Heterogeneous Photo-Fenton Reaction for Olive Mill Wastewater Treatment—Case of Reusable Catalyst
Abstract
:1. Introduction
2. Results and Discussions
2.1. OMW Characterization
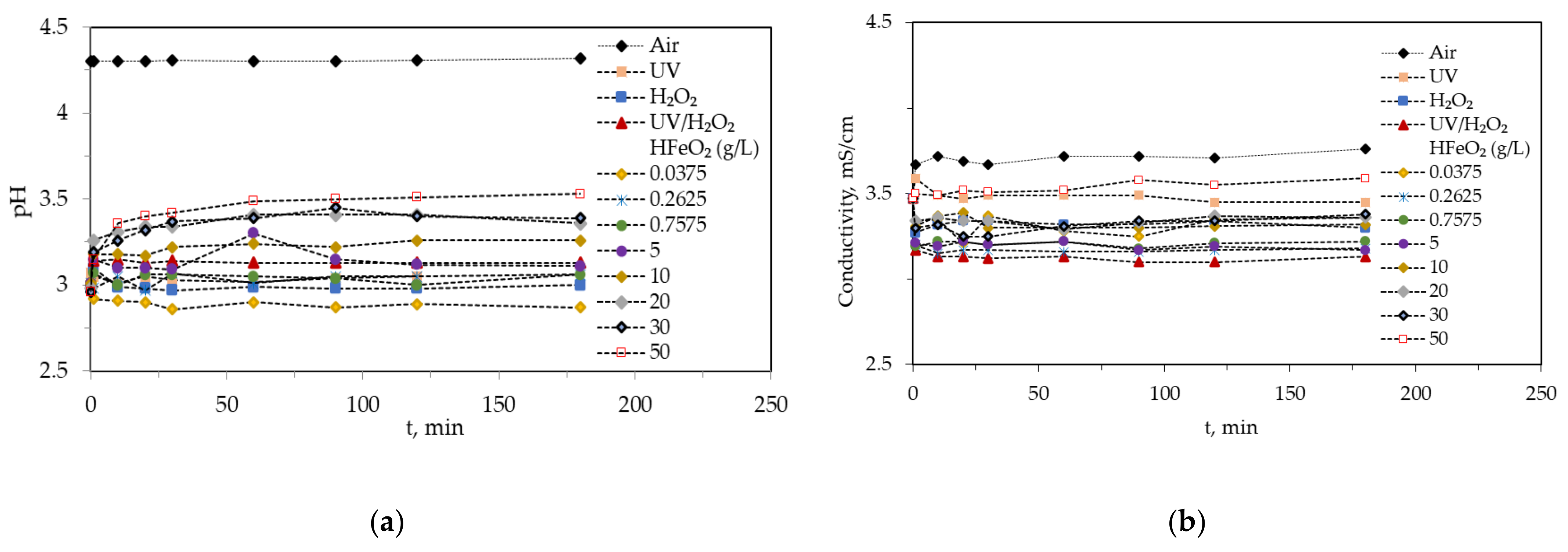
2.2. pH and Conductivity Variations
2.3. Organic Matter Degradation
2.4. Catalyst and Oxidant Agent Residue
2.5. Catalyst Reuse
3. Materials and Methods
3.1. Chemicals
3.2. Methodology
- Oxidation with only air.
- Oxidation with UV alone (photolysis).
- Oxidation with only H2O2.
- Oxidation with the system UV/H2O2.
3.3. Analytical Methods
3.4. Calculations and Kinetics
4. Conclusions
5. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azbar, N.; Bayram, A.; Filibeli, A.; Muezzinoglu, A.; Sengul, F.; Ozer, A. A Review of Waste Management Options in Olive Oil Production. Crit. Rev. Environ. Sci. Technol. 2004, 34, 209–247. [Google Scholar] [CrossRef]
- Niaounakis, M.; Halvadakis, C.P. Olive-Mill Waste Management–Literature Review and Patent Survey; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Niaounakis, M.; Halvadakis, C.P. Olive Processing Waste Management, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 5, pp. 23–64. [Google Scholar]
- Hodaifa, G.; Sanchez, S.; Martínez, M.E.; Órpez, R. Biomass production of Scenedesmus obliquus from mixtures of urban and olive-oil mill wastewaters used as culture medium. Appl. Energy 2013, 104, 345–352. [Google Scholar] [CrossRef]
- Hodaifa, G.; Martínez, M.E.; Sánchez, S. Use of industrial wastewater from olive-oil extraction for biomass production of Scenedesmus obliquus. Bioresour. Technol. 2008, 99, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Hodaifa, G.; Páez, A.; Agabo-García, C.; Ramos, J.; Gutiérrez, J.; Rosal, A. Flocculation on the Treatment of Olive Oil Mill Wastewater: Pretreatment. Int. J. Chem. Mol. Nuclear Mater. Metallur. Eng. 2015, 9, 645–650. [Google Scholar] [CrossRef]
- Bouknana, D.; Hammouti, B.; Salghi, R.; Jodeh, S.; Zarrouk, A.; Warad, I.; Aouniti, A.; Sbaa, M. Physicochemical characterization of olive oil mill wastewaters in the eastern region of Morocco. J. Mater. Environ. Sci. 2014, 5, 1039–1058. [Google Scholar]
- Vasileios, I.; Vaiopoulou, D.E.; Aivasidis, A. Fundamentals and applications of anaerobic digestion for sustainable treatment of food industry wastewater. In Utilization of By-Products and Treatment of Waste in the Food Industry; Oreopoulou, V., Russ, W., Eds.; Springer: Boston, MA, USA, 2007; pp. 73–98. [Google Scholar]
- Roig, A.; Cayuela, M.L.; Sánchez-Monedero, M.A. An overview on olive mill wastes and their valorisation methods. Waste Manag. 2006, 26, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Hodaifa, G.; Agabo-García, C.; Moya, A.; Pacheco, R.; Mateo, S. Treatment of Olive Oil Mill Wastewater by UV-Light and UV/H2O2 System. Int. J. Green Technol. 2015, 1, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Borja, R.; Raposo, F.; Rincón, B. Treatment technologies of liquid and solid wastes from two-phase olive oil mills. Grasas y Aceites 2006, 57, 32–46. [Google Scholar] [CrossRef]
- Mantzavinos, D.; Kalogerakis, N. Treatment of olive mill effluents. Environ. Int. 2005, 31, 289–295. [Google Scholar] [CrossRef]
- Paraskeva, P.; Diamadopoulos, E. Technologies for olive mill wastewater (OMW) treatment: A review. J. Chem. Technol. Biotechnol. 2006, 81, 1475–1485. [Google Scholar] [CrossRef]
- Tsagaraki, E.; Lazarides, H.N.; Petrotos, K.B. Olive Mill Wastewater Treatment. In Utilization of By-Products and Treatment of Waste in the Food Industry; Metzler, J.B., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 133–157. [Google Scholar]
- Khoufi, S.; Aloui, F.; Sayadi, S. Treatment of olive oil mill wastewater by combined process electro-Fenton reaction and anaerobic digestion. Water Res. 2006, 40, 2007–2016. [Google Scholar] [CrossRef]
- Cañizares, P.; Lobato, J.; Paz, R.; Rodrigo, M.A.; Saez, C. Advanced oxidation processes for the treatment of olive-oil mills wastewater. Chemosphere 2007, 67, 832–838. [Google Scholar] [CrossRef]
- Hodaifa, G.; García, C.A.; Borja, R. Study of Catalysts’ Influence on Photocatalysis/Photodegradation of Olive Oil Mill Wastewater. Determination of the Optimum Working Conditions. Catalyst 2020, 10, 554. [Google Scholar] [CrossRef]
- Hodaifa, G.; Gallardo, P.A.R.; García, C.A.; Kowalska, M.; Seyedsalehi, M. Chemical oxidation methods for treatment of real industrial olive oil mill wastewater. J. Taiwan Inst. Chem. Eng. 2019, 97, 247–254. [Google Scholar] [CrossRef]
- Benitez, F.J.; Beltran-Heredia, J.; Acero, J.L.; Pinilla, M.L. Simultaneous photodegradation and ozonation plus UV radiation of phenolic acids-major pollutants in agro-industrial wastewaters. J. Chem. Technol. Biotechnol. 1997, 70, 253–260. [Google Scholar] [CrossRef]
- Iboukhoulef, H.; Douani, R.; Amrane, A.; Chaouchi, A.; Elias, A. Heterogeneous Fenton like degradation of olive Mill wastewater using ozone in the presence of BiFeO3 photocatalyst. J. Photochem. Photobiol. A Chem. 2019, 383, 112012. [Google Scholar] [CrossRef]
- Pera-Titus, M.; García-Molina, V.; Baños, M.A.; Giménez, J.; Esplugas, S. Degradation of chlorophenols by means of advanced oxidation processes: A general review. Appl. Catal. B Environ. 2004, 47, 219–256. [Google Scholar] [CrossRef]
- Nieto, L.M.; Hodaifa, G.; Rodríguez, S.; Giménez, J.A.; Ochando, J. Degradation of organic matter in olive-oil mill wastewater through homogeneous Fenton-like reaction. Chem. Eng. J. 2011, 173, 503–510. [Google Scholar] [CrossRef]
- García, C.A.; Hodaifa, G. Real olive oil mill wastewater treatment by photo-Fenton system using artificial ultraviolet light lamps. J. Clean. Prod. 2017, 162, 743–753. [Google Scholar] [CrossRef]
- Alver, A.; Baştürk, E.; Kılıç, A.; Karataş, M. Use of advance oxidation process to improve the biodegradability of olive oil mill effluents. Process. Saf. Environ. Prot. 2015, 98, 319–324. [Google Scholar] [CrossRef]
- Michael, I.; Panagi, A.; Ioannou, L.; Frontistis, Z.; Fatta-Kassinos, D. Utilizing solar energy for the purification of olive mill wastewater using a pilot-scale photocatalytic reactor after coagulation-flocculation. Water Res. 2014, 60, 28–40. [Google Scholar] [CrossRef]
- Martínez, F.; Calleja, G.; Melero, J.; Molina, R. Heterogeneous photo-Fenton degradation of phenolic aqueous solutions over iron-containing SBA-15 catalyst. Appl. Catal. B Environ. 2005, 60, 181–190. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, J.; Fang, Y.; Cao, Z.; Chen, D.; Li, N.-J.; Xu, Q.-F.; Lu, J.-M. TiO2 Nanoparticles Anchored onto the Metal–Organic Framework NH2-MIL-88B(Fe) as an Adsorptive Photocatalyst with Enhanced Fenton-like Degradation of Organic Pollutants under Visible Light Irradiation. ACS Sustain. Chem. Eng. 2018, 6, 16186–16197. [Google Scholar] [CrossRef]
- Cong, Y.; Li, Z.; Zhang, Y.; Wang, Q.; Xu, Q. Synthesis of α-Fe2O3/TiO2 nanotube arrays for photoelectro-Fenton degradation of phenol. Chem. Eng. J. 2012, 191, 356–363. [Google Scholar] [CrossRef]
- Niu, L.; Wei, T.; Li, Q.; Zhang, G.; Xian, G.; Long, Z.; Ren, Z. Ce-based catalysts used in advanced oxidation processes for organic wastewater treatment: A review. J. Environ. Sci. 2020, 96, 109–116. [Google Scholar] [CrossRef]
- Giannakis, S.; Lin, K.-Y.A.; Ghanbari, F. A review of the recent advances on the treatment of industrial wastewaters by Sulfate Radical-based Advanced Oxidation Processes (SR-AOPs). Chem. Eng. J. 2021, 406, 127083. [Google Scholar] [CrossRef]
- Luo, H.; Zeng, Y.; He, D.; Pan, X. Application of iron-based materials in heterogeneous advanced oxidation processes for wastewater treatment: A review. Chem. Eng. J. 2021, 407, 127191. [Google Scholar] [CrossRef]
- Nieto, L.M.; Hodaifa, G.; Vives, S.R.; Casares, J.A.G.; Driss, S.B.; Grueso, R. Treatment of olive-mill wastewater from a two-phase process by chemical oxidation on an industrial scale. Water Sci. Technol. 2009, 59, 2017–2027. [Google Scholar] [CrossRef]
- Casadey, R.; Challier, C.; Altamirano, M.; Spesia, M.B.; Criado, S. Antioxidant and antimicrobial properties of tyrosol and derivative-compounds in the presence of vitamin B2. Assays of synergistic antioxidant effect with commercial food additives. Food Chem. 2021, 335, 127576. [Google Scholar] [CrossRef]
- Sánchez-Arévalo, C.M.; Jimeno-Jiménez, Á.; Carbonell-Alcaina, C.; Vincent-Vela, M.C.; Álvarez-Blanco, S. Effect of the operating conditions on a nanofiltration process to separate low-molecular-weight phenolic compounds from the sugars present in olive mill wastewaters. Process. Saf. Environ. Prot. 2021, 148, 428–436. [Google Scholar] [CrossRef]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive mill wastes: Biochemical characterizations and valorization strategies. Process. Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- Campos-Martin, J.M.; Blanco-Brieva, G.; Fierro, J.L.G. Hydrogen Peroxide Synthesis: An Outlook beyond the Anthraquinone Process. Angew. Chem. Int. Ed. 2006, 45, 6962–6984. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.; Schubert, D.; Amonette, J.; Nachimuthu, P.; Disselkamp, R. Ultraviolet stimulation of hydrogen peroxide production using aminoindazole, diaminopyridine, and phenylenediamine solid polymer complexes of Zn(II). J. Photochem. Photobiol. A Chem. 2008, 197, 245–252. [Google Scholar] [CrossRef]
- Patil, S.; Kumar, N. Sun light transmission through silica optical fibers for lighting: An experimental study. Mater. Today Proc. 2018, 5, 22943–22949. [Google Scholar] [CrossRef]
- DIN 38409-41. German Standard Methods for Examination of Water, Waste Water and Sludge; Summary Action and Material Characteristic Parameters (Group H); Determination of the Chemical Oxygen Demand (COD) in the Range over 15 mg/L (H41); German Institute for Standardisation (Deutsches Institut für Normung): Berlin, Germany, 1980. [Google Scholar]
- ISO 8466-1. Water Quality-Calibration and Evaluation of Analytical Methods and Estimation of Performance Characteristics—Part 1: Statistical Evaluation of the Linear Calibration Function; International Organization for Standardization: Genève, Switzerland, 1990. [Google Scholar]
- DIN 38402 A51. German Standard Methods for the Examination of Water, Waste Water and Sludge; General Information (Group A); Calibration of Analytical Methods, Evaluation of Analytical Results and Linear Calibration Functions Used to Determine the Performance Characteristics of Analytical Methods (A 51); German Institute for Standardisation (Deutsches Institut für Normung): Berlin, Germany, 1986. [Google Scholar]
- Dubber, D.; Gray, N.F. Replacement of chemical oxygen demand (COD) with total organic carbon (TOC) for monitoring wastewater treatment performance to minimize disposal of toxic analytical waste. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2010, 45, 1595–1600. [Google Scholar] [CrossRef]



| Parameters | Values | Spanish Maximum Values Allowed for Discharge into the Public Domain |
|---|---|---|
| pH | 4.37 | 5.5–9.5 |
| Electric conductivity (mS/cm) | 3.47 | - |
| Turbidity (FTU) | 3453 | - |
| Total solids (%) | 0.775 | - |
| Total suspended solids (%) | 0.699 | 30 |
| Organic matter (%) | 0.571 | - |
| Ashes (%) | 0.204 | - |
| Total phenolic compounds (mg/L) | 288 | 1 |
| Fat content (%) | 0.00360 | 40 |
| Dissolved O2 (mg/L) | 0.79 | - |
| BOD5 (mg O2/L) | 2030 | 300 |
| COD (mg O2/L) | 16586 | 500 |
| Total carbon (mg/L) | 4431.2 | - |
| Total organic carbon (mg/L) | 4252 | - |
| Inorganic carbon (mg/L) | 179 | - |
| Total nitrogen (mg/L) | 72.8 | - |
| Iron (mg/L) | 28.1 | 10 |
| Chlorides (mg/L) | 1132 | - |
| Sulphates (mg/L) | 29 | - |
| Na+ (mg/L) | 2.73 | - |
| NH4+ (mg/L) | 2.02 | - |
| K+ (mg/L) | 195 | - |
| Ca2+ (mg/L) | 8.00 | - |
| Oxidation System | HFeO2 (g/L) | Turbidity (FTU) | Residual H2O2 (g/L) | Residual Total-Fe (on OMW) (mg/L) |
|---|---|---|---|---|
| Air | - | 1469.5 | - | 0.431 |
| H2O2 | - | 930 | 4.58 | 0.199 |
| UV | - | 1111 | 1.06 | 0.368 |
| UV/H2O2 | - | 556 | 3.80 | 0.0650 |
| UV/H2O2/HFeO2 | 0.04 | 562 | 5.00 | 2.93 |
| 0.25 | 544 | 5.02 | 2.32 | |
| 0.75 | 569 | 4.99 | 2.66 | |
| 5.00 | 437 | 4.97 | 2.05 | |
| 10.0 | 469 | 4.78 | 2.32 | |
| 20.0 | 385 | 5.09 | 2.47 | |
| 30.0 | 447 | 4.94 | 3.12 | |
| 50.0 | 376 | 5.14 | 3.20 |
| Reutilization Number | % Amount Recovered After Each Use of HFeO2 |
|---|---|
| First | 87.6 |
| Second | 92.9 |
| Third | 91.0 |
| Parameter | Crude OMW | Sedimented OMW | Catalyst Reuse Number | %Average Final Removal | ||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||
| COD (mg O2/L) | 16,586 | 14,098 | 4913 | 4965 | 4666 | 65.6 |
| Total carbon (mg/L) | 4431.2 | 3776.5 | 1578 | 1333 | 1305 | 62.7 |
| TPCs (mg/L) | 288.0 | 279.4 | 30.4 | 16.4 | 20.1 | 92.0 |
| Turbidity (FTU) | 3453 | 3108 | 27.9 | 4.7 | 67.5 | 98.9 |
| Total iron (mg/L) | 28.1 | 26.9 | 1.9 | 2.0 | 1.6 | 93.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agabo-García, C.; Calderón, N.; Hodaifa, G. Heterogeneous Photo-Fenton Reaction for Olive Mill Wastewater Treatment—Case of Reusable Catalyst. Catalysts 2021, 11, 557. https://doi.org/10.3390/catal11050557
Agabo-García C, Calderón N, Hodaifa G. Heterogeneous Photo-Fenton Reaction for Olive Mill Wastewater Treatment—Case of Reusable Catalyst. Catalysts. 2021; 11(5):557. https://doi.org/10.3390/catal11050557
Chicago/Turabian StyleAgabo-García, Cristina, Naima Calderón, and Gassan Hodaifa. 2021. "Heterogeneous Photo-Fenton Reaction for Olive Mill Wastewater Treatment—Case of Reusable Catalyst" Catalysts 11, no. 5: 557. https://doi.org/10.3390/catal11050557
APA StyleAgabo-García, C., Calderón, N., & Hodaifa, G. (2021). Heterogeneous Photo-Fenton Reaction for Olive Mill Wastewater Treatment—Case of Reusable Catalyst. Catalysts, 11(5), 557. https://doi.org/10.3390/catal11050557







