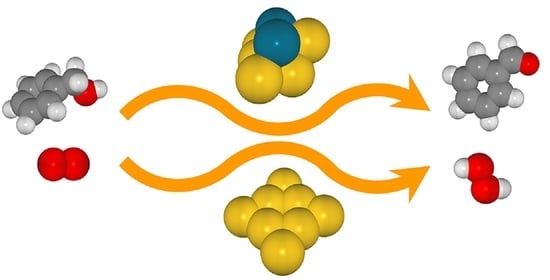
Catalytic Oxidation of Benzyl Alcohol to Benzaldehyde on Au8 and Au6Pd2 Clusters: A DFT Study on the Reaction Mechanism
Abstract
1. Introduction
2. Results and Discussion
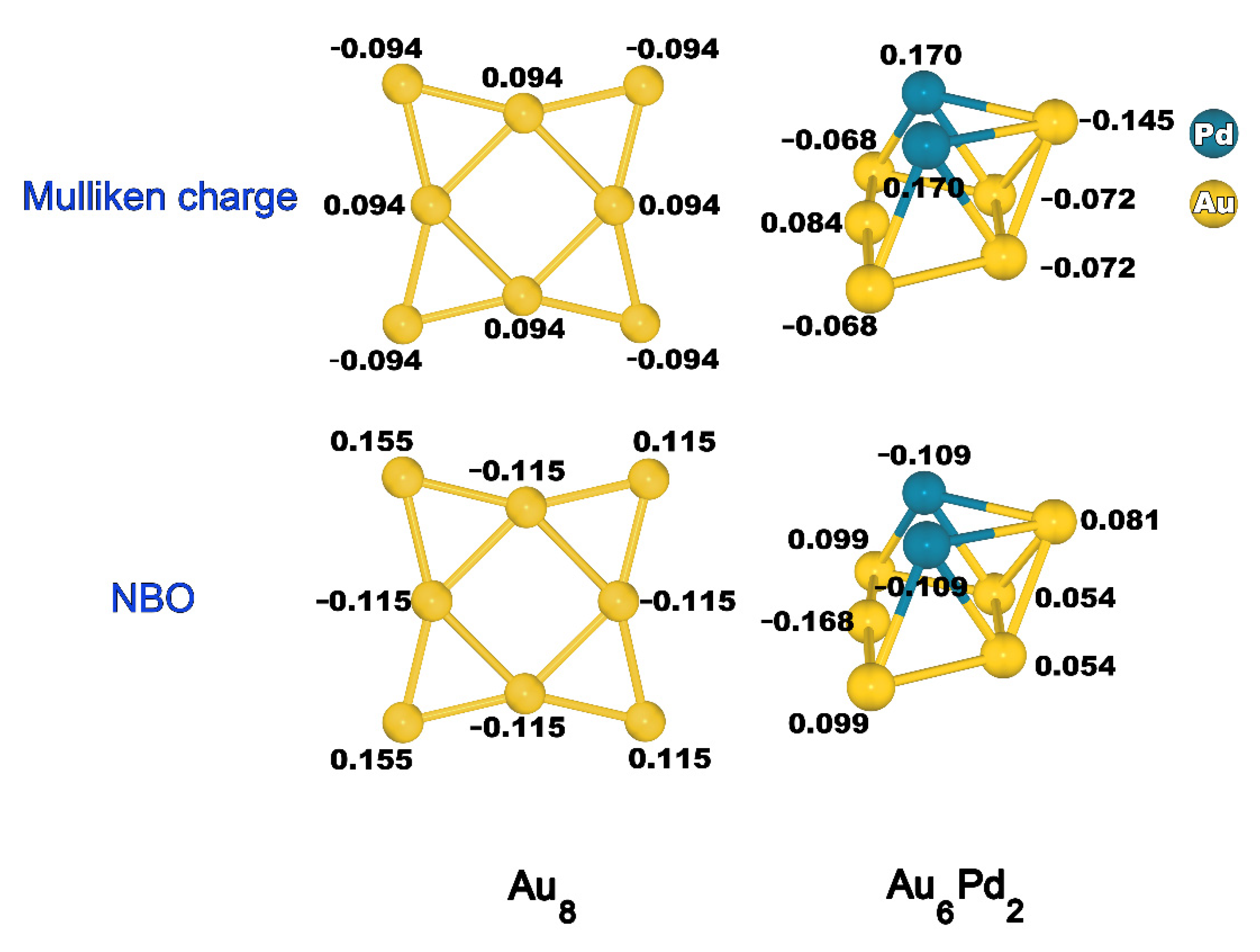
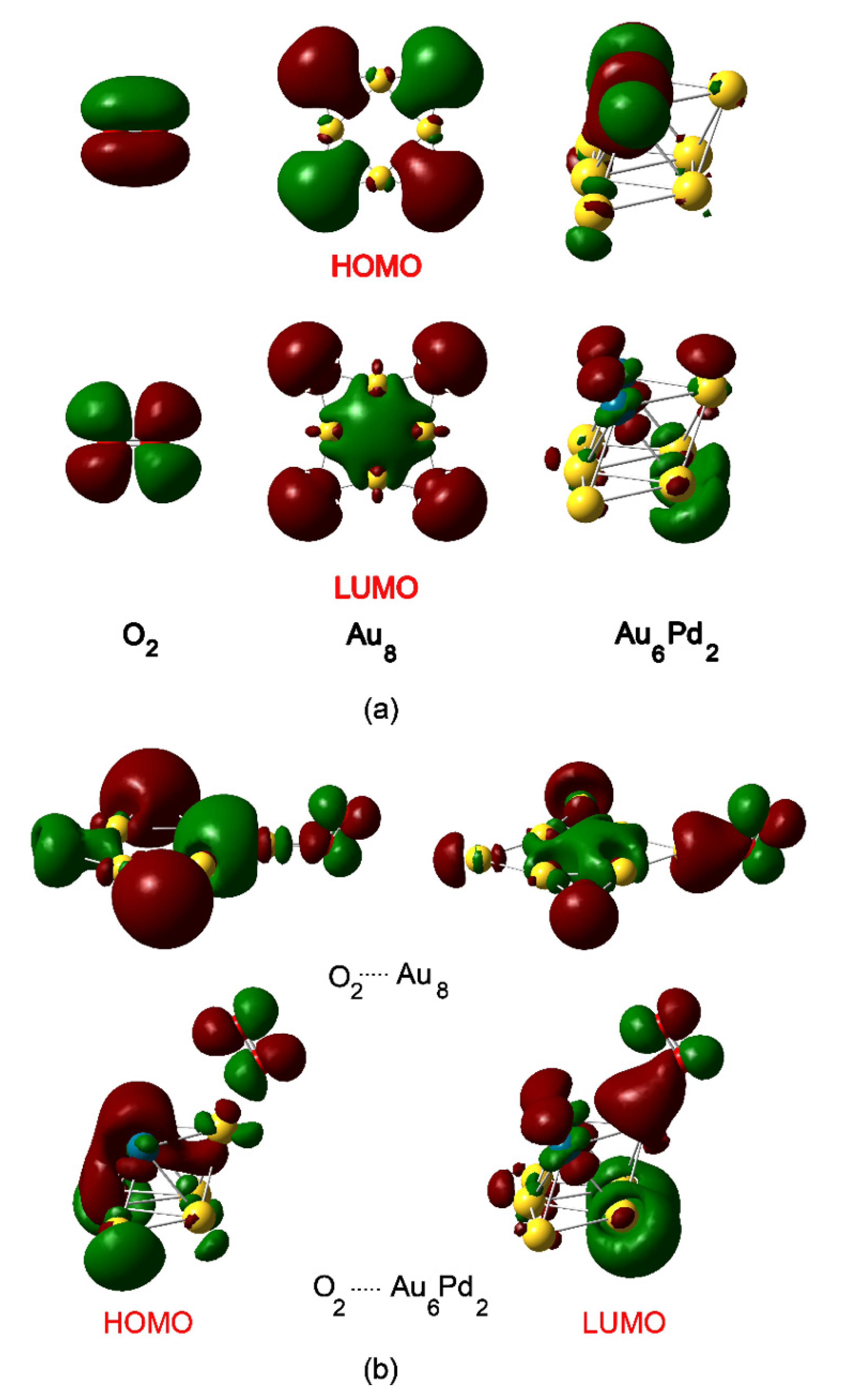
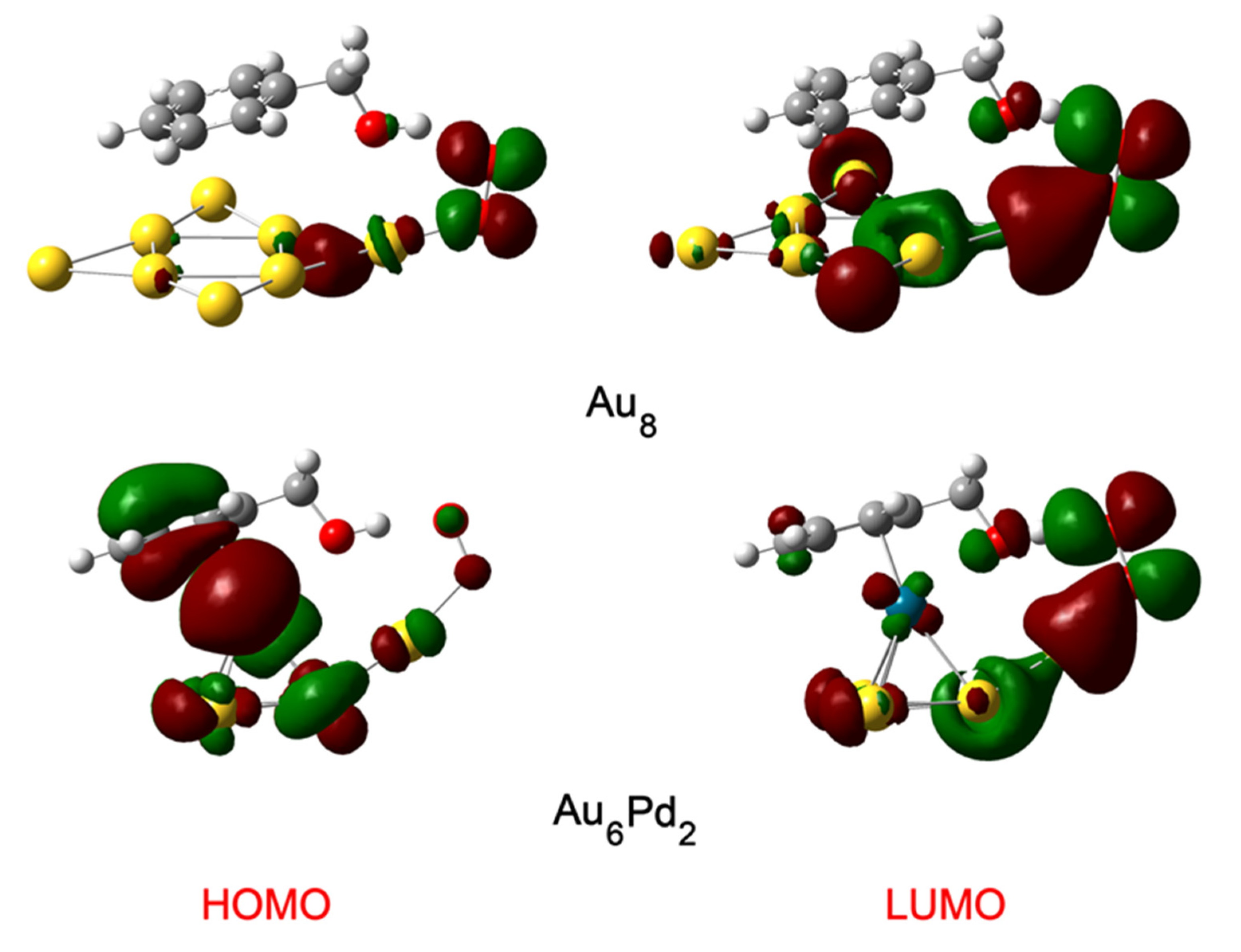
2.1. Structures of Au8 and Au6Pd2
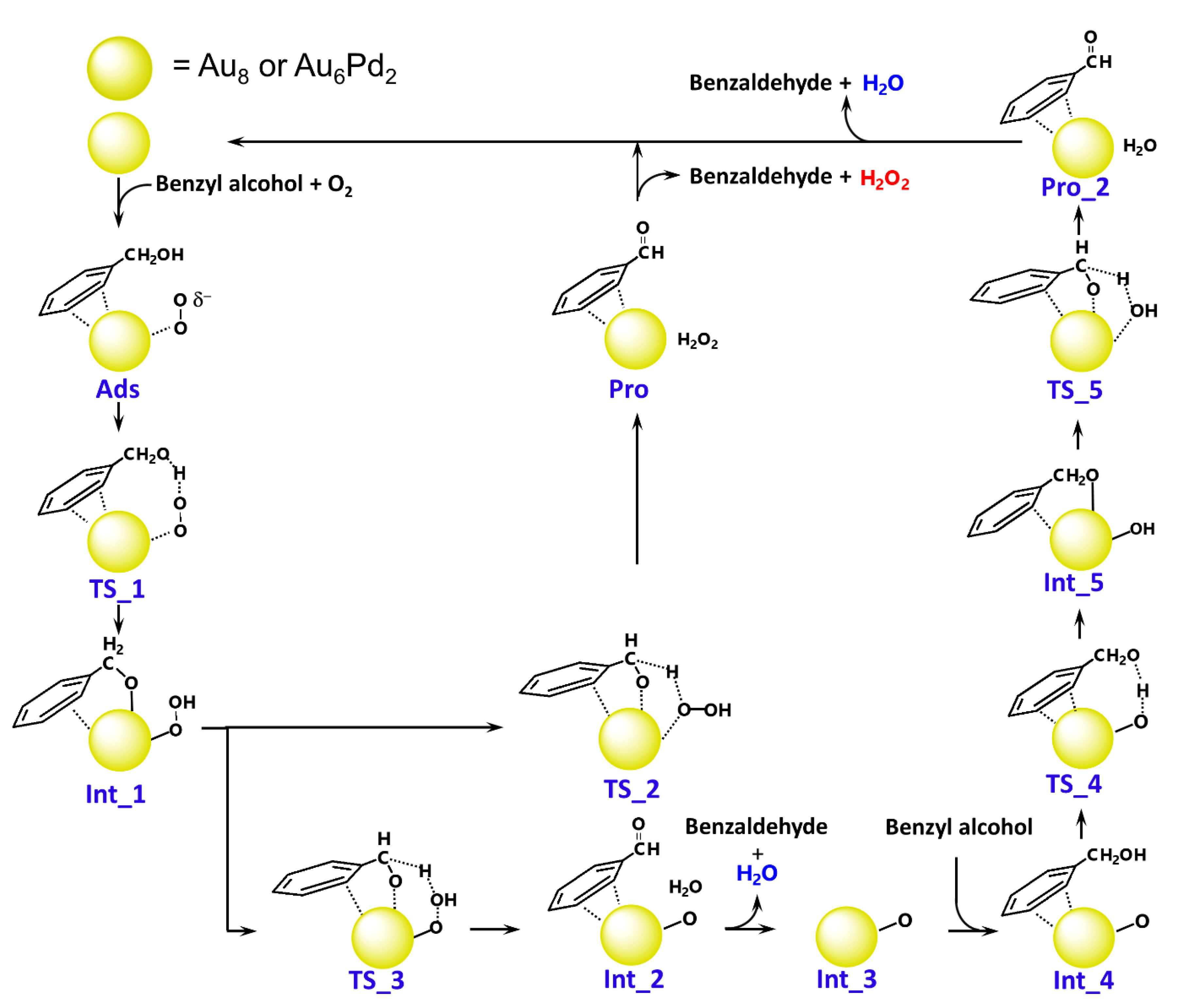
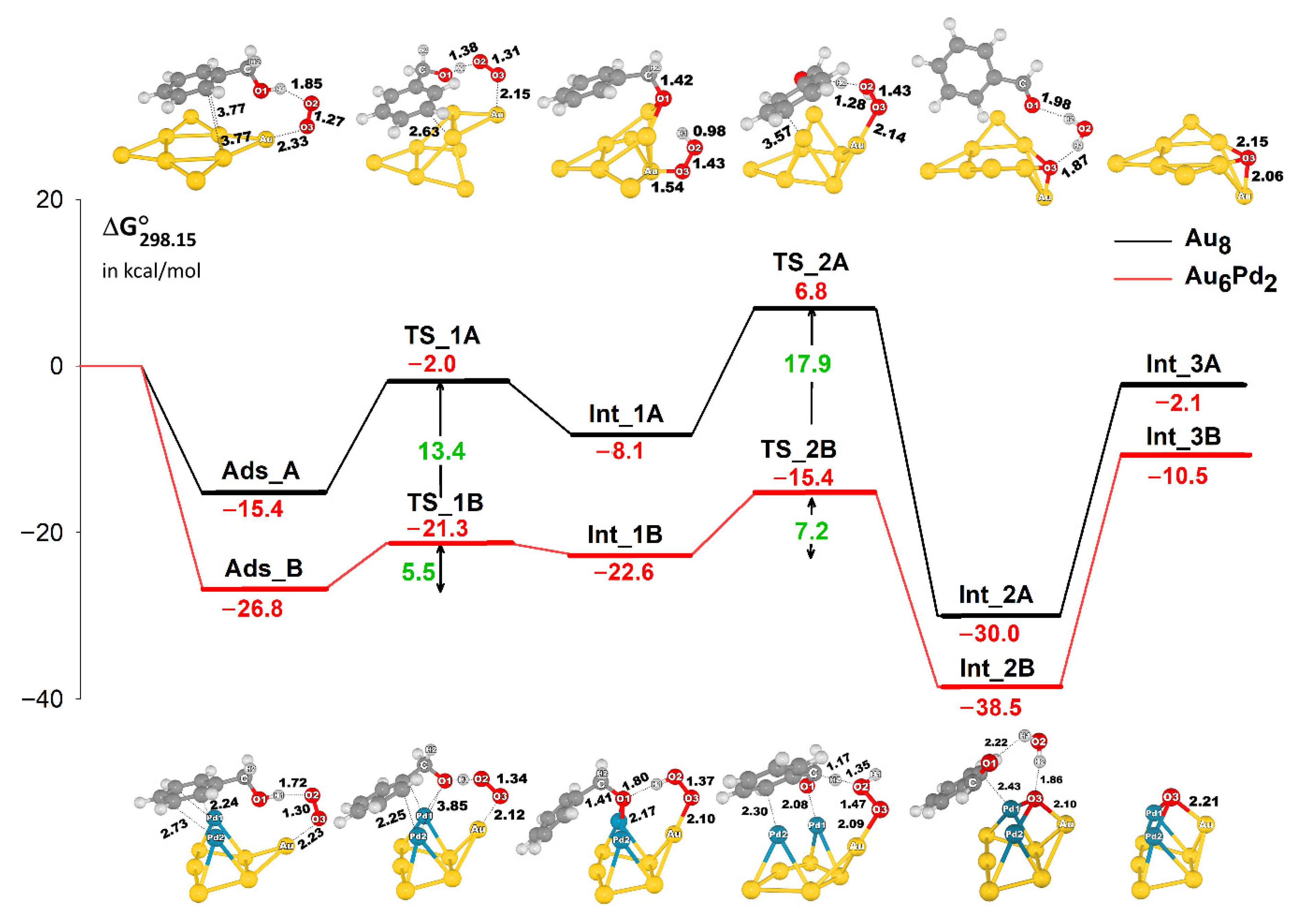
2.2. Pathways and Energetics: The Formation of Benzaldehyde and H2O
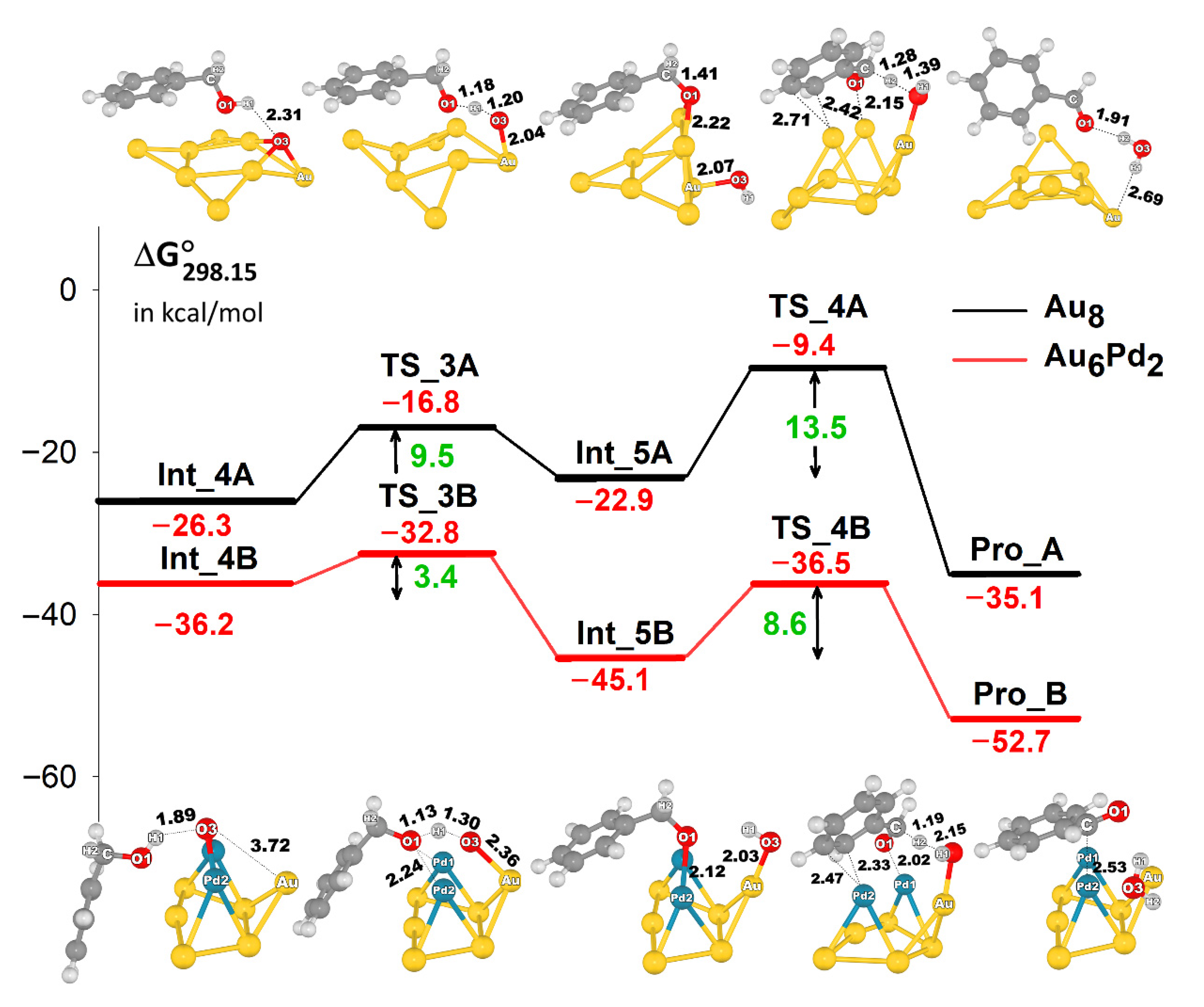
2.3. The Formation of Benzaldehyde and H2O2
3. Computational Details and Models
4. Conclusions
- (1)
- The reaction is initiated by the activation of the O2 molecule on a low-coordinated Au atom forming a peroxide-like species, followed by hydrogen abstraction from the hydroxyl group of benzyl alcohol. Subsequently, C–Hβ bond dissociation occurs to produce either benzaldehyde and H2O2 or benzaldehyde and H2O. The Au cluster is an effective catalyst, marking a significant improvement in the stability of the adsorbed substrates, as observed in the Au–Pd cluster.
- (2)
- The promotional roles of Pd in the Au-Pd cluster involve the enhancement in the electron distribution to the next-nearest-neighbor Au atom, which facilitates the activation of O2 and substantially affects the stability of the adsorption complex and transition states, yielding low-energy barriers compared with the Au cluster.
- (3)
- The reaction preferentially proceeds via deprotonation of the O–H bond of benzyl alcohol followed by C–Hβ bond dissociation, producing benzaldehyde and H2O. Another possible reaction pathway for H2O2 formation can also be responsible for the reasonable catalytic activity of this reaction because of the low energy barrier.
- (4)
- The calculated energy profiles show that the activation of the O–H bond of benzyl alcohol is significantly facilitated by the presence of active oxygen species bound to the catalyst clusters (superoxide-like and chemisorbed atomic oxygen species), whereas the effect is reduced by the barrier for C–Hβ bond dissociation that occurs on both Au and AuPd clusters. Thus, the latter step is considered to be the rate-determining step, which is also in agreement with experimental observations.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, M.; Li, C.; Fang, J. Noble-Metal Based Random Alloy and Intermetallic Nanocrystals: Syntheses and Applications. Chem. Rev. 2021, 121, 736–795. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, Z.; Gong, J. Shape-controlled synthesis of Au–Pd bimetallic nanocrystals for catalytic applications. Chem. Soc. Rev. 2016, 45, 3916–3934. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Liang, F. Au−Pd Nanostars with Low Pd Content: Controllable Preparation and Remarkable Performance in Catalysis. J. Phys. Chem. C 2020, 124, 7812–7822. [Google Scholar] [CrossRef]
- Sankar, M.; Dimitratos, N.; Miedziak, P.J.; Wells, P.P.; Kiely, C.J.; Hutchings, G.J. Designing bimetallic catalysts for a green and sustainable future. Chem. Soc. Rev. 2012, 41, 8099–8139. [Google Scholar] [CrossRef]
- Hutchings, G.J. Nanocrystalline gold and gold palladium alloy catalysts for chemical synthesis. Chem. Commun. 2008, 10, 1148–1164. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J.; Leppert, L.; Welz, H.; Polzer, F.; Wunder, S.; Wanderka, N.; Albrecht, M.; Lunkenbein, T.; Breu, J.; Kummel, S.; et al. Catalytic activity of nanoalloys from gold and palladium. Phys. Chem. Chem. Phys. 2012, 14, 6487–6495. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Jin, R. Heterogeneous catalysis by gold and gold-based bimetal nanoclusters. Nano Today 2018, 18, 86–102. [Google Scholar] [CrossRef]
- Edwards, J.K.; Freakley, S.J.; Carley, A.F.; Kiely, C.J.; Hutchings, G.J. Strategies for Designing Supported Gold–Palladium Bimetallic Catalysts for the Direct Synthesis of Hydrogen Peroxide. Acc. Chem. Res. 2014, 47, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Freakley, S.J.; Piccinini, M.; Edwards, J.K.; Ntainjua, E.N.; Moulijn, J.A.; Hutchings, G.J. Effect of Reaction Conditions on the Direct Synthesis of Hydrogen Peroxide with a AuPd/TiO2 Catalyst in a Flow Reactor. ACS Catal. 2013, 3, 487–501. [Google Scholar] [CrossRef]
- Edwards, J.K.; Pritchard, J.; Lu, L.; Piccinini, M.; Shaw, G.; Carley, A.F.; Morgan, D.J.; Kiely, C.J.; Hutchings, G.J. The Direct Synthesis of Hydrogen Peroxide Using Platinum-Promoted Gold–Palladium Catalysts. Angew. Chem. Int. Ed. 2014, 53, 2381–2384. [Google Scholar] [CrossRef] [PubMed]
- Ricciardulli, T.; Gorthy, S.; Adams, J.S.; Thompson, C.; Karim, A.M.; Neurock, M.; Flaherty, D.W. Effect of Pd Coordination and Isolation on the Catalytic Reduction of O2 to H2O2 over PdAu Bimetallic Nanoparticles. J. Am. Chem. Soc. 2021, 143, 5445–5464. [Google Scholar] [CrossRef]
- Neurock, M.; Tysoe, W.T. Mechanistic Insights in the Catalytic Synthesis of Vinyl Acetate on Palladium and Gold/Palladium Alloy Surfaces. Top. Catal. 2013, 56, 1314–1332. [Google Scholar] [CrossRef]
- Calaza, F.; Li, Z.; Garvey, M.; Neurock, M.; Tysoe, W.T. Reactivity and Selectivity in the Au/Pd(111) Alloy-Catalyzed Vinyl Acetate Synthesis. Catal. Lett. 2013, 143, 756–762. [Google Scholar] [CrossRef]
- Alshammari, H. Solvent-free aerobic oxidation of 1-octene using graphite-supported gold–palladium nanoparticle catalysts. Reac. Kinet. Mech. Cat. 2016, 119, 149–163. [Google Scholar] [CrossRef]
- Bawaked, S.; He, Q.; Dummer, N.F.; Carley, A.F.; Knight, D.W.; Bethell, D.; Kiely, C.J.; Hutchings, G.J. Selective oxidation of alkenes using graphite-supported gold-palladiumcatalysts. Catal. Sci. Technol. 2011, 1, 747–759. [Google Scholar] [CrossRef]
- Cattaneo, S.; Freakley, S.J.; Morgan, D.J.; Sankar, M.; Dimitratos, N.; Hutchings, G.J. Cinnamaldehyde hydrogenation using Au–Pd catalysts prepared by sol immobilisatio. Catal. Sci. Technol. 2018, 8, 1677–1685. [Google Scholar] [CrossRef]
- Liu, J.; Shan, J.; Lucci, F.R.; Cao, S.; Sykes, E.C.H.; Flytzani-Stephanopoulos, M. Palladium–gold single atom alloy catalysts for liquid phase selective hydrogenation of 1-hexyne. Catal. Sci. Technol. 2017, 7, 4276–4284. [Google Scholar] [CrossRef]
- Luneau, M.; Shirman, T.; Filie, A.; Timoshenko, J.; Chen, W.; Trimpalis, A.; Flytzani-Stephanopoulos, M.; Kaxiras, E.; Frenkel, A.I.; Aizenberg, J.; et al. Dilute Pd/Au Alloy Nanoparticles Embedded in Colloid-Templated Porous SiO2: Stable Au-Based Oxidation Catalysts. Chem. Mater. 2019, 31, 5759–5768. [Google Scholar] [CrossRef]
- Han, S.; Mullins, C.B. Catalytic Reactions on Pd–Au Bimetallic Model Catalysts. Acc. Chem. Res. 2021, 54, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Dhital, R.N.; Kamonsatikul, C.; Somsook, E.; Bobuatong, K.; Ehara, M.; Karanjit, S.; Sakurai, H. Low-Temperature Carbon-Chlorine Bond Activation by Bimetallic Gold/Palladium Alloy Nanoclusters: An Application to Ullmann Coupling. J. Am. Chem. Soc. 2012, 134, 20250–20253. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Sarina, S.; Jaatinen, E.; Jia, J.; Arnold, D.P.; Liu, H.; Zhu, H. Efficient photocatalytic Suzuki cross-coupling reactions on Au–Pd alloy nanoparticles under visible light irradiation. Green Chem. 2014, 16, 4272–4285. [Google Scholar] [CrossRef]
- Kadu, B.S. Suzuki–Miyaura cross coupling reaction: Recent advancements in catalysis and organic synthesis. Catal. Sci. Technol. 2021, 11, 1186–1221. [Google Scholar] [CrossRef]
- Meenakshisundaram, S.; Nowicka, E.; Miedziak, P.J.; Brett, G.L.; Jenkins, R.L.; Dimitratos, N.; Taylor, S.H.; Knight, D.W.; Bethell, D.; Hutchings, G.J. Oxidation of alcohols using supported gold and gold–palladium nanoparticles. Faraday Discuss. 2010, 145, 341–356. [Google Scholar] [CrossRef]
- Zhu, X.; Guo, Q.; Sun, Y.; Chen, S.; Wang, J.-Q.; Wu, M.; Fu, W.; Tang, Y.; Duan, X.; Chen, D.; et al. Optimising surface d charge of AuPd nanoalloy catalysts for enhanced catalytic activity. Nat. Commun. 2019, 10, 1428–1438. [Google Scholar] [CrossRef]
- McClure, J.P.; Boltersdorf, J.; Baker, D.R.; Farinha, T.G.; Dzuricky, N.; Villegas, C.E.P.; Rocha, A.R.; Leite, M.S. Structure–Property-Performance Relationship of Ultrathin Pd–Au Alloy Catalyst Layers for Low-Temperature Ethanol Oxidation in Alkaline Media. ACS Appl. Mater. Interfaces 2019, 11, 24919–24932. [Google Scholar] [CrossRef]
- Enache, D.I.; Edwards, J.K.; Landon, P.; Solsona-Espriu, B.; Carley, A.F.; Herzing, A.A.; Watanabe, M.; Kiely, C.J.; Knight, D.W.; Hutchings, G.J. Solvent-Free Oxidation of Primary Alcohols to Aldehydes Using Au-Pd/TiO2 Catalysts. Science 2006, 311, 362–365. [Google Scholar] [CrossRef]
- Evangelisti, C.; Schiavi, E.; Aronica, L.A.; Caporusso, A.M.; Vitulli, G.; Bertinetti, L.; Martra, G.; Balerna, A.; Mobilio, S. Bimetallic Gold–Palladium vapour derived catalysts: The role of structural features on their catalytic activity. J. Catal. 2012, 286, 224–236. [Google Scholar] [CrossRef]
- Li, X.; Feng, J.; Perdjon, M.; Oh, R.; Zhao, W.; Huang, X.; Liu, S. Investigations of supported Au-Pd nanoparticles on synthesized CeO2 with different morphologies and application in solvent-free benzyl alcohol oxidation. Appl. Surf. Sci. 2020, 505, 144473. [Google Scholar] [CrossRef]
- Tareq, S.; Yap, Y.H.T.; Saleh, T.A.; Abdullah, A.H.; Rashid, U.; Saiman, M.I. Synthesis of bimetallic gold-pallidum loaded on carbon as efficient catalysts for the oxidation of benzyl alcohol into benzaldehyde. J. Mol. Liq. 2018, 271, 885–891. [Google Scholar] [CrossRef]
- Wang, Z.; Song, Y.; Zou, J.; Li, L.; Yu, Y.; Wu, L. The cooperation effect in the Au-Pd/LDH for promoting photocatalytic selective oxidation of benzyl alcohol. Catal. Sci. Technol. 2018, 8, 268–275. [Google Scholar] [CrossRef]
- Cui, W.; Xiao, Q.; Sarina, S.; Ao, W.; Xie, M.; Zhu, H.; Bao, Z. Au–Pd alloy nanoparticle catalyzed selective oxidation of benzyl alcohol and tandem synthesis of imines at ambient conditions. Catal. Today 2014, 235, 152–159. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, Z.; Wang, J.; Fu, W.; Tan, R.; Yin, D. Selective Aerobic Oxidation of Alcohols over Gold-Palladium Alloy Catalysts Using Air at Atmospheric Pressure in Water. ChemCatChem 2019, 11, 1779–1788. [Google Scholar] [CrossRef]
- Lee, A.F.; Ellis, C.V.; Wilson, K.; Hondow, N.S. In situ studies of titania-supported Au shell–Pd core nanoparticles for the selective aerobic oxidation of crotyl alcohol. Catal. Today 2010, 157, 243–249. [Google Scholar] [CrossRef]
- Ksar, F.A.; Ramos, L.; Keita, B.; Nadjo, L.; Beaunier, P.; Remita, H. Bimetallic Palladium−Gold Nanostructures: Application in Ethanol Oxidation. Chem. Mater. 2009, 21, 3677–3683. [Google Scholar] [CrossRef]
- Brett, G.L.; He, Q.; Hammond, C.; Miedziak, P.J.; Dimitratos, N.; Sankar, M.; Herzing, A.A.; Conte, M.; Lopez-Sanchez, J.A.; Kiely, C.J.; et al. Selective Oxidation of Glycerol by Highly Active Bimetallic Catalysts at Ambient Temperature under Base-Free Conditions. Angew. Chem. Int. Ed. 2011, 50, 10136–10139. [Google Scholar] [CrossRef]
- Jiang, T.; Jia, C.; Zhang, L.; He, S.; Sang, Y.; Li, H.; Li, Y.; Xu, X.; Liu, H. Gold and gold–palladium alloy nanoparticles on heterostructured TiO2 nanobelts as plasmonic photocatalysts for benzyl alcohol oxidation. Nanoscale 2015, 7, 209–217. [Google Scholar] [CrossRef]
- Dhital, R.N.; Sakurai, H. Gold– and gold–palladium/poly(1-vinylpyrrolidin-2-one) nanoclusters as quasi-homogeneous catalyst for aerobic oxidation of glycerol. Tetrahedron Lett. 2011, 52, 2633–2637. [Google Scholar]
- Alshammari, H.M.; Alshammari, A.S.; Humaidi, J.R.; Alzahrani, S.A.; Alhumaimess, M.S.; Aldosari, O.F.; Hassan, H.M.A. Au-Pd Bimetallic Nanocatalysts Incorporated into Carbon Nanotubes (CNTs) for Selective Oxidation of Alkenes and Alcohol. Processes 2020, 8, 1380–1390. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Xu, M.; Kunal, P.; Trewyn, B.G. Aerobic oxidative esterification of primary alcohols over Pd-Au bimetallic catalysts supported on mesoporous silica nanoparticles. Catal. Today 2018, 306, 81–88. [Google Scholar] [CrossRef]
- Lee, D.G.; Spitzer, U.A. Aqueous dichromate oxidation of primary alcohols. J. Org. Chem. 1970, 35, 3589–3590. [Google Scholar] [CrossRef]
- Menger, F.M.; Lee, C. Oxidations with solid potassium permanganate. Tetrahedron Lett. 1981, 22, 1655–1656. [Google Scholar] [CrossRef]
- Keresszegi, C.; Bürgi, T.; Mallat, T.; Baiker, A. On the Role of Oxygen in the Liquid-Phase Aerobic Oxidation of Alcohols on Palladium. J. Catal. 2002, 211, 244–251. [Google Scholar] [CrossRef]
- Tsunoyama, H.; Sakurai, H.; Negishi, Y.; Tsukuda, T. Size-Specific Catalytic Activity of Polymer-Stabilized Gold Nanoclusters for Aerobic Alcohol Oxidation in Water. J. Am. Chem. Soc. 2005, 127, 9374–9375. [Google Scholar] [CrossRef]
- Corma, A.; Garcia, H. Supported gold nanoparticles as catalysts for organic reactions. Chem. Soc. Rev. 2008, 37, 2096–2126. [Google Scholar] [CrossRef]
- Pyykkö, P. Theoretical Chemistry of Gold. Angew. Chem. Int. Ed. 2004, 43, 4412–4456. [Google Scholar] [CrossRef]
- Pyykko, P. Theoretical chemistry of gold. III. Chem. Soc. Rev. 2008, 37, 1967–1997. [Google Scholar] [CrossRef]
- Coquet, R.; Howard, K.L.; Willock, D.J. Theory and simulation in heterogeneous gold catalysis. Chem. Soc. Rev. 2008, 37, 2046–2076. [Google Scholar] [CrossRef]
- Hammer, B.; Nørskov, J.K. Theoretical surface science and catalysis—Calculations and concepts. In Advances in Catalysis; Bruce, C., Gates, H.K., Eds.; Academic Press: Cambridge, MA, USA, 2000; Volume 45, pp. 71–129. [Google Scholar]
- Norskov, J.K.; Bligaard, T.; Rossmeisl, J.; Christensen, C.H. Towards the computational design of solid catalysts. Nat. Chem. 2009, 1, 37–46. [Google Scholar] [CrossRef]
- Li, J.; Ishihara, T.; Yoshizawa, K. Theoretical Revisit of the Direct Synthesis of H2O2 on Pd and Au@Pd Surfaces: A Comprehensive Mechanistic Study. J. Phys. Chem. C 2011, 115, 25359–25367. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, H.; Sullivan, M.B.; Lim, F.C.H.; Wu, P. Study of Pd–Au bimetallic catalysts for CO oxidation reaction by DFT calculations. Phys. Chem. Chem. Phys. 2009, 11, 1441–1446. [Google Scholar] [CrossRef]
- Atoa, Y.; Hayashi, A.; Koga, H.; Kawakami, T.; Yamanaka, S.; Okumura, M. Exploring a near-Hartree–Fock–Kohn–Sham approach to study electronic properties of azobenzene in interaction with gold: From clusters to the Au(111) surface. Chem. Phys. Lett. 2019, 724, 15–121. [Google Scholar]
- Miyazaki, R.; Jin, X.; Yoshii, D.; Yatabe, T.; Yabe, T.; Mizuno, N.; Yamaguchi, K.; Hasegawa, J.Y. Mechanistic study of C–H bond activation by O2 on negatively charged Au clusters: α,β-dehydrogenation of 1-methyl-4-piperidone by supported Au catalysts. Catal. Sci. Technol. 2021, 11, 3333–3346. [Google Scholar] [CrossRef]
- Varganov, S.A.; Olson, R.M.; Gordon, M.S.; Metiu, H. The interaction of oxygen with small gold clusters. J. Chem. Phys. 2003, 119, 2531–2537. [Google Scholar] [CrossRef]
- Madsen, G.K.H.; Hammer, B. Effect of subsurface Ti-interstitials on the bonding of small gold clusters on rutile TiO2(110). J. Chem. Phys. 2009, 130, 044704. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.; Ehara, M.; Piecuch, P. Aerobic oxidation of methanol to formic acid on Au8–: Benchmark analysis based on completely renormalized coupled-cluster and density functional theory calculations. J. Phys. Chem. A 2013, 117, 10416–10427. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. A new local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. J. Chem. Phys. 2006, 125, 194101. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Mantina, M.; Valero, R.; Truhlar, D.G. Validation study of the ability of density functionals to predict the planar-to-three-dimensional structural transition in anionic gold clusters. J. Chem. Phys. 2009, 131, 064705–064706. [Google Scholar] [CrossRef] [PubMed]
- Ferrighi, L.; Hammer, B.; Madsen, G.K.H. 2D−3D transition for cationic and anionic gold clusters: A kinetic energy density functional study. J. Am. Chem. Soc. 2009, 131, 10605–10609. [Google Scholar] [CrossRef]
- Bobuatong, K.; Karanjit, S.; Fukuda, R.; Ehara, M.; Sakurai, H. Aerobic oxidation of methanol to formic acid on Au20−: A theoretical study on the reaction mechanism. Phys. Chem. Chem. Phys. 2012, 14, 3103–3111. [Google Scholar] [CrossRef]
- Bobuatong, K.; Sakurai, H.; Ehara, M. Intramolecular hydroamination by a primary amine of an unactivated alkene on gold nanoclusters: A DFT study. ChemCatChem 2017, 9, 4490–4500. [Google Scholar]
- Ehara, M.; Priyakumar, U.D. Gold-Palladium nanocluster catalysts for homocoupling: Electronic structure and interface dynamics. Chem. Rec. 2019, 19, 947–959. [Google Scholar] [CrossRef]
- Karanjit, S.; Bobuatong, K.; Fukuda, R.; Ehara, M.; Sakurai, H. Mechanism of the aerobic oxidation of methanol to formic acid on Au8−: A DFT study. Int. J. Quantum Chem. 2012, 113, 428–436. [Google Scholar] [CrossRef]
- Oliver-Meseguer, J.; Cabrero-Antonino, J.R.; Irene Domínguez, I.; Leyva-Pérez, A.; Corma, A. Small Gold Clusters Formed in Solution Give Reaction Turnover Numbers of 107 at Room Temperature. Science 2012, 338, 1452–1455. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, S.; Chen, M.; Zhu1, Y. Unlocking the catalytic activity of an eight-atom gold cluster with a Pd atom. Nanoscale 2020, 12, 6020–6028. [Google Scholar] [CrossRef]
- Cordón, J.; Jiménez-Osés, G.; López-de-Luzuriaga, J.M.; Monge, M. The key role of Au-substrate interactions in catalytic gold subnanoclusters. Nat. Commun. 2017, 8, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Oliver-Meseguer, J.; Leyva-Pérez, A.; Corma, A. Very Small (3–6 Atoms) Gold Cluster Catalyzed Carbon–Carbon and Carbon–Heteroatom Bond-Forming Reactions in Solution. ChemCatChem 2013, 5, 3509–3515. [Google Scholar] [CrossRef]
- Landman, U.; Yoon, B.; Zhang, C.; Heiz, U.; Arenz, M. Factors in gold nanocatalysis: Oxidation of CO in the non-scalable size regime. Top. Catal. 2007, 44, 145–158. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, M.; Zhang, A.; Zhang, C. Graphene Oxide: An Ideal Support for Gold Nanocatalysts. J. Phys. Chem. C 2012, 116, 22336–22340. [Google Scholar] [CrossRef]
- Villa, A.; Janjic, N.; Spontoni, P.; Wang, D.; Su, D.S.; Prati, L. Au–Pd/AC as catalysts for alcohol oxidation: Effect of reaction parameters on catalytic activity and selectivity. Appl. Catal. A Gen. 2009, 364, 221–228. [Google Scholar] [CrossRef]
- Vijay, A.; Mills, G.; Metiu, H. Adsorption of gold on stoichiometric and reduced rutile TiO2 (110) surfaces. J. Chem. Phys. 2003, 118, 6536–6551. [Google Scholar] [CrossRef]
- Diefenbach, M.; Kim, K.S. Spatial structure of Au8: Importance of basis set completeness and geometry relaxation. J. Phys. Chem. B 2006, 110, 21639–21642. [Google Scholar] [CrossRef] [PubMed]
- Huber, K.P.; Herzberg, G. Molecular Spectra and Molecular Structure; Van Nostrand Reinhold: New York, NY, USA, 1979. [Google Scholar]
- Travers, M.J.; Cowles, D.C.; Ellison, G.B. Reinvestigation of the electron affinities of O2 and NO. Chem. Phys. Lett. 1989, 164, 449–455. [Google Scholar] [CrossRef]
- Tsunoyama, H.; Ichikuni, N.; Sakurai, H.; Tsukuda, T. Effect of electronic structures of Au clusters stabilized by Poly(N-vinyl-2-pyrrolidone) on aerobic oxidation catalysis. J. Am. Chem. Soc. 2009, 131, 7086–7093. [Google Scholar] [CrossRef]
- Tsukuda, T.; Tsunoyama, H.; Sakurai, H. Aerobic oxidations catalyzed by colloidal nanogold. Chem. Asian J. 2011, 6, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Pina, C.D.; Falletta, E.; Prati, L.; Rossi, M. Selective oxidation using gold. Chem. Soc. Rev. 2008, 37, 2077–2095. [Google Scholar] [CrossRef]
- Zope, B.N.; Hibbitts, D.D.; Neurock, M.; Davis, R.J. Reactivity of the Gold/Water Interface During Selective Oxidation Catalysis. Science 2010, 330, 74–78. [Google Scholar] [CrossRef]
- Boronat, M.; Corma, A. Molecular approaches to catalysis Naked gold nanoparticles as quasi-molecular catalysts for green processes. J. Catal. 2011, 284, 138–147. [Google Scholar] [CrossRef]
- Frisch, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A. 02; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Feller, D. The role of databases in support of computational chemistry calculations. J. Comp. Chem. 1996, 17, 1571–1586. [Google Scholar] [CrossRef]
- Schuchardt, K.L.; Didier, B.T.; Elsethagen, T.; Sun, L.; Gurumoorthi, V.; Chase, J.; Li, J.; Windus, T.L. Basis Set Exchange: A Community database for computational sciences. J. Chem. Inf. Model 2007, 47, 1045–1052. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kodchasee, J.; Chanloi, C.; Khemthong, P.; Uapipatanakul, B.; Ehara, M.; Bobuatong, K. Catalytic Oxidation of Benzyl Alcohol to Benzaldehyde on Au8 and Au6Pd2 Clusters: A DFT Study on the Reaction Mechanism. Catalysts 2021, 11, 720. https://doi.org/10.3390/catal11060720
Kodchasee J, Chanloi C, Khemthong P, Uapipatanakul B, Ehara M, Bobuatong K. Catalytic Oxidation of Benzyl Alcohol to Benzaldehyde on Au8 and Au6Pd2 Clusters: A DFT Study on the Reaction Mechanism. Catalysts. 2021; 11(6):720. https://doi.org/10.3390/catal11060720
Chicago/Turabian StyleKodchasee, Jitlada, Chanon Chanloi, Pongtanawat Khemthong, Boontida Uapipatanakul, Masahiro Ehara, and Karan Bobuatong. 2021. "Catalytic Oxidation of Benzyl Alcohol to Benzaldehyde on Au8 and Au6Pd2 Clusters: A DFT Study on the Reaction Mechanism" Catalysts 11, no. 6: 720. https://doi.org/10.3390/catal11060720
APA StyleKodchasee, J., Chanloi, C., Khemthong, P., Uapipatanakul, B., Ehara, M., & Bobuatong, K. (2021). Catalytic Oxidation of Benzyl Alcohol to Benzaldehyde on Au8 and Au6Pd2 Clusters: A DFT Study on the Reaction Mechanism. Catalysts, 11(6), 720. https://doi.org/10.3390/catal11060720