Core-Shell ZnO@Cu2O as Catalyst to Enhance the Electrochemical Reduction of Carbon Dioxide to C2 Products
Abstract
1. Introduction
2. Results and Discussion
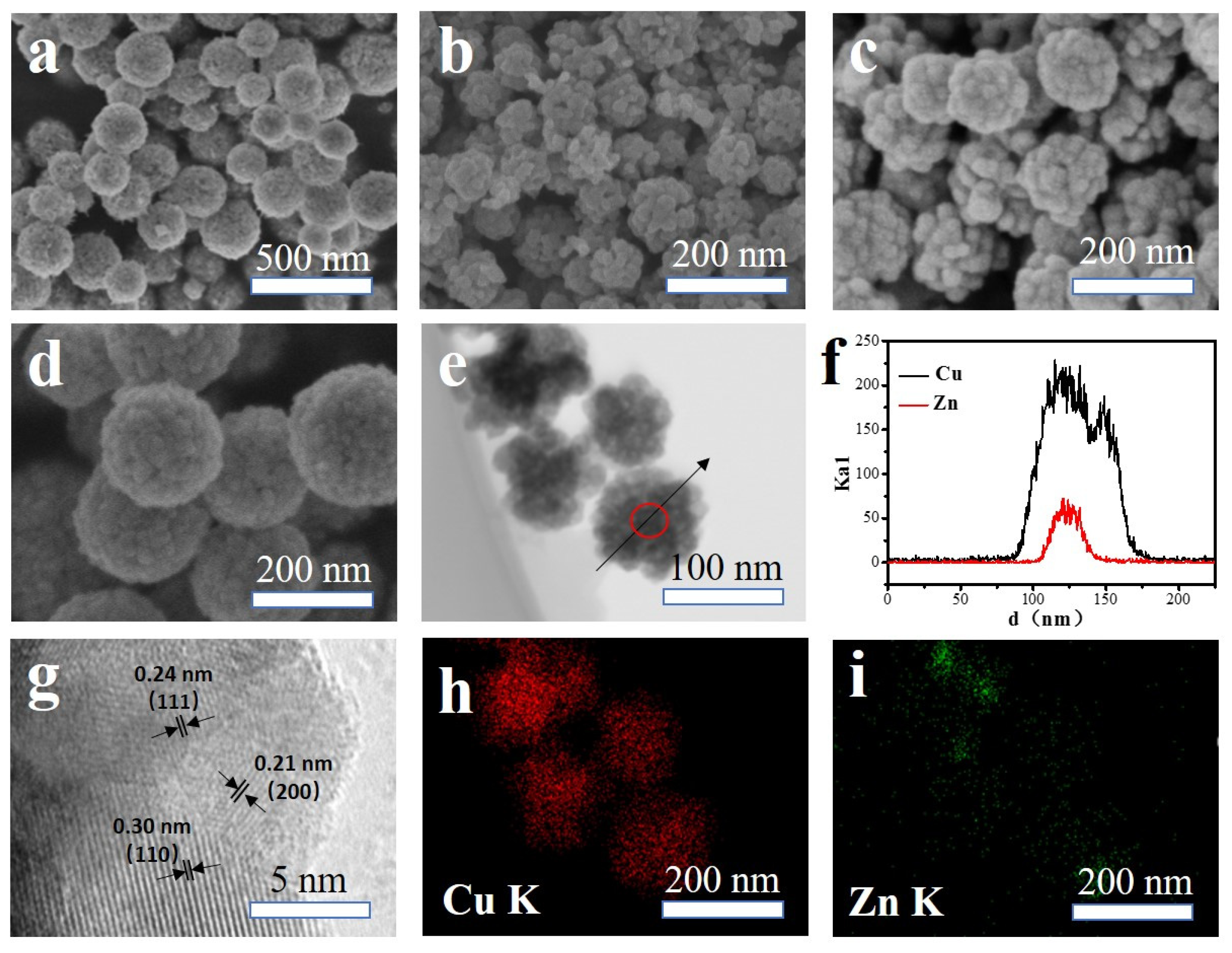
2.1. The Morphology and Structure of the Catalysts
2.1.1. ZnO Catalysts
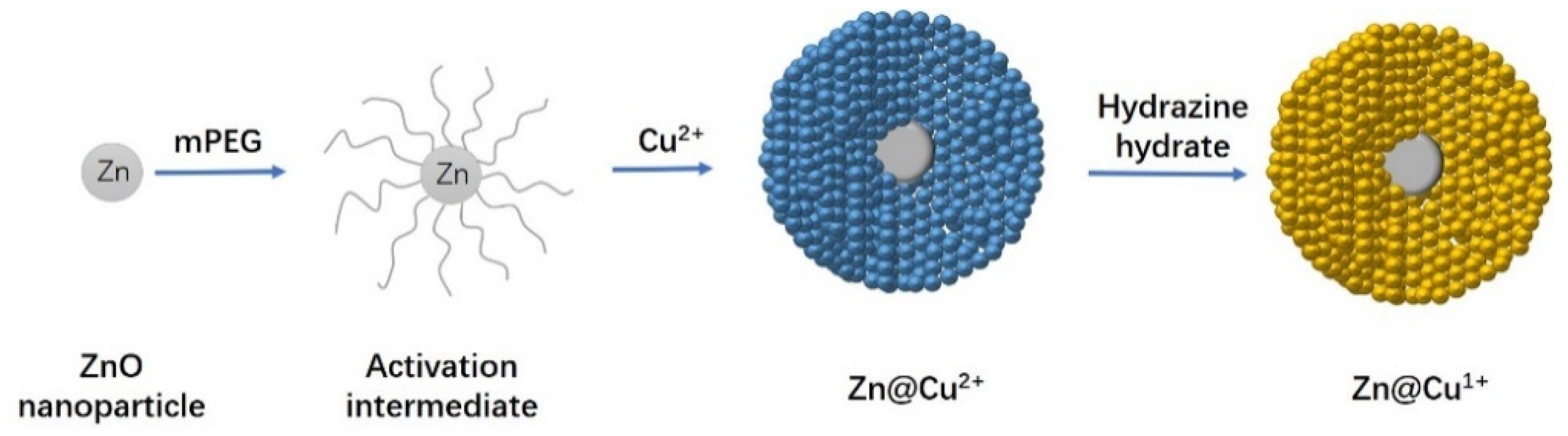
2.1.2. xZnO@yCu2O Catalyst
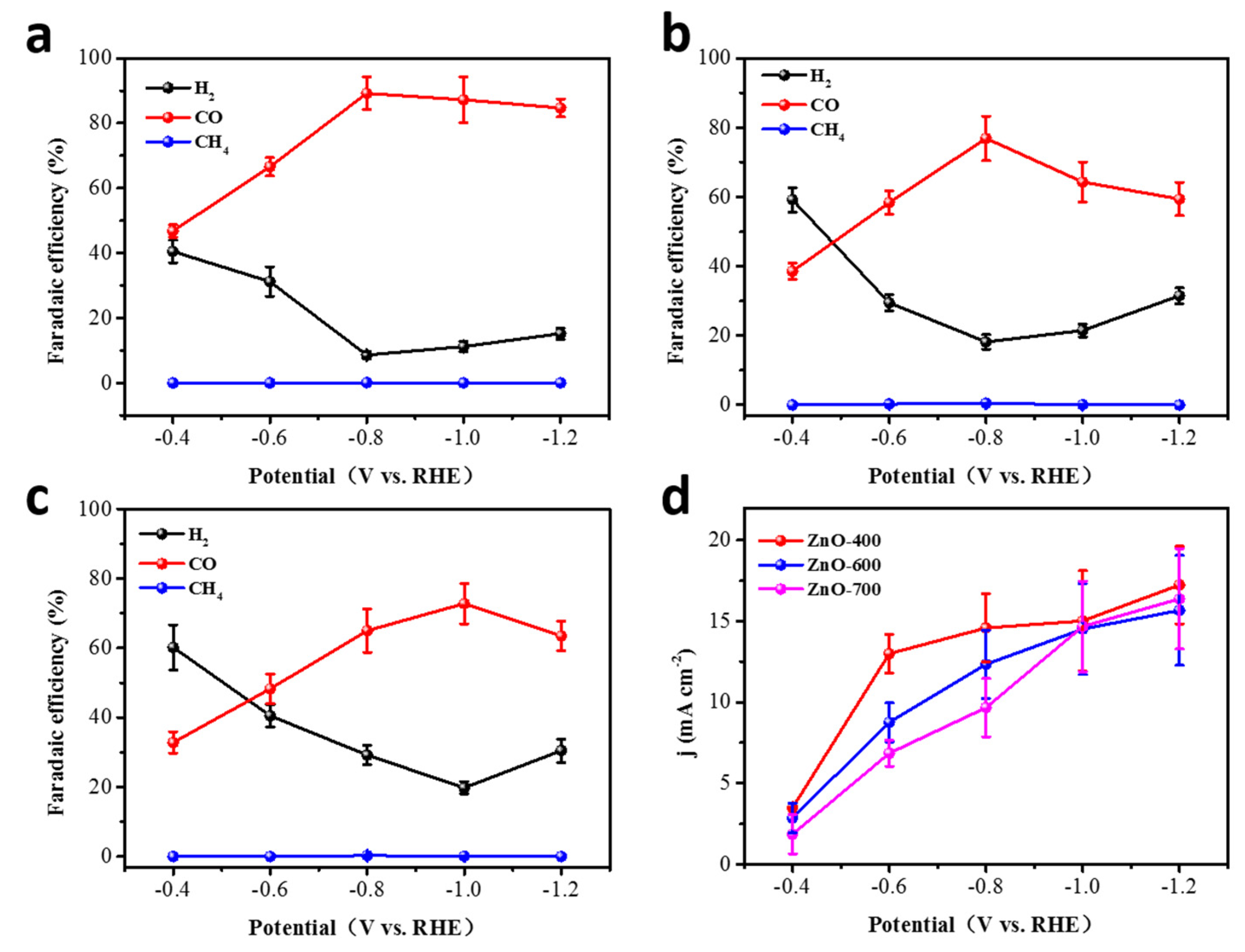
2.2. ECR Performance of ZnO Catalysts on the Catalyst
2.2.1. ZnO Catalysts
2.2.2. ZnO-400, Cu2O, and ZnO@4Cu2O Catalysts
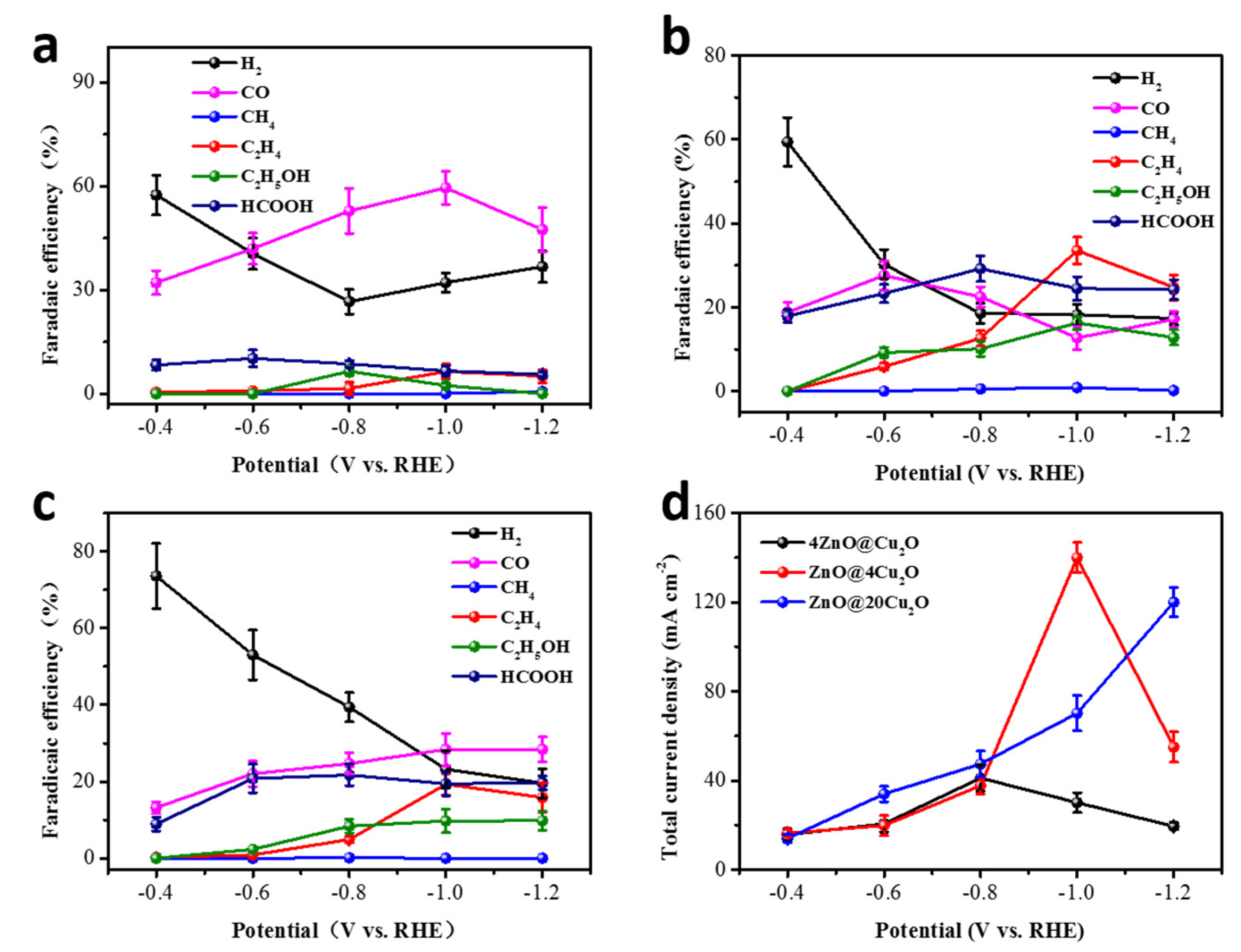
2.2.3. xZnO@yCu2O Catalysts
2.3. Electrochemical Characterization on xZnO@yCu2O Catalysts
3. Materials and Methods
3.1. Materials
3.2. Preparation of Catalysts
3.2.1. Synthesis of ZnO Catalysts
3.2.2. Preparation of the ZnO@Cu2O Catalyst
3.2.3. Synthesis of Cu2O Catalyst
3.2.4. Preparation of Gas Diffusion Electrode
3.3. Characterizations
3.4. Electrochemical CO2 Reduction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Goeppert, A.; Czaun, M.; Jones, J.P.; Prakash, G.K.S.; Olah, G.A. Recycling of carbon dioxide to methanol and derived products-closing the loop. Chem. Soc. Rev. 2014, 43, 7995–8048. [Google Scholar]
- Zhang, H.C.; Chang, X.X.; Chen, J.G.G.; Goddard, W.A.; Xu, B.J.; Cheng, M.J.; Lu, Q. Computational and experimental demonstrations of one-pot tandem catalysis for electrochemical carbon dioxide reduction to methane. Nat. Commun. 2019, 10, 3340. [Google Scholar] [CrossRef]
- Zhu, D.D.; Liu, J.L.; Qiao, S.Z. Recent advances in inorganic heterogeneous electrocatalysts for reduction of carbon dioxide. Adv. Mater. 2016, 28, 3423–3452. [Google Scholar] [CrossRef]
- Liu, Y.M.; Fan, X.F.; Nayak, A.; Wang, Y.; Shan, B.; Quan, X.; Meyer, T.J. Steering CO2 electroreduction toward ethanol production by a surface-bound Ru polypyridyl carbene catalyst on N-doped porous carbon. Proc. Natl. Acad. Sci. USA 2019, 116, 26353–26358. [Google Scholar] [CrossRef]
- Li, D.; Liu, T.T.; Huang, L.L.; Wu, J.J.; Li, N.; Zhen, L.; Feng, Y.J. Selective CO2–to–formate electrochemical conversion with core–shell structured Cu2O/Cu@C composites immobilized on nitrogen-doped graphene sheets. J. Mater. Chem. A 2020, 8, 18302–18309. [Google Scholar] [CrossRef]
- Li, J.C.; Kuang, Y.; Meng, Y.T.; Tian, X.; Hung, W.H.; Zhang, X.; Li, A.W.; Xu, M.Q.; Zhou, W.; Ku, C.S.; et al. Electroreduction of CO2 to formate on a copper-based electrocatalyst at high pressures with high energy conversion efficiency. J. Am. Chem. Soc. 2020, 142, 7276–7282. [Google Scholar] [CrossRef]
- Tan, Z.H.; Peng, T.Y.; Tian, X.J.; Wang, W.H.; Wang, X.S.; Yang, Z.X.; Ning, H.; Zhao, Q.S.; Wu, M.B. Controllable Synthesis of Leaf-Like CuO Nanosheets for Selective CO2 Electroreduction to Ethylene. ChemElectroChem 2020, 7, 2020–2025. [Google Scholar] [CrossRef]
- Raciti, D.; Livi, K.J.; Wang, C. Highly dense Cu nanowires for low-overpotential CO2 reduction. Nano Lett. 2015, 15, 6829–6835. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Sun, X.F.; Lu, L.; Yang, D.X.; Ma, J.; Zhu, Q.G.; Qian, Q.L.; Han, B.X. Efficient electroreduction of CO2 to C2 products over B-doped oxide-derived copper. Green Chem. 2018, 20, 4579–4583. [Google Scholar] [CrossRef]
- Wang, M.; Ren, X.N.; Yuan, G.; Niu, X.P.; Xu, Q.L.; Gao, W.L.; Zhu, S.K.; Wang, Q.F. Selective electroreduction of CO2 to CO over co-electrodeposited dendritic core-shell indium-doped Cu@Cu2O catalyst. J. CO2 Util. 2020, 37, 204–212. [Google Scholar] [CrossRef]
- Bai, X.W.; Li, Q.; Shi, L.; Niu, X.H.; Ling, C.Y.; Wang, J.L. Hybrid Cu0 and Cu x+ as atomic interfaces promote high-selectivity conversion of CO2 to C2H5OH at low potential. Small 2020, 16, 1901981. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Noh, Y.; Kim, Y.; Park, S.; Yoon, W.; Jang, D.; Choi, S.M.; Kim, W.B. Selective electrochemical CO2 conversion to multicarbon alcohols on highly efficient N-doped porous carbon-suported Cu catalysts. Green Chem. 2020, 22, 71–84. [Google Scholar] [CrossRef]
- Zhuang, T.T.; Liang, Z.Q.; Seifitokaldani, A.; Li, Y.; De Luna, P.; Burdyny, T.; Che, F.L.; Meng, F.; Min, Y.M.; Quintero-Bermudez, R. Steering post-C-C coupling selectivity enables high efficiency electroreduction of carbon dioxide to multi-carbon alcohols. Nat. Catal. 2018, 1, 421–428. [Google Scholar] [CrossRef]
- Kim, D.; Lee, S.; Ocon, J.D.; Jeong, B.; Lee, J.K.; Lee, J. Insights into an autonomously formed oxygen-evacuated Cu2O electrode for the selective production of C2H4 from CO2. Phys. Chem. Chem. Phys. 2015, 17, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Jung, H.; Kim, N.K.; Oh, H.S.; Min, B.K.; Hwang, Y.J. Mixed copper states in anodized Cu electrocatalyst for stable and selective ethylene production from CO2 reduction. J. Am. Chem. Soc. 2018, 140, 8681–8689. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Matios, E.; Wang, C.L.; Luo, J.M.; Lu, X.; Hu, X.F.; Li, W.Y. Rapid and scalable synthesis of cuprous halide-derived copper nano-architectures for selective electrochemical reduction of carbon dioxide. Nano Lett. 2019, 19, 3925–3932. [Google Scholar] [CrossRef] [PubMed]
- Loiudice, A.; Lobaccaro, P.; Kamali, E.A.; Thao, T.; Huang, B.H.; Ager, J.W.; Buonsanti, R. Tailoring copper nanocrystals towards C-2 Products in electrochemical CO2 Reduction. Angew. Chem. Int. Ed. 2016, 55, 5789–5792. [Google Scholar] [CrossRef]
- Ren, D.; Deng, Y.L.; Handoko, A.D.; Chen, C.S.; Malkhandi, S.; Yeo, B.S. Selective electrochemical reduction of carbon dioxide to ethylene and ethanol on copper(I) oxide catalysts. ACS Catal. 2015, 5, 2814–2821. [Google Scholar] [CrossRef]
- Li, C.W.; Kanan, M.W. CO2 Reduction at low overpotential on Cu electrodes resulting from the reduction of thick Cu2O films. J. Am. Chem. Soc. 2012, 134, 7231–7234. [Google Scholar] [CrossRef]
- Wang, W.H.; Ning, H.; Yang, Z.X.; Feng, Z.X.; Wang, J.L.; Wang, X.S.; Mao, Q.H.; Wu, W.T.; Zhao, Q.S.; Hu, H.; et al. Interface-induced controllable synthesis of Cu2O nanocubes for electroreduction CO2 to C2H4. Electrochim. Acta. 2019, 306, 360–365. [Google Scholar] [CrossRef]
- Su, X.S.; Sun, Y.M.; Jin, L.; Zhang, L.; Yang, Y.; Kerns, P.; Liu, B.; Li, S.Z.; He, J. Hierarchically porous Cu/Zn bimetallic catalysts for highly selective CO2 electroreduction to liquid C2 products. Appl. Catal. B Environ. 2020, 269, 118800. [Google Scholar] [CrossRef]
- Jimenez, C.; Garcia, J.; Martinez, F.; Camarillo, R.; Rincon, J. Cu nanoparticles deposited on CNT by supercritical fluid deposition for electrochemical reduction of CO2 in a gas phase GDE cell. Electrochim. Acta 2020, 337, 135663. [Google Scholar] [CrossRef]
- Ning, H.; Mao, Q.H.; Wang, W.H.; Yang, Z.X.; Wang, X.S.; Zhao, Q.S.; Song, Y.; Wu, M.B. N-doped reduced graphene oxide supported Cu2O nanocubes as high active catalyst for CO2 electroreduction to C2H4. J. Alloys Compd. 2019, 785, 7–12. [Google Scholar] [CrossRef]
- Feng, Y.H.; An, W.; Wang, Z.M.; Wang, Y.Q.; Men, Y.; Du, Y.Y. Electrochemical CO2 reduction reaction on M@Cu (211) bimetallic single-atom surface alloys: Mechanism, kinetics, and catalyst screening. ACS Sustain. Chem. Eng. 2020, 8, 210–222. [Google Scholar] [CrossRef]
- Gao, J.; Ren, D.; Guo, X.Y.; Zakeeruddin, S.M.; Gratzel, M. Sequential catalysis enables enhanced C–C coupling towards multi-carbon alkenes and alcohols in carbon dioxide reduction: A study on bifunctional Cu/Au electrocatalysts. Faraday Discuss 2019, 215, 282–296. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Ren, D.; Guo, X.Y.; Zakeeruddin, S.M.; Gratzel, M. Activation of bimetallic AgCu foam electrocatalysts for ethanol formation from CO2 by selective Cu oxidation/reduction. Faraday Discuss 2019, 215, 282–296. [Google Scholar] [CrossRef]
- Ren, D.; Gao, J.; Pan, L.F.; Wang, Z.W.; Luo, J.S.; Zakeeruddin, S.M.; Hagfeldt, A.; Gratzel, M. Atomic layer deposition of ZnO on CuO enables selective and efficient electroreduction of carbon dioxide to liquid fuels. Angew. Chem. Int. Ed. 2019, 58, 15036–15040. [Google Scholar] [CrossRef]
- Merino-Garcia, I.; Albo, J.; Solla-Gullon, J.; Montiel, V.; Lrabien, A. Cu oxide/ZnO-based surfaces for a selective ethylene production from gas-phase CO2 electroconversion. J. CO2 Util. 2019, 31, 135–142. [Google Scholar] [CrossRef]
- Payra, S.; Shenoy, S.; Chakraborty, C.; Tarafder, K.; Roy, S. Structure-sensitive electrocatalytic reduction of CO2 to methanol over carbon-supported intermetallic PtZn nano-alloys. ACS Appl. Mater. Inter. 2020, 12, 19402–19414. [Google Scholar] [CrossRef]
- Han, J.; Li, S.S.; Chen, J.Y.; Liu, Y.Q.; Geng, D.S.; Wang, D.W.; Zhang, L.C. Dendritic Ag/Pd Alloy Nanostructure Arrays for Electrochemical CO2 Reduction. ChemElectroChem 2020, 7, 2608–2613. [Google Scholar] [CrossRef]
- Zhi, X.; Jiao, Y.; Zheng, Y.; Vasileff, A.; Qiao, S.Z. Selectivity roadmap for electrochemical CO2 reduction on copper-based alloy catalysts. Nano Today 2020, 71, 104601. [Google Scholar] [CrossRef]
- Tsuji, M.; Yamaguchi, D.; Mstsunaga, M.; Alam, M.J. Epitaxial growth of Au@Cu core-shell nanocrystals prepared using the PVP-assisted polyol reduction method. Cryst. Growth DES 2010, 10, 5129–5135. [Google Scholar] [CrossRef]
- Tsai, Y.H.; Chanda, K.; Chu, Y.T.; Chiu, C.Y.; Huang, M.H. Direct formation of small Cu2O nanocubes, octahedra, and octapods for efficient synthesis of triazoles. Nanoscale 2014, 6, 8704–8709. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Yan, X.P.; Liu, S.J.; Wu, Y.H.; Wan, Q.; Sun, X.F.; Zhu, Q.G.; Liu, H.Z.; Ma, J.; Zheng, L.R.; et al. Highly efficient electroreduction of CO2 to C2+ alcohols on heterogeneous dual active sites. Angew. Chem. Int. Ed. 2020, 59, 16459–16464. [Google Scholar] [CrossRef] [PubMed]
- Schouten, K.J.P.; Qin, Z.S.; Gallent, E.P.; Koper, M.T.M. Two Pathways for the Formation of Ethylene in CO Reduction on Single-Crystal Copper Electrodes. J. Am. Chem. Soc. 2012, 134, 9864–9867. [Google Scholar] [CrossRef]
- Xu, H.P.; Rebollar, D.; He, H.Y.; Chong, L.N.; Liu, Y.Z.; Liu, C.; Sun, C.J.; Li, T.; Muntean, J.V.; Winans, R.E.; et al. Highly selective electrocatalytic CO(2) reduction to ethanol by metallic clusters dynamically formed from atomically dispersed copper. Nat. Energy 2020, 5, 623–632. [Google Scholar] [CrossRef]
- Song, H.; Song, J.T.; Kim, B.; Tan, Y.C.; Oh, J. Activation of C2H4 reaction pathways in electrochemical CO2 reduction under low CO2 partial pressure. Appl. Catal. B Environ. 2020, 272, 119049. [Google Scholar] [CrossRef]
- Shao, Q.; Wang, P.T.; Liu, S.H.; Huang, X.Q. Advanced engineering of core/shell nanostructures for electrochemical carbon dioxide reduction. J. Mater. Chem. A. 2019, 7, 20478–20493. [Google Scholar] [CrossRef]
- Liu, K.H.; Wang, J.Z.; Shi, M.M.; Yan, J.M.; Jiang, Q. Simultaneous Achieving of High Faradaic Efficiency and CO Partial Current Density for CO2 Reduction via Robust, Noble-Metal-Free Zn Nanosheets with Favorable Adsorption Energy. Adv. Energy Mater. 2019, 9, 1900276. [Google Scholar] [CrossRef]
- Birdja, Y.Y.; Perez-Gallent, E.; Figueiredo, M.C.; Gottle, A.J.; Calle-Vallejo, F.; Koper, M.T.M. Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels. Nat. Energy. 2019, 4, 732–745. [Google Scholar] [CrossRef]
- Tan, X.Y.; Yu, C.; Zhao, C.T.; Huang, H.W.; Yao, X.C.; Han, X.T.; Guo, W.; Cui, S.; Huang, H.L.; Qiu, J.S. Restructuring of Cu2O to Cu2O@Cu-Metal-Organic Frameworks for Selective Electrochemical Reduction of CO2. ACS Appl. Mater. Inter. 2019, 11, 9904–9910. [Google Scholar] [CrossRef]
- Suen, N.T.; Kong, Z.R.; Hsu, C.S.; Chen, H.C.; Tung, C.W.; Lu, Y.R.; Dong, C.L.; Shen, C.C.; Chung, J.C.; Chen, H.M. Morphology Manipulation of Copper Nanocrystals and Product Selectivity in the Electrocatalytic Reduction of Carbon Dioxide. ACS Catal. 2019, 9, 5217–5222. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, W.; Zuttel, A. Self-supported copper-based gas diffusion electrodes for CO2 electrochemical reduction. J. Mater. Chem. A 2019, 7, 26285–26292. [Google Scholar] [CrossRef]
- Reller, C.; Krause, R.; Volkova, E.; Schmid, B.; Neubauer, S.; Rucki, A.; Schuster, M.; Schmid, G. Selective Electroreduction of CO2 toward Ethylene on Nano Dendritic Copper Catalysts at High Current Density. Adv. Energy Mater. 2017, 7, 1602114. [Google Scholar] [CrossRef]
- Shen, S.B.; Peng, X.Y.; Song, L.D.; Qin, Y.; Li, C.; Zhuo, L.C.; He, J.; Ren, J.Q.; Liu, X.J.; Luo, J. AuCu Alloy Nanoparticle Embedded Cu Submicrocone Arrays for Selective Conversion of CO2 to Ethanol. Small 2019, 15, 1902229. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Peng, R.; Hensley, D.K.; Bonnesen, P.V.; Liang, L.B.; Wu, Z.L.; Meyer, H.M.; Chi, M.F.; Ma, C.; Sumpter, B.G.; et al. High-Selectivity Electrochemical Conversion of CO2 to Ethanol using a Copper Nanoparticle/N-Doped Graphene Electrode. Chemistryselect 2016, 1, 6055–6061. [Google Scholar] [CrossRef]
- Balamurugan, M.; Jeong, H.Y.; Choutipalli, V.S.K.; Hong, J.S.; Sao, H.; Saravanan, N.; Jang, J.H.; Lee, K.G.; Lee, Y.H.; Im, S.W.; et al. Electrocatalytic Reduction of CO2 to Ethylene by Molecular Cu-Complex Immobilized on Graphitized Mesoporous Carbon. Small 2020, 16, 2000955. [Google Scholar] [CrossRef]
- Wang, S.; Kou, T.; Baker, S.E.; Duoss, E.B.; Li, Y. Recent progress in electrochemical reduction of CO2 by oxide-derived copper catalysts. Mater. Today Nano 2020, 12, 100096. [Google Scholar] [CrossRef]
- Liu, Y.M.; Chen, S.; Quan, X.; Yu, H.T. Efficient Electrochemical Reduction of Carbon Dioxide to Acetate on Nitrogen-Doped Nanodiamond. J. Am. Chem. Soc. 2015, 137, 11631–11636. [Google Scholar] [CrossRef]
- Gao, Y.G.; Wu, Q.; Liang, X.Z.; Wang, Z.Y.; Zheng, Z.K.; Wang, P.; Liu, Y.Y.; Dai, Y.; Whangbo, M.H.; Huang, B.B. Cu2O Nanoparticles with Both {100} and {111} Facets for Enhancing the Selectivity and Activity of CO2 Electroreduction to Ethylene. Adv. Sci. 2020, 7, 1902820. [Google Scholar] [CrossRef]
- Liu, Z.F.; Jin, Z.G.; Li, W.; Qiu, J.J. Preparation of ZnO porous thin films by sol-gel method using PEG template. Mater. Lett. 2005, 59, 3620–3625. [Google Scholar] [CrossRef]
- Zhang, L.; Jing, H.; Boisvert, G.; He, J.Z.; Wang, H. Geometry Control and Optical Tunability of Metal-Cuprous Oxide Core-Shell Nanoparticles. ACS Nano 2012, 6, 3514–3527. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.C.; Sadakiyo, M.; Luo, R.; Heima, M.; Yamauchi, M.; Kenis, P.J.A. One-step electrosynthesis of ethylene and ethanol from CO2 in an alkaline electrolyzer. J. Power Sources 2016, 301, 219–228. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Ren, X.; Li, X.; Niu, X.; Wang, M.; Xu, S.; Wang, Z.; Han, Y.; Wang, Q. Core-Shell ZnO@Cu2O as Catalyst to Enhance the Electrochemical Reduction of Carbon Dioxide to C2 Products. Catalysts 2021, 11, 535. https://doi.org/10.3390/catal11050535
Zhu S, Ren X, Li X, Niu X, Wang M, Xu S, Wang Z, Han Y, Wang Q. Core-Shell ZnO@Cu2O as Catalyst to Enhance the Electrochemical Reduction of Carbon Dioxide to C2 Products. Catalysts. 2021; 11(5):535. https://doi.org/10.3390/catal11050535
Chicago/Turabian StyleZhu, Shuaikang, Xiaona Ren, Xiaoxue Li, Xiaopo Niu, Miao Wang, Shuang Xu, Zheyuan Wang, Yunxi Han, and Qingfa Wang. 2021. "Core-Shell ZnO@Cu2O as Catalyst to Enhance the Electrochemical Reduction of Carbon Dioxide to C2 Products" Catalysts 11, no. 5: 535. https://doi.org/10.3390/catal11050535
APA StyleZhu, S., Ren, X., Li, X., Niu, X., Wang, M., Xu, S., Wang, Z., Han, Y., & Wang, Q. (2021). Core-Shell ZnO@Cu2O as Catalyst to Enhance the Electrochemical Reduction of Carbon Dioxide to C2 Products. Catalysts, 11(5), 535. https://doi.org/10.3390/catal11050535





