Insights into the Structural Dynamics of Pt/CeO2 Single-Site Catalysts during CO Oxidation
Abstract
1. Introduction
2. Results and Discussion
2.1. Basic Characterization
2.2. Catalytic Activity
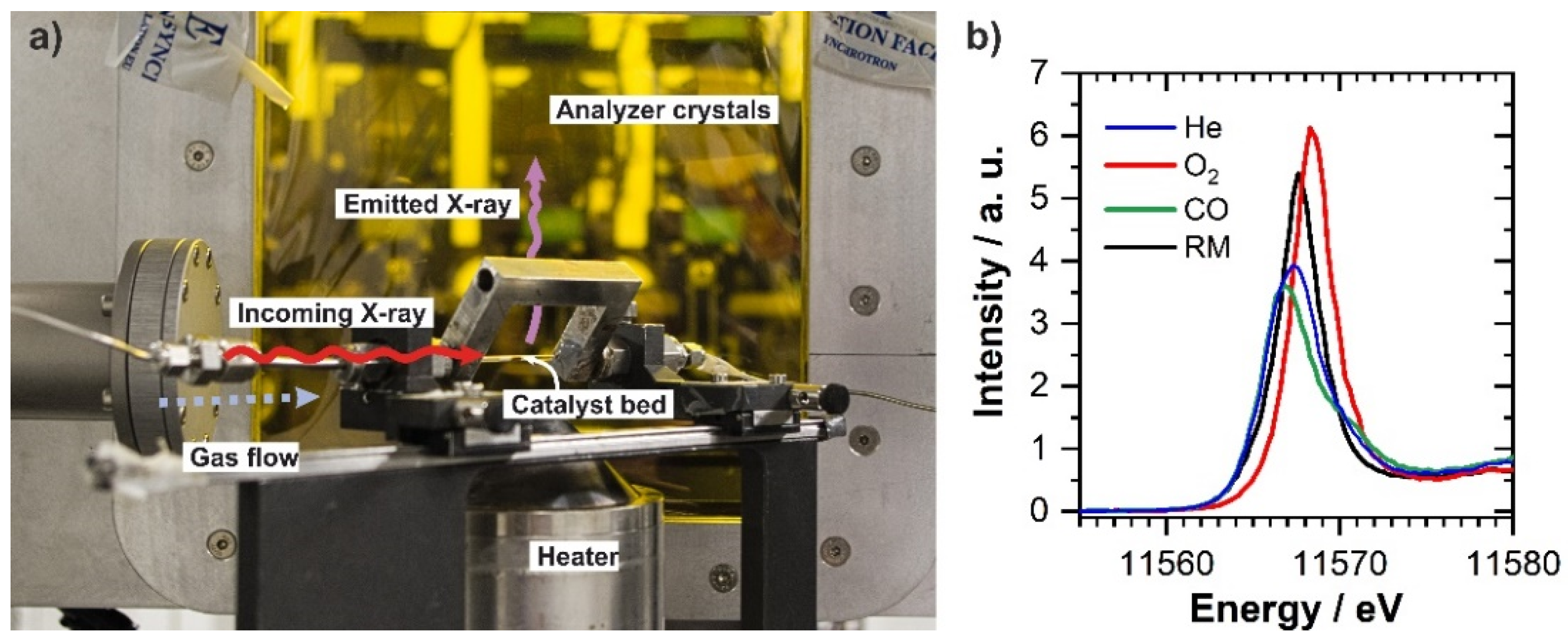
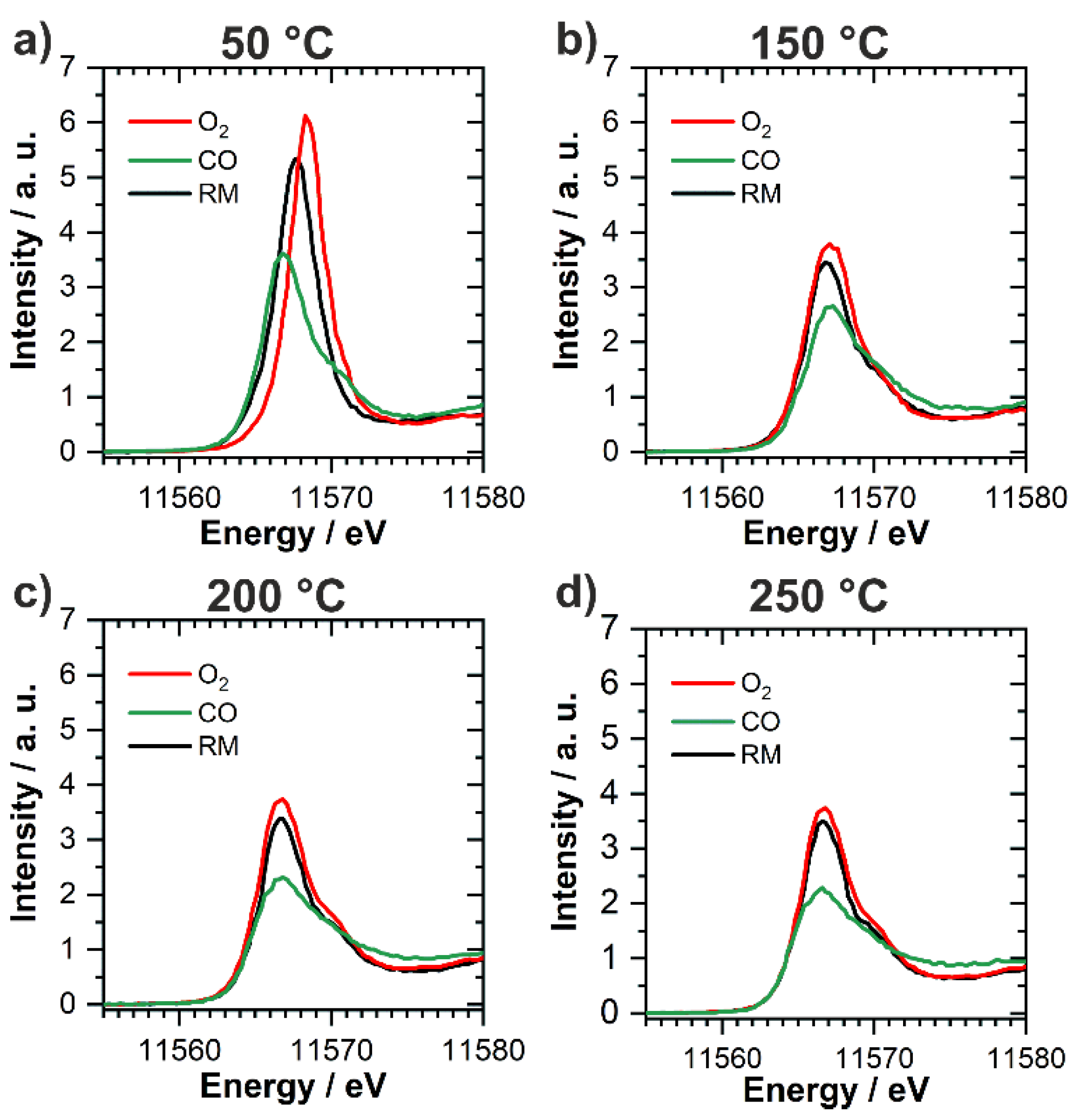
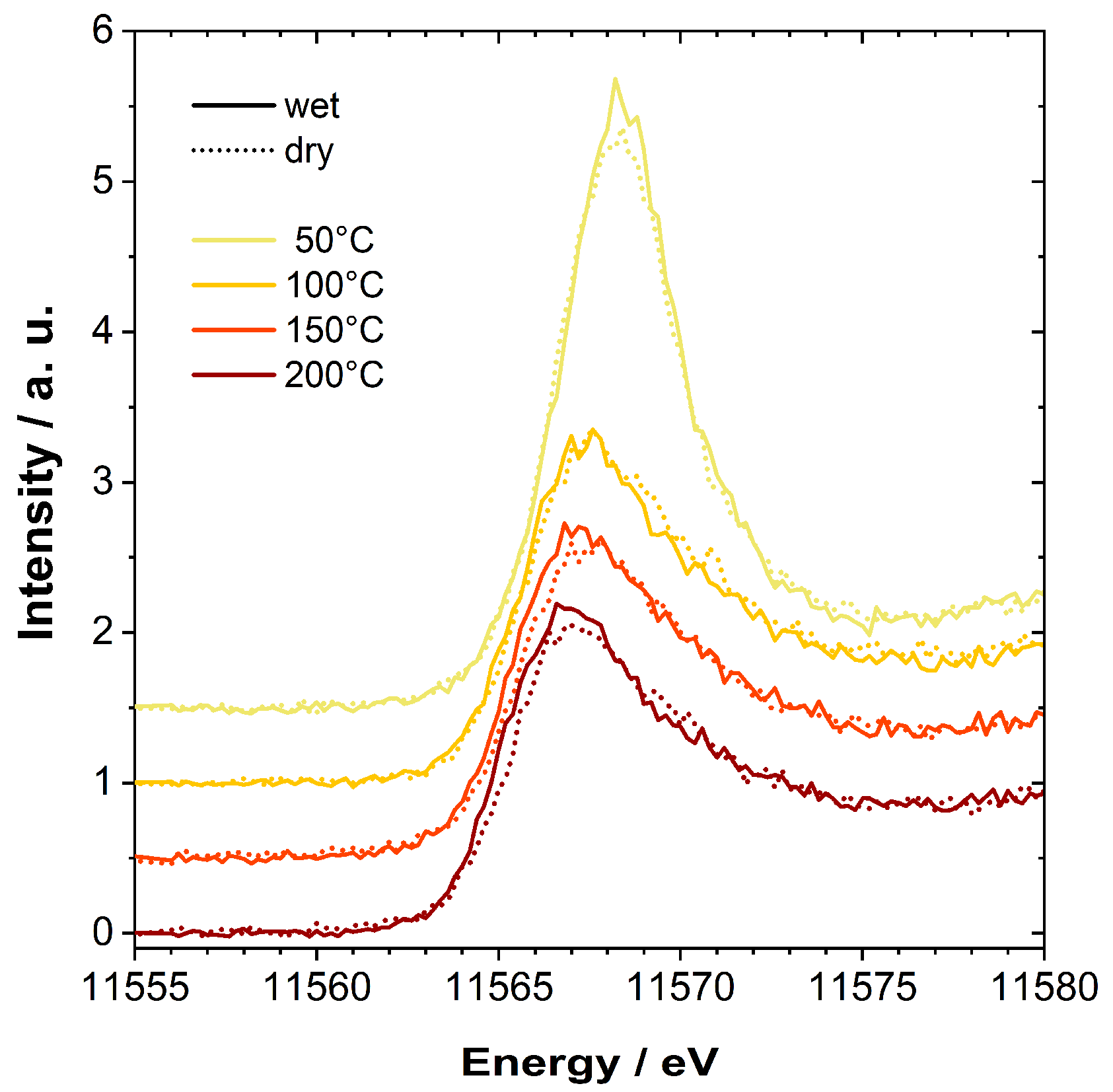
2.3. In Situ/Operando Characterization
- (i)
- (ii)
- Pt4+-“O” highly oxidized single sites located at four-fold hollow sites on the ceria surface by Pt–O–Ce bonds and involving strongly bound oxygen atoms; this species is predominant at low temperatures in the as prepared catalyst in inert atmosphere.
- (iii)
- Pt2+ located at four-fold hollow sites present or generated at the {211}/{221} edges or on the {110} facet of ceria support [14]; due to the high thermodynamic stability, this species emerges during single-site catalyst preparation at high temperature; under reaction conditions, bare Pt2+ forms during heating up to 200 °C upon desorption of oxygen from Pt4+-“O”, a process apparently not influenced by the CO presence (Figure S8); finally, this species appears at higher temperatures once CO oxidation front shifts towards the inlet of the catalyst bed.
- (iv)
- Ptδ+-CO forms by partial reduction of bare Pt2+ single sites; the appearance of this intermediate state is indicated by the slight decrease of the white line intensity, which is in line with the variation of the HERFD-XANES spectra profile during Pt/CeO2 reduction in 1000 ppm CO/He (Figure 4) as the temperature increases.
- (v)
- PtxΔ+-CO clusters, generated by nearby migration of the single atoms above 125 °C and emerging simultaneously with the CO oxidation onset.
3. Materials and Methods
3.1. Catalytic Activity Tests
3.2. Ex Situ Characterization
3.3. In Situ Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daelman, N.; Capdevila-Cortada, M.; López, N. Dynamic charge and oxidation state of Pt/CeO2 single-atom catalysts. Nat. Mater. 2019, 18, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Beniya, A.; Higashi, S. Towards dense single-atom catalysts for future automotive applications. Nat. Catal. 2019, 2, 590–602. [Google Scholar] [CrossRef]
- Nagai, Y.; Hirabayashi, T.; Dohmae, K.; Takagi, N.; Minami, T.; Shinjoh, H.; Matsumoto, S. Sintering inhibition mechanism of platinum supported on ceria-based oxide and Pt-oxide–support interaction. J. Catal. 2006, 242, 103–109. [Google Scholar] [CrossRef]
- Jones, J.; Xiong, H.; DeLaRiva, A.T.; Peterson, E.J.; Pham, H.; Challa, S.R.; Qi, G.; Oh, S.; Wiebenga, M.H.; Hernández, X.I.P.; et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 2016, 353, 150–154. [Google Scholar] [CrossRef]
- Gänzler, A.M.; Casapu, M.; Vernoux, P.; Loridant, S.; Aires, F.J.C.S.; Epicier, T.; Betz, B.; Hoyer, R.; Grunwaldt, J. Tuning the Structure of Platinum Particles on Ceria In Situ for Enhancing the Catalytic Performance of Exhaust Gas Catalysts. Angew. Chem. Int. Ed. 2017, 56, 13078–13082. [Google Scholar] [CrossRef] [PubMed]
- Golunski, S.E.; Hatcher, H.A.; Rajaram, R.R.; Truex, T.J. Origins of low-temperature three-way activity in Pt/CeO2. Appl. Catal. B Environ. 1995, 5, 367–376. [Google Scholar] [CrossRef]
- Moses-DeBusk, M.; Yoon, M.; Allard, L.F.; Mullins, D.R.; Wu, Z.; Yang, X.; Veith, G.; Stocks, G.M.; Narula, C.K. CO Oxidation on Supported Single Pt Atoms: Experimental and ab Initio Density Functional Studies of CO Interaction with Pt Atom on θ-Al2O3(010) Surface. J. Am. Chem. Soc. 2013, 135, 12634–12645. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Z.; Lin, F.; Wang, H.; Wang, Y. Single-atom Automobile Exhaust Catalysts. ChemNanoMat 2020, 6, 1659–1682. [Google Scholar] [CrossRef]
- Bera, P.; Priolkar, K.R.; Gayen, A.; Sarode, P.R.; Hegde, M.S.; Emura, S.; Kumashiro, R.; Jayaram, V.; Subbanna, G.N. Ionic Dispersion of Pt over CeO2 by the Combustion Method: Structural Investigation by XRD, TEM, XPS, and EXAFS. Chem. Mater. 2003, 15, 2049–2060. [Google Scholar] [CrossRef]
- Nie, L.; Mei, D.; Xiong, H.; Peng, B.; Ren, Z.; Hernandez, X.I.P.; DeLaRiva, A.; Wang, M.; Engelhard, M.H.; Kovarik, L.; et al. Activation of surface lattice oxygen in single-atom Pt/CeO2 for low-temperature CO oxidation. Science 2017, 358, 1419–1423. [Google Scholar] [CrossRef]
- Pereira-Hernández, X.I.; DeLaRiva, A.; Muravev, V.; Kunwar, D.; Xiong, H.; Sudduth, B.; Engelhard, M.; Kovarik, L.; Hensen, E.J.M.; Wang, Y.; et al. Tuning Pt-CeO2 interactions by high-temperature vapor-phase synthesis for improved reducibility of lattice oxygen. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Datye, A.K.; Guo, H. Single atom catalysis poised to transition from an academic curiosity to an industrially relevant technology. Nat. Commun. 2021, 12, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Dessal, C.; Len, T.; Morfin, F.; Rousset, J.-L.; Aouine, M.; Afanasiev, P.; Piccolo, L. Dynamics of Single Pt Atoms on Alumina during CO Oxidation Monitored by Operando X-ray and Infrared Spectroscopies. ACS Catal. 2019, 9, 5752–5759. [Google Scholar] [CrossRef]
- Maurer, F.; Jelic, J.; Wang, J.; Gänzler, A.; Dolcet, P.; Wöll, C.; Wang, Y.; Studt, F.; Casapu, M.; Grunwaldt, J.-D. Tracking the formation, fate and consequence for catalytic activity of Pt single sites on CeO2. Nat. Catal. 2020, 3, 824–833. [Google Scholar] [CrossRef]
- Cassinelli, W.H.; Martins, L.; Passos, A.R.; Pulcinelli, S.H.; Santilli, C.V.; Rochet, A.; Briois, V. Multivariate curve resolution analysis applied to time-resolved synchrotron X-ray Absorption Spectroscopy monitoring of the activation of copper alumina catalyst. Catal. Today 2014, 229, 114–122. [Google Scholar] [CrossRef]
- Nunes, C.A.; Resende, E.C.; Guimarães, I.R.; Anastácio, A.S.; Guerreiro, M.C. In-situ Monitoring of the Structure of a Goethite-Based Catalyst during Methane Oxidation by X-Ray Absorption Near-Edge Structure (XANES) Spectroscopy Assisted by Chemometric Methods. Appl. Spectrosc. 2011, 65, 692–697. [Google Scholar] [CrossRef]
- Rabeah, J.; Briois, V.; Adomeit, S.; La Fontaine, C.; Bentrup, U.; Brückner, A. Multivariate Analysis of Coupled Operando EPR/XANES/EXAFS/UV–Vis/ATR-IR Spectroscopy: A New Dimension for Mechanistic Studies of Catalytic Gas-Liquid Phase Reactions. Chem. A Eur. J. 2020, 26, 7395–7404. [Google Scholar] [CrossRef] [PubMed]
- Bergman, S.L.; Dahlin, S.; Mesilov, V.V.; Xiao, Y.; Englund, J.; Xi, S.; Tang, C.; Skoglundh, M.; Pettersson, L.J.; Bernasek, S.L. In-situ studies of oxidation/reduction of copper in Cu-CHA SCR catalysts: Comparison of fresh and SO2-poisoned catalysts. Appl. Catal. B Environ. 2020, 269, 118722. [Google Scholar] [CrossRef]
- Fahami, A.R.; Günter, T.; Doronkin, D.E.; Casapu, M.; Zengel, D.; Vuong, T.H.; Simon, M.; Breher, F.; Kucherov, A.V.; Brückner, A.; et al. The dynamic nature of Cu sites in Cu-SSZ-13 and the origin of the seagull NOx conversion profile during NH3-SCR. React. Chem. Eng. 2019, 4, 1000–1018. [Google Scholar] [CrossRef]
- Kerkeni, B.; Berthout, D.; Berthomieu, D.; Doronkin, D.E.; Casapu, M.; Grunwaldt, J.-D.; Chizallet, C. Copper Coordination to Water and Ammonia in CuII-Exchanged SSZ-13: Atomistic Insights from DFT Calculations and in Situ XAS Experiments. J. Phys. Chem. C 2018, 122, 16741–16755. [Google Scholar] [CrossRef]
- Gatla, S.; Aubert, D.; Flaud, V.; Grosjean, R.; Lunkenbein, T.; Mathon, O.; Pascarelli, S.; Kaper, H. Facile synthesis of high-surface area platinum-doped ceria for low temperature CO oxidation. Catal. Today 2019, 333, 105–112. [Google Scholar] [CrossRef]
- Casapu, M.; Fischer, A.; Gänzler, A.M.; Popescu, R.; Crone, M.; Gerthsen, D.; Türk, M.; Grunwaldt, J.-D. Origin of the Normal and Inverse Hysteresis Behavior during CO Oxidation over Pt/Al2O3. ACS Catal. 2017, 7, 343–355. [Google Scholar] [CrossRef]
- Abedi, A.; Hayes, R.; Votsmeier, M.; Epling, W.S. Inverse Hysteresis Phenomena During CO and C3H6 Oxidation over a Pt/Al2O3 Catalyst. Catal. Lett. 2012, 142, 930–935. [Google Scholar] [CrossRef]
- Safonova, O.V.; Tromp, M.; Van Bokhoven, J.A.; De Groot, F.M.F.; Evans, J.; Glatzel, P. Identification of CO Adsorption Sites in Supported Pt Catalysts Using High-Energy-Resolution Fluorescence Detection X-ray Spectroscopy. J. Phys. Chem. B 2006, 110, 16162–16164. [Google Scholar] [CrossRef] [PubMed]
- Grunwaldt, J.-D.; Clausen, B.S. Combining XRD and EXAFS with on-Line Catalytic Studies for in situ Characterization of Catalysts. Top. Catal. 2002, 18, 37–43. [Google Scholar] [CrossRef]
- Gänzler, A.M.; Casapu, M.; Boubnov, A.; Müller, O.; Conrad, S.; Lichtenberg, H.; Frahm, R.; Grunwaldt, J.-D. Operando spatially and time-resolved X-ray absorption spectroscopy and infrared thermography during oscillatory CO oxidation. J. Catal. 2015, 328, 216–224. [Google Scholar] [CrossRef]
- De Juan, A.; Jaumot, J.; Tauler, R. Multivariate Curve Resolution (MCR). Solving the mixture analysis problem. Anal. Methods 2014, 6, 4964–4976. [Google Scholar] [CrossRef]
- Jaumot, J.; de Juan, A.; Tauler, R. MCR-ALS GUI 2.0: New features and applications. Chemom. Intell. Lab. Syst. 2015, 140, 1–12. [Google Scholar] [CrossRef]
- Voronov, A.; Urakawa, A.; van Beek, W.; Tsakoumis, N.E.; Emerich, H.; Rønning, M. Multivariate curve resolution applied to in situ X-ray absorption spectroscopy data: An efficient tool for data processing and analysis. Anal. Chim. Acta 2014, 840, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Ferré, G.; Aouine, M.; Bosselet, F.; Burel, L.; Aires, F.J.C.S.; Geantet, C.; Ntais, S.; Maurer, F.; Casapu, M.; Grunwaldt, J.-D.; et al. Exploiting the dynamic properties of Pt on ceria for low-temperature CO oxidation. Catal. Sci. Technol. 2020, 10, 3904–3917. [Google Scholar] [CrossRef]
- Huang, M.; Fabris, S. Role of surface peroxo and superoxo species in the low-temperature oxygen buffering of ceria: Density functional theory calculations. Phys. Rev. B 2007, 75, 081404. [Google Scholar] [CrossRef]
- Gänzler, A.M.; Casapu, M.; Doronkin, D.E.; Maurer, F.; Lott, P.; Glatzel, P.; Votsmeier, M.; Deutschmann, O.; Grunwaldt, J.-D. Unravelling the Different Reaction Pathways for Low Temperature CO Oxidation on Pt/CeO2 and Pt/Al2O3 by Spatially Resolved Structure–Activity Correlations. J. Phys. Chem. Lett. 2019, 10, 7698–7705. [Google Scholar] [CrossRef] [PubMed]
- Kopelent, R.; Van Bokhoven, J.A.; Szlachetko, J.; Edebeli, J.; Paun, C.; Nachtegaal, M.; Safonova, O.V. Catalytically Active and Spectator Ce3+ in Ceria-Supported Metal Catalysts. Angew. Chem. 2015, 127, 8852–8855. [Google Scholar] [CrossRef]
- Ertl, G.; Neumann, M.; Streit, K. Chemisorption of CO on the Pt(111) surface. Surf. Sci. 1977, 64, 393–410. [Google Scholar] [CrossRef]
- Steininger, H.; Lehwald, S.; Ibach, H. On the adsorption of CO on Pt(111). Surf. Sci. 1982, 123, 264–282. [Google Scholar] [CrossRef]
- Gunasooriya, G.T.K.K.; Saeys, M. CO Adsorption Site Preference on Platinum: Charge Is the Essence. ACS Catal. 2018, 8, 3770–3774. [Google Scholar] [CrossRef]
- Gänzler, A.M.; Betz, B.; Baier-Stegmaier, S.; Belin, S.; Briois, V.; Votsmeier, M.; Casapu, M. Operando X-ray Absorption Spectroscopy Study During Conditioning of Pt-Based Catalysts and Its Implications for CO Oxidation. J. Phys. Chem. C 2020, 124, 20090–20100. [Google Scholar] [CrossRef]
- Meunier, F.C.; Cardenas, L.; Kaper, H.; Šmíd, B.; Vorokhta, M.; Grosjean, R.; Aubert, D.; Dembélé, K.; Lunkenbein, T. Synergy between Metallic and Oxidized Pt Sites Unravelled during Room Temperature CO Oxidation on Pt/Ceria. Angew. Chem. Int. Ed. 2021, 60, 3799–3805. [Google Scholar] [CrossRef]
- Kleist, W.; Grunwaldt, J.-D. Modern Applications of High Throughput R&D in Heterogeneous Catalysis; Hagemeyer, A., Volpe, A., Eds.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2014; ISBN 9781608058723. [Google Scholar]
- Grunwaldt, J.-D.; Baiker, A. In situ spectroscopic investigation of heterogeneous catalysts and reaction media at high pressure. Phys. Chem. Chem. Phys. 2005, 7, 3526–3539. [Google Scholar] [CrossRef]
- Landrot, G. FASTOSH: A Software to Process XAFS Data for Geochemical & Environmental Applications. In Proceedings of the Goldschmidt Abstracts, Boston, MA, USA, 12–17 August 2018; p. 1402. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolcet, P.; Maurer, F.; Casapu, M.; Grunwaldt, J.-D. Insights into the Structural Dynamics of Pt/CeO2 Single-Site Catalysts during CO Oxidation. Catalysts 2021, 11, 617. https://doi.org/10.3390/catal11050617
Dolcet P, Maurer F, Casapu M, Grunwaldt J-D. Insights into the Structural Dynamics of Pt/CeO2 Single-Site Catalysts during CO Oxidation. Catalysts. 2021; 11(5):617. https://doi.org/10.3390/catal11050617
Chicago/Turabian StyleDolcet, Paolo, Florian Maurer, Maria Casapu, and Jan-Dierk Grunwaldt. 2021. "Insights into the Structural Dynamics of Pt/CeO2 Single-Site Catalysts during CO Oxidation" Catalysts 11, no. 5: 617. https://doi.org/10.3390/catal11050617
APA StyleDolcet, P., Maurer, F., Casapu, M., & Grunwaldt, J.-D. (2021). Insights into the Structural Dynamics of Pt/CeO2 Single-Site Catalysts during CO Oxidation. Catalysts, 11(5), 617. https://doi.org/10.3390/catal11050617







