Preparation of MgCl2-Supported Ziegler-Natta Catalysts via New Surfactants Emulsion for Propylene Polymerization
Abstract
:1. Introduction
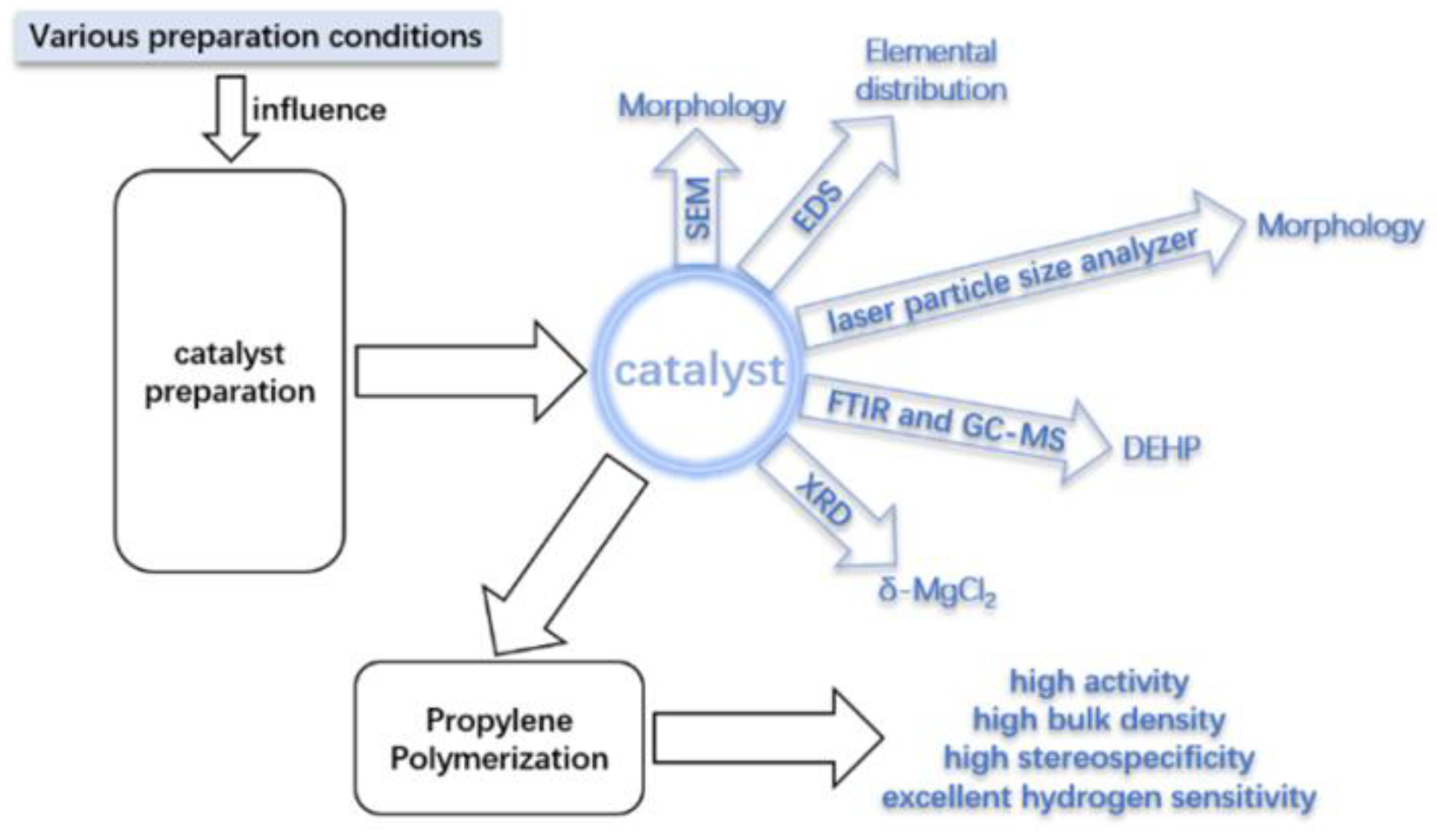
2. Results and Discussion
2.1. Effect of Catalyst Preparation Conditions
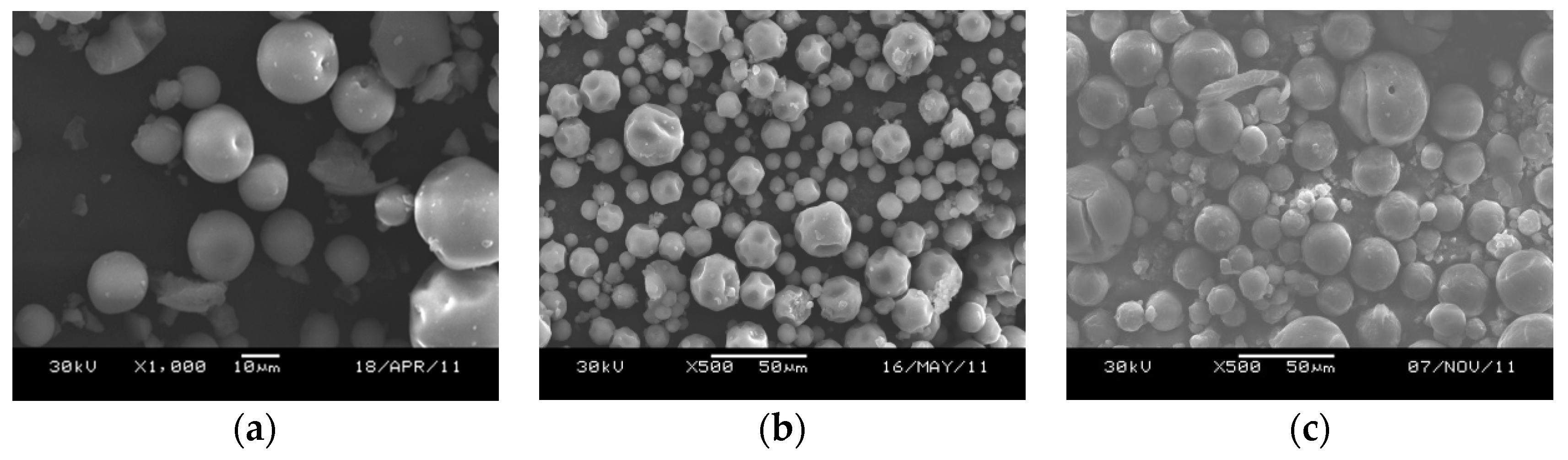
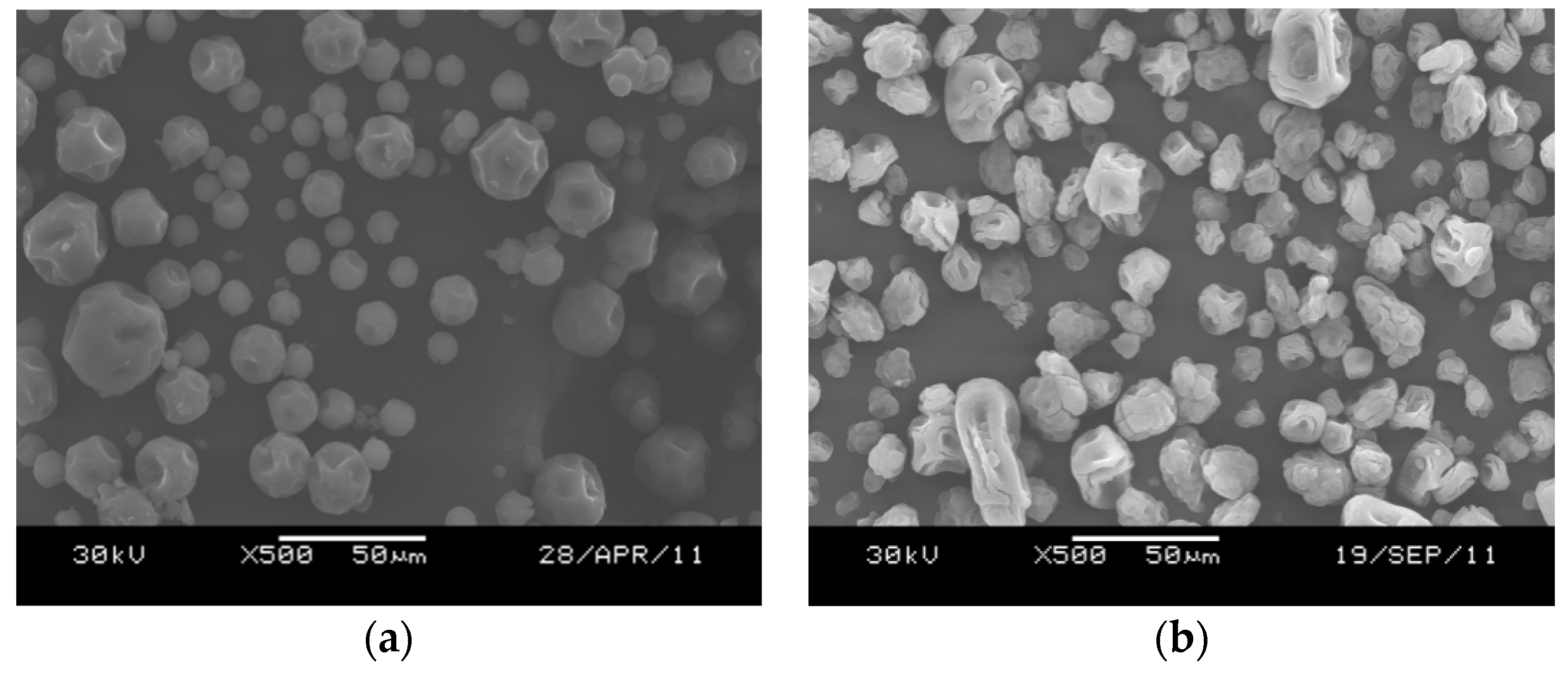
2.1.1. Influence of Surfactant
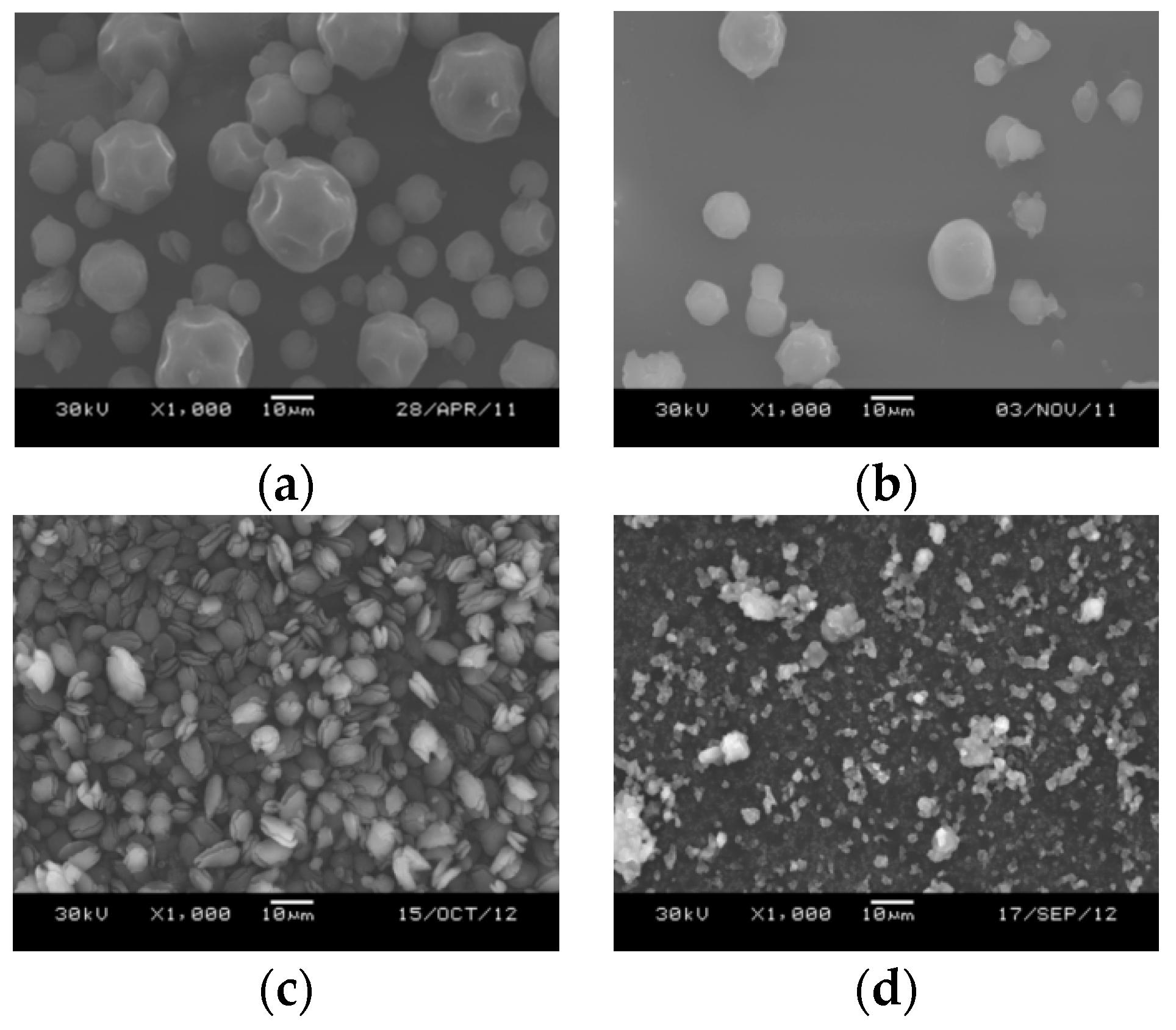
2.1.2. Influence of the TiCl4/Toluene Molar Ratio
2.1.3. Influence of TiCl4-Contacting Temperature
2.1.4. Influence of Phthaloyl Dichloride
2.1.5. Effect of the Stirring Rate
2.1.6. Influence of the Mixing Time of TiCl4/Toluene with Mg Complex
2.1.7. Influence of n-butyl Chloride
2.2. Characterization of the Catalyst
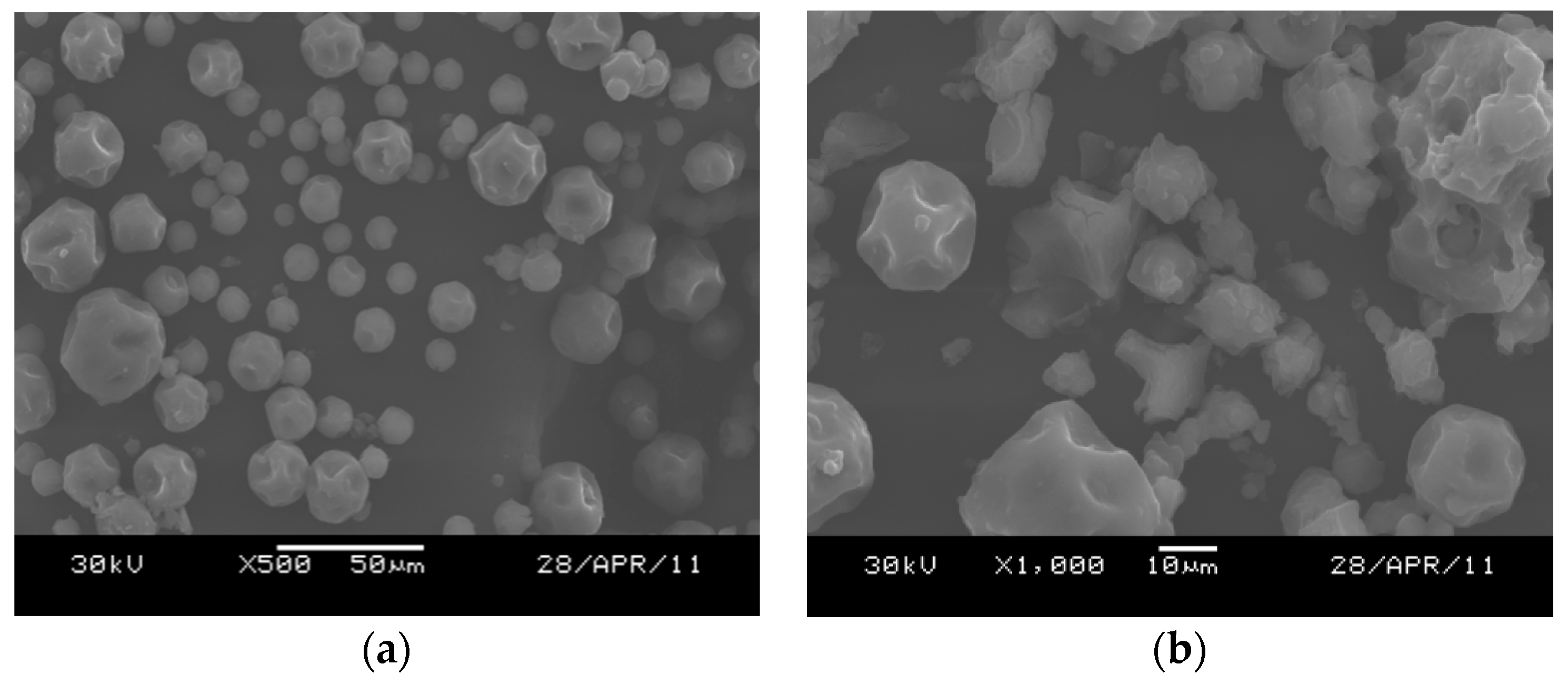
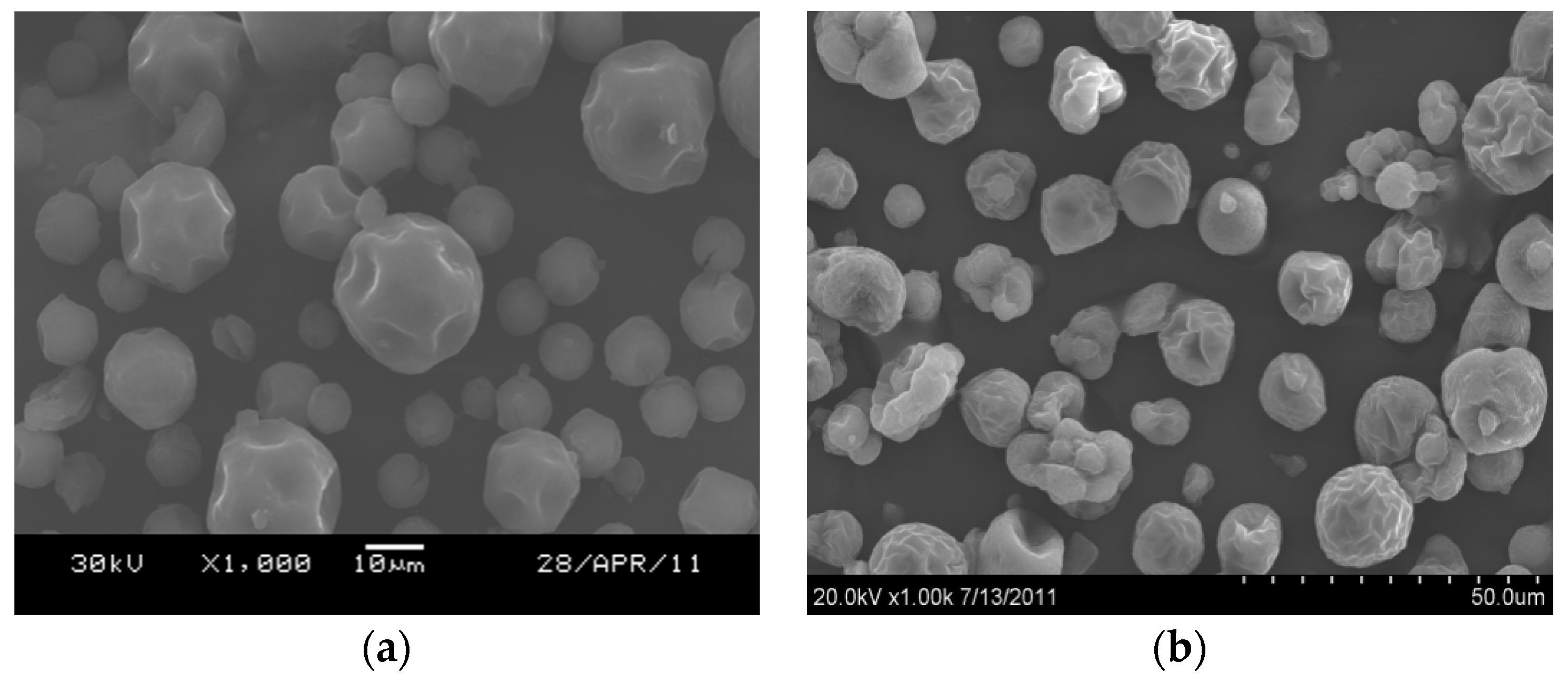
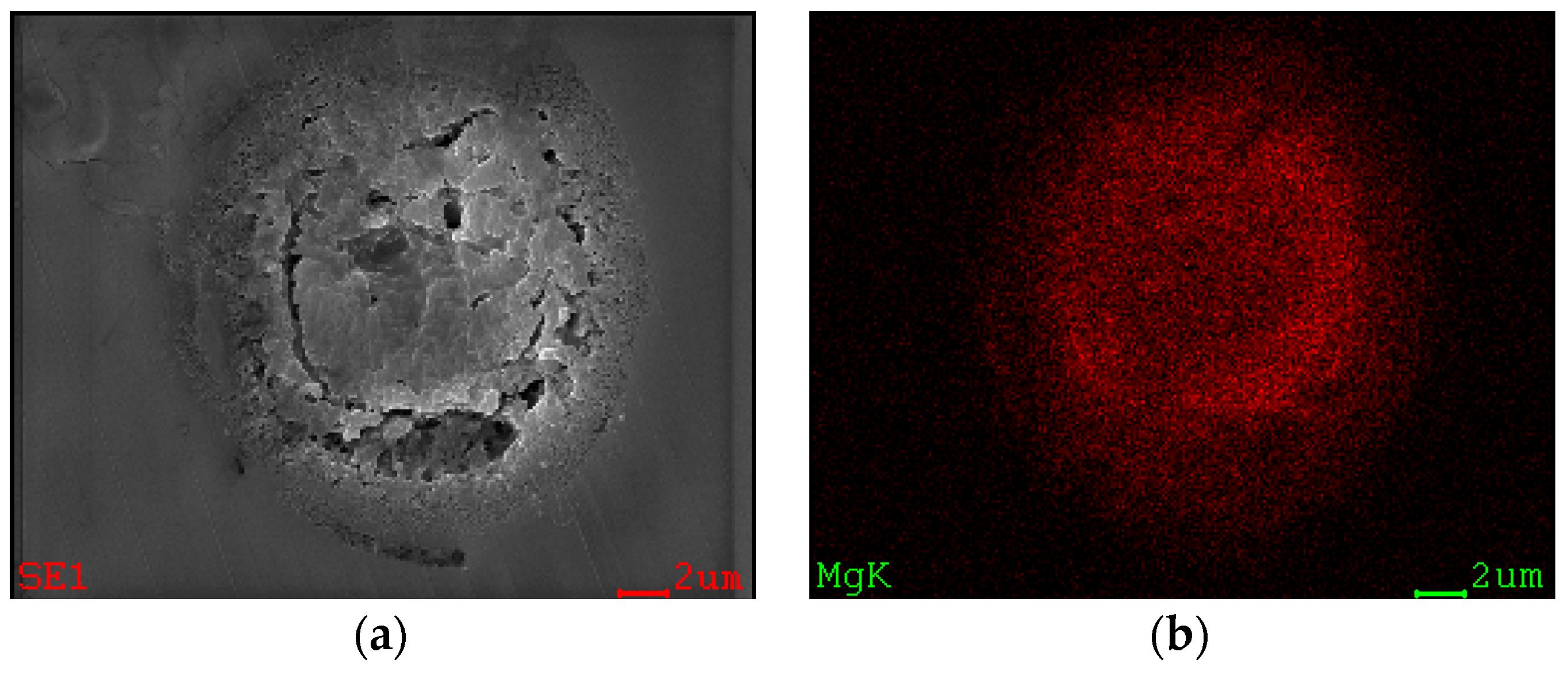
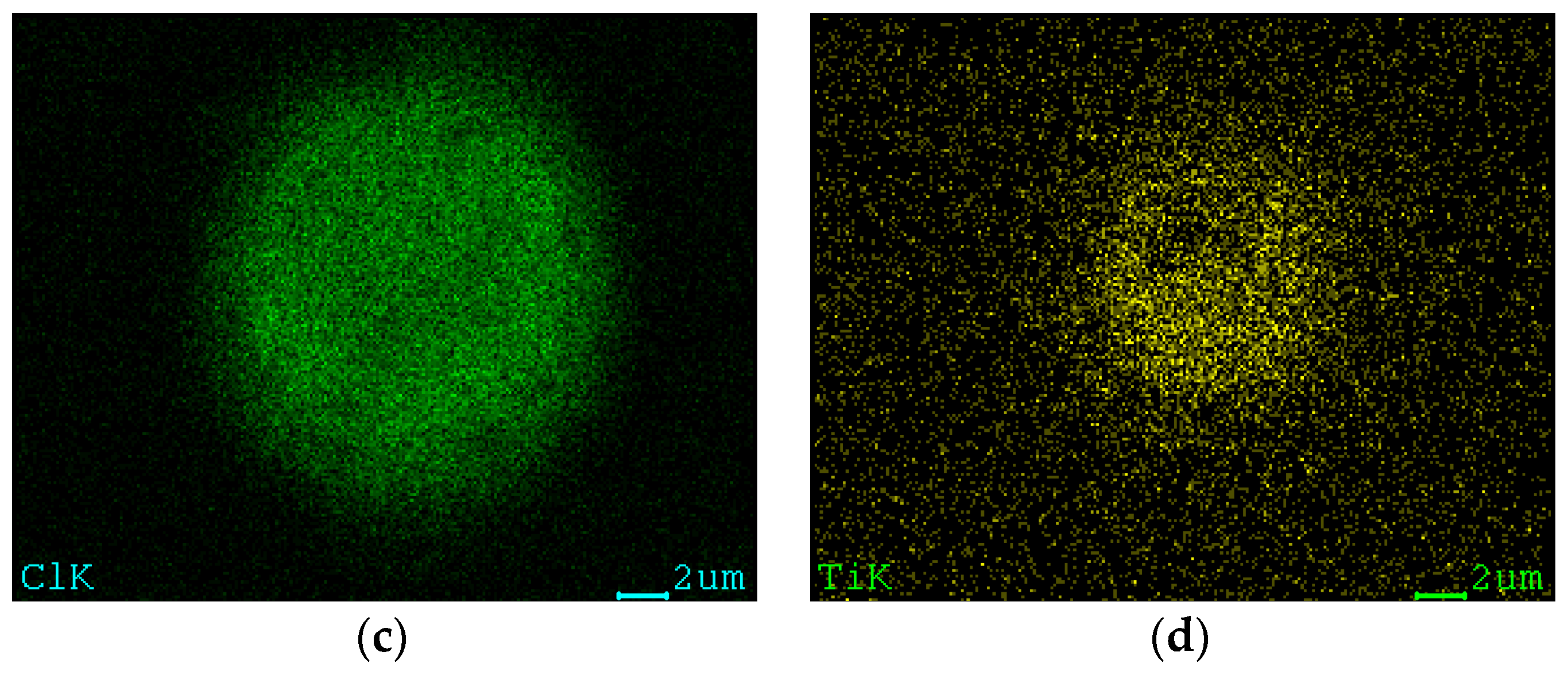
2.2.1. Elemental and Particle Size Analysis of the Catalyst
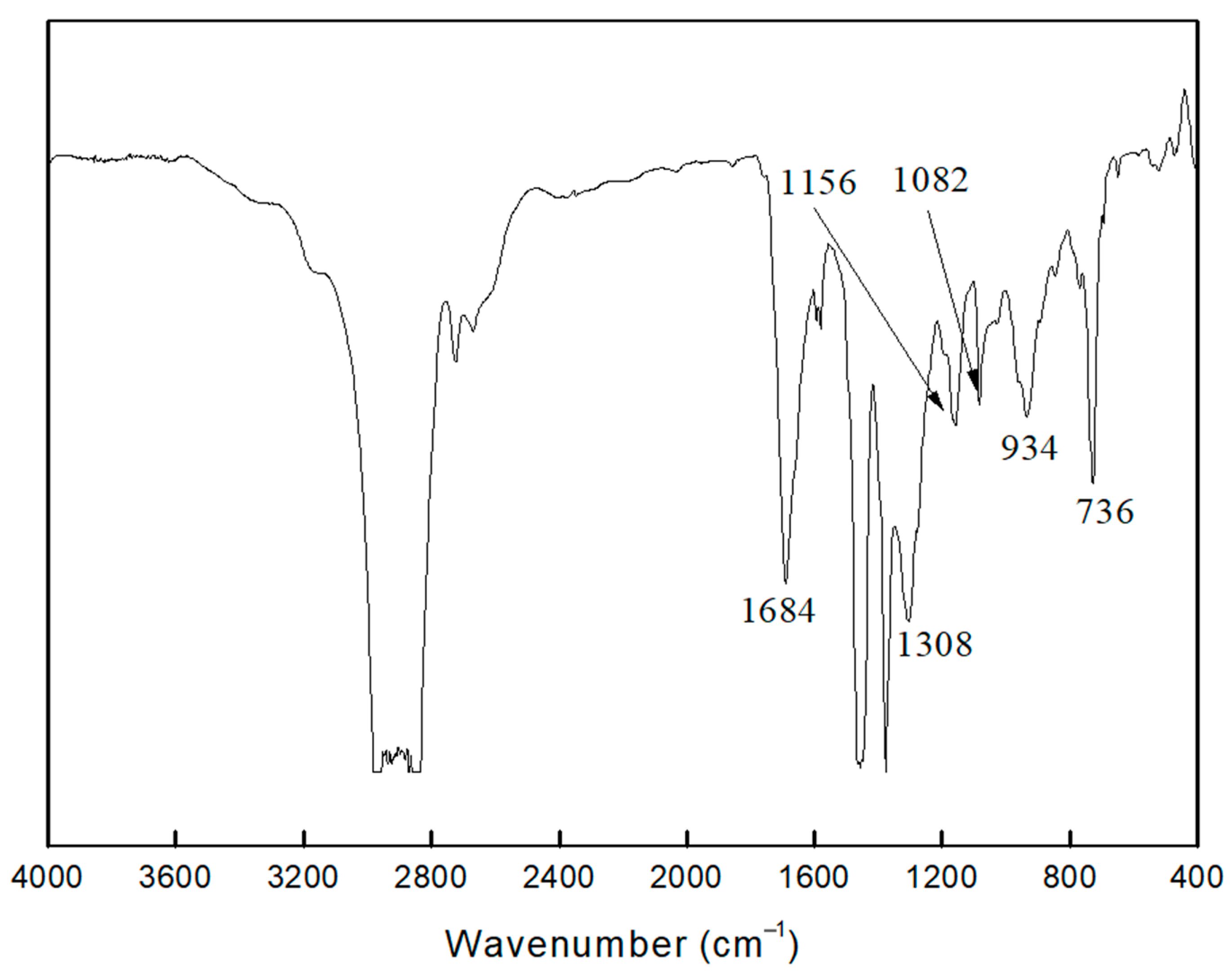
2.2.2. FTIR
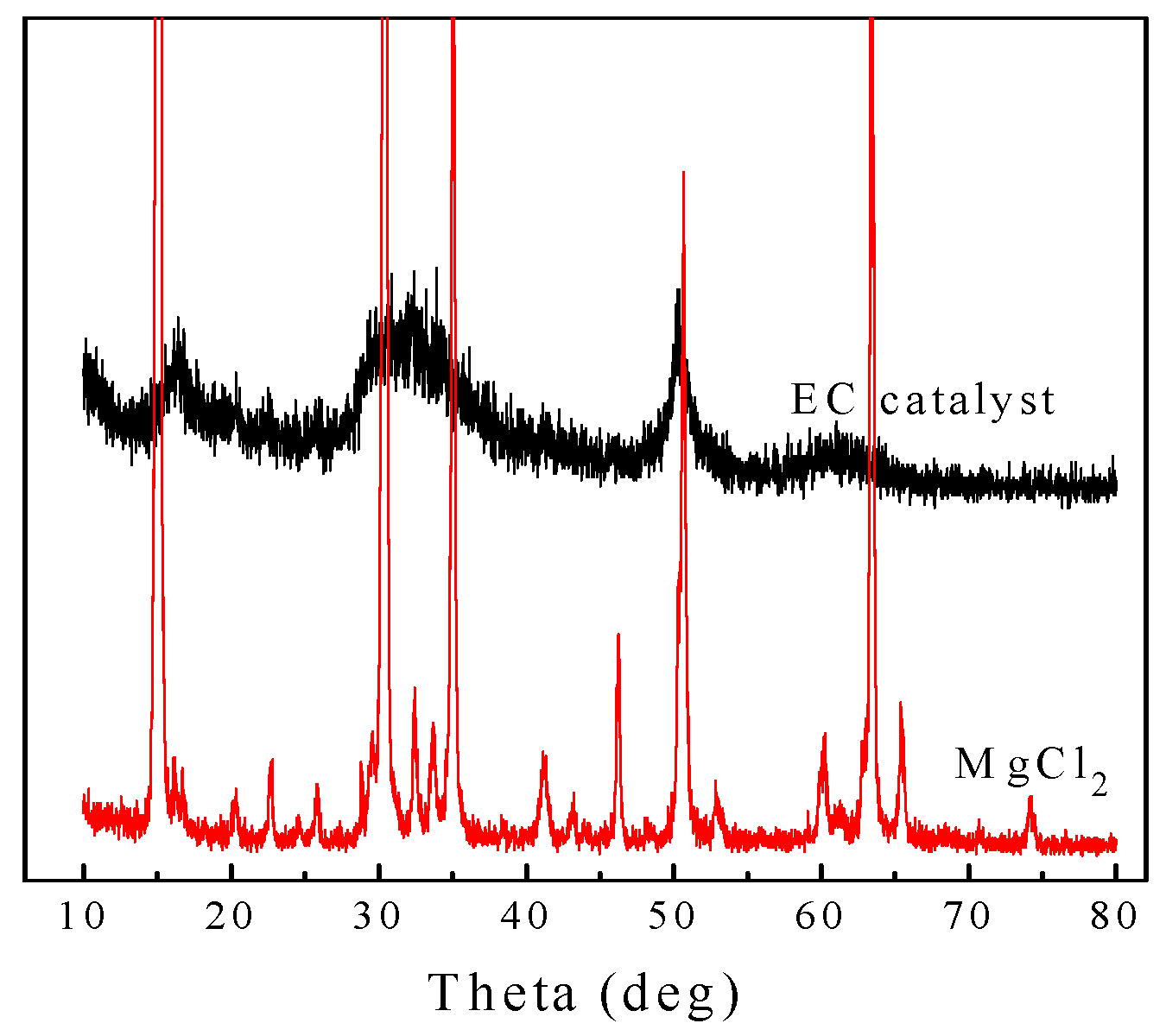
2.2.3. XRD
2.2.4. GC/MS
2.3. Propylene Polymerization
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Characterization
3.4. Propylene Polymerization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ziegler, K.; Holzkma, P.E.; Breil, H. Results and development of a discovery. Angew. Chem. 1955, 67, 541–547. [Google Scholar] [CrossRef]
- Natta, G. Catalysts and Polymerization. Polym. J. Sci. 1995, 16, 35. [Google Scholar]
- Ballard, D.G.H. Pi and Sigma Transition Metal Carbon Compounds as Catalysts for the Polymerization of Vinyl Monomers and Olefins. Adv. Catal. 1973, 23, 263–325. [Google Scholar]
- Zheng, W.; He, A.; Liu, C.; Shao, H.; Wang, R. The influences of alkylaluminium as cocatalyst on butene-1 polymerization with MgCl2-supported TiCl4 Ziegler-Natta catalysts. Polymer 2020, 210, 122998. [Google Scholar] [CrossRef]
- Galli, P.; Luciani, L.; Cecchin, G. Advances in the polymerization of polyolefins with coordination catalysts. Angew. Macromol. Chem. 1981, 94, 63–89. [Google Scholar] [CrossRef]
- Mikenas, T.B.; Koshevoy, E.I.; Zakharov, V.A. Effect of the Structure of Titanium–Magnesium Catalysts on the Morphology of Polyethylene Produced. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 2298–2308. [Google Scholar] [CrossRef]
- Gianini, U. Polymerization of olefins with high activity catalysts. Macromol. Chem. Suppl. 1981, 5, 216–229. [Google Scholar] [CrossRef]
- Fregonese, D.; Glisenti, A.; Mortara, S.; Rizzi, G.A.; Tondello, E.; Bresadola, S. MgCl2/TiC14/AlEt3 catalytic system for olefin polymerization: A XPS study. J. Mol. Catal. A Chem. 2002, 178, 115–123. [Google Scholar] [CrossRef]
- Kashiwa, N. The discovery and progress of MgCl2-Supported TiCl4 Catalysts. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 1–8. [Google Scholar] [CrossRef]
- Luo, Z.; Zheng, T.; Li, H.; Zhou, Q.; Wang, A.; Zhang, L.; Hu, Y. Asubmicron spherical polypropylene prepared by heterogeneous Ziegler–Natta catalyst. Ind. Eng. Chem. Res. 2015, 54, 11247–11250. [Google Scholar] [CrossRef]
- Zheng, W.P.; Ma, Y.P.; Du, D.L.; He, A.H.; Shao, H.F.; Liu, C.G. Polymerization Kinetics of Propylene with the MgCl2-Supported Ziegler-Natta Catalysts—Active Centers with Different Tacticity and Fragmentation of the Catalyst. Chin. J. Polym. Sci. 2021, 39, 70–80. [Google Scholar] [CrossRef]
- Kaminsky, W. Polymerization catalysis. Catal. Today 2000, 62, 23–34. [Google Scholar] [CrossRef]
- Cho, H.S.; Lee, W.Y. Synthesis of inorganic MgCl2-alcohol adduct via recrystallization method and its application in supported organometallic catalysts for the polymerization of ethylene with 1-hexene. J. Mol. Catal. A Chem. 2003, 191, 155–165. [Google Scholar] [CrossRef]
- Choi, J.H.; Chung, J.S.; Shin, H.W.; Song, I.K.; Lee, W.Y. The effect of alcohol treatment in the preparation of MgCl2 support by a recrystallization method on the catalytic activity and isotactic index for propylene polymerization. J. Catal. 1996, 32, 405–410. [Google Scholar] [CrossRef]
- Liu, B.; Nitta, T.; Nakatani, H.; Terano, M. Stereospecific Nature of Active Sites on TiCl4/MgCl2 Ziegler-Natta Catalyst in the Presence of an Internal Electron Donor. Macromol. Chem. Phys. 2003, 20, 395–402. [Google Scholar] [CrossRef]
- Lin, J.S.; Catlow, C.R.A. Quantum Mechanical study of TiCl4/MgCl2-supported Ziegler-Natta catalysts. J. Catal. 1995, 157, 145–152. [Google Scholar] [CrossRef]
- Koskinen, J.; Garoff, T.; Louhelainen, J.; Koskinen, J. Preparation of Solid Ziegler Catalyst by Using a Multifunctional, Pivoting, Inclinable Reactor and the Equipment. U.S. Patent 5,472,923, 5 December 1995. [Google Scholar]
- Koskinen, J.; Garoff, T. Method for the Preparation of a Particulate Carrier for a Polymerization Catalyst. U.S. Patent 5,468,698, 21 November 1995. [Google Scholar]
- Albizzati, E. Process for Preparing Spheroidally Shaped Products, Solid at Room Temperature. U.S. Patent 4,469,648, 4 September 1984. [Google Scholar]
- Udayakumar, S.; Ibrahim, N.; Chien, C.Y.; Wahab, S.A.R.S.A.; Noor, A.F.M.; Ramakrishnan, S. Synthesis of Ziegler-Natta Catalyst using Malaysian Ilmenite Derived TiCl4 via Recrystallization Method: A Statistical Approach. Bull. Chem. React. Eng. Catal. 2020, 15, 687–697. [Google Scholar] [CrossRef]
- Mori, H.; Higuchi, T.; Otsuka, N.; Terano, M. High resolution transmission electron microscope observation of industrial high performance Ziegler catalysts. Macromol. Chem. Phys. 2000, 201, 2789–2798. [Google Scholar] [CrossRef]
- Ratanasak, M.; Parasuk, V. Roles of malonate donor on activity and stereoselectivity of Ziegler–Natta catalyzed propylene polymerization. J. Organomet. Chem. 2015, 775, 6–11. [Google Scholar] [CrossRef]
- Zhong, C.; Gao, M.; Mao, B. Effect of high polymerization temperature on the microstructure of isotactic polypropylene prepared using heterogeneous TiCl4/MgCl2 catalysts. J. Appl. Polym. Sci. 2003, 90, 3980–3986. [Google Scholar] [CrossRef]
- Pater, J.T.; Weickert, G.; Van Swaaij, W.P. Polymerization of liquid propylene with a 4th generation Ziegler-Natta catalyst-influence of temperature, hydrogen and monomer concentration and prepolymerization method on polymerization kinetics. Chem. Eng. Sci. 2002, 57, 3461–3477. [Google Scholar] [CrossRef]
- Pater, J.T.; Weickert, G.; van Swaaij, W.P. Propene bulk polymerization kinetics: Role of prepolymerization and hydrogen. Chem. Eng. Sci. 2002, 57, 3461–3465. [Google Scholar] [CrossRef]
- Kissin, Y.V.; Zhou, Q.; Li, H.; Zhang, L. Active centers in propylene polymerization catalysts of the fourth generation. J. Catal. 2015, 332, 156–163. [Google Scholar] [CrossRef]
- Denifl, P.; Leinonen, T. Process for Preparing an Olefin Polymerization Catalyst Component. U.S. Patent 7271119B2, 18 September 2007. [Google Scholar]
- Rönkkö, H.L.; Knuuttila, H.; Denifl, P.; Leinonen, T.; Venäläinen, T. Structural studies on a solid self-supported Ziegler–Natta-type catalyst for propylene polymerization. J. Mol. Catal. A Chem. 2007, 278, 127–134. [Google Scholar] [CrossRef]
- Kunii, D.; Levenspiel, O. Fluidization Engineering; Wiley: New York, NY, USA, 1969; pp. 105–106. [Google Scholar]


| Ti (wt%) | Mg (wt%) | Cl (wt%) | DEHP (wt%) | D10 (µm) | D50 (µm) | D90 (µm) | Span Value |
|---|---|---|---|---|---|---|---|
| 0.95 | 17.62 | 39.75 | 27.72 | 6.8 | 19.8 | 29.8 | 1.16 |
| Run | T (°C) | Al/Ti | H2 (NL) | Activity 1 (kg PP/g cat) | Isotacticity (%) | Mw (104) | Tm (°C) |
|---|---|---|---|---|---|---|---|
| 1 | 50 | 200 | 1.5 | 21.22 | 98.61 | 39.32 | 161.7 |
| 2 | 60 | 200 | 1.5 | 26.01 | 98.50 | 36.65 | 161.4 |
| 3 | 70 | 200 | 1.5 | 34.71 | 98.22 | 33.93 | 160.9 |
| 4 | 80 | 200 | 1.5 | 34.59 | 97.98 | 28.33 | 160.3 |
| 5 | 70 | 200 | 2.0 | 34.67 | 97.98 | 30.86 | 160.4 |
| 6 | 70 | 200 | 4.0 | 35.09 | 97.88 | 23.40 | 158.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Li, J.; Ding, W.; Jiang, T. Preparation of MgCl2-Supported Ziegler-Natta Catalysts via New Surfactants Emulsion for Propylene Polymerization. Catalysts 2021, 11, 601. https://doi.org/10.3390/catal11050601
Wu Y, Li J, Ding W, Jiang T. Preparation of MgCl2-Supported Ziegler-Natta Catalysts via New Surfactants Emulsion for Propylene Polymerization. Catalysts. 2021; 11(5):601. https://doi.org/10.3390/catal11050601
Chicago/Turabian StyleWu, Yansong, Jian Li, Wei Ding, and Tao Jiang. 2021. "Preparation of MgCl2-Supported Ziegler-Natta Catalysts via New Surfactants Emulsion for Propylene Polymerization" Catalysts 11, no. 5: 601. https://doi.org/10.3390/catal11050601
APA StyleWu, Y., Li, J., Ding, W., & Jiang, T. (2021). Preparation of MgCl2-Supported Ziegler-Natta Catalysts via New Surfactants Emulsion for Propylene Polymerization. Catalysts, 11(5), 601. https://doi.org/10.3390/catal11050601





