Hexagonal WO3·0.33H2O Hierarchical Microstructure with Efficient Photocatalytic Degradation Activity
Abstract
1. Introduction
2. Results and Discussion
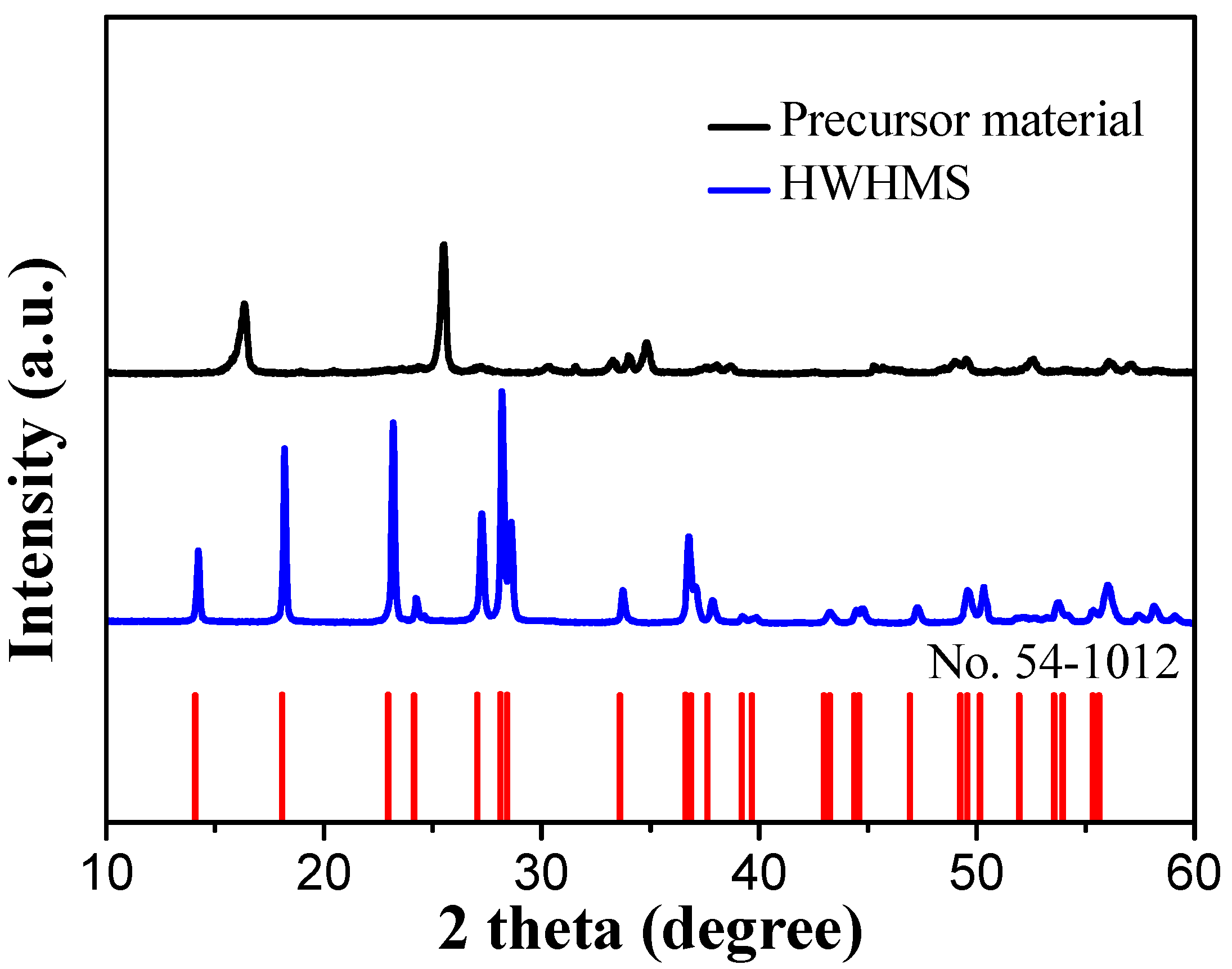
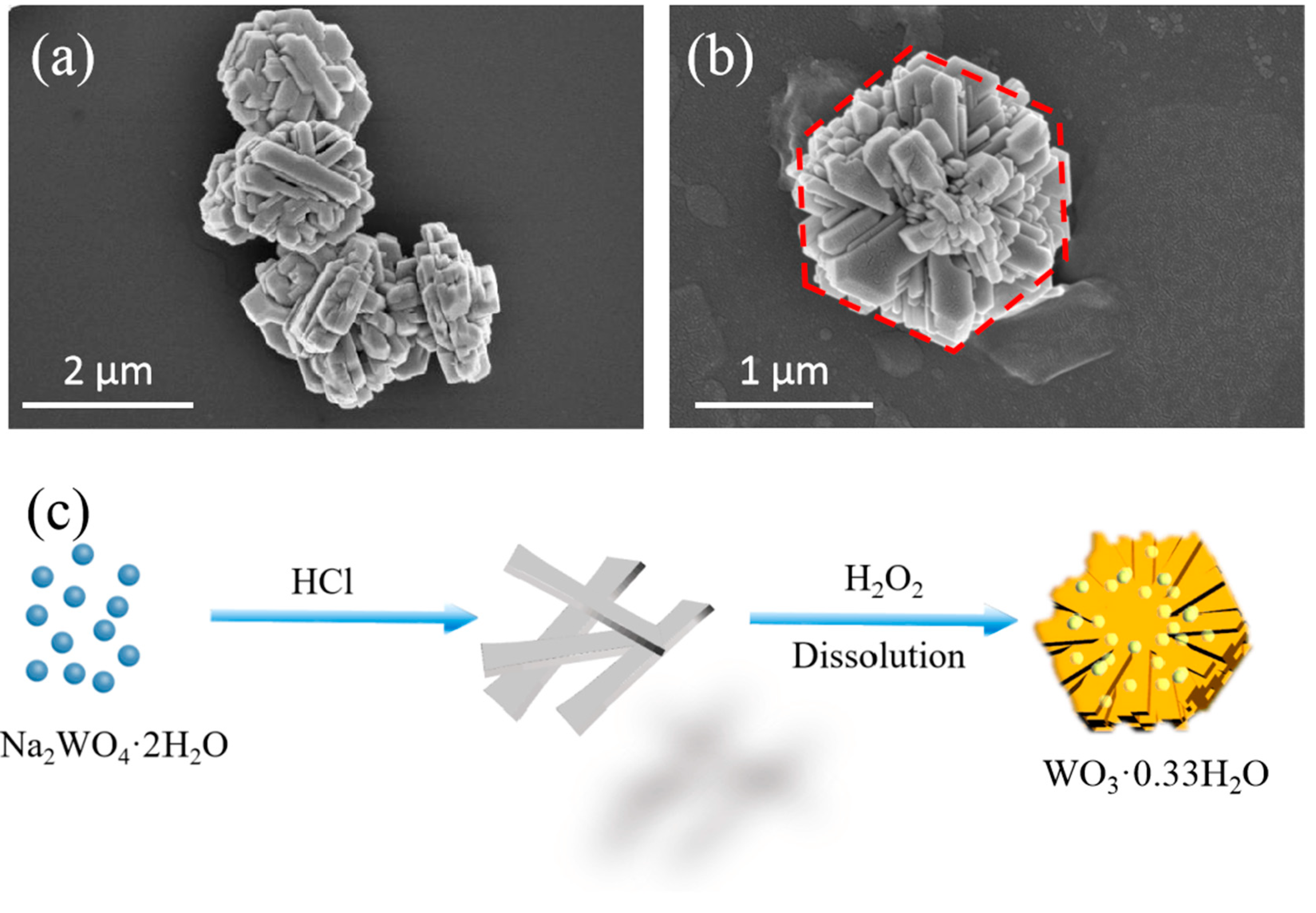
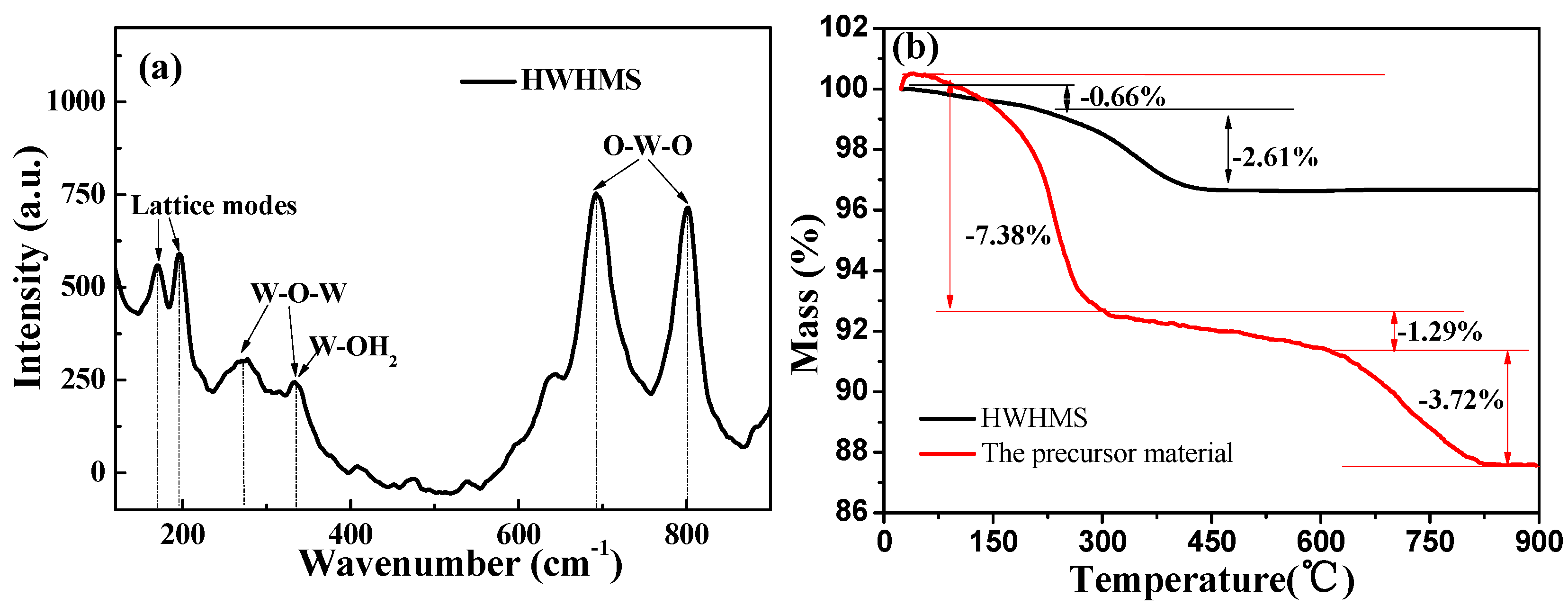
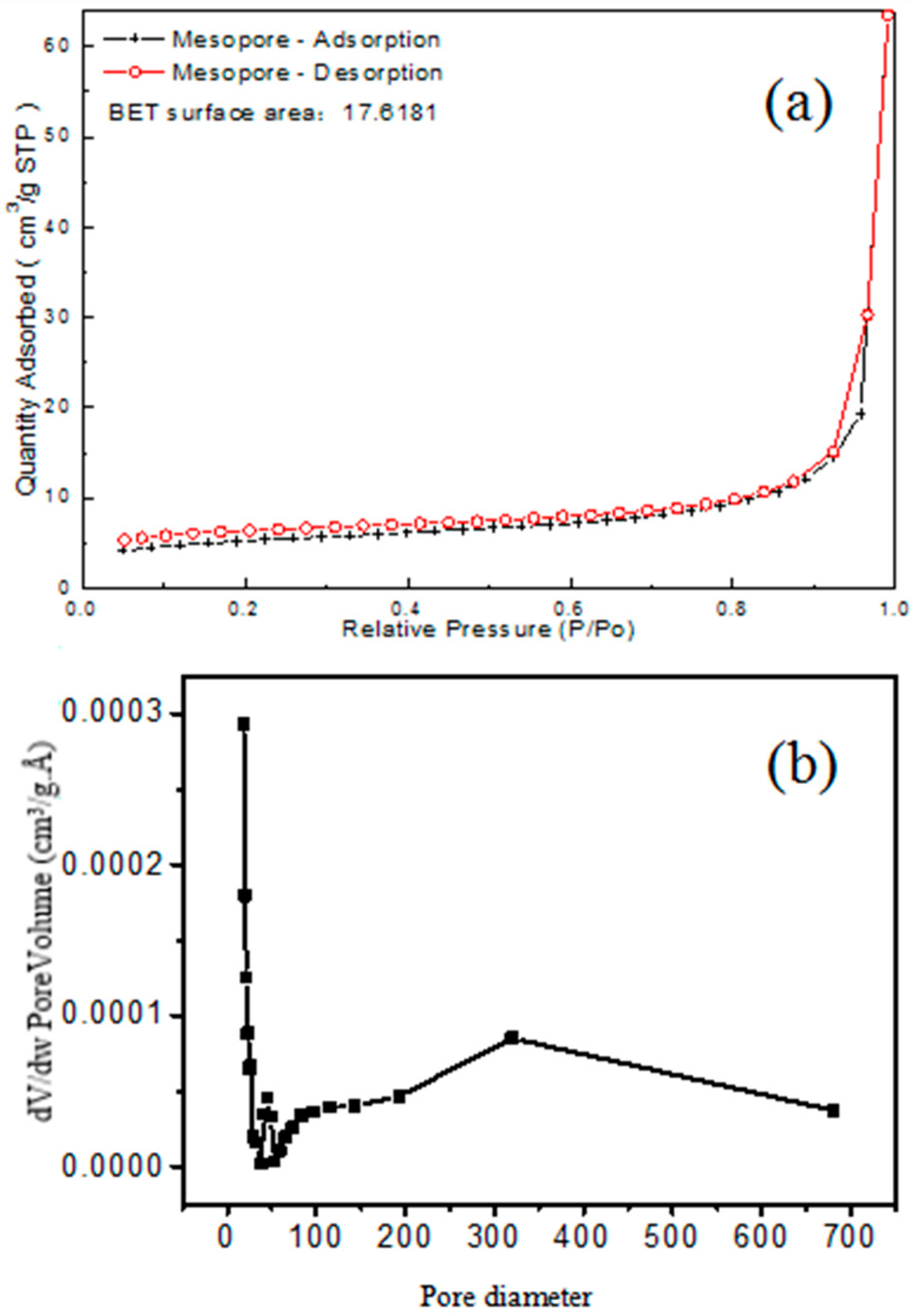
2.1. Characterization of the HWHMS
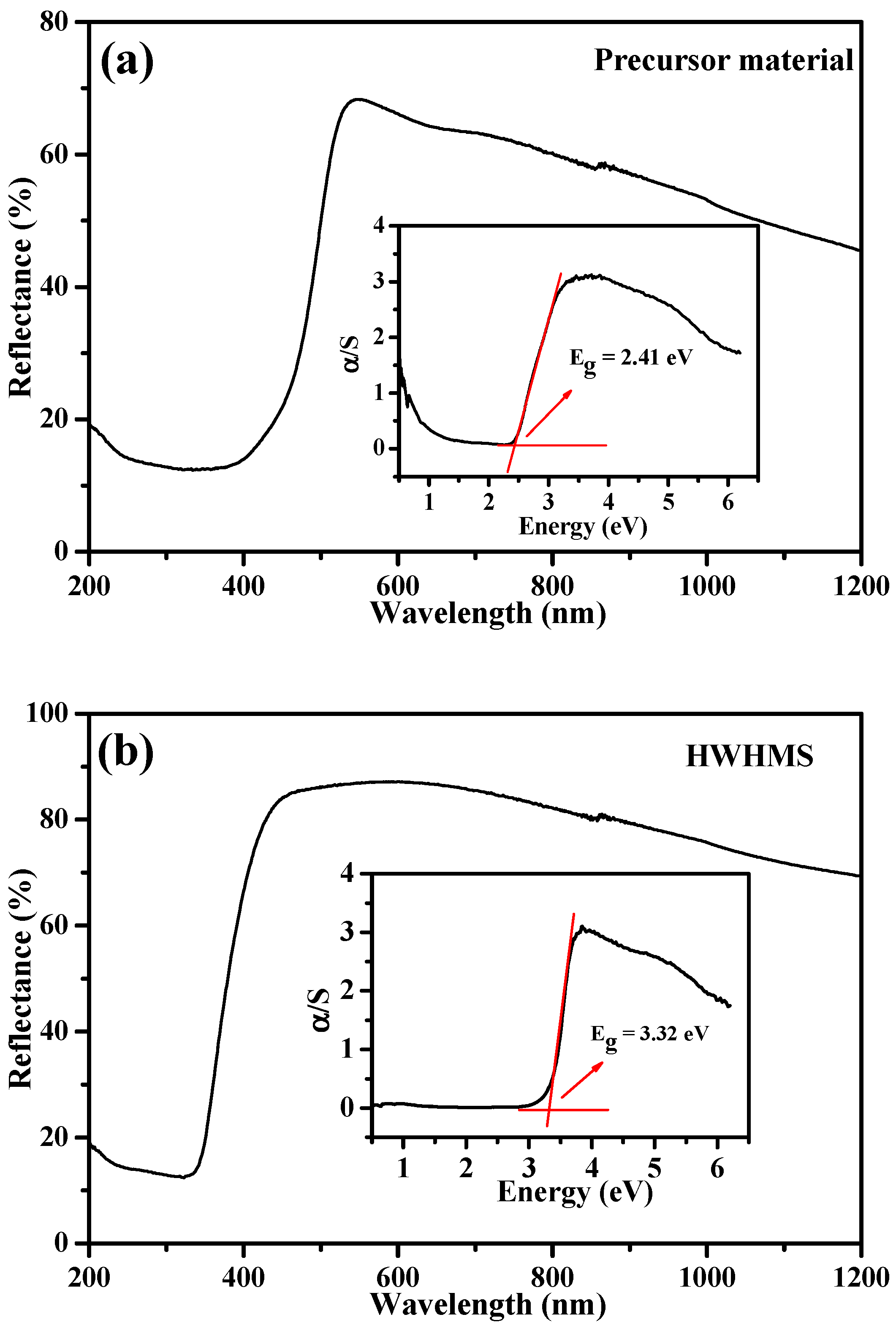
2.2. Optical Properties of the HWHMS
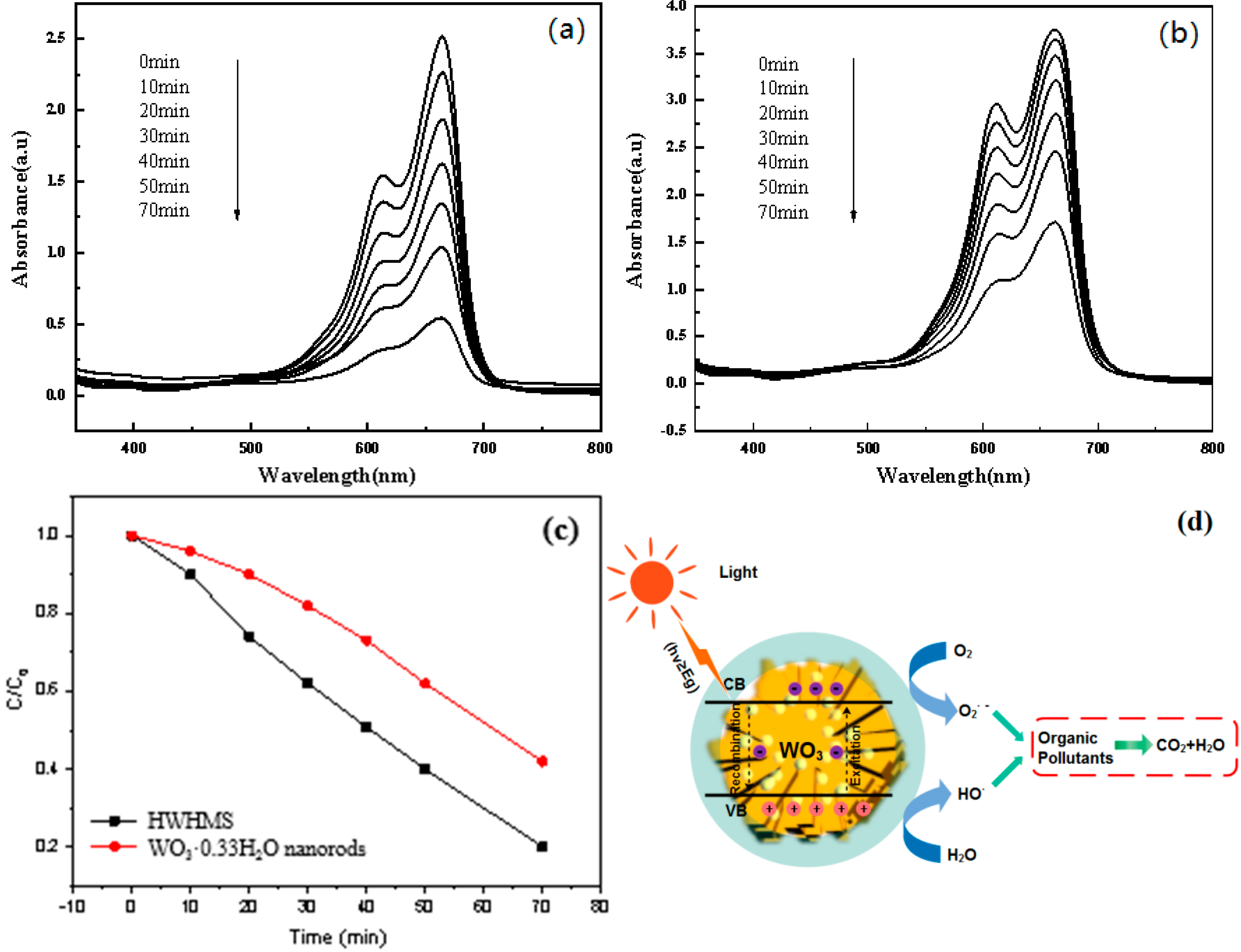
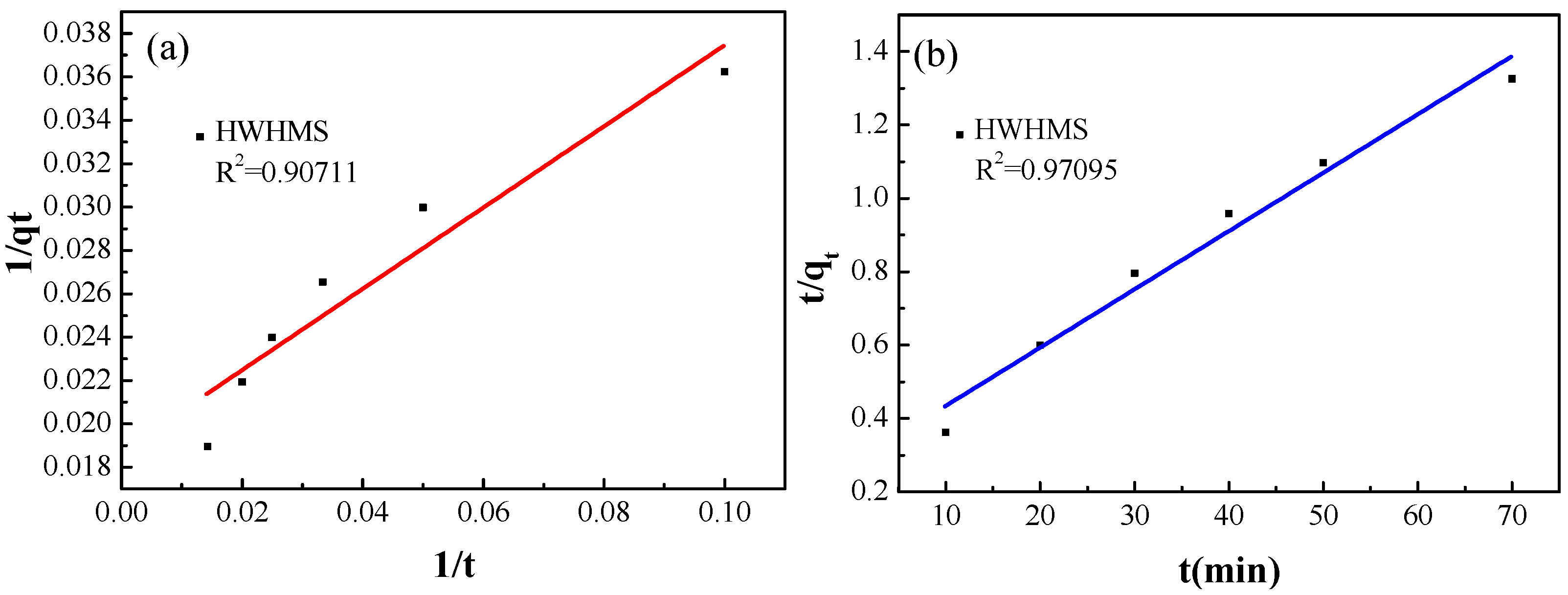
2.3. Photocatalytic Performance of the HWHMS
3. Materials and Methods
3.1. Preparation of the HWHMS
3.2. Characterization
3.3. Photocatalysis Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Adeel, M.; Saeed, M.; Khan, I.; Muneer, M. Synthesis and Characterization of Co-ZnO and Evaluation of Its Photocatalytic Activity for Photodegradation of Methyl Orange. ACS Omega 2021, 6, 1426–1435. [Google Scholar] [CrossRef]
- Daij, K.B.; Bellebia, S.; Bengharez, Z. Comparative experimental study on the COD removal in aqueous solution of pesticides by the electrocoagulation process using monopolar iron electrodes. Chem. Int. 2017, 3, 420–427. [Google Scholar]
- Djehaf, K.; Bouyakoub, A.Z.; Ouhib, R.; Benmansour, H.; Bentouaf, A.; Mahdad, A.; Moulay, N.; Bensaid, D.; Ameri, M. Textile Wastewater in Tlemcen (Western Algeria): Impact, Treatment by Combined Process. Chem. Int. 2017, 3, 414–419. [Google Scholar]
- Minas, F.; Chandravanshi, B.S.; Leta, S. Chemical Precipitation Method for Chromium Removal and Its Recovery from Tannery Wastewater in Ethiopia. Chem. Int. 2017, 3, 392–405. [Google Scholar]
- Ayach, A.; Fakhil, S.; Faiz, Z.; Bouih, A.; Malek, O.A.; Benkdad, A.; Benmansour, M.; Laissaoui, A.; Adjour, M.; Elbatal, Y.; et al. Adsorption of Methylene Blue on Bituminous Schists from Tarfaya-Boujdour. Chem. Int. 2017, 3, 442–451. [Google Scholar]
- Jafarinejad, S. Activated Sludge Combined with Powdered Activated Carbon (PACT Process) for the Petroleum Industry Wastewater Treatment: A Review. Chem. Int. 2017, 3, 368–374. [Google Scholar]
- Joseph, C.G.; Taufiq-Yap, Y.H.; Musta, B.; Sarjadi, M.S.; Elilarasi, L. Application of Plasmonic Metal Nanoparticles in TiO2-SiO2 Composite as an Efficient Solar-Activated Photocatalyst: A Review Paper. Front. Chem. 2021, 8, 1283. [Google Scholar] [CrossRef] [PubMed]
- Etacheri, V.; di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Reddy, P.L.; Kavitha, B.; Kim, K.-H. TiO2 -based photocatalytic disinfection of microbes in aqueous media: A review. Environ. Res. 2017, 154, 296–303. [Google Scholar] [CrossRef]
- Zhang, B.; He, X.; Ma, X.; Chen, Q.; Liu, G.; Zhou, Y.; Ma, D.; Cui, C.; Ma, J.; Xin, Y. In situ synthesis of ultrafine TiO2 nanoparticles modified g-C3N4 heterojunction photocatalyst with enhanced photocatalytic activity. Sep. Purif. Technol. 2020, 247, 116932. [Google Scholar] [CrossRef]
- Gomis-Berenguer, A.; Celorrio, V.; Iniesta, J.; Fermin, D.J.; Ania, C.O. Nanoporous carbon/WO3 anodes for an enhanced water photooxidation. Carbon 2016, 108, 471–479. [Google Scholar] [CrossRef]
- Gondal, M.A.; Adesida, A.A.; Rashid, S.G.; Shi, S.; Khan, R.; Yamani, Z.H.; Shen, K.; Xu, Q.Y.; Seddigi, Z.S.; Chang, X.F. Preparation of WO3/g-C3N4 composites and their enhanced photodegradation of contaminants in aqueous solution under visible light irradiation. React. Kinet. Mech. Cat. 2015, 114, 357–367. [Google Scholar] [CrossRef]
- Yu, C.L.; Chen, J.C.; Zhou, W.Q.; Wei, L.F.; Fan, Q.Z. Grinding calcination preparation of WO3/BiOCl heterostructures with enhanced visible light photocatalytic activity. Mater. Res. Innov. 2014, 19, 54–59. [Google Scholar] [CrossRef]
- Zhang, W.; Li, H.; Ma, Z.; Li, H.; Wang, H. Photocatalytic degradation of azophloxine on porous La2Ti2O7 prepared by sol-gel method. Solid State Sci. 2019, 87, 58–63. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, J.; Li, C. Role of thermal treatment on sol-gel preparation of porous cerium titanate: Characterization and photocatalytic degradation of ofloxacin. Mater. Sci. Semicond. Process. 2018, 85, 33–39. [Google Scholar] [CrossRef]
- Luo, J.Y.; Cao, Z.; Chen, F.; Li, L.; Lin, Y.R.; Liang, B.W.; Zeng, Q.G.; Zhang, M.; He, X.; Li, C. Strong aggregation adsorption of methylene blue from water using amorphous WO3 nanosheets. Appl. Surf. Sci. 2013, 287, 270–275. [Google Scholar] [CrossRef]
- Jeon, S.; Yong, K. Morphology-controlled synthesis of highly adsorptive tungsten oxide nanostructures and their application to water treatment. J. Mater. Chem. 2010, 20, 10146–10151. [Google Scholar] [CrossRef]
- Ou, H.; Wang, D.; Li, Y. How to select effective electrocatalysts: Nano or single atom? Nano Sel. 2021, 2, 492–511. [Google Scholar] [CrossRef]
- Pan, Y.; Shen, X.; Yao, L.; Bentalib, A.; Peng, Z. Active Sites in Heterogeneous Catalytic Reaction on Metal and Metal Oxide: Theory and Practice. Catalysts 2018, 8, 478. [Google Scholar] [CrossRef]
- Lin, H.; Fang, Q.; Wang, W.; Li, G.; Liu, F. Prussian Blue/PVDF Catalytic Membrane with Exceptional and Stable Fenton Oxidation Performance for Organic Pollutants Removal. Appl. Catal. B Environ. 2020, 273, 119047. [Google Scholar] [CrossRef]
- Chen, D.; Ye, J. Hierarchical WO3 Hollow Shells: Dendrite, Sphere, Dumbbell, and Their Photocatalytic Properties. Adv. Funct. Mater. 2008, 18, 1922–1928. [Google Scholar] [CrossRef]
- Huang, J.; Xu, X.; Gu, C.; Fu, G.; Wang, W.; Liu, J. Flower-like and hollow sphere-like WO3 porous nanostructures: Selective synthesis and their photocatalysis property. Mater. Res. Bull. 2012, 47, 3224–3232. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, G.; Yu, Y.G.; Sun, J.X. Template and surfactant free synthesis of hierarchical WO3·0.33H2O via a facile solvothermal route for photocatalytic RhB degradation. CrystEngComm 2014, 16, 6107. [Google Scholar] [CrossRef]
- Li, L.; Xu, J.; Lei, J.; Zhang, J.; McLarnon, F.; Wei, Z.; Li, N.; Pan, F. A one-step, cost-effective green method to in situ fabricate Ni(OH)2 hexagonal platelets on Ni foam as binder-free supercapacitor electrode materials. J. Mater. Chem. A 2015, 3, 1953–1960. [Google Scholar] [CrossRef]
- Liu, Z.; Li, P.; Dong, Y.; Wan, Q.; Zhai, F.; Volinsky, A.A.; Qu, X. Facile preparation of hexagonal WO3·0.33H2O/C nanostructures and its electrochemical properties for lithium-ion batteries. Appl. Surf. Sci. 2017, 394, 70–77. [Google Scholar] [CrossRef]
- Dursun, S.; Koyuncu, S.N.; Kaya, I.C.; Kaya, G.G.; Akyildiz, H. Production of CuO-WO3 hybrids and their dye removal capacity/performance from wastewater by adsorption/photocatalysis. J. Water Process. Eng. 2020, 36, 101390. [Google Scholar] [CrossRef]
- Najafi, A. A novel synthesis method of hierarchical mesoporous MgO nanoflakes employing carbon nanoparticles as the hard templates for photocatalytic degradation. Ceram. Int. 2017, 43, 5813–5818. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Wang, T.; Zhou, J.; Zhao, Y. Synthesis and magnetic properties of nanoporous Co3O4 nanoflowers. Microporous Mesoporous Mater. 2008, 114, 257–261. [Google Scholar] [CrossRef]
- Gooch, J.W. (Ed.) Kubelka–Munk equation. In Encyclopedic Dictionary of Polymers; Springer: New York, NY, USA, 2007. [Google Scholar]
- Shenoy, S.; Jang, E.; Park, T.J.; Gopinath, C.S.; Sridharan, K. Cadmium sulfide nanostructures: Influence of morphology on the photocatalytic degradation of erioglaucine and hydrogen generation. Appl. Surf. Sci. 2019, 483, 696–705. [Google Scholar] [CrossRef]
- Aminian, M.K.; Ye, J. Morphology influence on photocatalytic activity of tungsten oxide loaded by platinum nanoparticles. J. Mater. Res. 2010, 25, 141–148. [Google Scholar] [CrossRef]
- Wang, L.; Liu, S.; Wang, Z.; Zhou, Y.; Qin, Y.; Wang, Z.L. Piezotronic Effect Enhanced Photocatalysis in Strained Anisotropic ZnO/TiO2 Nanoplatelets via Thermal Stress. ACS Nano 2016, 10, 2636–2643. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Hu, C.; Yi, Q.; Wang, X.; Hua, H.; Li, X. Preparation and Improved Photocatalytic Activity of WO3·0.33H2O Nanonetworks. Catal. Lett. 2012, 142, 637–645. [Google Scholar] [CrossRef]
- He, X.Y.; Hu, C.; Xi, Y.; Zhang, K.Y.; Hua, H. Three-dimensional Ag2O/WO3·0.33H2O heterostructures for improving photocatalytic activity. Mater. Res. Bull. 2014, 50, 91–94. [Google Scholar] [CrossRef]
- Deblina, M.I.C.; Kalyan, M.; Somenath, R. Facet-Dependent Photodegradation of Methylene Blue Using Pristine CeO2 Nanostructures. ACS Omega 2019, 4, 4243–4251. [Google Scholar]
- Smazna, D.; Shree, S.; Polonskyi, O.; Lamaka, S.; Baum, M.; Zheludkevich, M.; Faupel, F.; Adelung, R.; Mishra, Y.K. Mutual interplay of ZnO micro- and nanowires and methylene blue during cyclic photocatalysis process. J. Environ. Chem. Eng. 2019, 7, 103016. [Google Scholar] [CrossRef]
- Huang, D.; Zheng, C.; Li, W.; Guo, Q.; Chen, W. Morphology related nonlinear optical response of tungsten trioxide nanostructures to nanosecond laser pulses. Opt. Mater. 2019, 88, 451–457. [Google Scholar] [CrossRef]
- Zhou, L.; Zou, J.; Yu, M.; Lu, P.; Wei, J.; Qian, Y.; Wang, Y.; Yu, C. Green Synthesis of Hexagonal-Shaped WO3·0.33H2O Nanodiscs Composed of Nanosheets. Cryst. Growth Des. 2008, 8, 3993–3998. [Google Scholar] [CrossRef]
- Guéry, C.; Choquet, C.; Dujeancourt, F.; Tarascon, J.M.; Lassègues, J.C. Infrared and X-ray studies of hydrogen intercalation in different tungsten trioxides and tungsten trioxide hydrates. J. Solid State Electrochem. 1997, 1, 199–207. [Google Scholar] [CrossRef]
- Wang, H.; Dai, F.; Yang, Y.; Li, Z.; Li, C.; Zhang, S. Catalyst Grading Optimization and Kinetic Simulation of the Shale Oil Hydrotreating Process. Energy Fuels 2017, 31, 4353–4360. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Wang, T.; Huang, D.; Zheng, C.; Lai, Y.; Xiao, X.; Cai, S.; Chen, W. Hexagonal WO3·0.33H2O Hierarchical Microstructure with Efficient Photocatalytic Degradation Activity. Catalysts 2021, 11, 496. https://doi.org/10.3390/catal11040496
Li W, Wang T, Huang D, Zheng C, Lai Y, Xiao X, Cai S, Chen W. Hexagonal WO3·0.33H2O Hierarchical Microstructure with Efficient Photocatalytic Degradation Activity. Catalysts. 2021; 11(4):496. https://doi.org/10.3390/catal11040496
Chicago/Turabian StyleLi, Wei, Tingting Wang, Dongdong Huang, Chan Zheng, Yuekun Lai, Xueqing Xiao, Shuguang Cai, and Wenzhe Chen. 2021. "Hexagonal WO3·0.33H2O Hierarchical Microstructure with Efficient Photocatalytic Degradation Activity" Catalysts 11, no. 4: 496. https://doi.org/10.3390/catal11040496
APA StyleLi, W., Wang, T., Huang, D., Zheng, C., Lai, Y., Xiao, X., Cai, S., & Chen, W. (2021). Hexagonal WO3·0.33H2O Hierarchical Microstructure with Efficient Photocatalytic Degradation Activity. Catalysts, 11(4), 496. https://doi.org/10.3390/catal11040496





