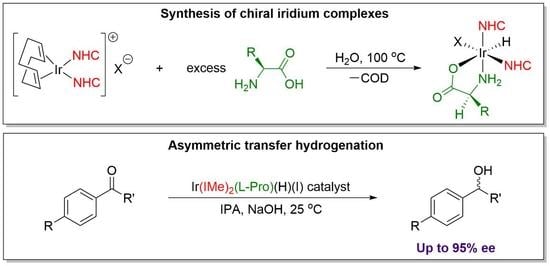
Design of Iridium N-Heterocyclic Carbene Amino Acid Catalysts for Asymmetric Transfer Hydrogenation of Aryl Ketones
Abstract
1. Introduction
2. Results and Discussion
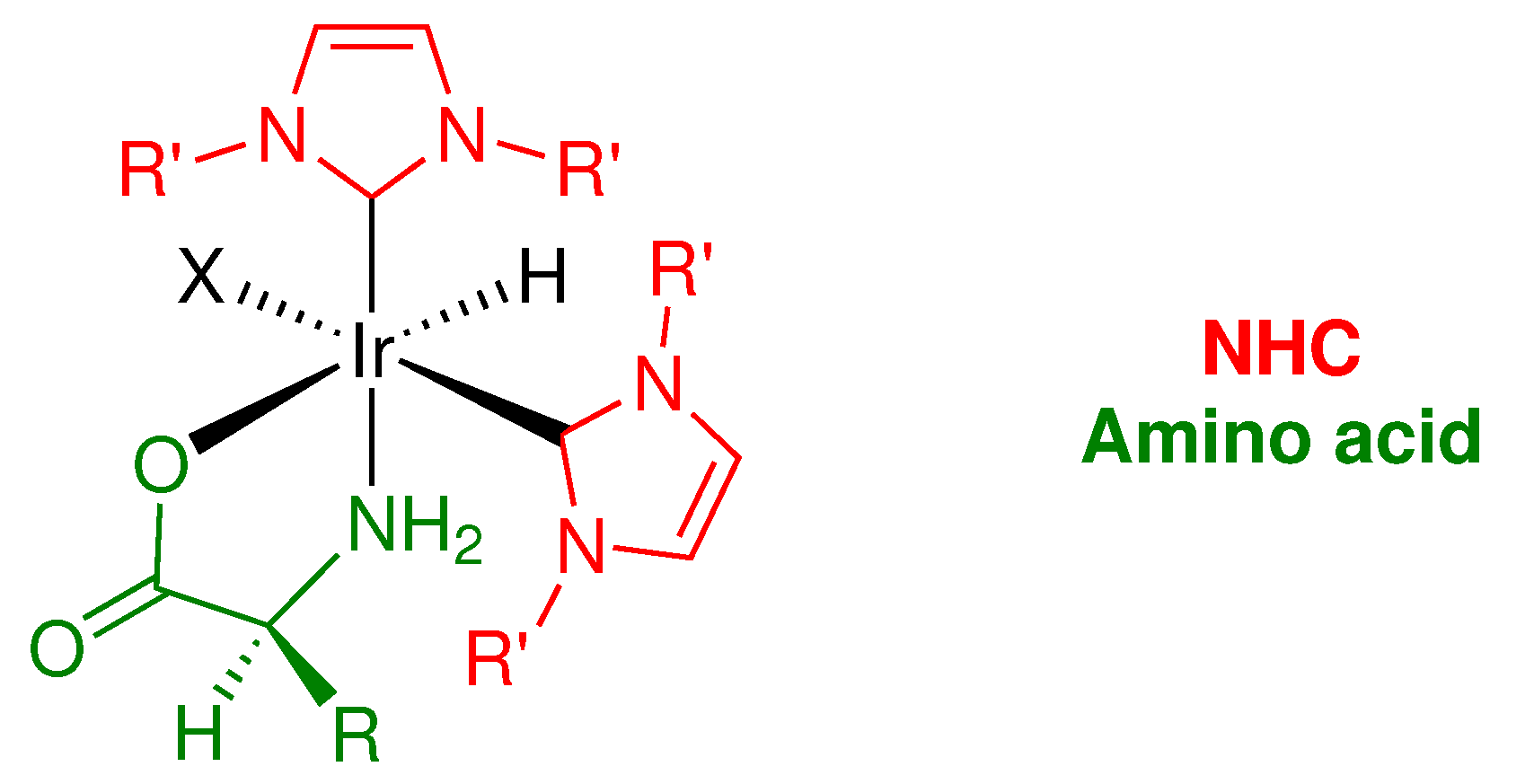
2.1. Synthesis
2.2. Characterization
2.2.1. X-ray Crystallography
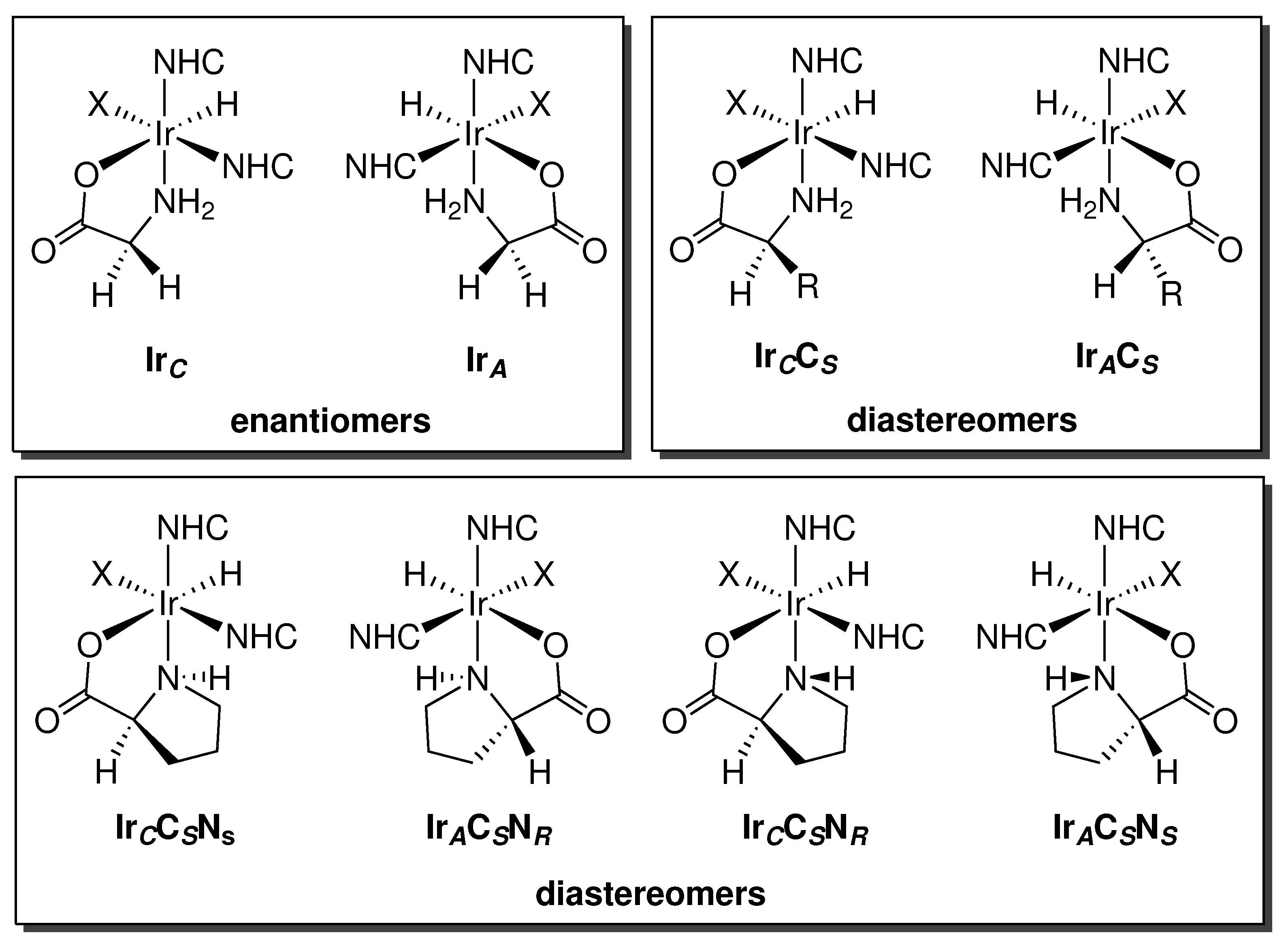
2.2.2. Stereochemistry
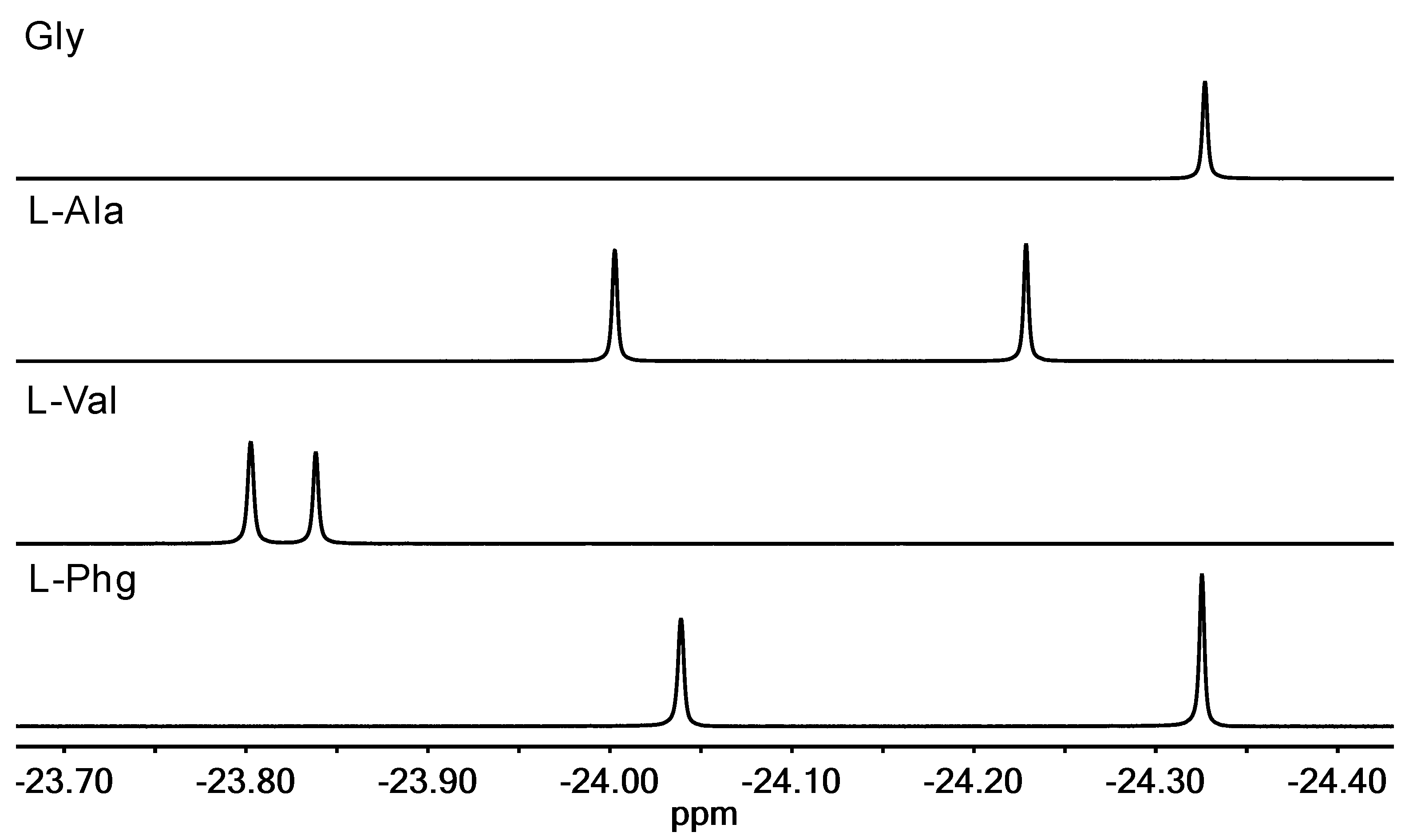
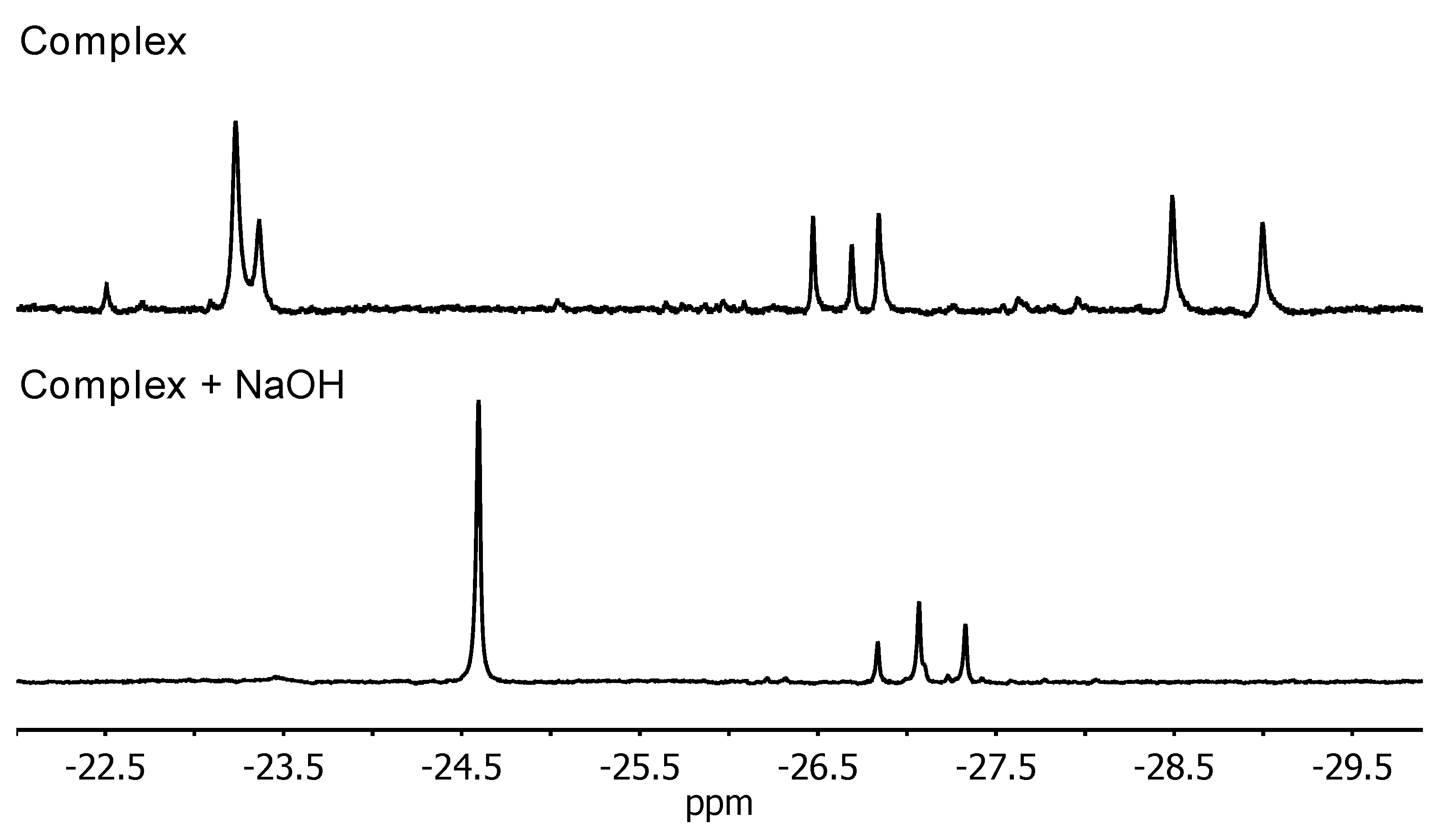
2.2.3. 1H NMR Spectroscopy
2.3. Asymmetric Transfer Hydrogenation of Ketones
2.3.1. Initial Catalytic Studies
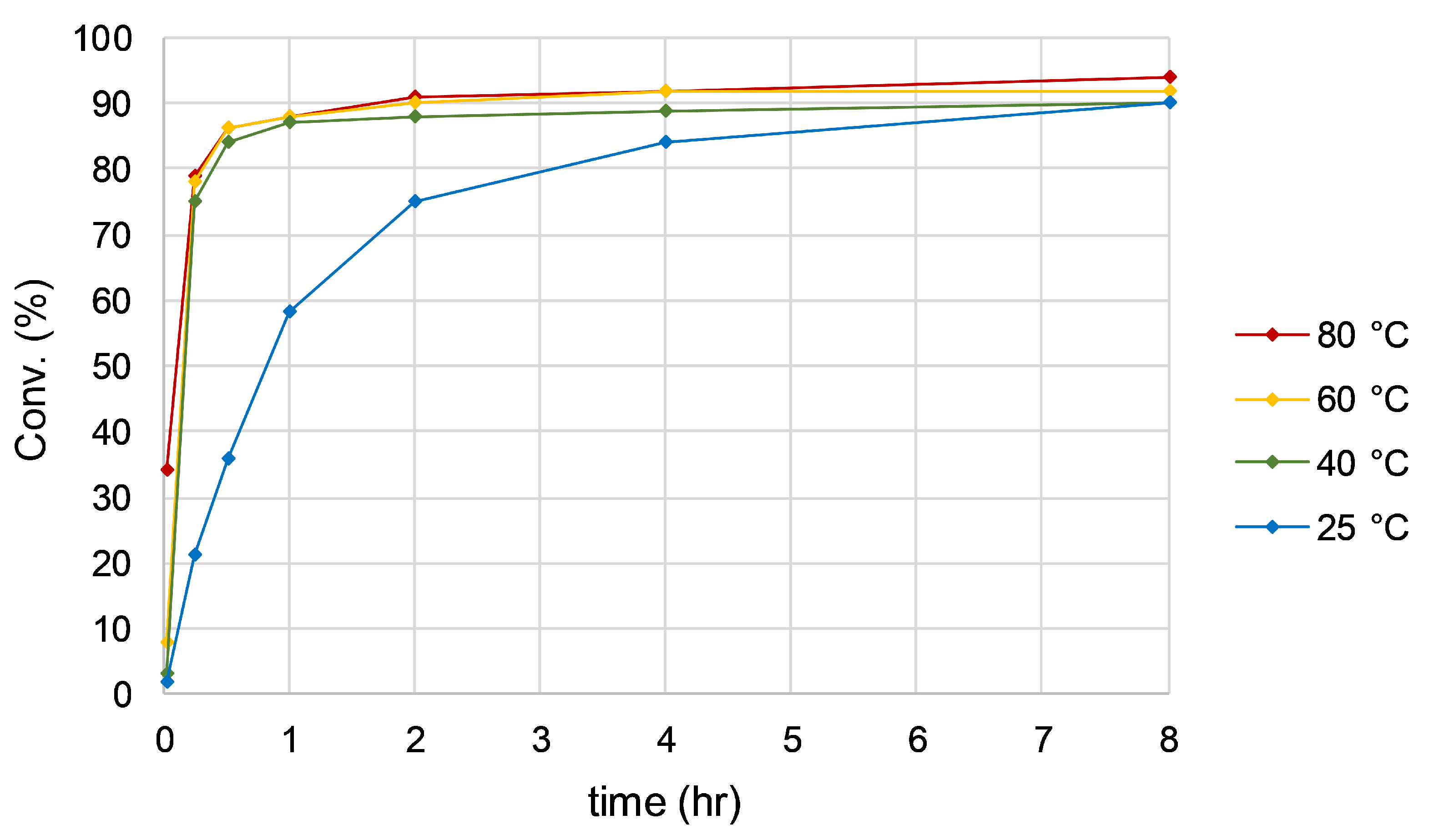
2.3.2. Optimization: Temperature
2.3.3. Optimization: Base
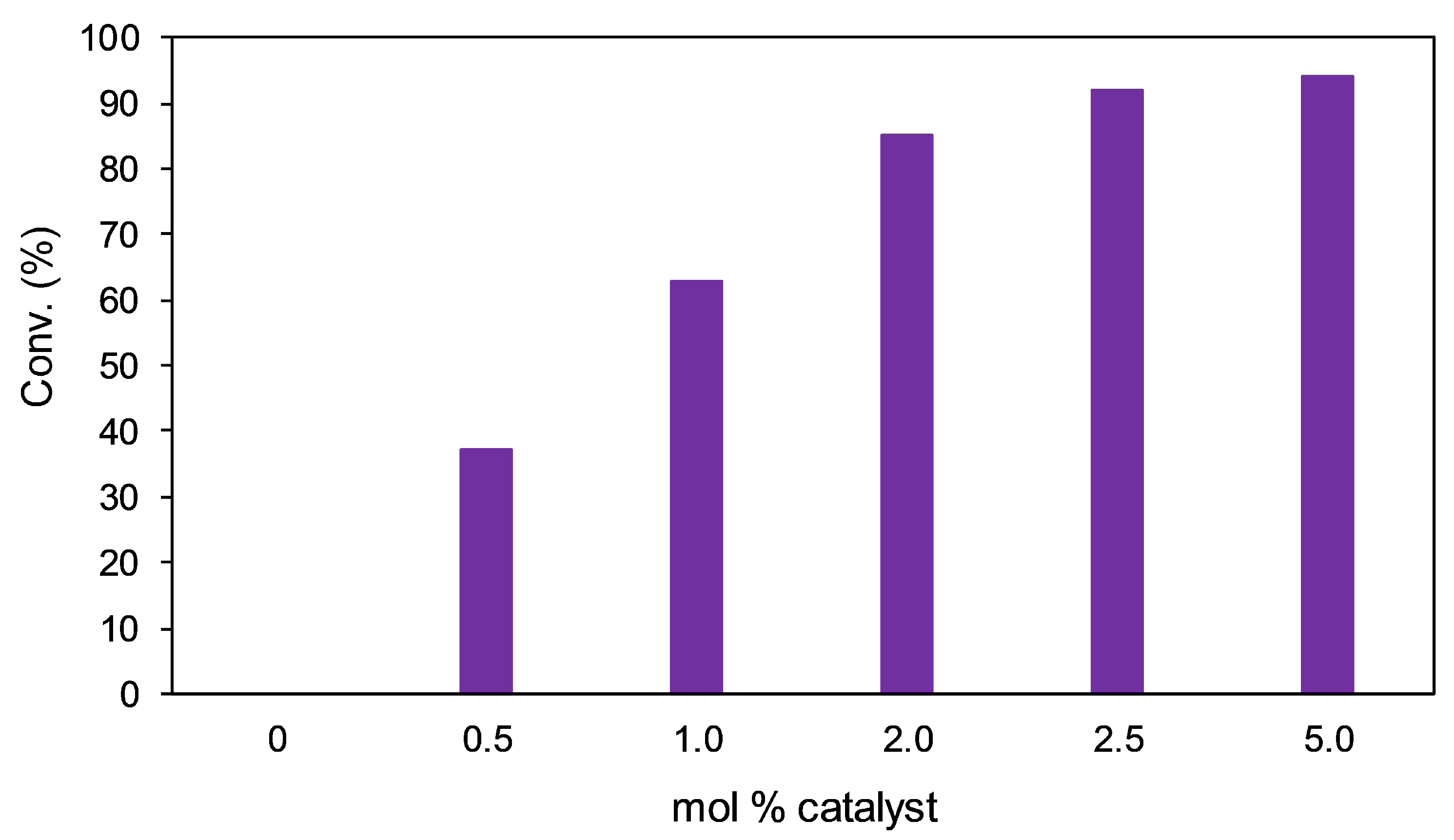
2.3.4. Optimization: Catalyst Loading
2.3.5. Optimized Results: Catalyst Scope
2.3.6. Optimized Results: Substrate Scope
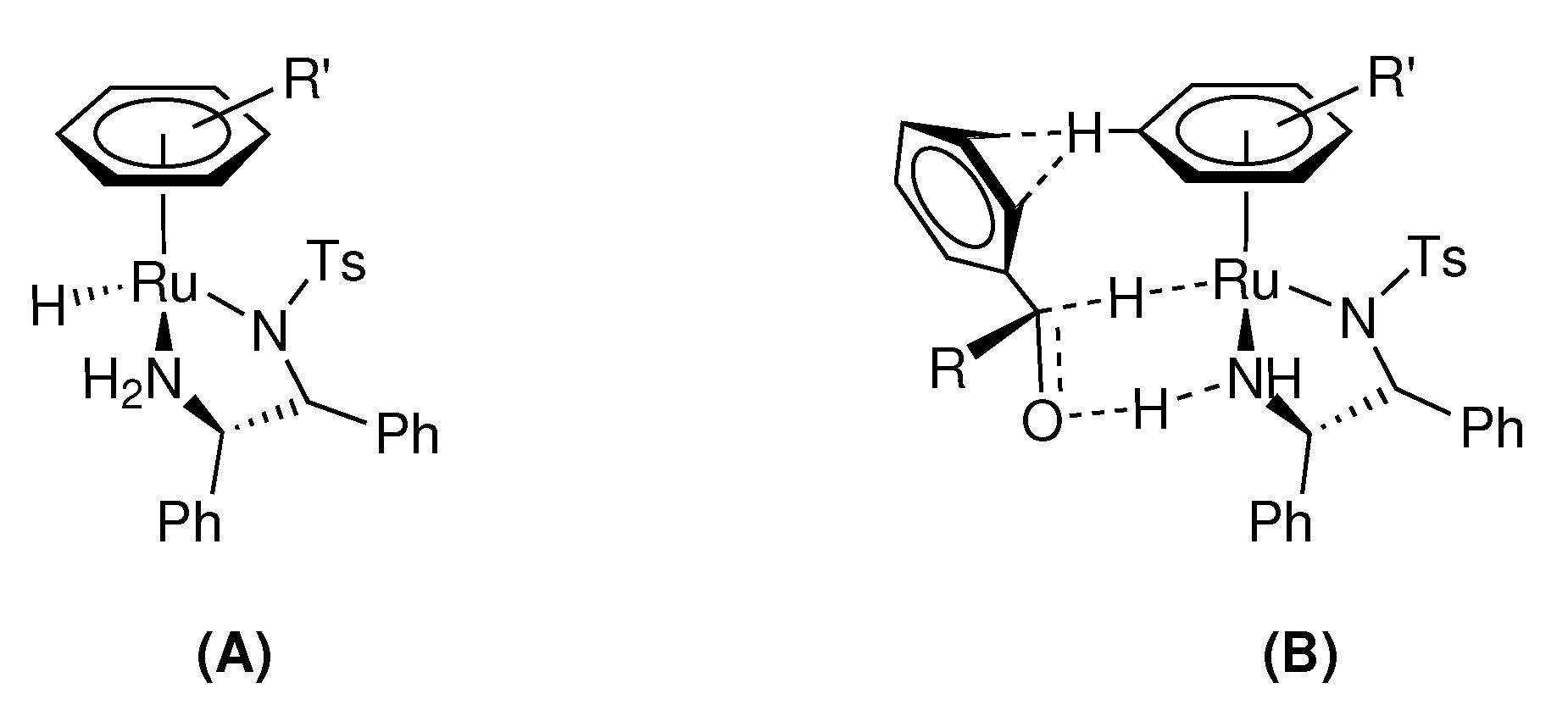
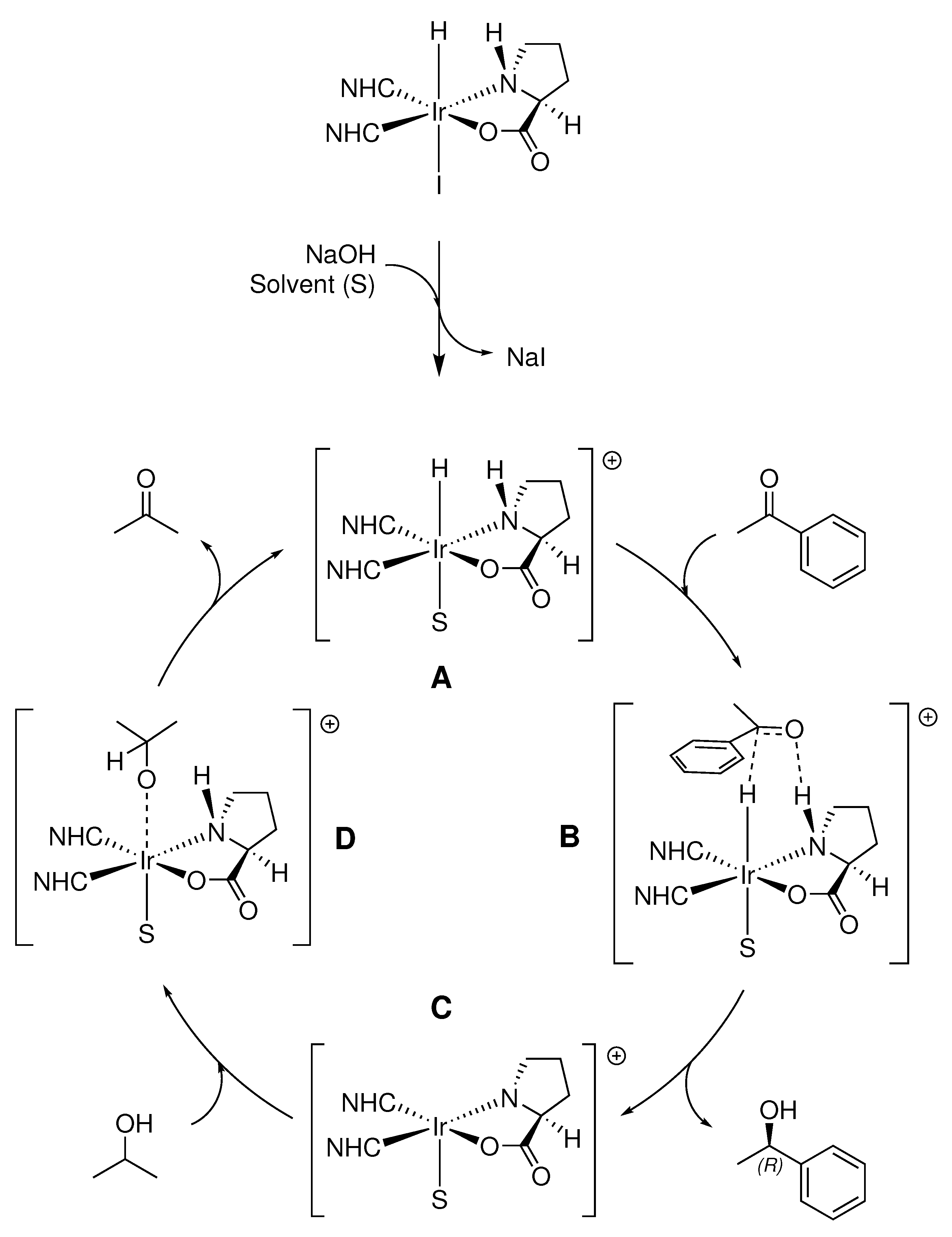
2.3.7. Proposed Catalytic Mechanism
3. Materials and Methods
3.1. Synthesis of Imidazolium Salts and Iridium Complex Precursors
3.2. General Procedure for the Synthesis of Ir(NHC)2(aa)(H)(X) Complexes
3.2.1. Synthesis of Ir(IMe)2(Gly)(H)(I)
3.2.2. Synthesis of Ir(IMe)2(l-Ala)(H)(I)
3.2.3. Synthesis of Ir(IMe)2(l-Val)(H)(I)
3.3. Synthesis of Ir(IMe)2(l-Phg)(H)(I)
3.3.1. Synthesis of Ir(IMe)2(l-Aze)(H)(I)
3.3.2. Synthesis of Ir(IMe)2(l-Pro)(H)(I)
3.3.3. Synthesis of Ir(IMe)2(d-Pro)(H)(I)
3.3.4. Synthesis of Ir(IMe)2(l-F-Pro)(H)(I)
3.3.5. Synthesis of Ir(IMe)2(l-Pip)(H)(I)
3.3.6. Synthesis of Ir(IEt)2(l-Pro)(H)(I)
3.3.7. Synthesis of Ir(IiPr)2(l-Pro)(H)(Cl)
3.4. General Procedure for Asymmetric Transfer Hydrogenation of Ketones
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| aa | Amino acid |
| Ala | Alanine |
| ATH | Asymmetric transfer hydrogenation |
| Aze | Azetidine-2-carboxylic acid |
| COD | 1,5-Cyclooctadiene |
| Cp* | Pentamethylcyclopentadienyl ligand |
| DMSO | Dimethyl sulfoxide |
| ee | Enantiomeric excess |
| F-Pro | cis-4-Fluoro-proline |
| FID | Flame ionization detector |
| GC | Gas chromatography |
| Gly | Glycine |
| HRMS/ESI+ | High resolution mass spectrometry/electrospray ionization |
| IiPr | 1,3-Diisopropylimidazol-2-ylidene |
| IEt | 1,3-Diethylimidazol-2-ylidene |
| IMe | 1,3-Dimethylimidazol-2-ylidene |
| IPA | Isopropyl alcohol |
| NHC | N-Heterocyclic carbene |
| NMR | Nuclear magnetic resonance |
| Phg | Phenylglycine |
| Pip | Pipecolic acid |
| Pro | Proline |
| TsDPEN | N-Tosyl-1,2-diphenylethylenediamine |
| Val | Valine |
References
- Breuer, M.; Ditrich, K.; Habicher, T.; Hauer, B.; Keßeler, M.; Stürmer, R.; Zelinski, T. Industrial methods for the production of optically active intermediates. Angew. Chem. Int. Ed. 2004, 43, 788–824. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Astruc, D. The golden age of transfer hydrogenation. Chem. Rev. 2015, 115, 6621–6686. [Google Scholar] [CrossRef]
- Hashiguchi, S.; Fujii, A.; Takehara, J.; Ikariya, T.; Noyori, R. Asymmetric transfer hydrogenation of aromatic ketones catalyzed by chiral ruthenium(II) complexes. J. Am. Chem. Soc. 1995, 117, 7562–7563. [Google Scholar] [CrossRef]
- Fujii, A.; Hashiguchi, S.; Uematsu, N.; Ikariya, T.; Noyori, R. Ruthenium(II)-catalyzed asymmetric transfer hydrogenation of ketones using a formic acid-Triethylamine mixture. J. Am. Chem. Soc. 1996, 118, 2521–2522. [Google Scholar] [CrossRef]
- Noyori, R.; Hashiguchi, S. Asymmetric transfer hydrogenation catalyzed by chiral ruthenium complexes. Acc. Chem. Res. 1997, 30, 97–102. [Google Scholar] [CrossRef]
- Noyori, R. Asymmetric catalysis: Science and opportunities (Nobel lecture). Angew. Chem. Int. Ed. 2002, 41, 2008. [Google Scholar] [CrossRef]
- Yamakawa, M.; Ito, H.; Noyori, R. The metal-ligand bifunctional catalysis: A theoretical study on the ruthenium(II)-catalyzed hydrogen transfer between alcohols and carbonyl compounds. J. Am. Chem. Soc. 2000, 122, 1466–1478. [Google Scholar] [CrossRef]
- Noyori, R.; Yamakawa, M.; Hashiguchi, S. Metal-ligand bifunctional catalysis: A nonclassical mechanism for asymmetric hydrogen transfer between alcohols and carbonyl compounds. J. Org. Chem. 2001, 66, 7931–7944. [Google Scholar] [CrossRef] [PubMed]
- Clapham, S.E.; Hadzovic, A.; Morris, R.H. Mechanisms of the H2-hydrogenation and transfer hydrogenation of polar bonds catalyzed by ruthenium hydride complexes. Coord. Chem. Rev. 2004, 248, 2201–2237. [Google Scholar] [CrossRef]
- Samec, J.S.M.; Bäckvall, J.E.; Andersson, P.G.; Brandt, P. Mechanistic aspects of transition metal-catalyzed hydrogen transfer reactions. Chem. Soc. Rev. 2006, 35, 237. [Google Scholar] [CrossRef] [PubMed]
- Eisenstein, O.; Crabtree, R.H. Outer sphere hydrogenation catalysis. New J. Chem. 2013, 37, 21–27. [Google Scholar] [CrossRef]
- Ikariya, T.; Blacker, A.J. Asymmetric transfer hydrogenation of ketones with bifunctional transition metal-based molecular catalysts. Acc. Chem. Res. 2007, 40, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Khusnutdinova, J.R.; Milstein, D. Metal-ligand cooperation. Angew. Chem. Int. Ed. 2015, 54, 12236–12273. [Google Scholar] [CrossRef] [PubMed]
- Dub, P.A.; Gordon, J.C. Metal–Ligand bifunctional catalysis: The “accepted” mechanism, the issue of concertedness, and the function of the ligand in catalytic cycles involving hydrogen atoms. ACS Catal. 2017, 7, 6635–6655. [Google Scholar] [CrossRef]
- Dub, P.A.; Gordon, J.C. The role of the metal-bound N–H functionality in Noyori-type molecular catalysts. Nat. Rev. Chem. 2018, 2, 396–408. [Google Scholar] [CrossRef]
- Agbossou-Niedercorn, F.; Michon, C. Bifunctional homogeneous catalysts based on first row transition metals in asymmetric hydrogenation. Coord. Chem. Rev. 2020, 425, 213523. [Google Scholar] [CrossRef]
- Gladiali, S.; Alberico, E. Asymmetric transfer hydrogenation: Chiral ligands and applications. Chem. Soc. Rev. 2006, 35, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, X.; Xiao, J. Broader, greener, and more efficient: Recent advances in asymmetric transfer hydrogenation. Chem. Asian J. 2008, 3, 1750–1770. [Google Scholar] [CrossRef] [PubMed]
- Stefane, B.; Pozgan, F. Asymmetric hydrogenation and transfer hydrogenation of ketones. In Hydrogenation; Karame, I., Ed.; InTechOpen: London, UK, 2012; Chapter 2. [Google Scholar] [CrossRef]
- Foubelo, F.; Nájera, C.; Yus, M. Catalytic asymmetric transfer hydrogenation of ketones: Recent advances. Tetrahedron Asymmetry 2015, 26, 769–790. [Google Scholar] [CrossRef]
- Baráth, E. Hydrogen transfer reactions of carbonyls, alkynes, and alkenes with noble metals in the presence of alcohols/ethers and amines as hydrogen donors. Catalysts 2018, 8, 671. [Google Scholar] [CrossRef]
- Matsunami, A.; Kayaki, Y. Upgrading and expanding the scope of homogeneous transfer hydrogenation. Tetrahedron Lett. 2018, 59, 504–513. [Google Scholar] [CrossRef]
- Seo, C.S.G.; Morris, R.H. Catalytic homogeneous asymmetric hydrogenation: Successes and opportunities. Organometallics 2019, 38, 47–65. [Google Scholar] [CrossRef]
- Ohta, T.; Nakahara, S.I.; Shigemura, Y.; Hattori, K.; Furukawa, I. α-Amino acid: An effective ligand for asymmetric catalysis of transfer hydrogenation of ketones. Appl. Organomet. Chem. 2001, 15, 699–709. [Google Scholar] [CrossRef]
- Carmona, D.; Lamata, M.P.; Oro, L.A. Half-sandwich complexes with aminocarboxylate ligands and their use as enantioselective hydrogen transfer catalysts. Eur. J. Inorg. Chem. 2002, 2002, 2239–2251. [Google Scholar] [CrossRef]
- Yim, A.S.; Wills, M. Asymmetric transfer hydrogenation using amino acid derivatives; further studies and a mechanistic proposal. Tetrahedron 2005, 61, 7994–8004. [Google Scholar] [CrossRef]
- Ahlford, K.; Ekström, J.; Zaitsev, A.B.; Ryberg, P.; Eriksson, L.; Adolfsson, H. Asymmetric transfer hydrogenation of ketones catalyzed by amino acid derived rhodium complexes: On the origin of enantioselectivity and enantioswitchability. Chem. Eur. J. 2009, 15, 11197–11209. [Google Scholar] [CrossRef] [PubMed]
- Paradowska, J.; Stodulski, M.; Mlynarski, J. Catalysts based on amino acids for asymmetric reactions in water. Angew. Chem. Int. Ed. 2009, 48, 4288–4297. [Google Scholar] [CrossRef] [PubMed]
- Nordin, M.; Liao, R.Z.; Ahlford, K.; Adolfsson, H.; Himo, F. Theoretical study of asymmetric transfer hydrogenation of ketones catalyzed by amino acid-derived rhodium complexes. ChemCatChem 2012, 4, 1095–1104. [Google Scholar] [CrossRef]
- Carmona, D.; Lahoz, F.J.; García-Orduña, P.; Oro, L.A.; Lamata, M.P.; Viguri, F. Half-sandwich complexes of osmium(II) with l-α-amino carboxylate ligands as asymmetric transfer hydrogenation catalysts. On the origin of the enantioselectivity. Organometallics 2012, 31, 3333–3345. [Google Scholar] [CrossRef]
- Carmona, D.; Viguri, F.; Pilar Lamata, M.; Ferrer, J.; Bardají, E.; Lahoz, F.J.; García-Orduña, P.; Oro, L.A. Ruthenium amino carboxylate complexes as asymmetric hydrogen transfer catalysts. Dalton Trans. 2012, 41, 10298. [Google Scholar] [CrossRef] [PubMed]
- Biancalana, L.; Abdalghani, I.; Chiellini, F.; Zacchini, S.; Pampaloni, G.; Crucianelli, M.; Marchetti, F. Ruthenium arene complexes with α-aminoacidato ligands: New insights into transfer hydrogenation reactions and cytotoxic behaviour. Eur. J. Inorg. Chem. 2018, 2018, 3041–3057. [Google Scholar] [CrossRef]
- Morris, D.M.; McGeagh, M.; De Peña, D.; Merola, J.S. Extending the range of pentasubstituted cyclopentadienyl compounds: The synthesis of a series of tetramethyl(alkyl or aryl)cyclopentadienes (Cp*R), their iridium complexes and their catalytic activity for asymmetric transfer hydrogenation. Polyhedron 2014, 84, 120–135. [Google Scholar] [CrossRef]
- Morris, D.M. Design and Modification of Half-Sandwich Ir(III), Rh(III), and Ru(II) Amino acid Complexes for Application in Asymmetric Transfer Hydrogenation Reactions. Ph.D. Thesis, Virginia Tech, Blacksburg, VA, USA, 2015. [Google Scholar]
- Herrmann, W.A. N-heterocyclic carbenes: A new concept in organometallic catalysis. Angew. Chem. Int. Ed. 2002, 41, 1290–1309. [Google Scholar] [CrossRef]
- Hopkinson, M.N.; Richter, C.; Schedler, M.; Glorius, F. An overview of N-heterocyclic carbenes. Nat. Chem. 2014, 510, 485–496. [Google Scholar] [CrossRef]
- César, V.; Bellemin-Laponnaz, S.; Gade, L.H. Chiral N-heterocyclic carbenes as stereodirecting ligands in asymmetric catalysis. Chem. Soc. Rev. 2004, 33, 619–636. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Candish, L.; Paul, D.; Glorius, F. N-heterocyclic carbenes in asymmetric hydrogenation. ACS Catal. 2016, 6, 5978–5988. [Google Scholar] [CrossRef]
- Janssen-Müller, D.; Schlepphorst, C.; Glorius, F. Privileged chiral N-heterocyclic carbene ligands for asymmetric transition-metal catalysis. Chem. Soc. Rev. 2017, 46, 4845–4854. [Google Scholar] [CrossRef] [PubMed]
- Hillier, A.C.; Lee, H.M.; Stevens, E.D.; Nolan, S.P. Cationic iridium complexes bearing imidazol-2-ylidene ligands as transfer hydrogenation catalysts. Organometallics 2001, 20, 4246–4252. [Google Scholar] [CrossRef]
- Albrecht, M.; Crabtree, R.H.; Mata, J.; Peris, E. Chelating bis-carbene rhodium(III) complexes in transfer hydrogenation of ketones and imines. Chem. Commun. 2002, 2, 32–33. [Google Scholar] [CrossRef]
- Poyatos, M.; Mata, J.A.; Falomir, E.; Crabtree, R.H.; Peris, E. New ruthenium(II) cnc-pincer bis(carbene) complexes: Synthesis and catalytic activity. Organometallics 2003, 22, 1110–1114. [Google Scholar] [CrossRef]
- Cross, W.B.; Daly, C.G.; Boutadla, Y.; Singh, K. Variable coordination of amine functionalised N-heterocyclic carbene ligands to Ru, Rh and Ir: C–H and N–H activation and catalytic transfer hydrogenation. Dalton Trans. 2011, 40, 9722. [Google Scholar] [CrossRef]
- Seo, H.; Kim, B.Y.; Lee, J.H.; Park, H.J.; Son, S.U.; Chung, Y.K. Synthesis of chiral ferrocenyl imidazolium salts and their rhodium(I) and iridium(I) complexes. Organometallics 2003, 22, 4783–4791. [Google Scholar] [CrossRef]
- Herrmann, W.A.; Baskakov, D.; Herdtweck, E.; Hoffmann, S.D.; Bunlaksananusorn, T.; Rampf, F.; Rodefeld, L. Chiral N-heterocyclic carbene ligands derived from 2,2′-bipiperidine and partially reduced biisoquinoline: Rhodium and iridium complexes in asymmetric catalysis. Organometallics 2006, 25, 2449–2456. [Google Scholar] [CrossRef]
- Dyson, G.; Frison, J.C.; Whitwood, A.C.; Douthwaite, R.E. Synthesis of rhodium(I) and iridium(I) complexes of chiral N-heterocyclic carbenes and their application to asymmetric transfer hydrogenation. Dalton Trans. 2009, 7141. [Google Scholar] [CrossRef]
- Diez, C.; Nagel, U. Chiral iridium(I) bis(NHC) complexes as catalysts for asymmetric transfer hydrogenation. Appl. Organomet. Chem. 2010, 24, 509–516. [Google Scholar] [CrossRef]
- Chiyojima, H.; Sakaguchi, S. Iridium complex bearing a chiral hydroxy-amide functionalized N-heterocyclic carbene: A catalyst precursor for asymmetric transfer hydrogenation. Tetrahedron Lett. 2011, 52, 6788–6791. [Google Scholar] [CrossRef]
- Sabater, S.; Baya, M.; Mata, J.A. Highly active cp*Ir catalyst at low temperatures bearing an N-heterocyclic carbene ligand and a chelated primary benzylamine in transfer hydrogenation. Organometallics 2014, 33, 6830–6839. [Google Scholar] [CrossRef]
- Ramasamy, B.; Kumar Gangwar, M.; Ghosh, P. Chiral oxazolidine-fused N-heterocyclic carbene complexes of rhodium and iridium and their utility in the asymmetric transfer hydrogenation of ketones. Eur. J. Inorg. Chem. 2017, 2017, 3253–3268. [Google Scholar] [CrossRef]
- Iglesias, M.; Oro, L.A. A leap forward in iridium–NHC catalysis: New horizons and mechanistic insights. Chem. Soc. Rev. 2018, 47, 2772–2808. [Google Scholar] [CrossRef]
- Ramasamy, B.; Gangwar, M.K.; Ghosh, P. Asymmetric transfer hydrogenation of α,β-unsaturated carbonyl compounds to saturated alcohols as catalyzed by iridium complexes of tricyclic bioxazoline-fused imidazole-derived N-heterocyclic carbene ligands. ChemistrySelect 2019, 4, 357–365. [Google Scholar] [CrossRef]
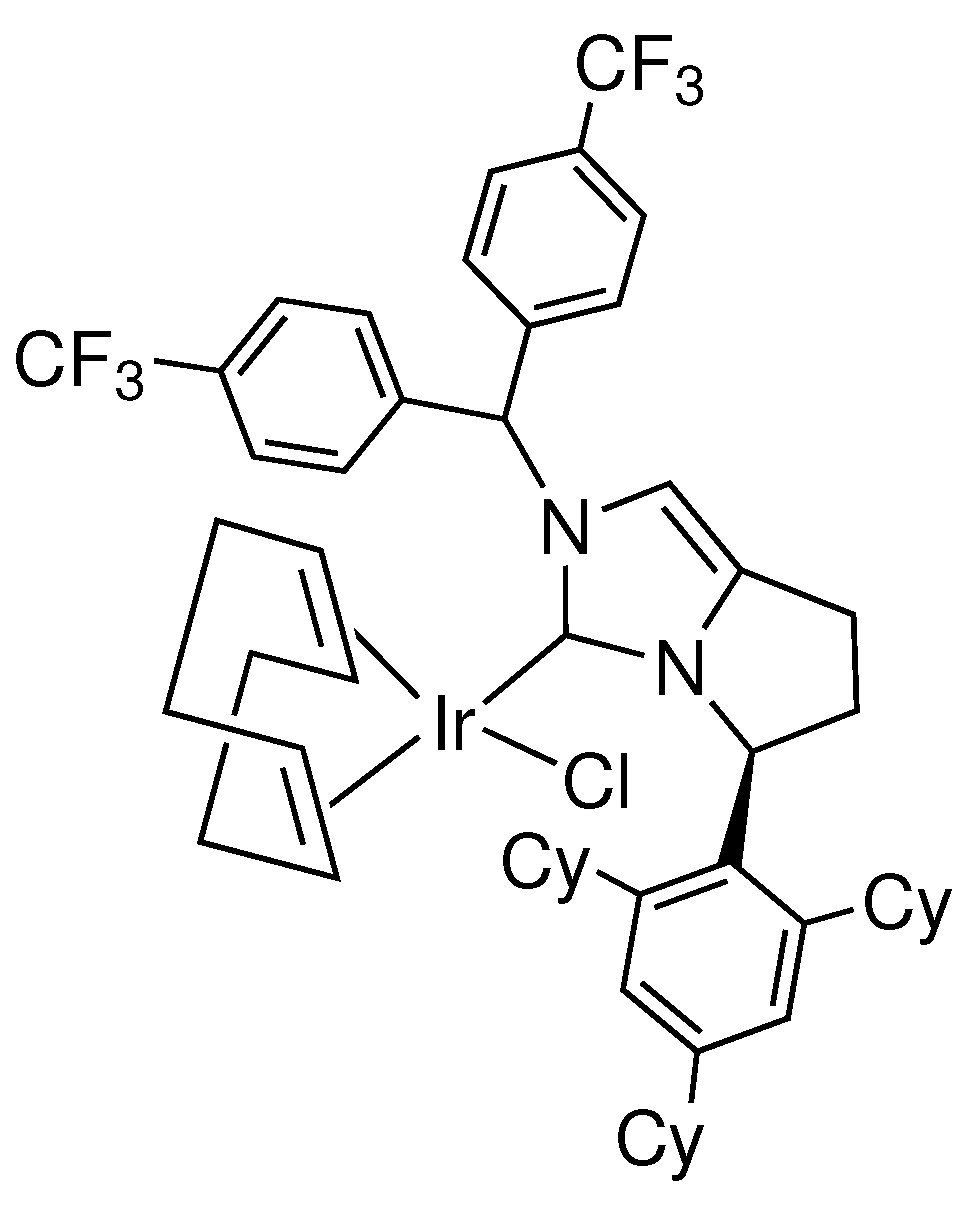
- Yoshida, K.; Kamimura, T.; Kuwabara, H.; Yanagisawa, A. Chiral bicyclic NHC/Ir complexes for catalytic asymmetric transfer hydrogenation of ketones. Chem. Commun. 2015, 51, 15442–15445. [Google Scholar] [CrossRef]
- CrysAlisPRO; Oxford Diffraction/Agilent Technologies UK Ltd.: Yarnton, UK, 2018.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Bruno, I.J.; Cole, J.C.; Edgington, P.R.; Kessler, M.; Macrae, C.F.; McCabe, P.; Pearson, J.; Taylor, R. New software for searching the Cambridge Structural Database and visualizing crystal structures. Acta Crystallogr. Sect. B Struct. Sci. 2002, 58, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Gardner, S.; Kawamoto, T.; Curran, D.P. Synthesis of 1,3-Dialkylimidazol-2-ylidene Boranes from 1,3-Dialkylimidazolium Iodides and Sodium Borohydride. J. Org. Chem. 2015, 80, 9794–9797. [Google Scholar] [CrossRef]
- Huang, S.; Qi, X.; Liu, T.; Wang, K.; Zhang, W.; Li, J.; Zhang, Q. Towards Safer Rocket Fuels: Hypergolic Imidazolylidene-Borane Compounds as Replacements for Hydrazine Derivatives. Chem. A Eur. J. 2016, 22, 10187–10193. [Google Scholar] [CrossRef] [PubMed]
- Baghurst, D.R.; Michael, D.; Mingos, P.; Watson, M.J. Application of microwave dielectric loss heating effects for the rapid and convenient synthesis of organometallic compounds. J. Organomet. Chem. 1989, 368, C43–C45. [Google Scholar] [CrossRef]
- Bernier, C.M.; DuChane, C.M.; Merola, J.S. Crystal structures of (η4 -cycloocta-1,5-diene)bis(1,3-dimethylimidazol-2-ylidene)iridium(I) iodide and (η4 -cycloocta-1,5-diene)bis(1,3-diethylimidazol-2-ylidene)iridium(I) iodide. Acta Crystallogr. Sect. E Crystallogr. Commun. 2020, 76, 611–614. [Google Scholar] [CrossRef] [PubMed]



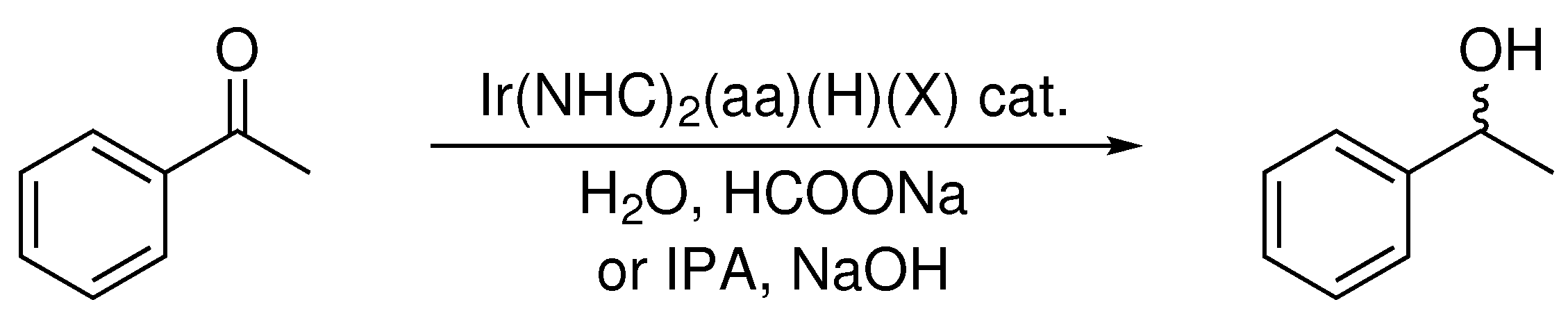



| NHC | Amino Acid | Yield (%) |
|---|---|---|
| IMe a | glycine | 53 |
| l-alanine | 78 | |
| l-valine | 87 | |
| l-phenylglycine | 66 | |
| l-azetidine-2-carboxylic acid | 55 | |
| l-proline | 92 | |
| d-proline | 72 | |
| cis-4-fluoro-l-proline | 44 | |
| l-pipecolic acid | 66 | |
| IEt a | l-proline | 68 |
| IiPr b | l-proline | 82 |
| Atoms | Bond Lengths (Å) |
| Ir1–C1 | 1.986 |
| Ir1–C4 | 2.004 |
| Ir1–O1 | 2.231 |
| Ir1–N5 | 2.157 |
| Ir1–I1 | 2.738 |
| Atoms | Bond Angles (°) |
| C1–Ir1–C4 | 89.39 |
| C1–Ir1–O1 | 91.49 |
| C1–Ir1–N5 | 90.53 |
| C4–Ir1–O1 | 101.52 |
| C4–Ir1–I1 | 93.28 |
| O1–Ir1–I1 | 91.60 |
| N5–Ir1–I1 | 86.85 |
| Solvent | NHC | Amino Acid | Conv. (%) d |
|---|---|---|---|
| H2O a | IMe c | glycine | 44 |
| l-alanine | 50 | ||
| l-proline | 59 | ||
| IPA b | IMe c | glycine | 76 |
| l-alanine | 86 | ||
| l-proline | 98 |
| NHC | Amino Acid | Conv. (%) b | ee (%) b | Conf. |
|---|---|---|---|---|
| IMe a | glycine | 75 | 0 | – |
| l-alanine | 81 | 3 | R | |
| l-valine | 74 | 5 | R | |
| l-phenylglycine | 77 | 6 | R | |
| l-azetidine-2-carboxylic acid | 83 | 17 | R | |
| l-proline | 98 | 68 | R | |
| d-proline | 95 | 68 | S | |
| l-pipecolic acid | 82 | 4 | S |
| NHC | Amino Acid | Conv. (%) | ee (%) | Conf. |
|---|---|---|---|---|
| IMe a | glycine | 46 ± 2 | 0 | – |
| l-alanine | 52 ± 1 | 9 ± 1 | R | |
| l-valine | 45 ± 3 | 6 ± 1 | R | |
| l-phenylglycine | 49 ± 2 | 8 ± 1 | R | |
| l-azetidine-2-carboxylic acid | 56 ± 1 | 67 ± 2 | R | |
| l-proline | 91 ± 1 | 92 ± 1 | R | |
| d-proline | 92 ± 2 | 92 ± 1 | S | |
| cis-4-fluoro-l-proline | 44 ± 2 | 89 ± 2 | R | |
| l-pipecolic acid | 55 ± 3 | 17 ± 1 | S | |
| IEt a | l-proline | 98 ± 1 | 55 ± 2 | R |
| IiPr b | l-proline | 98 ± 1 | 19 ± 1 | R |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernier, C.M.; Merola, J.S. Design of Iridium N-Heterocyclic Carbene Amino Acid Catalysts for Asymmetric Transfer Hydrogenation of Aryl Ketones. Catalysts 2021, 11, 671. https://doi.org/10.3390/catal11060671
Bernier CM, Merola JS. Design of Iridium N-Heterocyclic Carbene Amino Acid Catalysts for Asymmetric Transfer Hydrogenation of Aryl Ketones. Catalysts. 2021; 11(6):671. https://doi.org/10.3390/catal11060671
Chicago/Turabian StyleBernier, Chad M., and Joseph S. Merola. 2021. "Design of Iridium N-Heterocyclic Carbene Amino Acid Catalysts for Asymmetric Transfer Hydrogenation of Aryl Ketones" Catalysts 11, no. 6: 671. https://doi.org/10.3390/catal11060671
APA StyleBernier, C. M., & Merola, J. S. (2021). Design of Iridium N-Heterocyclic Carbene Amino Acid Catalysts for Asymmetric Transfer Hydrogenation of Aryl Ketones. Catalysts, 11(6), 671. https://doi.org/10.3390/catal11060671