Abstract
Enzymes displayed on the Bacillus subtilis spore coat have several features that are useful for biocatalysis. The enzyme is preimmobilized on an inert surface of the spore coat, which is due to the natural sporulation process. As a result, protein stability can be increased, and they are resistant to environmental changes. Next, they would not lyse under extreme conditions, such as in organic solvents. Furthermore, they can be easily removed from the reaction solution and reused. The laboratory evolved CotA laccase variant T480A-CotA was used to oxidize the following phenolic substrates: (+)-catechin, (−)-epicatechin, and sinapic acid. The kinetic parameters were determined and T480A-CotA had a greater Vmax/Km than wt-CotA for all substrates. The Vmax/Km for T480A-CotA was 4.1, 5.6, and 1.4-fold greater than wt-CotA for (+)-catechin, (−)-epicatechin, and sinapic acid, respectively. The activity of wt-CotA and T480A-CotA was measured at different concentrations from 0–70% in organic solvents (dimethyl sulfoxide, ethanol, methanol, and acetonitrile). The Vmax for T480A-CotA was observed to be greater than the wt-CotA in all organic solvents. Finally, the T480A-CotA was recycled 7 times over a 23-h period and up to 60% activity for (+)-catechin remained. The product yield was up to 3.1-fold greater than the wild-type.
1. Introduction
Enzymes are green and efficient catalysts because they operate at room temperature and in aqueous solutions. Furthermore, they have high substrate specificity that can produce chiral products with high enantiomeric excess. Therefore, enzymes suited for industrial applications continue to increase. However, they suffer several limitations [1,2,3]. For example, hydrophobic substrates are not soluble in aqueous solutions and an organic co-solvent must be added for the reaction to occur. In general, enzymes are not stable in organic solvents and they lose activity [4,5,6]. The protein structure is the result of the stability of the hydrophobic core, Van der Waals forces, electrostatic interactions, and hydrogen bonding. Organic solvent disrupts the hydrophobic core, leading to protein unfolding. Furthermore, organic solvents can strip water from the protein surface, which is essential for structure and function. Finally, the enzymatic activity would decrease in organic solvent because of thermodynamic reasons. In aqueous solution, the substrate is surrounded by water and oftentimes the active site is hydrophobic. It is energetically favorable for the substrate to be desolvated and partitioned from the hydrophobic active site. Hydrophobic substrates are thermodynamically stabilized in the organic solvent compared with water.
Enzyme immobilization has several advantages [7,8]. First, protein stability can be increased. Next, they are more resistant to environmental changes, which can enhance the lifetime in organic solvents. Furthermore, substrates are freely accessible to biocatalyst. Finally, the enzyme can be easily removed from reaction mixture for reuse [9]. Water-in-oil (W/O) microemulsion systems have been developed to solubilize hydrophobic substrates while retaining enzymatic stability and activity [10]. However, product separation and purification may be complicated because of the presence of compounds necessary to create the microemulsion. Next, enzymes associated with nanoparticles has also been used to maintain stability and activity [11]. One drawback is that protein immobilization process is a multistep procedure, which may increase expense. In addition, both methods require a purified protein, which also adds to the cost. Protein display on the surface of the microorganisms is one strategy to immobilize enzymes [12].
Traditional cell surface display systems, such as Escherichia coli and yeast, appear ideal; however, there are several issues [13]. Lysis may occur during the reaction and contents of the cells will enter the solution. As a result, this will add additional contamination, and separating the enzyme from the reaction solution may be required. Spore displayed proteins overcome these issues. Spores remain intact under extreme chemical and physical conditions [14,15]. As a result, enzymes displayed on their surface can tolerate extreme conditions [16]. They would not be lysed and can be easily removed from the reaction solution. In addition, protein folding problems associated with the target protein crossing cell membranes are eliminated. Furthermore, there is no need to overexpress, purify, and attach the protein to an inert surface. This is due to the natural sporulation process [17]. In addition, the whole cell biocatalyst can be recycled and reused, which reduces the overall cost associated with product yield.
Spores can be also used as a platform for protein engineering and optimization for extreme properties [18]. They remain viable and the genotype/phenotype connection is not lost during screening under harsh conditions. Traditional display methods suffer several issues. First, protein folding is a concern. For example, surface-displayed proteins of E. coli are expressed in a reducing environment and transported through several membranes into an oxidizing location. This hinders proper protein folding. Phage and yeast display also suffer from these hurdles. Next, viability is a problem. For example, if a cell lyses, then it cannot be cultured to retrieve the gene that is associated with improved protein. In short, the gene associated with the improved protein is detached.
Enzymes have been used in the food and process industries. Natural phenolic compounds have beneficial biological effects. They can act as antioxidants [19], antimutagens [20], and anticarcinogens [21]. For example, (+)-catechin (Figure 1a) and (−)-epicatechin (Figure 1b) are two phenolic isomers that can be found in green tea, and they are responsible for potential health benefits [22]; however, they affect the flavor and taste. Hence, these compounds can be removed by polymerization [23], and the sensory quality is enhanced [24]. Polycatechins can also be utilized to prevent neurodegenerative and cardiovascular diseases. Enzymes, such as laccases, have been used to polymerize phenolic compounds for a variety of applications [25,26]. Next, rapeseed meal (RSM) is widely utilized to feed all classes of livestock. Sinapic acid (Figure 1c) and the choline ester of sinapic acid (sinapine) are the major phenolic compounds found in RSM [27,28]. These compounds or their derivatives lower the nutritional value of RSM. In addition, they render RSM to be bitter and astringent [29,30]. Biocatalysts can be used to eliminate these antinutrients [31].
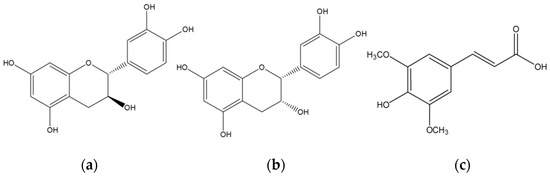
Figure 1.
Structure of substrates: (a) (+)-catechin; (b) (−)-epicatechin; and (c) sinapic acid.
Laccases (EC 1.10.3.2, benzenodiol:oxygen oxioreductase) catalyze the oxidation of a variety of phenolic and non-phenolic compounds. Substrate oxidation occurs at mononuclear copper site and dioxygen is reduced to water at a trinuclear copper center [32,33]. Laccase are found in a wide variety of organisms, which includes plants, fungi, and bacteria. They have applications in biological pulping and bleaching in the paper industry; waste detoxification and decontamination of industrial waste and contaminated soil or water, dye decolorization, and degradation of polyaromatic hydrocarbons; applications in the food and textile industries; and for biosensors and diagnostics [34,35,36,37].
Laccase (CotA; Gene ID 936023), which is found on the spore coat of Bacillus subtilis, was evolved by spore protein display for improved activity in organic solvents. A CotA variant was identified with a Thr480Ala (T480A-CotA) amino acid substitution after only one round of evolution [16]. This mutation was found on the side of the trinuclear copper center. The alanine mutation is on the surface of CotA and is approximately 18 Å away from the copper cluster (Figure 2). The screen was performed at 60% DMSO and it was 2.38 fold more active than the wild-type CotA (wt-CotA) with substrate 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS). T480A-CotA was more active from a range of 0–70% DMSO. In addition, the variant was more active in ethanol, methanol, and acetonitrile. In this study, we determined the potential of using spore display as a reusable whole cell biocatalysis in organic cosolvents. The catalytic properties of the spore displayed laccases, T480A-CotA and wt-CotA, were characterized with natural phenolic compounds.
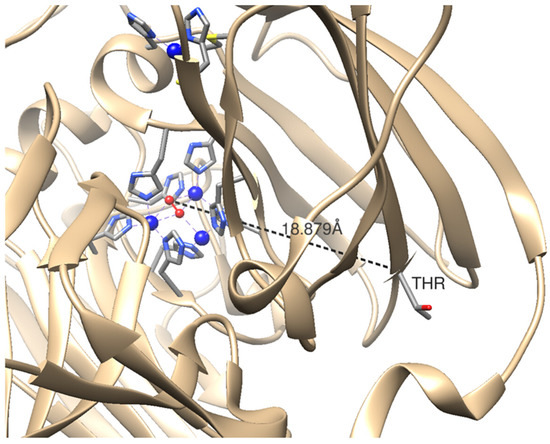
Figure 2.
The active site of CotA laccase. The model of CotA laccase (PDB ID: 3ZDW) is generated by UCSF Chimera (Resource for Biocomputing, Visualization, and Informatics (RBVI), UCSF). The ribbon of CotA laccase is light brown in color. Copper atoms are represented by spheres colored in blue. A molecule of dioxygen is represented by spheres colored in red. The amino acid residues that coordinate with copper atoms are represented by stick models. The threonine residue at site 480 (Thr480) is labeled and its distance from the center of trinuclear center is shown in black.
2. Results
2.1. Wt-CotA and T480A-CotA Activity Versus pH
The activity was measured for wt-CotA and T480A-CotA with (+)-catechin, (−)-epicatechin or sinapic acid in the pH range from 2 to 8 (Figure 3). The pH optimum is 7 for wt-CotA and T480A-CotA. The reactions were carried out in citrate phosphate buffer (100 mM, pH 7), methanol (20%), and CuCl2 (0.25 mM).
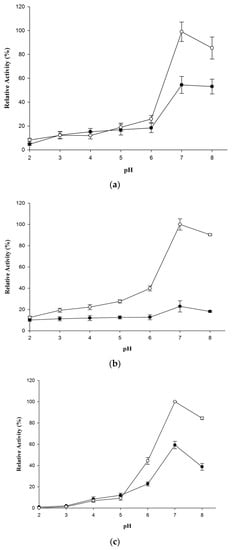
Figure 3.
Activity versus pH for wt-CotA (closed circles) and T480A-CotA (open circles): (a) (+)-catechin; (b) (−)-epicatechin; and (c) sinapic acid. Overall, 100% relative activity is at the pH with the greatest δA of 0.001 per min at 37 °C at 433, 440, or 512 nm for (+)-catechin, (−)-epicatechin, and sinapic acid, respectively.
2.2. Determination of Kinetic Parameters
Vmax and Km was determined for wt-CotA and T480A-CotA with (+)-catechin, (−)-epicatechin, and sinapic acid (Table 1; Figure A1a–c). The reactions were carried out in citrate phosphate buffer (100 mM, pH 7), methanol (20%), and CuCl2 (0.25 mM). In all experiments, T480A-CotA had higher Vmax/Km in comparison to wt-CotA.

Table 1.
Kinetic parameters of wt-CotA and T480A-CotA.
2.3. Activity of wt-CotA and T480A-CotA in Organic Solvent
The activity of wt-CotA and T480A-CotA was measured at different concentrations of DMSO, acetonitrile, methanol, and ethanol (Figure 4a–c; Table A1 Appendix A). T480A-CotA displayed increased Vmax for all the substrates and organic solvents that were evaluated when compared to wt-CotA. Both enzymes did not display appreciable activity in buffer alone for all organic solvents and substrates. T480A-CotA had increased Vmax for (+)-catechin of 1.44 to 2.99, 1.08 to 1.56, 1.53 to 3.42, and 1.15 to 2.41-fold in DMSO, acetonitrile, methanol, and ethanol, respectively (Figure 4a). Next, T480A-CotA had increased Vmax for (−)-epicatechin of 1.03 to 4.08, 1.04 to 4.18, 1.06 to 6.60, and 1.31 to 6.24-fold in DMSO, acetonitrile, methanol, and ethanol, respectively (Figure 4b). Finally, T480A-CotA had increased Vmax for sinapic acid of 1.5 to 3.4, 1.41 to 2.26, 1.50 to 6.17, and 1.50 to 5.10-fold in DMSO, acetonitrile, methanol, and ethanol, respectively (Figure 4c). In all cases, T480A-CotA had improved Vmax in comparison with wt-CotA.
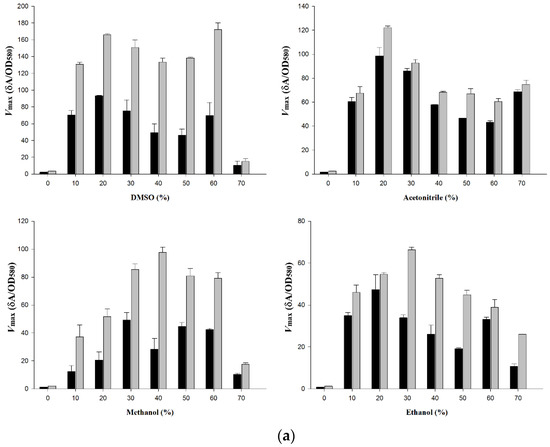
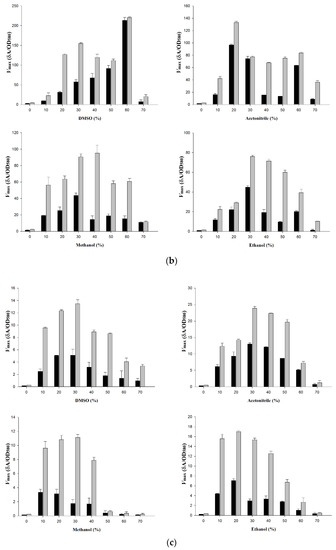
Figure 4.
Vmax (δA/OD580) of wt-CotA (black bars) and T480A-CotA (grey bars) in 0–70% organic solvents for substrate (a) (+)-catechin; (b) (−)-epicatechin; and (c) sinapic acid.
2.4. Wt-CotA and T480A-CotA Recycling in Organic Solvents
Wt-CotA and T480-CotA had catalyzed the oxidation of (+)-catechin in DMSO, acetonitrile, ethanol, and methanol. Then, the biocatalyst was filtered from the reaction and the reaction was repeated. The biocatalyst was recycled 7 times over a 23-h period. In all cases, the T480A-CotA has a higher product yield than the wt-CotA in all the organic solvents tested (Figure 5, Table A2). The total yield of T480A-CotA was 3.09 ± 0.32, 1.54 ± 0.09, 3.66 ± 0.39, and 1.79 ± 0.33-fold greater than that of the wt-CotA after each recycling period in DMSO, acetonitrile, methanol, and ethanol, respectively.
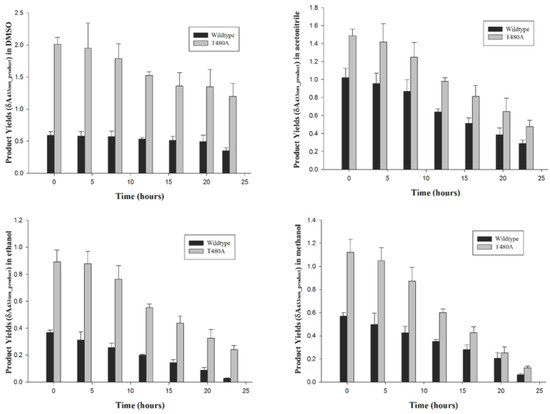
Figure 5.
The (+)-catechin products yields for wt-CotA (black bars) and T480A-CotA (grey bars) were determined for 7 cycles over a 23 h period.
3. Discussion
The pH activity trends were similar for wt-CotA and T480A-CotA toward (+)-catechin, (−)-epicatechin, and sinapic acid. For example, wt-CotA and T480A-CotA had a low activity at acidic pH and the activity increased to a maximum at approximately pH 7 for all the substrates. In addition, T480A-CotA always had a greater activity than the wt-CotA. The amino acid substitution did not change the pH optimum for both enzymes; however, the T480A-CotA displayed a higher Vmax [16]. A dramatic increase of laccase activity from pH 6 to 7 for the oxidation of (+)-catechin, (−)-epicatechin and sinapic acid was observed (Figure 3a–c). At pH 7, wt-CotA activity increases 2.9, 1.8, and 2.6-fold for substrate (+)-catechin, (−)-epicatechin, and sinapic acid, respectively, in comparison with that at pH 6. At pH 7, T480A-CotA activity increases 3.9, 2.5, and 2.3-fold for substrate (+)-catechin, (−)-epicatechin, and sinapic acid, respectively, compared with that at pH 6. In addition, the laccase activity dropped from pH 7 to 8. Therefore, the optimum pH values of wt-CotA and T480A-CotA for substrates (+)-catechin, (−)-epicatechin, and sinapic acid were pH 7. In addition, T480A-CotA showed 1.8, 4.4, and 1.7-fold higher activity than wt-CotA at pH 7 for substrates (+)-catechin, (−)-epicatechin, and sinapic acid, respectively. Therefore, T480A-CotA is more active than wt-CotA at their optimum pH for these substrates.
In general, the Vmax for T480A-CotA was greater than the wt-CotA in all organic solvents. Wt-CotA and T480A-CotA showed slight activity over the background in buffer alone. This is due to low solubility of the organic substrates in aqueous solution. Hence, an organic co-solvent is necessary to solubilize the substrate for enzymatic activity. For the substrates (+)-catechin and (−)-epicatechin, T480A-CotA has an optimum concentration of 60%, 20%, 40%, and 30% (v/v) for DMSO, acetonitrile, methanol, and ethanol, respectively (Figure 4a,b). For substrate (+)-catechin, T480A-CotA is 1.4–3.0, 1.1–3.0, 1.7–3.4, and 1.2–2.4 fold more active than wt-CotA in DMSO, acetonitrile, methanol, and ethanol, respectively. For substrate (−)-epicatechin, T480A-CotA is 1.0–4.1, 1.0–5.6, 1.1–7.9, and 1.3–6.2 fold more active in DMSO, acetonitrile, methanol, and ethanol, respectively. For substrate sinapic acid, T480A-CotA has an optimum concentration of 30%, 30%, 30%, and 20% (v/v) for DMSO, acetonitrile, methanol, and ethanol, respectively (Figure 4c). T480A-CotA is 2.4–4.8, 1.4–2.3, 1.5–6.4 and 1.5–5.2 fold more active in DMSO, acetonitrile, methanol, and ethanol, respectively.
Vmax and Km was determined for wt-CotA and T480A-CotA with (+)-catechin, (−)-epicatechin, and sinapic acid (Table 1; Figure A1a–c). T480A-CotA had a smaller Km for (+)-catechin and (−)-epicatechin than the wt-CotA. Wt-CotA has a Km 1.46-fold and 1.84-fold greater than T480A-CotA for (+)-catechin and (−)-epicatechin, respectively. On the other hand, T480A-CotA has a larger Km for sinapic acid. In all cases, T480A-CotA has a greater Vmax [16]. The Vmax/Km for T480A-CotA was 4.1, 5.6, and 1.4-fold greater than that of wt-CotA for (+)-catechin, (−)-epicatechin, and sinapic acid, respectively.
Enzyme recycling improves overall product yields by reusing the catalysts for several batches. This reduces the cost associated with product yield. Typically, the enzyme is expressed and purified, and attached to an inert particle. This is avoided in spore display because the enzyme is attached to the inert surface of the spore coat. CotA recycling was investigated, and the total product yield was determined for (+)-catechin over 23-h period for 7 cycles (Figure 5, Table A2). These percentages (v/v) were chosen at the maximum rates for the organic solvents, which were 60%, 20%, 40%, and 30% (v/v) for DMSO, acetonitrile, methanol, and ethanol, respectively. The trend of total product yield was similar for wt-CotA and T480A-CotA. The total product yield diminishes after recycling. The product yield retains 60%, 30%, and 10% from t = 0 to 23 h in DMSO, acetonitrile, and ethanol, respectively (Figure 5). In methanol, wt-CotA and T480A-CotA also has lowered product yield over time, which is 10% and 30%, respectively. The total product yield over 23-h period from T480A-CotA was 11.2, 7.1, 3.9, and 4.2 δA433nm_product in DMSO, acetonitrile, methanol, and ethanol, respectively. The optimum solvent was DMSO for the variant. Next, the total product yield over a 23-h period from wt-CotA was 3.6, 4.7, 1.4, and 2.4 δA433nm_product in DMSO, acetonitrile, methanol, and ethanol, respectively. In short, the total product yield was increased 3.1 to 1.5-fold depending on the solvent. Wt-CotA catalyzes most products in acetonitrile. In general, the biocatalysts have a greater product yield in polar aprotic solvents. The steady decrease in product yield may be due to inefficient recovery of the spores during centrifugation and discarding the supernatant. In addition, T480A-CotA outperforms the wt-CotA in all organic solvents.
4. Materials and Methods
4.1. Materials
Wt-CotA and T480A-CotA was expressed and purified on B. subtilis spores by following published procedures [16]. One unit is defined as a δA of 0.001 per min at 37 °C in a 300 μL reaction volume on a 96-well microplate at an absorbance at 433 nm (SpectraMax_M2) using (+)-catechin (433 nm), or absorbance at 440 nm using (−)-epicatechin, or absorbance at 512 nm using sinapic acid [38,39]. Dimethyl sulfoxide (DMSO), acetonitrile, methanol, ethanol, acetonitrile, (+)-catechin, (−)-epicatechin, and sinapic acid were procured from Sigma-Aldrich (St. Louis, MO, USA).
4.2. Wt-CotA and T480A-CotA Activity Versus pH
Substrate stocks were prepared for (+)-catechin (200 mM), (−)-epicatechin (200 mM), and sinapic acid (200 mM) in methanol. Each stock solution was prepared fresh before use. The purified spore pellets of wt-CotA or T480A-CotA were resuspended in aqueous CuCl2 (0.25 mM) for 60 min. Next, spore suspension was diluted in citric phosphate buffer (100 mM, 0.25 mM CuCl2).
The total volume of the reaction was 300 μL. The substrate (30 μL; 20 mM) was added to citrate phosphate buffer (250 μL; 100 mM) and methanol (10% v/v) solution containing CuCl2 (0.25 mM). The reaction was initiated with the spore resuspension (20 μL; OD580nm = 0.1–0.3). The pH was varied from 2 to 8 for the reactions. The absorbance for (+)-catechin (433 nm), (−)-epicatechin (440 nm), or sinapic acid (512 nm) was measured every 5 min for 60 min at 37 °C. Each reaction was done in triplicate. Control experiments were done with spores that had the cotA gene knockout (strain1S101, Bacillus Genetic Stock Center, The Ohio State University, Columbus, OH, USA).
4.3. Determination of Kinetic Parameters of wt-CotA and T480A-CotA
The total volume of the reaction was 300 μL. The substrate (30 μL; 0.05–6.0 mM) was added to citrate phosphate buffer (250 μL; 100 mM; pH 7) and methanol (20% v/v) solution containing CuCl2 (0.25 mM). The reaction was initiated with the spore resuspension (20 μL; OD580nm = 0.1–0.3). Km and Vmax were determined for both wt-CotA and T480A-CotA. The absorbance for (+)-catechin (433 nm), (−)-epicatechin (440 nm), or sinapic acid (512 nm) was measured every 5 min for 60 min at 37 °C. Each reaction was done in triplicate. Control experiments were done with spores that had the cotA gene knockout (strain1S101, Bacillus Genetic Stock Center, The Ohio State University, Columbus, OH, USA).
4.4. Wt-CotA and T480A-CotA Activity in Organic Solvents
The activity of wt-CotA and T480A-CotA was determined in DMSO, methanol, ethanol, and acetonitrile. The concentration of organic solvents used in the reaction media ranged from 0% to 70% (v/v). The substrates used were (+)-catechin, (−)-epicatechin, and sinapic acid and concentrations were 10 mM for each substrate. An OD580nm 0.1–0.3 was used for wt-CotA and T480A-CotA. Control experiments were done with spores that had the cotA gene knockout (strain1S101, Bacillus Genetic Stock Center, The Ohio State University, Columbus, OH, USA). The Vmax values were determined following the procedure above.
4.5. Wt-CotA and T480A-CotA Recycling in Organic Solvents
CotA was recycled and the total product yield was determined over a 23-h period. The spores were resuspended in aqueous CuCl2 (0.25 mM) solution for 60 min at 37 °C. Next, the spores were centrifuged, and the supernatant was discarded. The spores were resuspended in citrate phosphate buffer (100 mM, pH 7) with organic solvents (60%, 20%, 40%, and 30% (v/v) for DMSO, acetonitrile, methanol, and ethanol, respectively) at 37 °C with a final OD580 of 0.2. The spores were incubated for four hours and then the spores were centrifuged, and the supernatant was discarded. The reaction was initiated by resuspending the spores with 300 μL (+)-catechin (10 mM) in citrate phosphate buffer (100 mM, pH 7) with organic solvents (60%, 20%, 40%, and 30% (v/v) for DMSO, acetonitrile, methanol, and ethanol, respectively) and CuCl2 (0.25 mM) at 37 °C. The absorbance was taken at 433 nm to determine the product yield (δA433nm_product). A 60-min preincubation period with the CuCl2 containing reaction solution was not necessary. This cycle was repeated 7 times over a 23-h period. Control experiments were done with spores that had the cotA gene knockout (strain1S101, Bacillus Genetic Stock Center, The Ohio State University, Columbus, OH, USA).
5. Conclusions
The kinetic parameters and the relative activity were determined with wt-CotA and T480A-CotA on B. subtilis spore coat for substrate (+)-catechin, (−)-epicatechin, and sinapic acid in organic solvents. In general, the catalytic efficiency (Vmax/Km; (δA/OD580)/mM) of T480A-CotA is higher than wt-CotA for all the substrates. The Vmax for T480A-CotA was greater than the wt-CotA in all organic solvents used in this study. In addition, the catalyst can be easily removed from the reaction solution and reused. This allows for simpler recovery of the product from the enzyme. This investigation indicates that enzymes expressed on the spore coat can be utilized for industrial applications.
Author Contributions
Conceptualization, E.T.F.; methodology, E.T.F. and S.S.; software, S.S.; validation, E.T.F. and S.S.; formal analysis, E.T.F. and S.S.; investigation, E.T.F. and S.S.; resources E.T.F. and S.S.; data curation, E.T.F. and S.S.; writing—original draft preparation, E.T.F.; writing—review and editing, E.T.F. and S.S.; visualization, S.S.; supervision, E.T.F.; project administration, E.T.F.; funding acquisition, E.T.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ExxonMobil and National Science Foundation under MCB-0746078.
Data Availability Statement
The data are contained within the article or the Appendix A.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A
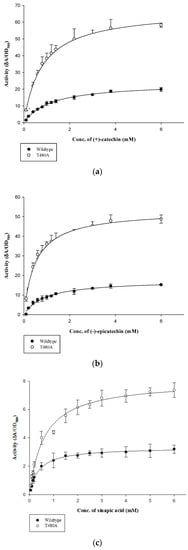
Figure A1.
Kinetics parameters of wt-CotA and T480A-CotA for substrate (a) (+)-catechin, (b) (−)-epicatechin, and (c) sinapic acid.

Table A1.
Activity of wt-CotA and T480A-CotA in 0–70% organic solvents for substrate (+)-catechin, (−)-epicatechin and sinapic acid.
Table A1.
Activity of wt-CotA and T480A-CotA in 0–70% organic solvents for substrate (+)-catechin, (−)-epicatechin and sinapic acid.
| a. Vmax (δA/OD580) of wt-CotA and T480A-CotA in 0–70% Organic Solvents for Substrate (+)-Catechin | ||||||||
| Conc. of Organic Solvents | Vmax (δA/OD580) | |||||||
| DMSO | Acetonitrile | Methanol | Ethanol | |||||
| wt | T480A | wt | T480A | wt | T480A | wt | T480A | |
| 0% | 2.3 ± 0.1 | 3.5 ± 0.2 | 1.6 ± 0.1 | 2.5 ± 0.1 | 1.32 ± 0.2 | 2.01 ± 0.1 | 0.9 ± 0.0 | 1.33 ± 0.1 |
| 10% | 70.4 ± 5.3 | 130.9 ± 2.3 | 60.5 ± 3.3 | 67.6 ± 5.3 | 12.4 ± 4.0 | 36.6 ± 8.4 | 34.9 ± 1.4 | 46.0 ± 3.4 |
| 20% | 93.5 ± 0.2 | 165.7 ± 1.7 | 98.7 ± 6.9 | 122 ± 1.6 | 20.3 ± 5.8 | 50.9 ± 5.4 | 47.4 ± 7 | 54.6 ± 1.0 |
| 30% | 75.3 ± 13.1 | 150.9 ± 8.7 | 86.0 ± 2.1 | 92.7 ± 2.9 | 48.6 ± 5.2 | 84.2 ± 3.8 | 33.8 ± 1.5 | 66.3 ± 1.4 |
| 40% | 49.1 ± 10.5 | 133.6 ± 4.3 | 57.7 ± 0.3 | 68.2 ± 0.9 | 28.1 ± 7.5 | 96.1 ± 3.9 | 26.1 ± 4.3 | 52.7 ± 1.7 |
| 50% | 46.3 ± 7.1 | 138.3 ± 0.8 | 46.6 ± 0.1 | 67.0 ± 4.2 | 44.0 ± 2.7 | 79.6 ± 5.2 | 19.2 ± 0.4 | 44.9 ± 2.2 |
| 60% | 69.7 ± 15.6 | 172.2 ± 8.0 | 43.3 ± 1.2 | 60.6 ± 2.6 | 41.8 ± 0.9 | 77.9 ± 4.0 | 33.2 ± 1.0 | 38.9 ± 3.7 |
| 70% | 10.5 ± 5.0 | 15.1 ± 3.5 | 68.6 ± 1.5 | 74.8 ± 3.5 | 10.2 ± 0.7 | 17.4 ± 1.0 | 10.8 ± 1.0 | 26.0 ± 0.1 |
| b. Vmax (δA/OD580) of wt-CotA and T480A-CotA in 0–70% Organic Solvents for Substrate (−)-Epicatechin | ||||||||
| Conc. of Organic Solvents | Vmax (δA/OD580) | |||||||
| DMSO | Acetonitrile | Methanol | Ethanol | |||||
| wt | T480A | wt | T480A | wt | T480A | Wt | T480A | |
| 0% | 2.9 ± 0.1 | 4.4 ± 0.2 | 1.7 ± 0.1 | 2.7 ± 0.1 | 1.6 ± 0.1 | 2.4 ± 0.1 | 1.0 ± 0.0 | 1.5 ± 0.1 |
| 10% | 9.2 ± 0.5 | 22.6 ± 6.9 | 16.2 ± 1.7 | 42.0 ± 3.4 | 19.3 ± 0.4 | 56.4 ± 9.6 | 11.7 ± 1.4 | 22.3 ± 3.2 |
| 20% | 31.0 ± 2.7 | 126.6 ± 1.6 | 96.8 ± 1.1 | 133 ± 1.9 | 25.4 ± 4.2 | 63.6 ± 3.8 | 22.2 ± 2.8 | 29.0 ± 1.0 |
| 30% | 57.4 ± 5.9 | 154.7 ± 2.3 | 74.5 ± 3.6 | 77.4 ± 1.0 | 43.6 ± 3.0 | 90.7 ± 3.6 | 44.9 ± 1.4 | 76.1 ± 1.3 |
| 40% | 67.8 ± 11.1 | 119.6 ± 7.8 | 15.6 ± 0.1 | 67.8 ± 0.6 | 14.4 ± 4.4 | 95.1 ± 9.9 | 19.4 ± 2.9 | 71.5 ± 1.6 |
| 50% | 91.2 ± 7.8 | 111.0 ± 4.3 | 13.4 ± 0.1 | 75.3 ± 2.8 | 18.7 ± 2.6 | 58.2 ± 3.4 | 9.6 ± 0.4 | 59.9 ± 2.1 |
| 60% | 213.6 ± 7.5 | 220.4 ± 2.0 | 63.5 ± 0.6 | 83.7 ± 1.7 | 15.4 ± 3.6 | 60.9 ± 3.5 | 20.1 ± 1.0 | 39.3 ± 3.5 |
| 70% | 7.4 ± 4.3 | 20.1 ± 5.0 | 8.7 ± 0.8 | 36.4 ± 2.3 | 10.9 ± 0.6 | 11.6 ± 0.9 | 1.3 ± 1.0 | 10.3 ± 0.1 |
| c. Vmax (δA/OD580) of wt-CotA and T480A-CotA in 0–70% Organic Solvents for Substrate Sinapic Acid | ||||||||
| Conc. of Organic Solvents | Vmax (δA/OD580) | |||||||
| DMSO | Acetonitrile | Methanol | Ethanol | |||||
| wt | T480A | wt | T480A | wt | T480A | Wt | T480A | |
| 0% | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.5 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.0 |
| 10% | 2.5 ± 0.4 | 9.6 ± 0.2 | 6.2 ± 0.6 | 12.3 ± 1.0 | 3.3 ± 0.4 | 9.6 ± 1.0 | 4.4 ± 0.0 | 15.6 ± 0.9 |
| 20% | 5.1 ± 0.0 | 12.3 ± 0.1 | 9.3 ± 1.3 | 14.2 ± 0.3 | 3.1 ± 0.6 | 10.8 ± 0.6 | 7.1 ± 0.4 | 17.0 ± 0.2 |
| 30% | 5.1 ± 1.0 | 13.5 ± 0.7 | 13.0 ± 0.4 | 23.9 ± 0.6 | 1.8 ± 0.6 | 11.1 ± 0.4 | 3.0 ± 0.4 | 15.3 ± 0.4 |
| 40% | 3.2 ± 0.8 | 8.9 ± 0.3 | 12.1 ± 0.0 | 22.4 ± 0.2 | 1.7 ± 0.8 | 7.9 ± 0.4 | 3.4 ± 0.6 | 12.6 ± 0.4 |
| 50% | 1.8 ± 0.5 | 8.7 ± 0.0 | 8.7 ± 0.0 | 19.7 ± 0.8 | 0.4 ± 0.3 | 0.6 ± 0.1 | 2.8 ± 0.1 | 6.7 ± 0.6 |
| 60% | 1.4 ± 1.2 | 4.1 ± 0.6 | 5.1 ± 0.2 | 7.2 ± 0.5 | 0.2 ± 0.1 | 0.4 ± 0.2 | 1.0 ± 0.3 | 2.7 ± 0.9 |
| 70% | 1.0 ± 0.4 | 3.4 ± 0.3 | 0.7 ± 0.3 | 1.2 ± 0.7 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.3 | 0.5 ± 0.0 |

Table A2.
The (+)-catechin products yields δA433nm_product for wt-CotA and T480A-CotA were determined for 7 cycles over a 23-h period.
Table A2.
The (+)-catechin products yields δA433nm_product for wt-CotA and T480A-CotA were determined for 7 cycles over a 23-h period.
| Time (Hours) | Product Yields (δA433nm_product) | |||||||
|---|---|---|---|---|---|---|---|---|
| DMSO | Acetonitrile | Methanol | Ethanol | |||||
| wt | T480A | wt | T480A | wt | T480A | wt | T480A | |
| 0 | 0.59 | 2.01 | 1.02 | 1.49 | 0.37 | 0.89 | 0.57 | 1.12 |
| 4 | 0.58 | 1.95 | 0.96 | 1.42 | 0.31 | 0.88 | 0.5 | 1.05 |
| 8 | 0.57 | 1.79 | 0.87 | 1.25 | 0.26 | 0.76 | 0.43 | 0.87 |
| 12 | 0.53 | 1.53 | 0.64 | 0.98 | 0.20 | 0.55 | 0.35 | 0.6 |
| 16 | 0.51 | 1.36 | 0.51 | 0.81 | 0.14 | 0.44 | 0.28 | 0.43 |
| 20 | 0.49 | 1.35 | 0.39 | 0.64 | 0.09 | 0.32 | 0.21 | 0.25 |
| 23 | 0.35 | 1.20 | 0.29 | 0.48 | 0.03 | 0.24 | 0.06 | 0.12 |
References
- Kumar, R.A.; Clark, D.S. High-throughput screening of biocatalytic activity: Applications in drug discovery. Curr. Opin. Chem. Biol. 2006, 10, 162–168. [Google Scholar] [CrossRef]
- Porter, J.L.; Rusli, R.A.; Ollis, D.L. Directed Evolution of Enzymes for Industrial Biocatalysis. ChemBioChem 2016, 17, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Wohlgemuth, R. Biocatalysis-key to sustainable industrial chemistry. Curr. Opin. Biotechnol. 2010, 21, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Klibanov, A. Why are enzymes less active in organic solvents than in water? Trends Biotechnol. 1997, 15, 97–101. [Google Scholar] [CrossRef]
- Doukyu, N.; Ogino, H. Organic solvent-tolerant enzymes. Biochem. Eng. J. 2010, 48, 270–282. [Google Scholar] [CrossRef]
- Stepankova, V.; Bidmanova, S.; Koudelakova, T.; Prokop, Z.; Chaloupkova, R.; Damborsky, J. Strategies for stabilization of enzymes in organic solvents. ACS Catal. 2013, 3, 2823–2836. [Google Scholar] [CrossRef]
- Dicosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef] [PubMed]
- Liese, A.; Hilterhaus, L. Evaluation of immobilized enzymes for industrial applications. Chem. Soc. Rev. 2013, 42, 6236–6249. [Google Scholar] [CrossRef]
- Binay, B.; Alagöz, D.; Yildirim, D.; Çelik, A.; Tükel, S.S. Highly stable and reusable immobilized formate dehydrogenases: Promising biocatalysts for in situ regeneration of NADH. Beilstein J. Org. Chem. 2016, 12, 271–277. [Google Scholar] [CrossRef]
- Mitsou, E.; Xenakis, A.; Zoumpanioti, M. Oxidation catalysis by enzymes in microemulsions. Catalysts 2017, 7, 52. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, Y.; Xue, Y.; Li, W.; Hu, Y. Laccase immobilized on magnetic nanoparticles modified by amino-functionalized ionic liquid via dialdehyde starch for phenolic compounds biodegradation. Chem. Eng. J. 2020, 391. [Google Scholar] [CrossRef]
- Shao, X.; Ni, H.; Lu, T.; Jiang, M.; Li, H.; Huang, X.; Li, L. An improved system for the surface immobilisation of proteins on Bacillus thuringiensis vegetative cells and spores through a new spore cortex-lytic enzyme anchor. New Biotechnol. 2012, 29, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Bessette, P.H.; Åslund, F.; Beckwith, J.; Georgiou, G. Efficient folding of proteins with multiple disulfide bonds in the Escherichia coli cytoplasm. Proc. Natl. Acad. Sci. USA 1999, 96, 13703–13708. [Google Scholar] [CrossRef]
- Chen, G.; Driks, A.; Tawfiq, K.; Mallozzi, M.; Patil, S. Bacillus anthracis and Bacillus subtilis spore surface properties and transport. Colloids Surf. B Biointerfaces 2010, 76, 512–518. [Google Scholar] [CrossRef]
- Moeller, R.; Setlow, P.; Reitz, G.; Nicholson, W.L. Roles of small, acid-soluble spore proteins and core water content in survival of Bacillus subtilis spores exposed to environmental solar UV radiation. Appl. Environ. Microbiol. 2009, 75, 5202–5208. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Lee, F.S.; Farinas, E.T. Bacillus subtilis spore display of laccase for evolution under extreme conditions of high concentrations of organic solvent. ACS Combi. Sci. 2014, 16, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Higgins, D.; Dworkin, J. Recent progress in Bacillus subtilis sporulation. FEMS Microbiol. Rev. 2012, 36, 131–148. [Google Scholar] [CrossRef]
- Gupta, N.; Farinas, E.T. Directed evolution of CotA laccase for increased substrate specificity using Bacillus subtilis spores. Protein Eng. Des. Sel. 2010, 23, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, H.; Parvez, S.; Rehman, H.; Banerjee, B.D.; Raisuddin, S. Catechin as an antioxidant in liver mitochondrial toxicity: Inhibition of tamoxifen-induced protein oxidation and lipid peroxidation. J. Biochem. Mol. Toxicol. 2007, 21, 110–117. [Google Scholar] [CrossRef]
- Sangwan, N.S.; Shanker, S.; Sangwan, R.S.; Kumar, S. Plant-derived products as antimutagens. Phytother. Res. 1998, 12, 389–399. [Google Scholar] [CrossRef]
- Suzuki, J.I.; Isobe, M.; Morishita, R.; Nagai, R. Effects of green tea catechin consumption on cardiovascular diseases. In Tea Consumption and Health; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2012; pp. 87–99. [Google Scholar]
- Wang, Y.F.; Shao, S.H.; X u, P.; Yang, X.Q.; Qian, L.S. Catechin-enriched green tea extract as a safe and effective agent for antimicrobial and anti-inflammatory treatment. Afr. J. Pharm. Pharmacol. 2011, 5, 1452–1461. [Google Scholar] [CrossRef]
- Han, Z.X.; Rana, M.M.; Liu, G.F.; Gao, M.J.; Li, D.X.; Wu, F.G.; Li, X.B.; Wan, X.C.; Wei, S. Green tea flavour determinants and their changes over manufacturing processes. Food Chem. 2016, 212, 739–748. [Google Scholar] [CrossRef]
- Papp, A.; Winnewisser, W.; Geiger, E.; Briem, F. Influence of (+)-catechin and ferulic acid on formation of beer haze and their removal through different polyvinylpolypyrollidone-types. J. Inst. Brew. 2001, 107, 55–60. [Google Scholar] [CrossRef]
- Kurisawa, M.; Chung, J.E.; Uyama, H.; Kobayashi, S. Laccase-catalyzed Synthesis and Antioxidant Property of Poly(catechin). Macromol. Biosci. 2003, 3, 758–764. [Google Scholar] [CrossRef]
- Madeira Junior, J.V., Jr.; Teixeira, C.B.; Macedo, G.A. Biotransformation and bioconversion of phenolic compounds obtainment: An overview. Crit. Rev. Biotechnol. 2015, 35, 75–81. [Google Scholar] [CrossRef]
- Krygier, K.; Sosulski, F.; Hogge, L. Free, esterified, and insoluble-bound phenolic acids. 2. Composition of phenolic acids in rapeseed flour and hulls. J. Agric. Food Chem. 1982, 30, 334–336. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, L.; Pu, H.; Li, C.; Hu, Q. Profile and distribution of soluble and insoluble phenolics in Chinese rapeseed (Brassica napus). Food Chem. 2012, 135, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Naczk, M.; Amarowicz, R.; Sullivan, A.; Shahidi, F. Current research developments on polyphenolics of rapeseed/canola: A review. Food Chem. 1998, 62, 489–502. [Google Scholar] [CrossRef]
- Shahidi, F.; Naczk, M. An overview of the phenolics of canola and rapeseed: Chemical, sensory and nutritional significance. J. Am. Oil Chem. Soc. 1992, 69, 917–924. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Hao, Z.; Xi, R.; Cai, Y.; Liao, X. Hydrogen peroxide-resistant CotA and YjqC of bacillus altitudinis spores are a promising biocatalyst for catalyzing reduction of sinapic acid and sinapine in rapeseed meal. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.M.; Solomon, E.I. Electron transfer and reaction mechanism of laccases. Cell. Mol. Life Sci. 2015, 72, 869–883. [Google Scholar] [CrossRef]
- Solomon, E.I.; Heppner, D.E.; Johnston, E.M.; Ginsbach, J.W.; Cirera, J.; Qayyum, M.; Kieber-Emmons, M.T.; Kjaergaard, C.H.; Hadt, R.G.; Tian, L. Copper active sites in biology. Chem. Rev. 2014, 114, 3659–3853. [Google Scholar] [CrossRef] [PubMed]
- Kües, U. Fungal enzymes for environmental management. Curr. Opin. Biotechnol. 2015, 33, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Pardo, I.; Camarero, S. Laccase engineering by rational and evolutionary design. Cell. Mol. Life Sci. 2015, 72, 897–910. [Google Scholar] [CrossRef]
- Santhanam, N.; Vivanco, J.M.; Decker, S.R.; Reardon, K.F. Expression of industrially relevant laccases: Prokaryotic style. Trends Biotechnol. 2011, 29, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Shraddha; Shekher, R.; Sehgal, S.; Kamthania, M.; Kumar, A. Laccase: Microbial sources, production, purification, and potential biotechnological applications. Enzyme Res. 2011, 2011. [Google Scholar] [CrossRef]
- Ma, H.L.; Kermasha, S.; Gao, J.M.; Borges, R.M.; Yu, X.z. Laccase-catalyzed oxidation of phenolic compounds in organic media. J. Mol. Catal. B Enzym. 2009, 57, 89–95. [Google Scholar] [CrossRef]
- Osman, A.M.; Wong, K.K.Y.; Fernyhough, A. The laccase/ABTS system oxidizes (+)-catechin to oligomeric products. Enzym. Microb. Technol. 2007, 40, 1272–1279. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).