Synthesis of Highly Porous Cu2O Catalysts for Efficient Ozone Decomposition
Abstract
1. Introduction
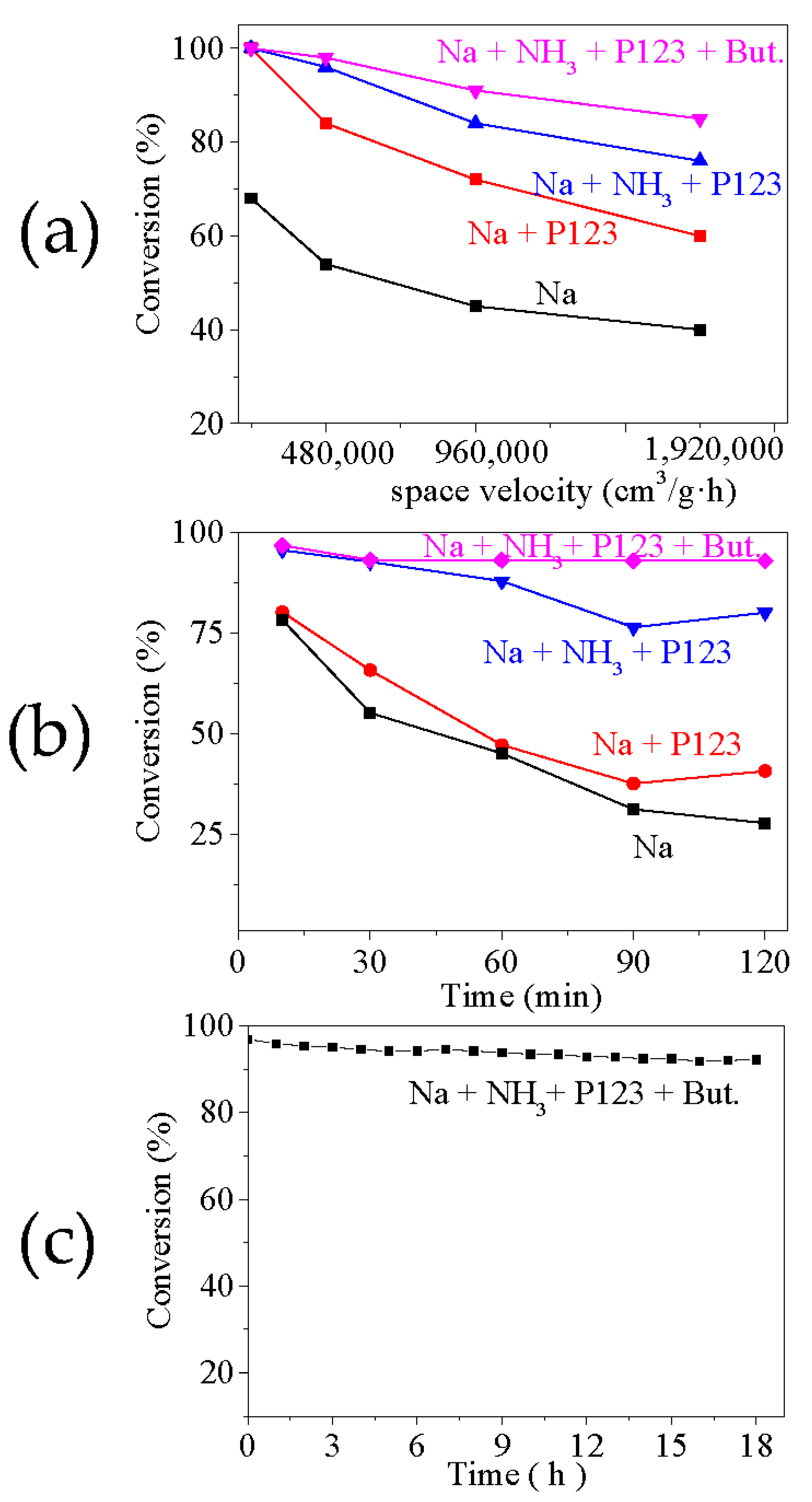
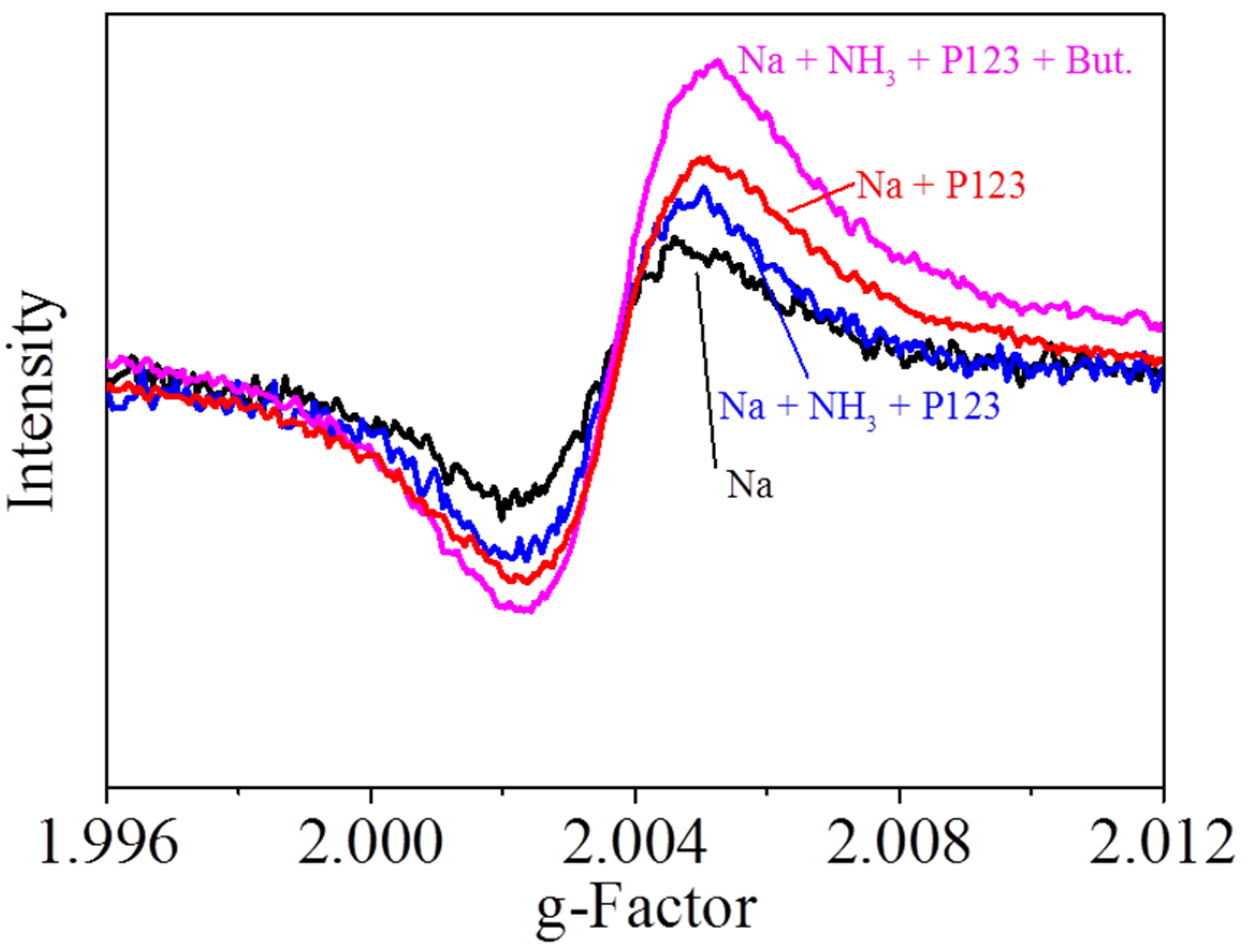
2. Results and Discussion
3. Experimental Procedure
3.1. Preparation of Catalysts
3.2. Characterization of Catalysts
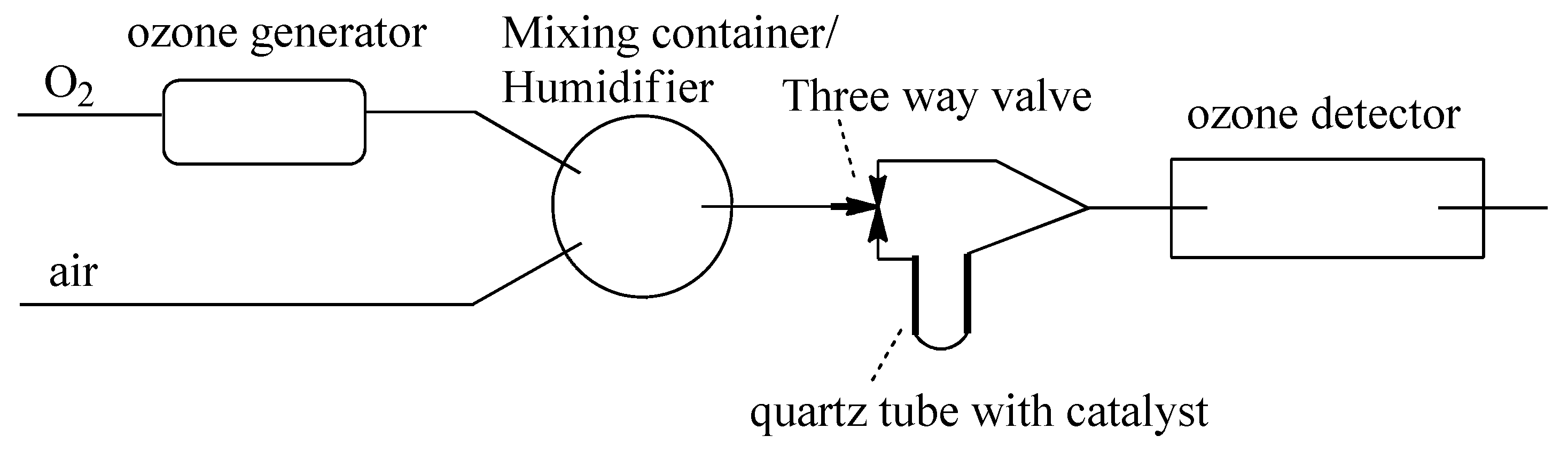
3.3. Ozone Catalytic Decomposition Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cleveland, W.S.; Graedel, T.E. Photochemical Air Pollution in the Northeast United States. Science 1979, 204, 1273–1278. [Google Scholar] [CrossRef]
- Liang, G.Y. Supported gold catalysts used for ozone decomposition and simultaneous elimination of ozone and carbon monoxide at ambient temperature. Appl. Catal. B Environ. 2001, 33, 217–222. [Google Scholar]
- Lu, X.; Zhang, L.; Wang, X.L.; Gao, M.; Li, K.; Zhang, Y.Z.; Yue, X.; Zhang, Y.H. Rapid Increases in Warm-Season Surface Ozone and Resulting Health Impact in China Since 2013. Environ. Sci. Technol. Lett. 2020, 7, 240–247. [Google Scholar] [CrossRef]
- Heisig, C.; Zhang, W.; Oyama, S.T. Decomposition of ozone using carbon-supported metal oxide catalysts. Appl. Catal. B Environ. 1997, 14, 117–129. [Google Scholar] [CrossRef]
- Li, X.; Ma, J.; He, H. Recent advances in catalytic decomposition of ozone. J. Environ. Sci. 2020, 94, 14–31. [Google Scholar] [CrossRef]
- Li, W.; Gibbs, G.V.; Oyama, S.T. Mechanism of ozone decomposition on a manganese oxide catalyst. I. In situ Raman spectroscopy and ab initio molecular orbital calculations. J. Am. Chem. Soc. 1998, 120, 9041–9046. [Google Scholar] [CrossRef]
- Dhandapani, B.; Oyama, S.T. Gas phase ozone decomposition catalysts. Appl. Catal. B Environ. 1997, 11, 129–166. [Google Scholar] [CrossRef]
- Li, X.T.; Ma, J.Z.; Yang, L.; He, G.Z.; Zhang, C.B.; Zhang, R.D.; He, H. Oxygen Vacancies Induced by Transition Metal Doping in γ-MnO2 for Highly Efficient Ozone Decomposition. Environ. Sci. Technol. 2018, 52, 12685–12696. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ma, J.Z.; Li, X.T.; Zhang, C.B.; He, H. Enhancing Oxygen Vacancies of Ce-OMS-2 via Optimized Hydrothermal Conditions to Improve Catalytic Ozone Decomposition. Ind. Eng. Chem. Res. 2020, 59, 118–128. [Google Scholar] [CrossRef]
- Hong, W.; Zhu, T.; Sun, Y.; Wang, H.; Li, X.; Shen, F. Enhancing Oxygen Vacancies by Introducing Na+ into OMS-2 Tunnels to Promote Catalytic Ozone Decomposition. Environ. Sci. Technol. 2019, 53, 13332–13343. [Google Scholar] [CrossRef]
- Queisser, H.J.; Haller, E.E. Defects in Semiconductors: Some Fatal, Some Vital. Science 1998, 281, 945–950. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based Photocatalytic Hydrogen Generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef]
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and Properties of Nanocrystals of Different Shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef] [PubMed]
- Hochbaum, A.I.; Yang, P. Semiconductor Nanowires for Energy Conversion. Chem. Rev. 2010, 110, 527–546. [Google Scholar] [CrossRef]
- Wang, H.; Rogach, A.L. Hierarchical SnO2 Nanostructures: Recent Advances in Design, Synthesis, and Applications. Chem. Mater. 2014, 26, 123–133. [Google Scholar] [CrossRef]
- Nolan, M.; Elliott, S.D. The p-type conduction mechanism in Cu2O: A first principles study. Phys. Chem. Chem. Phys. 2006, 8, 5350–5358. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, M.G.; Wang, A.Q.; Ma, G.J.; Han, N.; Chen, Y.F. A one-pot synthesis of a monolithic Cu2O/Cu catalyst for efficient ozone decomposition. RSC Adv. 2020, 10, 40916–40922. [Google Scholar] [CrossRef]
- Gong, S.Y.; Li, W.H.; Xie, Z.; Ma, X.; Liu, H.D.; Han, N.; Chen, Y.F. Low temperature decomposition of ozone by facilely synthesized cuprous oxide catalyst. New J. Chem. 2017, 41, 4828–4834. [Google Scholar] [CrossRef]
- Gong, S.; Chen, J.; Wu, X.; Han, N.; Chen, Y. In-situ synthesis of Cu2O/reduced graphene oxide composite as effective catalyst for ozone decomposition. Catal. Commun. 2018, 106, 25–29. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Zhang, X.; Qu, F. Template-Free Synthesis of Porous Cu2O Nanospheres at Room Temperature and Investigation on Their Adsorption Property. J. Nanomater. 2013, 2013, 378919. [Google Scholar] [CrossRef]
- Gong, S.Y.; Wang, A.Q.; Zhang, J.L.; Guan, J.; Han, N.; Chen, Y.F. Gram-scale synthesis of ultra-fine Cu2O for highly efficient ozone decomposition. RSC Adv. 2020, 10, 5212–5219. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Z.; Han, H.; Wu, D.; Xu, F.; Wang, H.; Jiang, K. Mesoporous Cu2O submicro-spheres, facile synthesis and the selective adsorption properties. Chem. Eng. J. 2012, 185–186, 151–159. [Google Scholar] [CrossRef]
- Zoolfakar, A.S.; Rani, R.A.; Morfa, A.J.; O’Mullane, A.P.; Kalantar-Zadeh, K. Nanostructured copper oxide semiconductors: A perspective on materials, synthesis methods and applications. J. Mater. Chem. C 2014, 2, 5247–5270. [Google Scholar] [CrossRef]
- Kuo, C.H.; Huang, M.H. Morphologically controlled synthesis of Cu2O nanocrystals and their properties. Nano Today 2010, 5, 106–116. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Jiao, X.L.; Chen, D.R. PEG-Assisted Preparation of Single-Crystalline Cu2O Hollow Nanocubes. J. Phys. Chem. C 2008, 112, 16769–16773. [Google Scholar] [CrossRef]
- Chen, K.F.; Xue, D.F. pH-assisted crystallization of Cu2O: Chemical reactions control the evolution from nanowires to polyhedra. Crystengcomm 2012, 14, 8068–8075. [Google Scholar] [CrossRef]
- Leng, M.; Liu, M.Z.; Zhang, Y.B.; Wang, Z.Q.; Yu, C.; Yang, X.G.; Zhang, H.J.; Wang, C. Polyhedral 50-Facet Cu2O Microcrystals Partially Enclosed by {311} High-Index Planes: Synthesis and Enhanced Catalytic CO Oxidation Activity. J. Am. Chem. Soc. 2010, 132, 17084–17087. [Google Scholar] [CrossRef]
- Kleitz, F.; Solovyov, L.A.; Anilkumar, G.M.; Choi, S.H.; Ryoo, R. Transformation of highly ordered large pore silica mesophases (Fm3m, Im3m and p6mm) in a ternary triblock copolymer-butanol-water system. Chem. Commun 2004, 1536–1537. [Google Scholar] [CrossRef]
- Kleitz, F.; Kim, T.W.; Ryoo, R. Phase domain of the cubic im3m mesoporous silica in the EO106PO70EO106-butanol-H2O system. Langmuir 2006, 22, 440–445. [Google Scholar] [CrossRef]
- Sun, S.; Kong, C.; You, H.; Song, X.; Ding, B.; Yang, Z. Facet-selective growth of Cu–Cu2O heterogeneous architectures. CrystEngComm 2012, 14, 40–43. [Google Scholar] [CrossRef]
- Parmigiani, F.; Pacchioni, G.; Illas, F.; Bagus, P.S. Studies of the Cu2O bond in cupric oxide by X-ray photoelectron spectroscopy and ab initio electronic structure models. J. Electron. Spectrosc. Relat. Phenom. 1992, 59, 255–269. [Google Scholar] [CrossRef]
- Komarneni, M.; Shan, J.; Chakradhar, A.; Kadossov, E.; Cabrini, S.; Burghaus, U. Adsorption Dynamics of CO on Silica Supported CuOx Clusters: Utilizing Electron Beam Lithography To Study Methanol Synthesis Model Systems. J. Phys. Chem. C 2012, 116, 5792–5801. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, P.; Chen, L. Catalytic decomposition of gaseous ozone over manganese dioxides with different crystal structures. Appl. Catal. B Environ. 2016, 189, 210–218. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, W.; Zhong, L.; Liu, D.; Cao, X.; Cui, F. Oxygen vacancy-rich 2D/2D BiOCl-g-C3N4 ultrathin heterostructure nanosheets for enhanced visible-light-driven photocatalytic activity in environmental remediation. Appl. Catal. B Environ. 2018, 220, 290–302. [Google Scholar] [CrossRef]
- Bi, W.; Ye, C.; Xiao, C.; Tong, W.; Zhang, X.; Shao, W.; Xie, Y. Spatial Location Engineering of Oxygen Vacancies for Optimized Photocatalytic H2 Evolution Activity. Small 2014, 10, 2820–2825. [Google Scholar] [CrossRef]
- Wang, A.; Zhang, L.; Rahimi, M.G.; Gong, S.; Nie, L.; Han, N.; Chen, Y. Defect engineering of ZnO for electron transfer in O3 catalytic decomposition. Appl. Catal. B Environ. 2020, 277, 119223. [Google Scholar] [CrossRef]

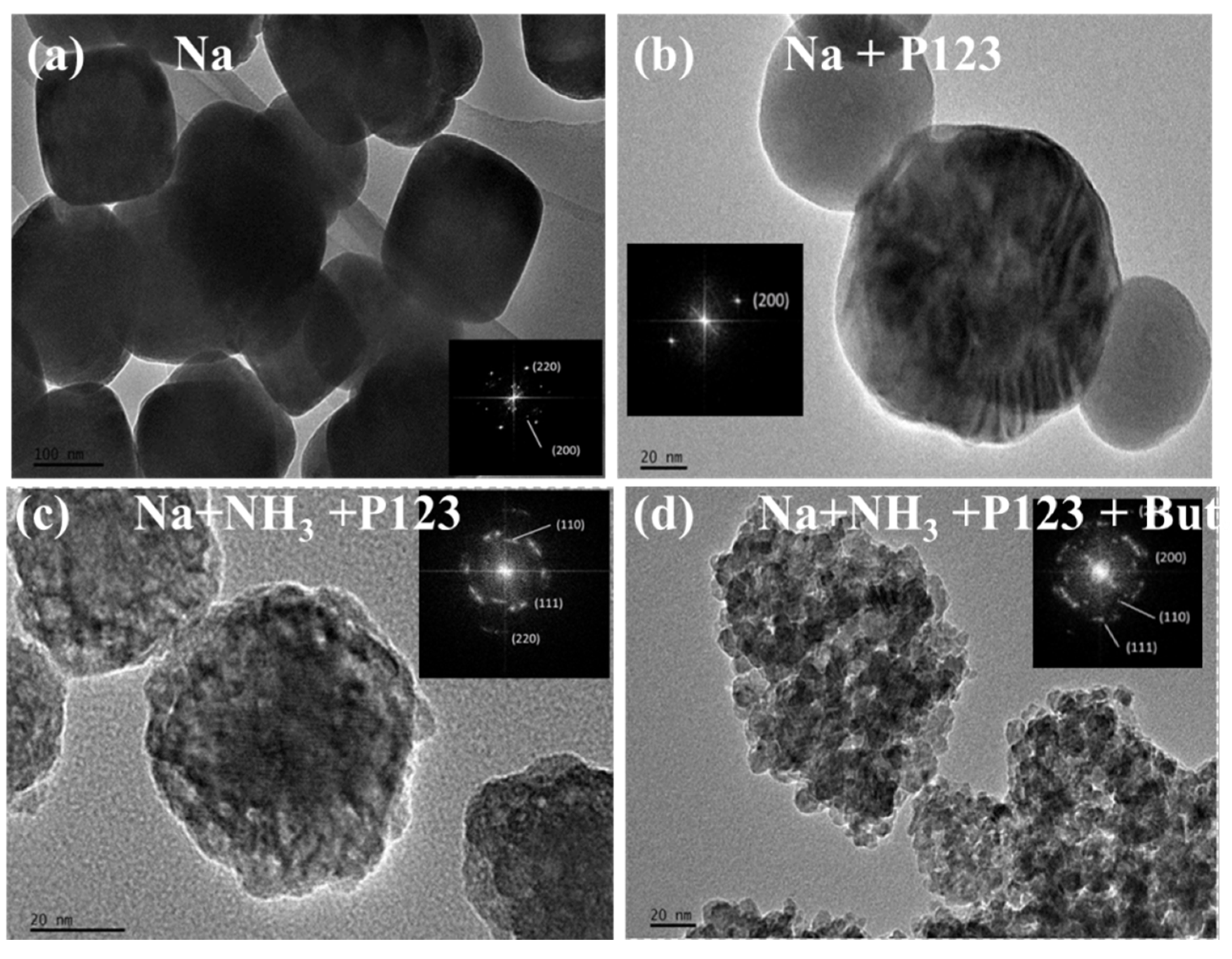


| Catalyst | Alkali | Surfactant | Morphology aa | BET (m2·g−1) | Barrett-Joyner-Halenda (nm) | Crystallite-Size (nm) 1 |
|---|---|---|---|---|---|---|
| -- | NH3 | P123 | No precipitation | |||
| Na | NaOH | -- | Cube | 4.8 | 89 | 45.4 |
| -- | NaOH | N-butanol | Cube2 | 7.4 | 9.6 | 42.8 |
| Na + P123 | NaOH | P123 | Sphere | 13.2 | 1.7 | 34.4 |
| Na + NH3 + P123 | NaOH + NH3 | P123 | Porous sphere | 41.5 | 6.4 | 5.5 |
| Na + NH3 + P123 + But. | NaOH + NH3 | P123 + N-butanol | Amorphous granules | 79.5 | 3.7 | 2.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Chen, J.; Zhao, X.; Ma, G. Synthesis of Highly Porous Cu2O Catalysts for Efficient Ozone Decomposition. Catalysts 2021, 11, 600. https://doi.org/10.3390/catal11050600
Jiang Y, Chen J, Zhao X, Ma G. Synthesis of Highly Porous Cu2O Catalysts for Efficient Ozone Decomposition. Catalysts. 2021; 11(5):600. https://doi.org/10.3390/catal11050600
Chicago/Turabian StyleJiang, Yishan, Juna Chen, Xin Zhao, and Guojun Ma. 2021. "Synthesis of Highly Porous Cu2O Catalysts for Efficient Ozone Decomposition" Catalysts 11, no. 5: 600. https://doi.org/10.3390/catal11050600
APA StyleJiang, Y., Chen, J., Zhao, X., & Ma, G. (2021). Synthesis of Highly Porous Cu2O Catalysts for Efficient Ozone Decomposition. Catalysts, 11(5), 600. https://doi.org/10.3390/catal11050600







