Lipase Immobilization in Mesoporous Silica Nanoparticles for Biofuel Production
Abstract
1. Introduction
2. Lipases
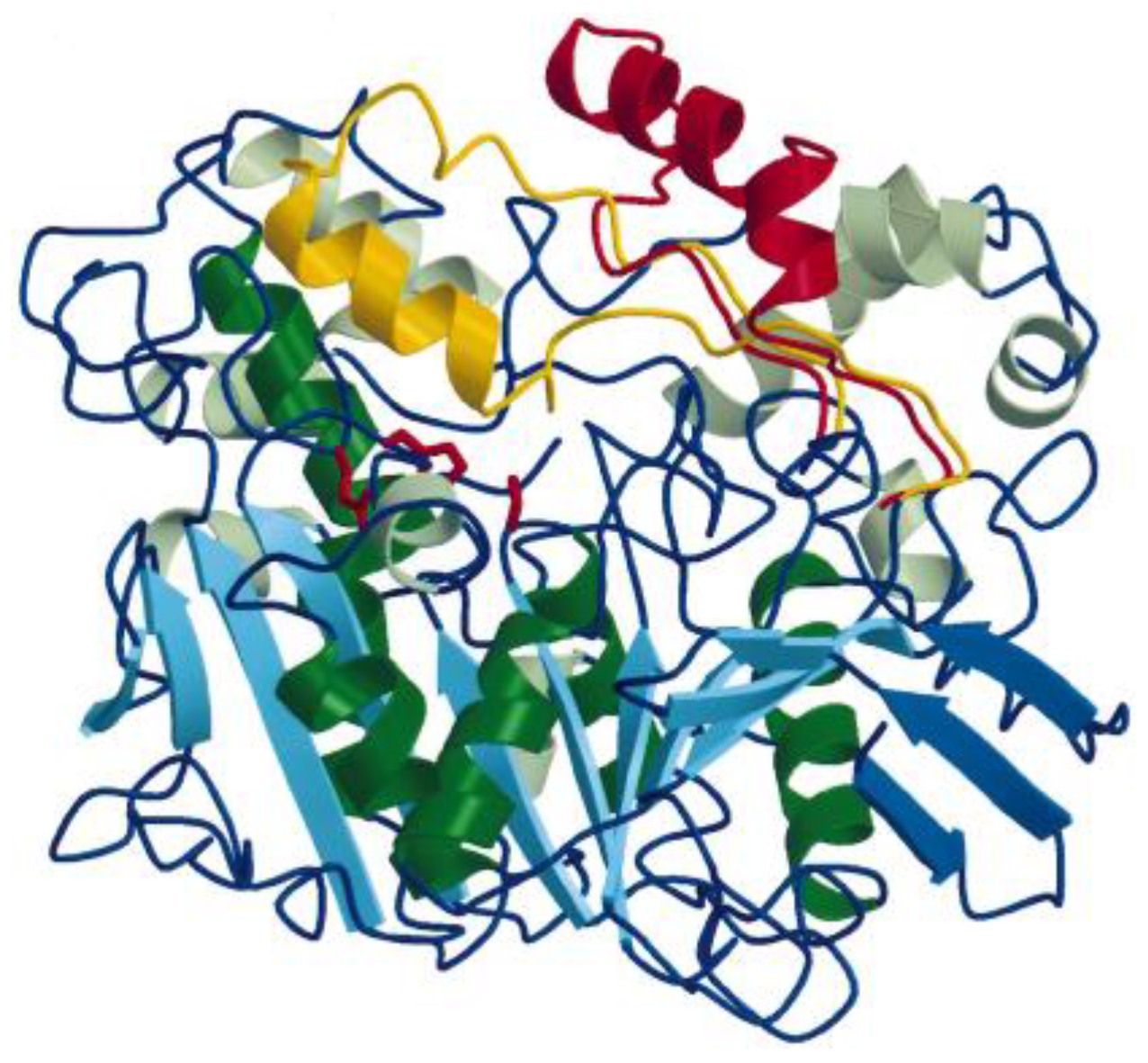
2.1. Three-Dimensional Structure
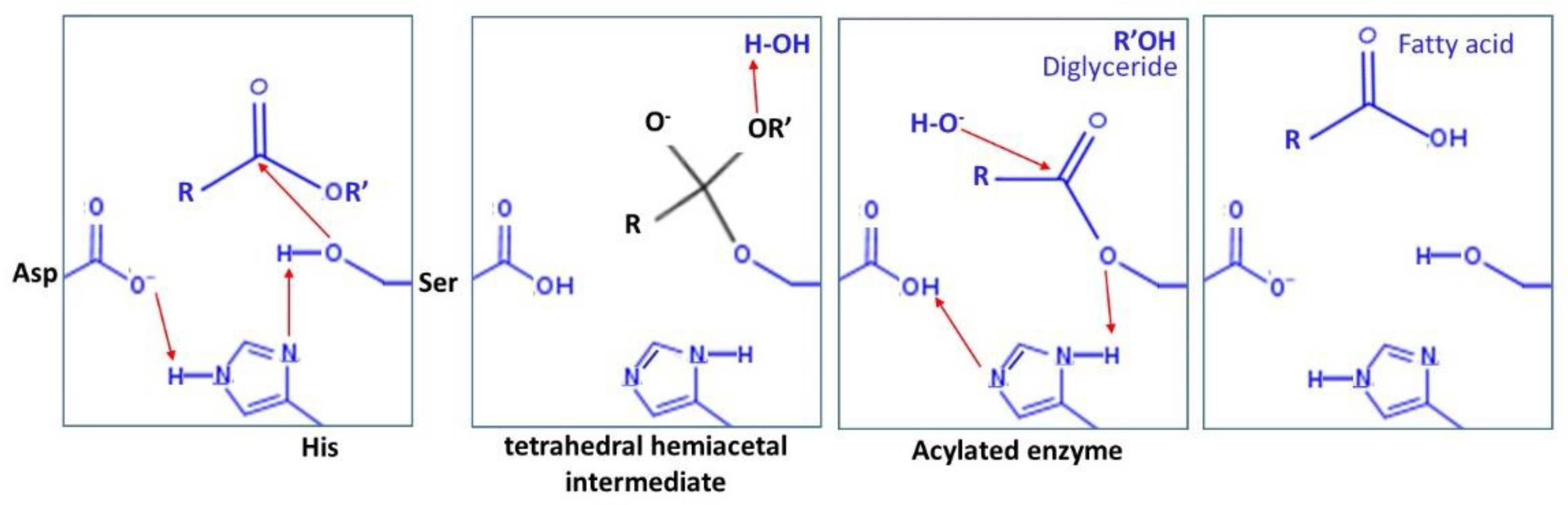
2.2. Mechanism of Catalysis
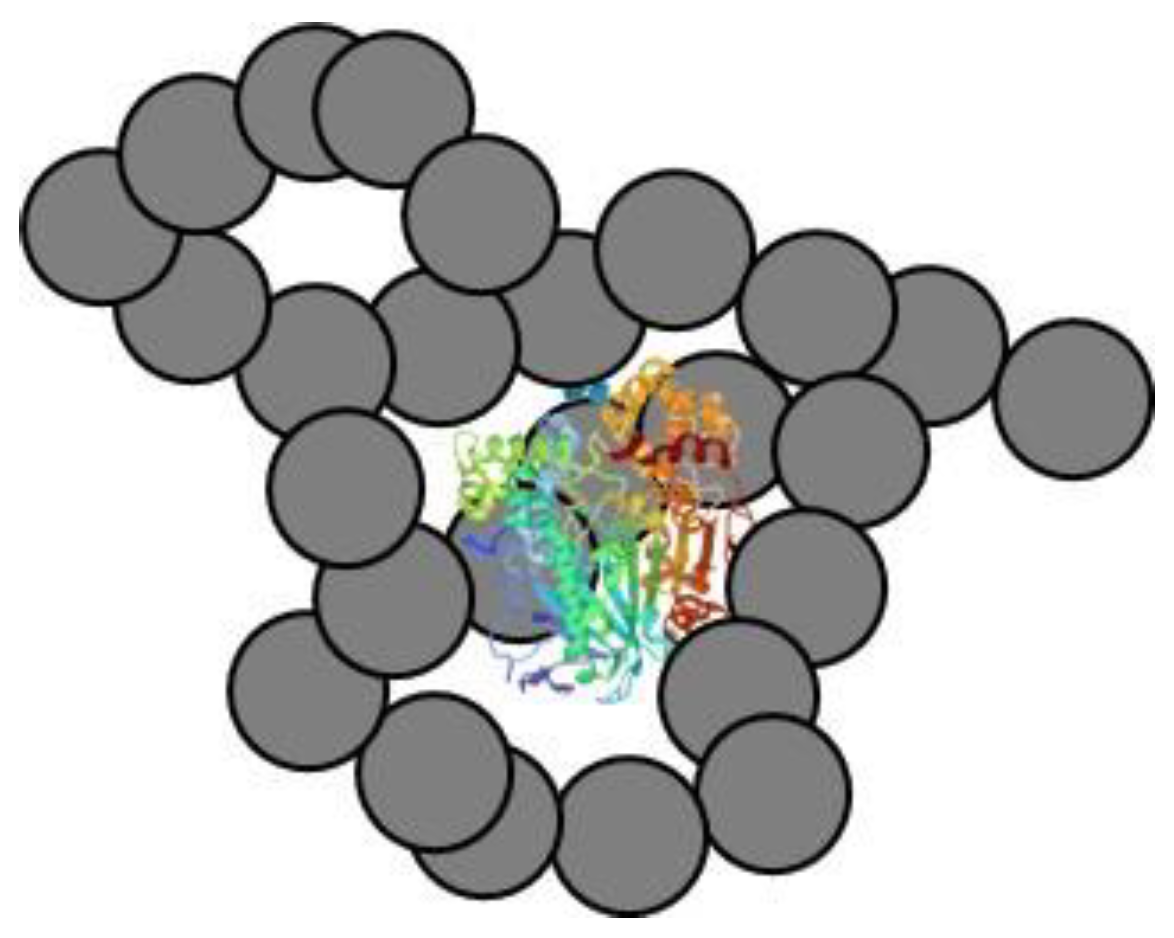
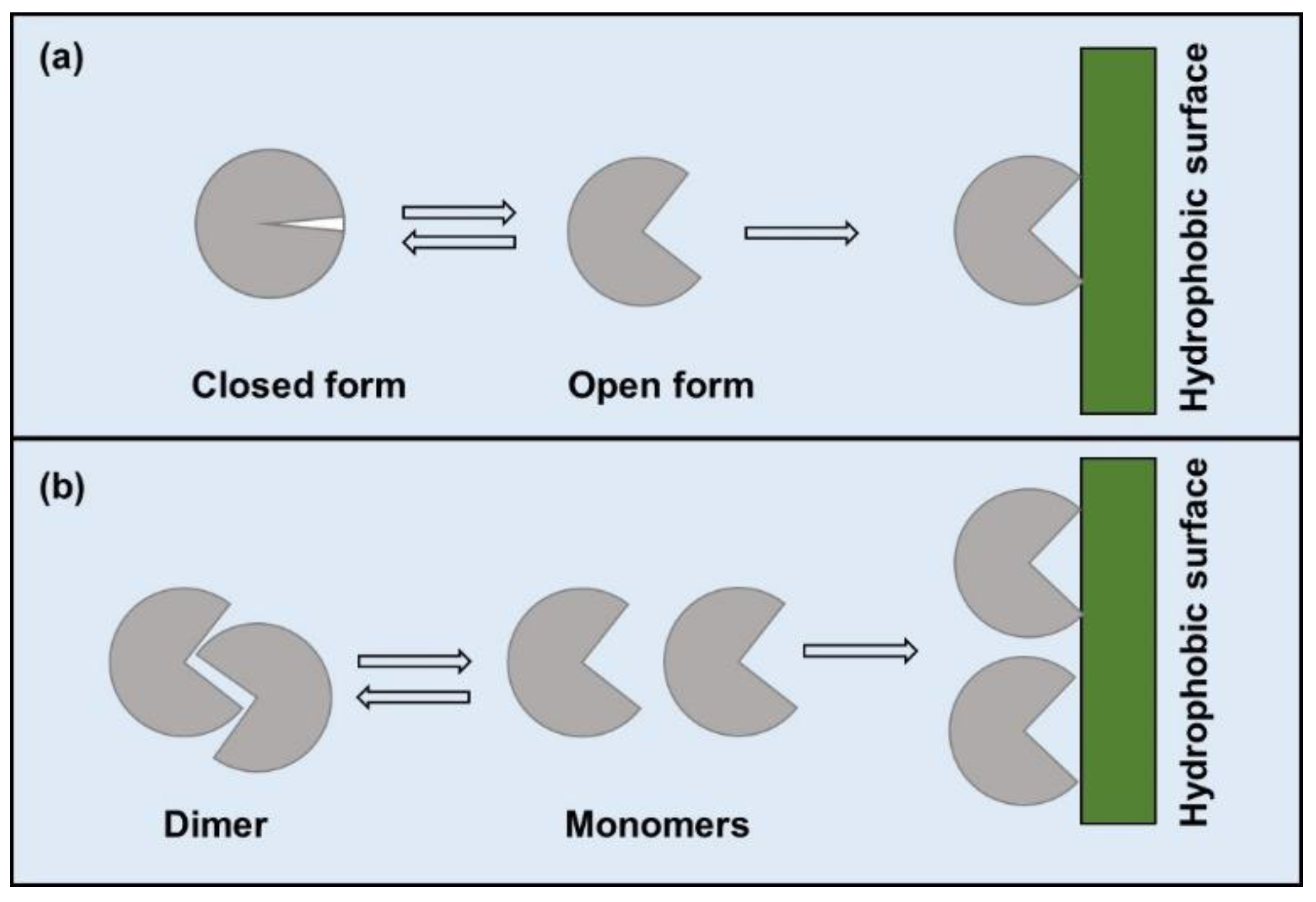
2.3. Interfacial Activation
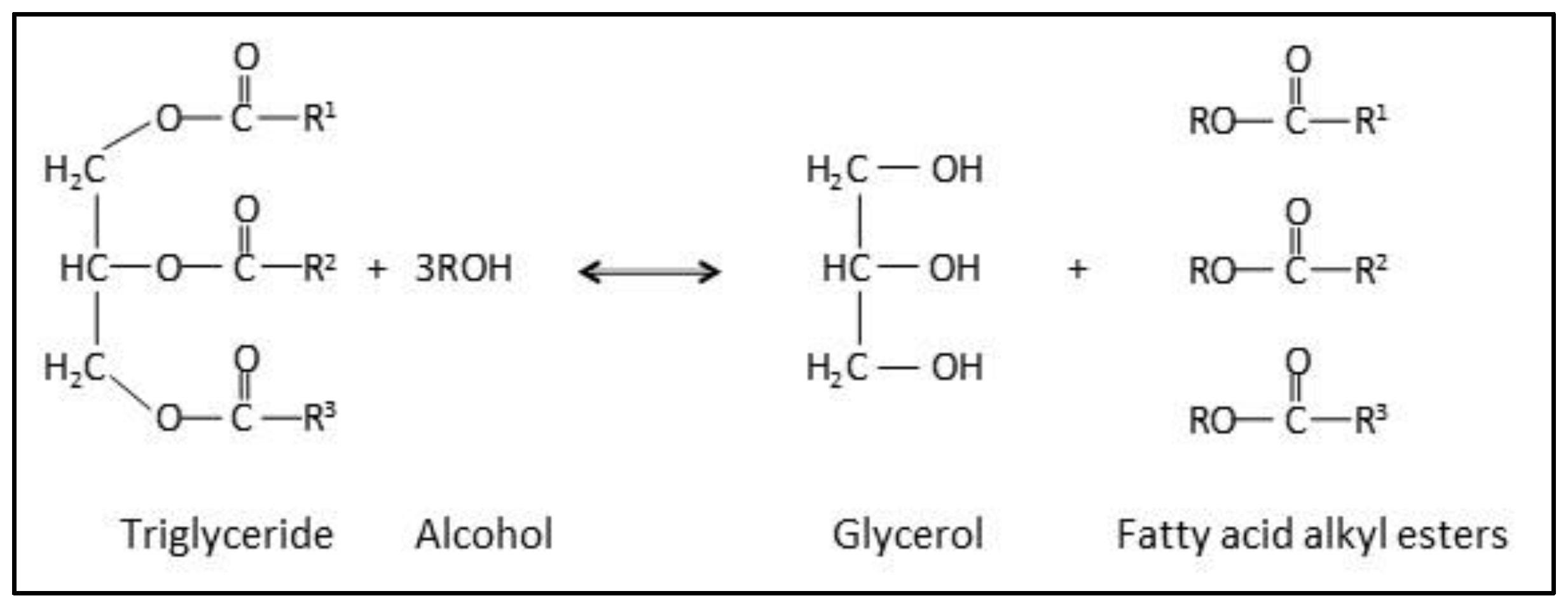
2.4. Enzymatic Biodiesel Production
3. Mesoporous Silica Supports
3.1. Sol-Gel Silica
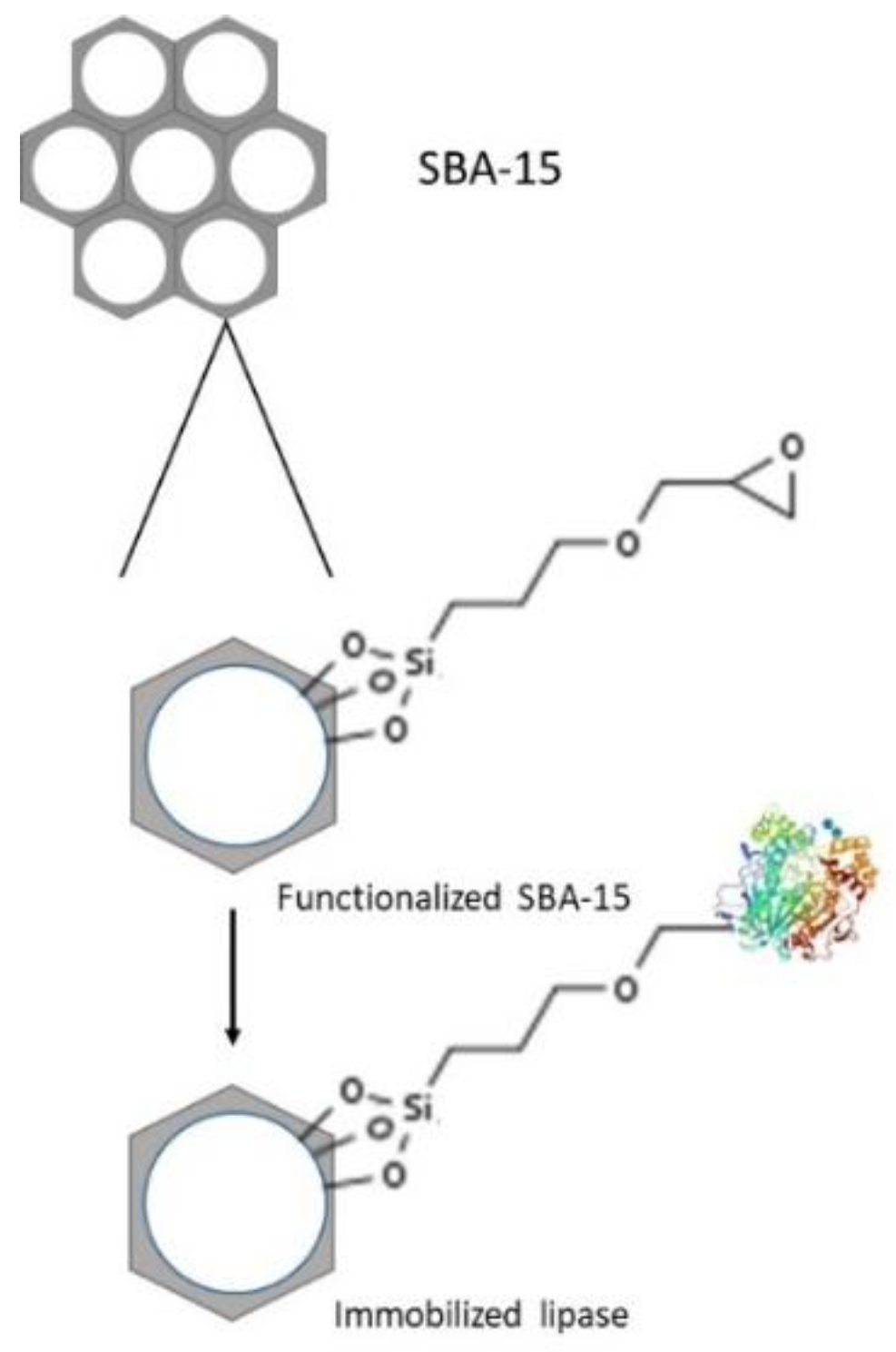
3.2. Ordered Mesoporous Silica (OMS)
3.3. Mesocellular Silica Foam
3.4. Biomimetic Silica
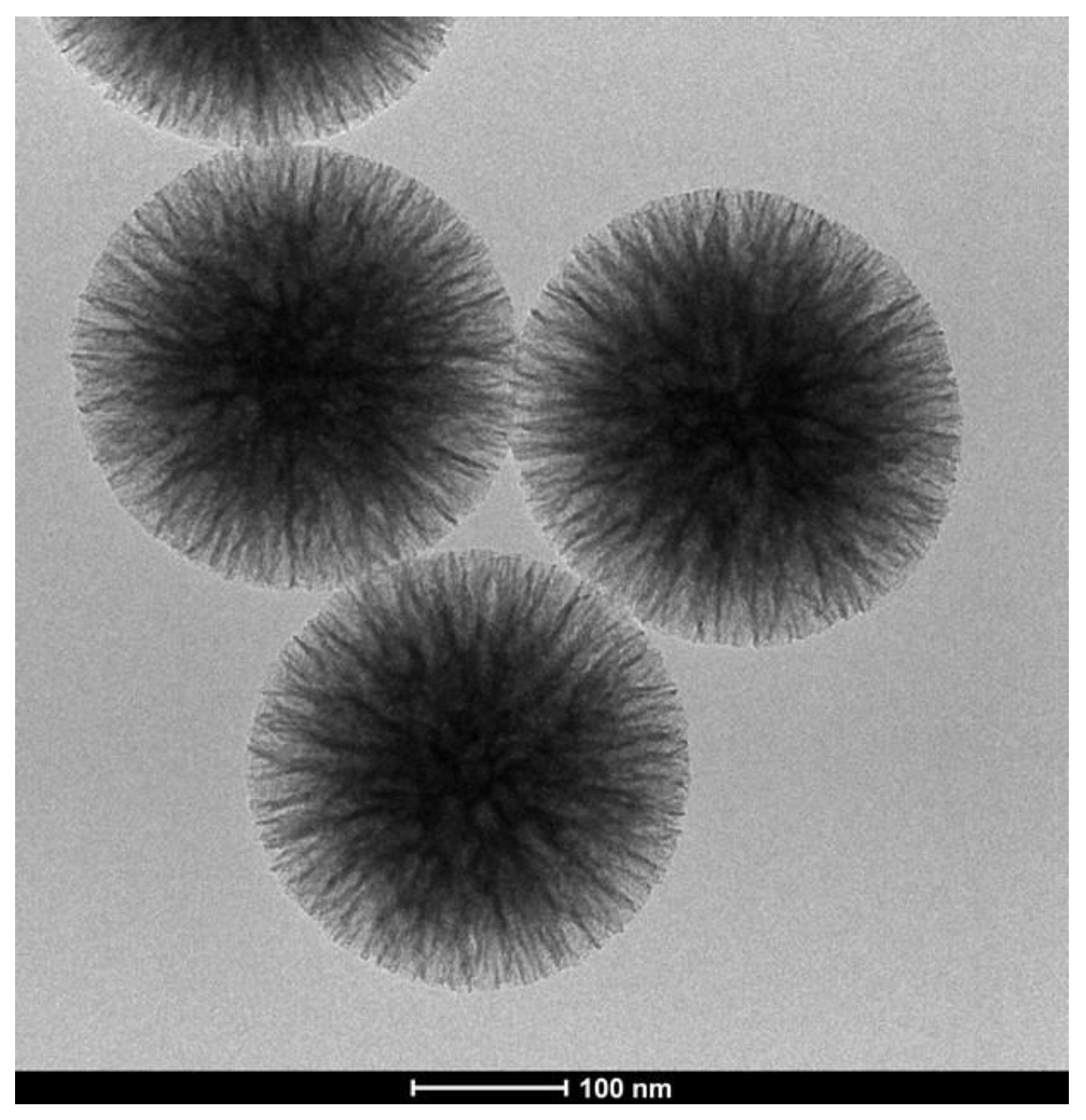
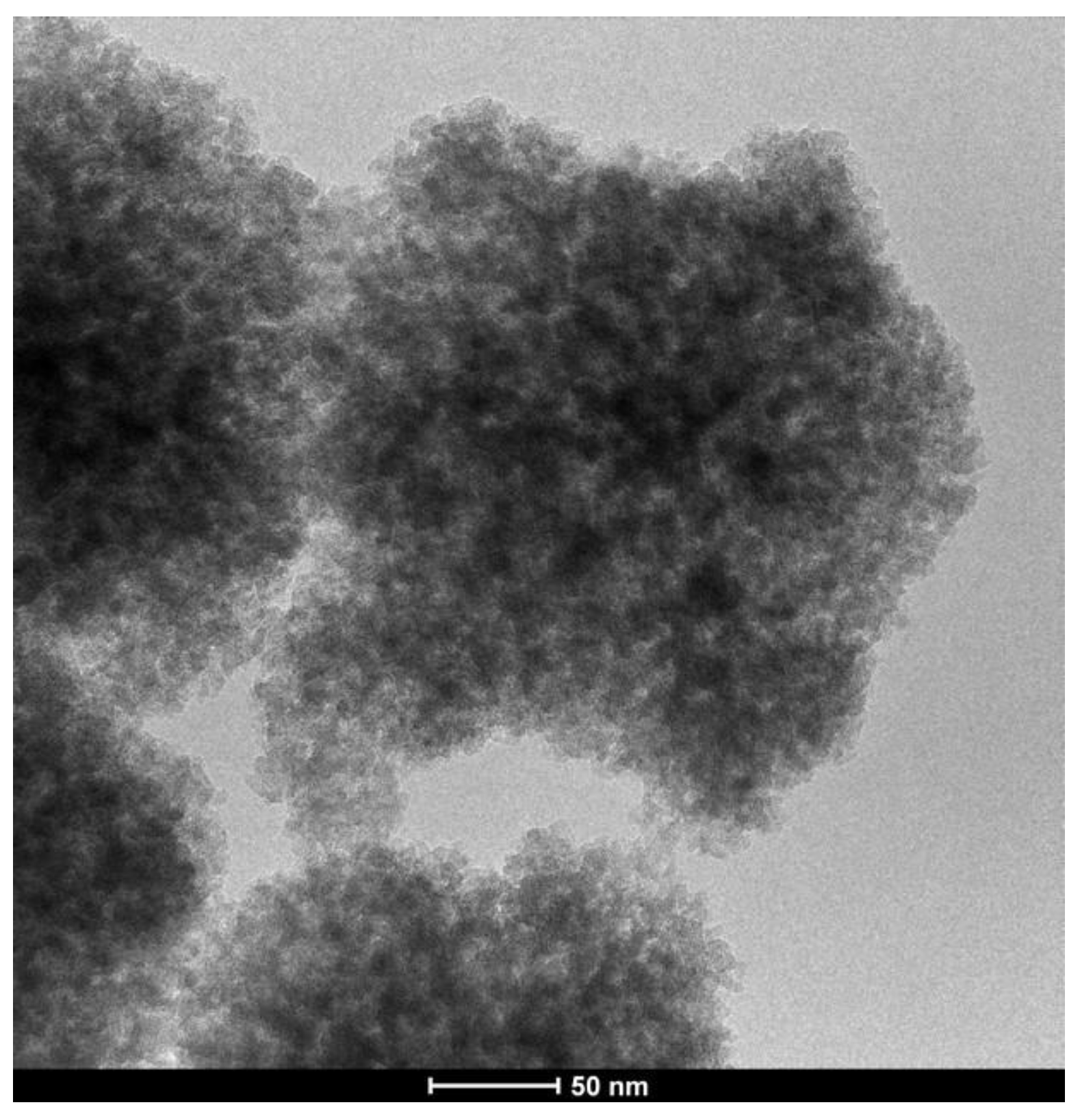
3.5. Silica Nanoflowers
3.6. Non-Surfactant Template Mesoporous Silica
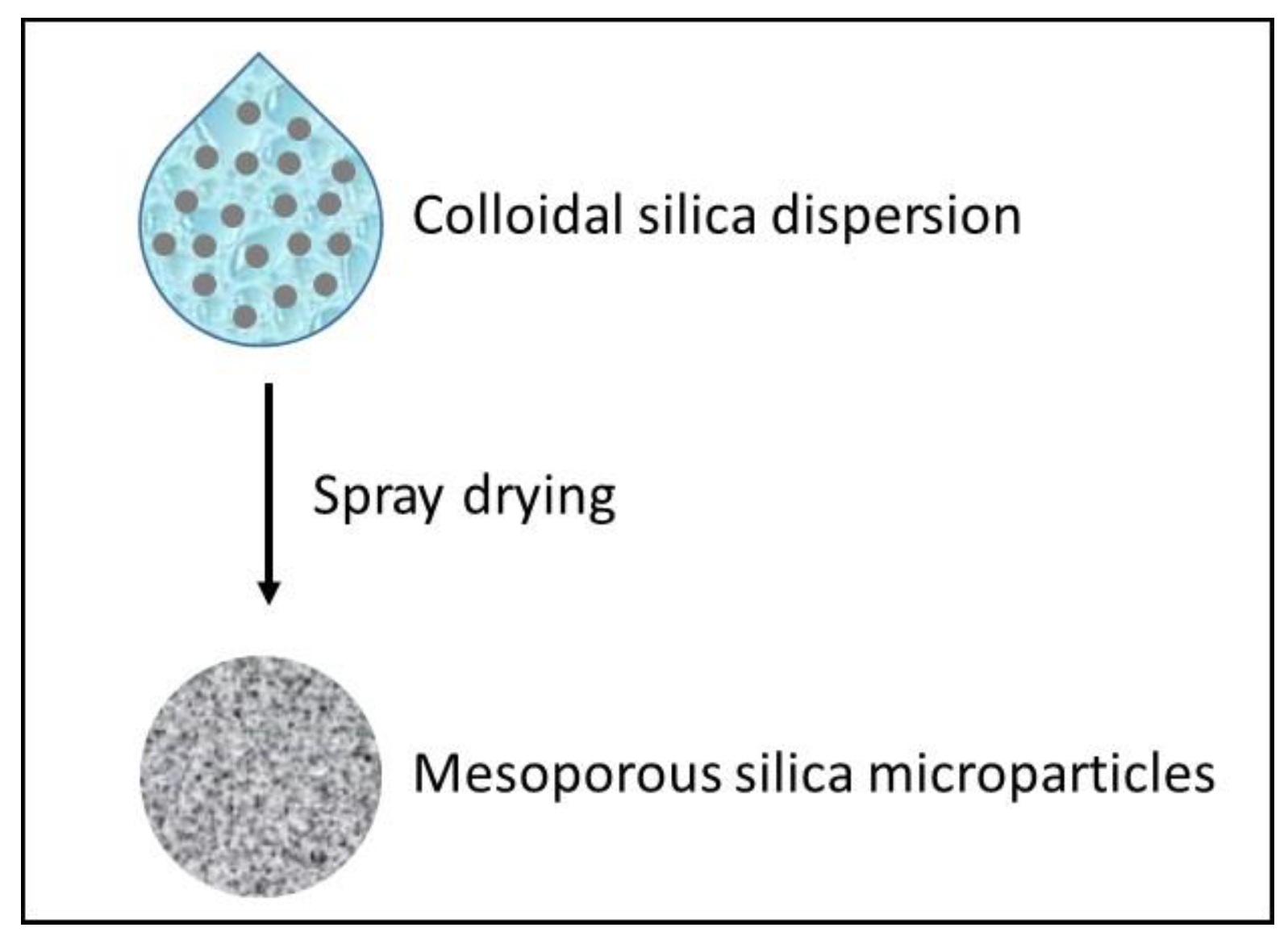
3.7. Spray-Drying Mesoporous Microparticles
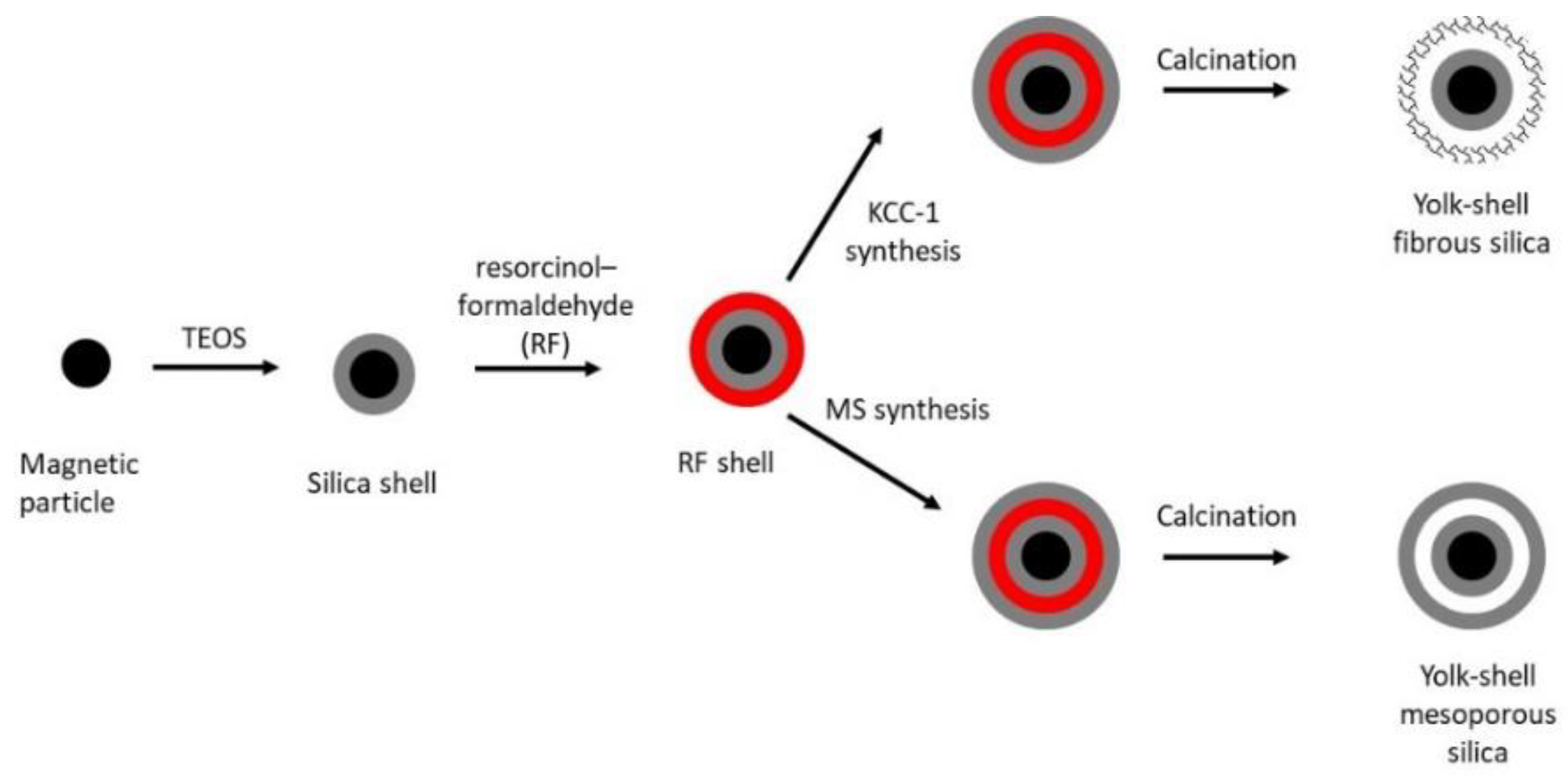
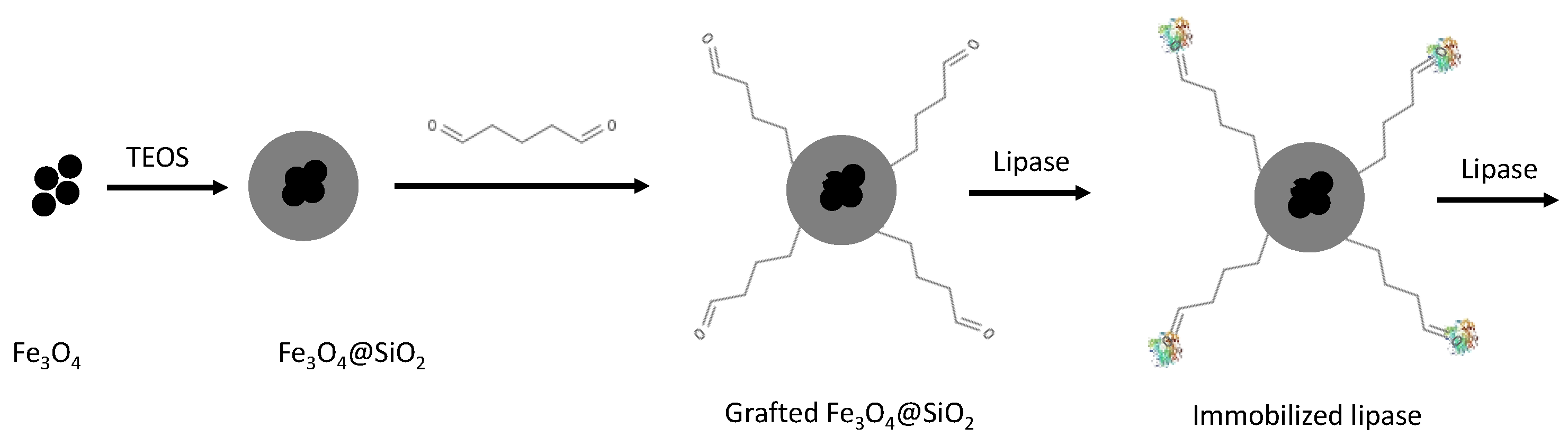
3.8. Core-Shell Magnetic Silica Particles
4. Lipase Immobilization
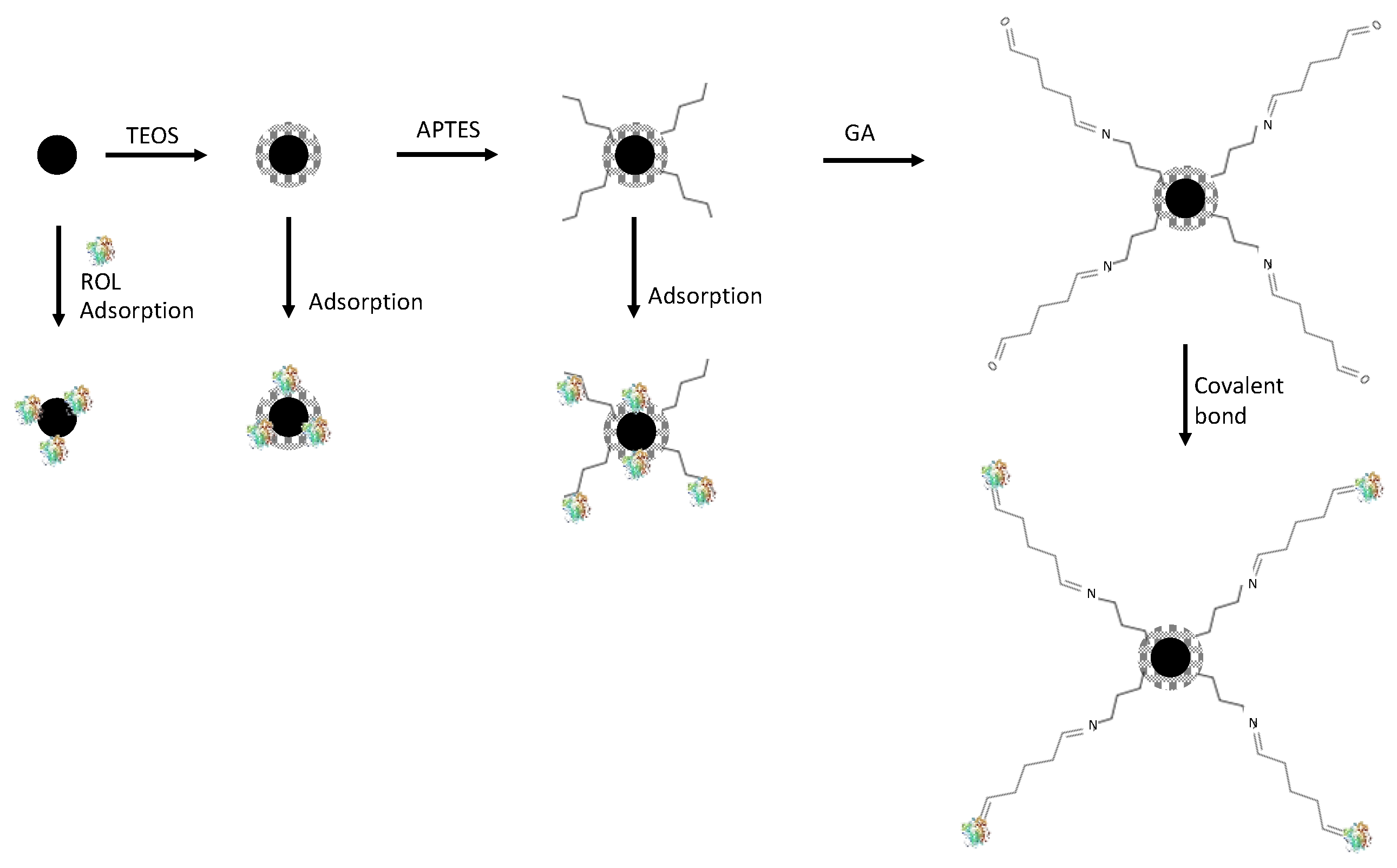
4.1. Adsorption
4.2. Covalent Immobilization
4.3. Entrapment
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cygler, M.; Schrag, J.D. Structure and conformational flexibility of Candida rugosa lipase. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 1999, 1441, 205–214. [Google Scholar] [CrossRef]
- Aguieiras, E.C.; De Barros, D.S.; Fernandez-Lafuente, R.; Freire, D.M. Production of lipases in cottonseed meal and application of the fermented solid as biocatalyst in esterification and transesterification reactions. Renew. Energy 2019, 130, 574–581. [Google Scholar] [CrossRef]
- Löfgren, J.; Görbe, T.; Oschmann, M.; Svedendahl Humble, M.; Bäckvall, J.E. Transesterification of a tertiary alcohol by engi-neered Candida antarctica lipase A. ChemBioChem 2019, 20, 1438–1443. [Google Scholar] [CrossRef]
- Sivakanthan, S.; Jayasooriya, A.P.; Madhujith, T. Optimization of the production of structured lipid by enzymatic interesteri-fication from coconut (Cocos nucifera) oil and sesame (Sesamum indicum) oil using Response Surface Methodology. LWT Food Sci. Technol. 2019, 101, 723–730. [Google Scholar] [CrossRef]
- Reis, P.; Holmberg, K.; Watzke, H.; Leser, M.; Miller, R. Lipases at interfaces: A review. Adv. Colloid Interface Sci. 2009, 147–148, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Melani, N.B.; Tambourgi, E.B.; Silveira, E. Lipases: From Production to Applications. Sep. Purif. Rev. 2020, 49, 143–158. [Google Scholar] [CrossRef]
- Sarmah, N.; Revathi, D.; Sheelu, G.; Rani, K.Y.; Sridhar, S.; Mehtab, V.; Sumana, C. Recent advances on sources and industrial applications of lipases. Biotechnol. Prog. 2018, 34, 5–28. [Google Scholar] [CrossRef]
- Di Iorio, S.; Magno, A.; Mancaruso, E.; Vaglieco, B.; Arnone, L.; Dal Bello, L. Engine Performance and Emissions of a Small Diesel Engine Fdueled with Various Diesel/RME Blends; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2014. [Google Scholar] [CrossRef]
- Meher, L.; Sagar, D.V.; Naik, S. Technical aspects of biodiesel production by transesterification—A review. Renew. Sustain. Energy Rev. 2006, 10, 248–268. [Google Scholar] [CrossRef]
- Califano, V.; Bloisi, F.; Perretta, G.; Aronne, A.; Ausanio, G.; Costantini, A.; Vicari, L. Frozen Microemulsions for MAPLE Immobilization of Lipase. Molecules 2017, 22, 2153. [Google Scholar] [CrossRef]
- Malcata, F.X.; Reyes, H.R.; Garcia, H.S.; Hill, C.G., Jr.; Amundson, C.H. Immobilized lipase reactors for modification of fats and oils—A review. J. Am. Oil Chem. Soc. 1990, 67, 890–910. [Google Scholar] [CrossRef]
- Knezevic, Z.; Siler-Marinkovic, S.; Mojovic, L. Immobilized lipases as practical catalysts. Acta Period. Technol. 2004, 35, 151–164. [Google Scholar] [CrossRef]
- Manoel, E.A.; dos Santos, J.C.; Freire, D.M.; Rueda, N.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports involves the open form of the enzyme. Enzym. Microb. Technol. 2015, 71, 53–57. [Google Scholar] [CrossRef]
- Tran, N.D.; Balkus, K.J. Perspective of recent progress in immobilization of enzymes. ACS Catal. 2011, 1, 956–968. [Google Scholar] [CrossRef]
- Zhao, X.; Bao, X.Y.; Guo, W.; Lee, F.Y. Immobilizing catalysts on porous materials. Mater. Today 2006, 9, 32–39. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R.; Armisén, P.; Sabuquillo, P.; Fernández-Lorente, G.; Guisán, J.M. Immobilization of lipases by selective adsorption on hydrophobic supports. Chem. Phys. Lipids 1998, 93, 185–197. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Virgen-Ortíz, J.J.; Dos Santos, J.C.; Berenguer-Murcia, Á.; Alcantara, A.R.; Barbosa, O.; Ortiz, C.; Fernan-dez-Lafuente, R. Immobilization of lipases on hydrophobic supports: Immobilization mechanism, advantages, problems, and solutions. Biotechnol. Adv. 2019, 37, 746–770. [Google Scholar] [CrossRef]
- De María, P.D.; Sánchez-Montero, J.M.; Sinisterra, J.V.; Alcántara, A.R. Understanding Candida rugosa lipases: An overview. Biotechnol. Adv. 2006, 24, 180–196. [Google Scholar] [CrossRef]
- Ollis, D.L.; Cheah, E.; Cygler, M.; Dijkstra, B.W.; Frolow, F.; Franken, S.M.; Harel, M.; Remington, S.J.; Silman, I.; Schrag, J.D.; et al. The alpha/beta hydrolase fold. Protein Eng. 1992, 5, 197–211. [Google Scholar] [CrossRef]
- Mendes, A.A.; Oliveira, P.C.; de Castro, H.F. Properties and biotechnological applications of porcine pancreatic lipase. J. Mol. Catal. B Enzym. 2012, 78, 119–134. [Google Scholar] [CrossRef]
- Uppenberg, J.; Hansen, M.T.; Patkar, S.; Jones, T. The sequence, crystal structure determination and refinement of two crystal forms of lipase B from Candida antarctica. Structure 1994, 2, 293–308. [Google Scholar] [CrossRef]
- Carrasco-López, C.; Godoy, C.; Rivas, B.D.L.; Fernández-Lorente, G.; Palomo, J.M.; Guisán, J.M.; Fernández-Lafuente, R.; Martínez-Ripoll, M.; Hermoso, J.A. Activation of Bacterial Thermoalkalophilic Lipases Is Spurred by Dramatic Structural Rearrangements. J. Biol. Chem. 2009, 284, 4365–4372. [Google Scholar] [CrossRef] [PubMed]
- Christopher, L.P.; Kumar, H.; Zambare, V.P. Enzymatic biodiesel: Challenges and opportunities. Appl. Energy 2014, 119, 497–520. [Google Scholar] [CrossRef]
- Lee, K.W.; Min, K.; Park, K.; Yoo, Y.J. Development of an amphiphilic matrix for immobilization of Candida antartica lipase B for biodiesel production. Biotechnol. Bioprocess Eng. 2010, 15, 603–607. [Google Scholar] [CrossRef]
- Shieh, C.-J.; Liao, H.-F.; Lee, C.-C. Optimization of lipase-catalyzed biodiesel by response surface methodology. Bioresour. Technol. 2003, 88, 103–106. [Google Scholar] [CrossRef]
- Sim, J.H.; Kamaruddin, A.H.; Bhatia, S. Biodiesel (FAME) Productivity, Catalytic Efficiency and Thermal Stability of Lipozyme TL IM for Crude Palm Oil Transesterification with Methanol. J. Am. Oil Chem. Soc. 2010, 87, 1027–1034. [Google Scholar] [CrossRef]
- Salis, A.; Pinna, M.; Monduzzi, M.; Solinas, V. Biodiesel production from triolein and short chain alcohols through biocatalysis. J. Biotechnol. 2005, 119, 291–299. [Google Scholar] [CrossRef]
- Liu, T.; Liu, Y.; Wang, X.; Li, Q.; Wang, J.; Yan, Y. Improving catalytic performance of Burkholderia cepacia lipase immobilized on macroporous resin NKA. J. Mol. Catal. B Enzym. 2011, 71, 45–50. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, B.; Yan, Y. Cloning and Expression of Pseudomonas fluorescens 26-2 Lipase Gene in Pichia pastoris and Characterizing for Transesterification. Appl. Biochem. Biotechnol. 2008, 159, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Meng, X.; He, J.; Sun, P. Analysis of immobilized Candida rugosa lipase catalyzed preparation of biodiesel from rapeseed soapstock. Food Bioprod. Process. 2008, 86, 283–289. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Wang, Y.; Wang, Y.; Wang, F.; Jiang, J. Expression and characterization of recombinant Rhizopus oryzae lipase for enzymatic biodiesel production. Bioresour. Technol. 2011, 102, 9810–9813. [Google Scholar] [CrossRef]
- Zhang, K.-P.; Lai, J.-Q.; Huang, Z.-L.; Yang, Z. Penicillium expansum lipase-catalyzed production of biodiesel in ionic liquids. Bioresour. Technol. 2011, 102, 2767–2772. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yan, Y.; Liu, S.; Hu, J.; Wang, G. Preparation of cross-linked lipase-coated micro-crystals for biodiesel production from waste cooking oil. Bioresour. Technol. 2011, 102, 4755–4758. [Google Scholar] [CrossRef]
- Lv, L.; Dai, L.; Du, W.; Liu, D. Progress in Enzymatic Biodiesel Production and Commercialization. Processes 2021, 9, 355. [Google Scholar] [CrossRef]
- Zhou, Z.; Hartmann, M. Progress in enzyme immobilization in ordered mesoporous materials and related applications. Chem. Soc. Rev. 2013, 42, 3894–3912. [Google Scholar] [CrossRef]
- Talbert, J.N.; Goddard, J.M. Enzymes on material surfaces. Colloids Surf. B Biointerfaces 2012, 93, 8–19. [Google Scholar] [CrossRef]
- Silvestri, B.; Guarnieri, D.; Luciani, G.; Costantini, A.; Netti, P.A.; Branda, F. Fluorescent (rhodamine), folate decorated and doxorubicin charged, PEGylated nanoparticles synthesis. J. Mater. Sci. Mater. Electron. 2012, 23, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- Milea, C.A.; Bogatu, C.; Duta, A. The Influence of Parameters in Silica Sol-Gel Process. Eng. Sci. 2011, 4, 59–66. [Google Scholar]
- Soares, C.M.F.; Dos Santos, O.A.; De Castro, H.F.; De Moraes, F.F.; Zanin, G.M. Studies on Immobilized Lipase in Hydrophobic Sol-Gel. Appl. Biochem. Biotechnol. 2004, 113–116, 307–319. [Google Scholar] [CrossRef]
- Ficanha, A.M.M.; Antunes, A.; Oro, C.E.D.; Valduga, A.T.; Moreira, C.M.; Dallago, R.M.; Mignoni, M. Study of Drying Conditions of the Aerogel Obtained by the Sol-Gel Technique for Immobilization In Situ of Lipase Candida antarctica B. Ind. Biotechnol. 2019, 15, 350–356. [Google Scholar] [CrossRef]
- Vidinha, P.; Augusto, V.; Almeida, M.; Fonseca, I.; Fidalgo, A.; Ilharco, L.; Cabral, J.M.S.; Barreiros, S. Sol–gel encapsulation: An efficient and versatile immobilization technique for cutinase in non-aqueous media. J. Biotechnol. 2006, 121, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Zarcula, C.; Kiss, C.; Corîci, L.; Croitoru, R.; Csunderlik, C.; Peter, F. Combined sol-gel entrapment and adsorption method to obtain solid-phase lipase biocatalyst. Rev. Chim. 2009, 60, 922–927. [Google Scholar]
- Tomin, A.; Weiser, D.; Hellner, G.; Bata, Z.; Corici, L.; Péter, F.; Koczka, B.; Poppe, L. Fine-tuning the second generation sol–gel lipase immobilization with ternary alkoxysilane precursor systems. Process. Biochem. 2011, 46, 52–58. [Google Scholar] [CrossRef]
- Barbosa, A.S.; Lisboa, J.A.; Silva, M.A.O.; Carvalho, N.B.; Pereira, M.M.; Fricks, A.T.; Mattedi, S.; Lima, Á.S.; Franceschi, E.; Soares, C.M.F. The novel mesoporous silica aerogel modified with protic ionic liquid for lipase immobilization. Quím. Nova 2016, 39, 415–422. [Google Scholar] [CrossRef]
- De Souza, R.L.; de Faria, E.L.P.; Figueiredo, R.T.; Freitas, L.D.S.; Iglesias, M.; Mattedi, S.; Zanin, G.M.; dos Santos, O.A.A.; Coutinho, J.A.; Lima, Á.S.; et al. Protic ionic liquid as additive on lipase immobilization using silica sol–gel. Enzym. Microb. Technol. 2013, 52, 141–150. [Google Scholar] [CrossRef]
- Zou, B.; Song, C.; Xu, X.; Xia, J.; Huo, S.; Cui, F. Enhancing stabilities of lipase by enzyme aggregate coating immobilized onto ionic liquid modified mesoporous materials. Appl. Surf. Sci. 2014, 311, 62–67. [Google Scholar] [CrossRef]
- Barbosa, A.D.S.; Silva, M.A.D.O.; Carvalho, N.B.; Mattedi, S.; Iglesias, M.A.; Fricks, A.T.; Lima, Á.S.; Franceschi, E.; Soares, C.M.F. Immobilization of lipase by encapsulation in silica aerogel. Quím. Nova 2014, 37, 969–976. [Google Scholar] [CrossRef]
- Beck, J.S.; Calabro, D.C.; McCullen, S.B.; Pelrine, B.P.; Schmitt, K.D.; Vartuli, J.C. Catalytic Conversion over Modified Synthetic Mesoporous Crystalline Material. U.S. Patent 5,200,058, 6 April 1993. [Google Scholar]
- Beck, J.S.; Calabro, D.C.; McCullen, S.B.; Pelrine, B.P.; Schmitt, K.D.; Vartuli, J.C. Method for Functionalizing Synthetic Mesoporous Crystalline Material. U.S. Patent 2,069,722, 27 May 1992. [Google Scholar]
- Silvestri, B.; Pezzella, A.; Luciani, G.; Costantini, A.; Tescione, F.; Branda, F. Heparin conjugated silica nanoparticle synthesis. Mater. Sci. Eng. C 2012, 32, 2037–2041. [Google Scholar] [CrossRef]
- Parida, K.; Dash, S.S. Manganese containing MCM-41: Synthesis, characterization and catalytic activity in the oxidation of ethylbenzene. J. Mol. Catal. A Chem. 2009, 306, 54–61. [Google Scholar] [CrossRef]
- Alothman, Z.A.; Apblett, A.W. Metal ion adsorption using polyamine-functionalized mesoporous materials prepared from bromopropyl-functionalized mesoporous silica. J. Hazard. Mater. 2010, 182, 581–590. [Google Scholar] [CrossRef]
- Di Renzo, F.; Cambon, H.; Dutarte, R. A 28-year-old synthesis of micelle-templated mesoporous silica. Microporous Mater. 1997, 10, 283–286. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, S.; Qiu, Z.; Tian, G.; Feng, Y.; Xiao, F.-S. Stable Ordered Mesoporous Silica Materials Templated by High-Temperature Stable Surfactant Micelle in Alkaline Media. J. Phys. Chem. B 2004, 108, 4696–4700. [Google Scholar] [CrossRef]
- Zhao, D.Y.; Feng, J.L.; Huo, Q.S.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50–300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.L.; Barrow, C.J.; Puri, M. Nanobiotechnology as a novel paradigm for enzyme immobilisation and stabilisation with potential applications in biodiesel production. Appl. Microbiol. Biotechnol. 2013, 97, 23–39. [Google Scholar] [CrossRef]
- Takahashi, H.; Li, B.; Sasaki, T.; Miyazaki, C.; Kajino, T.; Inagaki, S. Immobilized enzymes in ordered mesoporous silica ma-terials and improvement of their stability and catalytic activity in an organic solvent. Microporous Mesoporous Mater. 2001, 44, 755–762. [Google Scholar] [CrossRef]
- Schmidt-Winkel, P.; Lukens, W.W.; Yang, P.; Margolese, D.I.; Lettow, J.S.; Ying, J.Y.; Stucky, G.D. Microemulsion Templating of Siliceous Mesostructured Cellular Foams with Well-Defined Ultralarge Mesopores. Chem. Mater. 2000, 12, 686–696. [Google Scholar] [CrossRef]
- Han, Y.-J.; Watson, J.T.; Stucky, G.D.; Butler, A. Catalytic activity of mesoporous silicate-immobilized chloroperoxidase. J. Mol. Catal. B Enzym. 2002, 17, 1–8. [Google Scholar] [CrossRef]
- Thananukul, N.; Phongphut, A.; Prichanont, S.; Thanachayanont, C.; Fearn, S.; Chayasombat, B. A comparative study on mesocellular foam silica with different template removal methods and their effects on enzyme immobilization. J. Porous Mater. 2018, 26, 1059–1068. [Google Scholar] [CrossRef]
- Jung, D.; Paradiso, M.; Hartmann, M. Formation of cross-linked glucose oxidase aggregates in mesocellular foams. J. Mater. Sci. 2009, 44, 6747–6753. [Google Scholar] [CrossRef]
- Betancor, L.; Luckarift, H.R. Bioinspired enzyme encapsulation for biocatalysis. Trends Biotechnol. 2008, 26, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Begum, G.; Goodwin, W.B.; Deglee, B.M.; Sandhage, K.H.; Kröger, N. Compartmentalisation of enzymes for cascade reactions through biomimetic layer-by-layer mineralization. J. Mater. Chem. B 2015, 3, 5232–5240. [Google Scholar] [CrossRef]
- Wang, Y.; Du, X.; Liu, Z.; Shi, S.; Lv, H. Dendritic fibrous nano-particles (DFNPs): Rising stars of mesoporous materials. J. Mater. Chem. A 2019, 7, 5111–5152. [Google Scholar] [CrossRef]
- Califano, V.; Costantini, A.; Silvestri, B.; Venezia, V.; Cimino, S.; Sannino, F. The effect of pore morphology on the catalytic performance of β-glucosidase immobilized into mesoporous silica. Pure Appl. Chem. 2019, 91, 1583–1592. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, L.-L.; Jiang, J.-G.; Calin, N.; Lam, K.-F.; Zhang, S.-J.; Wu, H.-H.; Wu, G.-D.; Albela, B.; Bonneviot, L.; et al. Facile Large-Scale Synthesis of Monodisperse Mesoporous Silica Nanospheres with Tunable Pore Structure. J. Am. Chem. Soc. 2013, 135, 2427–2430. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Cha, D.K.; Zhang, X.; Basset, J.M. High-Surface-Area Silica Nanospheres (KCC-1) with a Fibrous Morphology. Angew. Chem. Int. Ed. 2010, 49, 9652–9656. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.-S.; Lee, J.-K. Tunable Synthesis of Hierarchical Mesoporous Silica Nanoparticles with Radial Wrinkle Structure. Langmuir 2012, 28, 12341–12347. [Google Scholar] [CrossRef]
- Costantini, A.; Venezia, V.; Pota, G.; Bifulco, A.; Califano, V.; Sannino, F. Adsorption of cellulase on wrinkled silica nanopar-ticles with enhanced inter-wrinkle distance. Nanomaterials 2020, 10, 1799. [Google Scholar] [CrossRef]
- Califano, V.; Sannino, F.; Costantini, A.; Avossa, J.; Cimino, S.; Aronne, A. Wrinkled Silica Nanoparticles: Efficient Matrix for β-Glucosidase Immobilization. J. Phys. Chem. C 2018, 122, 8373–8379. [Google Scholar] [CrossRef]
- Sannino, F.; Costantini, A.; Ruffo, F.; Aronne, A.; Venezia, V.; Califano, V. Covalent Immobilization of β-Glucosidase into Mesoporous Silica Nanoparticles from Anhydrous Acetone Enhances Its Catalytic Performance. Nanomaterials 2020, 10, 108. [Google Scholar] [CrossRef]
- Wei, Y.; Xu, J.; Dong, H.; Dong, J.H.; Qiu, K.; Jansen-Varnum, S.A. Preparation and Physisorption Characterization of d-Glucose-Templated Mesoporous Silica Sol−Gel Materials. Chem. Mater. 1999, 11, 2023–2029. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Shimizu, T.; Kuroda, K.; Kato, C. The Preparation of Alkyltrimethylammonium–Kanemite Complexes and Their Conversion to Microporous Materials. Bull. Chem. Soc. Jpn. 1990, 63, 988–992. [Google Scholar] [CrossRef]
- Baú, L.; Bártová, B.; Arduini, M.; Mancin, F. Surfactant-free synthesis of mesoporous and hollow silicananoparticles with an inorganic template. Chem. Commun. 2009, 7584–7586. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zharov, I. Large Pore Mesoporous Silica Nanoparticles by Templating with a Nonsurfactant Molecule, Tannic Acid. Chem. Mater. 2014, 26, 2030–2037. [Google Scholar] [CrossRef]
- Gao, J.-K.; Zhang, Z.-J.; Jiang, Y.-J.; Chen, Y.; Gao, S.-F. Biomimetic-Functionalized, Tannic Acid-Templated Mesoporous Silica as a New Support for Immobilization of NHase. Molecules 2017, 22, 1597. [Google Scholar] [CrossRef]
- Venezia, V.; Sannino, F.; Costantini, A.; Silvestri, B.; Cimino, S.; Califano, V. Mesoporous silica nanoparticles for β-glucosidase immobilization by templating with a green material: Tannic acid. Microporous Mesoporous Mater. 2020, 302, 110203. [Google Scholar] [CrossRef]
- Iskandar, F.; Gradon, L.; Okuyama, K. Control of the morphology of nanostructured particles prepared by the spray drying of a nanoparticle sol. J. Colloid Interface Sci. 2003, 265, 296–303. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Choi, S.H.; Kang, Y.C.; Lee, J.-K. Large-scale aerosol-assisted synthesis of biofriendly Fe2O3yolk–shell particles: A promising support for enzyme immobilization. Nanoscale 2016, 8, 6728–6738. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhu, J.; Xu, Z. Template-Assisted Synthesis of Mesoporous Magnetic Nanocomposite Particles. Adv. Funct. Mater. 2004, 14, 345–351. [Google Scholar] [CrossRef]
- Deng, Y.; Qi, D.; Deng, C.; Zhang, A.X.; Zhao, D. Superparamagnetic High-Magnetization Microspheres with an Fe3O4@SiO2Core and Perpendicularly Aligned Mesoporous SiO2Shell for Removal of Microcystins. J. Am. Chem. Soc. 2008, 130, 28–29. [Google Scholar] [CrossRef]
- Sen, T.; Bruce, I.J. Mesoporous silica–magnetite nanocomposites: Fabrication, characterisation and applications in biosciences. Microporous Mesoporous Mater. 2009, 120, 246–251. [Google Scholar] [CrossRef]
- Tran, D.-T.; Chen, C.-L.; Chang, J.-S. Immobilization of Burkholderia sp. lipase on a ferric silica nanocomposite for biodiesel production. J. Biotechnol. 2012, 158, 112–119. [Google Scholar] [CrossRef]
- Shuai, W.; Das, R.K.; Naghdi, M.; Brar, S.K.; Verma, M. A review on the important aspects of lipase immobilization on na-nomaterials. Biotechnol. Appl. Biochem. 2017, 64, 496–508. [Google Scholar] [CrossRef]
- Ismail, A.R.; Baek, K.-H. Lipase immobilization with support materials, preparation techniques, and applications: Present and future aspects. Int. J. Biol. Macromol. 2020, 163, 1624–1639. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, sta-bility and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Knežević, Z.; Mojović, L.; Adnađević, B. Immobilization of lipase on a hydrophobic zeolite type Y. J. Serb. Chem. Soc. 1998, 63, 257–264. [Google Scholar]
- Palomo, J.M.; Fuentes, M.; Fernández-Lorente, G.; Mateo, C.; Guisan, J.M.; Fernández-Lafuente, R. General Trend of Lipase to Self-Assemble Giving Bimolecular Aggregates Greatly Modifies the Enzyme Functionality. Biomacromolecules 2003, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Palomo, J.M.; Muñoz, G.; Fernández-Lorente, G.; Mateo, C.; Fernández-Lafuente, R.; Guisán, J.M. Interfacial adsorption of lipases on very hydrophobic support (octadecyl–Sepabeads): Immobilization, hyperactivation and stabilization of the open form of lipases. J. Mol. Catal. B Enzym. 2002, 19-20, 279–286. [Google Scholar] [CrossRef]
- Fernandez-Lorente, G.; Rocha-Martín, J.; Guisán, J.M. Immobilization of Lipases by Adsorption on Hydrophobic Supports: Modulation of Enzyme Properties in Biotransformations in Anhydrous Media. Methods Mol. Biol. 2020, 2100, 143–158. [Google Scholar]
- Serra, E.; Mayoral, Á.; Sakamoto, Y.; Blanco, R.M.; Díaz, I. Immobilization of lipase in ordered mesoporous materials: Effect of textural and structural parameters. Microporous Mesoporous Mater. 2008, 114, 201–213. [Google Scholar] [CrossRef]
- Carteret, C.; Jacoby, J.; Blin, J. Using factorial experimental design to optimize biocatalytic biodiesel production from Mucor Miehei Lipase immobilized onto ordered mesoporous materials. Microporous Mesoporous Mater. 2018, 268, 39–45. [Google Scholar] [CrossRef]
- Katiyar, M.; Abida, K.; Ali, A. Candida rugosa lipase immobilization over SBA-15 to prepare solid biocatalyst for cotton seed oil transesterification. Mater. Today Proc. 2021, 36, 763–768. [Google Scholar] [CrossRef]
- Jacoby, J.; Pasc, A.; Carteret, C.; Dupire, F.; Stébé, M.; Coupard, V.; Blin, J.-L. Ordered mesoporous materials containing Mucor Miehei Lipase as biocatalyst for transesterification reaction. Process. Biochem. 2013, 48, 831–837. [Google Scholar] [CrossRef]
- Kalantari, M.; Yu, M.; Yang, Y.; Strounina, E.; Gu, Z.; Huang, X.; Zhang, J.; Song, H.; Yu, C. Tailoring mesoporous-silica na-noparticles for robust immobilization of lipase and biocatalysis. Nano Res. 2017, 10, 605–617. [Google Scholar] [CrossRef]
- Kalantari, M.; Yu, M.; Liu, Y.; Huang, X.; Yu, C. Engineering mesoporous silica microspheres as hyper-activation supports for continuous enzymatic biodiesel production. Mater. Chem. Front. 2019, 3, 1816–1822. [Google Scholar] [CrossRef]
- Deon, M.; Ricardi, N.C.; De Andrade, R.C.; Hertz, P.F.; Nicolodi, S.; Costa, T.M.H.; Bussamara, R.; Benvenutti, E.V.; De Menezes, E.W. Designing a Support for Lipase Immobilization Based On Magnetic, Hydrophobic, and Mesoporous Silica. Langmuir 2020, 36, 10147–10155. [Google Scholar] [CrossRef] [PubMed]
- Lisboa, M.C.; Rodrigues, C.A.; Barbosa, A.S.; Mattedi, S.; Freitas, L.S.; Mendes, A.A.; Dariva, C.; Franceschi, E.; Lima, Á.S.; Soares, C.M.F. New perspectives on the modification of silica aerogel particles with ionic liquid used in lipase immobilization with platform in ethyl esters production. Process Biochem. 2018, 75, 157–165. [Google Scholar] [CrossRef]
- Virgen-Ortiz, J.J.; Pedrero, S.G.; Fernandez-Lopez, L.; Lopez-Carrobles, N.; Gorines, B.C.; Otero, C.; Fernandez-Lafuente, R. Desorption of Lipases Immobilized on Octyl-Agarose Beads and Coated with Ionic Polymers after Thermal Inactivation. Stronger Adsorption of Polymers/Unfolded Protein Composites. Molecules 2017, 22, 91. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of Different Enzyme Immobilization Strategies to Improve Enzyme Performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef]
- Jin, W.; Xu, Y.; Yu, X.-W. Preparation of lipase cross-linked enzyme aggregates in octyl-modified mesocellular foams. Int. J. Biol. Macromol. 2019, 130, 342–347. [Google Scholar] [CrossRef]
- Bi, Y.; Yu, M.; Zhou, H.; Zhou, H.; Wei, P. Biosynthesis of oleyl oleate in solvent-free system by Candida rugosa Lipase (CRL) immobilized in macroporous resin with cross-linking of aldehyde-dextran. J. Mol. Catal. B Enzym. 2016, 133, 1–5. [Google Scholar] [CrossRef]
- Sandoval, G.; Marty, A. Screening methods for synthetic activity of lipases. Enzym. Microb. Technol. 2007, 40, 390–393. [Google Scholar] [CrossRef]
- Liu, J.; Peng, J.; Shen, S.; Jin, Q.; Li, C.; Yang, Q. Enzyme entrapped in polymer-modified nanopores: The effects of macromo-lecular crowding and surface hydrophobicity. Chemistry 2013, 19, 2711–2719. [Google Scholar] [CrossRef]
- Jin, W.; Xu, Y.; Yu, X.-W. Email Formation lipase cross-linked enzyme aggregates on octyl-modified mesocellular foams with oxidized sodium alginate. Colloids Surf. B Biointerfaces 2019, 184, 110501. [Google Scholar] [CrossRef] [PubMed]
- Malar, C.G.; Seenuvasan, M.; Kumar, K.S.; Kumar, A.; Parthiban, R. Review on surface modification of nanocarriers to overcome diffusion limitations: An enzyme immobilization aspect. Biochem. Eng. J. 2020, 158, 107574. [Google Scholar] [CrossRef]
- Jiang, Y.; Zheng, P.; Zhou, L.; Kong, W.; Gao, J.; Wang, J.; Gu, J.; Zhang, X.; Wang, X. Immobilization of lipase in hierarchically ordered macroporous/mesoporous silica with improved catalytic performance. J. Mol. Catal. B Enzym. 2016, 130, 96–103. [Google Scholar] [CrossRef]
- Pang, J.; Zhou, G.; Liu, R.; Li, T. Esterification of oleic acid with methanol by immobilized lipase on wrinkled silica nanoparticles with highly ordered, radially oriented mesochannels. Mater. Sci. Eng. C 2016, 59, 35–42. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, W.; Zhou, L.; Ma, L.; He, Y.; Gao, J. Improved Performance of Lipase Immobilized on Tannic Acid-Templated Mesoporous Silica Nanoparticles. Appl. Biochem. Biotechnol. 2016, 179, 1155–1169. [Google Scholar] [CrossRef]
- Xiang, X.; Ding, S.; Suo, H.; Xu, C.; Gao, Z.; Hu, Y. Fabrication of chitosan-mesoporous silica SBA-15 nanocomposites via functional ionic liquid as the bridging agent for PPL immobilization. Carbohydr. Polym. 2018, 182, 245–253. [Google Scholar] [CrossRef]
- Babaki, M.; Yousefi, M.; Habibi, Z.; Mohammadi, M.; Yousefi, P.; Mohammadi, J.; Brask, J. Enzymatic production of biodiesel using lipases immobilized on silica nanoparticles as highly reusable biocatalysts: Effect of water, t-butanol and blue silica gel contents. Renew. Energy 2016, 91, 196–206. [Google Scholar] [CrossRef]
- Siódmiak, T.; Ziegler-Borowska, M.; Marszałł, M.P. Lipase-immobilized magnetic chitosan nanoparticles for kinetic resolution of (R,S)-ibuprofen. J. Mol. Catal. B Enzym. 2013, 94, 7–14. [Google Scholar] [CrossRef]
- Wang, P. Nanoscale biocatalyst systems. Curr. Opin. Biotechnol. 2006, 17, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Mehrasbi, M.R.; Mohammadi, J.; Peyda, M.; Mohammadi, M. Covalent immobilization of Candida antarctica lipase on core-shell magnetic nanoparticles for production of biodiesel from waste cooking oil. Renew. Energy 2017, 101, 593–602. [Google Scholar] [CrossRef]
- Ali, Z.; Tian, L.; Zhang, B.; Ali, N.; Khan, M.; Zhang, Q. Synthesis of fibrous and non-fibrous mesoporous silica magnetic yolk–shell microspheres as recyclable supports for immobilization of Candida rugosa lipase. Enzym. Microb. Technol. 2017, 103, 42–52. [Google Scholar] [CrossRef]
- Ali, Z.; Tian, L.; Zhang, B.; Ali, N.; Zhang, Q. Synthesis of paramagnetic dendritic silica nanomaterials with fibrous pore structure (Fe3O4@ KCC-1) and their application in immobilization of lipase from Candida rugosa with enhanced catalytic activity and stability. N. J. Chem. 2017, 41, 8222–8231. [Google Scholar] [CrossRef]
- Esmi, F.; Nematian, T.; Salehi, Z.; Khodadadi, A.A.; Dalai, A.K. Amine and aldehyde functionalized mesoporous silica on magnetic nanoparticles for enhanced lipase immobilization, biodiesel production, and facile separation. Fuel 2021, 291, 120126. [Google Scholar] [CrossRef]
- Khoobbakht, G.; Kheiralipour, K.; Yuan, W.; Seifi, M.R.; Karimi, M. Desirability function approach for optimization of enzy-matic transesterification catalyzed by lipase immobilized on mesoporous magnetic nanoparticles. Renew. Energy 2020, 158, 253–262. [Google Scholar] [CrossRef]
- Shao, Y.B.; Jing, T.; Tian, J.Z.; Zheng, Y.J.; Shang, M.H. Characterization and optimization of mesoporous magnetic nanopar-ticles for immobilization and enhanced performance of porcine pancreatic lipase. Chem. Pap. 2015, 69, 1298–1311. [Google Scholar] [CrossRef]
- Khoobi, M.; Motevalizadeh, S.F.; Asadgol, Z.; Forootanfar, H.; Shafiee, A.; Faramarzi, M.A. Synthesis of functionalized poly-ethylenimine-grafted mesoporous silica spheres and the effect of side arms on lipase immobilization and application. Biochem. Eng. J. 2014, 88, 131–141. [Google Scholar] [CrossRef]
- Ye, P.; Jiang, J.; Xu, Z.-K. Adsorption and activity of lipase from Candida rugosa on the chitosan-modified poly(acrylonitrile-co-maleic acid) membrane surface. Colloids Surf. B Biointerfaces 2007, 60, 62–67. [Google Scholar] [CrossRef]
- Carvalho, N.B.; Vidal, B.T.; Barbosa, A.S.; Pereira, M.M.; Mattedi, S.; Freitas, L.D.S.; Lima, Á.S.; Soares, C.M. Lipase immo-bilization on silica xerogel treated with protic ionic liquid and its application in biodiesel production from different oils. Int. J. Mol. Sci. 2018, 19, 1829. [Google Scholar] [CrossRef]
- Katiyar, M.; Ali, A. One-pot lipase entrapment within silica particles to prepare a stable and reusable biocatalyst for trans-esterification. J. Am. Oil Chem. Soc. 2015, 92, 623–632. [Google Scholar] [CrossRef]
- Ficanha, A.M.M.; Antunes, A.; Oro, C.E.D.; Dallago, R.M.; Mignoni, M.L. Immobilization of candida antarctica b (calb) in silica aerogel: Morphological characteristics and stability. Biointerfaces Res. Appl. Chem. 2020, 10, 6744–6756. [Google Scholar]
- Cazaban, D.; Illanes, A.; Wilson, L.; Betancor, L. Bio-inspired silica lipase nanobiocatalysts for the synthesis of fatty acid methyl esters. Process. Biochem. 2018, 74, 86–93. [Google Scholar] [CrossRef]
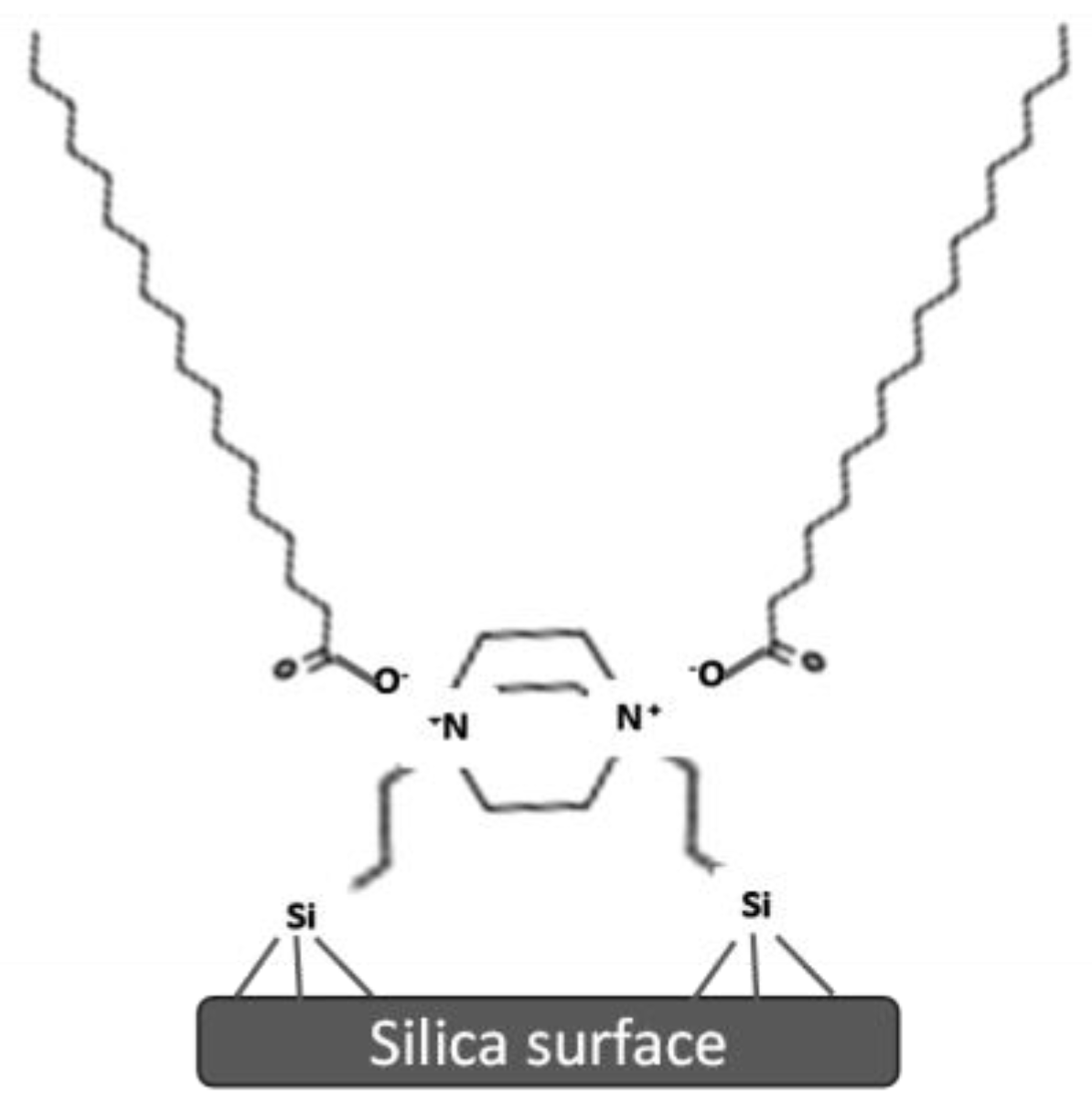
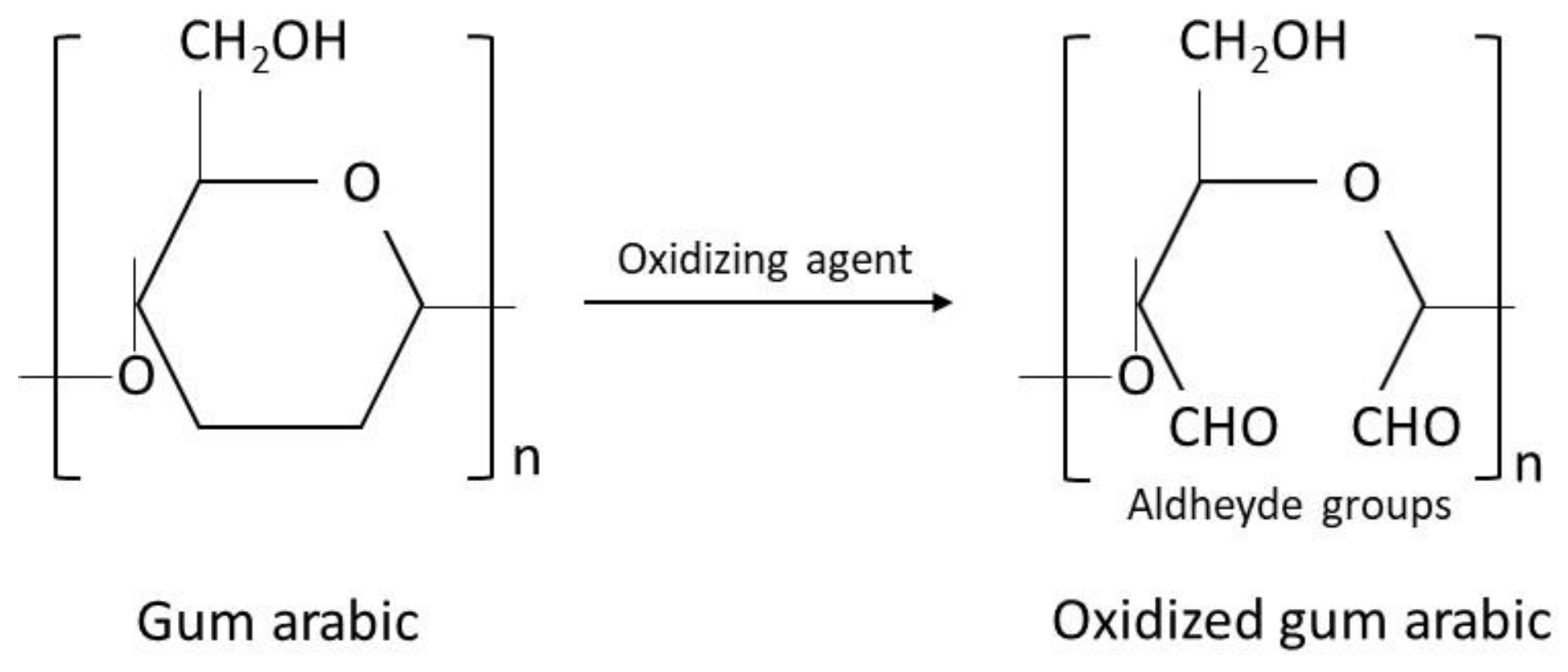

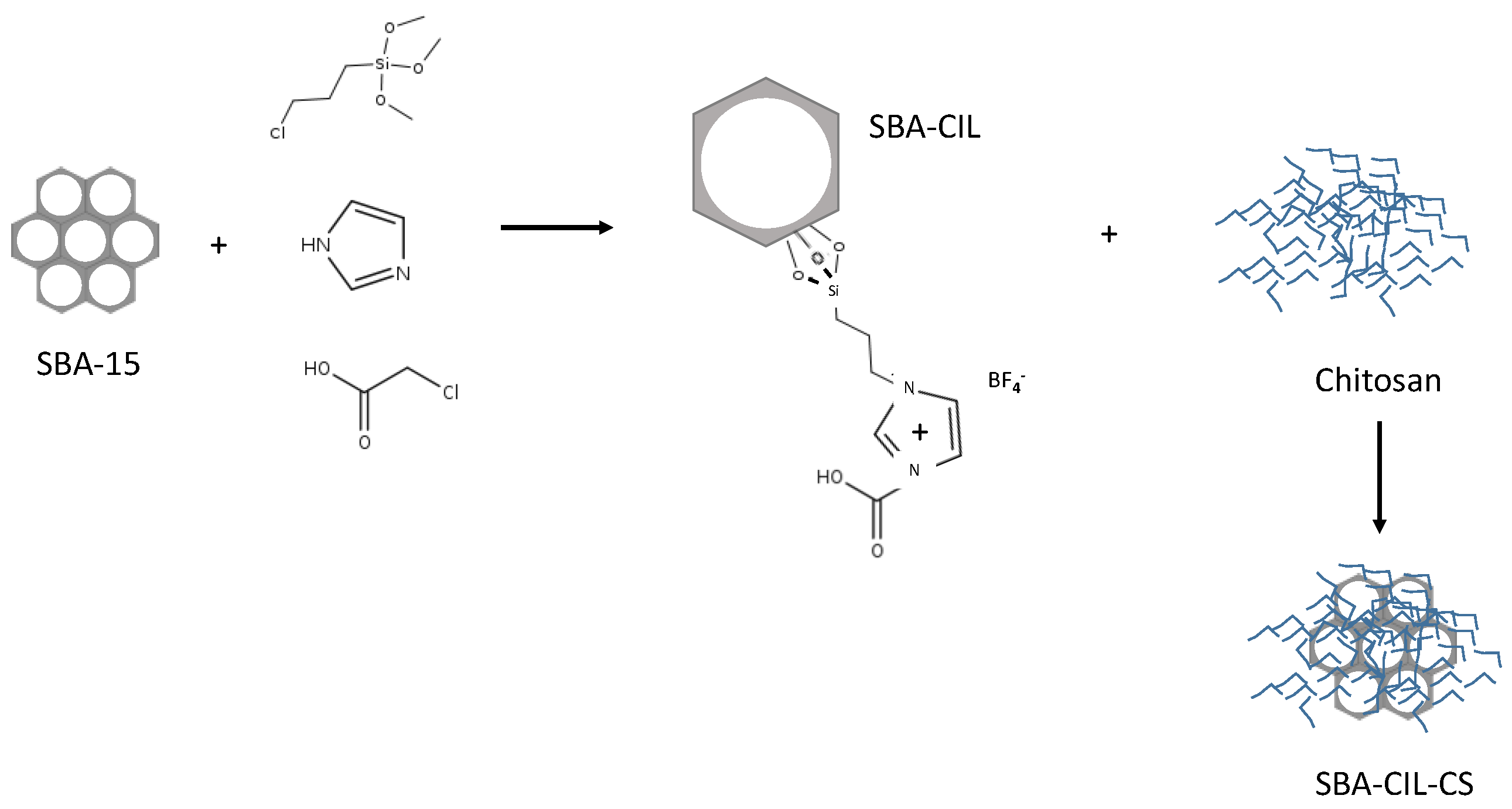
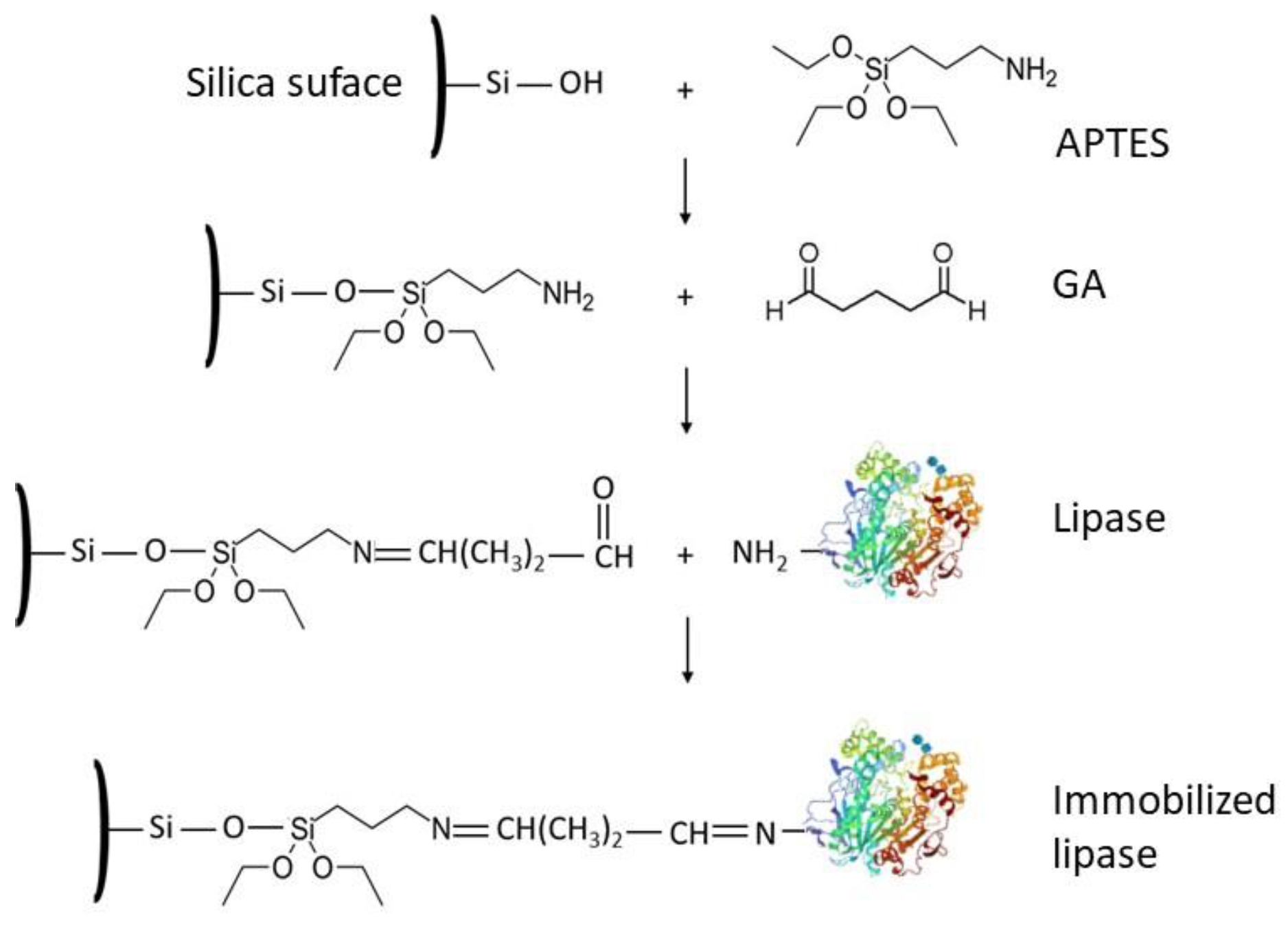
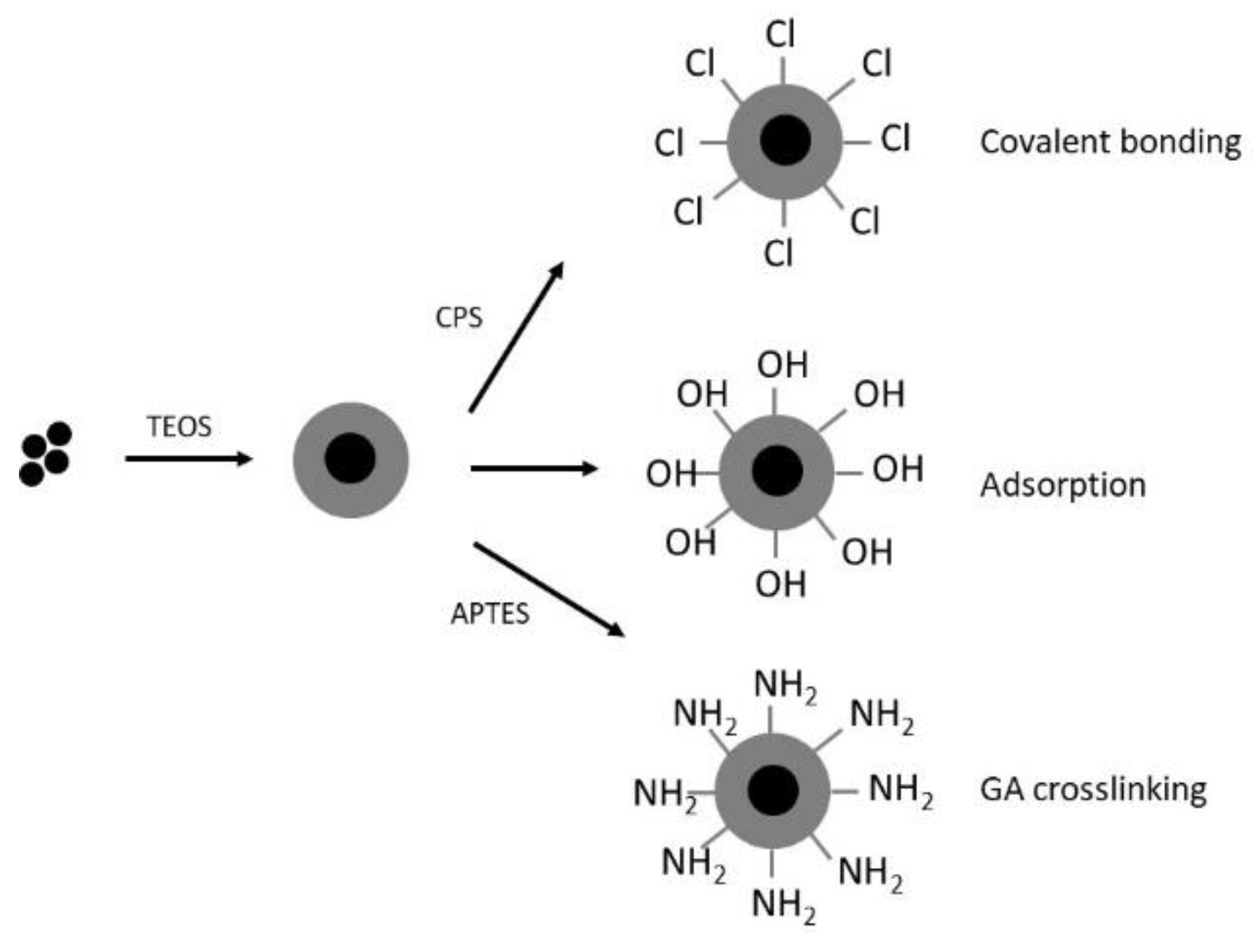

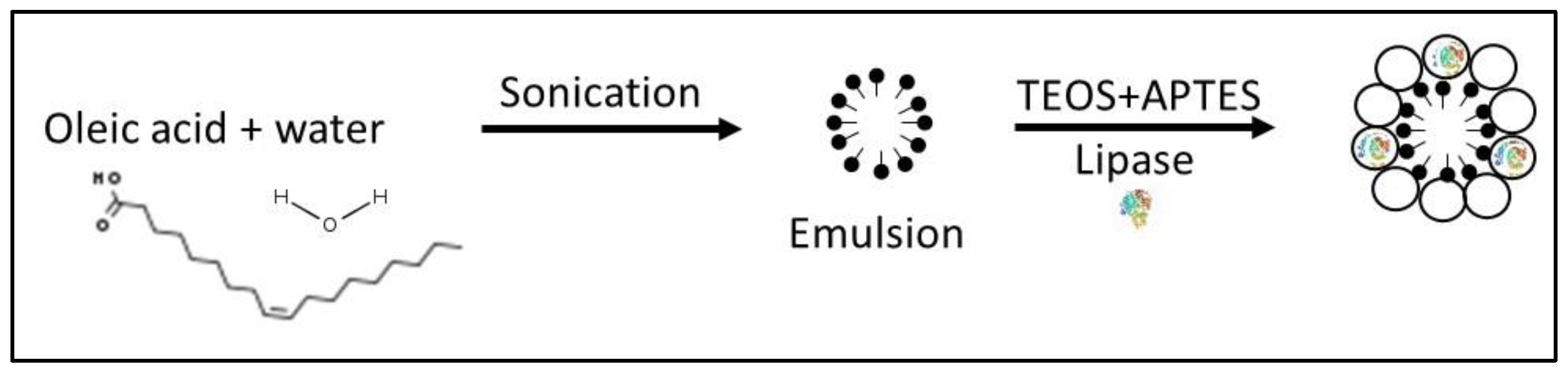
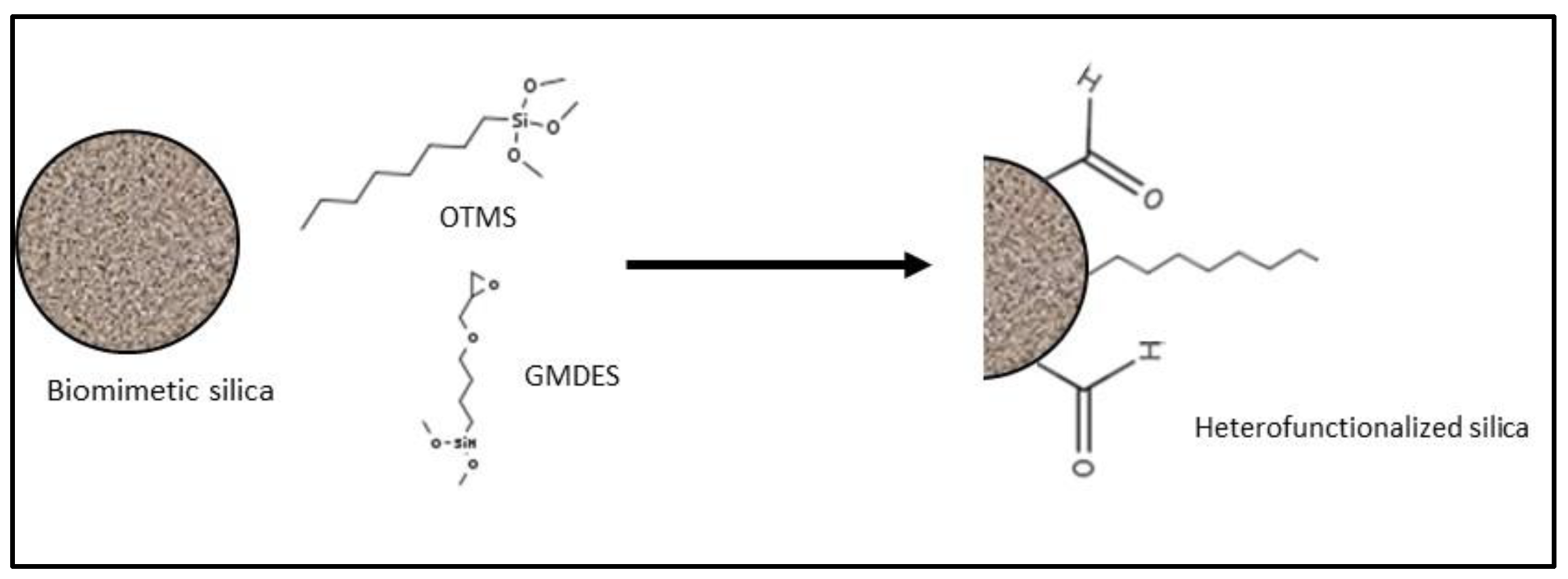
| Source | Brand Name | Reference |
|---|---|---|
| Candida antartica | Novozym® 435 | [24] |
| Mucor miehei | Lipozyme IM | [25] |
| Thermomyces lanuginosus | Lipozyme TL IM | [26] |
| Rhizomucor miehei | Lipozyme RM IM | [27] |
| Burkholderia cepacia | - | [28] |
| Pseudomonas fluorescens | - | [29] |
| Candida rugose | - | [30] |
| Rhizopus oryzae | - | [31] |
| Penicillium expansum | - | [32] |
| Geotrichum sp. | - | [33] |
| Pore Diameter (nm) | Specific Activity with Respect to Soluble Enzyme (times) | Residual Activity after 5 Reuses (%) |
|---|---|---|
| 1.6 | 4.51 | 11 |
| 2.0 | 4.39 | 12 |
| 4.8 | 5.23 | 93 |
| 6.9 | 5.70 | 68 |
| 8.0 | 5.94 | 54 |
| 13 | 6.12 | 49 |
| Support | Reaction | Reactants | Solvent | Reference |
|---|---|---|---|---|
| SBA-15 | Transesterification | Rapeseed oil + methanol | - | [93] |
| SBA-15 | Transesterification | Cotton seed oil + methanol | - | [94] |
| C18-MSN | Hydrolysis | 4-nitrophenyl palmitate (pNPP) | Water | [96] |
| C18-Silica microspheres | Transesterification | Corn oil + methanol | - | [97] |
| Aerogel-IL | Hydrolysis Transesterification | Olive oil emulsion Coconut oil + ethanol | - | [99] |
| Gum Arabic/MCF | Esterification | n-caprylic acid+ethanol | Heptane | [103] |
| Sodium alginate/MCF | Transesterification | Soybean oil + methanol | - | [107] |
| Core shell magnetic silica | Hydrolysis | 4-nitrophenyl palmitate (pNPP) | Water/isopropanol | [98] |
| Hierarchically ordered macro/meso | Esterification | Fatty acid + alcohols | Cyclohexane | [109] |
| Wrinkled silica | Esterification | Oleic acid + methanol | - | [110] |
| TA-MSN | Esterification Transesterification | Oleic acid + ethanol Soybean oil + ethanol | n-hexane n-hexane | [111] |
| SBA-15/Chitosan | Hydrolysis | Triacetin | Water | [112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costantini, A.; Califano, V. Lipase Immobilization in Mesoporous Silica Nanoparticles for Biofuel Production. Catalysts 2021, 11, 629. https://doi.org/10.3390/catal11050629
Costantini A, Califano V. Lipase Immobilization in Mesoporous Silica Nanoparticles for Biofuel Production. Catalysts. 2021; 11(5):629. https://doi.org/10.3390/catal11050629
Chicago/Turabian StyleCostantini, Aniello, and Valeria Califano. 2021. "Lipase Immobilization in Mesoporous Silica Nanoparticles for Biofuel Production" Catalysts 11, no. 5: 629. https://doi.org/10.3390/catal11050629
APA StyleCostantini, A., & Califano, V. (2021). Lipase Immobilization in Mesoporous Silica Nanoparticles for Biofuel Production. Catalysts, 11(5), 629. https://doi.org/10.3390/catal11050629






