyst, which is obtained by dynamic impregnation.
This study highlights a sustainable route for renewable materials, with the resulting materials suitable for various applications, including catalysis.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/catal11070815/s1, Figure S1: TEM images for MCM-41r (a), Ni-MCM-41r (b) and Fe-MCM-41r (c), Figure S2: EDS mapping for MCM-41r, Figure S3: EDS mapping for Ni-MCM-41r, Figure S4: EDS mapping for Fe-MCM-41r, Table S1: Structural properties derived from TEM analysis.
Author Contributions
V.-C.N.: conceptualization, methodology, investigation, data curation, supervision, writing, reviewing, and editing. M.S.R.: visualization, investigation, data curation, and validation. All authors discussed the results and contributed to the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Part of this research was financially supported by the Romanian Ministry of Research, Innovation, and Digitization, under NUCLEU Program–Financing Contract no. 9N/2019, under Project PN 19 11 03 01; and the other part of the research was financed by the Romanian Ministry Education and Research, UEFISCDI Authority, under Project PN-III-P1-1.2-PCCDI-2017-0776/No. 36 PCCDI/15.03.2018.
Acknowledgments
The authors would like to express their gratitude to Amalia Soare, Daniela Ebrasu-Ion, Stanica Enache, Adriana Marinoiu, Claudia Sandru, and Marius Constantinescu with the National R&D Institute for Cryogenic and Isotopic Technologies–ICSI Ramnicu Valcea, Romania, for the scanning electron microscopy, thermogravimetric, Raman, specific area, and pore distribution measurements, metal content, and elemental analysis. We also express our gratitude to Lucian Barbu-Tudoran, from National R&D Institute of Isotopic and Molecular Technologies, Cluj-Napoca for TEM and EDS measurements.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Hossain, S.K.S.; Mathur, L.; Roy, P.K. Rice husk/rice husk ash as an alternative source of silica in ceramics: A review. J. Asian Ceram. Soc. 2018, 6, 299–313. [Google Scholar] [CrossRef]
- Schwanke, A.J.; Melo, D.M.A.; Silva, A.O.; Pergher, S.B.C. Use of rice husk ash as only source of silica in the formation of mesoporous materials. Ceramica 2013, 59, 181–185. [Google Scholar] [CrossRef]
- Filote, C.; Felseghi, R.A.; Raboaca, M.S.; Aşchilean, I. Environmental impact assessment of green energy systems for power supply of electric vehicle charging station. Int. J. Energy Res. 2020, 44, 10471–10494. [Google Scholar] [CrossRef]
- Available online: https://www.statista.com/statistics/255937/leading-rice-producers-worldwide/ (accessed on 19 April 2021).
- Sarangi, M.; Bhattacharyya, S.; Behera, R.C. Effect of temperature on morphology and phase transformations of nano-crystalline silica obtained from rice husk. Phase Transit. 2009, 82, 377–386. [Google Scholar] [CrossRef]
- Ding, T.P.; Ma, G.R.; Shui, M.X.; Wan, D.F.; Li, R.H. Silicon isotope study on rice plants from the Zhejiang province, China. Chem. Geol. 2005, 218, 41–50. [Google Scholar] [CrossRef]
- Asuncion, M.Z.; Hasegawa, I.; Kampf, J.W.; Laine, R.M. The selective dissolution of rice hull ash to form [OSiO1.5]8[R4N]8 (R = Me, CH2CH2OH) octasilicates. Basic nanobuilding blocks and possible models of intermediates formed during biosilicification processes. J. Mater. Chem. 2005, 15, 2114–2121. [Google Scholar] [CrossRef]
- Nicolae, V.; Neamtu, B.; Picu, O.; Stefanache, M.A.M.; Cioranu, V.S.I. The comparative evaluation of salivary biomarkers (Calcium, Phosphate, Salivary pH) in Cario-resistance versus Cario-activity. Rev. Chim. 2016, 67, 821–824. [Google Scholar]
- Torres-Carrasco, M.; Reinosa, J.J.; de la Rubia, M.A.; Reyes, E.; Alonso Peralta, F.; Fernández, J.F. Critical aspects in the handling of reactive silica in cementitious materials: Effectiveness of rice husk ash vs nano-silica in mortar dosage. Constr. Build. Mater. 2019, 223, 360–367. [Google Scholar] [CrossRef]
- Prasara-A, J.; Gheewala, S.H. Sustainable utilization of rice husk ash from power plants: A review. J. Clean. Prod. 2018, 167, 1020–1028. [Google Scholar] [CrossRef]
- Pode, R. Potential applications of rice husk ash waste from rice husk biomass power plant. Renew. Sustain. Energy Rev. 2016, 53, 1468–1485. [Google Scholar] [CrossRef]
- Rata, M.; Rata, G.; Filote, C.; Raboaca, M.S.; Graur, A.; Afanasov, C.; Felseghi, A.R. The electricalvehicle simulator for charging station in mode 3 of IEC 61851-1 standard. Energies 2019, 13, 176. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Zhao, P.; Shao, Q. Porous silica and carbon derived materials from rice husk pyrolysis char. Microporous Mesoporous Mater. 2014, 188, 46–76. [Google Scholar] [CrossRef]
- Shen, Y. Rice husk silica derived nanomaterials for sustainable applications. Renew. Sustain. Energy Rev. 2017, 80, 453–466. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Pramada, P.N.; Majeed, J. Effect of calcination temperature and heating rate on the optical properties and reactivity of rice husk ash. J. Mater. Sci. 2006, 41, 7926–7933. [Google Scholar] [CrossRef]
- Sapei, L.; Nöske, R.; Strauch, P.; Paris, O. Isolation of mesoporous biogenic silica from the perennial plant Equisetum hyemale. Chem. Mater. 2008, 20, 2020–2025. [Google Scholar] [CrossRef]
- Soltani, N.; Bahrami, A.; Pech-Canul, M.I.; González, L.A. Review on the physicochemical treatments of rice husk for production of advanced materials. Chem. Eng. J. 2015, 264, 899–935. [Google Scholar] [CrossRef]
- Krishnarao, R.V.; Mahajan, Y.R. Formation of SiC whiskers from raw rice husks in argon atmosphere. Ceram. Int. 1996, 22, 353–358. [Google Scholar] [CrossRef]
- Chumee, J.; Grisdanurak, N.; Neramittagapong, S.; Wittayakun, J. Characterization of alMCM-41 synthesized with rice husk silica and utilization as supports for platinum-iron catalysts. Braz. J. Chem. Eng. 2009, 26, 367–373. [Google Scholar] [CrossRef]
- Grisdanurak, N.; Chiarakorn, S.; Wittayakun, J. Utilization of mesoporous molecular sieves synthesized from natural source husk silica to chlorinated volatile organic compounds (CVOCs) adsorption. Korean J. Chem. Eng. 2003, 20, 950–955. [Google Scholar] [CrossRef]
- Matsumoto, A.; Chen, H.; Tsutsumi, K.; Grün, M.; Unger, K. Novel route in the synthesis of MCM-41 containing framework aluminum and its characterization. Microporous Mesoporous Mater. 1999, 32, 55–62. [Google Scholar] [CrossRef]
- Sankaralingam, M.; Balamurugan, M.; Palaniandavar, M. Alkane and alkene oxidation reactions catalyzed by nickel(II) complexes: Effect of ligand factors. Coord. Chem. Rev. 2020, 403, 213085. [Google Scholar] [CrossRef]
- Chen, Y.; Qiu, B.; Liu, Y.; Zhang, Y. An active and stable nickel-based catalyst with embedment structure for CO2 methanation. Appl. Catal. B Environ. 2020, 269, 118801. [Google Scholar] [CrossRef]
- Fedorova, Z.A.; Danilova, M.M.; Zaikovskii, V.I. Porous nickel-based catalysts for tri-reforming of methane to synthesis gas: Catalytic activity. Mater. Lett. 2020, 261, 127087. [Google Scholar] [CrossRef]
- Sun, S.; Wang, W.; Shang, M.; Ren, J.; Zhang, L. Efficient catalytic oxidation of tetraethylated rhodamine over ordered mesoporous manganese oxide. J. Mol. Catal. A Chem. 2010, 320, 72–78. [Google Scholar] [CrossRef]
- Zhu, L.; Meng, Z.D.; Park, C.Y.; Ghosh, T.; Oh, W.C. Characterization and relative sonocatalytic efficiencies of a new MWCNT and CdS modified TiO2 catalysts and their application in the sonocatalytic degradation of rhodamine B. Ultrason. Sonochem. 2013, 20, 478–484. [Google Scholar] [CrossRef]
- Yu, J.X.; Li, B.H.; Sun, X.M.; Jun, Y.; Chi, R.A. Adsorption of methylene blue and rhodamine B on baker’s yeast and photocatalytic regeneration of the biosorbent. Biochem. Eng. J. 2009, 45, 145–151. [Google Scholar] [CrossRef]
- Tilli, S.; Ciullini, I.; Scozzafava, A.; Briganti, F. Differential decolorization of textile dyes in mixtures and the joint effect of laccase and cellobiose dehydrogenase activities present in extracellular extracts from Funalia trogii. Enzym. Microb. Technol. 2011, 49, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.C.; Docal, C.; Gonsalves, A.M.D.A.R. Efficient azo dye degradation by hydrogen peroxide oxidation with metalloporphyrins as catalysts. J. Mol. Catal. A Chem. 2005, 238, 192–198. [Google Scholar] [CrossRef]
- Javaid, R.; Qazi, U.Y.; Kawasaki, S.I. Highly efficient decomposition of Remazol Brilliant Blue R using tubular reactor coated with thin layer of PdO. J. Environ. Manag. 2016, 180, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Aguedach, A.; Brosillon, S.; Morvan, J.; Lhadi, E.K. Photocatalytic degradation of azo-dyes reactive black 5 and reactive yellow 145 in water over a newly deposited titanium dioxide. Appl. Catal. B Environ. 2005, 57, 55–62. [Google Scholar] [CrossRef]
- Dong, Y.; He, K.; Zhao, B.; Yin, Y.; Yin, L.; Zhang, A. Catalytic ozonation of azo dye active brilliant red X-3B in water with natural mineral brucite. Catal. Commun. 2007, 8, 1599–1603. [Google Scholar] [CrossRef]
- Shang, N.C.; Chen, Y.H.; Yang, Y.P.; Chang, C.H.; Yu, Y.H. Ozonation of dyes and textile wastewater in a rotating packed bed. J. Environ. Sci. Health Part A Toxic/Hazardous Subst. Environ. Eng. 2006, 41, 2299–2310. [Google Scholar] [CrossRef] [PubMed]
- Ince, N.H. Ultrasound-assisted advanced oxidation processes for water decontamination. Ultrason. Sonochem. 2018, 40, 97–103. [Google Scholar] [CrossRef]
- Gandhi, S.; Thandavan, K.; Sethuraman, S.; Krishnan, U.M. Investigation of the photodegradation properties of iron oxide doped mesoporous SBA-15 silica. J. Porous Mater. 2013, 20, 1009–1015. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, B.; Zhao, Y.; Yang, T. Fabrication of the magnetic mesoporous silica Fe-MCM-41-A as efficient adsorbent: Performance, kinetics and mechanism. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Tahir, M.A.; Bhatti, H.N.; Hussain, I.; Bhatti, I.A.; Asghar, M. Sol-Gel Synthesis of Mesoporous Silica-Iron Composite: Kinetics, Equilibrium and Thermodynamics Studies for the Adsorption of Turquoise-Blue X-GB Dye. Z. Phys. Chem. 2020, 234, 233–253. [Google Scholar] [CrossRef]
- Niculescu, V.; Ene, R.; Iordache, I.; Parvulescu, V. Progress of Cryogenics and Isotopes Separation. Prog. Cryog. Isot. Sep. 2012, 15, 37–42. [Google Scholar]
- Miricioiu, M.G.; Iacob, C.; Nechifor, G.; Niculescu, V.C. High selective mixed membranes based on mesoporous MCM-41 and MCM-41-NH2 particles in a polysulfone matrix. Front. Chem. 2019, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Li, Y. Iron-Impregnated Nanosized Silica and Alumina: Implications for Contaminant Transformation. 2014. Available online: http://hdl.handle.net/2346/60677 (accessed on 28 June 2020).
- Constantinescu, M.; Bucura, F.; Ionete, R.E.; Niculescu, V.C.; Ionete, E.I.; Zaharioiu, A.; Oancea, S.; Miricioiu, M.G. Comparative study on plastic materials as a new source of energy. Mater. Plast. 2019, 56, 41–46. [Google Scholar] [CrossRef]
- Marinoiu, A.; Jianu, C.; Cobzaru, C.; Raceanu, M.; Capris, C.; Soare, A.; Petreanu, I.; Carcadea, E. Facile synthesis of well dispersed au nanoparticles on reduced graphene oxide. Prog. Cryog. Isot. Sep. 2017, 20, 5–14. [Google Scholar]
- Spiridon, S.I.; Ionete, E.I.; Monea, B.F.; Sofilca, N.; Ebrasu-Ion, D.; Enache, S.; Vaseashta, A. Synthesis, Characterization and Applications of Single Walled Carbon Nanotube–Pt–P2O5 Sensors for Absolute Humidity Measurements. Surf. Eng. Appl. Electrochem. 2018, 54, 623–630. [Google Scholar] [CrossRef]
- Chicea, D.; Neamtu, B.; Chicea, R.; Chicea, L.M. The application of AFM for biological samples imaging. Dig. J. Nanomater. Biostruct. 2010, 5, 1015–1022. [Google Scholar]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. International union of pure commission on colloid and surface chemistry including catalysis * Reporting physisorption data for gas/solid systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Allen, T. Particle Size Measurement, 3rd ed.; Springer: New York, NY, USA, 1981; ISBN 978-1-4899-3063-7. [Google Scholar]
- Kunal, B.; Bahurudeen, A.; Mohammed Haneefa, K.; Mahalingam, B. Microstructural Characterization of Rice Husk and Residual Ash for the Production of Superior Blended Concrete. Int. J. Res. Eng. Technol. 2015, 4, 327–332. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Sakamoto, Y.; Terasaki, O.; Ryoo, R.; Ko, C.H. Determination of Pore Size and Pore Wall Structure of MCM-41 by Using Nitrogen Adsorption, Transmission Electron Microscopy, and X-ray Diffraction. J. Phys. Chem. B 2000, 104, 292–301. [Google Scholar] [CrossRef]
- Hoang, V.D.; Dang, T.P.; Dinh, Q.K.; Nguyen, H.P.; Vu, A.T. The synthesis of novel hybrid thiol-functionalized nano-structured SBA-15. Adv. Nat. Sci. Nanosci. Nanotechnol. 2010, 1, 035011. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Xu, Y.; Sun, G.; Wang, L.; Sun, Y.; Qin, X. Preparation, characterization of thiol-functionalized silica and application for sorption of Pb2+ and Cd2+. Colloids Surfaces A Physicochem. Eng. Asp. 2009, 349, 61–68. [Google Scholar] [CrossRef]
- Viana, R.B.; Da Silva, A.B.F.; Pimentel, A.S. Infrared spectroscopy of anionic, cationic, and zwitterionic surfactants. Adv. Phys. Chem. 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- Shu, Y.; Shao, Y.; Wei, X.; Wang, X.; Sun, Q.; Zhang, Q.; Li, L. Synthesis and characterization of Ni-MCM-41 for methyl blue adsorption. Microporous Mesoporous Mater. 2015, 214, 88–94. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, M.; Mei, Q.; Tang, J.; Fei, Z.; Chen, X.; Liu, Q.; Cui, M.; Qiao, X. Iron-doped mesoporous silica, Fe-MCM-41, as an active Lewis acid catalyst for acidolysis of benzyl chloride with fatty acid. J. Porous Mater. 2019, 26, 261–269. [Google Scholar] [CrossRef]
- Abdel Salam, M.S.; Betiha, M.A.; Shaban, S.A.; Elsabagh, A.M.; Abd El-Aal, R.M.; El kady, F.Y. Synthesis and characterization of MCM-41-supported nano zirconia catalysts. Egypt. J. Pet. 2015, 24, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Liu, L.; Xiong, G. Effect of zeolite precursor on the formation of MCM-41 molecular sieve containing zeolite y building units. Phys. Chem. Chem. Phys. 2011, 13, 11248–11253. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, L.; Zheng, L.; Xian, Y.; Jin, L. Photoelectrocatalytic degradation of rhodamine B using Ti/TiO2 electrode prepared by laser calcination method. Electrochim. Acta 2006, 51, 4942–4949. [Google Scholar] [CrossRef]
- Chen, F.; Xie, S.; Huang, X.; Qiu, X. Ionothermal synthesis of Fe3O4 magnetic nanoparticles as efficient heterogeneous Fenton-like catalysts for degradation of organic pollutants with H2O2. J. Hazard. Mater. 2017, 322, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Hu, Y.B.; Huang, L.Z.; Zhang, R.; Xu, T.; Zhao, J. pH-sensitive nanocarriers for Ganoderma applanatum polysaccharide release via host–guest interactions. J. Mater. Sci. 2018, 53, 7963–7975. [Google Scholar] [CrossRef]
- Baldev, E.; MubarakAli, D.; Ilavarasi, A.; Pandiaraj, D.; Ishack, K.A.S.S.; Thajuddin, N. Degradation of synthetic dye, Rhodamine B to environmentally non-toxic products using microalgae. Colloids Surf. B Biointerfaces 2013, 105, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.P.; Rath, D.; Nanda, B.; Parida, K.M. Transition metal/metal oxide modified MCM-41 for pollutant degradation and hydrogen energy production: A review. RSC Adv. 2015, 5, 83707–83724. [Google Scholar] [CrossRef]
- Ding, C.; Wang, J.; Li, Y.; Ma, Q.; Ma, L.; Guo, J.; Ma, Z.; Liu, P.; Zhang, K. The role of active sites location in partial oxidation of methane to syngas for MCM-41 supported ni nanoparticles. Catalysts 2019, 9, 606. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Liu, C.; Zhuo, J.; Yao, Q. Investigation of a Ni-modified MCM-41 catalyst for the reduction of oxygenates and carbon deposits during the co-pyrolysis of cellulose and polypropylene. ACS Omega 2020, 5, 20299–20310. [Google Scholar] [CrossRef]
- Venkatathri, N.; Santhanaraj, D.; Shanthi, K. Synthesis and characterization of a novel mesoporous Mn-organophosphate molecular sieve. J. Indian Chem. Soc. 2011, 88, 225–230. [Google Scholar] [CrossRef]
- Raboaca, M.S.; Bizon, N.; Grosu, O.V. Energy management strategies for hybrid electric vehicles - vosviwer bibliometric analysis. In Proceedings of the 12th International Conference on Electronics, Computers and Artificial Intelligence (ECAI), Bucharest, Romania, 25–27 June 2020; 20062137. pp. 1–8. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, L.; Shang, J.; Gao, H. A low cost synthesis of fly ash-based mesoporous nanocomposites for production of hydrogen by photocatalytic water-splitting. J. Mater. Sci. 2013, 48, 5571–5578. [Google Scholar] [CrossRef]
- Shah, B.A.; Patel, A.V.; Bagia, M.I.; Shah, A.V. Green approach towards the synthesis of MCM-41 from siliceous sugar industry waste. Int. J. Appl. Chem. 2017, 13, 497–514. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).