3.1. Promoter Pt-Loading
The impact of Pt-loading on the activity of a typical CO promoter is demonstrated using the example of the reference catalysts 1–3 (Pt/Al
2O
3) with different Pt-loadings (see
Table 1). Overall, there appears to be essentially no difference between the three chosen Pt-loadings when the overall catalyst design is identical. At first, this result may be somewhat surprising as this observation would suggest that a large fraction of the precious metal is apparently not contributing to the catalytic activity. However, the observation may relate to the chosen synthetic pathway and the resulting relative size of the Pt-clusters on the support surface. From prior reports on spectroscopic studies of precious metal catalysts [
9,
10], in a 1 nm Pt particle the ratio of surface Pt to Pt forming the core of the particle is ~1:1. With growing particle size, the average Pt coordination number will grow and, as a result, in a 20 nm Pt particle the surface Pt atoms account for approximately 5% of the total metal forming the said particle. Considering the particle size for a typical industrial catalyst, such as those presented here, increasing Pt loading on the support further leads to ever-diminishing returns as the available Pt surface area is essentially unchanged as the diameter of the Pt clusters is increased. Therefore, while some refineries may choose to increase the precious metal levels to combat growing CO emissions, the benefit of such an approach is limited and inherently inefficient.
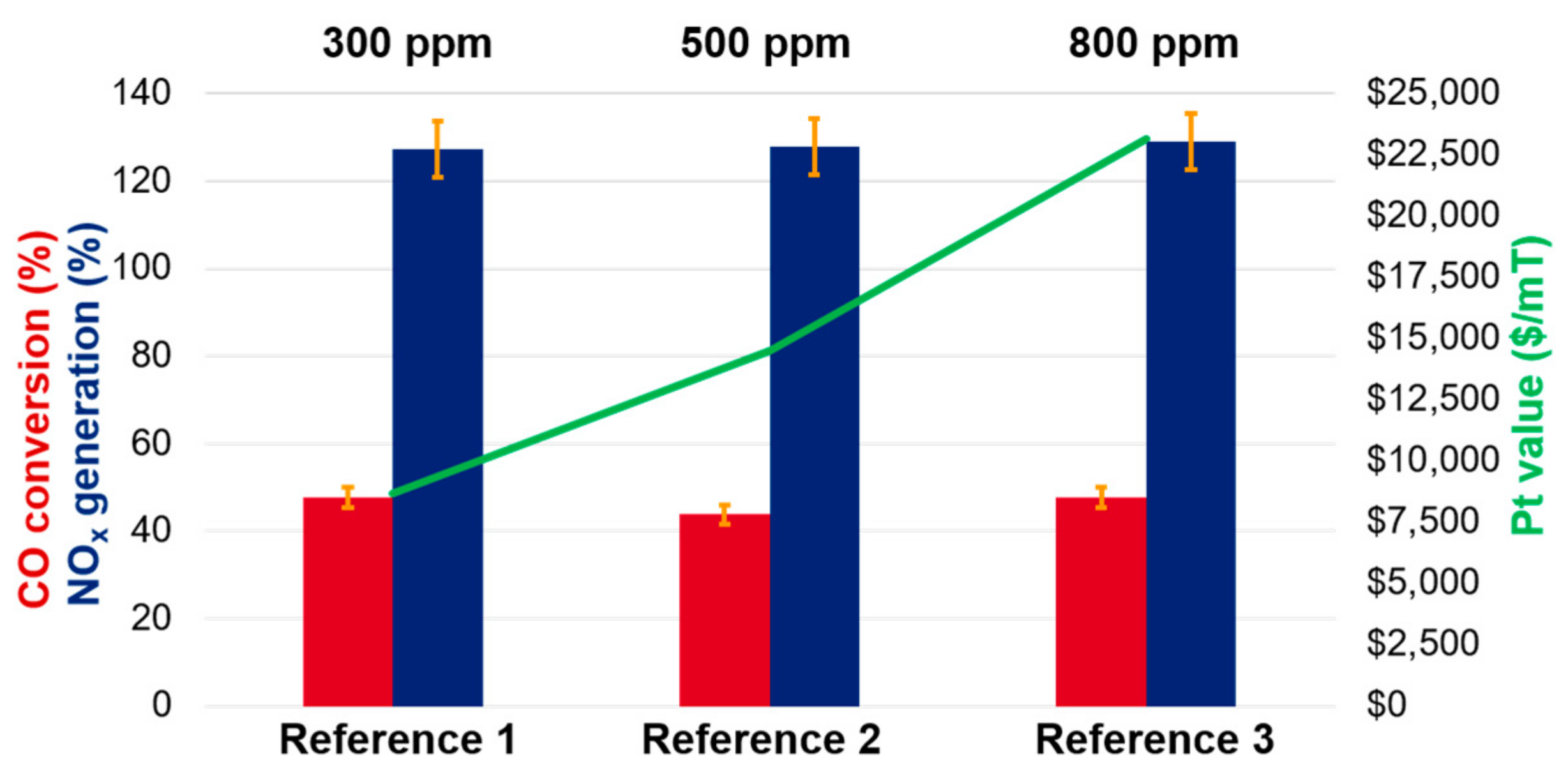
An example of this is demonstrated using reference catalysts 1–3, which differ only in the amount of Pt deposited onto the support. From
Figure 2, within the experimental uncertainty, the efficiency for CO conversion as well as NO
x generation in the fresh state is similar in all cases. The key difference is in the actual cost to the refiner (
$/mT) of Pt used, which ranges from ~8700
$/mT to ~23000
$/mT (at 900
$/oz t Pt market price, a reasonable average value in the 2016–2020 timeframe) [
11]. Note that the financial impact increases with the growing (USD ~1200/oz t) Pt market price, as has been experienced in late 2020 and early 2021 [
11].
Therefore, while a refinery may be under the impression that superior performance is obtained through a higher amount of metal, the actual value proposition diminishes substantially with increasing Pt loading. That is, on a relative basis, when the support material is identical in all cases, the oxidation of a CO molecule to CO2 is ~2.6-times more cost-effective with reference-1 than with reference-3, when the value of the Pt is considered. These considerations, of course, are true when the total concentration of the CO promoter component in an FCC blend is constant and only the PGM amount allocated on this component is increased. An alternative scenario would be for a refinery to increase the total Pt content by increasing the amount of CO promoter in the blend. For example, instead of using 0.25 wt % CO promoter with 1000 ppm Pt, the FCC blend could be formulated using 0.5 wt % CO promoter with 500 ppm Pt. In this case, the benefit of increasing the total Pt in the FCC unit is more pronounced, and in certain cases may lead to the desired improvement of the apparent CO conversion. However, such an approach will typically also lead to a significant NOx emission increase, a much undesired side-effect that in certain areas with very tight emissions regulations, e.g., California, would not be a viable solution. Beyond the NOx concern, most of the precious metal in such an FCC blend would still remain inactive since it forms the core of the PGM-particles. Overall, such an approach could be considered a better of the two poor solutions. Indeed, there are many other, cost-efficient, means to consider before the Pt content is increased, as we will discuss in subsequent paragraphs.
We note at this point that the support used for the catalyst adds further, in some cases significant, costs to the overall Pt promoter design. As Pt loading has limited benefit, we hence focus on the maximization of Pt utilization through support tuning, which is a far more efficient and cost-effective way to reduce CO emissions when the total refinery spend on environmental compliance is considered.
3.2. Promoter Doping
To demonstrate the potential of catalyst support modification towards improved activity, we explore the impact of support doping. In this work, the impact of catalyst doping with ceria is demonstrated using the 300 ppm Pt reference-1 as well as a series of catalysts A–D composed of 300 ppm Pt supported on alumina with different levels of CeO
2 (see
Table 1 for details). The reference CO promoter achieves a 47.6% conversion of CO to CO
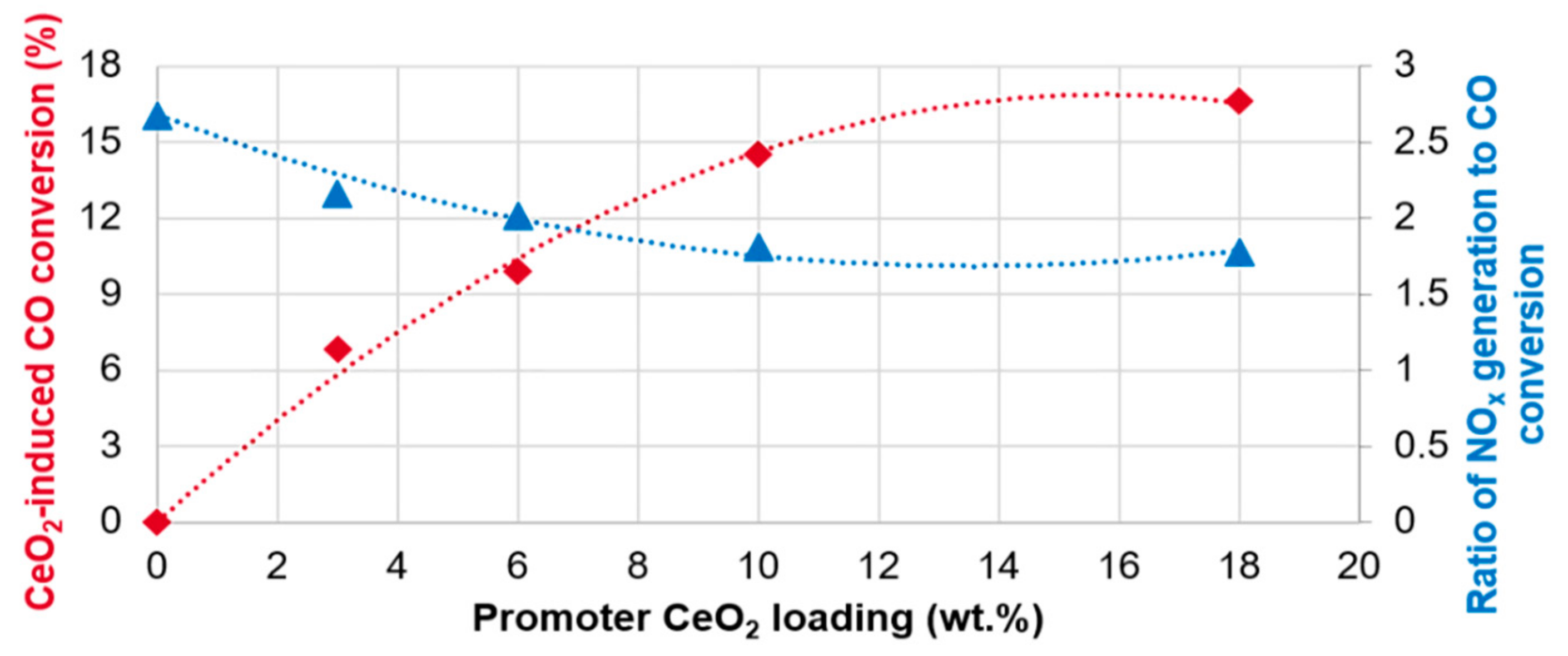
2 under chosen experimental conditions. This will be used as baseline for further assessment of the impact of ceria as an improvement of the CO conversion is observed with increasing amounts of ceria. These results are summarized in
Figure 3.
We observe a nearly linear CO conversion improvement between 0% and ~10% CeO
2 doping of the CO promoter. Beyond ~10%, the impact of ceria is greatly diminished with only minor improvement in ever-increasing CeO
2 loading. There are at least two possible reasons why the addition of ceria is beneficial to the promoter performance when measured in fresh state. First, ceria itself is known as an oxidation catalyst [
12,
13] that can convert CO to CO
2 at elevated temperatures, either by itself or as a mixture with further oxides, e.g., CuO [
14]. Second, the chosen aging and testing conditions may be quite favorable for the doped CO promoter. The testing temperature of 700 °C, when the thermal stability of ceria is considered, is quite mild and does not affect the ability of ceria to serve as an oxygen storage component (OSC) [
15]. The addition of OSC, whether in the form of ceria/zirconia or ceria by itself, to PGM is a well-known concept in the field of mobile emissions control [
16] and has been shown to allow for optimized surface O
2 concentration on the PGM surface, thus maximizing the metal efficacy for CO oxidation [
17].
The diminishing benefit of increasing ceria content above ~10% is explained by several factors: (1) kinetic limitation of the reaction under chosen testing conditions and (2) the way the discussed promoters were prepared. As an example, the degree of the OSC capacity utilization is expected to decrease with increasing CeO2 content due to mass transfer limitations and the inaccessibility of a portion of ceria in the CeO2-particle core.
Another important observation for the presented sample set is that the addition of ceria leads to a decrease in NO
x generation by as much as ~10% (
Table 1). This feature is important as some refineries, especially those with a high Nelson complexity index (NCI), prefer to view the CO promoter performance as a ratio of CO conversion benefit balanced by the NO
x penalty. That is, one can assess promoter efficiency based on how much NO
x is generated per molecule CO that is oxidized. The ratio of NO
x generation to CO conversion for the studied sample-set is reported in
Figure 3. Interestingly, while for every 1% of CO conversion the reference, ceria-free, promoter generates ~2.7% additional NO
x emission, the addition of 10% ceria allows one to decrease the NO
x generation to ~1.8% per 1% CO conversion. Therefore, the addition of ceria has not only allowed boosting the absolute CO conversion levels, but also to decrease the relative NO
x emissions by as much as ~33%, a very significant value for most oil refineries. Again, it should be noted that, similar to CO conversion, the NO
x benefit becomes marginal beyond ~10% CeO
2 content.
While there is a multitude of factors affecting the NO
x generation, there are at least two that we propose relevant to explain the observations in this work. Suggested causes are surmised and are the focus of an ongoing research project outside the frame of the current contribution. First, the addition of ceria is suggested to affect the rates of CO and NO oxidation shifting the balance in favor of CO. Second, under chosen reaction conditions ceria can contribute to the apparently lower NO
x emissions by trapping a portion of the NO
2, a product of NO oxidation, in the form of ceria-nitrate. Examples of NO
x-traps using PGM/ceria components have been previously reported in the field of mobile emissions control [
18,
19]. Such a mechanism is suggested favorable for a CO promoter geared towards applications in the oil refining industry. As an example, the promoter can trap a portion of the generated NO
x in the regenerator. Subsequently, as the promoter circulates through the FCC unit, cerium nitrate can be regenerated in the riser to ceria while releasing NO
x, which in turn can be converted to ammonia and thus removed at the top of the riser column. The regenerated ceria is then ready for the subsequent regenerator cycle. It is worth noting that while the effect is likely significant to help boost CO conversion, the typical amounts of promoter in the FCC catalyst blends (often around 1 wt %) are generally insufficient to dramatically shift the refinery NO
x emission. Therefore, the proposed NO
x emissions reduction is generally considered less efficient than the installation of a stationary NO
x-scrubber that operates via selective catalytic reduction (SCR) of NO
x with ammonia [
20].
3.3. NOx Emission Suppression
To supplement the NO
x observations discussed in the previous chapter, a series of experiments were performed using formulations that in addition to ceria also incorporated further NO
x-trapping components, similar to those typically reported for Lean NO
x Trap (LNT) diesel applications in mobile emissions control [
21]. Two additional samples, catalyst E (catalyst C doped with 2.6% SrO) and catalyst F (catalyst C doped with 4.7% SrO), see
Table 1, were prepared and compared in their CO conversion as well as NO
x generation activity to catalyst C, which is one of more active promoter designs discussed in present work. The CO conversion and NO
x generation values for these catalysts are reported in
Table 1. Sr, specifically in the form of SrO, has been widely proposed to manage NO
x emissions in the past and is hence a suitable example of such chemistry [
22,
23]. Further comparison of the impact of SrO on overall activity is shown in
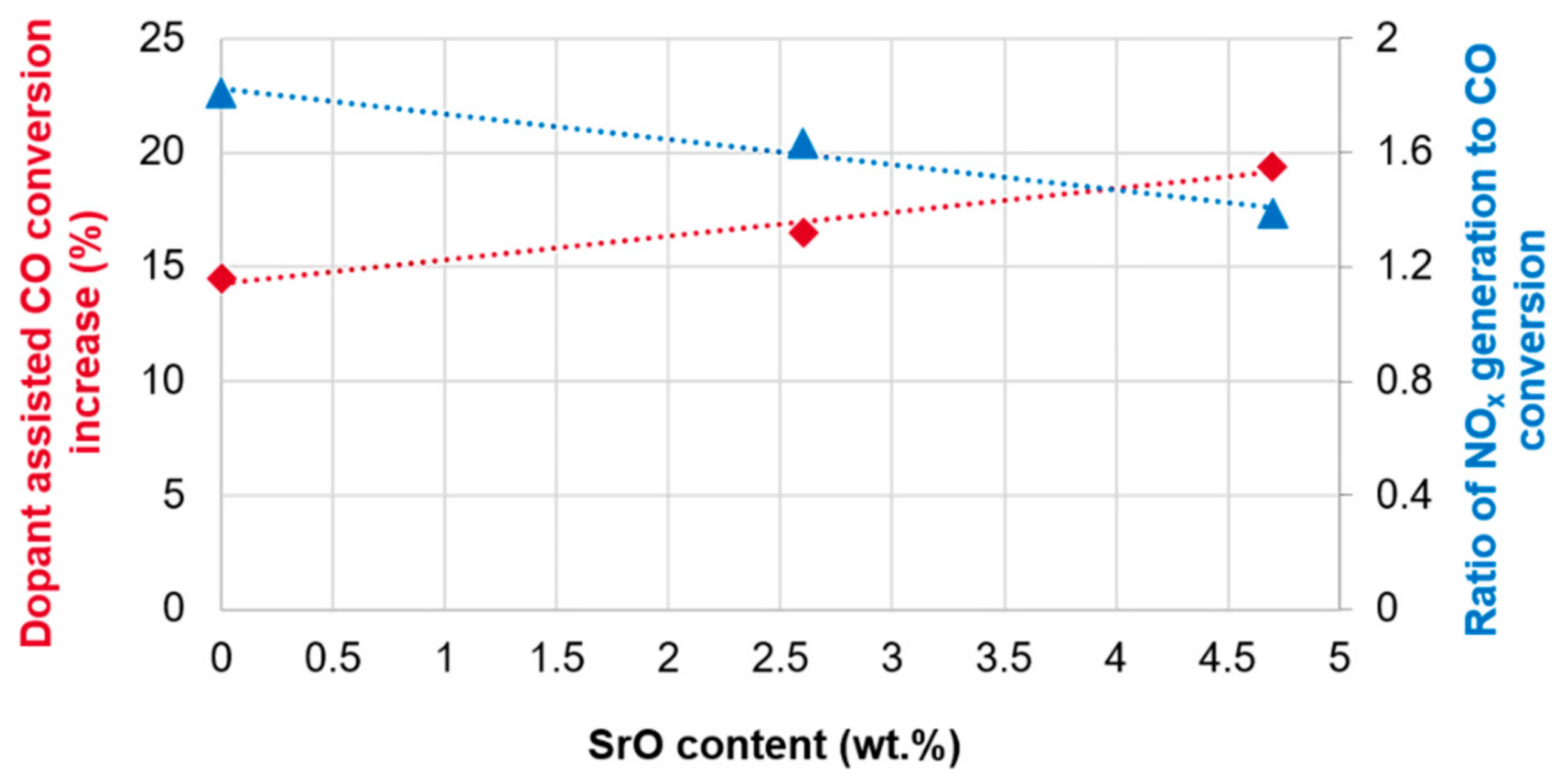
Figure 4.
It should be noted that the addition of SrO to catalyst C leads to a near-linear improvement (
Figure 4) in both CO conversion increase and NO
x generation reduction, which suggests these reactions are closely coupled. The observation is explained by the ability of SrO to trap NO
2 in the form of strontium nitrate, which can then be decomposed in the riser to yield ammonia and the regenerated SrO, much like the mechanism described for CeO
2 in the previous chapter. The key difference here is that SrO, due to its nature, cannot contribute to CO oxidation. In turn, this means that while SrO selectively stores NO
2, a larger amount of the ceria remains available to oxidize CO rather than be “deactivated” by NO
2 adsorption. This operation is very similar to that of a typical LNT design for diesel emissions system applications, where the NO
x-trapping function is partially decoupled from CO and hydrocarbon oxidation to maximize catalytic efficiency [
24].
With the potential NOx emissions suppression in mind, it is not obvious that the addition of a dedicated NOx-trapping component is the most practical approach towards NOx management in a refinery since the necessary amount of such SrO-based trap would need to comprise a substantial amount, e.g., 10–20%, of the FCC formulation, which is not a viable approach towards NOx mitigation in most existing FCC units.
3.4. Impact of Aging
Two catalysts are chosen for further testing to assess the impact of promoter aging on performance under conditions typical of an FCC unit. Specifically, two designs are compared here, these are reference-2, a 500 ppm Pt containing promoter that is similar to those designs often used in commercial applications, and catalyst C, 300 ppm Pt and 10% CeO
2 containing promoter design that has shown optimal NO
x/CO ratio, as discussed in previous chapter. Additional information on these two catalysts is provided in
Table 1. It is important to note that while at first glance the comparison may not seem direct or fair, we focus on a real-life type of application, where a refinery would consider one or two catalysts at most when selecting the CO promoter for their application. Ultimately, what is most critical, is the highest efficiency per unit catalyst, i.e., lb or kg, rather than specific elemental composition, as this approach allows for a minimization of the monthly CO promoter spend at the refinery.
There are two types of aging tests that were developed for this workstream. First, a steam-aging, which is used to simulate the regenerator portion of the FCC operation cycle. Here, we chose a 12 h aging duration with a T
max = 787 °C using a sealed steam reactor that is filled with a 90% H
2O-steam/10% N
2 mixture. Second, Lean/Rich (L/R) aging, which is more typical for a mobile emissions control testing setup, e.g., for a very lean diesel engine, that mimics the full FCC unit operation cycle. This aging is also 12 h in duration, reaches a T
max = 780 °C and is performed using a flow-through reactor that cycles with 10 min Air, followed by 10 min N
2, followed by 10 min H
2, followed by 10 min N
2, while at a constant 10% H
2O-steam in every step. Due to powder bed penetration difference between air and H
2, the 10 min H
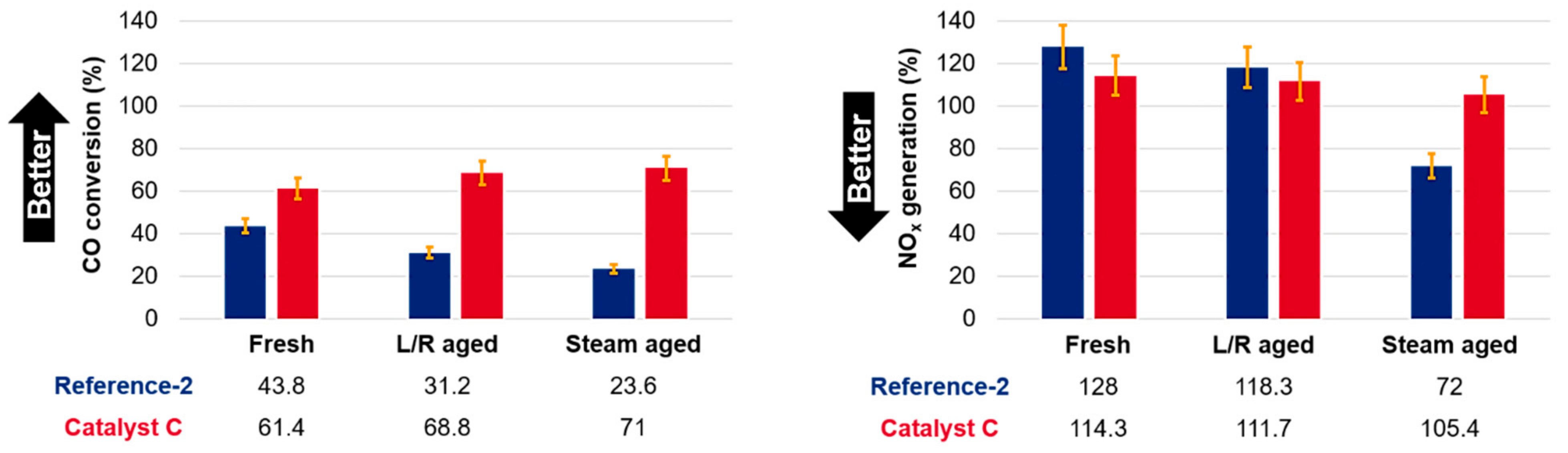
2 reduction is equivalent to ~1 min reduction using hydrocarbons. This point is important as it simulates the relative time a CO promoter spends in the FCC unit, i.e., ~90% of the lifetime in the regenerator and ~10% lifetime in the riser, where the promoter can be regenerated. The comparison of the said two samples in fresh as well as aged form is shown in
Figure 5.
The superiority of catalyst C to the reference-2, regardless of the lower Pt content in the experimental sample was expected based on fresh testing. However, the extent of the aging on the said catalysts was not obvious prior to testing. For CO conversion, the CO activity of the reference-2 design is decreased by 25–50% depending on the aging type, with steam aging being particularly harmful.
At this point we believe it is necessary to provide a side note on the nature of Pt aging as often observed in mobile emissions control, e.g., for diesel-type emissions mitigation systems. Pt is quite mobile in the oxide form and stable in metallic form when it comes to sintering or Ostwald ripening [
25,
26]. What this translates to is an elevated degree of mobility on the surface that ultimately leads to a higher extent of agglomeration upon aging in the absence of reductants. Therefore, while both aging protocols in this work had a nominal duration of 12 h, the actual time Pt was subject to oxidation was different, with more favorable conditions in the L/R aging case. Since Pt was offered the occasional regeneration step, that in turn has led to an apparent higher degree of CO activity after the 12 h L/R aging cycle compared to steam aging. The key learning here is that the promoter aging protocol must be chosen such to best suit the actual refinery operation. In turn, this approach not only allows the correct promoter dosing and durability estimate, but also a fair comparison of competing catalyst technologies (e.g., as part of catalyst vendor bids).
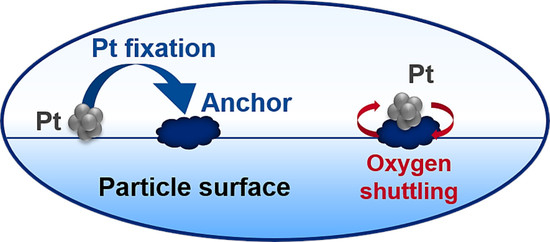
With the above in mind, the remarkable stability of catalyst C after both aging protocols deserves a further explanation as the performance appears to improve slightly with aging. There are two key reasons for this observation. First, the CeO
2 that is present in catalyst C will exhibit some CO activity on its own, as discussed previously. The aging conditions chosen in this work are generally below temperatures that are considered harmful to ceria, i.e., cause structural collapse and stop oxygen mobility [
27]. Therefore, the CeO
2-contribution to CO oxidation is essentially unaffected. Second, likely even more prominent, ceria when introduced to the promoter design in an appropriate manner, can serve as an anchoring point for Pt as the affinity of Pt to CeO
2 is known to exceed that of Pt to Al
2O
3 [
28]. Therefore, as the aging begins, Pt that may be randomly distributed on the surface of the promoter particle, is quickly anchored on the surface of ceria domains. This in turn means that Pt is given little opportunity to find further Pt particles and sinter into larger Pt clusters on the promoter surface. The large number of small anchored Pt particles offers a significantly larger apparent Pt surface area that is available for the CO oxidation reaction. Additionally, ceria can stabilize Pt at atomic dispersion which is active for CO oxidation but not for dehydrogenation chemistry [
29,
30]. Finally, while it is not possible to completely exclude some degree of Pt encapsulation with ceria, the relatively mild aging temperatures [
31], as compared to some of the most aggressive environmental catalysis type aging conditions [
32], lead us to surmise that the anchoring of Pt with ceria has a significant net-benefit effect over a simpler Pt/Al
2O
3 type design under operation conditions typical of a refinery FCC unit.
As for NOx generation, the same general observations and explanations as those discussed for CO conversion are still valid. The decrease of the Pt surface area due to sintering leads to diminishing NOx emissions. For Catalyst C, the NOx generation is much more stable, regardless of aging, which serves as an indication of the excellent stability and durability of this promoter design. The latter observation is critical when it comes to real-life applications: refiners are interested in a stable mode of operation with little to no fluctuation of the emissions performance. A promoter such as reference-2 would need to be continuously dosed, either via loader or as part of additional FCC catalyst to maintain average CO conversion activity. In contrast, a design such as catalyst C will allow the refinery to reduce the overall tonnage of the promoter needed monthly. Specifically, for the case exemplified here, one could use ~20% less CO promoter on a weight basis when switching from reference-2 to catalyst C without a sacrifice in CO activity. At the same time, the NOx emissions would be reduced by ~10–15%. Most importantly, not only would the price per unit promoter ($/kg) be reduced by 40% based on the Pt-loading related value, but the overall cost of CO promotion would be reduced as a function of lower monthly consumption. Furthermore, the improved durability of the promoter would reduce the risk of non-compliance and the amount of effort, especially in regard to monitoring as well as the actual staffing necessary to maintain promoter levels in the unit, thus simplifying the day-to-day operations for the refinery.