Facile Redox Synthesis of Novel Bimetallic Crn+/Pd0 Nanoparticles Supported on SiO2 and TiO2 for Catalytic Selective Hydrogenation with Molecular Hydrogen
Abstract
1. Introduction
2. Results and Discussion
- Preliminary H2 adsorption from a gas flow on a supported Pd nanoparticle
- Subsequent redox reaction in aqueous slurry
- Permanent H2 adsorption in an aqueous slurry on a supported Pd nanoparticle
- Simultaneous redox reaction in slurry
- Preliminary CrO42− chemisorption from solution in the slurry
- Permanent H2 adsorption in an aqueous slurry on supported Pd nanoparticle
- Simultaneous reduction in aqueous slurry
2.1. Elemental Composition, Nanoparticle Size and Morphology
2.2. TPR-H2 Experiments
2.3. Catalytic Activity
3. Experimental
3.1. Catalyst Synthesis
3.2. Catalyst Characterization
3.3. Catalytic Activity Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arnold, H.; Döbert, F.; Gaube, J. Hydrogenation reactions. In Handbook of Heterogeneous Catalysis, 2nd ed.; Ertl, G., Knözinger, H., Schüth, F., Weitkamp, J., Eds.; Wiley-VCH Verlag GmbH&Co. KGaA: Weinheim, Germany, 2008; Volume 7, pp. 3266–3359. [Google Scholar]
- Nasrollahzadeh, M.; Sajjadi, M.; Shokouhimehr, M.; Varma, R.S. Recent Developments in Palladium (Nano)Catalysts Supported on Polymers for Selective and Sustainable Oxidation Processes. Coord. Chem. Rev. 2019, 397, 54–75. [Google Scholar] [CrossRef]
- Trzeciak, A.M.; Augustyniak, A.W. The Role of Palladium Nanoparticles in Catalytic C–C Cross-Coupling Reactions. Coord. Chem. Rev. 2019, 384, 1–20. [Google Scholar] [CrossRef]
- Alonso, D.; Baeza, A.; Chinchilla, R.; Gómez, C.; Guillena, G.; Pastor, I.; Ramón, D. Solid-Supported Palladium Catalysts in Sonogashira Reactions: Recent Developments. Catalysts 2018, 8, 202. [Google Scholar] [CrossRef]
- Xu, X.; Shuai, K.; Xu, B. Review on Copper and Palladium Based Catalysts for Methanol Steam Reforming to Produce Hydrogen. Catalysts 2017, 7, 183. [Google Scholar] [CrossRef]
- Louis, C.; Delannoy, L. Selective hydrogenation of polyunsaturated hydrocarbons and unsaturated aldehydes over bimetallic catalysts. In Advances in Catalysis; Elsevier: Amsterdam, The Netherlands, 2019; Volume 64, pp. 1–88. ISBN 978-0-12-817099-1. [Google Scholar]
- Dasgupta, A.; Rioux, R.M. Intermetallics in Catalysis: An Exciting Subset of Multimetallic Catalysts. Catal. Today 2019, 330, 2–15. [Google Scholar] [CrossRef]
- Marakatti, V.S.; Peter, S.C. Synthetically Tuned Electronic and Geometrical Properties of Intermetallic Compounds as Effective Heterogeneous Catalysts. Prog. Solid State Chem. 2018, 52, 1–30. [Google Scholar] [CrossRef]
- Coq, B.; Figueras, F. Bimetallic Palladium Catalysts: Influence of the Co-Metal on the Catalyst Performance. J. Mol. Catal. Chem. 2001, 173, 117–134. [Google Scholar] [CrossRef]
- Mashkovsky, I.S.; Markov, P.V.; Bragina, G.O.; Baeva, G.N.; Rassolov, A.V.; Bukhtiyarov, A.V.; Prosvirin, I.P.; Bukhtiyarov, V.I.; Stakheev, A.Y. PdZn/α-Al2O3 Catalyst for Liquid-Phase Alkyne Hydrogenation: Effect of the Solid-State Alloy Transformation into Intermetallics. Mendeleev Commun. 2018, 28, 152–154. [Google Scholar] [CrossRef]
- Mashkovsky, I.S.; Smirnova, N.S.; Markov, P.V.; Baeva, G.N.; Bragina, G.O.; Bukhtiyarov, A.V.; Prosvirin, I.P.; Stakheev, A.Y. Tuning the Surface Structure and Catalytic Performance of PdIn/Al2O3 in Selective Liquid-Phase Hydrogenation by Mild Oxidative-Reductive Treatments. Mendeleev Commun. 2018, 28, 603–605. [Google Scholar] [CrossRef]
- Pinna, F.; Selva, M.; Signoretto, M.; Strukul, G.; Boccuzzi, F.; Benedetti, A.; Canton, P.; Fagherazzi, G. Pd-Fe/SiO2 Catalysts in the Hydrogenation of 2,4-Dinitrotoluene. J. Catal. 1994, 150, 356–367. [Google Scholar] [CrossRef]
- Juszczyk, W.; Pielaszek, J.; Karpiński, Z.; Pinna, F. Reaction of 2,2-Dimethylpropane with Dihydrogen over Silica-Supported PdFe Catalysts. Appl. Catal. Gen. 1996, 144, 281–291. [Google Scholar] [CrossRef]
- Yang, J.; Li, S.; Zhang, L.; Liu, X.; Wang, J.; Pan, X.; Li, N.; Wang, A.; Cong, Y.; Wang, X.; et al. Hydrodeoxygenation of Furans over Pd-FeOx/SiO2 Catalyst under Atmospheric Pressure. Appl. Catal. B Environ. 2017, 201, 266–277. [Google Scholar] [CrossRef]
- Redina, E.A.; Kirichenko, O.A.; Shesterkina, A.A.; Kustov, L.M. Unusual Behavior of Bimetallic Nanoparticles in Catalytic Processes of Hydrogenation and Selective Oxidation. Pure Appl. Chem. 2020, 92, 989–1006. [Google Scholar] [CrossRef]
- Shesterkina, A.A.; Kustov, L.M.; Strekalova, A.A.; Kazansky, V.B. Heterogeneous Iron-Containing Nanocatalysts—Promising Systems for Selective Hydrogenation and Hydrogenolysis. Catal. Sci. Technol. 2020, 10, 3160–3174. [Google Scholar] [CrossRef]
- Ouchaib, T.; Moraweck, B.; Massardier, J.; Renouprez, A. Charcoal Supported Palladium and Palladium-Chromium Catalysts: A Comparison in the Hydrogenation of Dienes with Silica Supported Metals. Catal. Today 1990, 7, 191–198. [Google Scholar] [CrossRef]
- Borgna, A. New Supported Palladium-Chromium Catalysts: Characterization and Catalytic Properties. J. Catal. 1991, 128, 99–112. [Google Scholar] [CrossRef]
- Hu, L.; Xia, G.; Qu, L.; Li, M.; Li, C.; Xin, Q.; Li, D. The Effect of Chromium on Sulfur Resistance of Pd/HY–Al2O3 Catalysts for Aromatic Hydrogenation. J. Catal. 2001, 202, 220–228. [Google Scholar] [CrossRef]
- Kustov, L.M.; Tarasov, A.L.; Kirichenko, O.A. Microwave-Activated Dehydrogenation of Perhydro-N-Ethylcarbazol over Bimetallic Pd-M/TiO2 Catalysts as the Second Stage of Hydrogen Storage in Liquid Substrates. Int. J. Hydrog. Energy 2017, 42, 26723–26729. [Google Scholar] [CrossRef]
- Kirichenko, O.; Strekalova, A.; Kapustin, G.; Shesterkina, A.; Redina, E.; Kustov, L. Redox Behavior of Novel FeOx/Pd/SiO2 Catalytic Nanomaterials. J. Therm. Anal. Calorim. 2019, 138, 1913–1922. [Google Scholar] [CrossRef]
- Richard, F.C.; Bourg, A.C.M. Aqueous Geochemistry of Chromium: A Review. Water Res. 1991, 25, 807–816. [Google Scholar] [CrossRef]
- Pfennig, A. Kirk-Othmer Encyclopedia of Chemical Technology, Vol. 1. Herausgeg. von J. I. Kroschwitz und M. Howe-Grant. John Wiley & Sons, New York—Chichester—Toronto 1991.4. Aufl., XXXII, 1087 S., zahlr. Abb. u. Tab., geb., Subskriptionspreis US-$ 225,-. Chem. Ing. Tech. 1992, 64, 1134. [Google Scholar] [CrossRef]
- Barbier, J. Handbook of Heterogeneous Catalysis; Ertl, G., Knözinger, H., Weitkamp, J., Eds.; Wiley-VCH: Weinheim, Germany, 1997; p. 257. [Google Scholar]
- Barbier, J. Redox methods for preparation of bimetallic catalysts. In Preparation of Solid Catalysts; Ertl, G., Knozinger, H., Weitkamp, J., Eds.; WILEY-VCH Verlag GmbH: Weinheim, Germany, 1999; pp. 526–540. [Google Scholar]
- Redina, E.A.; Kirichenko, O.A.; Greish, A.A.; Kucherov, A.V.; Tkachenko, O.P.; Kapustin, G.I.; Mishin, I.V.; Kustov, L.M. Preparation of Bimetallic Gold Catalysts by Redox Reaction on Oxide-Supported Metals for Green Chemistry Applications. Catal. Today 2015, 246, 216–231. [Google Scholar] [CrossRef]
- Kosmulski, M. PH-Dependent Surface Charging and Points of Zero Charge. IV. Update and New Approach. J. Colloid Interface Sci. 2009, 337, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, J.; Li, Y.; Tang, J.; Zhang, X. Microstructure Characterization and Formation Mechanism of Colloid Palladium for Activation Treatment on the Surface of PPTA Fibers. Appl. Surf. Sci. 2020, 516, 146134. [Google Scholar] [CrossRef]
- Guo, J.; Mao, L.; Zhang, J.; Feng, C. Role of Cl− Ions in Photooxidation of Propylene on TiO2 Surface. Appl. Surf. Sci. 2010, 256, 2132–2137. [Google Scholar] [CrossRef]
- John, F.; Moulder William, F.; Stickle Peter, E.; Sobol Kenneth, D. Handbook of X-ray Photoelectron Spectroscopy; Chastain, J., Ed.; Perkin-Elmer: Eden Prairie, MN, USA, 1992; Volume 118. [Google Scholar]
- Aronniemi, M.; Sainio, J.; Lahtinen, J. Chemical State Quantification of Iron and Chromium Oxides Using XPS: The Effect of the Background Subtraction Method. Surf. Sci. 2005, 578, 108–123. [Google Scholar] [CrossRef]
- Vasconcelos Borges Pinho, P.; Chartier, A.; Moussy, J.-B.; Menut, D.; Miserque, F. Crystal Field Effects on the Photoemission Spectra in Cr2O3 Thin Films: From Multiplet Splitting Features to the Local Structure. Materialia 2020, 12, 100753. [Google Scholar] [CrossRef]
- Liu, B.; Šindelář, P.; Fang, Y.; Hasebe, K.; Terano, M. Correlation of Oxidation States of Surface Chromium Species with Ethylene Polymerization Activity for Phillips CrOx/SiO2 Catalysts Modified by Al-Alkyl Cocatalyst. J. Mol. Catal. Chem. 2005, 238, 142–150. [Google Scholar] [CrossRef]
- Xu, L.; Zuo, Y.; Tang, J.; Tang, Y.; Ju, P. Chromium–Palladium Films on 316L Stainless Steel by Pulse Electrodeposition and Their Corrosion Resistance in Hot Sulfuric Acid Solutions. Corros. Sci. 2011, 53, 3788–3795. [Google Scholar] [CrossRef]
- Keller, P.; Strehblow, H.-H. XPS Investigations of Electrochemically Formed Passive Layers on Fe/Cr-Alloys in 0.5 M H2SO4. Corros. Sci. 2004, 46, 1939–1952. [Google Scholar] [CrossRef]
- Liotta, L.F.; Venezia, A.M.; Pantaleo, G.; Deganello, G.; Gruttadauria, M.; Noto, R. Chromia on Silica and Zirconia Oxides as Recyclable Oxidizing System: Structural and Surface Characterization of the Active Chromium Species for Oxidation Reaction. Catal. Today 2004, 91–92, 231–236. [Google Scholar] [CrossRef]
- Lear, T.; Marshall, R.; Antonio Lopez-Sanchez, J.; Jackson, S.D.; Klapötke, T.M.; Bäumer, M.; Rupprechter, G.; Freund, H.-J.; Lennon, D. The Application of Infrared Spectroscopy to Probe the Surface Morphology of Alumina-Supported Palladium Catalysts. J. Chem. Phys. 2005, 123, 174706. [Google Scholar] [CrossRef] [PubMed]
- Garbowski, E.; Feumi-Jantou, C.; Mouaddib, N.; Primet, M. Catalytic Combustion of Methane over Palladium Supported on Alumina Catalysts: Evidence for Reconstruction of Particles. Appl. Catal. Gen. 1994, 109, 277–291. [Google Scholar] [CrossRef]
- Boujana, S.; Demri, D.; Cressely, J.; Kiennemann, A.; Hindermann, J.P. FT-IR and TPD Studies on Support-Metal and Promoter-Metal Interaction. Pt and Pd Catalysts. Catal. Lett. 1991, 7, 359–366. [Google Scholar] [CrossRef]
- Pinna, F.; Signoretto, M.; Strukul, G.; Polizzi, S.; Pernicone, N. Pd-SiO2 Catalysts. Stability of β-PdHx as a Function of Pd Dispersion. React. Kinet. Catal. Lett. 1997, 60, 9–13. [Google Scholar] [CrossRef]
- Shesterkina, A.A.; Kirichenko, O.A.; Kozlova, L.M.; Kapustin, G.I.; Mishin, I.V.; Strelkova, A.A.; Kustov, L.M. Liquid-Phase Hydrogenation of Phenylacetylene to Styrene on Silica-Supported Pd–Fe Nanoparticles. Mendeleev Commun. 2016, 26, 228–230. [Google Scholar] [CrossRef]
- Kirichenko, O.; Kapustin, G.; Nissenbaum, V.; Mishin, I.; Kustov, L. Evaluation of Stability of Silica-Supported Fe–Pd and Fe–Pt Nanoparticles in Aerobic Conditions Using Thermal Analysis. J. Therm. Anal. Calorim. 2014, 118, 749–758. [Google Scholar] [CrossRef]
- Kirichenko, O.A.; Strekalova, A.A.; Kapustin, G.I.; Shesterkina, A.A. A New Redox Method for Depositing FeOx on the Surface of Pd(0)/SiO2 Nanoparticles—Catalysts for Selective Phenylacetylene Hydrogenation. Russ. J. Phys. Chem. A 2018, 92, 2396–2398. [Google Scholar] [CrossRef]
- Ouyang, L.; Tian, P.; Da, G.; Xu, X.-C.; Ao, C.; Chen, T.; Si, R.; Xu, J.; Han, Y.-F. The Origin of Active Sites for Direct Synthesis of H2O2 on Pd/TiO2 Catalysts: Interfaces of Pd and PdO Domains. J. Catal. 2015, 321, 70–80. [Google Scholar] [CrossRef]
- Zaki, M.I.; Fouad, N.E.; Bond, G.C.; Tahir, S.F. Temperature-Programmed Reduction of Calcined Chromia-Coated Alumina and Silica Catalysts: Probing Chromium (VI)-Oxygen Species. Thermochim. Acta 1996, 285, 167–179. [Google Scholar] [CrossRef]
- Markov, P.V.; Mashkovsky, I.S.; Bragina, G.O.; Wärnå, J.; Bukhtiyarov, V.I.; Stakheev, A.Y.; Murzin, D.Y. Experimental and Theoretical Analysis of Particle Size Effect in Liquid-Phase Hydrogenation of Diphenylacetylene. Chem. Eng. J. 2021, 404, 126409. [Google Scholar] [CrossRef]
- Jóźwiak, W.K.; Maniecki, T.P. Influence of Atmosphere Kind on Temperature Programmed Decomposition of Noble Metal Chlorides. Thermochim. Acta 2005, 435, 151–161. [Google Scholar] [CrossRef]
- Kachala, V.V.; Khemchyan, L.L.; Kashin, A.S.; Orlov, N.V.; Grachev, A.A.; Zalesskiy, S.S.; Ananikov, V.P. Target-Oriented Analysis of Gaseous, Liquid and Solid Chemical Systems by Mass Spectrometry, Nuclear Magnetic Resonance Spectroscopy and Electron Microscopy. Russ. Chem. Rev. 2013, 82, 648–685. [Google Scholar] [CrossRef]
- Yates, D. The Catalytic Activity of Rhodium in Relation to Its State of Dispersion. J. Catal. 1967, 8, 348–358. [Google Scholar] [CrossRef]
- Kirichenko, O.; Kapustin, G.; Nissenbaum, V.; Strelkova, A.; Shuvalova, E.; Shesterkina, A.; Kustov, L. Thermal Decomposition and Reducibility of Silica-Supported Precursors of Cu, Fe and Cu–Fe Nanoparticles. J. Therm. Anal. Calorim. 2018, 134, 233–251. [Google Scholar] [CrossRef]
- Kirichenko, O.A.; Nissenbaum, V.D.; Kapustin, G.I.; Kustov, L.M. Thermal Analysis of Ammonium Trioxalatometallate Complexes Supported on Titania and Reducibility of Their Decomposition Products. Thermochim. Acta 2009, 494, 35–39. [Google Scholar] [CrossRef]
- Shesterkina, A.A.; Kozlova, L.M.; Kirichenko, O.A.; Kapustin, G.I.; Mishin, I.V.; Kustov, L.M. Influence of the Thermal Treatment Conditions and Composition of Bimetallic Catalysts Fe—Pd/SiO2 on the Catalytic Properties in Phenylacetylene Hydrogenation. Russ. Chem. Bull. 2016, 65, 432–439. [Google Scholar] [CrossRef]
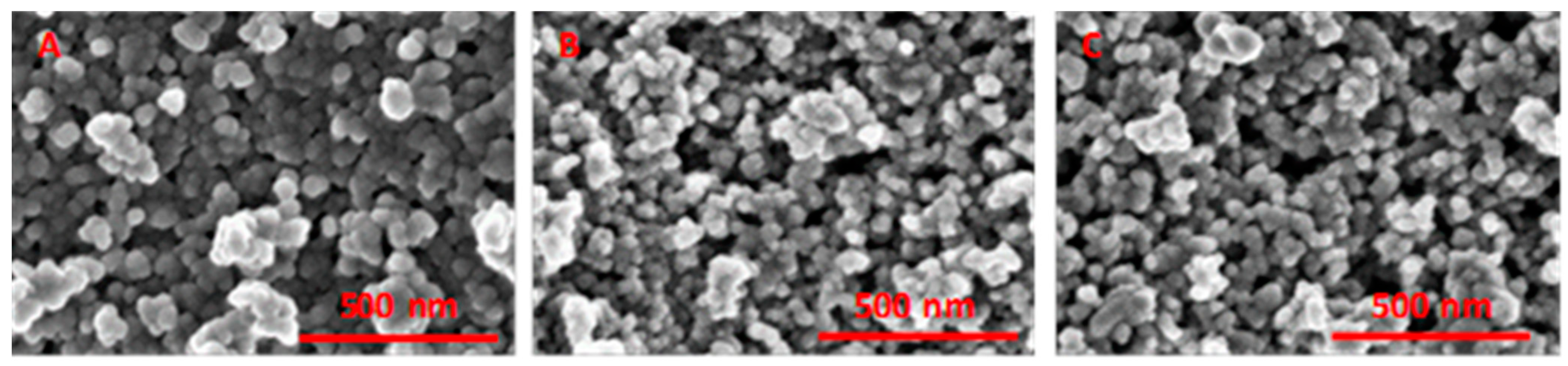
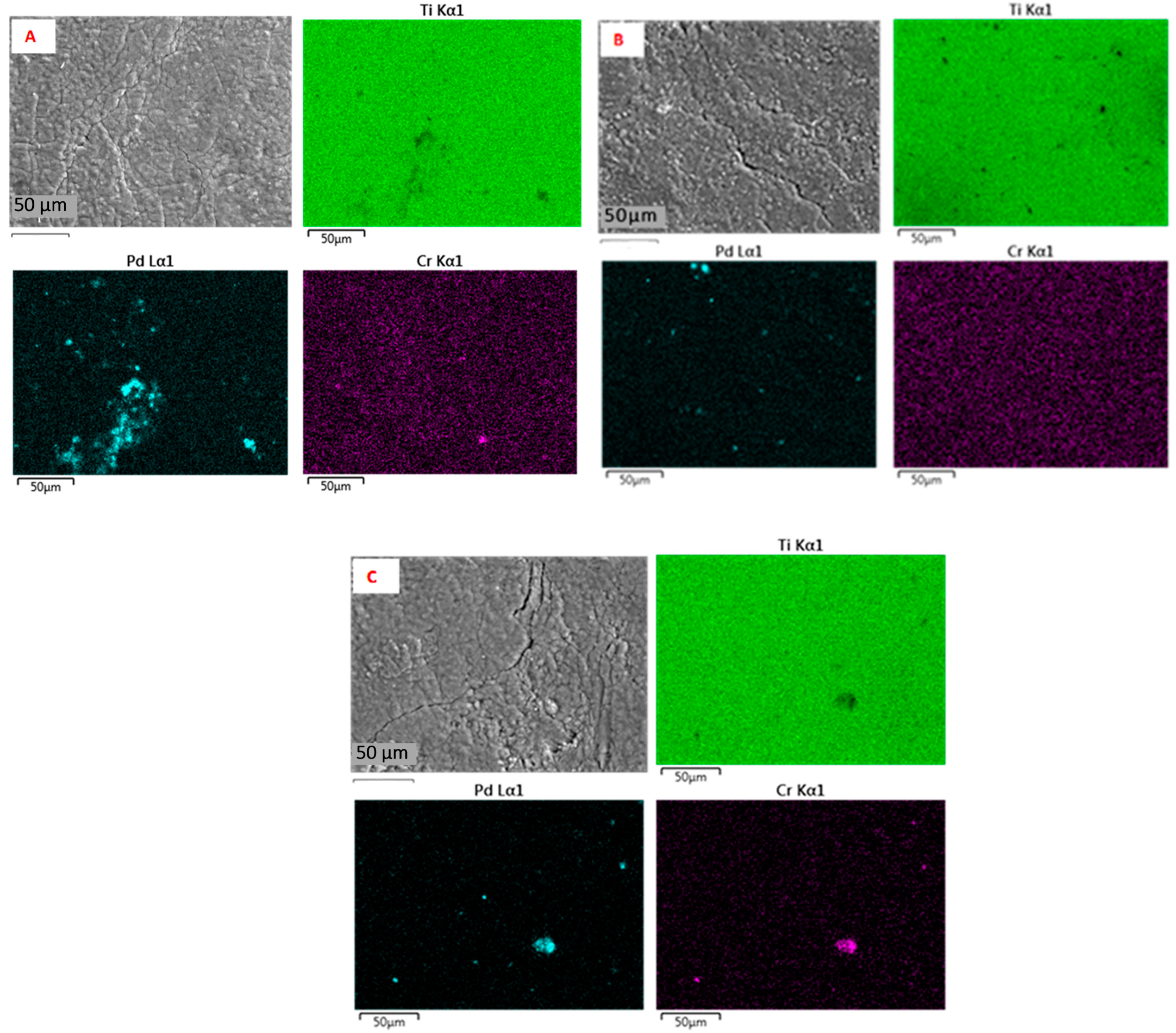
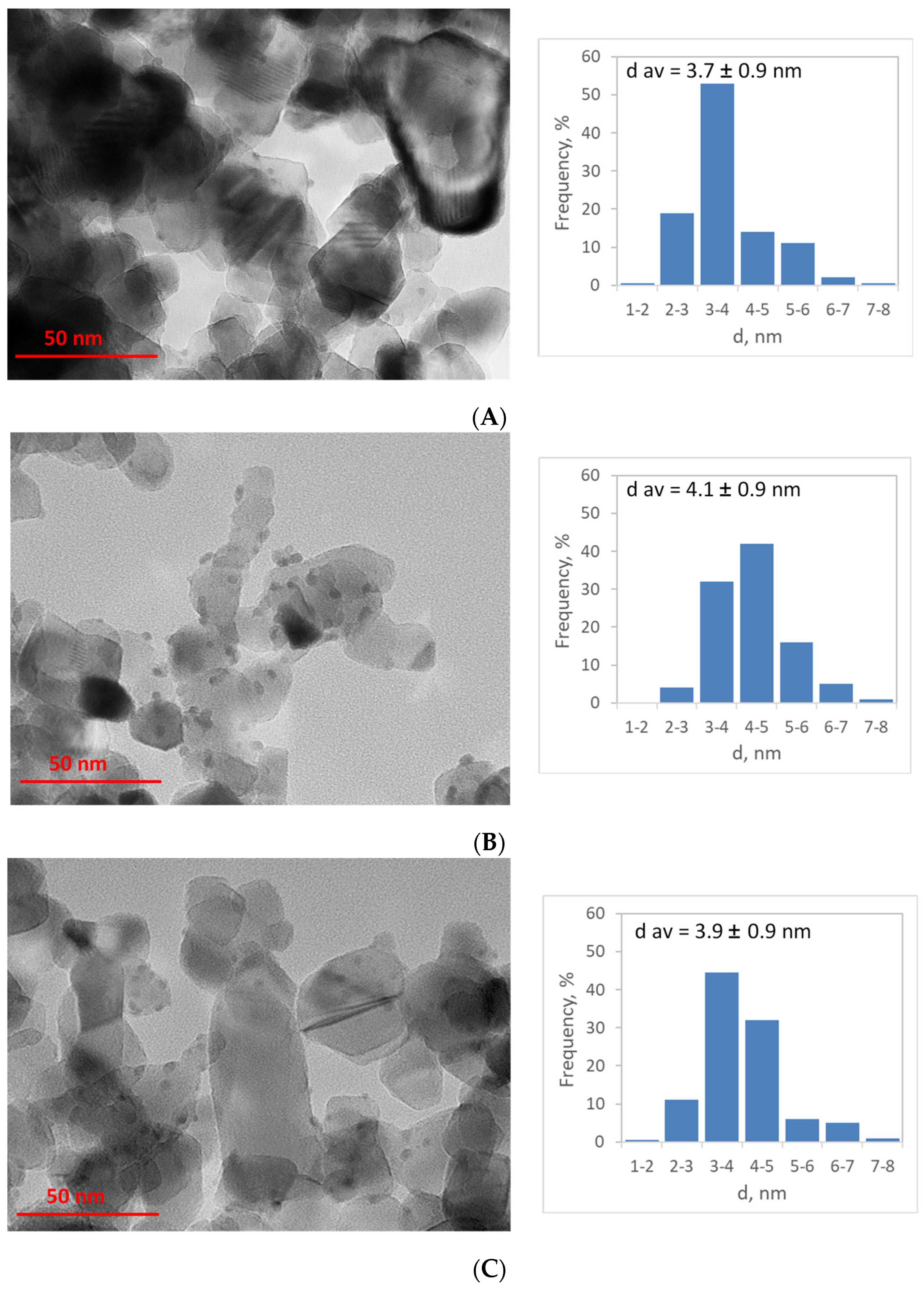
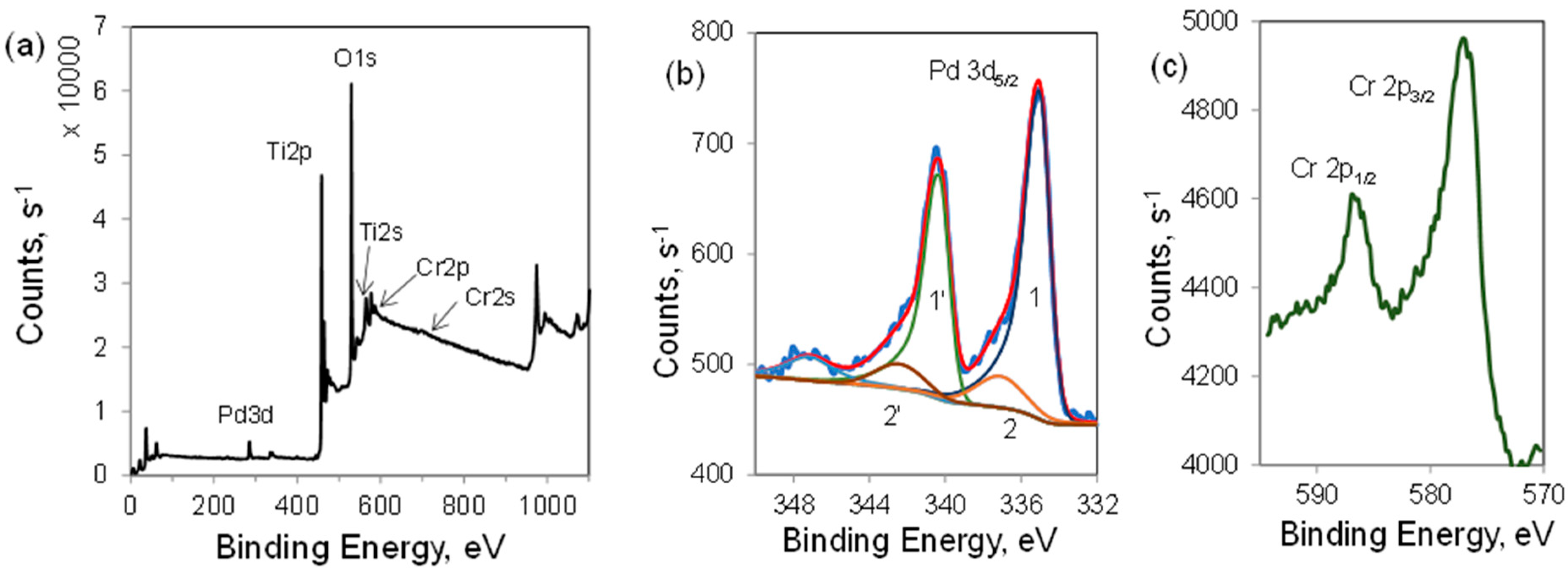
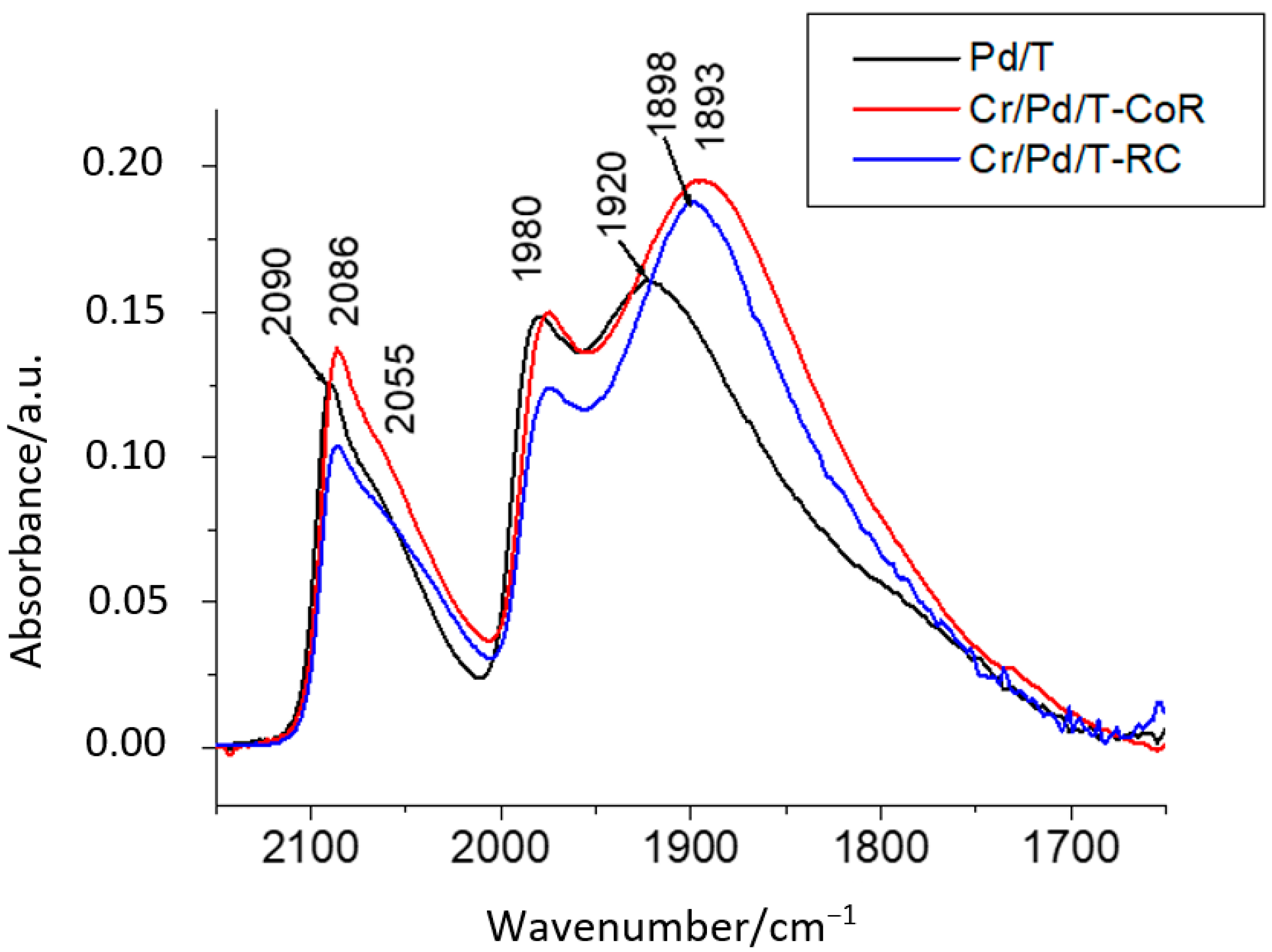
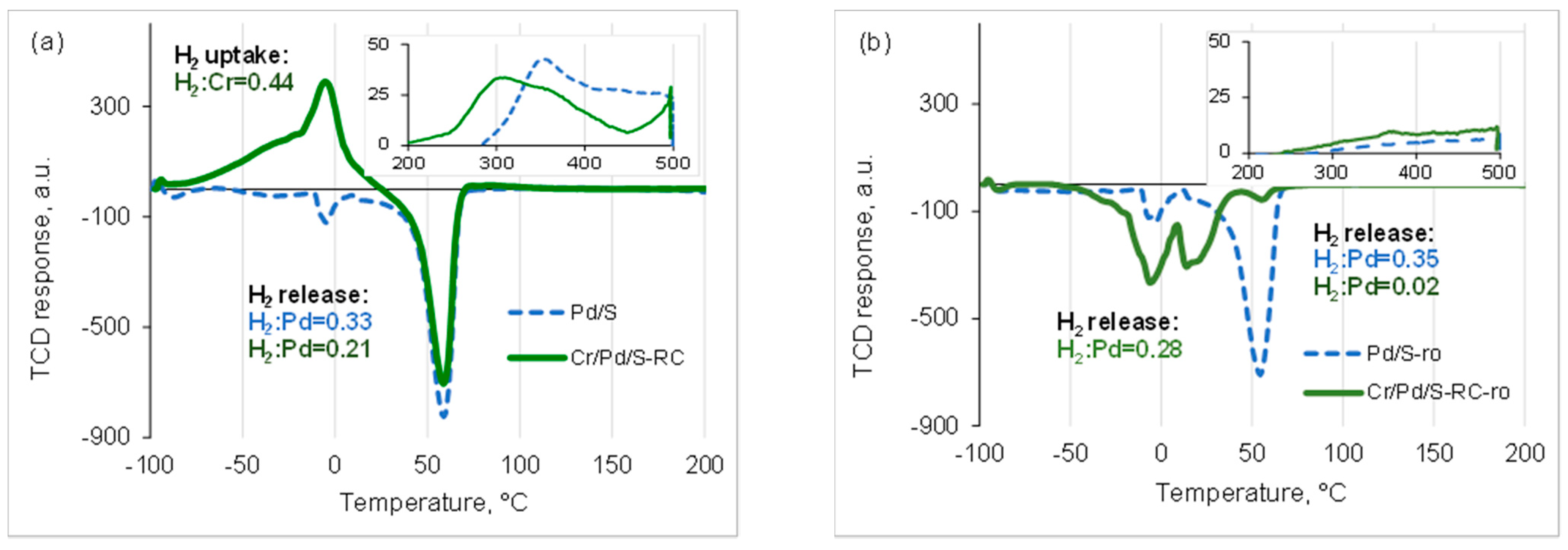
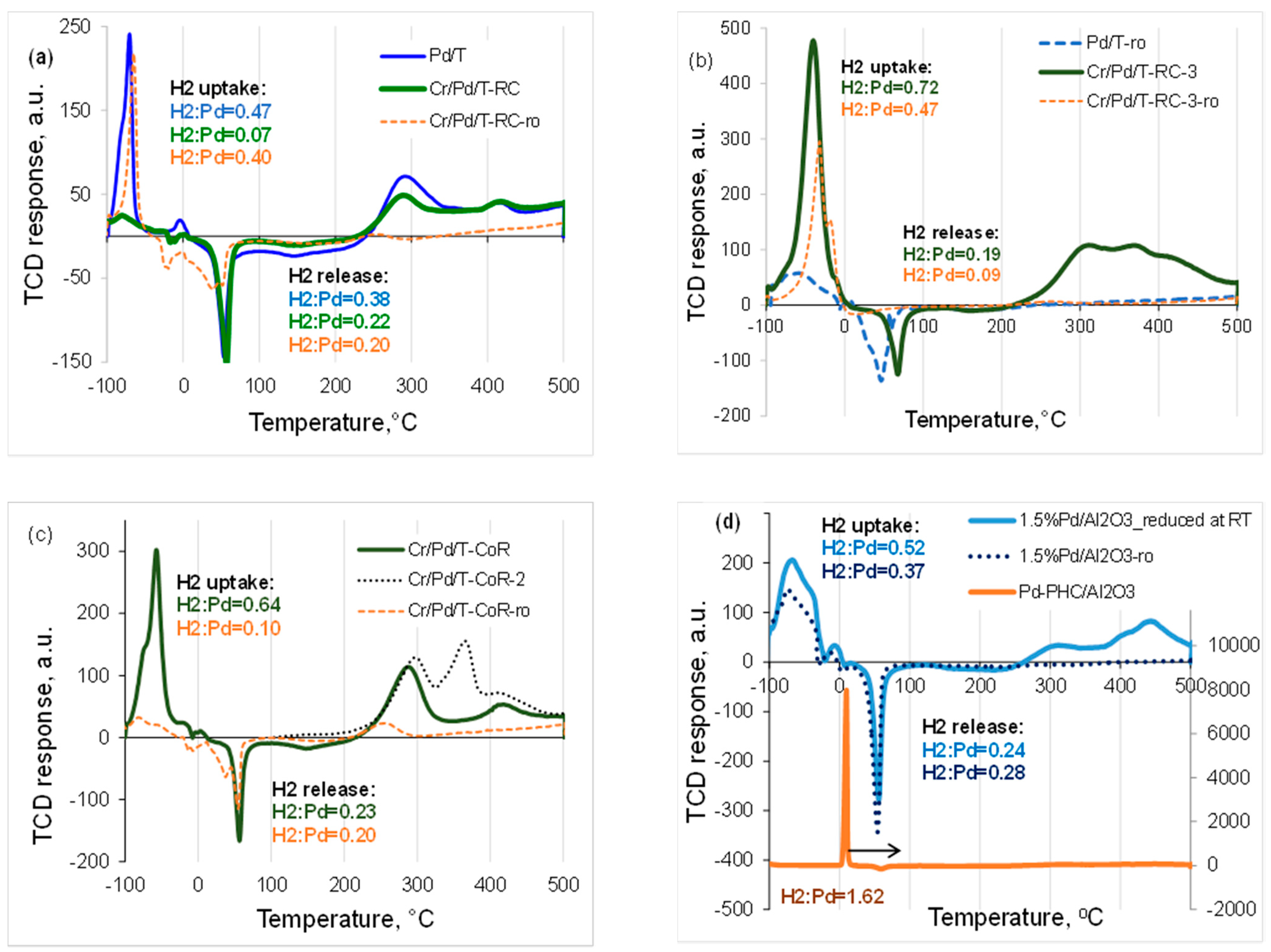
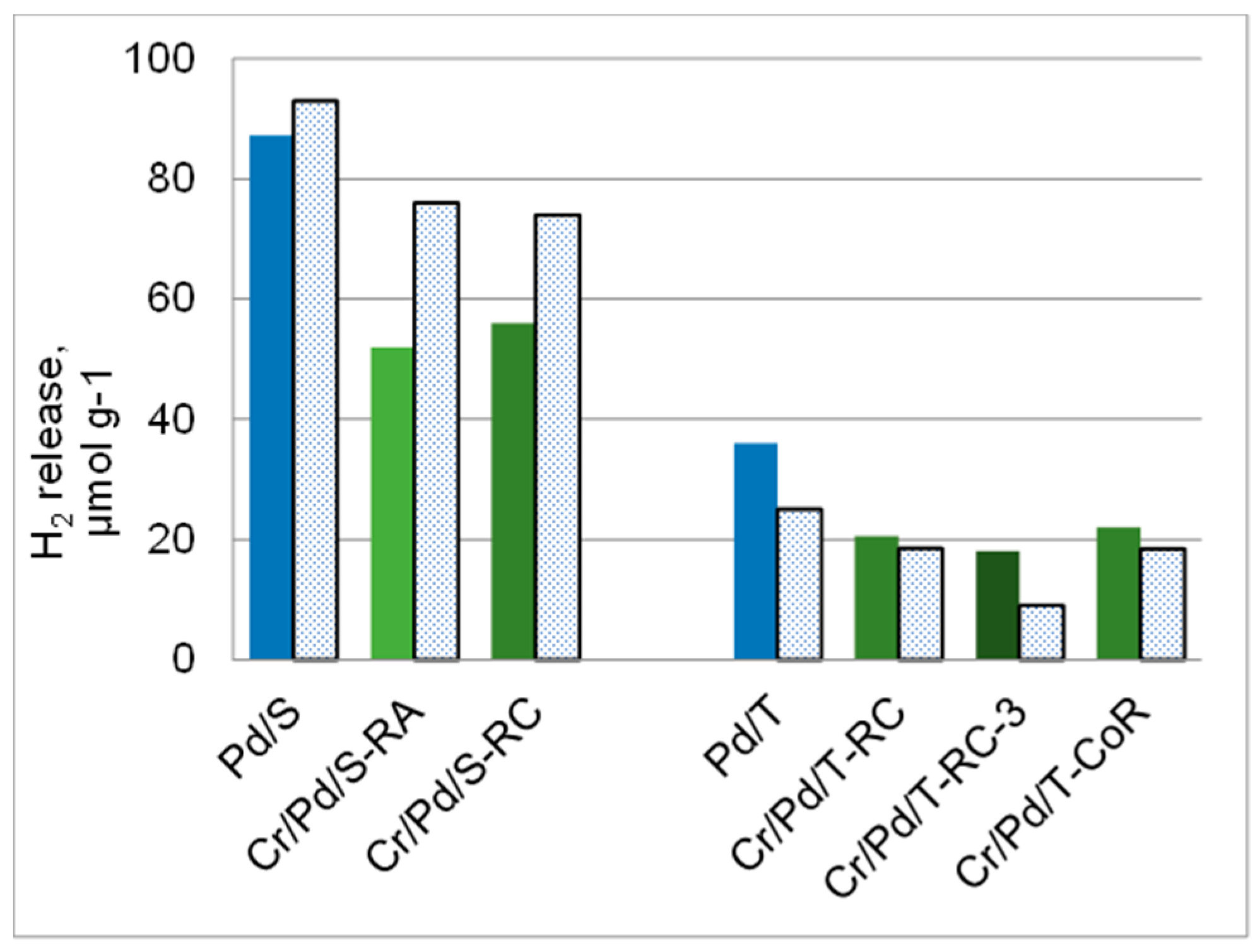
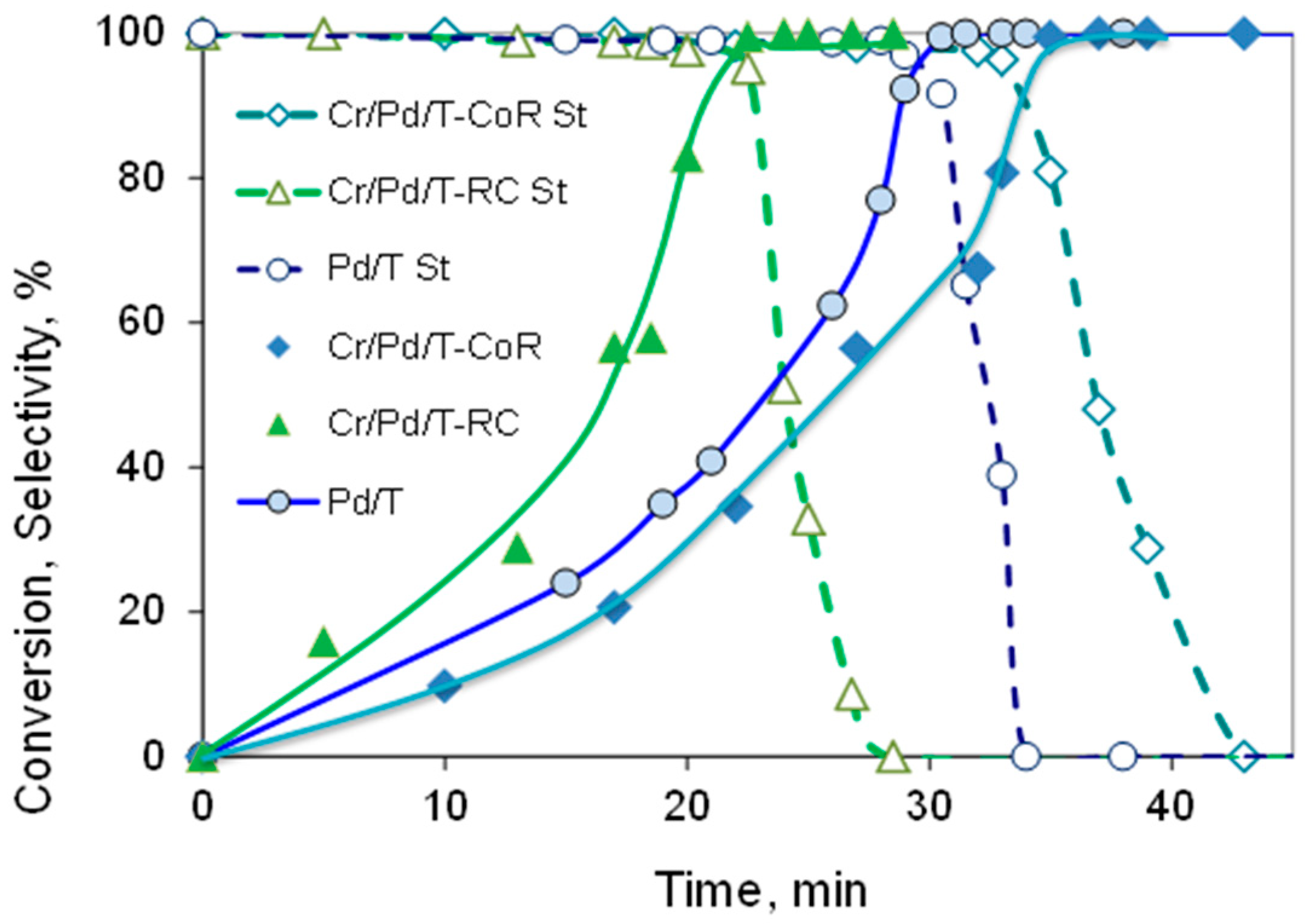
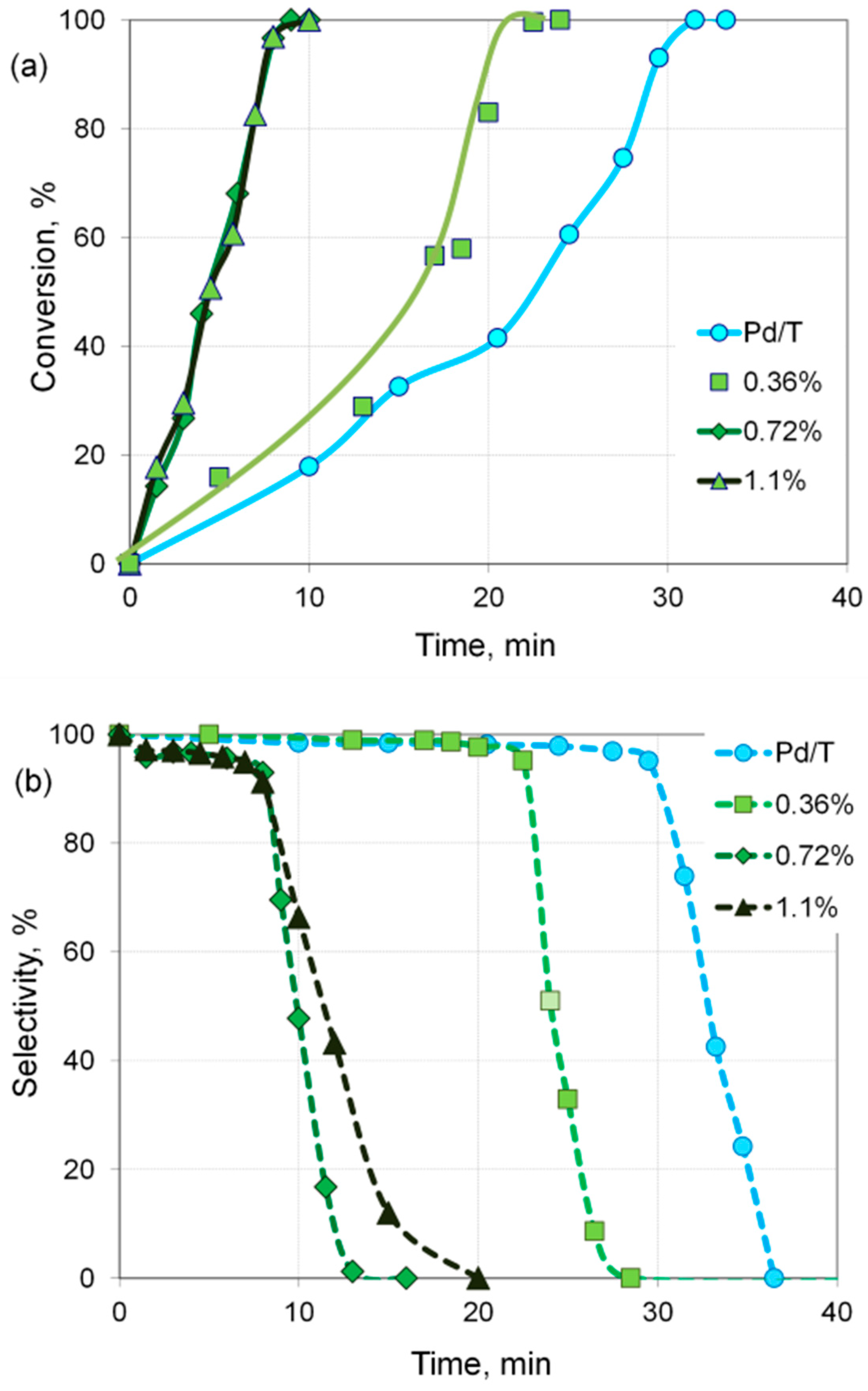
| Sample | Cr, wt.% | Cr:Pdv | [Cr6+], mmol L−1 | τRR, h | pHi | pHms |
|---|---|---|---|---|---|---|
| Cr/Pd/S-RA | 0.026 | 0.018 | 2.6 | 2 | - | - |
| Cr/Pd/S-RA | 0.260 | 0.180 | 26.0 | 18 | 4.4 | - |
| Cr/Pd/S-RC | 0.360 | 0.740 | 1.5 | 25 | 4.4 | 8.3 |
| Cr/Pd/T-RC | 0.360 | 0.740 | 5.9 | 5 | 2.8 | 8.3 |
| Cr/Pd/T-RC-2 | 0.720 | 1.50 | 12.0 | 3 | 2.7 | 10.3 |
| Cr/Pd/T-RC-3 | 1.10 | 2.20 | 18.0 | 3 | 2.6 | 10.7 |
| Cr/Pd/T-CoR | 0.360 | 0.740 | 2.9 | 6 | 5.7 | 9.7 |
| Cr/Pd/T-CoR-2 | 0.430 | 0.870 | 3.5 | 6 | 5.3 | 9.5 |
| Sample | O 1s | Ti 2p | C 1s | Pd 3d | Cr 2p1/2 | O/Ti | C/Ti |
|---|---|---|---|---|---|---|---|
| Cr/Pd/T-CoR | 60.5 | 23.5 | 14.5 | 0.6 | - | 2.6 | 0.6 |
| Cr/Pd/T-RC | 60.4 | 23.4 | 15.4 | 0.6 | - | 2.6 | 0.7 |
| Cr/Pd/T-RC-3 | 61.0 | 23.1 | 13.0 | 0.5 | 1.4 | 2.6 | 0.6 |
| Catalyst | Spectral Parameters | Pd 3d | ||
|---|---|---|---|---|
| 1-1′ Pd0 | 2-2′ Pd2+ | Satellite Pd0 | ||
| Cr/Pd/T-CoR | BE, eV | 335.1 | 337.1 | 347.4 |
| Half width, eV | 1.3 | 3.0 | 3.0 | |
| Intensity, % | 76 | 14 | 10 | |
| Cr/Pd/T-RC-2 | BE, eV | 335.1 | 337.2 | 347.2 |
| Half width, eV | 1.3 | 3.0 | 3.0 | |
| Intensity, % | 77 | 16 | 7 | |
| Cr/Pd/T-RC-3 | BE, eV | 335.1 | 337.2 | 347.2 |
| Half width, eV | 1.3 | 3.0 | 3.0 | |
| Intensity, % | 79 | 14 | 7 | |
| Sample | Cr, % | Cr:Pdv | nCO, mmol g−1Pd | SF | SPd, m2 g−1 | γPd, % | Cr:Pds | dPd, nm | dTEM, nm |
|---|---|---|---|---|---|---|---|---|---|
| Pd/S | 0 | - | 0.26 | 1.80 | 24 | 5.3 | - | 21 | 22 (XRD) |
| Pd/T | 0 | - | 2.1 | 1.80 | 180 | 40 | - | 2.8 ± 0.3 | - |
| Cr/Pd/T-RC | 0.36 | 0.74 | 1.9 | 1.82 | 160 | 37 | 1.9 | 3.1 ± 0.3 | 4.1 ± 0.9 |
| Cr/Pd/T-CoR | 0.36 | 0.74 | 1.9 | 1.81 | 160 | 36 | 1.9 | 3.1 ± 0.3 | 3.7 ± 0.9 |
| Sample | Cr, wt.% | Cr:Pdv | T, °C | r0(H2) | τPhA, min | S99St, % | τSt, min |
|---|---|---|---|---|---|---|---|
| Pd/S | - | - | 400 (H2) | 0.58 | 3.5 | 91 | 3 |
| Cr/Pd/S-RA | 0.026 | 0.018 | 60 (air) | 0.24 | 11 | 78 | 10 |
| Cr/Pd/S-RA | 0.026 | 0.018 | 400 (air) | 0.40 | 5 | 73 | 6 |
| Cr/Pd/S-RA | 0.026 | 0.018 | 400 (H2) | 0.33 | 6 | 81 | 8 |
| Cr/Pd/S-RA | 0.26 | 0.18 | 500 (H2) | 0.051 | 72 | 73 | 180 |
| Sample | Cr, % wt. | Cr:Pdv | r0(H2)c | τPhAa, min | S99St b, % | τSta, min |
|---|---|---|---|---|---|---|
| Pd/T | - | - | 0.14 | 32 | 92 | 2.5 |
| Cr/Pd/T-RC | 0.36 | 0.74 | 0.45 | 24 | 95 | 10 |
| Cr/Pd/T-RC-2 | 0.72 | 1.50 | 0.70 | 9 | 83 | 7 |
| Cr/Pd/T-RC-3 | 1.10 | 2.20 | 0.56 | 9 | 85 | 11 |
| Cr/Pd/T-CoR | 0.36 | 0.74 | 0.14 | 35 | 81 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirichenko, O.A.; Redina, E.A.; Kapustin, G.I.; Chernova, M.S.; Shesterkina, A.A.; Kustov, L.M. Facile Redox Synthesis of Novel Bimetallic Crn+/Pd0 Nanoparticles Supported on SiO2 and TiO2 for Catalytic Selective Hydrogenation with Molecular Hydrogen. Catalysts 2021, 11, 583. https://doi.org/10.3390/catal11050583
Kirichenko OA, Redina EA, Kapustin GI, Chernova MS, Shesterkina AA, Kustov LM. Facile Redox Synthesis of Novel Bimetallic Crn+/Pd0 Nanoparticles Supported on SiO2 and TiO2 for Catalytic Selective Hydrogenation with Molecular Hydrogen. Catalysts. 2021; 11(5):583. https://doi.org/10.3390/catal11050583
Chicago/Turabian StyleKirichenko, Olga A., Elena A. Redina, Gennady I. Kapustin, Marina S. Chernova, Anastasiya A. Shesterkina, and Leonid M. Kustov. 2021. "Facile Redox Synthesis of Novel Bimetallic Crn+/Pd0 Nanoparticles Supported on SiO2 and TiO2 for Catalytic Selective Hydrogenation with Molecular Hydrogen" Catalysts 11, no. 5: 583. https://doi.org/10.3390/catal11050583
APA StyleKirichenko, O. A., Redina, E. A., Kapustin, G. I., Chernova, M. S., Shesterkina, A. A., & Kustov, L. M. (2021). Facile Redox Synthesis of Novel Bimetallic Crn+/Pd0 Nanoparticles Supported on SiO2 and TiO2 for Catalytic Selective Hydrogenation with Molecular Hydrogen. Catalysts, 11(5), 583. https://doi.org/10.3390/catal11050583








