Structural Requirements for Chemoselective Ammonolysis of Ethylene Glycol to Ethanolamine over Supported Cobalt Catalysts
Abstract
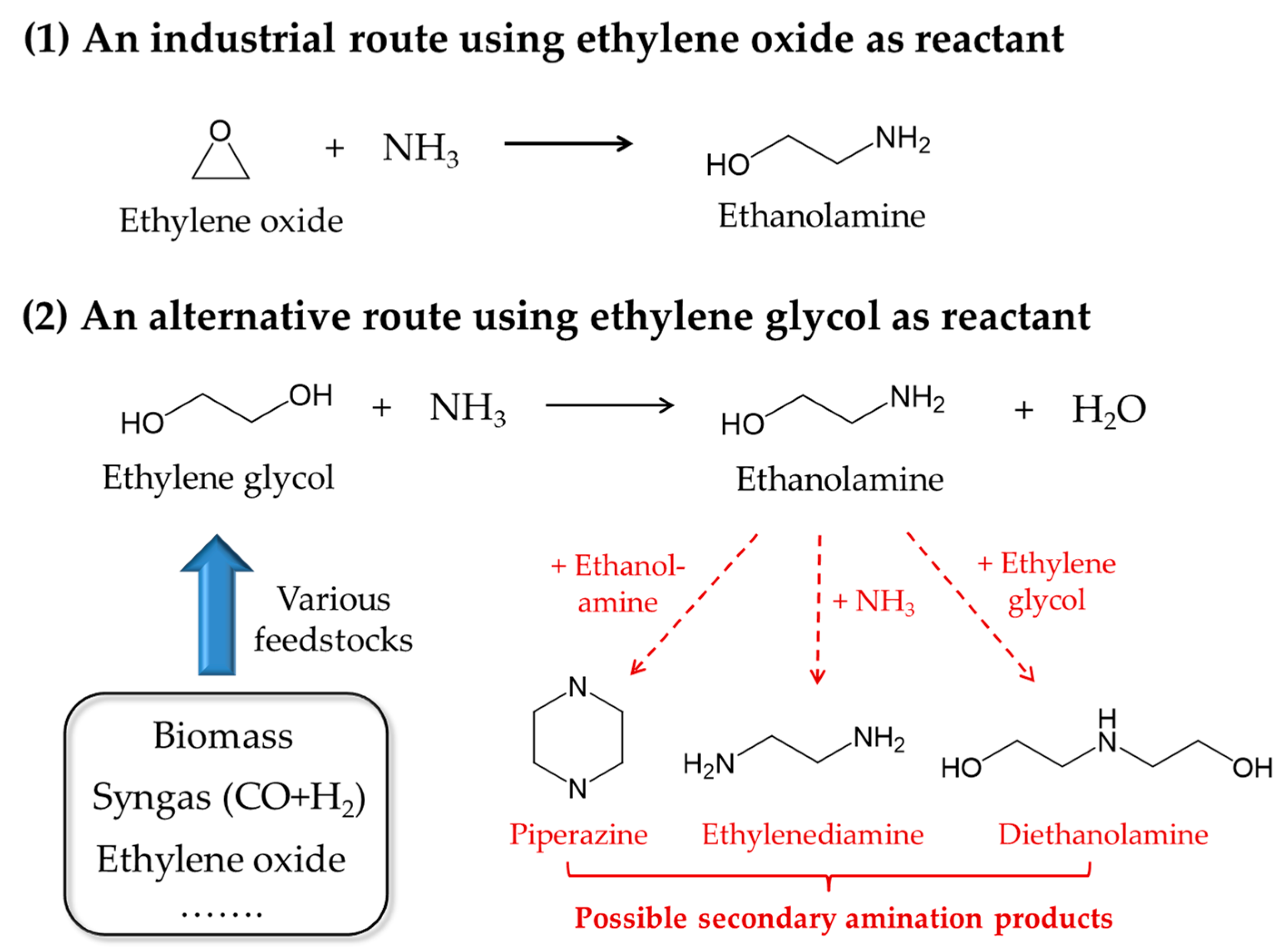
1. Introduction
2. Results and Discussion
2.1. Preparation and Caharacterization of Co/γ-Al2O3 Catalysts with Different Co Particle Sizes
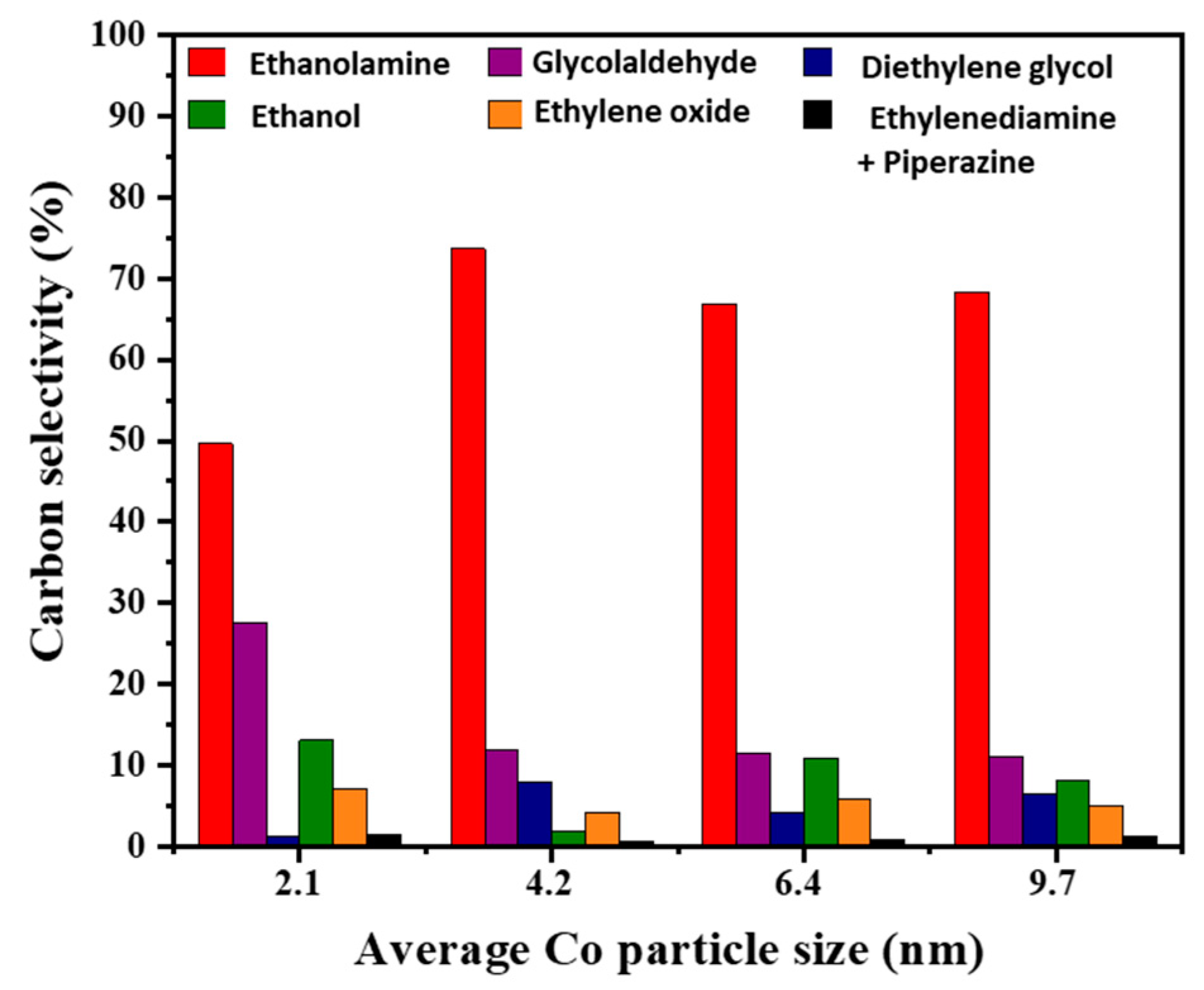
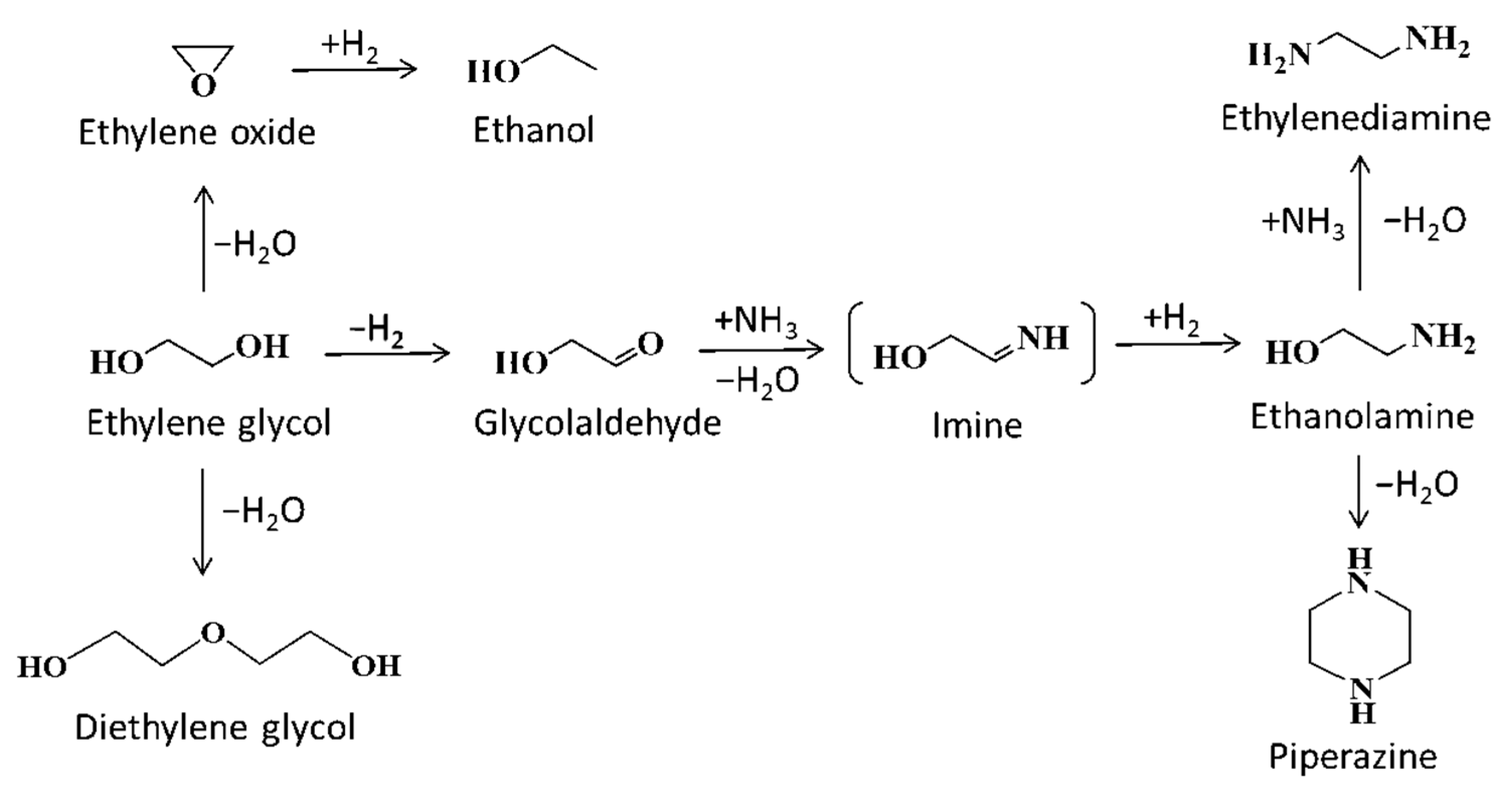
2.2. Effects of Co Particle Size for Co/γ-Al2O3 Catalysts in Ammonolysis of Ethylene Glycol
2.3. Preparation and Caharacterization of Co/γ-Al2O3 Catalysts Doped by a Second Metal
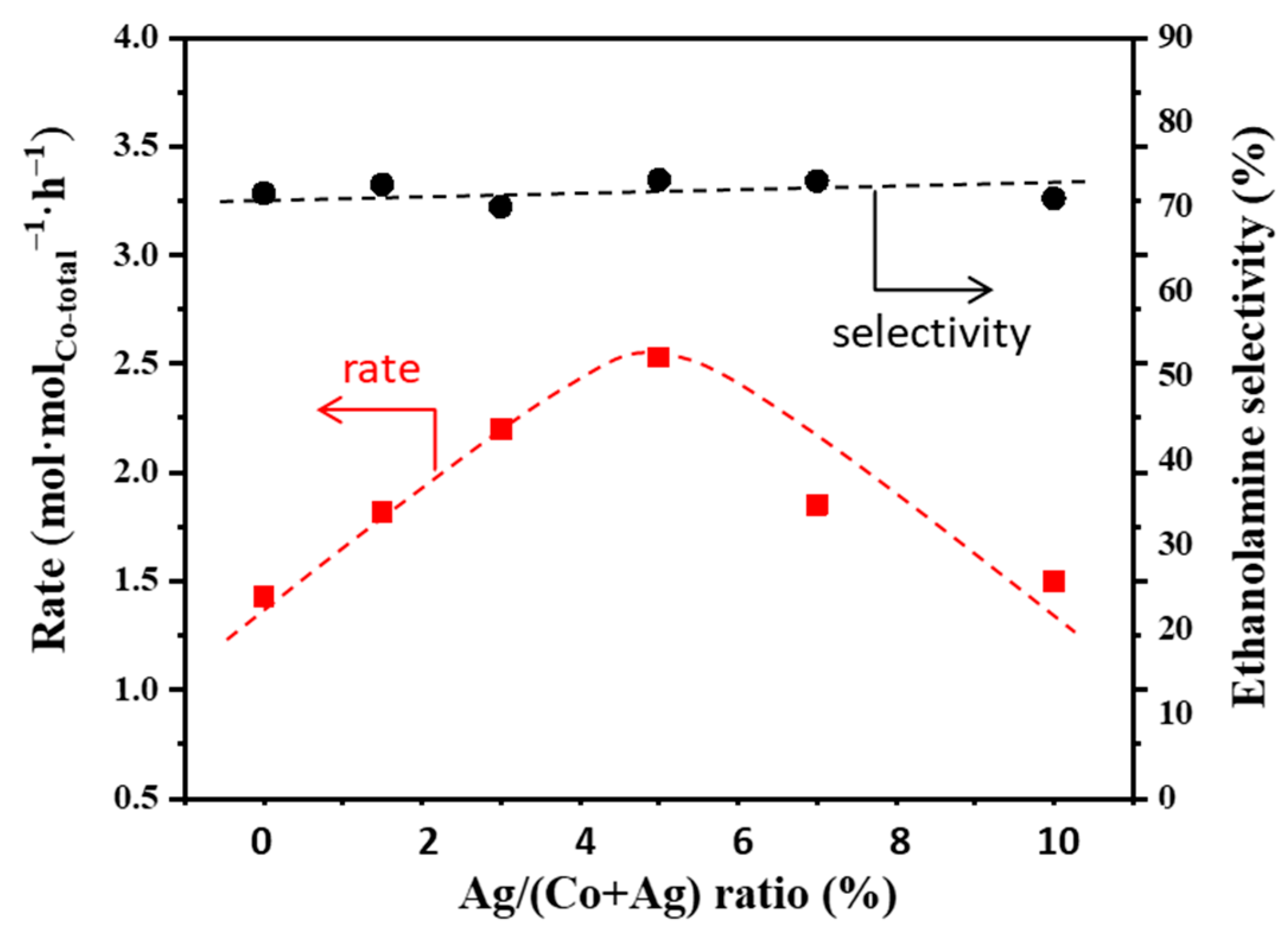
2.4. Effects of Doping a Second Metal for Co/γ-Al2O3 Catalysts in Ammonolysis of Ethylene Glycoy
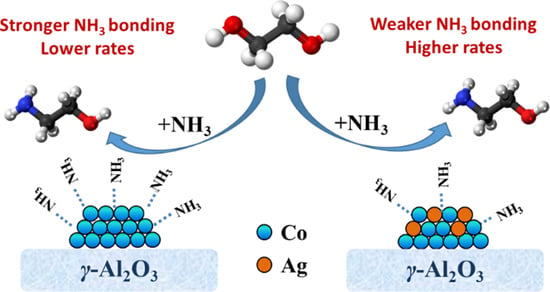
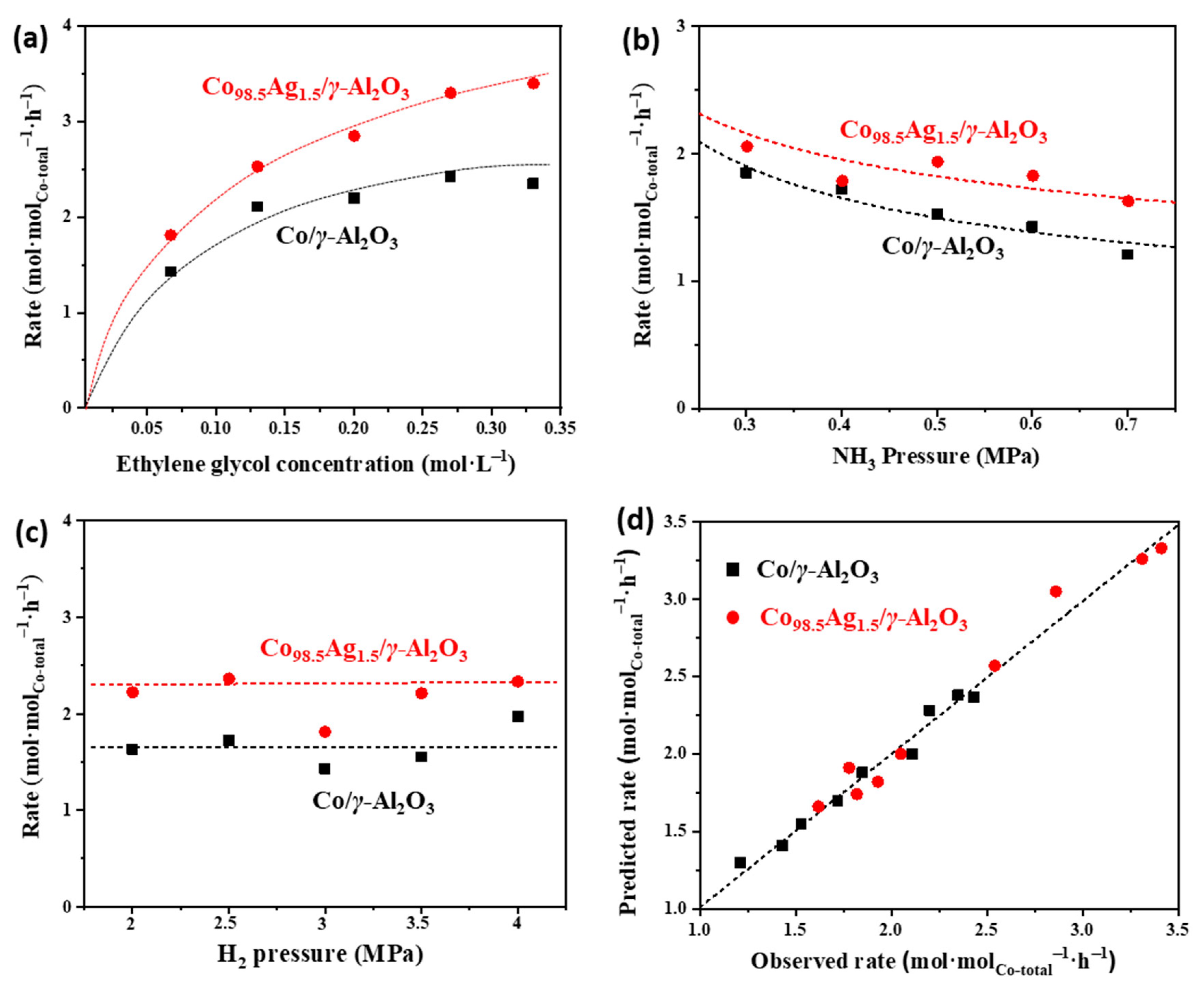
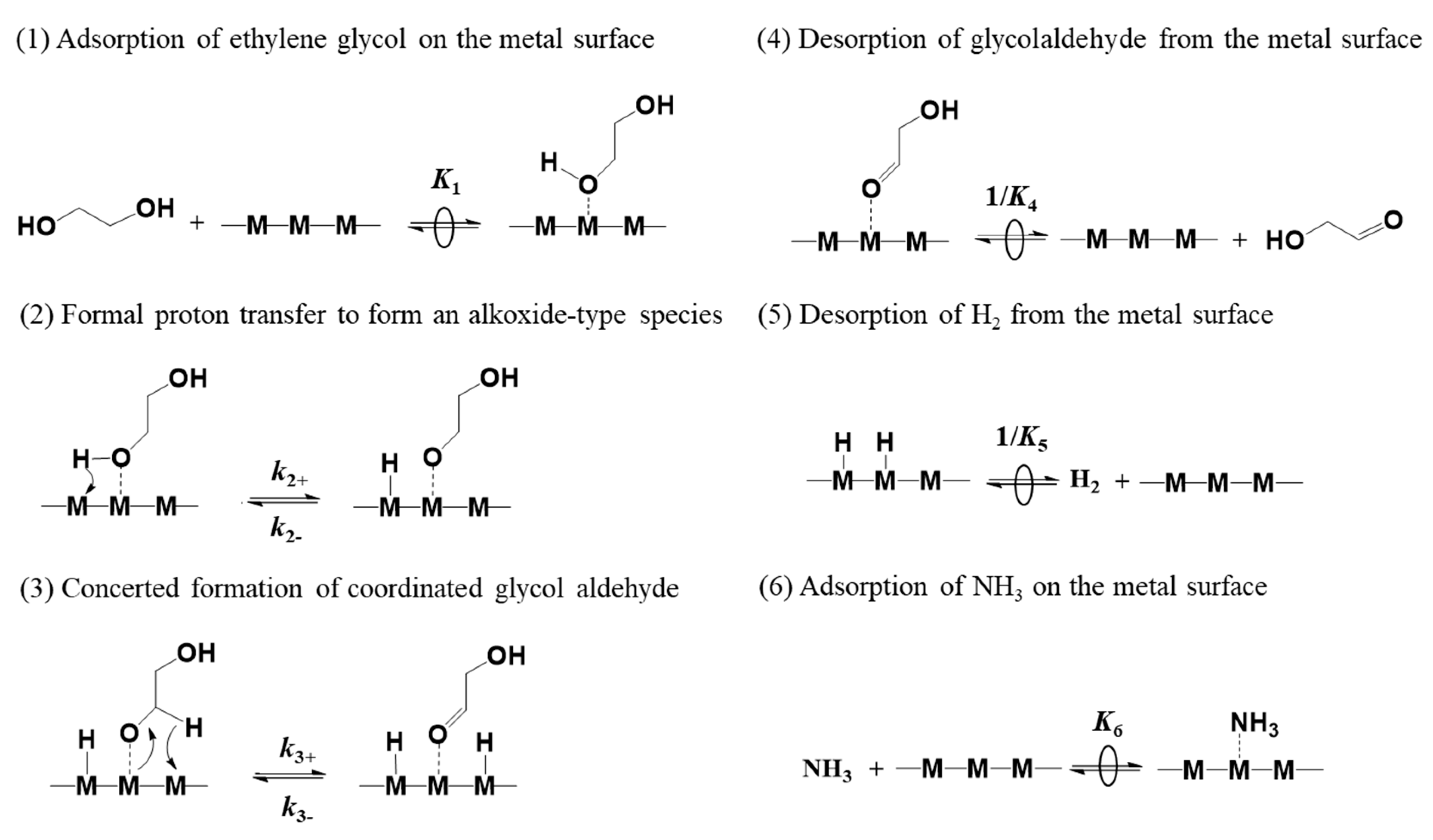
2.5. Kinetic Insights into the Ag-Doping Effects on Co/γ-Al2O3 Catalysts
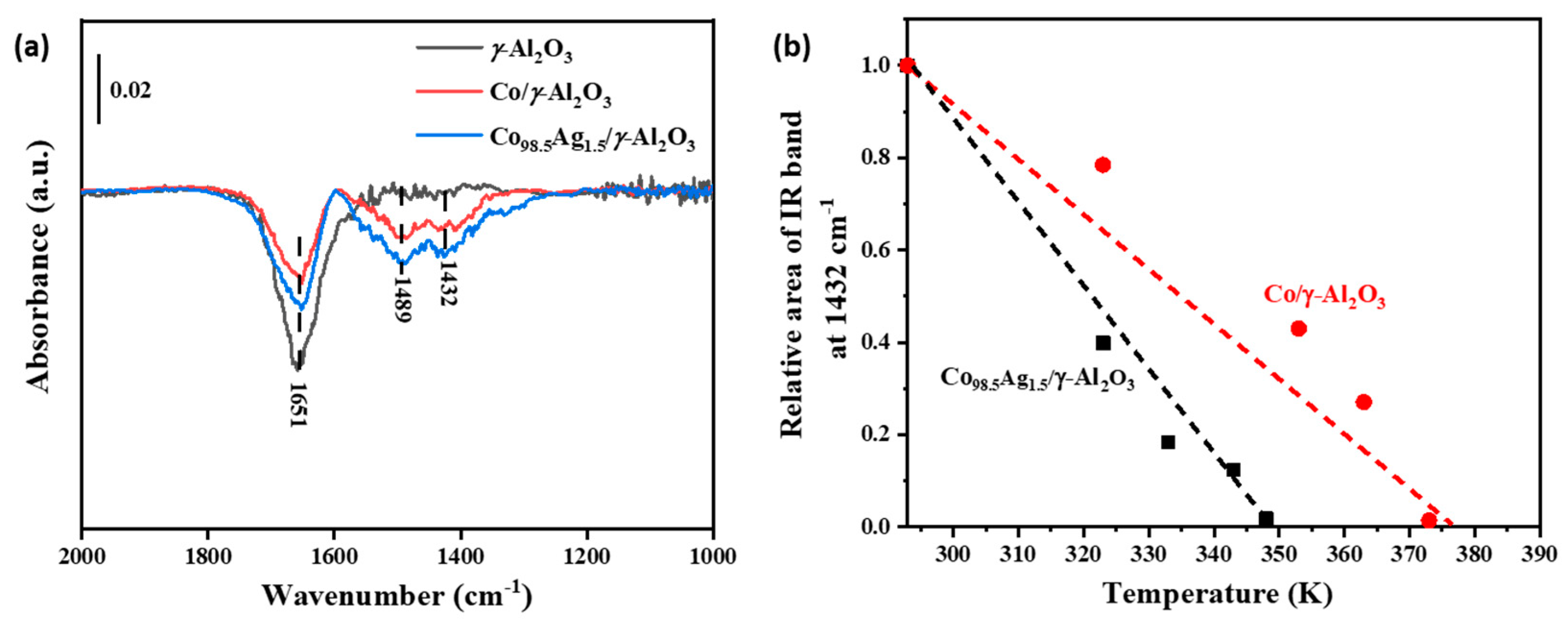
2.6. Spectroscopic Evidence for the Ag-Doping Effect on the Stability of Adsorbed NH3 Species
3. Experimental
3.1. Preparation of Supported Co Catalysts
3.2. Catalyst Characterization
3.3. Catalytic Ammonolysis of Ethylene Glycol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yue, H.; Zhao, Y.; Ma, X.; Gong, J. Ethylene glycol: Properties, synthesis, and applications. Chem. Soc. Rev. 2012, 41, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, A.N.; Oncken, M.P.; Filho, R.M.; Maciel, M.R.W. A roadmap for renewable C2–C3 glycols production: A process engineering approach. Green Chem. 2019, 21, 5168–5194. [Google Scholar] [CrossRef]
- Wang, Z.-Q.; Sun, J.; Xu, Z.-N.; Guo, G.-C. CO direct esterification to dimethyl oxalate and dimethyl carbonate: The key functional motifs for catalytic selectivity. Nanoscale 2020, 12, 20131–20140. [Google Scholar] [CrossRef]
- Faveere, W.H.; van Praet, S.; Vermeeren, B.; Dumoleijn, K.N.R.; Moonen, K.; Taarning, E.; Sels, B.F. Toward replacing ethylene oxide in a sustainable world: Glycolaldehyde as bio-based C2 platform molecule. Angew. Chem. Int. Ed. 2020, 59, 2–22. [Google Scholar]
- Liang, G.; Wang, A.; Li, L.; Xu, G.; Yan, N.; Zhang, T. Production of Primary Amines by Reductive Amination of Biomass-Derived Aldehydes/Ketones. Angew. Chem. Int. Ed. 2017, 56, 3050–3054. [Google Scholar] [CrossRef]
- Fischer, A.; Mallat, T.; Baiker, A. Amination of diols and polyols to acyclic amines. Catal. Today 1997, 37, 167–189. [Google Scholar] [CrossRef]
- Pelckmans, M.; Renders, T.; Van de Vyver, S.; Sels, B.F. Bio-based amines through sustainable heterogeneous catalysis. Green Chem. 2017, 19, 5303–5331. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Shi, F. 7-Alcohol Amination for N-Alkyl Amine Synthesis with Heterogeneous Catalysts. Process. Chem. 2020, 32, 162–178. [Google Scholar]
- Wu, Y.; Yuan, H.; Shi, F. Sustainable Catalytic Amination of Diols: From Cycloamination to Monoaminatio. ACS Sustain. Chem Eng. 2018, 6, 1061–1067. [Google Scholar] [CrossRef]
- Ho, C.R.; Defalque, V.; Zheng, S.; Bell, A.T. Propanol Amination over Supported Nickel Catalysts: Reaction Mechanism and Role of the Support. ACS Catalysis 2019, 9, 2931–2939. [Google Scholar] [CrossRef]
- Shimizu, K.-I.; Kon, K.; Onodera, W.; Yamazaki, H.; Kondo, J. Heterogeneous Ni Catalyst for Direct Synthesis of Primary Amines from Alcohols and Ammonia. ACS Catalysis 2012, 3, 112–117. [Google Scholar] [CrossRef]
- Bassili, V.A.; Baiker, A. Catalytic amination of 1-methoxy-2-propanol over silica supported nickel: Study of the influence of the reaction parameters. Appl. Catal. 1990, 65, 293–308. [Google Scholar] [CrossRef]
- Pera-Titus, M.; Shi, F. Catalytic Amination of Biomass-Based Alcohols. ChemSusChem 2014, 7, 720–722. [Google Scholar] [CrossRef] [PubMed]
- Bähn, S.; Imm, S.; Neubert, L.; Zhang, M.; Neumann, H.; Beller, M. The Catalytic Amination of Alcohols. ChemCatChem 2011, 3, 1853–1864. [Google Scholar] [CrossRef]
- Guillena, G.; Ramon, D.J.; Yus, M. Hydrogen Autotransfer in the N-Alkylation of Amines and Related Compounds using Al-cohols and Amines as Electrophiles. Chem. Rev. 2010, 110, 1611–1641. [Google Scholar] [CrossRef] [PubMed]
- Hamid, M.H.S.A.; Slatford, P.A.; Williams, J.M.J. Borrowing Hydrogen in the Activation of Alcohols. Adv. Synth. Catal. 2007, 349, 1555–1575. [Google Scholar] [CrossRef]
- Jagadeesh, R.V.; Stemmler, T.; Surkus, A.E.; Bauer, M.; Pohl, M.M.; Radnik, J.; Junge, K.; Junge, H.; Bruckner, A.; Beller, M. Co-balt-based nanocatalysts for green oxidation and hydrogenation processes. Nat. Protoc. 2015, 10, 916–926. [Google Scholar] [CrossRef]
- Jagadeesh, R.V.; Murugesan, K.; Alshammari, A.S.; Neumann, H.; Pohl, M.-M.; Radnik, J.; Beller, M. MOF-derived cobalt na-noparticles catalyze a general synthesis of amines. Science 2017, 358, 326–332. [Google Scholar] [CrossRef]
- Murugesan, K.; Chandrashekhar, V.; Senthamarai, T.; Jagadeesh, R.V.; Beller, M. Reductive amination using cobalt-based nanoparticles for synthesis of amines. Nat. Protoc. 2020, 15, 1313–1337. [Google Scholar] [CrossRef]
- Hahn, G.; Kunnas, P.; De Jonge, N.; Kempe, R. General synthesis of primary amines via reductive amination employing a reusable nickel catalyst. Nat. Catal. 2018, 2, 71–77. [Google Scholar] [CrossRef]
- Murugesan, K.; Beller, M.; Jagadeesh, R.V. Reusable Nickel Nanoparticles-Catalyzed Reductive Amination for Selective Synthesis of Primary Amines. Angew. Chem. Int. Ed. 2019, 58, 5064–5068. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Du, X.; Wang, H.; Wu, Z.; Li, Y.; Chen, L. Reductive Amination of Triacetoneamine with n-Butylamine Over Cu–Cr–La/γ-Al2O3. Catal. Lett. 2011, 141, 1703–1708. [Google Scholar] [CrossRef]
- Liang, G.; Zhou, Y.; Zhao, J.; Khodakov, A.Y.; Ordomsky, V.V. Structure-Sensitive and Insensitive Reactions in Alcohol Ami-nation over Nonsupported Ru Nanoparticles. ACS Catalysis 2018, 8, 11226–11234. [Google Scholar] [CrossRef]
- Xie, C.; Song, J.; Hua, M.; Hu, Y.; Huang, X.; Wu, H.; Yang, G.; Han, B. Ambient-Temperature Synthesis of Primary Amines via Reductive Amination of Carbonyl Compounds. ACS Catalysis 2020, 10, 7763–7772. [Google Scholar] [CrossRef]
- Furukawa, S.; Suzuki, R.; Komatsu, T. Selective Activation of Alcohols in the Presence of Reactive Amines over Intermetallic PdZn: Efficient Catalysis for Alcohol-Based N-Alkylation of Various Amines. ACS Catalysis 2016, 6, 5946–5953. [Google Scholar] [CrossRef]
- Tong, T.; Guo, W.; Liu, X.; Guo, Y.; Pao, C.-W.; Chen, J.-L.; Hu, Y.; Wang, Y. Dual functions of CoOx decoration in PtCo/CeO2 catalysts for the hydrogen-borrowing amination of alcohols to primary amines. J. Catal. 2019, 378, 392–401. [Google Scholar] [CrossRef]
- Wu, Y.; Gui, W.; Liu, X.; Zhang, L.; Wang, S.; Wang, Z.; Zhang, C. Promotional Effect of Cu for Catalytic Amination of Diethylene Glycol with Tertiarybutylamine over Ni–Cu/Al2O3 Catalysts. Catal. Lett. 2020, 150, 2427–2436. [Google Scholar] [CrossRef]
- Ibáñez, J.; Araque, M.; Paul, S.; Pera-Titus, M. Direct amination of 1-octanol with NH3 over Ag-Co/Al2O3: Promoting effect of the H2 pressure on the reaction rate. Chem. Eng. J. 2019, 358, 1620–1630. [Google Scholar] [CrossRef]
- Wang, T.; Ibañez, J.; Wang, K.; Fang, L.; Sabbe, M.; Michel, C.; Paul, S.; Pera-Titus, M.; Sautet, P. Rational design of selective metal catalysts for alcohol amination with ammonia. Nat. Catal. 2019, 2, 773–779. [Google Scholar] [CrossRef]
- Niu, F.; Bahri, M.; Ersen, O.; Yan, Z.; Kusema, B.T.; Khodakov, A.Y.; Ordomsky, V.V. A multifaceted role of a mobile bismuth promoter in alcohol amination over cobalt catalysts. Green Chem. 2020, 22, 4270–4278. [Google Scholar] [CrossRef]
- Ma, L.; Yan, L.; Lu, A.-H.; Ding, Y. Effects of Ni particle size on amination of monoethanolamine over Ni-Re/SiO2 catalysts. Chin. J. Catal. 2019, 40, 567–579. [Google Scholar] [CrossRef]
- Zhang, C.; Li, S.; Wu, C.; Li, X.; Yan, X. Preparation and Characterization of Pt@Au/Al2O3 Core-Shell Nanoparticles for Toluene Oxidation Reaction. Acta Physico-Chimica Sinica 2020, 36, 1907057. [Google Scholar] [CrossRef]
- Niu, F.; Xie, S.; Yan, Z.; Kusema, B.T.; Ordomsky, V.V.; Khodakov, A.Y. Alcohol amination over titania-supported ruthenium nanoparticles. Catal. Sci. Technol. 2020, 10, 4396–4404. [Google Scholar] [CrossRef]
- Ibáñez, J.; Kusema, B.T.; Paul, S.; Pera-Titus, M.; Abad, J.I. Ru and Ag promoted Co/Al2O3 catalysts for the gas-phase amination of aliphatic alcohols with ammonia. Catal. Sci. Technol. 2018, 8, 5858–5874. [Google Scholar] [CrossRef]
- Jongsomjit, B.; Panpranot, J.; Goodwin, J.G. Co-Support Compound Formation in Alumina-Supported Cobalt Catalysts. J. Catal. 2001, 204, 98–109. [Google Scholar] [CrossRef]
- Najafabadi, A.T.; Khodadadi, A.A.; Parnian, M.J.; Mortazavi, Y. Atomic layer deposited Co/γ-Al2O3 catalyst with enhanced cobalt dispersion and Fischer–Tropsch synthesis activity and selectivity. Appl. Catal. A Gen. 2016, 511, 31–46. [Google Scholar] [CrossRef]
- Van der Grift, C.J.G.; Wielers, A.F.H.; Joghi, B.P.J.; van Beijnum, J.; de Boer, M.; Versluijs-Helder, M.; Geus, J.W. Effect of the reduction treatment on the structure and reactivity of silica-supported copper particles. J. Catal. 1991, 131, 178–189. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, J.; Jia, Y.; Liu, H.; Xia, C.; Liu, H. Selective hydrogenolysis of xylitol to ethylene glycol and propylene glycol over copper catalysts. Appl. Catal. B Environ. 2014, 147, 377–386. [Google Scholar] [CrossRef]
- Wang, Y.; Furukawa, S.; Fu, X.; Yan, N. Organonitrogen Chemicals from Oxygen-Containing Feedstock over Heterogeneous Catalysts. ACS Catalysis 2020, 10, 311–335. [Google Scholar] [CrossRef]
- Baiker, A.; Caprez, W.; Holstein, W.L. Catalytic amination of aliphatic alcohols in the gas and liquid phases: Kinetics and reaction pathway. Ind. Eng. Chem. Prod. Res. Dev. 1983, 22, 217–225. [Google Scholar] [CrossRef]
- Runeberg, J.; Baiker, A.; Kijenski, J. Copper catalyzed amination of ethylene glycol. Appl. Catal. 1985, 17, 309–319. [Google Scholar] [CrossRef]
- Dumon, A.S.; Wang, T.; Ibanez, J.; Tomer, A.; Yan, Z.; Wischert, R.; Sautet, P.; Pera-Titus, M.; Michel, C. Direct n-octanol amination by ammonia on supported Ni and Pd catalysts: Activity is enhanced by “spectator” ammonia adsorbates. Catal. Sci. Technol. 2018, 8, 611–621. [Google Scholar] [CrossRef]
- Yue, C.-J.; Di, K.; Gu, L.-P.; Zhang, Z.-W.; Ding, L.-L. Selective amination of 1,2-propanediol over Co/La3O4 catalyst prepared by liquid-phase reduction. Mol. Catal. 2019, 477, 110539. [Google Scholar] [CrossRef]
- Fu, X.-P.; Han, P.; Wang, Y.-Z.; Wang, S.; Yan, N. Insight into the roles of ammonia during direct alcohol amination over supported Ru catalysts. J. Catal. 2021, 399, 121–131. [Google Scholar] [CrossRef]
- Mamontov, G.V.; Grabchenko, M.V.; Sobolev, V.I.; Zaikovskii, V.I.; Vodyankina, O.V. Ethanol dehydrogenation over Ag-CeO2/SiO2 catalyst: Role of Ag-CeO2 interface. Appl. Catal. A 2016, 528, 161–167. [Google Scholar] [CrossRef]
- Liu, H.; Tan, H.-R.; Tok, E.S.; Jaenicke, S.; Chuah, G.-K. Dehydrogenation of Alcohols over Alumina-Supported Silver Catalysts: The Role of Oxygen in Hydrogen Formation. ChemCatChem 2016, 8, 968–975. [Google Scholar] [CrossRef]
- Hu, H.; Cai, S.; Li, H.; Huang, L.; Shi, L.; Zhang, D. In Situ DRIFTs Investigation of the Low-Temperature Reaction Mechanism over Mn-Doped Co3O4 for the Selective Catalytic Reduction of NOx with NH3. J. Phys. Chem. C 2015, 119, 22924–22933. [Google Scholar] [CrossRef]
- Meng, D.; Zhan, W.; Guo, Y.; Guo, Y.; Wang, L.; Lu, G. A Highly Effective Catalyst of Sm-MnOx for the NH3-SCR of NOx at Low Temperature: Promotional Role of Sm and Its Catalytic Performance. ACS Catalysis 2015, 5, 5973–5983. [Google Scholar] [CrossRef]
- Kim, K.; Park, A.H.K.; Kim, N.H. Silver-Particle-Based Surface-Enhanced Raman Scattering Spectroscopy for Biomolecular Sensing and Recognition. Langmuir 2006, 22, 3421–3427. [Google Scholar] [CrossRef]
- Tada, S.; Yokoyama, M.; Kikuchi, R.; Haneda, T.; Kameyama, H. N2O Pulse Titration of Ni/α-Al2O3 Catalysts: A New Technique Applicable to Nickel Surface-Area Determination of Nickel-Based Catalysts. J. Phys. Chem. C 2013, 117, 14652–14658. [Google Scholar] [CrossRef]
- Jones, R.D.; Bartholomew, C.H. Improved flow technique for measurement of hydrogen chemisorption on metal catalysts. Appl. Catal. 1988, 39, 77–88. [Google Scholar] [CrossRef]
- Martínez, A.; López, C.; Márquez, F.; Díaz, I. Fischer–Tropsch synthesis of hydrocarbons over mesoporous Co/SBA-15 catalysts: The influence of metal loading, cobalt precursor, and promoters. J. Catal. 2003, 220, 486–499. [Google Scholar] [CrossRef]
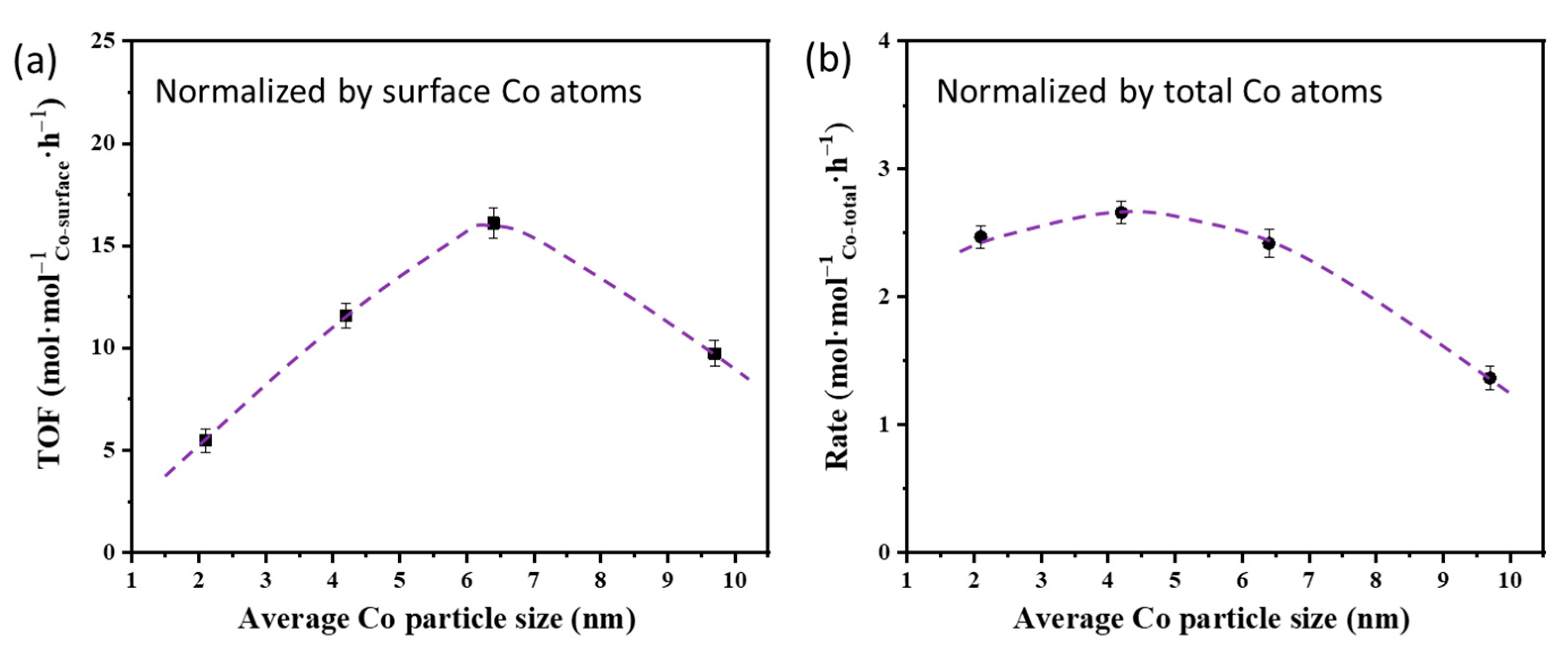
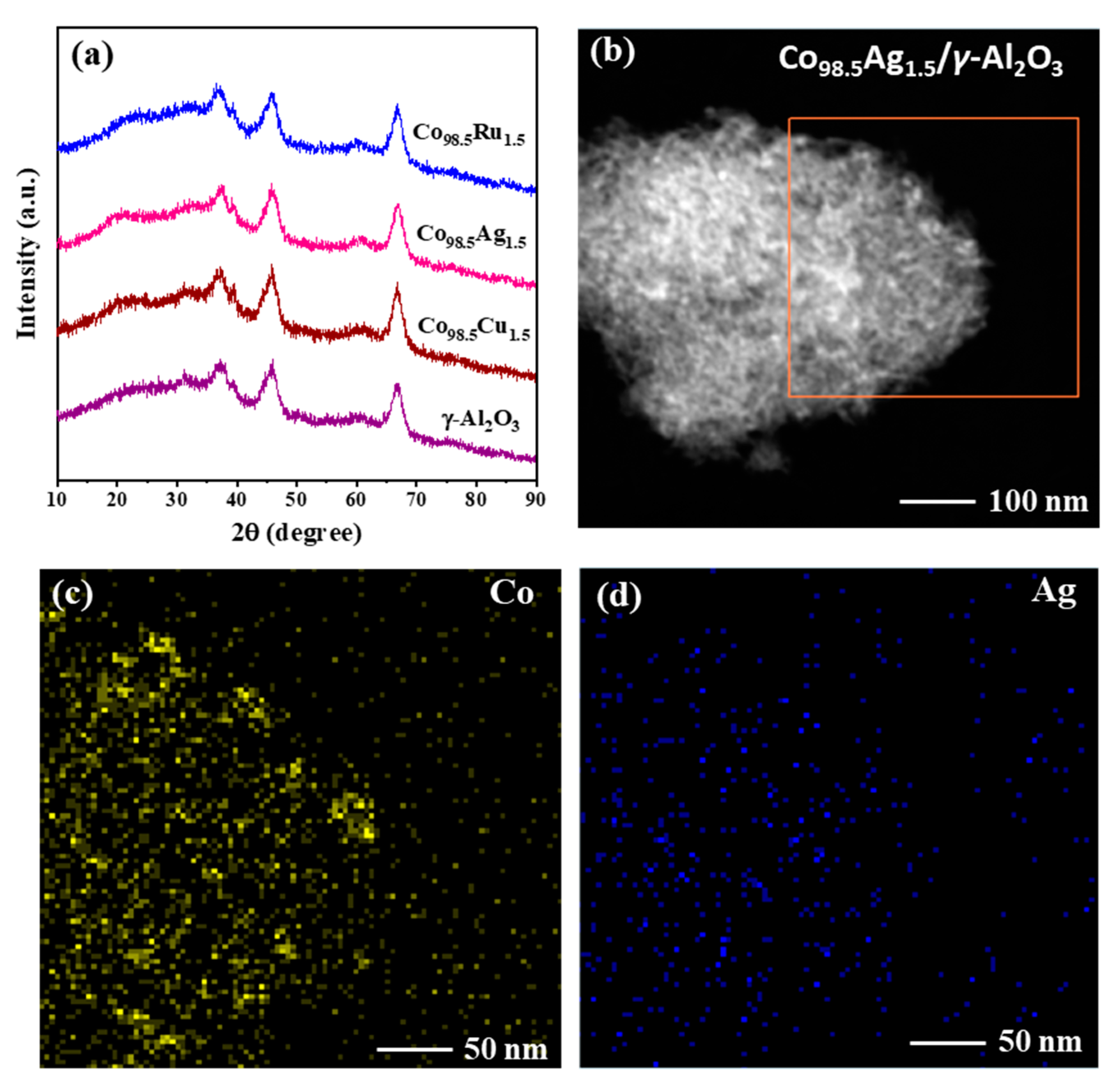
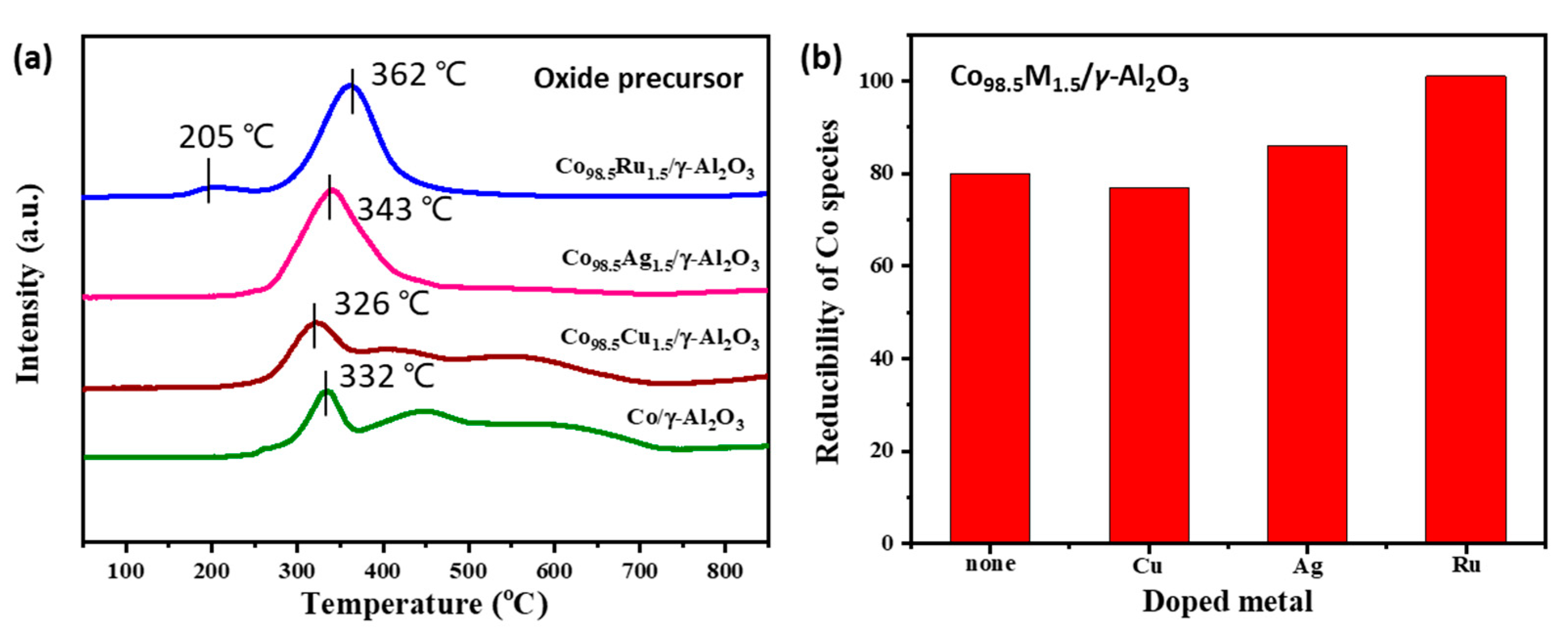
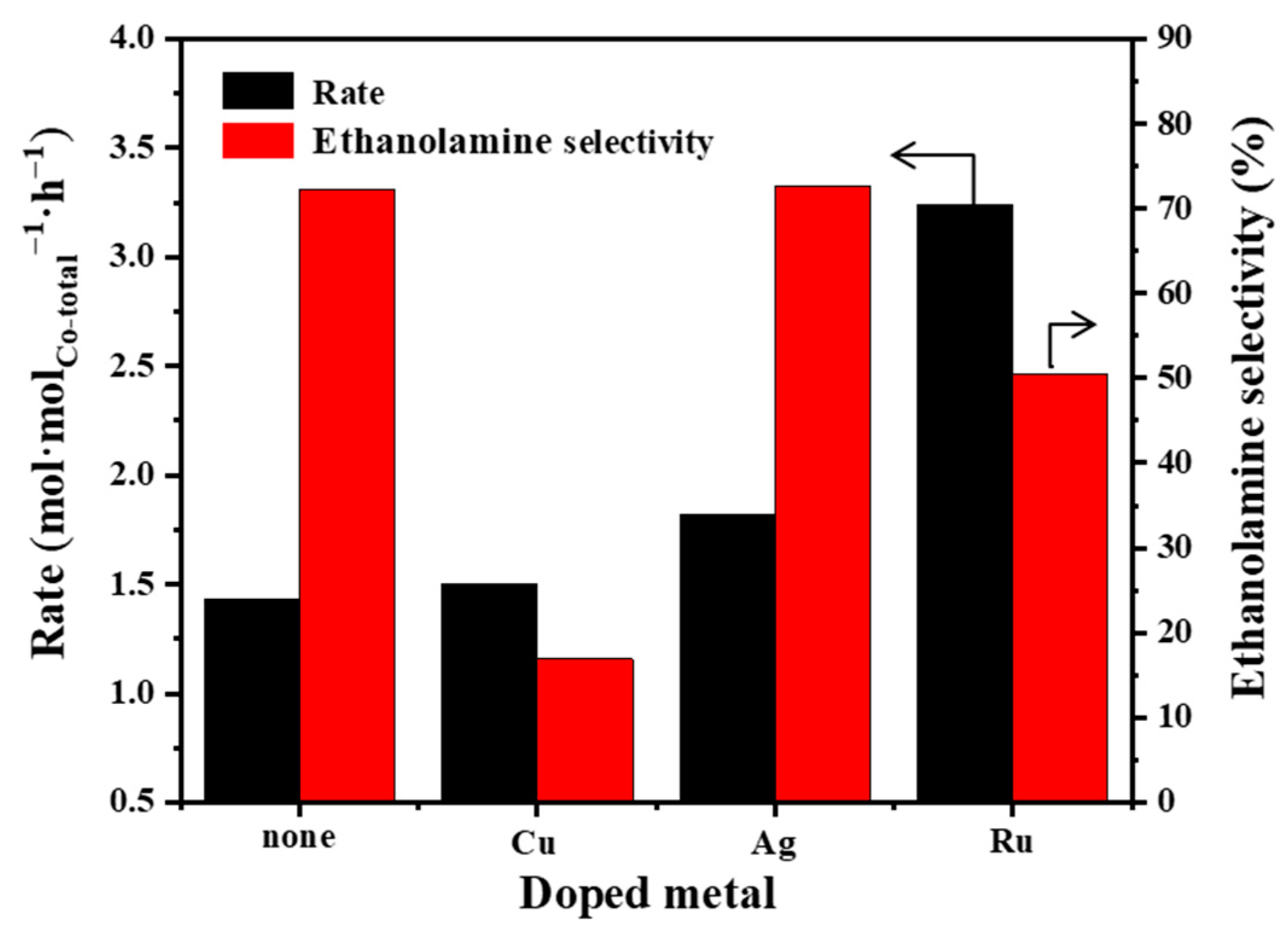
| Catalyst | Co Loading a (wt%) | Co Reducibility b (%) | Co Dispersion c (%) | Average Co Particle Size c (nm) |
|---|---|---|---|---|
| 2 wt% Co/γ-Al2O3 | 1.7 | 40.2 | 44.9 | 2.1 |
| 5 wt% Co/γ-Al2O3 | 4.9 | 80.1 | 23.1 | 4.2 (4.3 ± 0.2 d) |
| 10 wt% Co/γ-Al2O3 | 9.8 | 85.3 | 14.9 | 6.4 |
| 15 wt% Co/γ-Al2O3 | 14.7 | 87.1 | 9.8 | 9.7 |
| Catalyst | k2+ (h−1) | K1 (L∙mol−1) | K6 (MPa−1) | k2 +K1 (L∙mol−1∙h−1) |
|---|---|---|---|---|
| Co/γ-Al2O3 | 1.4 | 4.9 | 8.0 | 6.9 |
| Co98.5Ag1.5/γ-Al2O3 | 1.6 | 3.2 | 3.3 | 5.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, X.; Gu, G.; Hu, Y.; Wang, H.; Zhang, Z.; Wang, S. Structural Requirements for Chemoselective Ammonolysis of Ethylene Glycol to Ethanolamine over Supported Cobalt Catalysts. Catalysts 2021, 11, 736. https://doi.org/10.3390/catal11060736
Lei X, Gu G, Hu Y, Wang H, Zhang Z, Wang S. Structural Requirements for Chemoselective Ammonolysis of Ethylene Glycol to Ethanolamine over Supported Cobalt Catalysts. Catalysts. 2021; 11(6):736. https://doi.org/10.3390/catal11060736
Chicago/Turabian StyleLei, Xianchi, Guoding Gu, Yafei Hu, Haoshang Wang, Zhaoxia Zhang, and Shuai Wang. 2021. "Structural Requirements for Chemoselective Ammonolysis of Ethylene Glycol to Ethanolamine over Supported Cobalt Catalysts" Catalysts 11, no. 6: 736. https://doi.org/10.3390/catal11060736
APA StyleLei, X., Gu, G., Hu, Y., Wang, H., Zhang, Z., & Wang, S. (2021). Structural Requirements for Chemoselective Ammonolysis of Ethylene Glycol to Ethanolamine over Supported Cobalt Catalysts. Catalysts, 11(6), 736. https://doi.org/10.3390/catal11060736