Ionothermal Synthesis of Triclinic SAPO-34 Zeolites
Abstract
:1. Introduction
2. Results and Discussion
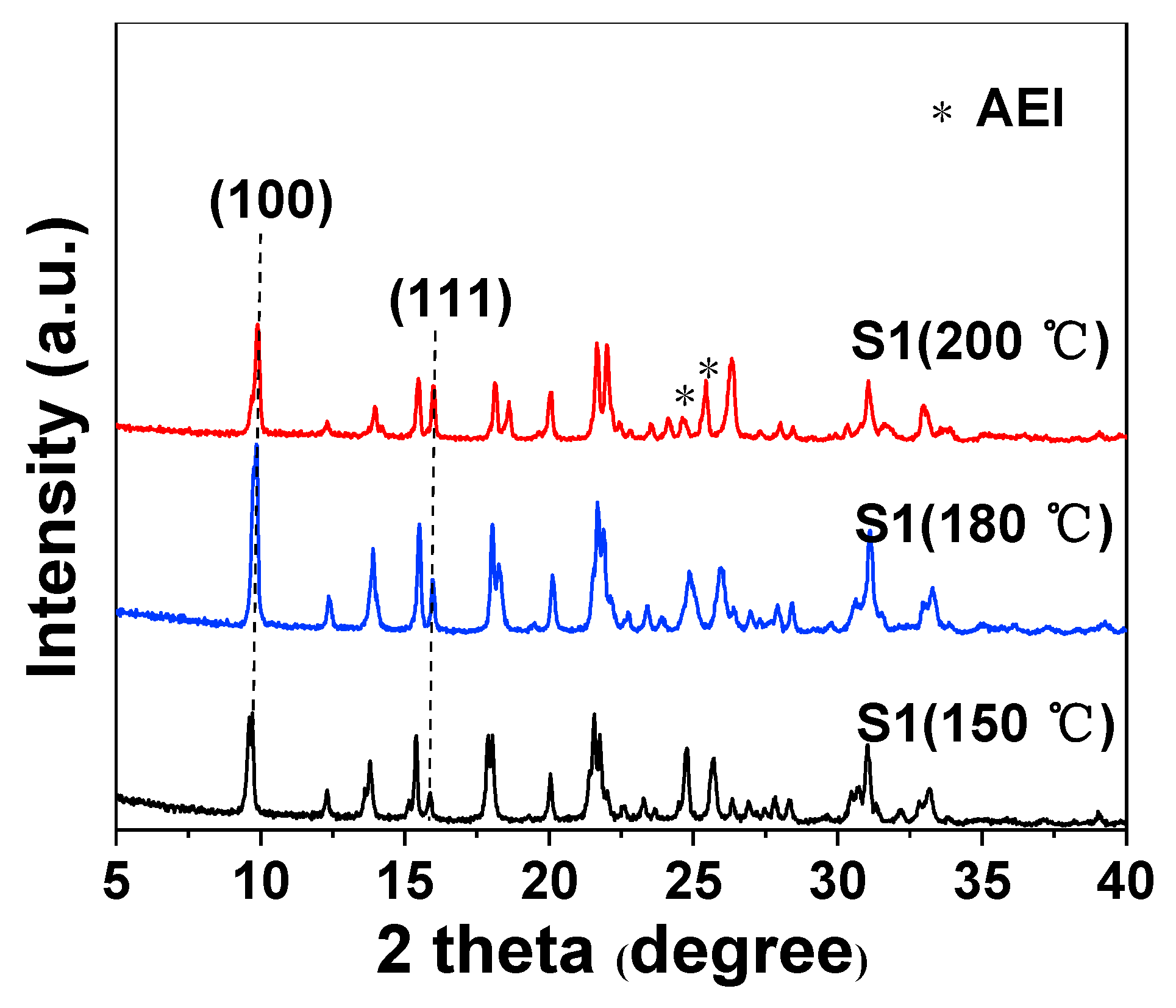
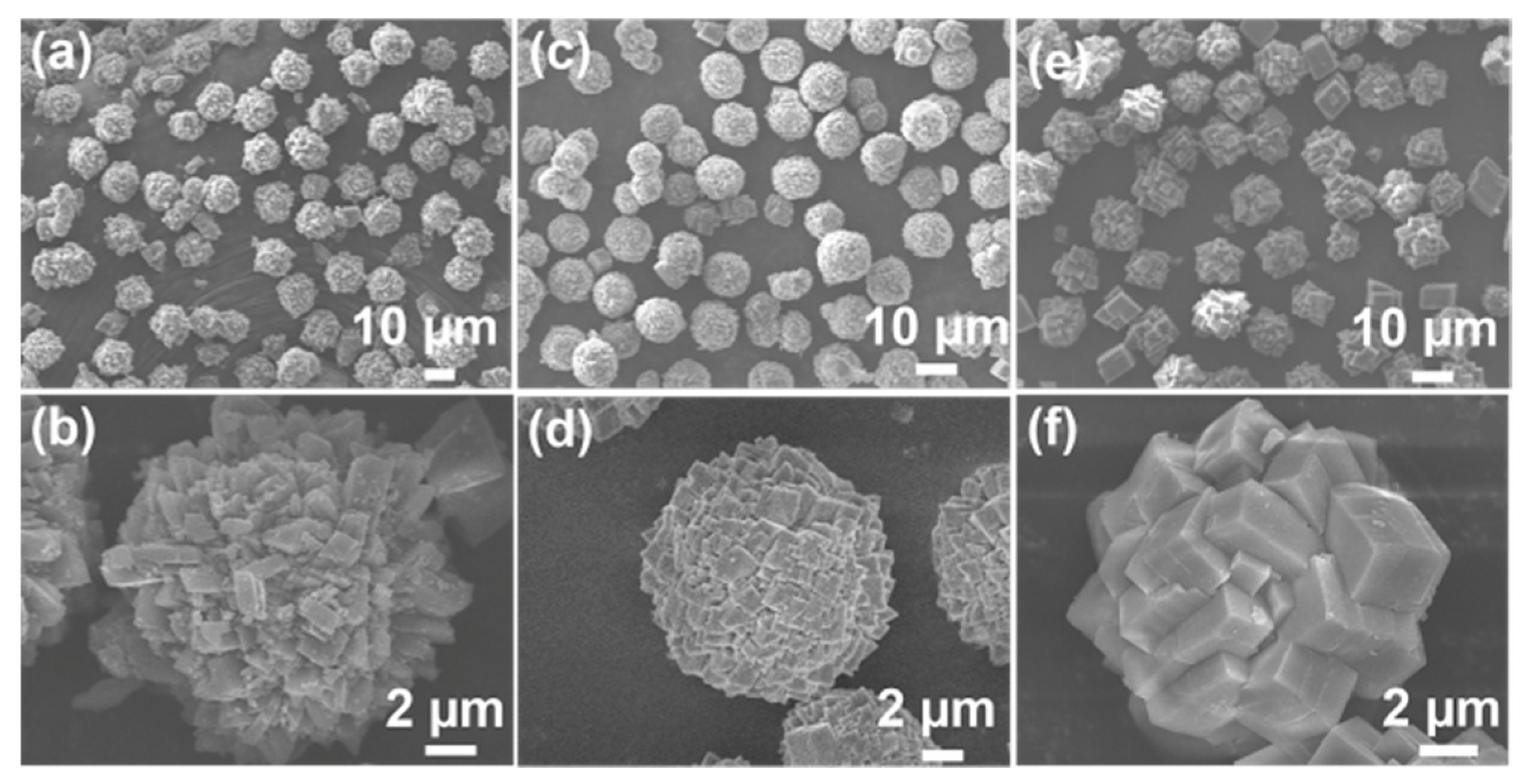
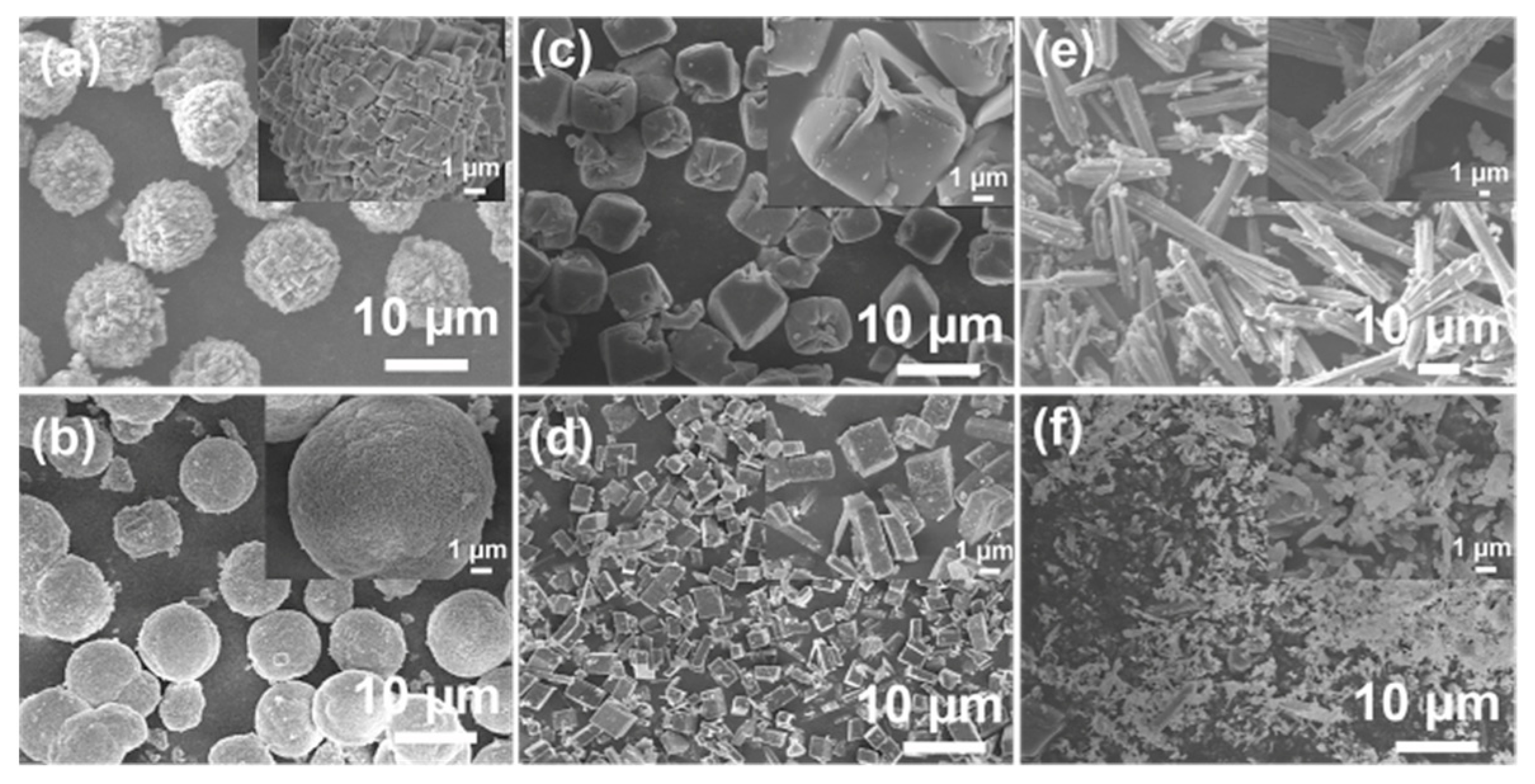
2.1. Effect of Crystallization Temperature
2.2. Synthesis of SAPO-34 in [EMIm]Cl
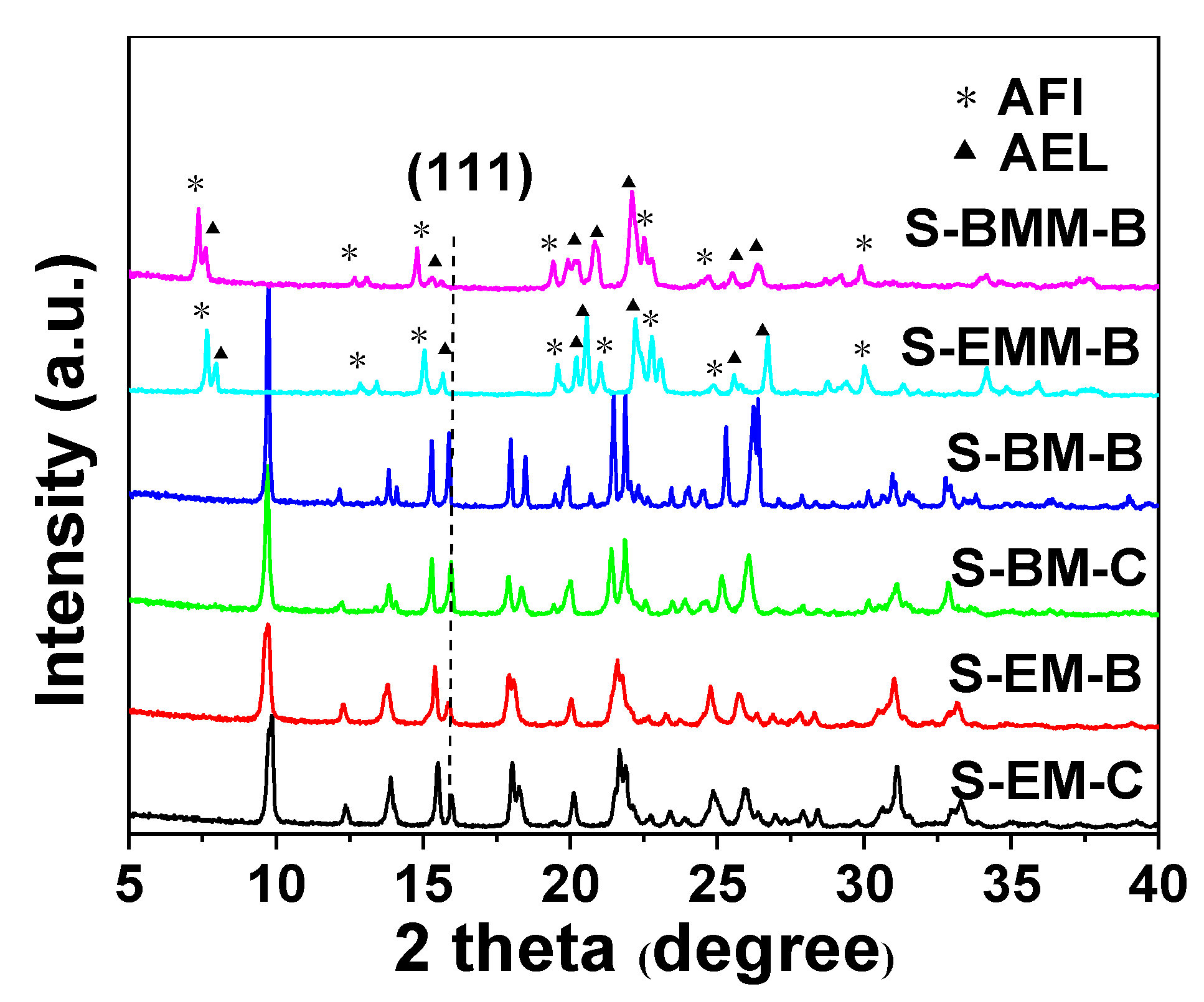
2.3. Effect of Types of Ionic Liquid
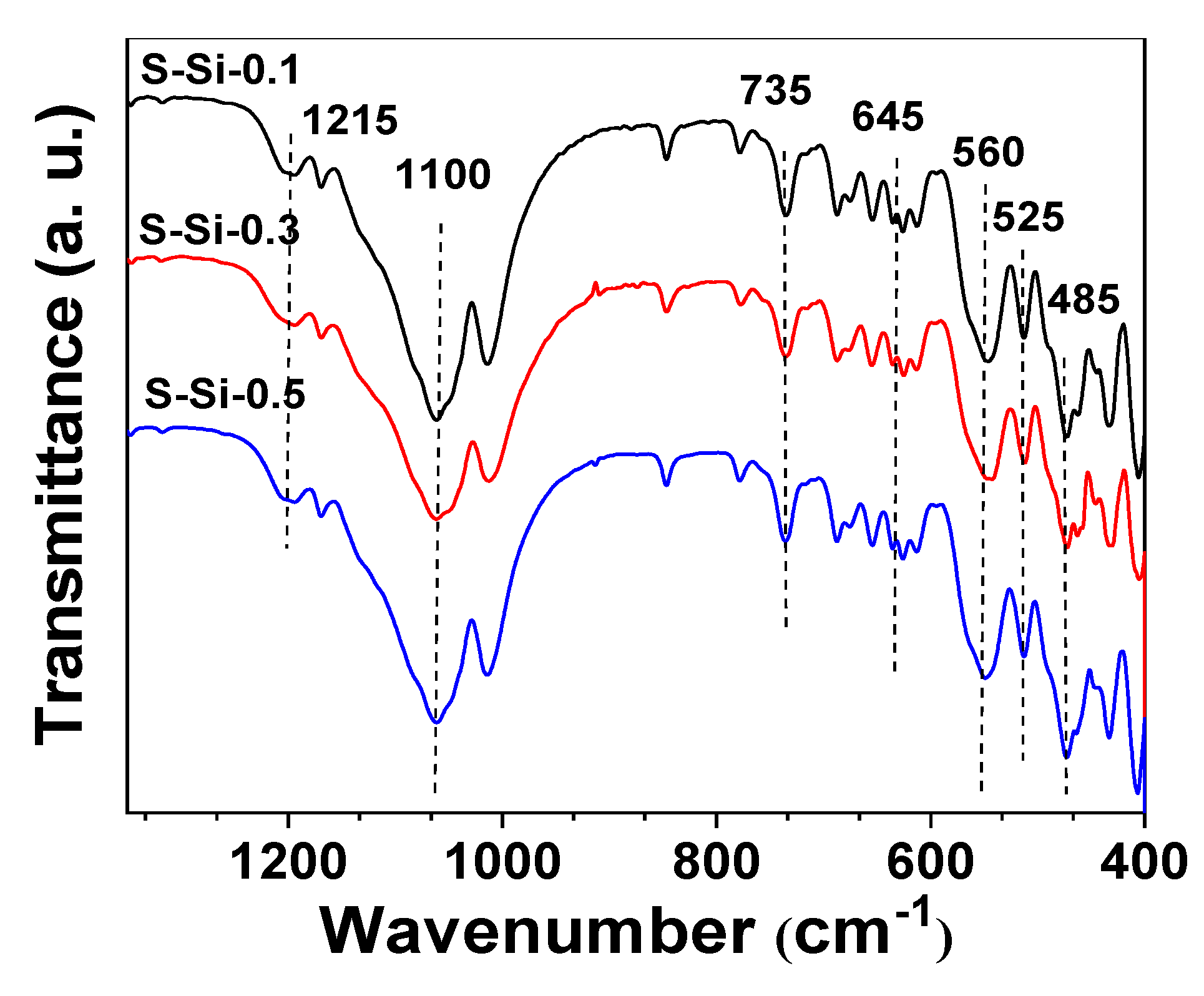
2.4. Effect of Organic Amines
2.5. Catalyst Test
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
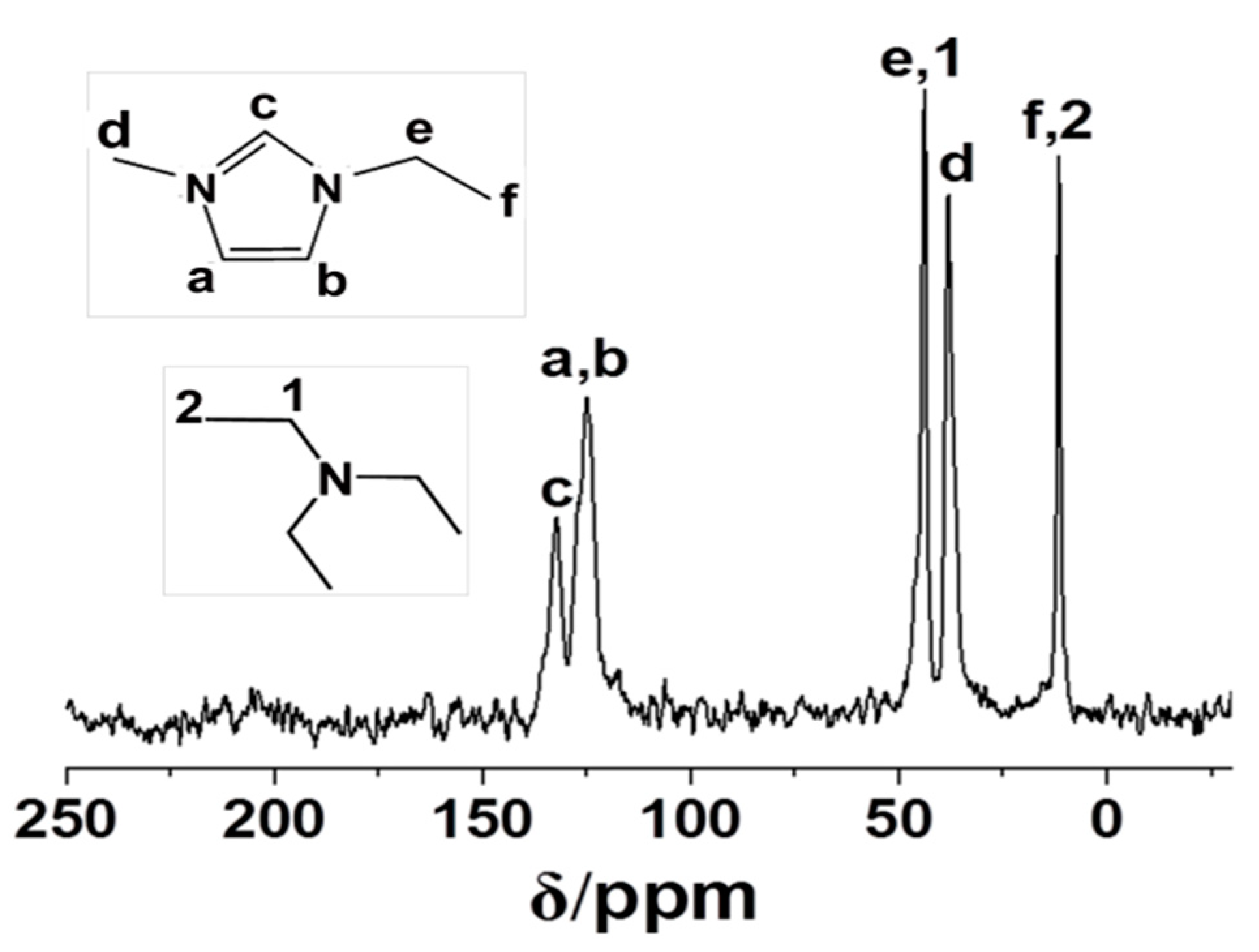
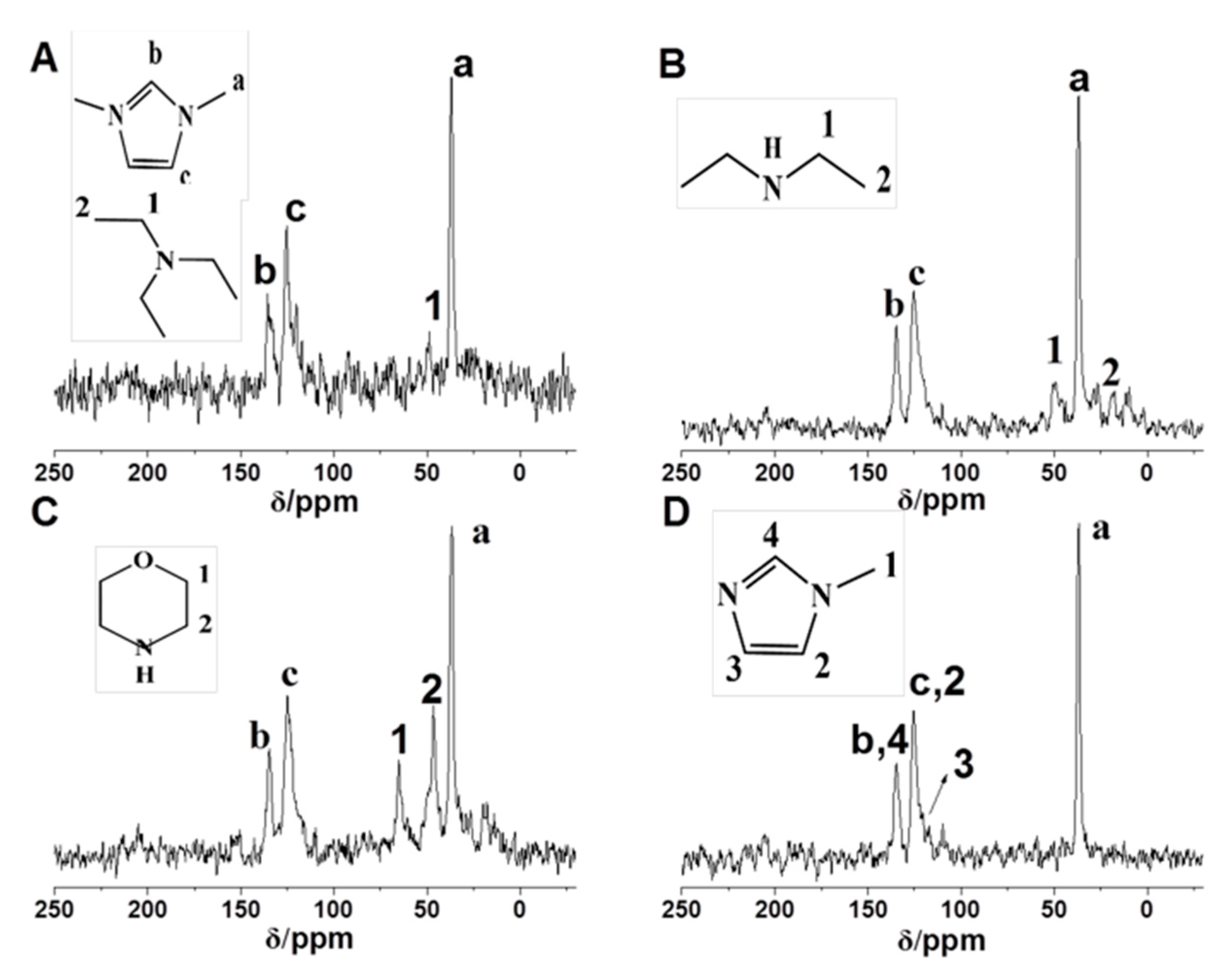
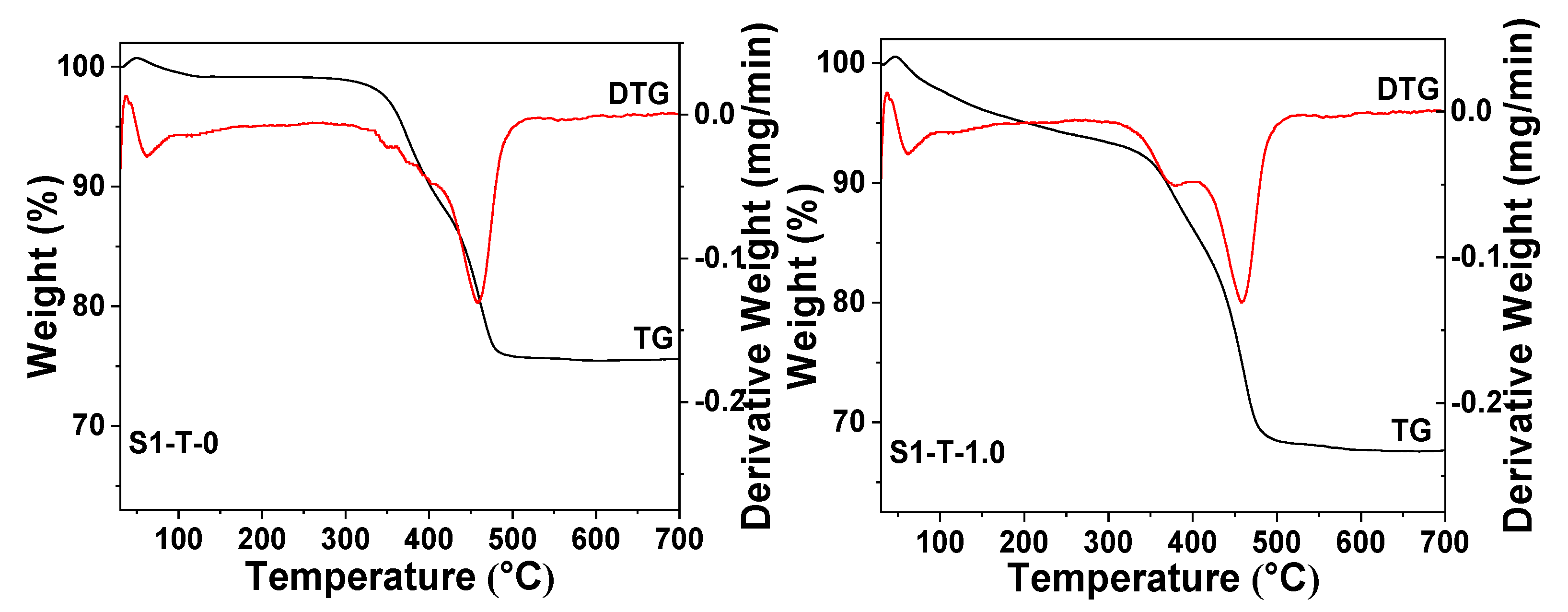
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Davis, M.E. Ordered porous materials for emerging applications. Nature 2002, 417, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Ackley, M.W.; Rege, S.U.; Saxena, H. Application of natural zeolites in the purification and separation of gases. Microporous Mesoporous Mater. 2003, 61, 25–42. [Google Scholar] [CrossRef]
- Davis, M.E. Zeolites from a Materials Chemistry Perspective. Chem. Mater. 2013, 26, 239–245. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, N.; Guo, G.Q.; Chen, X.X.; Yu, J. Synthesis of tri-level hierarchical SAPO-34 zeolite with intracrystalline micro-meso-macroporosity showing superior MTO performance. J. Mater. Chem. A 2015, 3, 19783–19789. [Google Scholar] [CrossRef]
- Xi, D.Y.; Sun, Q.M.; Xu, J.; Cho, M.; Cho, H.S.; Asahina, S.; Li, Y.; Deng, F.; Terasaki, O.; Yu, J. In situ growth-etching approach to the preparation of hierarchically macroporous zeolites with high MTO catalytic activity and selectivity. J. Mater. Chem. A. 2014, 2, 17994–18004. [Google Scholar] [CrossRef]
- Kim, S.; Liu, Y.J.; Moore, J.S.; Dixit, R.S.; Pendergast, J.G.; Sholl, D.; Jones, C.W.; Nair, S. Thin Hydrogen-Selective SAPO-34 Zeolite Membranes for Enhanced Conversion and Selectivity in Propane Dehydrogenation Membrane Reactors. Chem. Mater. 2016, 28, 4397–4402. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.H.; Bai, L.; Chang, N.; Nan, G.Z.; Hu, D.; Zhang, Y.F.; Wei, W. Solvent-free synthesis of SAPO-34 nanocrystals with reduced template consumption for methanol-to-olefins process. Appl. Catal. A Gen. 2017, 531, 203–211. [Google Scholar] [CrossRef]
- Wu, P.F.; Yang, M.; Sun, L.; Zeng, S.; Xu, S.; Tian, P.; Liu, Z.M. Synthesis of nanosized SAPO-34 with the assistance of bifunctional amine and seeds. Chem. Commun. 2018, 54, 11160–11163. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Zhang, J.; Chen, L.; Xu, H. A layered mesoporous SAPO-34 prepared by using as-synthesized SBA-15 as silica source. Microporous Mesoporous Mater. 2011, 145, 150–156. [Google Scholar] [CrossRef]
- Morris, R.E.; Weigel, S.J. The synthesis of molecular sieves from non-aqueous solvents. Chem. Soc. Rev. 1997, 26, 309–317. [Google Scholar] [CrossRef]
- Cooper, E.R.; Andrews, C.D.; Wheatley, P.S.; Webb, P.B.; Wormald, P.; Morris, R.E. Ionic liquids and eutectic mixtures as solvent and template in synthesis of zeolite analogues. Nature 2004, 430, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Parnham, E.R.; Morris, R.E. 1-Alkyl-3-methyl Imidazolium Bromide Ionic Liquids in the Ionothermal Synthesis of Aluminium Phosphate Molecular Sieves. Chem. Mater. 2006, 18, 4882–4887. [Google Scholar] [CrossRef]
- Taubert, A.; Li, Z. Inorganic materials from ionic liquids. Dalton Trans. 2007, 7, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Seddon, K. Ionic liquids A taste of the future. Nat. Mater. 2003, 2, 363–365. [Google Scholar] [CrossRef]
- Greaves, T.L.; Drummond, C.J. Solvent nanostructure, the solvophobic effect and amphiphile self-assembly in ionic liquids. Chem. Soc. Rev. 2013, 42, 1096–1120. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.E. Ionothermal synthesis-ionic liquids as functional solvents in the preparation of crystalline materials. Chem. Commun. 2009, 21, 2990–2998. [Google Scholar] [CrossRef]
- Ngo, H.L.; Lecompte, K.; Hargens, L.; Mcewen, A.B. Thermal properties of imidazolium ionic liquids. Thermochim. Acta 2000, 357, 97–102. [Google Scholar] [CrossRef]
- Fortas, W.; Djelad, A.; Hasnaoui, M.A.; Sassi, M.; Bengueddach, A. Adsorption of gentian violet dyes in aqueous solution on microporous AlPOs molecular sieves synthesized by ionothermal method. Mater. Res. Express 2018, 5, 25018. [Google Scholar] [CrossRef]
- Li, X.; Li, K.; Ma, H.; Xu, R.; Tao, S.; Tian, Z. Ionothermal synthesis of a CHA-type aluminophosphate molecular sieve membrane and its formation mechanism. Microporous Mesoporous Mater. 2015, 217, 54–62. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, E.J.; López-Arbeloa, F.; Hong, S.B.; Camblor, M.A. Microporous aluminophosphates synthesized with 1,2,3-trimethylimidazolium and fluoride. Dalton. Trans. 2016, 45, 7616–7626. [Google Scholar] [CrossRef]
- Lin, Y.; Wei, Y.; Zhang, L.; Guo, K.; Wang, M.; Huang, P.; Meng, X.; Zhang, R. Facile ionothermal synthesis of SAPO-LTA zeotypes with high structural stability and their catalytic performance in MTO reaction. Microporous Mesoporous Mater. 2019, 28, 109611. [Google Scholar] [CrossRef]
- Zhao, X.; Wen, J.; Zhao, J.; Li, A.; Li, G.; Wang, X. Hierarchically structured SAPO-5 molecular sieve catalysts with tailored mesoporosity for alkylation reaction. J. Porous Mater. 2015, 22, 577–584. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, M.; Romero, Á.A.; Pinilla-Herrero, I.; Sastre, E. Ionothermal preparation of triclinic SAPO-34 and its catalytic performance in the MTO process. Catal. Today 2017, 296, 239–246. [Google Scholar] [CrossRef]
- Azim, M.M.; Stark, A. The First Systematic Study on the Conditions Affecting the Ionothermal Synthesis of Silicoaluminophosphates. ChemistrySelect 2018, 3, 12495–12503. [Google Scholar] [CrossRef]
- Tan, J.; Liu, Z.; Bao, X.; Liu, X.; Han, X.; He, C.; Zhai, R. Crystallization and Si incorporation mechanisms of SAPO-34. Microporous Mesoporous Mater. 2002, 53, 97–108. [Google Scholar] [CrossRef]
- Blasco, T.; Chica, A.; Corma, A.; Murphy, W.J.; Agúndez-Rodríguez, J.; Pérez-Pariente, J. Changing the Si distribution in SAPO-11 by synthesis with surfactants improves the hydroisomerization/dewaxing properties. J. Catal. 2006, 242, 153–161. [Google Scholar] [CrossRef]
- Wang, Y.S.; Peng, X.Y.; Tian, Z.J.; Lin, L.W. Research Progress in Ionothermal Synthesis of Molecular Sieves. Chin. J. Catal. 2012, 33, 39–50. [Google Scholar] [CrossRef]
- Tian, Z.J.; Liu, H. Ionothermal synthesis of zeolitic molecular sieves: Properties, progress and prospect. Chem. Ind. Eng. Prog. 2015, 34, 1501–1510. [Google Scholar]
- Pei, R.; Tian, Z.; Wei, Y.; Li, K.; Xu, Y.; Wang, L.; Ma, H. Ionothermal synthesis of AlPO4-34 molecular sieves using heterocyclic aromatic amine as the structure directing agent. Mater. Lett. 2010, 64, 2384–2387. [Google Scholar] [CrossRef]
- Wang, C.; Yang, M.; Tian, P.; Xu, S.; Yang, Y.; Wang, D.; Yuan, Y.; Liu, Z. Dual template-directed synthesis of SAPO-34 nanosheet assemblies with improved stability in the methanol to olefins reaction. J. Mater. Chem. A 2015, 3, 5608–5616. [Google Scholar] [CrossRef]
- Feng, J.; Guo, L.L.; Wang, Z.; Wang, B.S.; Wang, J.F.; Lu, T.L.; Xu, J.; Zhan, Y.Z.; Rawal, A.; Zhao, C.; et al. Effect of ionothermal synthesis on the acidity and catalytic performance of a SAPO-5 molecular sieve. ChemistrySelect 2019, 35, 10520–10524. [Google Scholar] [CrossRef]



| Sample | Time | Conversion/% | Yield/% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | C2= | C2 | C3= | C3 | DME | C4= | C4 | C5 | C2=~C4= | |||
| S0 | 1 h | 100 | 26.7 | 44.7 | 0.5 | 21.0 | 0.6 | 0 | 3.3 | 1.7 | 1.5 | 69 |
| S1-Si-0.3 | 1 h | 100 | 17.4 | 3.7 | 0.6 | 4.1 | 0.3 | 72.2 | 0.7 | 0.6 | 0.4 | 8.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, L.; Yan, X.; Guo, L.; Duan, Y.; Wang, Z.; Lu, T.; Xu, J.; Zhan, Y.; Wang, J. Ionothermal Synthesis of Triclinic SAPO-34 Zeolites. Catalysts 2021, 11, 616. https://doi.org/10.3390/catal11050616
Han L, Yan X, Guo L, Duan Y, Wang Z, Lu T, Xu J, Zhan Y, Wang J. Ionothermal Synthesis of Triclinic SAPO-34 Zeolites. Catalysts. 2021; 11(5):616. https://doi.org/10.3390/catal11050616
Chicago/Turabian StyleHan, Li, Xinxin Yan, Lulu Guo, Yanan Duan, Zheng Wang, Tianliang Lu, Jun Xu, Yuzhong Zhan, and Jianfeng Wang. 2021. "Ionothermal Synthesis of Triclinic SAPO-34 Zeolites" Catalysts 11, no. 5: 616. https://doi.org/10.3390/catal11050616
APA StyleHan, L., Yan, X., Guo, L., Duan, Y., Wang, Z., Lu, T., Xu, J., Zhan, Y., & Wang, J. (2021). Ionothermal Synthesis of Triclinic SAPO-34 Zeolites. Catalysts, 11(5), 616. https://doi.org/10.3390/catal11050616






