Recovery/Reuse of Heterogeneous Supported Spent Catalysts
Abstract
1. Introduction
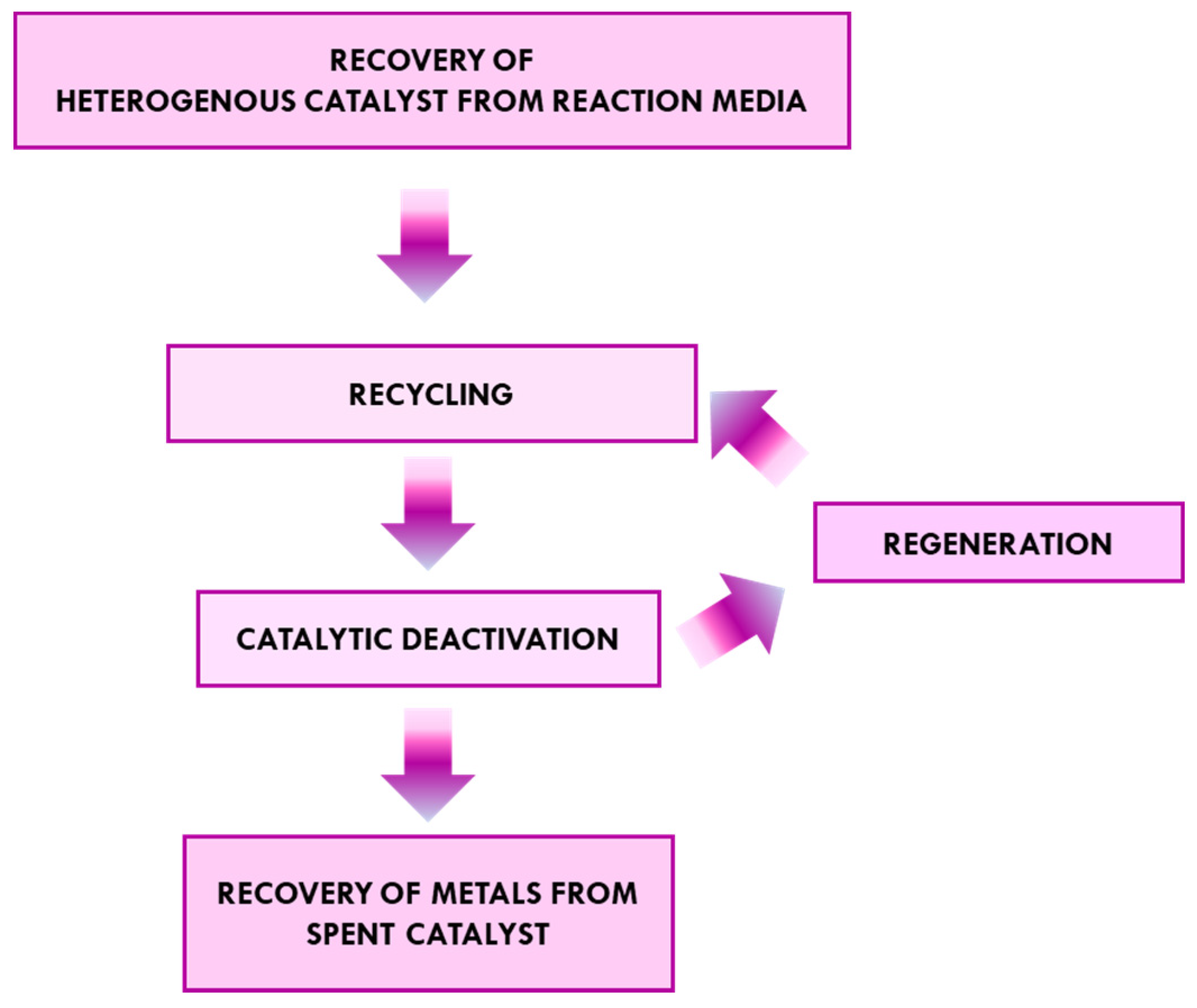
2. The Catalyst’s Life Cycle
3. Methods for Recycling Heterogenous Catalysts
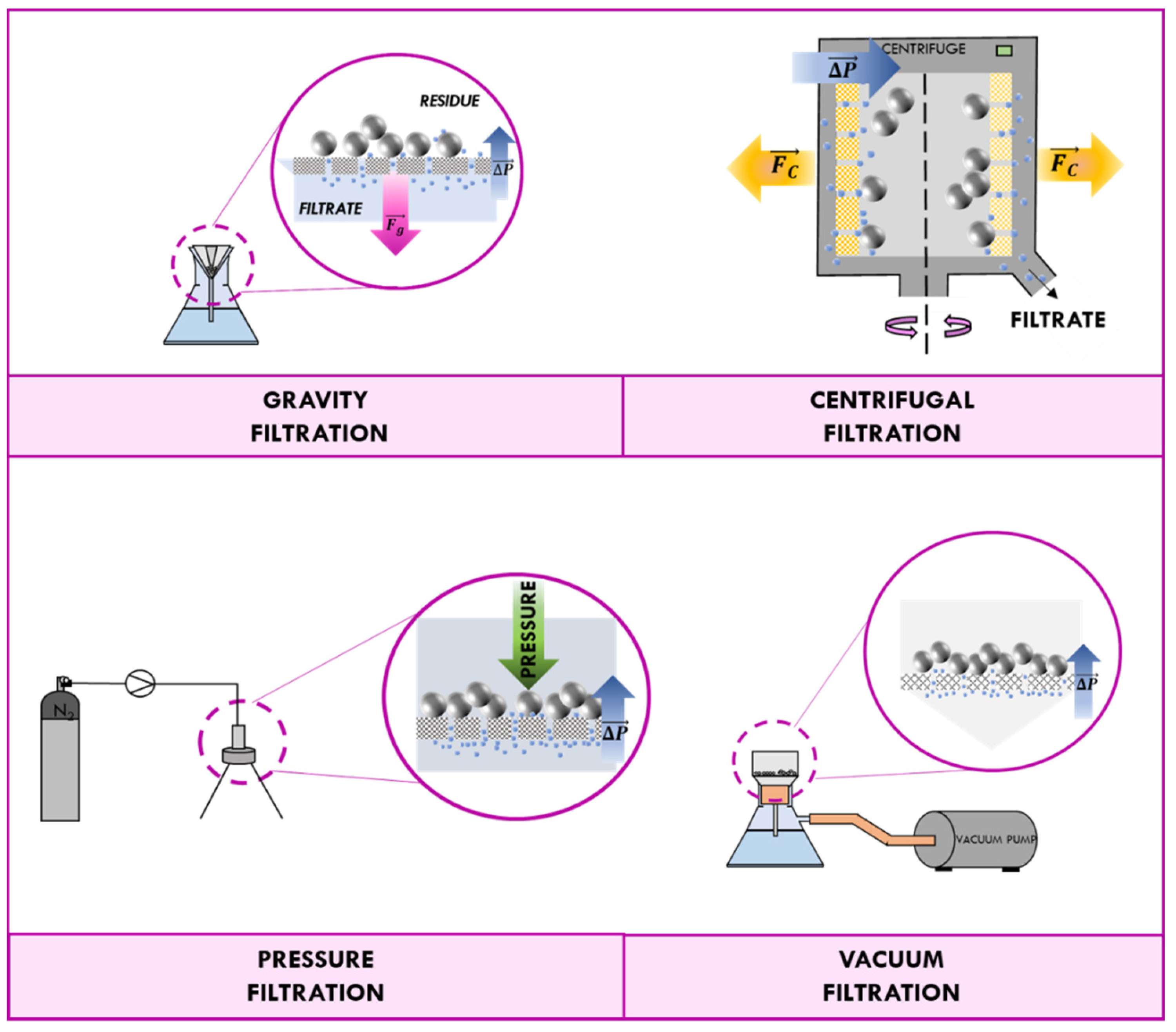
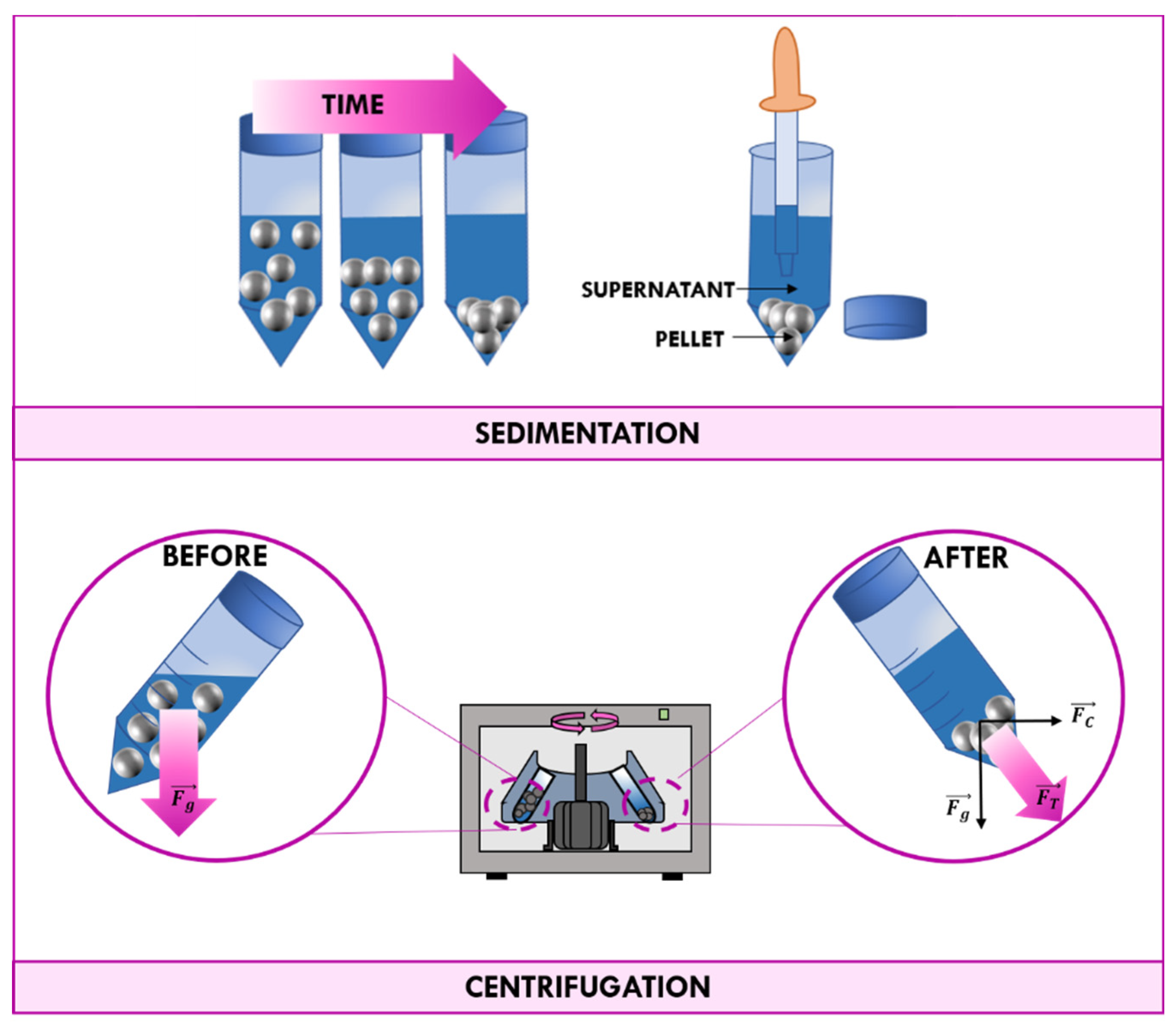
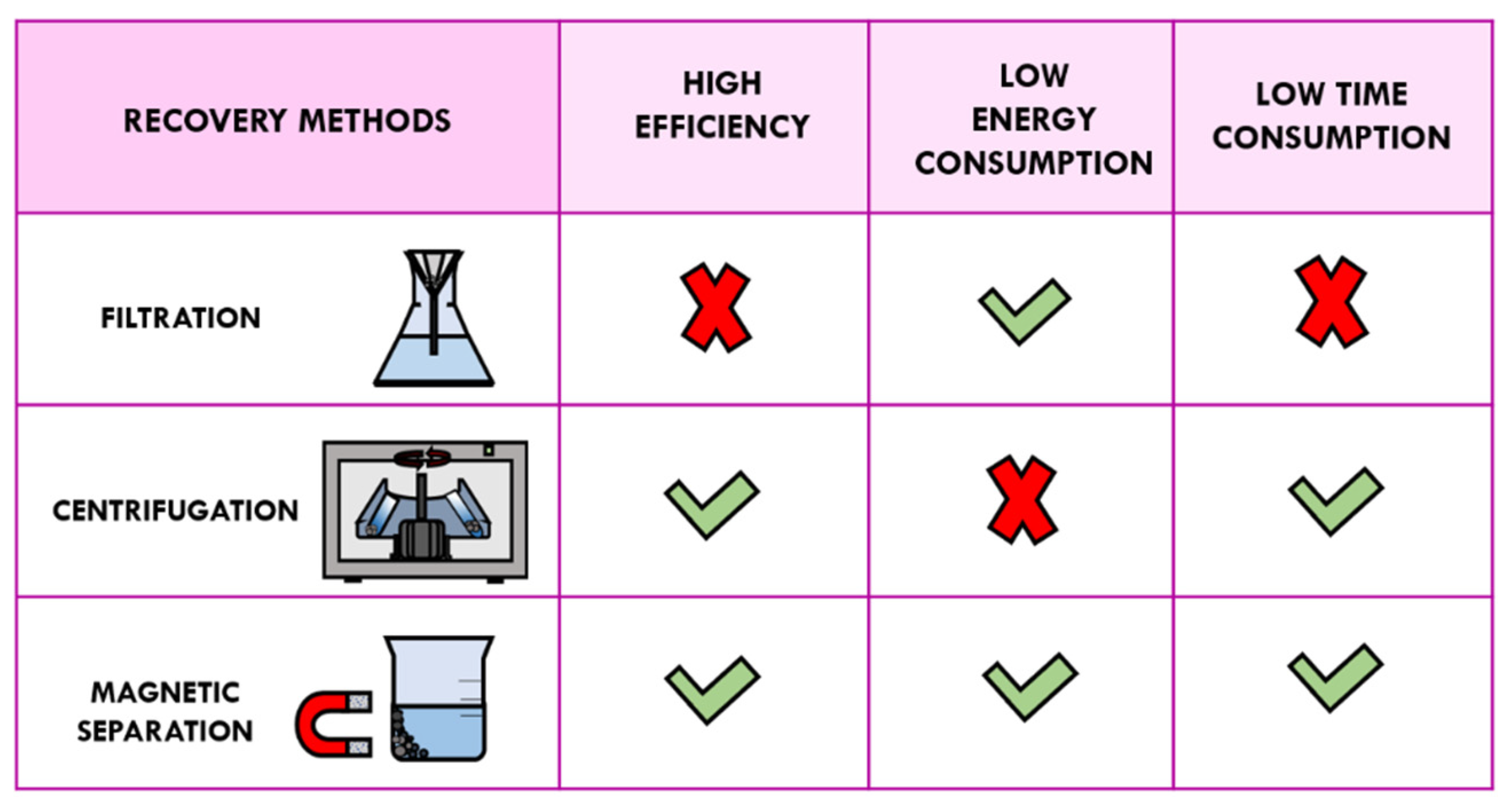
3.1. Traditional Methods of Recovery: Filtration and Centrifugation
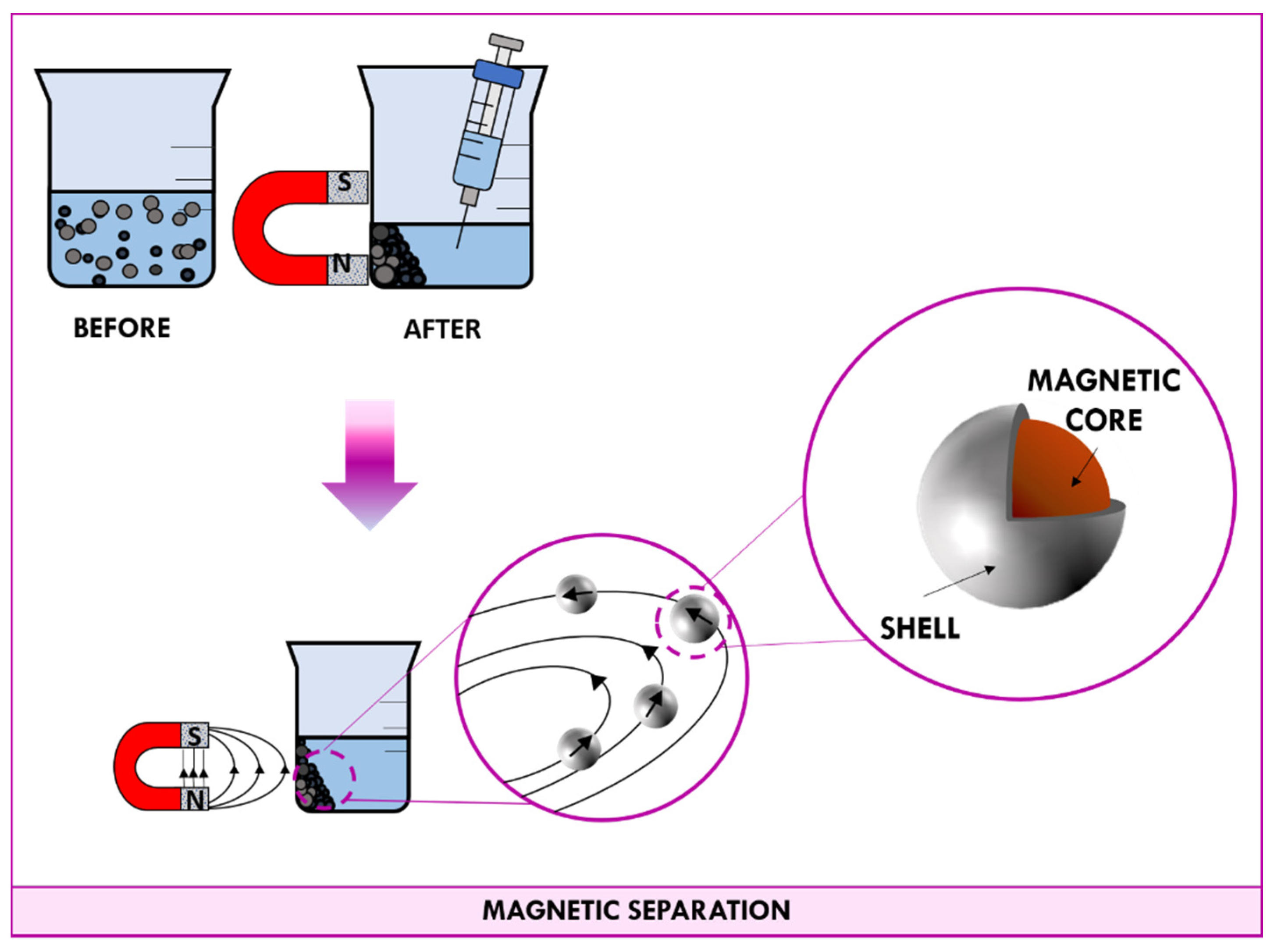
3.2. Magnetic Separation
3.2.1. Magnetic Nanoparticles (MNPs)
3.2.2. Magnetically Recoverable Transition Metal Ferrite Nanoparticles
3.2.3. Magnetically Recoverable Orthoferrites of Rare-Earth Nanoparticles
3.2.4. Surface Modification of Magnetic Nanoparticles
3.2.5. Ionic Liquid Coated Magnetic Nanoparticles
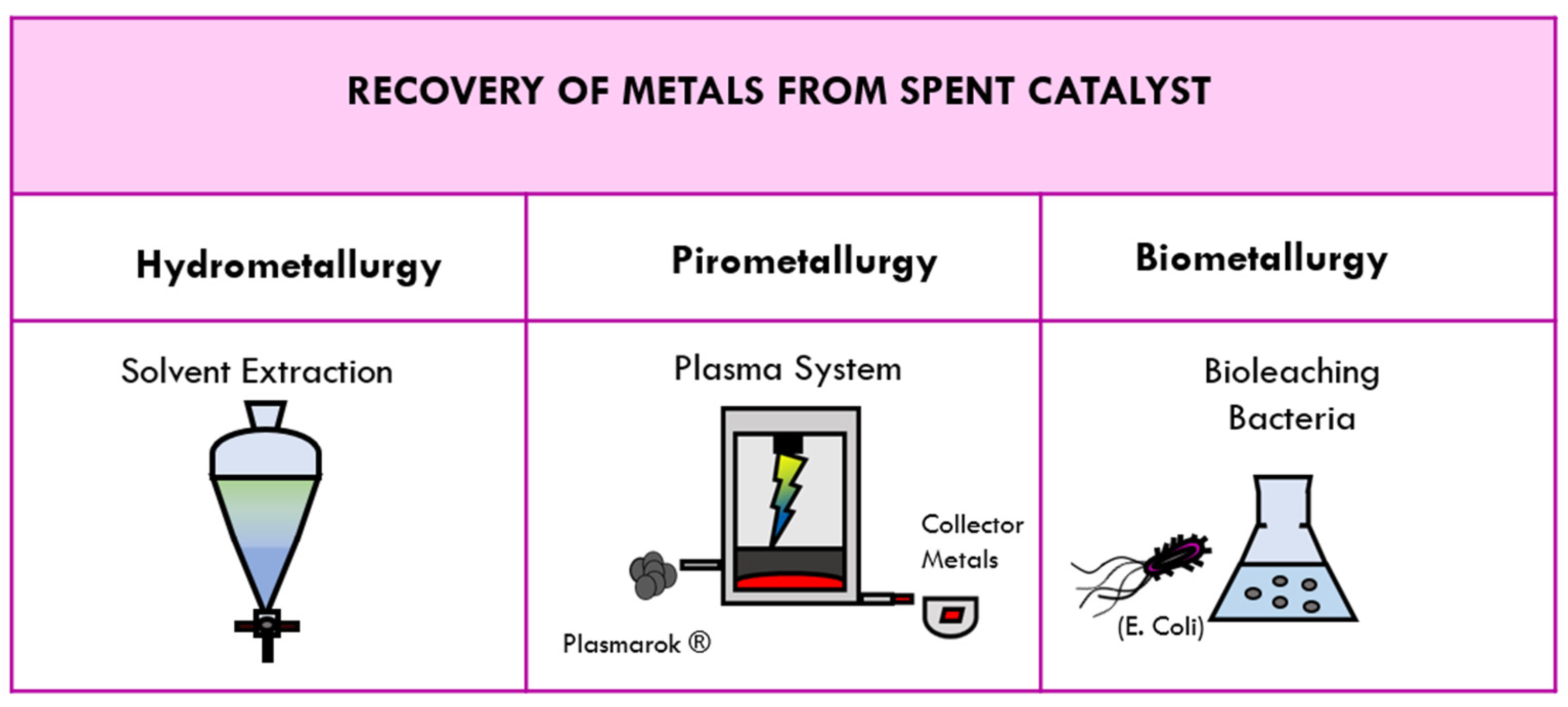
4. Methods for Recovering Metals from Spent Catalysts
4.1. Traditional Methods of Recovery: Hydrometallurgy and Pirometallurgy
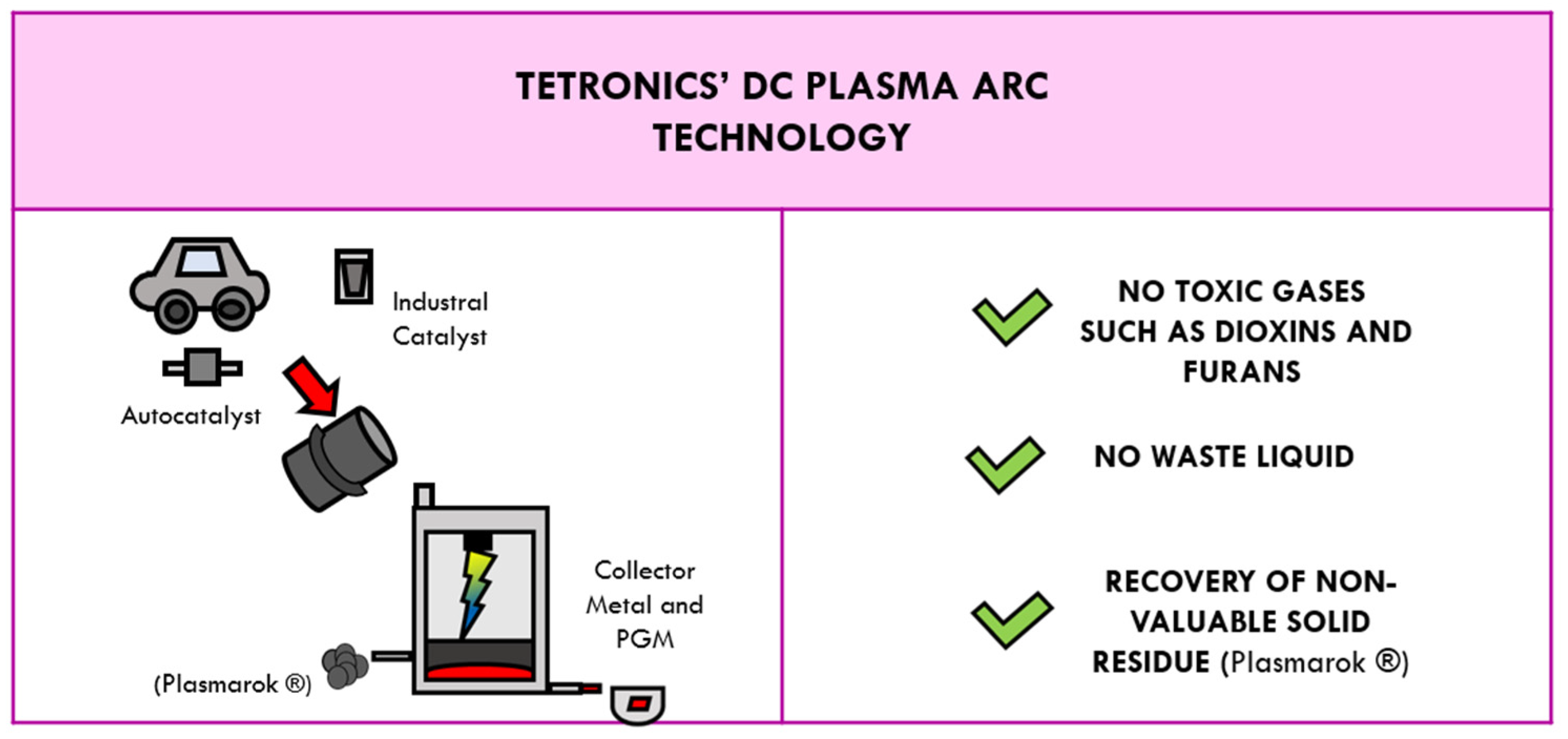
4.2. Tetronics’ DC Plasma Arc Technology
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Parrino, F.; Bellardita, M.; Garcia-Lopez, E.I.; Marci, G.; Loddo, V.; Palmisano, L. Heterogeneous Photocatalysis for Selective Formation of High-Value-Added Molecules: Some Chemical and Engineering Aspects. ACS Catal. 2018, 8, 11191–11225. [Google Scholar] [CrossRef]
- Dosa, M.; Piumetti, M.; Bensaid, S.; Andana, T.; Galletti, C.; Fino, D.; Russo, N. Photocatalytic Abatement of Volatile Organic Compounds by TiO2 Nanoparticles Doped with Either Phosphorous or Zirconium. Materials 2019, 12, 2121. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Padrón, D.; Puente-Santiago, A.R.; Balu, A.M.; Muñoz-Batista, M.J.; Luque, R. Environmental Catalysis: Present and Future. ChemCatChem 2019, 11, 18–38. [Google Scholar] [CrossRef]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef] [PubMed]
- Frontera, P.; Malara, A.; Stelitano, S.; Leonardi, S.G.; Bonavita, A.; Fazio, E.; Antonucci, P.; Neri, G.; Neri, F.; Santangelo, S. Characterisation and H2O2 sensing properties of TiO2-CNTs/Pt electro-catalysts. Mater. Chem. Phys. 2016, 170, 129–137. [Google Scholar] [CrossRef]
- Kisszékelyi, P.; Nagy, S.; Fehér, Z.; Huszthy, P.; Kupai, J. Membrane-Supported Recovery of Homogeneous Organocatalysts: A Review. Chemistry 2020, 2, 48. [Google Scholar] [CrossRef]
- Xu, C.; Paone, E.; Rodríguez-Padrón, D.; Luque, R.; Mauriello, F. Reductive catalytic routes towards sustainable production of hydrogen, fuels and chemicals from biomass derived polyols. Renew. Sustain. Energy Rev. 2020, 127, 109852. [Google Scholar] [CrossRef]
- Malara, A.; Paone, E.; Bonaccorsi, L.; Mauriello, F.; Macario, A.; Frontera, P. Pd/Fe3O4 Nanofibers for the Catalytic Conversion of Lignin-Derived Benzyl Phenyl Ether under Transfer Hydrogenolysis Conditions. Catalysts 2019, 10, 20. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Malara, A.; Santangelo, S.; Triolo, C.; Crea, F.; Antonucci, P. Trimetallic Ni-Based Catalysts over Gadolinia-Doped Ceria for Green Fuel Production. Catalysts 2018, 8, 435. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Malara, A.; Modafferi, V.; Mascolo, M.C.; Candamano, S.; Crea, F.; Antonucci, P. CO2 and CO hydrogenation over Ni-supported materials. Funct. Mater. Lett. 2018, 11, 1850061. [Google Scholar] [CrossRef]
- Frontera, P.; Malara, A.; Modafferi, V.; Antonucci, V.; Antonucci, P.; Macario, A. Catalytic activity of Ni-Co supported metals in carbon dioxides methanation. Can. J. Chem. Eng. 2020, 98, 1924–1934. [Google Scholar] [CrossRef]
- Malara, A.; Frontera, P.; Antonucci, P.; Macario, A. Smart recycling of carbon oxides: Current status of methanation reaction. Curr. Opin. Green Sustain. Chem. 2020, 26, 100376. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Malara, A.; Antonucci, V.; Modafferi, V.; Antonucci, P.L. Simultaneous methanation of carbon oxides on nickel-iron catalysts supported on ceria-doped gadolinia. Catal. Today 2020, 357, 565–572. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P. Supported Catalysts for CO2 Methanation: A Review. Catalysts 2017, 7, 59. [Google Scholar] [CrossRef]
- Boaro, M.; Colussi, S.; Trovarelli, A. Ceria-Based Materials in Hydrogenation and Reforming Reactions for CO2 Valorization. Front. Chem. 2019, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Kesavan, J.K.; Luisetto, I.; Tuti, S.; Meneghini, C.; Iucci, G.; Battocchio, C.; Mobilio, S.; Casciardi, S.; Sisto, R. Nickel supported on YSZ: The effect of Ni particle size on the catalytic activity for CO2 methanation. J. CO2 Util. 2018, 23, 200–211. [Google Scholar] [CrossRef]
- Santos, J.L.; Megías-Sayago, C.; Ivanova, S.; Centeno, M.Á.; Odriozola, J.A. Functionalized biochars as supports for Pd/C catalysts for efficient hydrogen production from formic acid. Appl. Catal. B Environ. 2021, 282, 119615. [Google Scholar] [CrossRef]
- Palliyarayil, A.; Prakash, P.S.; Nandakumar, S.; Kumar, N.S.; Sil, S. Palladium nanoparticles impregnated activated carbon material for catalytic oxidation of carbon monoxide. Diam. Relat. Mater. 2020, 107, 107884. [Google Scholar] [CrossRef]
- Yam, K.M.; Guo, N.; Jiang, Z.; Li, S.; Zhang, C. Graphene-Based Heterogeneous Catalysis: Role of Graphene. Catalysts 2020, 10, 53. [Google Scholar] [CrossRef]
- Paone, E.; Beneduci, A.; Corrente, G.; Malara, A.; Mauriello, F. Hydrogenolysis of aromatic ethers under lignin-first conditions. Mol. Catal. 2020, 497, 111228. [Google Scholar] [CrossRef]
- Gumina, B.; Mauriello, F.; Pietropaolo, R.; Galvagno, S.; Espro, C. Hydrogenolysis of sorbitol into valuable C3-C2 alcohols at low H2 pressure promoted by the heterogeneous Pd/Fe3O4 catalyst. Mol. Catal. 2018, 446, 152–160. [Google Scholar] [CrossRef]
- Santos, J.L.; Mäki-Arvela, P.; Monzón, A.; Murzin, D.Y.; Centeno, M. Ángel Metal catalysts supported on biochars: Part I synthesis and characterization. Appl. Catal. B Environ. 2020, 268, 118423. [Google Scholar] [CrossRef]
- Ren, Y.; Li, H.; Yang, W.; Shi, D.; Wu, Q.; Zhao, Y.; Feng, C.; Liu, H.; Jiao, Q. Alkaline Ionic Liquids Immobilized on Protective Copolymers Coated Magnetic Nanoparticles: An Efficient and Magnetically Recyclable Catalyst for Knoevenagel Condensation. Ind. Eng. Chem. Res. 2019, 58, 2824–2834. [Google Scholar] [CrossRef]
- Wang, D.; Astruc, D. Fast-Growing Field of Magnetically Recyclable Nanocatalysts. Chem. Rev. 2014, 114, 6949–6985. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, G. Catalysis: Concepts and Green Applications; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Baig, R.B.N.; Varma, R.S. Magnetically retrievable catalysts for organic synthesis. Chem. Commun. 2013, 49, 752–770. [Google Scholar] [CrossRef] [PubMed]
- Vono, L.L.R.; Damasceno, C.C.; Matos, J.R.; Jardim, R.F.; Landers, R.; Masunaga, S.H.; Rossi, L.M. Separation technology meets green chemistry: Development of magnetically recoverable catalyst supports containing silica, ceria, and titania. Pure Appl. Chem. 2018, 90, 133–141. [Google Scholar] [CrossRef]
- Sádaba, I.; Granados, M.L.; Riisager, A.; Taarning, E. Deactivation of solid catalysts in liquid media: The case of leaching of active sites in biomass conversion reactions. Green Chem. 2015, 17, 4133–4145. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef]
- Chiranjeevi, T.; Pragya, R.; Gupta, S.; Gokak, D.; Bhargava, S. Minimization of Waste Spent Catalyst in Refineries. Procedia Environ. Sci. 2016, 35, 610–617. [Google Scholar] [CrossRef]
- Molnár, Á.; Papp, A. Catalyst recycling—A survey of recent progress and current status. Coord. Chem. Rev. 2017, 349, 1–65. [Google Scholar] [CrossRef]
- Ripperger, S.; Gösele, W.; Alt, C.; Loewe, T. Filtration, 1. Fundamentals. Ullmann’s Encycl. Ind. Chem. 2013, 1–38. [Google Scholar] [CrossRef]
- Amirsoleimani, M.; Khalilzadeh, M.A.; Zareyee, D. Preparation and catalytic evaluation of a palladium catalyst deposited over modified clinoptilolite (Pd@MCP) for chemoselective N-formylation and N-acylation of amines. J. Mol. Struct. 2021, 1225, 129076. [Google Scholar] [CrossRef]
- Majekodunmi, S.O. A review on centrifugation in the pharmaceutical industry. Am. J. Biomed. Eng. 2015, 5, 67–78. [Google Scholar]
- Nasresfahani, Z.; Kassaee, M.Z. Nickel−Copper bimetallic mesoporous nanoparticles: As an efficient heterogeneous catalyst for N -alkylation of amines with alcohols. Appl. Organomet. Chem. 2021, 35, e6032. [Google Scholar] [CrossRef]
- Li, H.; Ma, Y. Magnetically separable Fe3O4-supported Ru–Ni bimetallic catalysts for diformyltricyclodecanes hydrogenation to value-added fine chemicals. Prog. React. Kinet. Mech. 2020, 45. [Google Scholar] [CrossRef]
- Wang, A.; Sudarsanam, P.; Xu, Y.; Zhang, H.; Li, H.; Yang, S. Functionalized magnetic nanosized materials for efficient biodiesel synthesis via acid–base/enzyme catalysis. Green Chem. 2020, 22, 2977–3012. [Google Scholar] [CrossRef]
- Li, F.; Zhu, W.; Jiang, S.; Wang, Y.; Song, H.; Li, C. Catalytic transfer hydrogenation of furfural to furfuryl alcohol over Fe3O4 modified Ru/Carbon nanotubes catalysts. Int. J. Hydrog. Energy 2020, 45, 1981–1990. [Google Scholar] [CrossRef]
- Alaei, S.; Haghighi, M.; Toghiani, J.; Vahid, B.R. Magnetic and reusable MgO/MgFe2O4 nanocatalyst for biodiesel production from sunflower oil: Influence of fuel ratio in combustion synthesis on catalytic properties and performance. Ind. Crop. Prod. 2018, 117, 322–332. [Google Scholar] [CrossRef]
- Martinson, K.; Kondrashkova, I.; Omarov, S.; Sladkovskiy, D.; Kiselev, A.; Kiseleva, T.; Popkov, V. Magnetically recoverable catalyst based on porous nanocrystalline HoFeO3 for processes of n-hexane conversion. Adv. Powder Technol. 2020, 31, 402–408. [Google Scholar] [CrossRef]
- Wahajuddin, A.S. Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. Int. J. Nanomed. 2012, 7, 3445–3471. [Google Scholar] [CrossRef]
- Kneller, E.F.; Luborsky, F.E. Particle Size Dependence of Coercivity and Remanence of Single-Domain Particles. J. Appl. Phys. 1963, 34, 656–658. [Google Scholar] [CrossRef]
- Kooti, M.; Nasiri, E. Synthesis of a novel magnetic nanocatalyst based on rhodium complex for transfer hydrogenation of ketone. Appl. Organomet. Chem. 2019, 33, e4886. [Google Scholar] [CrossRef]
- Karimi-Chayjani, R.; Daneshvar, N.; Shirini, F.; Tajik, H. New magnetic nanocatalyst containing a bis-dicationic ionic liquid framework for Knoevenagel condensation reaction. Res. Chem. Intermed. 2019, 45, 2471–2488. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Carregal-Romero, S.; Casula, M.F.; Gutiérrez, L.; Morales, M.P.; Böhm, I.B.; Heverhagen, J.T.; Prosperi, D.; Parak, W.J. Biological applications of magnetic nanoparticles. Chem. Soc. Rev. 2012, 41, 4306–4334. [Google Scholar] [CrossRef] [PubMed]
- Sasikala, A.R.K.; GhavamiNejad, A.; Unnithan, A.R.; Thomas, R.G.; Moon, M.; Jeong, Y.Y.; Park, C.H.; Kim, C.S. A smart magnetic nanoplatform for synergistic anticancer therapy: Manoeuvring mussel-inspired functional magnetic nanoparticles for pH responsive anticancer drug delivery and hyperthermia. Nanoscale 2015, 7, 18119–18128. [Google Scholar] [CrossRef] [PubMed]
- SJiang, S.; Eltoukhy, A.A.; Love, K.T.; Langer, R.; Anderson, D.G. Lipidoid-Coated Iron Oxide Nanoparticles for Efficient DNA and siRNA delivery. Nano Lett. 2013, 13, 1059–1064. [Google Scholar] [CrossRef]
- Zhai, Q.; Du, B.; Feng, R.; Xu, W.; Wei, Q. A highly sensitive gas sensor based on Pd-doped Fe3O4nanoparticles for volatile organic compounds detection. Anal. Methods 2014, 6, 886–892. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, T.; Yang, X.; Zhang, L.; Leng, K.; Huang, Y.; Chen, Y. A high-performance supercapacitor-battery hybrid energy storage device based on graphene-enhanced electrode materials with ultrahigh energy density. Energy Environ. Sci. 2013, 6, 1623–1632. [Google Scholar] [CrossRef]
- Chen, S.; Bai, B.; He, Y.; Hu, N.; Wang, H.; Suo, Y. Controllable conversion of Prussian blue@yeast bio-template into 3D cage-like magnetic Fe3O4@N-doped carbon absorbent and its cohesive regeneration by persulfate activation. RSC Adv. 2019, 9, 1151–1164. [Google Scholar] [CrossRef]
- Malara, A.; Pantò, F.; Santangelo, S.; Antonucci, P.L.; Fiore, M.; Longoni, G.; Ruffo, R.; Frontera, P. Comparative life cycle assessment of Fe2O3-based fibers as anode materials for sodium-ion batteries. Environ. Dev. Sustain. 2020, 23, 6786–6799. [Google Scholar] [CrossRef]
- Wang, Z.; Zhai, S.; Lv, J.; Qi, H.; Zheng, W.; Zhai, B.; An, Q. Versatile hierarchical Cu/Fe3O4 nanocatalysts for efficient degradation of organic dyes prepared by a facile, controllable hydrothermal method. RSC Adv. 2015, 5, 74575–74584. [Google Scholar] [CrossRef]
- Abdullah, N.H.; Shameli, K.; Abdullah, E.C.; Abdullah, L.C. Solid matrices for fabrication of magnetic iron oxide nanocomposites: Synthesis, properties, and application for the adsorption of heavy metal ions and dyes. Compos. Part B Eng. 2019, 162, 538–568. [Google Scholar] [CrossRef]
- Silva, V.A.J.; Andrade, P.L.; Silva, M.P.C.; Valladares, L.D.L.S.; Aguiar, J.A. Synthesis and characterization of Fe3O4 nanoparticles coated with fucan polysaccharides. J. Magn. Magn. Mater. 2013, 343, 138–143. [Google Scholar] [CrossRef]
- Lian, S.; Kang, Z.; Wang, E.; Jiang, M.; Hu, C.; Xu, L. Convenient synthesis of single crystalline magnetic Fe3O4 nanorods. Solid State Commun. 2003, 127, 605–608. [Google Scholar] [CrossRef]
- Neoh, K.G.; Kang, E.T. Surface modification of magnetic nanoparticles for stem celllabeling. Soft Matter 2011, 8, 2057–2069. [Google Scholar] [CrossRef]
- Zhu, N.; Ji, H.; Yu, P.; Niu, J.; Farooq, M.U.; Akram, M.W.; Udego, I.O.; Li, H.; Niu, X. Surface Modification of Magnetic Iron Oxide Nanoparticles. Nanomaterials 2018, 8, 810. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liang, B.; Li, L.; Yang, X.; Huang, Y.; Wang, A.; Wang, X.; Zhang, T. Cerium-Oxide-Modified Nickel as a Non-Noble Metal Catalyst for Selective Decomposition of Hydrous Hydrazine to Hydrogen. ACS Catal. 2015, 5, 1623–1628. [Google Scholar] [CrossRef]
- Akbayrak, S.; Taneroğlu, O.; Özkar, S. Nanoceria supported cobalt(0) nanoparticles: A magnetically separable and reusable catalyst in hydrogen generation from the hydrolysis of ammonia borane. New J. Chem. 2017, 41, 6546–6552. [Google Scholar] [CrossRef]
- Ali, A.; Hira Zafar, M.Z.; ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef]
- Vahidian, M.; Elhamifar, D.; Shaker, M. Protected, Email Core–shell structured magnetic mesoporous silica-titania: A novel, powerful and recoverable nanocatalyst. Polyhedron 2020, 178, 114326. [Google Scholar] [CrossRef]
- Hamad, H.A.; El-Latif, M.M.A.; Kashyout, A.B.; Sadik, W.A.; Feteha, M.Y. Study on synthesis of superparamagnetic spinel cobalt ferrite nanoparticles as layered double hydroxides by co-precipitation method. Russ. J. Gen. Chem. 2014, 84, 2205–2210. [Google Scholar] [CrossRef]
- Amiri, M.; Eskandari, K.; Salavati-Niasari, M. Magnetically retrievable ferrite nanoparticles in the catalysis application. Adv. Colloid Interface Sci. 2019, 271, 101982. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.M.; Elkatory, M.R.; Hamad, H.A. Highly active and stable magnetically recyclable CuFe2O4 as a heterogenous catalyst for efficient conversion of waste frying oil to biodiesel. Fuel 2020, 268, 117297. [Google Scholar] [CrossRef]
- Sun, X.; Yuan, K.; Zhang, Y. Advances and prospects of rare earth metal-organic frameworks in catalytic applications. J. Rare Earths 2020, 38, 801–818. [Google Scholar] [CrossRef]
- Jyothi, R.K.; Thenepalli, T.; Ahn, J.W.; Parhi, P.K.; Chung, K.W.; Lee, J.-Y. Review of rare earth elements recovery from secondary resources for clean energy technologies: Grand opportunities to create wealth from waste. J. Clean. Prod. 2020, 267, 122048. [Google Scholar] [CrossRef]
- Zeng, Z.; Xu, Y.; Zhang, Z.; Gao, Z.; Luo, M.; Yin, Z.; Zhang, C.; Xu, J.; Huang, B.; Luo, F.; et al. Rare-earth-containing perovskite nanomaterials: Design, synthesis, properties and applications. Chem. Soc. Rev. 2020, 49, 1109–1143. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, L.; Arena, F.; Granados, M.L.; Ojeda, M.; Fierro, J.; Frusteri, F. Metal–support interactions and reactivity of Co/CeO2 catalysts in the Fischer–Tropsch synthesis reaction. J. Catal. 2005, 234, 451–462. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Monforte, G.; Bonura, G.; Ferraro, M.; Dispenza, G.; Antonucci, V.; Aricò, A.; Antonucci, P. The role of Gadolinia Doped Ceria support on the promotion of CO2 methanation over Ni and Ni Fe catalysts. Int. J. Hydrog. Energy 2017, 42, 26828–26842. [Google Scholar] [CrossRef]
- Xie, W.; Wang, H. Immobilized polymeric sulfonated ionic liquid on core-shell structured Fe3O4/SiO2 composites: A magnetically recyclable catalyst for simultaneous transesterification and esterifications of low-cost oils to biodiesel. Renew. Energy 2020, 145, 1709–1719. [Google Scholar] [CrossRef]
- Shifrina, Z.B.; Bronstein, L.M. Magnetically Recoverable Catalysts: Beyond Magnetic Separation. Front. Chem. 2018, 6, 298. [Google Scholar] [CrossRef]
- Ratti, R. Ionic Liquids: Synthesis and Applications in Catalysis. Adv. Chem. 2014, 2014, 729842. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Zhuang, Q.; Zhang, M.; Wang, H.; Gao, Z.; Sun, J.-K.; Yuan, J. Poly(ionic liquid) composites. Chem. Soc. Rev. 2020, 49, 1726–1755. [Google Scholar] [CrossRef] [PubMed]
- Sorkhi, S.E.S.; Hashemi, M.M.; Ezabadi, A. Introduction of a novel dicationic Brönsted acidic ionic liquid based on pyrazine and its application in the synthesis of xanthenediones and 3, 4-dihydropyrimidin-2(1H)-ones under solvent-free conditions. Res. Chem. Intermed. 2020, 46, 2229–2246. [Google Scholar] [CrossRef]
- Cano, I.; Martin, C.; Fernandes, J.A.; Lodge, R.W.; Dupont, J.; Casado-Carmona, F.A.; Lucena, R.; Cardenas, S.; Sans, V.; de Pedro, I. Paramagnetic ionic liquid-coated SiO2@Fe3O4 nanoparticles—The next generation of magnetically recoverable nanocatalysts applied in the glycolysis of PET. Appl. Catal. B Environ. 2020, 260, 118110. [Google Scholar] [CrossRef]
- Campisciano, V.; Giacalone, F.; Gruttadauria, M. Supported Ionic Liquids: A Versatile and Useful Class of Materials. Chem. Rec. 2017, 17, 918–938. [Google Scholar] [CrossRef]
- Arghan, M.; Koukabi, N.; Kolvari, E. Sulfonated-polyvinyl amine coated on Fe3O4 nanoparticles: A high-loaded and magnetically separable acid catalyst for multicomponent reactions. J. Iran. Chem. Soc. 2019, 16, 2333–2350. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Hosseini, S.H.; Aghayeemeibody, S.A.; Hosseini, S.T. Poly(basic ionic liquid) coated magnetic nanoparticles: High-loaded supported basic ionic liquid catalyst. Comptes Rendus Chim. 2013, 16, 906–911. [Google Scholar] [CrossRef]
- Rezaei, F.; Amrollahi, M.A.; Khalifeh, R. Brønsted Acidic Dicationic Ionic Liquid Immobilized on Fe3O4/SiO2 Nanoparticles as an Efficient and Magnetically Separable Catalyst for the Synthesis of Bispyrazoles. ChemistrySelect 2020, 5, 1760–1766. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, C.; Wan, H.; Wang, L.; Li, Z.; Li, B.; Guo, Q.; Guan, G. Fabrication of Magnetic NH2-MIL-88B (Fe) Confined Brønsted Ionic Liquid as an Efficient Catalyst in Biodiesel Synthesis. Energy Fuels 2016, 30, 10739–10746. [Google Scholar] [CrossRef]
- Xie, W.; Wang, H. Synthesis of heterogenized polyoxometalate-based ionic liquids with Brönsted-Lewis acid sites: A magnetically recyclable catalyst for biodiesel production from low-quality oils. J. Ind. Eng. Chem. 2020, 87, 162–172. [Google Scholar] [CrossRef]
- Fornalczyk, A. Industrial catalysts as a source of valuable metals. JAMME 2012, 55, 864–868. [Google Scholar]
- Eijsbouts, S.; Battiston, A.A.; Van Leerdam, G.C. Life cycle of hydroprocessing catalysts and total catalyst management. Catal. Today 2008, 130, 361–373. [Google Scholar] [CrossRef]
- Innocenzi, V.; Ippolito, N.M.; De Michelis, I.; Prisciandaro, M.; Medici, F.; Vegliò, F. A review of the processes and lab-scale techniques for the treatment of spent rechargeable NiMH batteries. J. Power Sources 2017, 362, 202–218. [Google Scholar] [CrossRef]
- Hu, J.; Wang, X.; Xiao, L.; Song, S.; Zhang, B. Removal of vanadium from molybdate solution by ion exchange. Hydrometallurgy 2009, 95, 203–206. [Google Scholar] [CrossRef]
- Cai, Y.; Ma, L.; Xi, X.; Nie, Z.; Nie, Z. Separation of tungsten and molybdenum using selective precipitation with manganese sulfate assisted by cetyltrimethyl ammonium bromide (CTAB). Hydrometallurgy 2020, 198, 105494. [Google Scholar] [CrossRef]
- Wu, W.-C.; Tsai, T.-Y.; Shen, Y.-H. Tungsten Recovery from Spent SCR Catalyst Using Alkaline Leaching and Ion Exchange. Minerals 2016, 6, 107. [Google Scholar] [CrossRef]
- Changming, D.; Chao, S.; Gong, X.; Ting, W.; Xiange, W. Plasma methods for metals recovery from metal–containing waste. Waste Manag. 2018, 77, 373–387. [Google Scholar] [CrossRef]
- Marafi, M.; Rana, M.S. Refinery waste: The spent hydroprocessing catalyst and its recycling options. WIT Trans. Ecol. Environ. 2016, 202, 219–230. [Google Scholar]
- Wiecka, Z.; Rzelewska-Piekut, M.; Cierpiszewski, R.; Staszak, K.; Regel-Rosocka, M. Hydrometallurgical Recovery of Cobalt(II) from Spent Industrial Catalysts. Catalyst 2020, 10, 61. [Google Scholar] [CrossRef]
- Amiri, F.; Yaghmaei, S.; Mousavi, S.; Sheibani, S. Recovery of metals from spent refinery hydrocracking catalyst using adapted Aspergillus niger. Hydrometallurgy 2011, 109, 65–71. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miceli, M.; Frontera, P.; Macario, A.; Malara, A. Recovery/Reuse of Heterogeneous Supported Spent Catalysts. Catalysts 2021, 11, 591. https://doi.org/10.3390/catal11050591
Miceli M, Frontera P, Macario A, Malara A. Recovery/Reuse of Heterogeneous Supported Spent Catalysts. Catalysts. 2021; 11(5):591. https://doi.org/10.3390/catal11050591
Chicago/Turabian StyleMiceli, Mariachiara, Patrizia Frontera, Anastasia Macario, and Angela Malara. 2021. "Recovery/Reuse of Heterogeneous Supported Spent Catalysts" Catalysts 11, no. 5: 591. https://doi.org/10.3390/catal11050591
APA StyleMiceli, M., Frontera, P., Macario, A., & Malara, A. (2021). Recovery/Reuse of Heterogeneous Supported Spent Catalysts. Catalysts, 11(5), 591. https://doi.org/10.3390/catal11050591







