Abstract
Guaiacol (2-methoxyphenol), an important fine chemical intermediate, is conventionally synthesized by liquid-phase processes with expensive, corrosive and toxic methylating agents such as dimethyl sulphate and dimethyl iodide. Recently, vapour-phase alkylation of catechol (1, 2-dihydroxybenzene) with methanol for the synthesis of guaiacol in the presence of heterogeneous catalysts has received more attention, as the route is economical and environmentally friendly. However, most of the investigated catalysts exhibited unsatisfactory catalytic performance for industrial applications. In this study, five metal phosphates M-P-O (M = La, Ce, Mg, Al, Zn) catalysts were synthesized and tested in the selective O-methylation of catechol with methanol. Among these catalysts, cerium phosphate (CP) showed the highest catalytic activity and guaiacol yield. Lanthanum phosphate (LP) was the second most active, which was still obviously better than magnesium phosphate (MP) and aluminium phosphate (ALP). Zinc phosphate (ZP) was not active in the reaction. Relevant samples were characterized by X-ray diffraction (XRD), Fourier transform infrared (FT-IR), scanning electron microscopy (SEM), N2 adsorption-desorption, temperature programmed desorption of NH3 or CO2 (NH3-TPD, CO2-TPD). The suitable acid-base properties contribute to the superior catalytic performance of CP. Long-term stability and regeneration tests were also studied.
1. Introduction
Guaiacol (2-methoxyphenol) is a significant intermediate in fine chemical production, especially for the synthesis of vanillin (4-hydroxy-3-methoxybenzaldehyde) [1,2]. Traditionally, guaiacol is prepared in liquid-phase by using dimethyl sulphate or alkyl halides as methylating agents and sodium hydroxide as homogeneous catalyst, however the methylating agents are expensive, corrosive, and toxic [3]. Currently, vapour-phase methylation of catechol (1, 2-dihydroxybenzene) with non-toxic methanol or dimethyl carbonate (DMC) has attracted more attention because of its economical and environmentally friendly [4,5]. From the point of practical application, methanol is more suitable as methylating agent than DMC due to its lower cost.
Various heterogeneous catalysts have been investigated in vapour-phase methylation of catechol with methanol, including metal oxides [6,7], sulphates [2], metal phosphates [5,8,9], and supported heteropoly acids [10]. In general, the catalytic performance of these catalysts is greatly affected by the operation conditions, catalyst structures, acid and/or base properties. However, a common problem is that most of the investigated catalysts exhibited unsatisfactory catalytic performance.
Metal phosphates are a family of metal salts with high thermal stability and acid-base properties, which can be used as catalysts directly for oxidative dehydrogenation of isobutane (2-methylpropane) [11] or ethylbenzene [12], hydrolysis [13,14] or decomposition [15,16] of fluoride, vapor-phase O-alkylation of phenol [17], and selective catalytic reduction of NO with NH3 [18]. Alternatively, metal phosphates can also be used to prepare supported catalysts such as Ru/CePO4 for the aerobic oxidation of alcohols [19], Pd/AlPO4 for hydrogenation of naphthalene [20], metal phosphate-supported Au or Pt for CO oxidation [21,22], metal phosphate-supported RuOx catalysts for N2O decomposition [23].
Zhang and co-workers [9] found that the acid-base properties of aluminium phosphate with lower Ti content were weaker, favouring guaiacol formation and catalytic stability. In addition, the catalytic performance of aluminophosphates could be enhanced by adjusting P/Al ratio [24] or coating microporous aluminophosphate on mesoporous SBA-15 [25], further confirming that suitable acid-base properties were crucial to vapour-phase methylation of catechol with methanol. However, Jafari et al. [5] found that catechol conversion and product distribution depended highly on acidity of Ti or Cs modified lanthanum phosphate. Thus, the reaction mechanism is still controversial.
Previous studies mainly focused on a particular metal phosphate. The objective of the current work is to systematically study the catalytic performance of different metal phosphates M-P-O (M = La, Ce, Mg, Al, Zn) in vapour-phase methylation of catechol with methanol. Among these catalysts, cerous phosphate (CP) is the most active one. Reasons for the observations were elucidated through detailed characterization.
2. Results
2.1. Basic Physicochemical Properties of Metal Phosphates Samples
XRD patterns of the as-synthesized products are shown in Figure 1. According to the literatures [26,27,28,29], the characteristic diffraction peaks are obtained for crystallized CP, LP, MP and ZP samples. ALP show a broad peak between 20° and 30° corresponding to the characteristic of amorphous phase, and two small sharp peaks (around 20°) corresponding to crystalline aluminium phosphate [30]. FT-IR test was carried out to further identify the products.
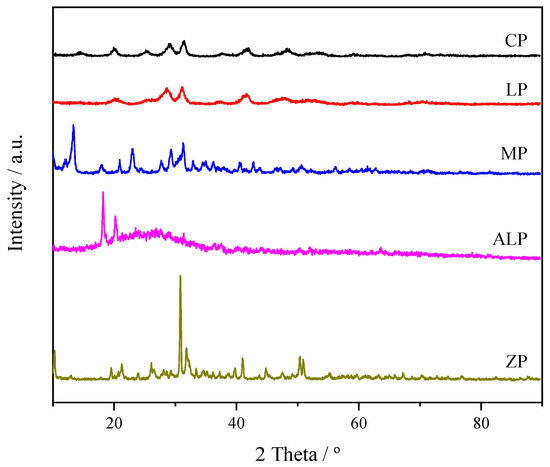
Figure 1.
XRD patterns of different metal phosphate catalysts.
Figure 2 shows the FT-IR spectra of different M-P-O samples. As shown for all metal phosphates, the absorption band at 3430 cm−1 and 1628 cm−1 are ascribed to O–H stretching vibration and O–H bending vibration of adsorbed water molecules, respectively [31,32]. The band at ca. 1046 cm−1 and 615 cm−1 are attributed to P–O asymmetric stretch vibration and O=P–O bending vibration, and the bands at ca. 538 cm−1 are due to O–P–O bending vibration typical for PO43− [32,33]. These observations confirm the formation of metal phosphates, thus supporting the XRD results.
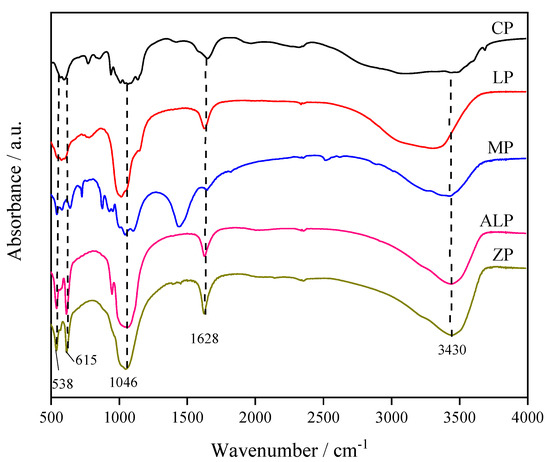
Figure 2.
FT-IR spectra of different metal phosphate catalysts.
The morphology of M-P-O samples was investigated with SEM analysis. As shown in Figure 3 and Figure S1, metal phosphates have irregular particles except LP. LP is mainly composed of small cubic column with a length of about 5 μm. The particle size distribution of CP and ALP are mainly in range of 1–5 μm, while that of ZP is in range of 10–50 μm. The average particles sizes of MP are about 2 μm. The degree of agglomeration is increased from CP to LP, and further to MP. However, ALP and ZP are highly dispersed.
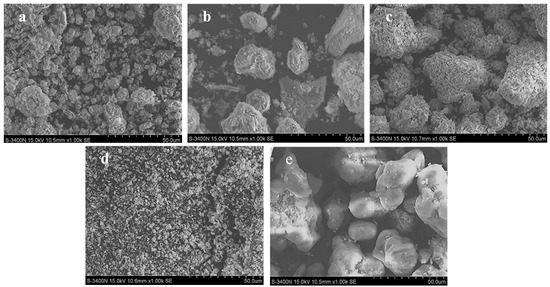
Figure 3.
SEM images of (a) CP, (b) LP, (c) MP, (d) ALP, and (e) ZP.
Table 1 summarizes the textual properties of M-P-O samples obtained by the N2 adsorption-desorption data. The BET surface areas of catalysts show the sequence of LP > CP > MP > ALP > ZP. As shown in Figure 3, an increase in particle size of ZP may lead to a significant decrease in the BET surface area. The pore volumes of LP and MP are much larger than the others, which is probably owing to secondary pores formed by obvious aggregation.

Table 1.
Textural properties of different metal phosphate catalysts.
2.2. Catalytic Performance
The catalytic activity and selectivity for the selective O-methylation of catechol had been investigated over the M-P-O catalysts. As presented in Figure 4a, for the CP sample, an initial catechol conversion of 80.3% and falls to 65.3% after 11 h on stream. The catechol conversion observed for the LP catalyst keeps constant at about 40.4% during 11 h on stream. However, the initial catechol conversion on MP (10.5%) and ALP (7.2%) are obvious lower than CP and LP, and deactivation is rather fast. ZP shows almost no conversion of catechol, indicating it is inactive in selective O-methylation of catechol. Apparently, the catechol conversion on catalysts follows the sequence of CP > LP > MP > ALP > ZP.
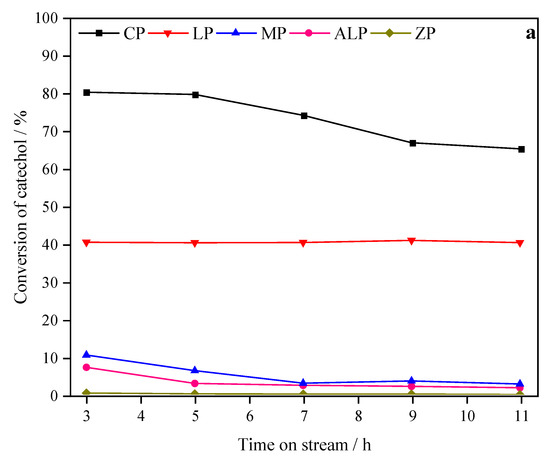
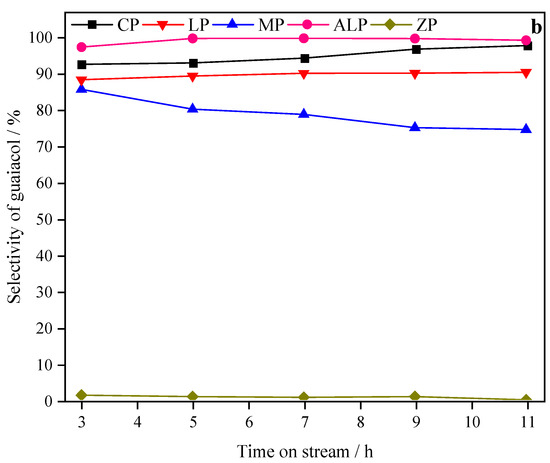
Figure 4.
Effect of time on stream on catechol conversion (a) and guaiacol selectivity (b) over different metal phosphate catalysts. Reaction conditions: atmospheric pressure; T = 270 °C; WHSV = 0.4 h−1.
The effects of time on stream on the selectivity of guaiacol for each catalyst is shown in Figure 4b. As shown, the initial guaiacol selectivity of CP, LP, MP, and ALP are 92.6%, 88.4%, 85.7%, 97.4%, respectively. However, ZP shows no selectivity. The guaiacol selectivity of CP, LP, and ALP increased slightly with time. After 11 h on stream, the guaiacol selectivity of ALP and CP was as high as 99.3% and 97.8%, respectively. In contrast, the guaiacol selectivity of MP decreased with time. The guaiacol selectivity follows the sequence of ALP > CP > LP > MP > ZP.
2.3. TPD Characterization
For selective O-methylation of catechol and some other alkylation reactions, the catalytic activity is correlated to the acidity and basicity of catalysts [25,32,34].
The acid strength and the amounts of acid sites on M-P-O catalysts were characterized by NH3-TPD (Figure 5, Table 2). As shown, M-P-O catalysts show two types of peaks below and above 300 °C, corresponding to NH3 desorbed from the weak and strong acid sites, respectively [35]. The temperature corresponding to the desorption peak of weak acid sites follows the sequence of ZP < MP < CP < ALP < LP, consistent with the sequence of weak acid strength. The area of NH3 desorption peak follows the sequence of CP > LP > ALP >MP > ZP, indicating that the amount of weak acid sites of CP is the highest. For strong acid sites, the desorption temperature follows the sequence of CP = ALP < ZP < MP, and the area of NH3 desorption peak follows the sequence of MP ≈ ZP > ALP > CP. LP have no desorption of NH3 above 300 °C, indicating the lack of strong acid sites.
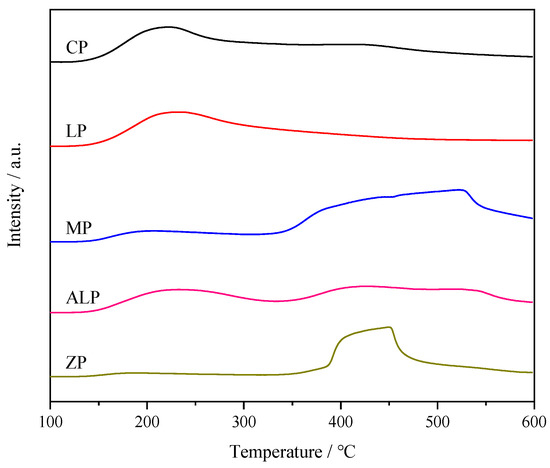
Figure 5.
NH3-TPD curves of different metal phosphate catalysts.

Table 2.
Textural properties of different metal phosphate catalysts.
The basicity of the catalysts is evaluated by CO2-TPD (Figure 6, Table 3). The strength and the amounts of base sites were related to the temperature and area of CO2 desorption peak, respectively. Figure 6 shows CP, LP and ALP have obvious peaks corresponding to CO2 desorption, while MP have very limited desorption of CO2. The CO2 desorption temperature of CP, MP and ALP are very close, and higher than that of LP, indicating LP has relatively weak basicity. On the other hand, ZP shows no CO2 desorption peak, indicating the absence of base sites on these catalysts. The amounts of base sites of these catalysts follow the sequence of MP < CP < LP < ALP (Table 3). The interpretation of the TPD data will be presented in Section 4.
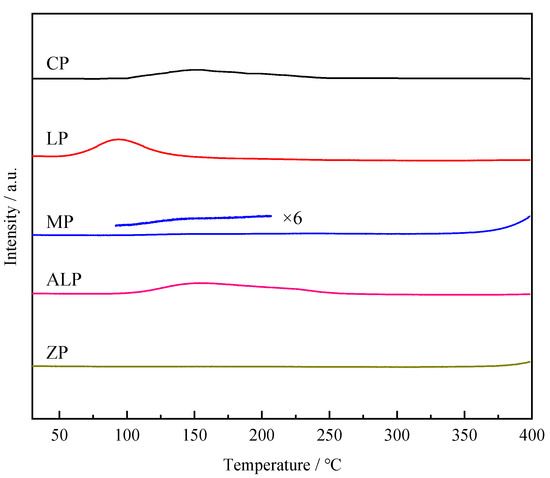
Figure 6.
CO2-TPD curves of different metal phosphate catalysts.

Table 3.
CO2-TPD results of different metal phosphate catalysts.
2.4. Catalytic Stability and Catalyst Regeneration
Figure 7 shows the catalytic performance of the most active CP catalyst in two-cycle experiments. In the first cycle, the catechol conversion of CP decreased from 71.7% to 43.3% and gradually stabilized after 72 h on stream (Figure 7a). Then the catalyst was regenerated, and its catechol conversion recovered from 43.3% to 66.5%. The catechol conversion of regenerated CP in the second cycle decreased from 66.5% to 37.8% with the time on stream, consistent with the trend seen in the first cycle.
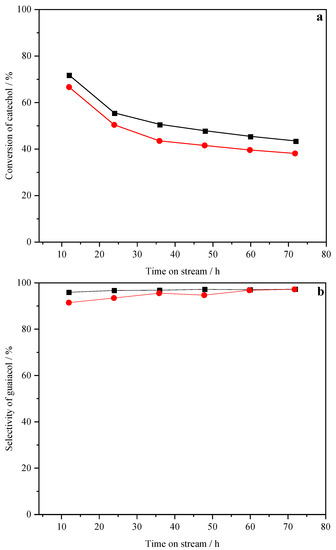
Figure 7.
Catechol conversion (a) and guaiacol selectivity (b) of CP catalyst in cycle experiment, (■) the first cycle, (●) the second cycle.
As shown in Figure 7b, the initial guaiacol selectivity of the regenerated CP was slightly lower than that of fresh CP, but increased with the reaction time, and finally close to the guaiacol selectivity of fresh CP (97.6%).
3. Discussion
In this work, metal phosphates were prepared by precipitation method. According to XRD data (Figure 1) and FT-IR spectra (Figure 2), all as-synthesized samples were metal phosphates in nature. Among them, CP, LP, MP and ZP were in crystallized phase, whereas ALP was in amorphous phase. We found that the initial catalytic activities (conversion of catechol) of metal phosphates follow the sequence of CP > LP > MP > ALP > ZP (Figure 4a), while the initial guaiacol selectivity follows the sequence of ALP > CP > LP > MP > ZP (Figure 4b), thus resulting in the initial guaiacol yield follows the sequence of CP (74.4%) > LP (39.4%) > MP (9.0%) > ALP (6.4%) > ZP (0%).
The selective O-methylation of catechol with methanol is commonly regarded as an acid-base catalytic reaction for the production of guaiacol [4,9,10,24,25]. Therefore, the different acid-base properties of metal phosphates may have an effect on their catalytic performance. From NH3-TPD data (Figure 5), it can be seen that there are two kinds of acid sites in metal phosphates samples, i.e., weak and strong acid sites. Generally, coke deposition easily occurs on strong acid sites [4,9]. The amount of strong acid sites on MP, ALP, ZP was much higher than CP and LP (Table 2), thus coke rapidly formed and blocked acid sites of MP, ALP and ZP, further causing their low initial activities and quick deactivation in the reaction. Additionally, MP, ALP and ZP samples were black in colour after 11 h reaction, indicating the fast coke formation.
The catalytic activity in O-methylation of catechol is correlated with the weak acid properties of metal phosphates [8,24]. The weaker strength and larger amount of the weak acid sites (Figure 5) might be responsible for the higher catechol conversion of CP. However, due to the presence of small amount of strong acid sites on CP, the catechol conversion decreased by 15.0% after 11 h reaction. In contrast, the catechol conversion of LP kept constant because of the absence of strong acid sites.
The weak base sites on the catalyst are beneficial for forming O-methylated products [2,6,24]. By comparing ALP, CP, and MP with similar base strength, it is noteworthy that the guaiacol selectivity is associated with the amount of base sites, i.e., the selectivity increases as the amount of base sites increases (Figure 4b or Figure 6). Though CP and LP possess comparable amounts of base sites, the guaiacol selectivity of CP is higher because of its suitable base strength (Figure 1). ZP shows no guaiacol selectivity, probably due to its strong acid sites and the absence of weak basic sites. From the above results, it can be concluded that the high catalytic activity and selectivity of CP own to its suitable weak acid-base sites. Moreover, it further confirms the acid-base co-operation mechanism in selective O-methylation of catechol with methanol [2,9].
The most active CP is compared with other acid and/or basic catalysts. As shown in Figures S2–S4, SAPO-34 and HAP samples exhibits poor catalytic performance, which is related to their high amount of strong acid sites and the presence of strong basic sites (CO2 desorbed above 250 °C). Similar to the strong acid sites, coke is easily formed on strong basic sites [9]. Besides, the blockage of SAPO-34 pores also results in the decrease of catalytic performance [4]. Therefore, irregular pores created by the agglomeration of CP and MP may be blocked by oligomeric products and coke (Figure 3, Table 1), this is probably another reason for deactivation of CP and MP.
In the cycle experiment (Figure 7), although the catalytic activity of the fresh and regenerated CP both decrease with the time on stream, most of the activity of the used CP can be recovered by regeneration, indicating that coke deposition is the main reason for the loss of catalytic activity of CP. It is noteworthy that the guaiacol selectivity of fresh and regenerated CP keep constant at 97.6% as the reaction proceeded, which was significantly higher than that in the literature [2,5,25]. High guaiacol selectivity facilitates the separation and purification of the products.
4. Materials and Methods
4.1. Materials
The chemical reagents used included catechol, methanol, aqueous ammonia, phosphoric acid, La(NO3)3·6H2O (lanthanum nitrate hydrate), Ce(NO3)3·6H2O (cerium (III) nitrate hexahydrate), Mg(NO3)2 (magnesium nitrate), Al(NO3)3·9H2O (aluminum nitrate nonahydrate), and Zn(NO3)2·6H2O (zinc nitrate hexahydrate). All the above reagents of analytical grade were obtained from Adamas and used as received.
4.2. Sample Preparation
Metal phosphates M-P-O (M = La, Ce, Mg, Al, Zn) catalysts were prepared by precipitation method. In a typical synthesis, 8.702 g La(NO3)3·6H2O was dissolved in 1000 mL deionized water, then 4.980 g phosphoric acid solution was added dropwise with vigorous stirring. The pH of the gel was adjusted to 8.0 by adding concentrated aqueous ammonia. After stirring continuously at room temperature for 24 h, the product was separated by filtration and washed with water, dried at 100 °C overnight, and calcinated at 400 °C for 4 h. The obtained sample is denoted as LP.
Other metal phosphates M-P-O (M = Ce, Mg, Al, Zn) samples were prepared following the same synthesis method as LP. M-P-O prepared from Ce(NO3)3·6H2O, Mg(NO3)2, Al(NO3)3·9H2O, and Zn(NO3)2·6H2O are denoted as CP, MP, ALP, and ZP, respectively.
4.3. Catalyst Characterization
X-ray diffraction (XRD) patterns were recorded on a Rigaku Ultima IV diffractometer (Rigaku, Tokyo, Japan) with Cu Kα radiation, operated at 40 kV and 40 mA.
Fourier transform infrared (FT-IR) spectra were recorded on a Nicolet iS5 spectrometer (Thermo Scientific, Baltimore, MD, USA). A sample was mixed with dry KBr and compressed at 10 MPa for 5 min. Measurements were conducted at a resolution of 0.8 cm−1, using the spectrum of pure KBr tablet as the background.
Scanning electron microscopy (SEM) experiments were conducted on a Hitachi S-4000 instrument (Hitachi, Tokyo, Japan).
Textural properties were determined with a Micrometrics ASAP 2020 M+C adsorption apparatus (Micromeritics, Norcross, GA, USA) at liquid N2 temperature (−196 °C), using N2 as adsorbate. Before measurement, all the samples were degassed at 200 °C for 10 h.
Temperature programmed desorption of NH3 or CO2 was performed on a conventional flow apparatus. Prior to adsorption, 0.15 mg sample (40–60 mesh) was pretreated in flowing N2 (30 mL/min) at 300 °C for 2 h, cooled down to 30 °C. Then the sample was exposed to 30 mL/min of 10% NH3/N2 or 10% CO2/N2 flow for 30 min and swept with N2 (30 mL/min) to remove physically adsorbed NH3 or CO2. NH3 or CO2 desorption was conducted in flowing N2 (30 mL/min) by raising the temperature at a heating rate of 10 °C/min. TCD or MS ()(Pfeiffer Omnistar, Germany) was used to detect desorbed NH3 and CO2, respectively.
4.4. Catalytic Evaluation
Vapour-phase selective O-methylation of catechol with methanol was carried out in a continuous-flow, fixed-bed microreactor under atmospheric pressure. A 1.5 g catalyst (40–60 mesh) was placed in a quartz tube (i.d. = 10 mm), and pretreated in flowing N2 (50 mL/min) at 400 °C for 2 h. After the catalyst cooled down to 270 °C, the reactant mixture (catechol: methanol = 1:6, molar ratio) was pumped through a piston pump (WHSV of reactant mixture was 0.4 h−1). The effluent products were condensed using a cold trap, and then analysed via an FLGC9720 gas chromatograph (Fuli, Zhejiang, China) with a flame ionization detector (FID). The catechol conversion was calculated as ([catechol]in − [catechol]out)/[catechol]in × 100%, where [catechol]in is the catechol content in the reactant mixture (no reaction occurs), and [catechol]out is the catechol content in the effluent products.
Long-term stability and catalyst regeneration tests were conducted, they were measured as function of time on stream at 270 °C. After the first cycle of stability test, the catalyst was regenerated in flowing air (40 mL/min) at 400 °C for 6 h, then cooled down to 270 °C, the reactant mixture was fed again (denoted as the second cycle).
5. Conclusions
Different metal phosphates M-P-O (M = La, Ce, Mg, Al, Zn) catalysts were prepared by precipitation method. MP, ALP and ZP with high amount of strong acid sites showed low catalytic activity in the selective O-methylation of catechol with methanol. The stable catalytic activity of LP can be ascribed to its absence of strong acid sites. The higher activity of CP can be related to the weaker strength and higher amount of the weak acid sites. In addition, the suitable base strength and larger amount of weak base sites contribute to the higher guaiacol selectivity of CP. Most of the catalytic activity of used CP can be recovered by regeneration. Although here we only studied the selective O-methylation of catechol with methanol, we believe that potential uses of metal phosphates can be explored in other alkylation reactions in the future.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/catal11050531/s1, Figure S1: SEM images of (a) CP, (b) LP, (c) MP, (d) ALP, and (e) ZP. (a magnification of 2000), Figure S2: Effect of time on stream on catechol conversion (a) and guaiacol selectivity (b) over CP, SAPO-34, HAP catalysts. Reaction conditions: atmospheric pressure; T=270 °C; WHSV=0.4 h−1, Figure S3: NH3-TPD curves of CP, SAPO-34, HAP catalysts, Figure S4: CO2-TPD curves of CP, SAPO-34, HAP catalysts.
Author Contributions
Investigation, H.M., X.L., F.X., Z.X., W.Z. and T.M.; supervision, H.M. and T.M.; writing—original draft, X.L.; writing—review and editing, H.M. and T.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- Dorothea, G.; Barbara, E. Ullmann´s Encyclopaedia of Industrial Chemistry; Verlagsgesellschaft: Weinheim, Germany, 1991; Volume A19. [Google Scholar]
- Vishwanathan, V.; Balakrishna, G.; Rajesh, B.; Jayasri, V.; Sikhwivhilu, L.M.; Coville, N.J. Alkylation of catechol with methanol to give guaiacol over sulphate-modified zirconia solid acid catalysts: The influence of structural modification of zirconia on catalytic performance. Catal. Commun. 2008, 9, 2422–2427. [Google Scholar] [CrossRef]
- Stoochnoff, B.A.; Benoiton, N.L. The methylation of some phenols and alcohols with sodium hydride / methyl iodide in tetrahydrofuran at room temperature. Tetrahedron Lett. 1973, 14, 21–24. [Google Scholar] [CrossRef]
- Luque, R.; Campelo, J.M.; Conesa, T.D.; Luna, D.; Marinas, J.M.; Romero, A.A. Catechol O-methylation with dimethyl carbonate over different acid-base catalysts. New J. Chem. 2006, 30, 1228–1234. [Google Scholar] [CrossRef]
- Jafari, A.A.; Khodadadi, A.; Mortazavi, Y. Vapor-phase selective O-alkylation of catechol with methanol over lanthanum phosphate and its modified catalysts with Ti and Cs. J. Mol. Catal. A 2013, 372, 79–83. [Google Scholar] [CrossRef]
- Vishwanathan, V.; Ndou, S.; Sikhwivhilu, L.; Plint, N.; Raghavan, K.V.; Coville, N.J. Evidence for weak base site participation in the vapour phase methylation of catechol over solid base catalysts. Chem. Commun. 2001, 10, 893–894. [Google Scholar] [CrossRef]
- Fu, Z.H.; Yu, Y.; Yin, D.L.; Xu, Y.Z.; Liu, H.P.; Liao, H.Y.; Xu, Q.; Tan, F.Q.; Wang, J. Vapor-phase highly selective O-methylation of catechol with methanol over ZnCl2 modified gamma-Al2O3 catalysts. J. Mol. Catal. A 2005, 232, 69–75. [Google Scholar] [CrossRef]
- Li, X.M.; Zhang, W.X.; Liu, G.; Jiang, L.; Zhu, X.M.; Pan, C.L.; Jiang, D.Z.; Tang, A.Q. Effect of the P/Al ratio of Al-P-O on the catalytic activity of O-methylation of catechol with methanol. React. Kinet. Catal. Lett. 2003, 79, 365–371. [Google Scholar] [CrossRef]
- Zhu, X.M.; Li, X.M.; Jia, M.J.; Liu, G.; Zhang, W.X.; Jiang, D.Z. Vapour-phase selective O-methylation of catechol with methanol over Ti-containing aluminium phosphate catalysts. Appl. Catal. A 2005, 282, 155–161. [Google Scholar] [CrossRef]
- Zhu, X.M.; Li, X.M.; Zou, X.J.; Wang, Y.L.; Jia, M.J.; Zhang, W.X. Supported ammonium metatungstate as highly efficient catalysts for the vapour-phase O-methylation of catechol with methanol. Catal. Commun. 2006, 7, 579–582. [Google Scholar] [CrossRef]
- Takita, Y.; Qing, X.; Takami, A.; Nishiguchi, H.; Nagaoka, K. Oxidative dehydrogenation of isobutane to isobutene III reaction mechanism over CePO4 catalyst. Appl. Catal. A 2005, 296, 63–69. [Google Scholar] [CrossRef]
- Bautista, F.M.; Campelo, J.M.; Luna, D.; Marinas, J.M.; Quiros, R.A.; Romero, A.A. Screening of amorphous metal-phosphate catalysts for the oxidative dehydrogenation of ethylbenzene to styrene. Appl. Catal. B 2007, 70, 611–620. [Google Scholar] [CrossRef]
- Takita, Y.; Moriyama, J.; Yoshinaga, Y.; Nishiguchi, H.; Ishihara, T.; Yasuda, S.; Ueda, Y.; Kubo, M.; Miyamoto, A. Adsorption of water vapor on the AlPO4-based catalysts and reaction mechanism for CFCs decomposition. Appl. Catal. A 2004, 271, 55–60. [Google Scholar] [CrossRef]
- Takubo, T.; Hirose, Y.; Kashiwagi, D.; Inoue, T.; Yamada, H.; Nagaoka, K.; Takita, Y. Metal phosphate and fluoride catalysts active for hydrolysis of NF3. Catal. Commun. 2009, 11, 147–150. [Google Scholar] [CrossRef]
- Takita, Y.; Tanabe, T.; Ito, M.; Ogura, M.; Muraya, T.; Yasuda, S.; Nishiguchi, H.; Ishihara, T. Decomposition of CH2FCF3 (134a) over metal phosphate catalysts. Ind. Eng. Chem. Res. 2002, 41, 2585–2590. [Google Scholar] [CrossRef]
- Kashiwagi, D.; Takai, A.; Takubo, T.; Nagaoka, K.; Inoue, T.; Takita, Y. Metal phosphate catalysts effective for degradation of sulfur hexafluoride. Ind. Eng. Chem. Res. 2009, 48, 632–640. [Google Scholar] [CrossRef]
- Devi, G.S.; Giridhar, D.; Reddy, B.M. Vapour phase O-alkylation of phenol over alkali promoted rare earth metal phosphates. J. Mol. Catal. A 2002, 181, 173–178. [Google Scholar] [CrossRef]
- Li, F.; Zhang, Y.B.; Xiao, D.H.; Wang, D.Q.; Pan, X.Q.; Yang, X.G. Hydrothermal method prepared Ce-P-O catalyst for the selective catalytic reduction of NO with NH3 in a broad temperature range. ChemCatChem 2010, 2, 1416–1419. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Wang, J.H.; Zhang, T. Novel Ca-doped CePO4 supported ruthenium catalyst with superior catalytic performance for aerobic oxidation of alcohols. Chem. Commun. 2011, 47, 5307–5309. [Google Scholar] [CrossRef]
- Danjo, Y.; Kikuchi, I.; Ino, Y.; Ohshima, M.; Kurokawa, H.; Miura, H. Support effect of Pd/AlPO4 catalyst in hydrogen storage of organic hydride method in the presence of CO. React. Kinet. Mech. Cat. 2012, 105, 381–389. [Google Scholar] [CrossRef]
- Ma, Z.; Yin, H.; Overbury, S.H.; Dai, S. Metal phosphates as a new class of supports for gold nanocatalysts. Catal. Lett. 2008, 126, 20–30. [Google Scholar] [CrossRef]
- Qian, X.S.; Qin, H.M.; Meng, T.; Lin, Y.; Ma, Z. Metal phosphate-supported Pt catalysts for CO oxidation. Materials 2014, 7, 8105–8130. [Google Scholar] [CrossRef]
- Cui, Y.W.; Liu, H.; Lin, Y.; Ma, Z. Metal phosphate-supported RuOx catalysts for N2O decomposition. J. Taiwan Inst. Chem. E. 2016, 67, 254–262. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Z.; Jia, M.; Zou, X.; Zhu, X.; Zhang, W.; Jiang, D. Thermally stable amorphous mesoporous aluminophosphates with controllable P/Al ratio: Synthesis, characterization, and catalytic performance for selective O-methylation of catechol. J. Phys. Chem. B 2006, 110, 16953–16960. [Google Scholar] [CrossRef]
- Liao, X.Z.; Zhou, Z.; Wang, Z.L.; Zou, X.J.; Liu, G.; Jia, M.J.; Zhang, W.X. Preformed precursor of microporous aluminophosphate coating on mesoporous SBA-15: Synthesis, characterization, and catalytic property for selective O-methylation of catechol. J. Colloid Interface Sci. 2007, 308, 176–181. [Google Scholar] [CrossRef]
- Rajesh, K.; Mukundan, P.; Pillai, P.K.; Nair, V.R.; Warrier, K.G.K. High-surface-area nanocrystalline cerium phosphate through aqueous sol-gel route. Chem. Mater. 2004, 16, 2700–2705. [Google Scholar] [CrossRef]
- Rajesh, K.; Shajesh, P.; Seidel, O.; Mukundan, P.; Warrier, K.G.K. A facile sol-gel strategy for the synthesis of rod-shaped, nanocrystalline, high-surface-area lanthanum phosphate powders and nanocoatings. Adv. Funct. Mater. 2007, 17, 1682–1690. [Google Scholar] [CrossRef]
- Qi, C.; Zhu, Y.J.; Wu, C.T.; Sun, T.W.; Chen, F.; Wu, J. Magnesium phosphate pentahydrate nanosheets: Microwave-hydrothermal rapid synthesis using creatine phosphate as an organic phosphorus source and application in protein adsorption. J. Colloid Interface Sci. 2016, 462, 297–306. [Google Scholar] [CrossRef]
- Zhou, X.M.; Du, H.J.; Ma, H.; Sun, L.N.; Cao, R.; Li, H.S.; Zhang, P.X. Facile preparation and characterization of zinc phosphate with self-assembled flower-like micro-nanostructures. J. Phys. Chem. Solids 2015, 78, 1–7. [Google Scholar] [CrossRef]
- Campelo, J.M.; Jaraba, M.; Luna, D.; Luque, R.; Marinas, J.M.; Romero, A.A.; Navio, J.A.; Macias, M. Effect of phosphate precursor and organic additives on the structural and catalytic properties of amorphous mesoporous AlPO4 materials. Chem. Mater. 2003, 15, 3352–3364. [Google Scholar] [CrossRef]
- Srilakshmi, C.; Lingaiah, N.; Nagaraju, P.; Prasad, P.S.S.; Narayana, K.V.; Martin, A.; Luecke, B. Studies on bulk metal phosphate catalysts for the ammoxidation of 2-methylpyrazine. Appl. Catal. A 2006, 309, 247–253. [Google Scholar] [CrossRef]
- Chada, R.R.; Ketike, T.; Boilla, A.R.; Gangadharam, S.D.; Kamaraju, S.R.R.; Burri, D.R. Preparation and characterization of lanthanum phosphate catalysts for O-methylation of phenol to anisole in gas phase. Mol. Catal. 2017, 438, 224–229. [Google Scholar] [CrossRef]
- Lin, Y.; Meng, T.; Ma, Z. Catalytic decomposition of N2O over RhOx supported on metal phosphates. J. Ind. Eng. Chem. 2015, 28, 138–146. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S.; Ryoo, R.; Choi, M. Revisiting side-chain alkylation of toluene to styrene: Critical role of microporous structures in catalysts. J. Catal. 2019, 373, 25–36. [Google Scholar] [CrossRef]
- Nguyen, T.T.N.; Ruaux, V.; Massin, L.; Lorentz, C.; Afanasiev, P.; Mauge, F.; Belliere-Baca, V.; Rey, P.; Millet, J.M.M. Synthesis, characterization and study of lanthanum phosphates as light alcohols dehydration catalysts. Appl. Catal. B 2015, 166, 432–444. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).