Experimental and Physico-Chemical Comparison of ZnO Nanoparticles’ Activity for Photocatalytic Applications in Wastewater Treatment
Abstract
1. Introduction
2. Results and Discussion
2.1. Physico-Chemical Characterization
2.2. Photocatalytic Results
3. Materials and Methods
3.1. ZnO Syntheses
3.1.1. Zn(NO3)2∙6H2O Precursor
3.1.2. Zn(CH3COOH)2 Precursor
3.2. Characterization Techniques
3.3. Photocatalytic Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pietrelli, L.; Ferro, S.; Reverberi, A.P.; Vocciante, M. Removal and recovery of heavy metals from tannery sludge subjected to plasma pyro-gasification process. J. Clean. Prod. 2020, 273, 123166. [Google Scholar] [CrossRef]
- Soltani, R.D.C.; Jorfi, S.; Safari, M.; Rajaei, M.S. Enhanced sonocatalysis of textile wastewater using benton-ite-supported ZnO nanoparticles: Response surface methodological approach. J. Environ. Manag. 2016, 179, 47–57. [Google Scholar] [CrossRef]
- Vocciante, M.; Finocchi, A.; De Folly D’Auris, A.; Conte, A.; Tonziello, J.; Pola, A.; Reverberi, A.P. Enhanced oil spill remedi-ation by adsorption with interlinked multilayered graphene. Materials 2019, 12, 2231. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Vocciante, M.; De Folly D’Auris, A.; Finocchi, A.; Tagliabue, M.; Bellettato, M.; Ferrucci, A.; Reverberi, A.P.; Ferro, S. Ad-sorption of ammonium on clinoptilolite in presence of competing cations: Investigation on groundwater remediation. J. Clean. Prod. 2018, 198, 480–487. [Google Scholar] [CrossRef]
- Pietrelli, L.; Ippolito, N.M.; Ferro, S.; Dovì, V.G.; Vocciante, M. Removal of Mn and As from drinking water by red mud and pyrolusite. J. Environ. Manag. 2019, 237, 526–533. [Google Scholar] [CrossRef]
- Bel Hadjltaiefa, H.; Ben Zina, M.; Galvez, M.E.; Da Costa, P. Photocatalytic degradation of methyl green dye in aqueous so-lution over natural clay-supported ZnO-TiO2 catalysts. J. Photochem. Photobiol. A Chem. 2016, 315, 25–33. [Google Scholar] [CrossRef]
- Bloh, J.Z.; Dillert, R.; Bahnemann, D.W. Designing Optimal Metal-Doped Photocatalysts: Correlation between Photocatalytic Activity, Doping Ratio, and Particle Size. J. Phys. Chem. C 2012, 116, 25558–25562. [Google Scholar] [CrossRef]
- De Franco, M.A.E.; da Silva, W.L.; Bagnara, M.; Lansarin, M.A.; dos Santos, J.H.Z. Photocatalytic degradation of nicotine in an aqueous solution using unconventional supported catalysts and commercial ZnO/TiO2 under ultraviolet irradiation. Sci. Total Environ. 2014, 494, 97–103. [Google Scholar] [CrossRef]
- Toccafondi, C.; Dante, S.; Reverberi, A.P.; Salerno, M. Biomedical Applications of Anodic Porous Alumina. Curr. Nanosci. 2015, 11, 572–580. [Google Scholar] [CrossRef]
- Pascariu, V.; Avadanei, O.; Gasner, P.; Stoica, I.; Reverberi, A.P.; Mitoseriu, L. Preparation and characterization of PbTiO3–epoxy resin compositionally graded thick films. Phase Transit. 2013, 86, 715–725. [Google Scholar] [CrossRef]
- McCluskey, M.; Jokela, S. Sources of n-type conductivity in ZnO. Phys. B Condens. Matter 2007, 401, 355–357. [Google Scholar] [CrossRef]
- Jiang, Y.; O’Neill, A.J.; Ding, Y. Zinc oxide nanoparticle-coated films: Fabrication, characterization, and antibacterial properties. J. Nanoparticle Res. 2015, 17, 180. [Google Scholar] [CrossRef]
- Van de Walle, C.G. Hydrogen as a cause of doping in zinc oxide. Phys. Rev. Lett. 2000, 85, 1012–1015. [Google Scholar] [CrossRef] [PubMed]
- Janotti, A.; Van de Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 1–29. [Google Scholar] [CrossRef]
- Reverberi, A.P.; Salerno, M.; Lauciello, S.; Fabiano, B. Synthesis of copper nanoparticles in ethylene glycol by chemical reduc-tion with vanadium (+2) salts. Materials 2016, 9, 809. [Google Scholar] [CrossRef]
- Janotti, A.; Van de Walle, C.G. Hydrogen multicentre bonds. Nat. Mater. 2007, 6, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, A.; Alberti, S.; Caratto, V.; Lova, P.; Locardi, F.; Pampararo, G.; Villa, S.; Ferretti, M. Structural studies on copper and nitrogen doped nanosized anatase. Z. Krist. Cryst. Mater. 2018, 233, 867–876. [Google Scholar] [CrossRef]
- Caratto, V.; Locardi, F.; Alberti, S.; Villa, S.; Sanguineti, E.; Martinelli, A.; Balbi, T.; Canesi, L.; Ferretti, M. Different sol–gel preparations of iron-doped TiO2 nanoparticles: Characterization, photocatalytic activity and cytotoxicity. J. Sol Gel Sci. Technol. 2016, 80, 152–159. [Google Scholar] [CrossRef]
- Humánez, M.F.A.; Vides, L.A.M.; Almanza-Montero, O.A. Sol-gel synthesis of zinc oxide nanoparticle at three different temperatures and its characterization via XRD, IR and EPR. DYNA 2016, 83, 224–228. [Google Scholar] [CrossRef]
- Alberti, S.; Ferretti, M.; Vicini, S.; Castellano, M.; Caratto, V. Porous polydimethylsiloxane membranes loaded with low-temperature crystallized TiO2 NPs for detachable antibacterial films. J. Mater. Sci. 2018, 54, 1665–1676. [Google Scholar] [CrossRef]
- Alberti, S.; Locardi, F.; Sturini, M.; Speltini, A.; Maraschi, F.; Costa, G.A.; Ferretti, M.; Caratto, V. Photocatalysis in darkness: Optimization of sol-gel synthesis of NP-TiO2 supported on a persistent luminescence material and its application for the removal of ofloxacin from water. J. Nanomed. Nanotechnol. 2018, 9, 501. [Google Scholar] [CrossRef]
- Alberti, S.; Caratto, V.; Peddis, D.; Belviso, C.; Ferretti, M. Synthesis and characterization of a new photocatalyst based on TiO2 nanoparticles supported on a magnetic zeolite obtained from iron and steel industrial waste. J. Alloys Compd. 2019, 797, 820–825. [Google Scholar] [CrossRef]
- Sampaio, M.J.; Bacsa, R.; Benyounes, A.; Axet, R.; Serp, P.; Silva, C.; Silva, A.; Faria, J. Synergistic effect between carbon nanomaterials and ZnO for photocatalytic water decontamination. J. Catal. 2015, 331, 172–180. [Google Scholar] [CrossRef]
- Kumar, P.P.N.V.; Shameem, U.; Kollu, P.; Kalyani, R.L.; Pammi, S.V.N. Green Synthesis of Copper Oxide Nanoparticles Using Aloe vera Leaf Extract and Its Antibacterial Activity Against Fish Bacterial Pathogens. BioNanoScience 2015, 5, 135–139. [Google Scholar] [CrossRef]
- Senthilkumar, S.R.; Sivakumar, T. Green tea (Camelia Sinensis) mediated synthesis of zinc oxide (ZnO) nanoparticles and studies on their antimicrobial activities. Int. J. Pharm. Pharm. Sci. 2014, 6, 461–465. [Google Scholar]
- Balasooriya, E.R.; Jayasinghe, C.D.; Jayawardena, U.A.; Ruwanthika, R.W.D.; Mendis de Silva, R.; Udagama, P.V. Honey mediated green synthesis of nanoparticles: New era of safe technology. Hindawi J. Nanomater. 2017, 5919836. [Google Scholar] [CrossRef]
- Azizi, S.; Ahmad, M.B.; Namvar, F.; Mohamad, R. Green biosynthesis and characterization of zinc oxide nanoparticles using brown marine macroalga Sargassum muticum aqueous extract. Mater. Lett. 2014, 116, 275–277. [Google Scholar] [CrossRef]
- Fardood, S.T.; Ramazani, A.; Moradi, S.; Asiabi, P.A. Green synthesis of zinc oxide nanoparticles using arabic gum and photocatalytic degradation of direct blue 129 dye under visible light. J. Mater. Sci. Mater. Electron. 2017, 28, 13596–13601. [Google Scholar] [CrossRef]
- Vigneshwaran, N.; Kumar, S.; A Kathe, A.; Varadarajan, P.V.; Prasad, V. Functional finishing of cotton fabrics using zinc oxide–soluble starch nanocomposites. Nanotechnology 2006, 17, 5087–5095. [Google Scholar] [CrossRef]
- Sun, D.; Wong, M.; Sun, L.; Li, Y.; Miyatake, N.; Sue, H.-J. Purification and stabilization of colloidal ZnO nanoparticles in methanol. J. Sol-Gel Sci. Technol. 2007, 43, 237–243. [Google Scholar] [CrossRef]
- Znaidi, L. Sol-gel deposited ZnO thin films: A review. Mater. Sci. Eng. B 2010, 174, 18–30. [Google Scholar] [CrossRef]
- Xu, L.; Gu, F.; Su, J.; Chen, Y.; Li, X.; Wang, X. The evolution behavior of structures and photoluminescence of K-doped ZnO thin films under different annealing temperatures. J. Alloys Compd. 2011, 509, 2942–2947. [Google Scholar] [CrossRef]
- Zak, A.K.; Abrishami, M.E.; Majid, W.A.; Yousefi, R.; Hosseini, S. Effects of annealing temperature on some structural and optical properties of ZnO nanoparticles prepared by a modified sol–gel combustion method. Ceram. Int. 2011, 37, 393–398. [Google Scholar] [CrossRef]
- Villa, S.; Caratto, V.; Locardi, F.; Alberti, S.; Sturini, M.; Speltini, A.; Maraschi, F.; Canepa, F.; Ferretti, M. Enhancement of TiO2 NPs Activity by Fe3O4 Nano-Seeds for Removal of Organic Pollutants in Water. Materials 2016, 9, 771. [Google Scholar] [CrossRef]
- Alberti, S.; Villa, S.; Singh, G.; Seland, F.; Martinelli, A.; Ferretti, M.; Canepa, F.; Caratto, V. Systematic study on TiO2 crystal-lization via hydrothermal synthesis in the presence of different ferrite nanoparticles as nucleation seeds. J. Nanosci. Nanotechnol. 2019, 19, 4994–4999. [Google Scholar] [CrossRef] [PubMed]
- Sofianos, V.; Lee, J.; Silvester, D.; Samanta, P.; Paskevicius, M.; English, N.; Buckley, C. Diverse morphologies of zinc oxide nanoparticles and their electrocatalytic performance in hydrogen production. J. Energy Chem. 2021, 56, 162–170. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Xiong, S.F.; Yin, Z.L.; Yuan, Z.F.; Yan, W.B.; Yang, W.Y.; Liu, J.J.; Zhang, F. Dual frequency (20/40 kHz) ultra-sonic assisted photocatalysis for degradation of methylene blue effluent: Synergistic effect and kinetic study. Ultrason. Sono Chem. 2012, 19, 756–761. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L. Green synthesis of zinc oxide nanoparticles using Phoenix dactylifera waste as bioreductant for effective dye degradation and antibacterial performance in wastewater treatment. J. Hazard. Mater. 2021, 402, 123560. [Google Scholar] [CrossRef]
- Kurbanov, S.; Yang, W.C.; Kang, T.W. Kelvin probe force microscopy of defects in ZnO nanocrystals associated with emis-sion at 3.31 eV. Appl. Phys. Express 2011, 4, 021101. [Google Scholar] [CrossRef]
- López, R.; Gómez, R. Band-gap energy estimation from diffuse reflectance measurements on sol–gel and commercial TiO2: A comparative study. J. Sol. Gel Sci. Technol. 2012, 61, 1–7. [Google Scholar] [CrossRef]
- Lova, P.; Manfredi, G.; Boarino, L.; Laus, M.; Urbinati, G.; Losco, T.; Marabelli, F.; Caratto, V.; Ferretti, M.; Castellano, M.; et al. Hybrid ZnO:polystyrene nanocomposite for all-polymer photonic crystals. Phys. Status Solidi C 2015, 12, 158–162. [Google Scholar] [CrossRef]
- Stefan, M. More than just light. Solutions in ultraviolet light. In 100 Years of Innovation Osram; OSRAM GmbH: Munich, Germany, 2016; p. 24. [Google Scholar]
- Mahfoudh, I.; Principi, P.; Fioretti, R.; Safi, M. Experimental studies on the effect of using phase change material in a salinity-gradient solar pond under a solar simulator. Sol. Energy 2019, 186, 335–346. [Google Scholar]
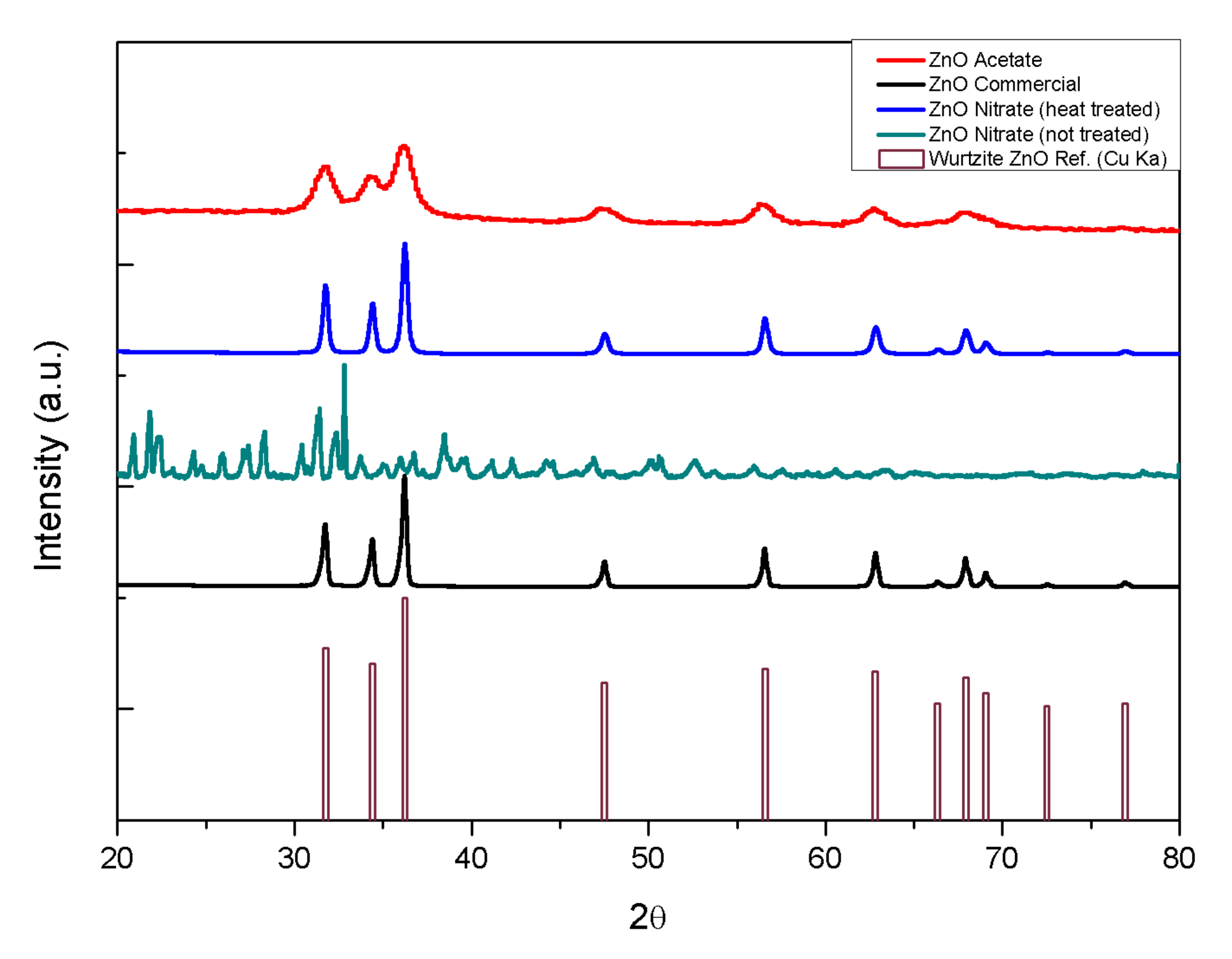

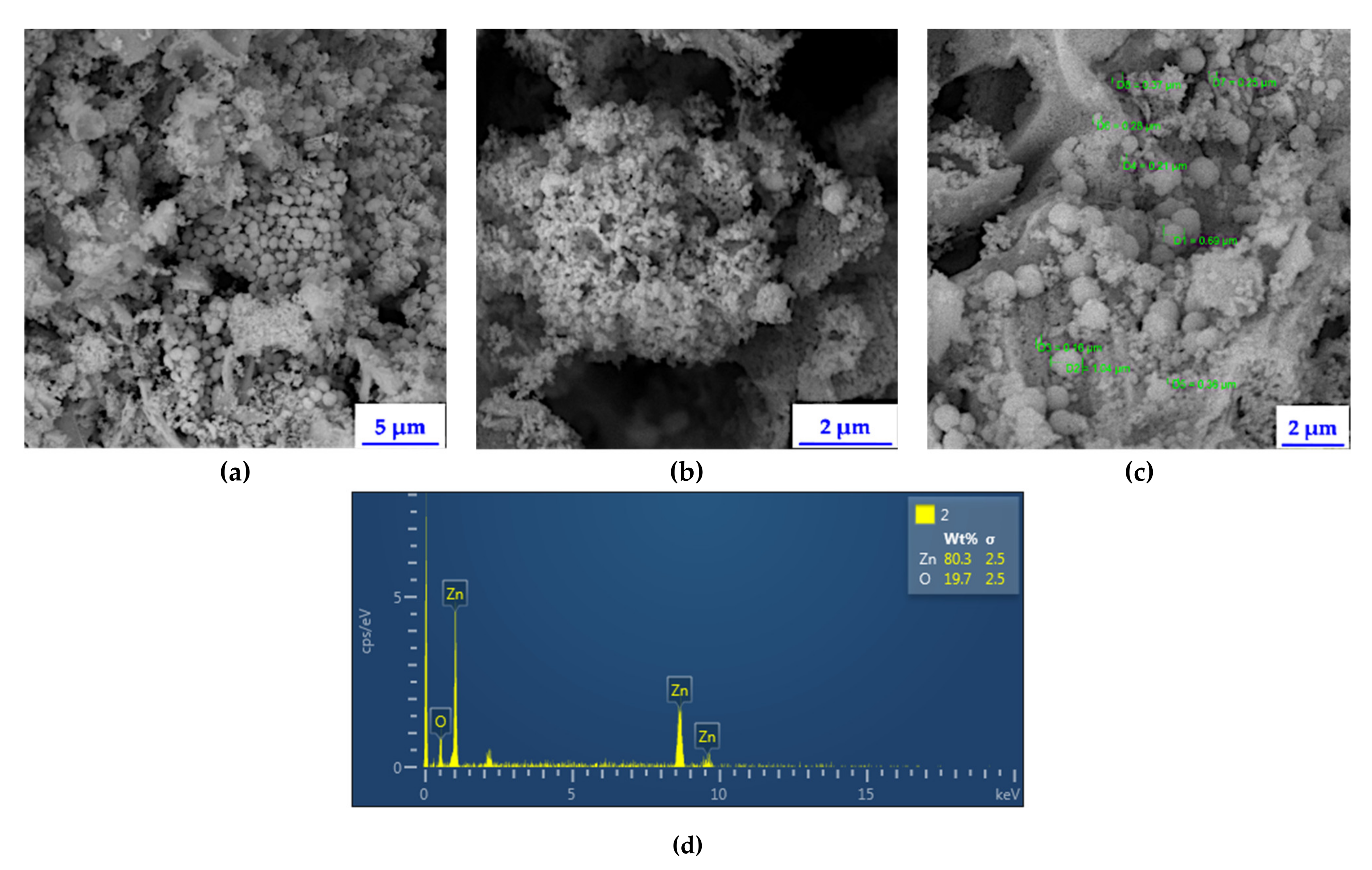
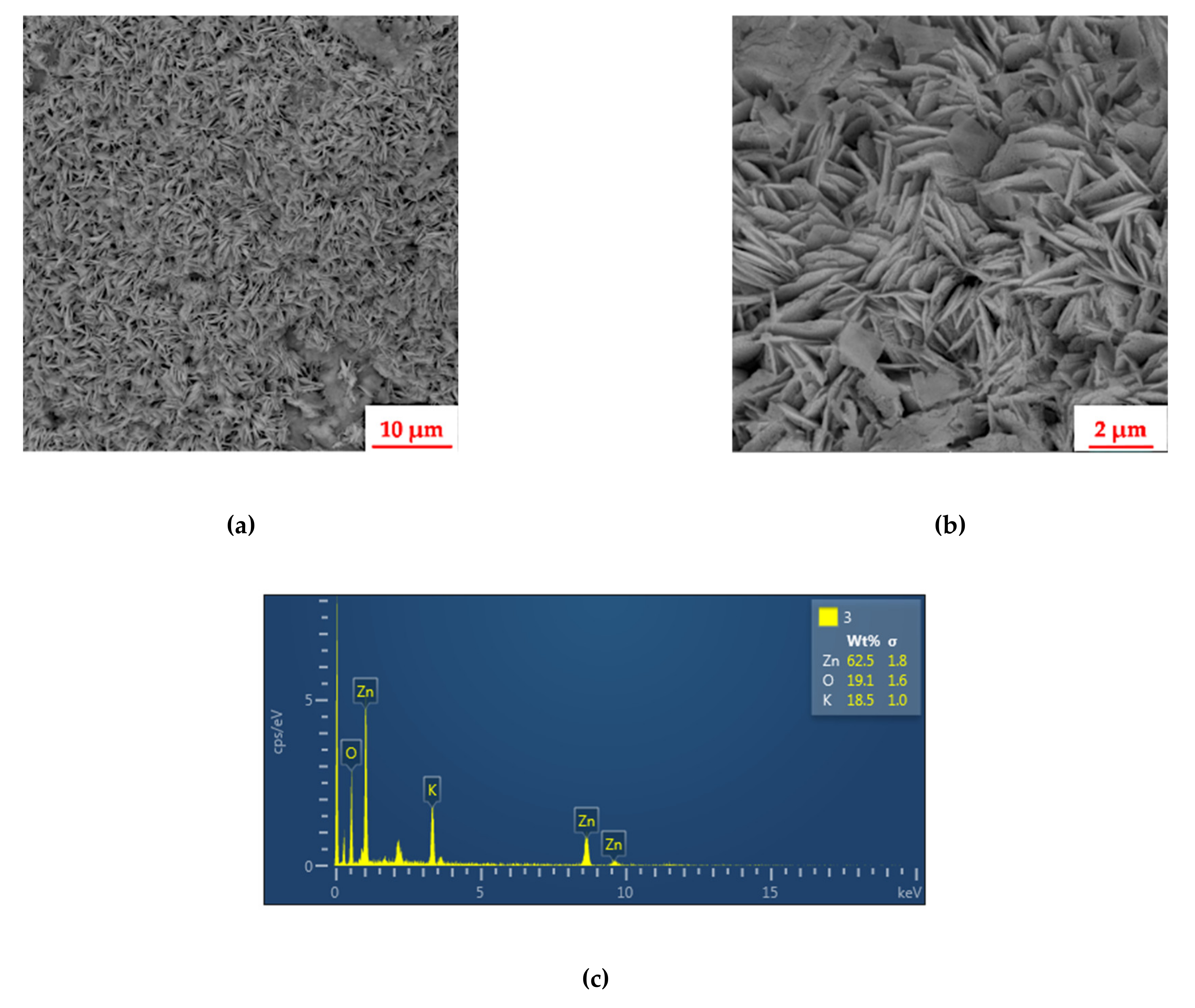
| Sample | Mean Crystallite Size |
|---|---|
| ZnO Acetate route | 6.84 ± 1.18 nm |
| ZnO Commercial | 29.85 ± 2.59 nm |
| ZnO Nitrate (heat-treated) route | 24.48 ± 2.09 nm |
| Samples | MB Dark Absorption (20 min) | MB% Degradation | Dev. st. (n = 3) | k (I) |
|---|---|---|---|---|
| MB Photolysis | n.c. | 8% | 2.5 | 0.002 min−1 |
| ZnO Acetate route | 0.9% | 78% | 2.0 | 0.054 min−1 |
| ZnO Commercial | 5.2% | 100%* | 0.5 | 0.162 min−1 |
| ZnO Nitrate (heat treated) route | 2.3% | 93% | 0.9 | 0.085 min−1 |
| Samples | Egap Values | λ Absorption |
|---|---|---|
| ZnO Nitrate (heat treated) route | 3.27 eV | 379 nm |
| ZnO Commercial | 3.40 eV | 364 nm |
| ZnO Acetate route | 3.31 eV | 374 nm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alberti, S.; Basciu, I.; Vocciante, M.; Ferretti, M. Experimental and Physico-Chemical Comparison of ZnO Nanoparticles’ Activity for Photocatalytic Applications in Wastewater Treatment. Catalysts 2021, 11, 678. https://doi.org/10.3390/catal11060678
Alberti S, Basciu I, Vocciante M, Ferretti M. Experimental and Physico-Chemical Comparison of ZnO Nanoparticles’ Activity for Photocatalytic Applications in Wastewater Treatment. Catalysts. 2021; 11(6):678. https://doi.org/10.3390/catal11060678
Chicago/Turabian StyleAlberti, Stefano, Irene Basciu, Marco Vocciante, and Maurizio Ferretti. 2021. "Experimental and Physico-Chemical Comparison of ZnO Nanoparticles’ Activity for Photocatalytic Applications in Wastewater Treatment" Catalysts 11, no. 6: 678. https://doi.org/10.3390/catal11060678
APA StyleAlberti, S., Basciu, I., Vocciante, M., & Ferretti, M. (2021). Experimental and Physico-Chemical Comparison of ZnO Nanoparticles’ Activity for Photocatalytic Applications in Wastewater Treatment. Catalysts, 11(6), 678. https://doi.org/10.3390/catal11060678