One-Pot Bi-Enzymatic Cascade Synthesis of Novel Ganoderma Triterpenoid Saponins
Abstract
1. Introduction
2. Results
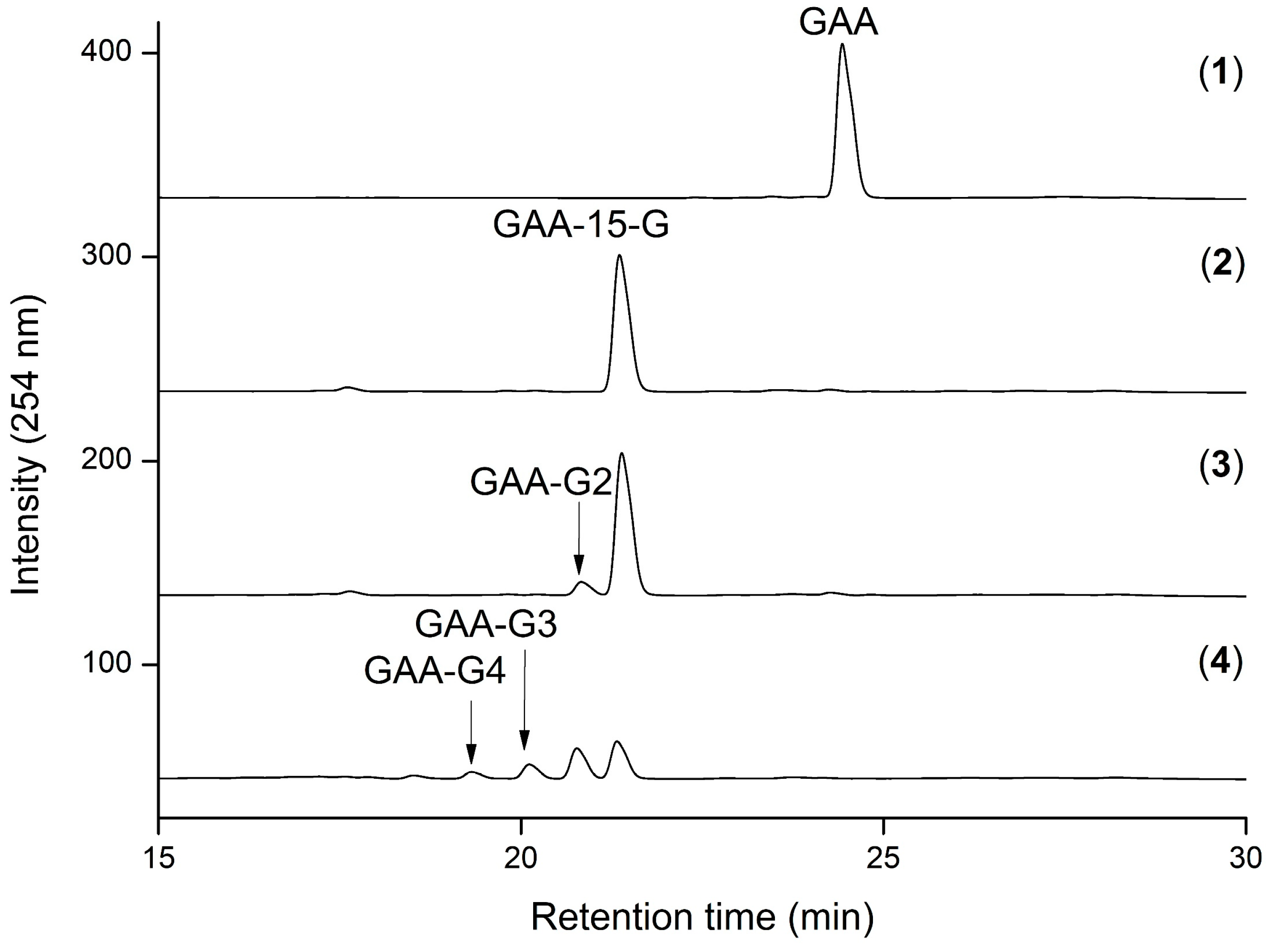
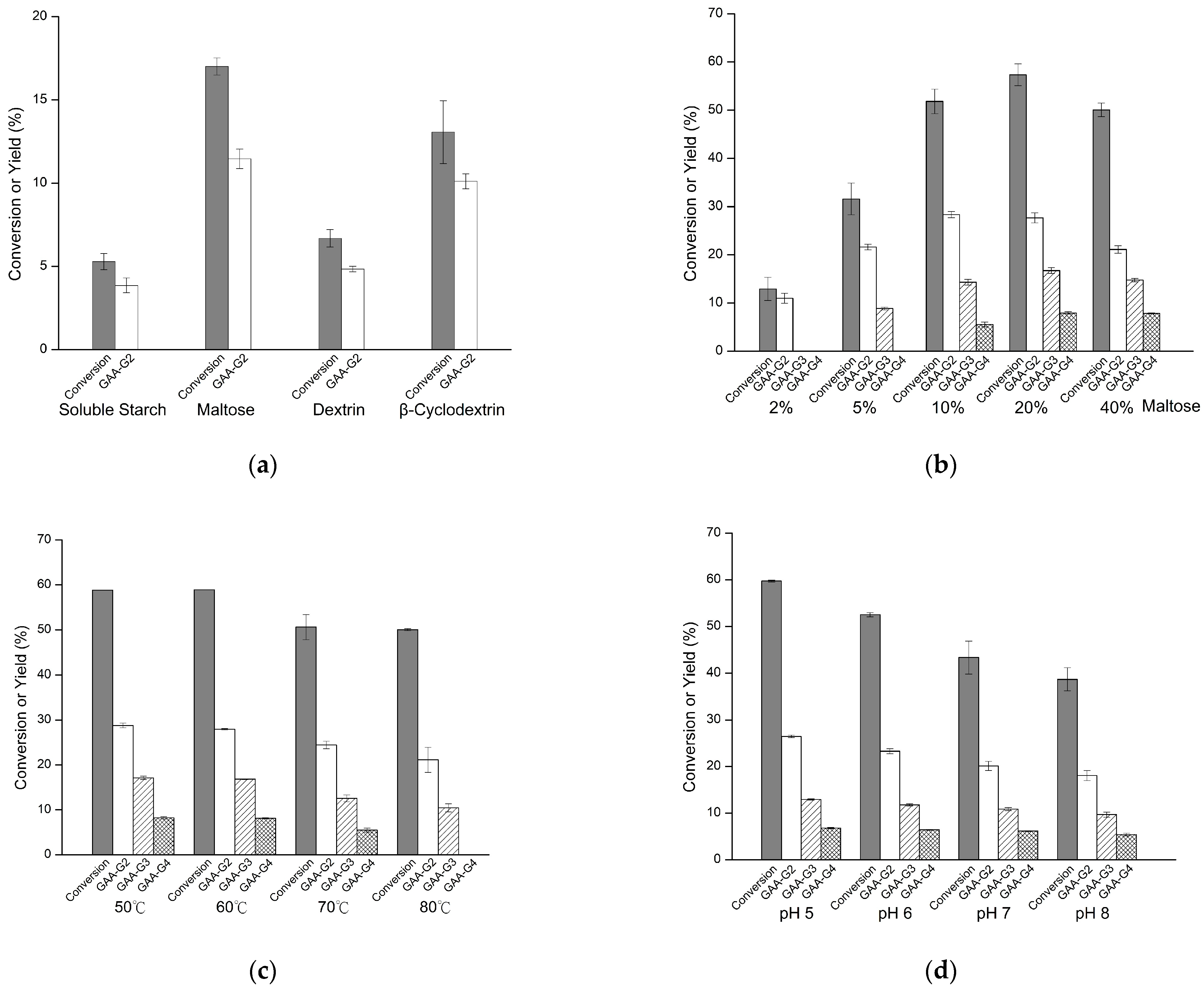
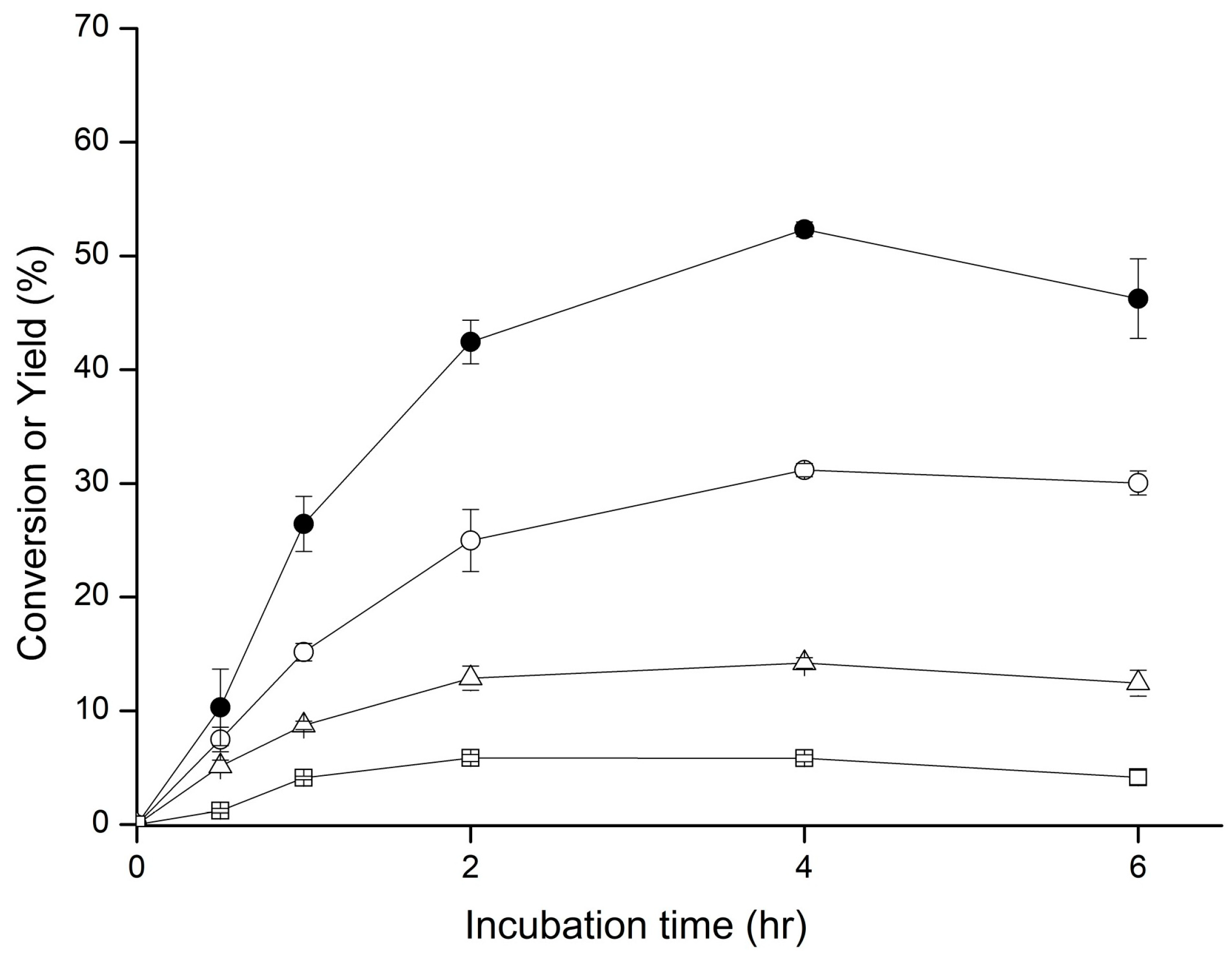
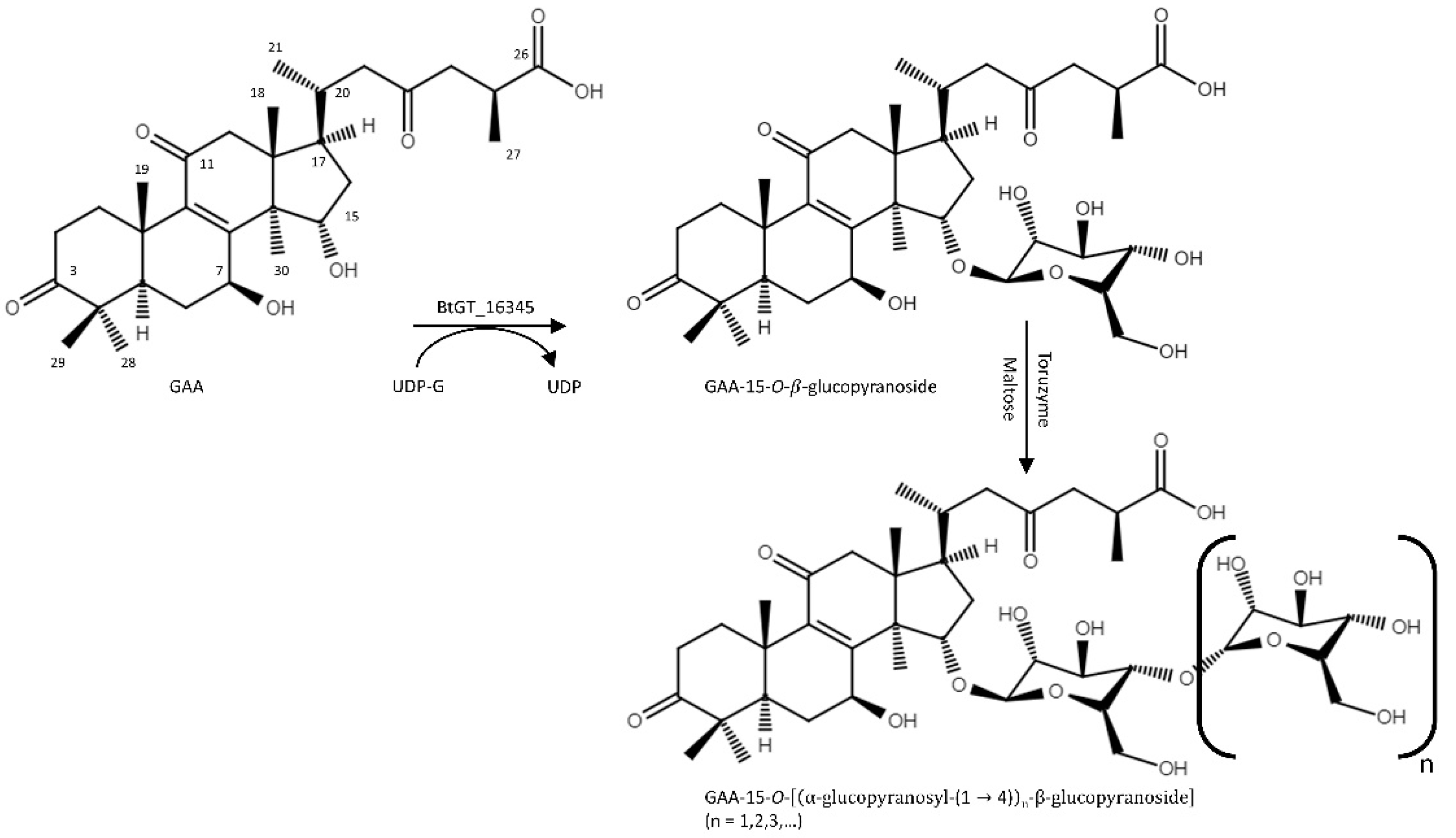
2.1. Biotransformation of GAA
2.2. Purification and Identification of the Biotransformed Products
2.3. Determination of Aqueous Solubility of the Products
3. Discussion
4. Materials and Methods
4.1. Chemicals and Enzymes
4.2. Biotransformation
4.3. High-Performance Liquid Chromatography (HPLC)
4.4. Purification and Identification of the Glycosylated Products
4.5. Determination of Aqueous Solubility of the Glycosylated Products
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmad, M.F. Ganoderma lucidum: Persuasive biologically active constituents and their health endorsement. Biomed. Pharmacother. 2018, 107, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-W.; Zhao, W.; Zhong, J.-J. Biotechnological production and application of ganoderic acids. Appl. Microbiol. Biotechnol. 2010, 87, 457–466. [Google Scholar]
- Wen, G.; Li, T.; He, H.; Zhou, X.; Zhu, J. Ganoderic acid A inhibits bleomycin-induced lung fibrosis in mice. Pharmarcology 2020, 105, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Wan, B.; Li, Y.; Sun, S.; Yang, Y.; Lv, Y.; Wang, L.; Song, M.; Chen, M. Ganoderic acid A attenuates lipopolysaccharide-induced lung injury in mice. Biosci. Rep. 2019, 39, BSR20190301. [Google Scholar] [CrossRef]
- Meng, J.; Wang, S.Z.; He, J.Z.; Zhu, S.Z.; Huang, B.Y.; Wang, S.Y.; Li, M.; Zhou, H.; Lin, S.Q.; Yang, B.X. Ganoderic acid A is the effective ingredient of Ganoderma triterpenes in retarding renal cyst development in polycystic kidney disease. Acta Pharmacol. Sin. 2020, 41, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.R.; Jia, W.H.; Liu, C.; Wang, H.Q.; Yang, H.G.; He, G.R.; Chen, R.Y.; Du, G.H. Ganoderic acid A protects neural cells against NO stress injury in vitro via stimulating β adrenergic receptors. Acta Pharmacol. Sin. 2020, 41, 516–522. [Google Scholar] [CrossRef]
- Yao, L.; Lu, J.; Wang, J.; Gao, W.Y. Advances in biosynthesis of triterpenoid saponins in medicinal plants. Chin. J. Nat. Med. 2020, 18, 417–424. [Google Scholar] [CrossRef]
- Shi, Z.Y.; Zeng, J.Z.; Wong, A.S.T. Chemical structures and pharmacological profiles of Ginseng saponins. Molecules 2019, 24, 2443. [Google Scholar] [CrossRef]
- Huang, G.; Lv, M.; Hu, J.; Huang, K.; Xu, H. Glycosylation and activities of natural products. Mini Rev. Med. Chem. 2016, 16, 1013–1016. [Google Scholar] [CrossRef]
- Shimoda, K.; Hamada, H.; Hamada, H. Synthesis of xylooligosaccharides of daidzein and their anti-oxidant and anti-allergic activities. Int. J. Mol. Sci. 2011, 12, 5616–5625. [Google Scholar] [CrossRef]
- Chiang, C.M.; Wang, T.Y.; Yang, S.Y.; Wu, J.Y.; Chang, T.S. Production of new isoflavone glucosides from glycosylation of 8-hydroxydaidzein by glycosyltransferase from Bacillus subtilis ATCC 6633. Catalysts 2018, 8, 387. [Google Scholar] [CrossRef]
- Chang, T.S.; Wang, T.Y.; Yang, S.Y.; Kao, Y.H.; Wu, J.Y.; Chiang, C.M. Potential industrial production of a well-soluble, alkaline-stable, and anti-inflammatory isoflavone glucoside from 8-hydroxydaidzein glucosylated by recombinant amylosucrase of Deinococcus geothermalis. Molecules 2019, 24, 2236. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.H.; Yu, X.Q.; Zhang, Y.X.; Yang, J.W.; Hu, X.Q.; Zhang, H.B. Transglycosylation improved caffeic acid phenethyl ester anti-inflammatory activity and water solubility by Leuconostoc mesenteroides dextransucrase. J. Agric. Food Chem. 2019, 67, 4505–4512. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, J.; Xie, Y. Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: An overview. Int. J. Pharm. 2019, 570, 118642. [Google Scholar] [CrossRef]
- Fu, J.; Wu, Z.; Zhang, L. Clinical applications of the naturally occurring or synthetic glycosylated low molecular weight. Prog. Mol. Biol. Transl. Sci. 2019, 163, 487–522. [Google Scholar]
- Mestrom, L.; Przypis, M.; Kowalczykiewicz, D.; Pollender, A.; Kumpf, A.; Marsden, S.R.; Bento, I.; Jarzebski, A.B.; Szymanska, K.; Chrusciel, A.; et al. Leloir glycosyltransferases in applied biocatalysis: A multidisciplinary approach. Int. J. Mol. Sci. 2019, 20, 5263. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Sangwan, R.S.; Sangwan, N.S. Plant secondary metabolism linked glycosyltransferases: An update on expanding knowledge and scopes. Biotechnol. Adv. 2016, 34, 716–739. [Google Scholar] [CrossRef] [PubMed]
- Hofer, B. Recent developments in the enzymatic O-glycosylation of flavonoids. Appl. Microbiol. Biotechnol. 2016, 100, 4269–4281. [Google Scholar] [CrossRef]
- Sordon, S.; Poplonski, J.; Huszcza, E. Microbial glycosylation of flavonoids. Pol. J. Microbiol. 2016, 65, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Zhang, H.; Sun, X.; Zhao, H.; Wu, L.; Zhu, D.; Yang, G.; Shao, Y.; Zhang, X.; Mao, X.; et al. A comprehensive review of the structure elucidation and biological activity of triterpenoids from Ganoderma spp. Molecules 2014, 19, 17478–17535. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Zuo, J.; Gon, X.; Yi, F.; Zhu, W.; Li, L. Advances in research on the active constituents and physiological effects of Ganoderma lucidum. Biomed. Dermatol. 2019, 3, 6. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, Z.; Zhang, L.; Wang, J.; Wu, C. Glycosyltransferase GT1 family: Phylogenetic distribution, substrates coverage, and representative structural features. Comput. Struct. Biotechnol. J. 2020, 18, 1383–1390. [Google Scholar] [CrossRef]
- Chang, T.S.; Wang, T.Y.; Chiang, C.M.; Lin, Y.J.; Chen, H.L.; Wu, Y.W.; Ting, H.J.; Wu, J.Y. Biotransformation of celastrol to a novel, well-soluble, low-toxic and anti-oxidative celastrol-29-O-β-glucoside by Bacillus glycosyltransferases. J. Biotech. Bioeng. 2021, 131, 176–182. [Google Scholar]
- Chang, T.S.; Wang, T.Y.; Hsueh, T.Y.; Lee, Y.W.; Chuang, H.M.; Cai, W.X.; Wu, J.Y.; Chiang, C.M.; Wu, Y.W. A genome-centric approach reveals a novel glycosyltransferase from the GA A07 strain of Bacillus thuringiensis responsible for catalyzing 15-O-glycosylation of ganoderic acid A. Int. J. Mol. Sci. 2019, 20, 5192. [Google Scholar] [CrossRef] [PubMed]
- Moulis, C.; Guieysse, D.; Morel, S.; Severac, E.; Remaud-Simeon, M. Natural and engineered transglycosylases: Green tools for the enzyme-based synthesis of glycoproducts. Curr. Opin. Chem. Biol. 2021, 61, 96–106. [Google Scholar] [CrossRef]
- Zhou, W.B.; Feng, B.; Huang, H.Z.; Qin, Y.J.; Wang, Y.Z.; Kang, L.P.; Zhao, Y.; Wang, X.N.; Cai, Y.; Tan, D.W.; et al. Enzymatic synthesis of α-glucosyl-timosaponin BII catalyzed by the extremely thermophilic enzyme: Toruzyme 3.0 L. Carbohyd. Res. 2010, 345, 1752–1759. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Alfonso, J.L.; Leemans, L.; Poveda, A.; Jimenez-Barbero, J.; Ballesteros, A.O.; Plou, F.J. Efficient α-glucosylation of epigallocatechin catalyzed by cyclodextrin glucanotransferase from Thermoanaerobacter species. J. Agric. Food Chem. 2018, 66, 7402–7408. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Alfonso, J.L.; Rodrigo-Frutos, D.; Belmonte-Reche, E.; Penalver, P.; Poveda, A.; Jimenez-Barbero, J.; Ballesteros, A.O.; Hirose, Y.; Polaina, J.; Morales, J.C.; et al. Enzymatic synthesis of a novel pterostilbene α-glucoside by the combination of cyclodextrin glucanotransferase and amyloglucosidase. Molecules 2018, 23, 1271. [Google Scholar] [CrossRef] [PubMed]
- González-Alfonso, J.L.; Miguez, N.; Padilla, D.; Leemans, L.; Poveda, A.; Jiménez-Barbero, J.; Ballesteros, A.O.; Sandoval, G.; Plou, F.J. Optimization of regioselective α-glucosylation of hesperetin catalyzed by cyclodextrin glucanotransferase. Molecules 2018, 23, 2885. [Google Scholar] [CrossRef]
- Choung, W.J.; Hwang, S.H.; Ko, D.S.; Kim, S.B.; Kim, S.H.; Jeon, S.H.; Choi, H.D.; Lim, S.S.; Shim, J.H. Enzymatic synthesis of a novel kaempferol-3-O-β-d-glucopyranosyl-(1-4)-O-α-d-glucopyranoside using cyclodextrin glucanotransferase and its inhibitory effects on aldose reductase, inflammation, and oxidative stress. J. Agric. Food Chem. 2017, 65, 2760–2767. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Feng, B.; Huang, H.Z.; Kang, L.P.; Cong, Y.; Zhou, W.B.; Zou, P.; Cong, Y.W.; Spng, X.B.; Ma, B.P. Glucosylation of steroidal saponins by cyclodextrin glucanotransferase. Plan. Med. 2010, 76, 1724–1731. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.S.; Lee, H.J.; Mathiyalagan, R.; Kim, Y.J.; Yang, D.U.; Lee, D.Y.; Min, J.W.; Jimenez, Z.; Yang, D.C. Synthesis of a novel α-glucosyl ginsenoside F1 by cyclodextrin glucanotransferase and its in vitro cosmetic applications. Biomolecules 2018, 8, 142. [Google Scholar] [CrossRef] [PubMed]
- Rha, C.S.; Kim, E.R.; Kim, Y.J.; Jug, Y.S.; Kim, D.O.; Park, C.S. Simple and efficient production of highly soluble daidzin glycosides by amylosucrase from Deinococcus geothermalis. J. Agric. Food Chem. 2019, 67, 12824–12832. [Google Scholar] [CrossRef] [PubMed]
- Rha, C.S.; Choi, J.M.; Jung, Y.S.; Kim, E.R.; Ko, M.J.; Seo, D.H.; Kim, D.O.; Park, C.S. High-efficiency enzymatic production of α-isoquercitrin glucosides by amylosucrase from Deinococcus geothermalis. Enz. Micro. Technol. 2019, 120, 84–90. [Google Scholar] [CrossRef]
- Chang, T.S.; Chiang, C.M.; Wu, J.Y.; Tsai, Y.L.; Ting, H.J. Production of a new triterpenoid disaccharide saponin from sequential glycosylation of ganoderic acid A by two novel Bacillus glycosyltransferases. Biosci. Biotechnol. Biochem. 2021, 85, 687–690. [Google Scholar] [CrossRef]
- Koh, D.W.; Park, M.O.; Choi, S.W.; Lee, B.H.; Yoo, S.H. Efficient biocatalytic production of cyclodextrins by combined action of amylosucrase and cyclodextrin glucanotransferase. J. Agric. Food Chem. 2016, 64, 4371–4375. [Google Scholar] [CrossRef]
- Shimoda, K.; Kubota, N.; Hamada, H.; Hamada, H. Synthesis of resveratrol glycosides by plant glucosyltransferase and cyclodextrin glucanotransferase and their neuroprotective activity. Nat. Prod. Commun. 2015, 10, 995–996. [Google Scholar] [CrossRef]
- Zhang, T.; Gong, T.; Hu, Z.; Gu, A.; Yang, J.; Zhu, P. Enzymatic synthesis of unnatural ginsenosides using a promiscuous UDP-glucosyltransferase from Bacillus subtilis. Molecules 2018, 23, 2797. [Google Scholar] [CrossRef]
- Liang, H.; Hu, Z.; Zhang, T.; Gong, T.; Chen, J.; Zhu, P.; Li, Y.; Yang, J. Production of a bioactive unnatural ginsenoside by metabolically engineered yeasts based on a new UDP-glycosyltransferase from Bacillus subtilis. Metab. Eng. 2017, 44, 60–69. [Google Scholar] [CrossRef]
- Dai, L.; Li, J.; Yang, J.; Zhu, Y.; Men, Y.; Zeng, Y.; Cai, Y.; Dong, C.; Dai, Z.; Zhang, X.; et al. Use of a promiscuous glycosyltransferase from Bacillus subtilis 168 for the enzymatic synthesis of novel protopanaxatriol-type Ginsenosides. J. Agric. Food. Chem. 2018, 66, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Chen, Y.; Shi, J.; Wang, R.; Yang, Y.; Yang, L.; Zhao, S.; Wang, Z. Biosynthesis of rare 20(R)-protopanaxadiol/protopanaxatriol type ginsenosides through Escherichia coli engineered with uridine diphosphate glycosyltransferase genes. J. Ginseng Res. 2019, 43, 116–124. [Google Scholar] [CrossRef] [PubMed]
| Compound | Aqueous Solubility (mg/L) | Fold 1 | Reference |
|---|---|---|---|
| GAA | 15.37 ± 0.36 | 1 | [35] |
| GAA-15-G | 920.17 ± 44.97 | 59.9 | [35] |
| GAA-15,26-diglucoside | 15,743 ± 1270.39 | 1024.4 | [35] |
| GAA-G2 | >70,000 | >4554.3 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, T.-S.; Chiang, C.-M.; Wang, T.-Y.; Tsai, Y.-L.; Wu, Y.-W.; Ting, H.-J.; Wu, J.-Y. One-Pot Bi-Enzymatic Cascade Synthesis of Novel Ganoderma Triterpenoid Saponins. Catalysts 2021, 11, 580. https://doi.org/10.3390/catal11050580
Chang T-S, Chiang C-M, Wang T-Y, Tsai Y-L, Wu Y-W, Ting H-J, Wu J-Y. One-Pot Bi-Enzymatic Cascade Synthesis of Novel Ganoderma Triterpenoid Saponins. Catalysts. 2021; 11(5):580. https://doi.org/10.3390/catal11050580
Chicago/Turabian StyleChang, Te-Sheng, Chien-Min Chiang, Tzi-Yuan Wang, Yu-Li Tsai, Yu-Wei Wu, Huei-Ju Ting, and Jiumn-Yih Wu. 2021. "One-Pot Bi-Enzymatic Cascade Synthesis of Novel Ganoderma Triterpenoid Saponins" Catalysts 11, no. 5: 580. https://doi.org/10.3390/catal11050580
APA StyleChang, T.-S., Chiang, C.-M., Wang, T.-Y., Tsai, Y.-L., Wu, Y.-W., Ting, H.-J., & Wu, J.-Y. (2021). One-Pot Bi-Enzymatic Cascade Synthesis of Novel Ganoderma Triterpenoid Saponins. Catalysts, 11(5), 580. https://doi.org/10.3390/catal11050580








