Visible-Light Enhanced Catalytic Wet Peroxide Oxidation of Natural Organic Matter in the Presence of Al/Fe-Pillared Clay
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Feautures of the Al/Fe-PILC Clay Catalyst
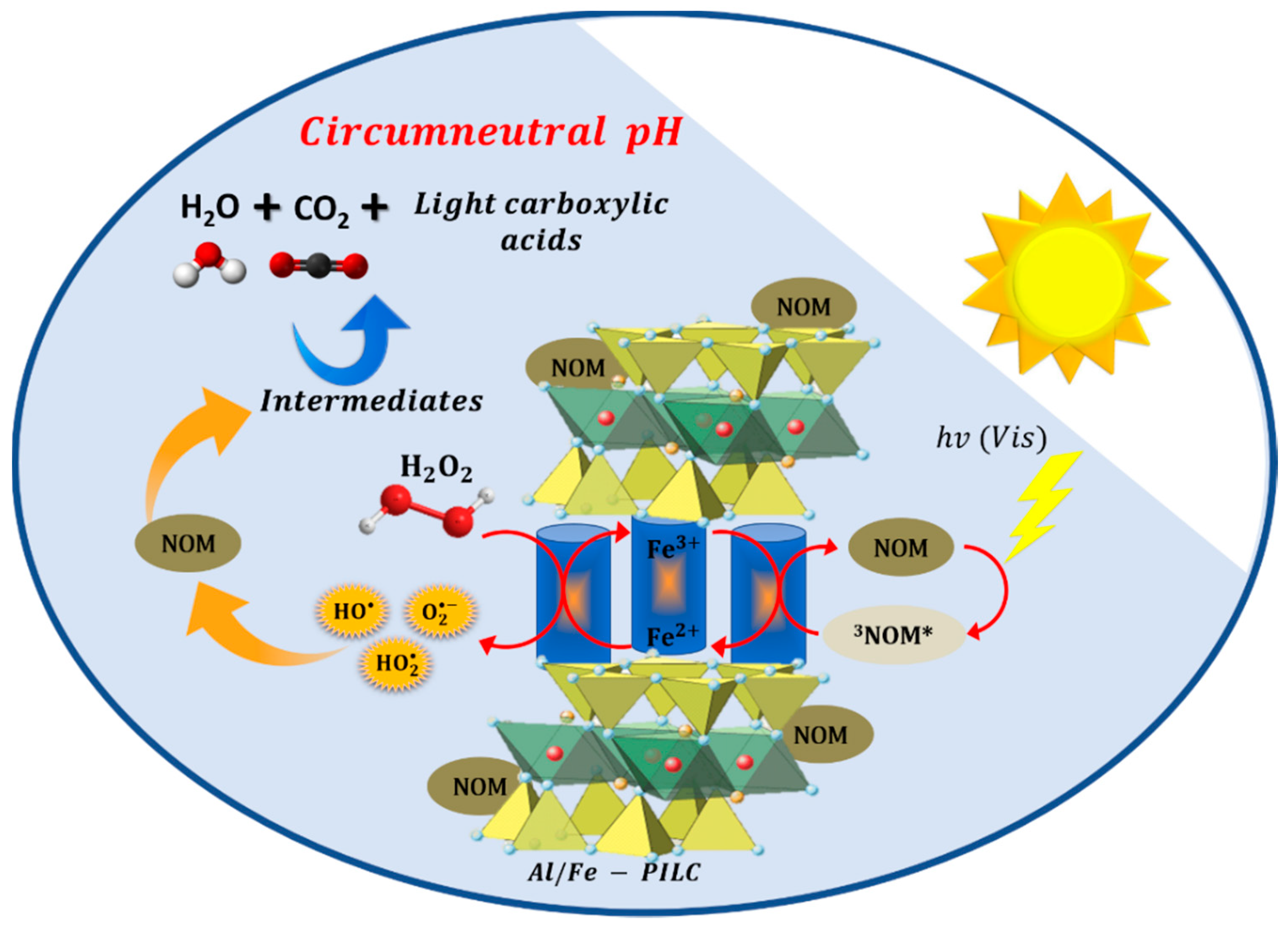
2.2. Photo-CWPO Degradation of Natural Organic Matter
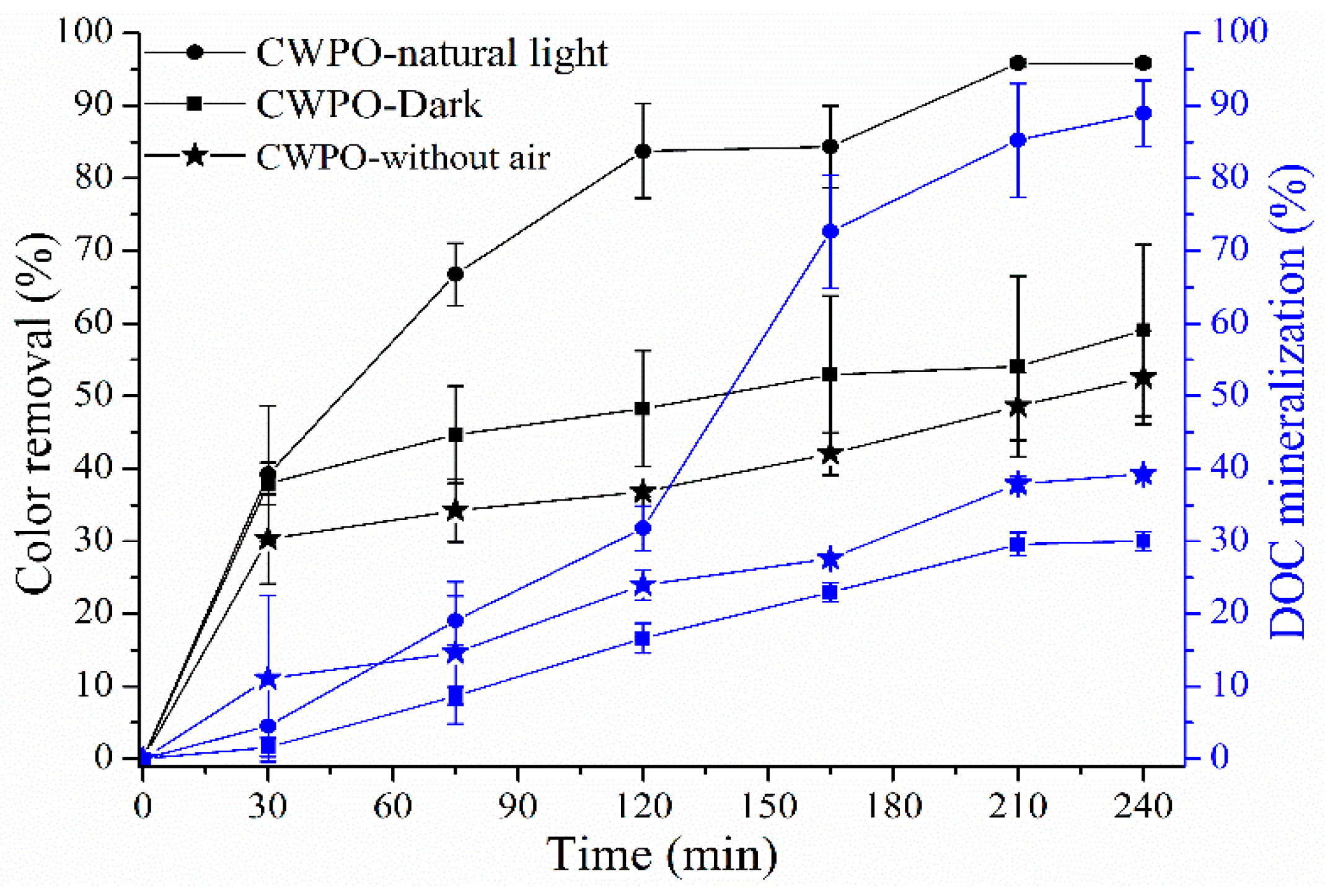
2.2.1. CWPO Degradation of NOM under Optimal Conditions
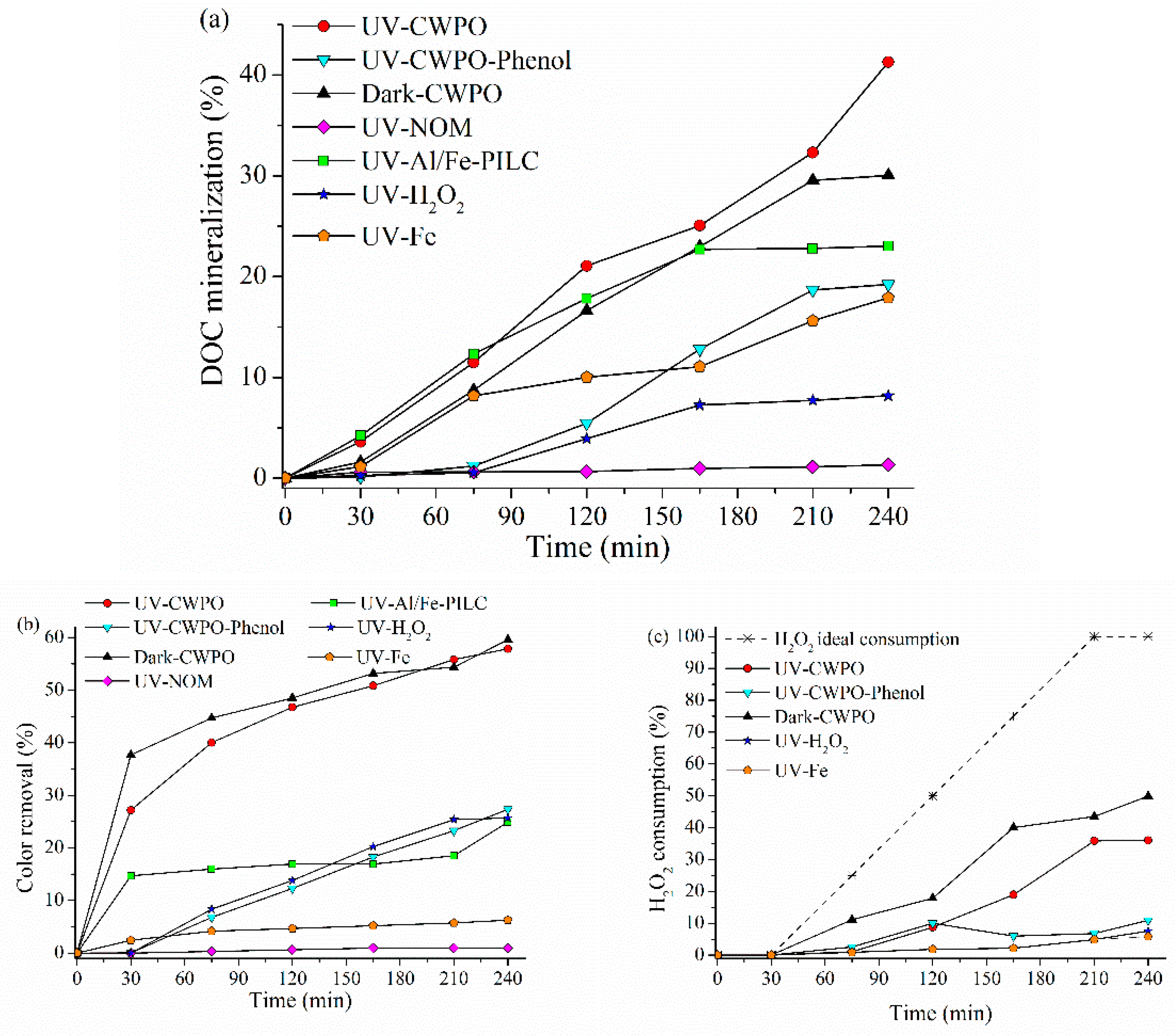
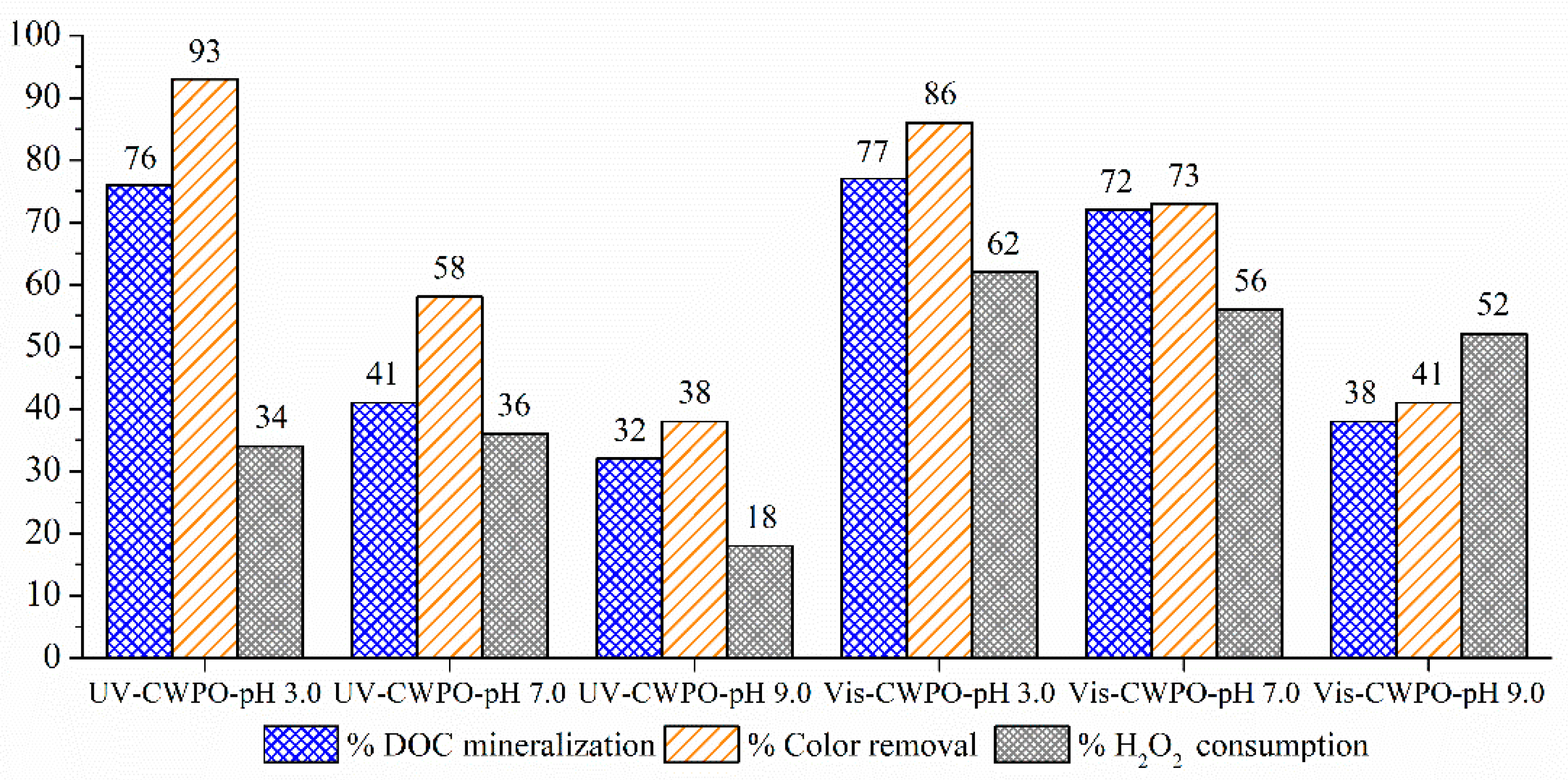
2.2.2. Photo-CWPO under Ultraviolet (UV) Irradiation
2.2.3. Photo-CWPO under Visible (Vis) Irradiation
2.2.4. PH Effect in the Visible-Light Enhanced CWPO
3. Materials and Methods
3.1. Materials
3.2. Al/Fe-PILC Catalyst
Physicochemical Characterization of the Al/Fe-PILC Catalyst
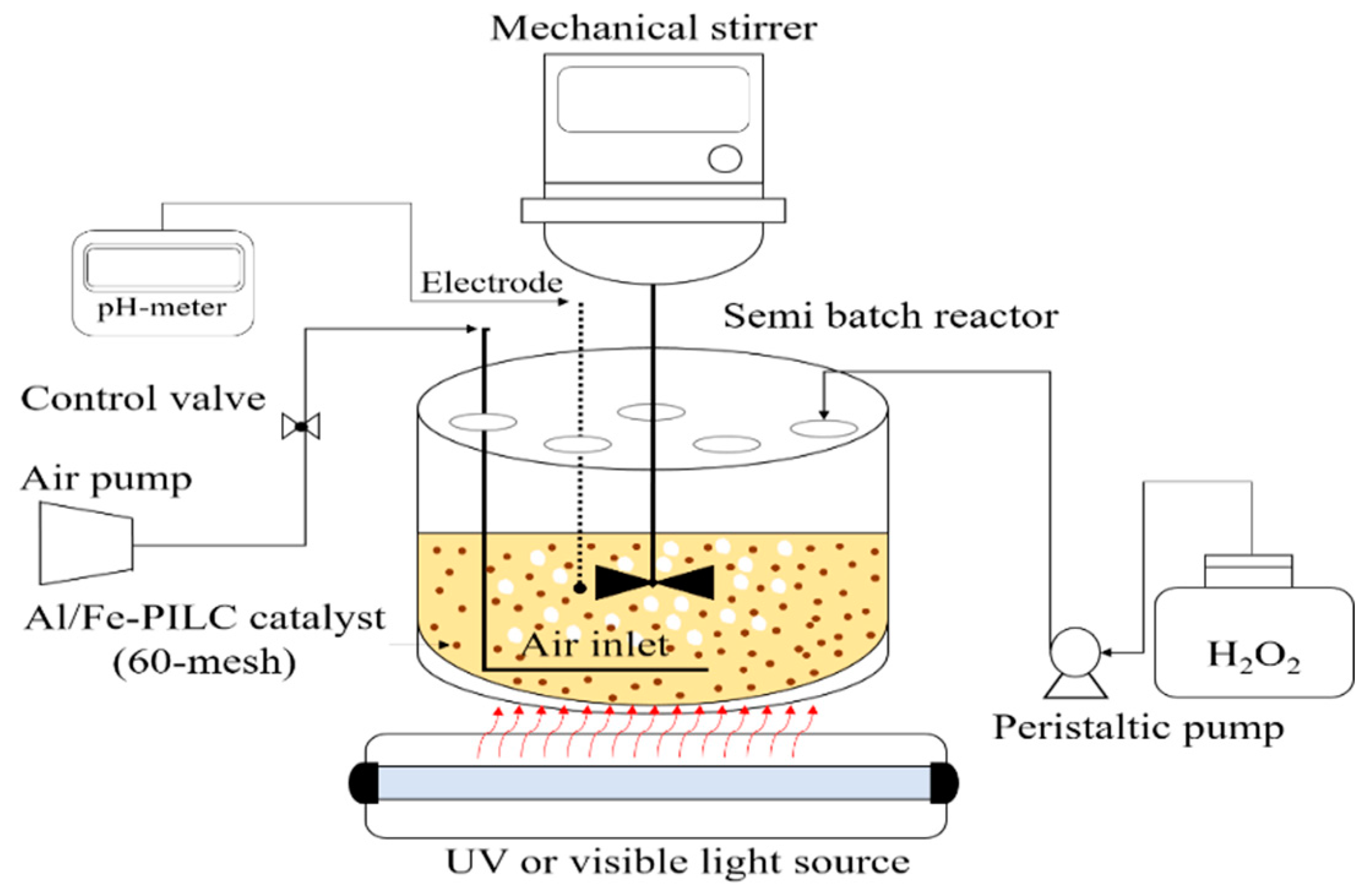
3.3. Photo-CWPO Tests
3.4. Analytic Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sorlini, S. Natural Organic Matter: Characterization and Removal by AOPs to Assist Drinking Water Facilities. In Applications of Advanced Oxidation Processes (AOPs) in Drinking Water Treatment, 1st ed.; Gil, A., Galeano, L.A., Vicente, M.A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; Volume 67, pp. 53–68. [Google Scholar] [CrossRef]
- Valencia, S.; Marín, J.; Restrepo, G. Photocatalytic Degradation of Humic Acids with Titanium Dioxide Embedded into Polyethylene Pellets to Enhance the Postrecovery of Catalyst. Environ. Eng. Sci. 2017, 35, 185–193. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Thomas, N.; Dionysiou, D.D.; Pillai, S.C. Heterogeneous Fenton catalysts: A review of recent advances. J. Hazard. Mater. 2021, 404, 124082. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Li, T.; Zhang, C.-Z.; Fan, X.; Li, Y.; Song, M. Degradation of oxidized multi-walled carbon nanotubes in water via photo-Fenton method and its degradation mechanism. Chem. Eng. J. 2017, 323, 37–46. [Google Scholar] [CrossRef]
- Vilhunen, S.H.; Sillanpää, M.E.T. Ultraviolet light emitting diodes and hydrogen peroxide in the photodegradation of aqueous phenol. J. Hazard. Mater. 2009, 161, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Sillanpää, M.; Ncibi, M.C.; Matilainen, A. Advanced oxidation processes for the removal of natural organic matter from drinking water sources: A comprehensive review. J. Environ. Manage. 2018, 208, 56–76. [Google Scholar] [CrossRef] [PubMed]
- Martínez, F.; Calleja, G.; Melero, J.A.; Molina, R. Iron species incorporated over different silica supports for the heterogeneous photo-Fenton oxidation of phenol. Appl. Catal. B Environ. 2007, 70, 452–460. [Google Scholar] [CrossRef]
- González-Bahamón, L.F.; Hoyos, D.F.; Benítez, N.; Pulgarín, C. New Fe-immobilized natural bentonite plate used as photo-Fenton catalyst for organic pollutant degradation. Chemosphere 2011, 82, 1185–1189. [Google Scholar] [CrossRef]
- Lu, M.; Wang, J.; Wang, Y.; He, Z. Heterogeneous Photo-Fenton Catalytic Degradation of Practical Pharmaceutical Wastewater by Modified Attapulgite Supported Multi-Metal Oxides. Water 2021, 13, 156. [Google Scholar] [CrossRef]
- Truong, H.B.; Huy, B.T.; Ray, S.K.; Lee, Y.-I.; Cho, J.; Hur, J. H2O2-assisted photocatalysis for removal of natural organic matter using nanosheet C3N4-WO3 composite under visible light and the hybrid system with ultrafiltration. Chem. Eng. J. 2020, 399, 125733. [Google Scholar] [CrossRef]
- Gil, A. Materiales porosos basados en arcillas pilareadas: Control de su estructura para aplicaciones medioambientales y energéticas. Av. Cienc. Ing. 2012, 3, 137–148. [Google Scholar]
- Muñoz, H.-J.; Vallejo, C.; Blanco, C.; Gil, A.; Vicente, M.-Á.; Ramírez, J.-H.; Galeano, L.-A. 10 kg scaled-up preparation of Al/Fe-pillared clay CWPO catalysts from concentrated precursors. Green Chem. 2018, 20, 5196–5208. [Google Scholar] [CrossRef]
- Vallejo, C.A.; Galeano, L.A.; Trujillano, R.; Vicente, M.Á.; Gil, A. Preparation of Al/Fe-PILC clay catalysts from concentrated precursors: Enhanced hydrolysis of pillaring metals and intercalation. RSC Adv. 2020, 10, 40450–40460. [Google Scholar] [CrossRef]
- Ramírez, J.H.; Galeano, L.A.; Pinchao, G.; Bedoya, R.A.; Hidalgo, A. Optimized CWPO phenol oxidation in CSTR reactor catalyzed by Al/Fe-PILC from concentrated precursors at circumneutral pH. J. Environ. Chem. Eng. 2018, 6, 2429–2441. [Google Scholar] [CrossRef]
- Garcia-Mora, A.M.; Torres-Palma, R.A.; García, H.; Hidalgo-Troya, A.; Galeano, L.-A. Removal of dissolved natural organic matter by the Al/Fe pillared clay-activated-catalytic wet peroxide oxidation: Statistical multi-response optimization. J. Water Process Eng. 2021, 39, 101755. [Google Scholar] [CrossRef]
- Ordoñez-Ordoñez, A.; Revelo-Romo, D.M.; Garcia-Mora, A.M.; Hidalgo-Troya, A.; Galeano, L.-A. MS2 coliphage inactivation by Al/Fe PILC-activated Catalytic Wet Peroxide Oxidation: Multiresponse statistical optimization. Heliyon 2019, 5, e01892. [Google Scholar] [CrossRef]
- Khankhasaeva, S.T.; Badmaeva, S.V. Removal of p-aminobenzenesulfanilamide from water solutions by catalytic photo-oxidation over Fe-pillared clay. Water Res. 2020, 185, 116212. [Google Scholar] [CrossRef] [PubMed]
- Truong, H.B.; Huy, B.T.; Ly, Q.V.; Lee, Y.-I.; Hur, J. Visible light-activated degradation of natural organic matter (NOM) using zinc-bismuth oxides-graphitic carbon nitride (ZBO-CN) photocatalyst: Mechanistic insights from EEM-PARAFAC. Chemosphere 2019, 224, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Q.; Li, J.-M.; Liu, W.-B. Effect of F, V and Mn co-doping on the catalytic performance of TiO2-pillared bentonite in the photocatalytic denitration. J. Fuel Chem. Technol. 2020, 48, 1131–1139. [Google Scholar] [CrossRef]
- Hadjltaief, H.B.; Zina, M.B.; Galvez, M.E.; Da Costa, P. Photo-Fenton oxidation of phenol over a Cu-doped Fe-pillared clay. C. R. Chim. 2015, 18, 1161–1169. [Google Scholar] [CrossRef]
- Guimarães, V.; Teixeira, A.R.; Lucas, M.S.; Silva, A.M.T.; Peres, J.A. Pillared interlayered natural clays as heterogeneous photocatalysts for H2O2-assisted treatment of a winery wastewater. Sep. Purif. Technol. 2019, 228, 115768. [Google Scholar] [CrossRef]
- Parker, K.M.; Pignatello, J.J.; Mitch, W.A. Influence of Ionic Strength on Triplet-State Natural Organic Matter Loss by Energy Transfer and Electron Transfer Pathways. Environ. Sci. Technol. 2013, 47, 10987–10994. [Google Scholar] [CrossRef]
- Richard, C.; Canonica, S. Aquatic Phototransformation of Organic Contaminants Induced by Coloured Dissolved Natural Organic Matter. In Environmental Photochemistry Part II; Boule, P., Bahnemann, D.W., Robertson, P.K.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 299–323. [Google Scholar]
- Li, Y.; Pan, Y.; Lian, L.; Yan, S.; Song, W.; Yang, X. Photosensitized degradation of acetaminophen in natural organic matter solutions: The role of triplet states and oxygen. Water Res. 2017, 109, 266–273. [Google Scholar] [CrossRef]
- Muñoz, H.-J.; Blanco, C.; Gil, A.; Vicente, M.-Á.; Galeano, L.-A. Preparation of Al/Fe-Pillared Clays: Effect of the Starting Mineral. Materials 2017, 10, 1364. [Google Scholar] [CrossRef] [PubMed]
- Kooli, F. Pillared montmorillontes from unusual antiperspirant aqueous solutions: Characterization and catalytic tests. Microporous Mesoporous Mater. 2013, 167, 228–236. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 9–10. [Google Scholar] [CrossRef]
- Galeano, L.-A.; Vicente, M.Á.; Gil, A. Catalytic Degradation of Organic Pollutants in Aqueous Streams by Mixed Al/M-Pillared Clays (M = Fe, Cu, Mn). Catal. Rev. 2014, 56, 239–287. [Google Scholar] [CrossRef]
- García Mora, A.M.; Portilla Delgado, C.S.; Torres Palma, R.A.; Hidalgo Troya, A.; Galeano, L.A. Catalytic wet peroxide oxidation of natural organic matter to enhance the treatment of real surface water at urban and rural drinking water plants. J. Water Process Eng. 2021. accepted. [Google Scholar]
- Rezaee, R.; Maleki, A.; Jafari, A.; Mazloomi, S.; Zandsalimi, Y.; Mahvi, A.H. Application of response surface methodology for optimization of natural organic matter degradation by UV/H2O2 advanced oxidation process. J. Environ. Health Sci. Eng. 2014, 12, 67. [Google Scholar] [CrossRef]
- Santos-Juanes, L.; Sánchez, J.L.G.; López, J.L.C.; Oller, I.; Malato, S.; Sánchez Pérez, J.A. Dissolved oxygen concentration: A key parameter in monitoring the photo-Fenton process. Appl. Catal. B Environ. 2011, 104, 316–323. [Google Scholar] [CrossRef]
- Jung, H.-J.; Hong, J.-S.; Suh, J.-K. A comparison of fenton oxidation and photocatalyst reaction efficiency for humic acid degradation. J. Ind. Eng. Chem. 2013, 19, 1325–1330. [Google Scholar] [CrossRef]
- O’Dowd, K.; Pillai, S.C. Photo-Fenton disinfection at near neutral pH: Process, parameter optimization and recent advances. J. Environ. Chem. Eng. 2020, 8, 104063. [Google Scholar] [CrossRef]
- Sarathy, S.; Mohseni, M. The fate of natural organic matter during UV/H2O2 advanced oxidation of drinking water. Can. J. Civ. Eng. 2008, 36, 160–169. [Google Scholar] [CrossRef]
- Cheng, M.; Song, W.; Ma, W.; Chen, C.; Zhao, J.; Lin, J.; Zhu, H. Catalytic activity of iron species in layered clays for photodegradation of organic dyes under visible irradiation. Appl. Catal. B Environ. 2008, 77, 355–363. [Google Scholar] [CrossRef]
- De León, M.A.; Castiglioni, J.; Bussi, J.; Sergio, M. Catalytic activity of an iron-pillared montmorillonitic clay mineral in heterogeneous photo-Fenton process. Catal. Today 2008, 133–135, 600–605. [Google Scholar] [CrossRef]
- Lei, X.; Pan, J.; Devlin, A.T. Characteristics of Absorption Spectra of Chromophoric Dissolved Organic Matter in the Pearl River Estuary in Spring. Remote Sens. 2019, 11, 1533. [Google Scholar] [CrossRef]
- Wenk, J.; von Gunten, U.; Canonica, S. Effect of Dissolved Organic Matter on the Transformation of Contaminants Induced by Excited Triplet States and the Hydroxyl Radical. Environ. Sci. Technol. 2011, 45, 1334–1340. [Google Scholar] [CrossRef]
- Page, S.E.; Arnold, W.A.; McNeill, K. Assessing the Contribution of Free Hydroxyl Radical in Organic Matter-Sensitized Photohydroxylation Reactions. Environ. Sci. Technol. 2011, 45, 2818–2825. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Yang, W.; You, L.; Li, J.; Chen, J.; Zhou, K. Simultaneous reduction of Cr (VI) and degradation of tetracycline hydrochloride by a novel iron-modified rectorite composite through heterogeneous photo-Fenton processes. Chem. Eng. J. 2020, 393, 124758. [Google Scholar] [CrossRef]
- Xu, H.; Cooper, W.J.; Jung, J.; Song, W. Photosensitized degradation of amoxicillin in natural organic matter isolate solutions. Water Res. 2011, 45, 632–638. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.; Helal, S.R.; Latch, D.E.; Arnold, W.A. Quantifying photo-production of triplet excited states and singlet oxygen from effluent organic matter. Water Res. 2019, 156, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Canonica, S. Oxidation of Aquatic Organic Contaminants Induced by Excited Triplet States. CHIMIA Int. J. Chem. 2007, 61, 641–644. [Google Scholar] [CrossRef]
- Sels, B.F.; De Vos, D.E.; Jacobs, P.A. Kinetics of the Oxygenation of Unsaturated Organics with Singlet Oxygen Generated from H2O2 by a Heterogeneous Molybdenum Catalyst. J. Am. Chem. Soc. 2007, 129, 6916–6926. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yan, S.; Song, W. Photochemically Induced Formation of Reactive Oxygen Species (ROS) from Effluent Organic Matter. Environ. Sci. Technol. 2014, 48, 12645–12653. [Google Scholar] [CrossRef]
- Sun, L.; Qian, J.; Blough, N.V.; Mopper, K. Insights into the Photoproduction Sites of Hydroxyl Radicals by Dissolved Organic Matter in Natural Waters. Environ. Sci. Technol. Lett. 2015, 2, 352–356. [Google Scholar] [CrossRef]
- Bodhipaksha, L.C.; Sharpless, C.M.; Chin, Y.-P.; Sander, M.; Langston, W.K.; MacKay, A.A. Triplet Photochemistry of Effluent and Natural Organic Matter in Whole Water and Isolates from Effluent-Receiving Rivers. Environ. Sci. Technol. 2015, 49, 3453–3463. [Google Scholar] [CrossRef] [PubMed]
- Zhan, M.; Yang, X.; Xian, Q.; Kong, L. Photosensitized degradation of bisphenol A involving reactive oxygen species in the presence of humic substances. Chemosphere 2006, 63, 378–386. [Google Scholar] [CrossRef]
- Garg, S.; Rose, A.L.; Waite, T.D. Photochemical production of superoxide and hydrogen peroxide from natural organic matter. Geochim. Cosmochim. Acta 2011, 75, 4310–4320. [Google Scholar] [CrossRef]
- Merino, C.; Kuzyakov, Y.; Godoy, K.; Jofré, I.; Nájera, F.; Matus, F. Iron-reducing bacteria decompose lignin by electron transfer from soil organic matter. Sci. Total Environ. 2021, 761, 143194. [Google Scholar] [CrossRef]
- Poggenburg, C.; Mikutta, R.; Schippers, A.; Dohrmann, R.; Guggenberger, G. Impact of natural organic matter coatings on the microbial reduction of iron oxides. Geochim. Cosmochim. Acta 2018, 224, 223–248. [Google Scholar] [CrossRef]
- Orsetti, S.; Laskov, C.; Haderlein, S.B. Electron Transfer between Iron Minerals and Quinones: Estimating the Reduction Potential of the Fe (II)-Goethite Surface from AQDS Speciation. Environ. Sci. Technol. 2013, 47, 14161–14168. [Google Scholar] [CrossRef]
- Golanoski, K.S.; Fang, S.; Del Vecchio, R.; Blough, N.V. Investigating the Mechanism of Phenol Photooxidation by Humic Substances. Environ. Sci. Technol. 2012, 46, 3912–3920. [Google Scholar] [CrossRef]
- Bauer, I.; Kappler, A. Rates and Extent of Reduction of Fe (III) Compounds and O2 by Humic Substances. Environ. Sci. Technol. 2009, 43, 4902–4908. [Google Scholar] [CrossRef]
- Elsner, M.; Schwarzenbach, R.P.; Haderlein, S.B. Reactivity of Fe (II)-Bearing Minerals toward Reductive Transformation of Organic Contaminants. Environ. Sci. Technol. 2004, 38, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Carena, L.; Näsi, M.-T.; Vähätalo, A.V. Superoxide-driven autocatalytic dark production of hydroxyl radicals in the presence of complexes of natural dissolved organic matter and iron. Water Res. 2020, 177, 115782. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Subdiaga, E.; Haderlein, S.B.; Knicker, H.; Kappler, A. High-pH and anoxic conditions during soil organic matter extraction increases its electron-exchange capacity and ability to stimulate microbial Fe (III) reduction by electron shuttling. Biogeosciences 2020, 17, 683–698. [Google Scholar] [CrossRef]
- Sundman, A.; Byrne, J.M.; Bauer, I.; Menguy, N.; Kappler, A. Interactions between magnetite and humic substances: Redox reactions and dissolution processes. Geochem. Trans. 2017, 18, 6. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Clarizia, L.; Russo, D.; Di Somma, I.; Marotta, R.; Andreozzi, R. Homogeneous photo-Fenton processes at near neutral pH: A review. Appl. Catal. B Environ. 2017, 209, 358–371. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Y.; Hu, C. Impacts of bacteria and corrosion on removal of natural organic matter and disinfection byproducts in different drinking water distribution systems. Int. Biodeterior. Biodegrad. 2017, 117, 52–59. [Google Scholar] [CrossRef]
- Baird, R.B. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association (APHA): Washington, DC, USA; American Water Works Association (AWWA): Denver, CO, USA; Water Environment Federation (WEF): Alexandria, VA, USA, 2017. [Google Scholar]
- Kiassen, N.; Marchington, D.; McGowant, H. H2O2 Determination by the Is- Method and by KMnO4 Titration. Anal. Chem. 1994, 66, 2921–2925. [Google Scholar] [CrossRef]
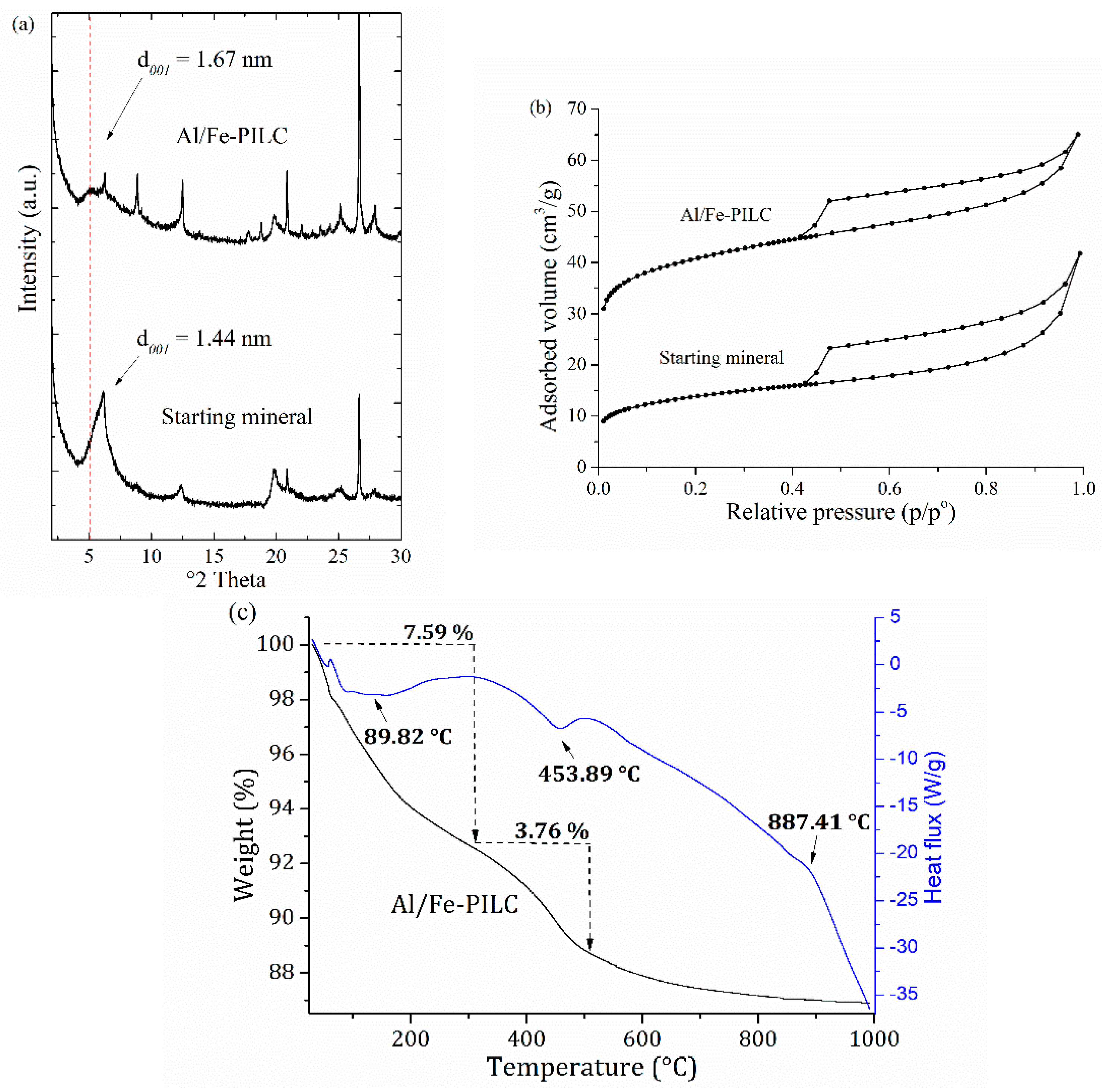

| Material | Physicochemical Properties | |||||
|---|---|---|---|---|---|---|
| d001 (nm) a | SBET (m2/g) b | Sμp (m2/g) c | Fecontent (% w/w) | Feincorporated (% w/w) d | CEC (meq/100 g) e | |
| Starting mineral | 1.44 | 51 | 26 | 9.96 | NA | 256 |
| Al/Fe-PILC | 1.67 | 153 | 110 | 11.68 | 1.72 | 70 |
| Test a | DOC Mineralization (%) | Color Removal (%) | H2O2 Consumption (%) |
|---|---|---|---|
| Dark-CWPO-SM | 9 | 27 | 2 |
| UV-SM-NOM | 4 | 6 | NA |
| Vis-SM-NOM | 5 | 5 | NA |
| UV-SM-H2O2 | NA | NA | ~0 |
| Vis-SM-H2O2 | NA | NA | ~0 |
| UV-CWPO-SM-pH 3.0 | 11 | 78 | 0 |
| Vis-CWPO-SM-pH 3.0 | 12 | 90 | 4 |
| UV-CWPO-SM-pH 7.0 | 3 | 34 | 5 |
| Vis-CWPO-SM-pH 7.0 | 13 | 24 | 16 |
| UV-CWPO-SM-pH 9.0 | 5 | 28 | 17 |
| Vis-CWPO-SM-pH 9.0 | 4 | 29 | 18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portilla-Delgado, C.S.; García-Mora, A.M.; Dappozze, F.; Guillard, C.; Galeano, L.A. Visible-Light Enhanced Catalytic Wet Peroxide Oxidation of Natural Organic Matter in the Presence of Al/Fe-Pillared Clay. Catalysts 2021, 11, 637. https://doi.org/10.3390/catal11050637
Portilla-Delgado CS, García-Mora AM, Dappozze F, Guillard C, Galeano LA. Visible-Light Enhanced Catalytic Wet Peroxide Oxidation of Natural Organic Matter in the Presence of Al/Fe-Pillared Clay. Catalysts. 2021; 11(5):637. https://doi.org/10.3390/catal11050637
Chicago/Turabian StylePortilla-Delgado, Cristian S., Ana M. García-Mora, Frederic Dappozze, Chantal Guillard, and Luis A. Galeano. 2021. "Visible-Light Enhanced Catalytic Wet Peroxide Oxidation of Natural Organic Matter in the Presence of Al/Fe-Pillared Clay" Catalysts 11, no. 5: 637. https://doi.org/10.3390/catal11050637
APA StylePortilla-Delgado, C. S., García-Mora, A. M., Dappozze, F., Guillard, C., & Galeano, L. A. (2021). Visible-Light Enhanced Catalytic Wet Peroxide Oxidation of Natural Organic Matter in the Presence of Al/Fe-Pillared Clay. Catalysts, 11(5), 637. https://doi.org/10.3390/catal11050637