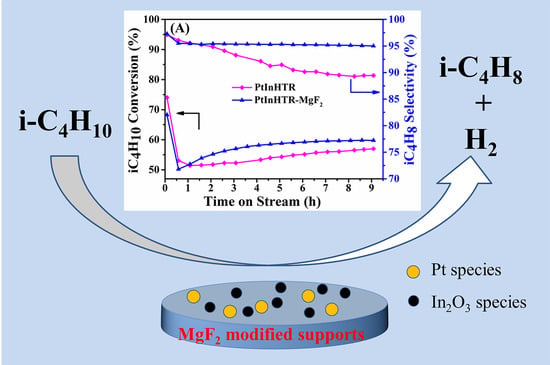
MgF2-Modified Hydrotalcite-Derived Composites Supported Pt-In Catalysts for Isobutane Direct Dehydrogenation
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Composite Supports and Catalysts
2.1.1. The X-Ray Diffraction (XRD)
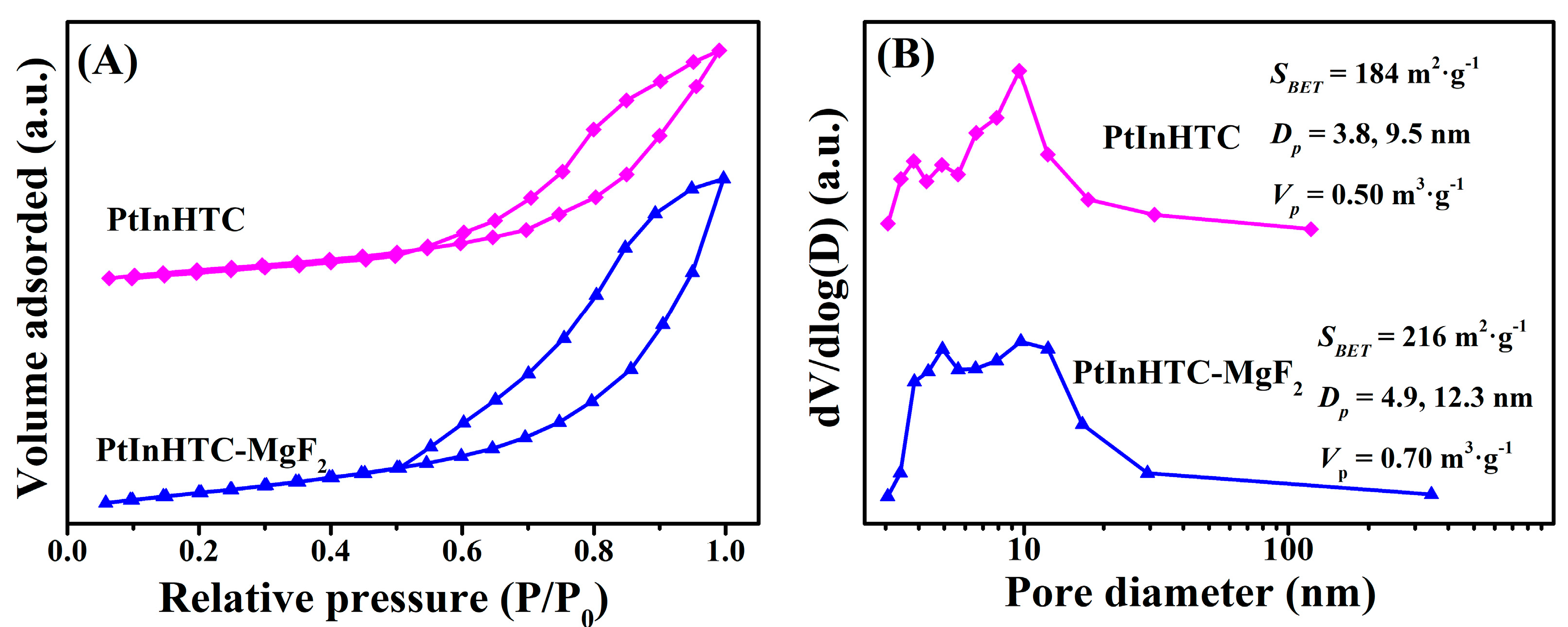
2.1.2. N2-Adsorption–Desorption Isotherms
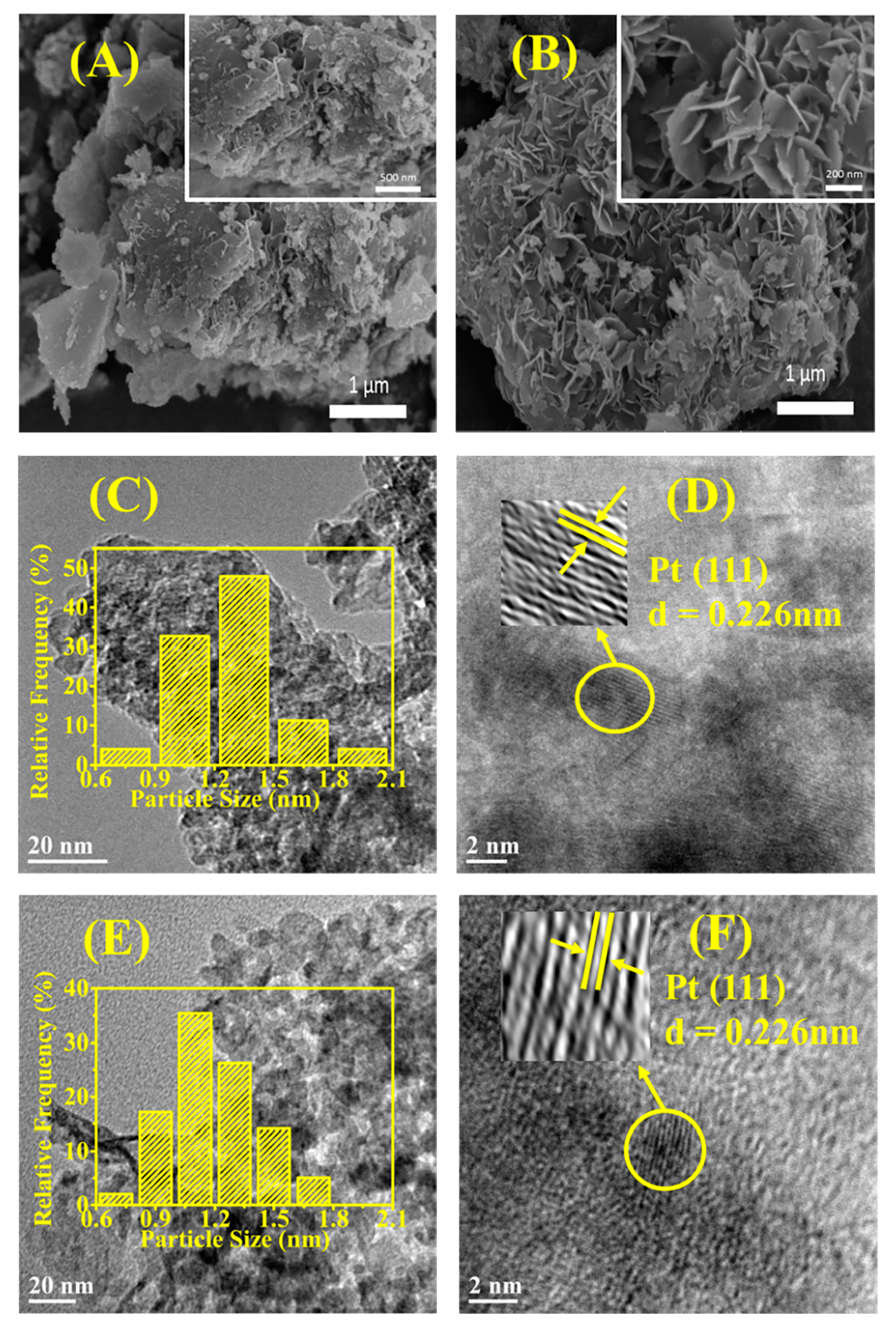
2.1.3. The Scanning Electron Microscopy (SEM) and the Transmission Electron Microscopy (TEM)
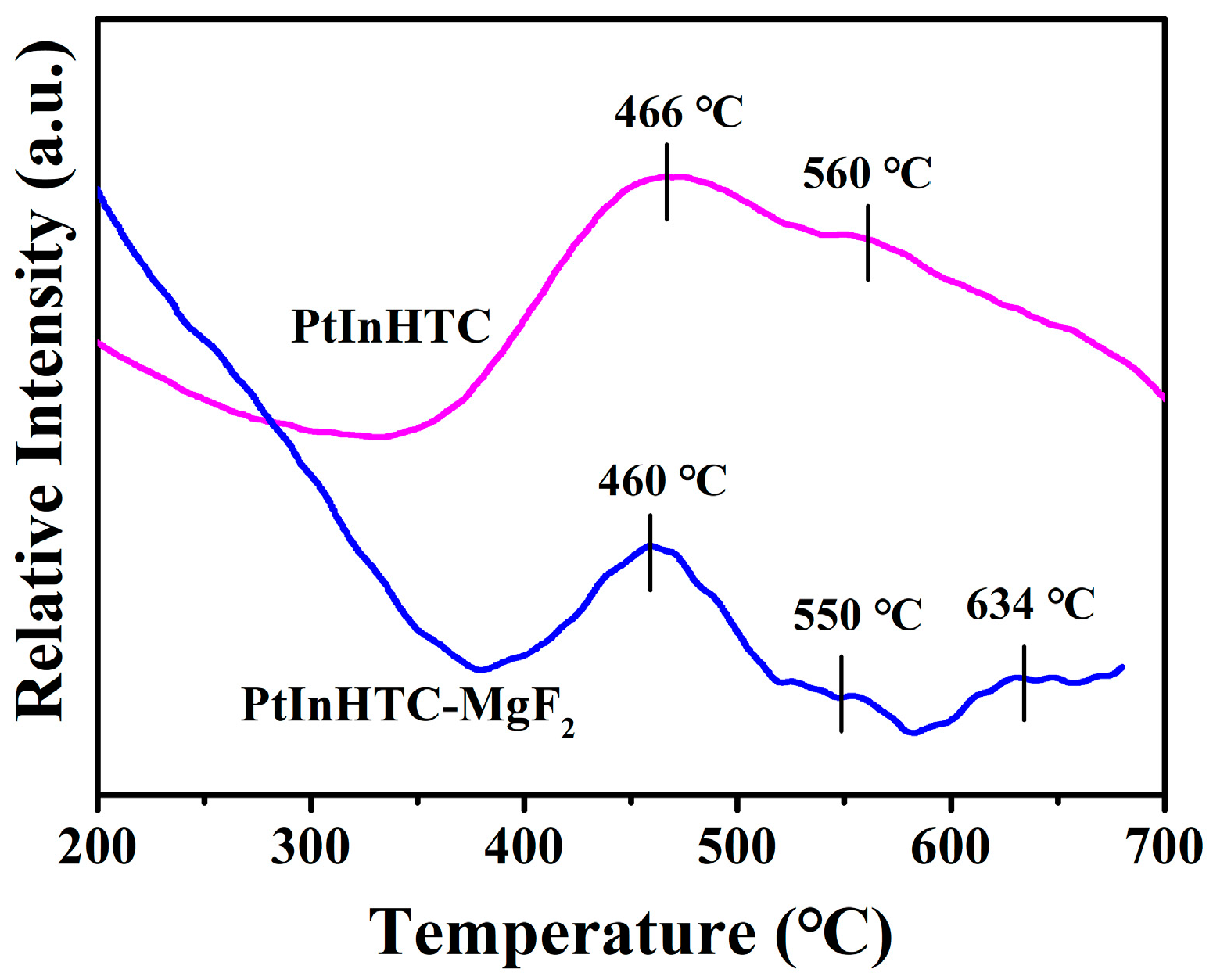
2.1.4. The Temperature-Programmed Reduction (H2-TPR)
2.1.5. X-Ray Photoelectron Spectroscopy (XPS)
2.2. Catalytic Dehydrogenation Performance of Catalysts
2.3. Characterization of the Spent Catalysts
2.3.1. Thermogravimetric Analysis (TG-DTA) and the X-Ray Diffraction (XRD)
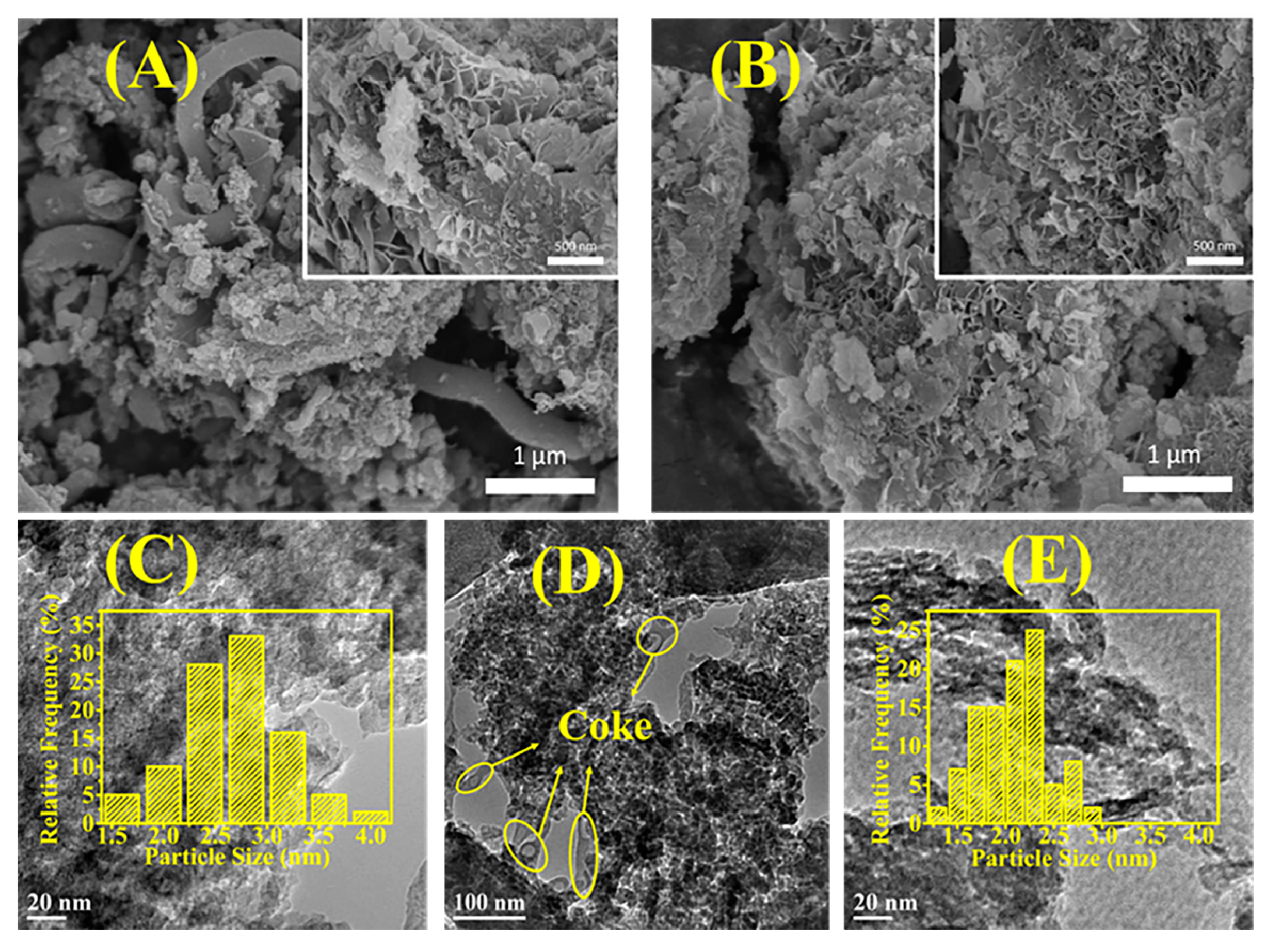
2.3.2. SEM and TEM
3. Materials and Methods
3.1. Materials Used
3.2. Synthesis of Composites and Precursors
3.3. Synthesis of Catalysts
3.4. Precursors, Composites and Catalysts Characterization
3.5. Catalytic Dehydrogenation Performance Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, G.; Li, P.; Zhao, F.; Song, H.; Xia, C. Selective aromatization of biomass derived diisobutylene to p-xylene over supported non-noble metal catalysts. Catal. Today 2016, 276, 105–111. [Google Scholar] [CrossRef]
- Sharma, R.K.; Mohanty, S.; Gupta, V. Advances in butyl rubber synthesis via cationic polymerization: An overview. Polym. Int. 2021. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, S.M.; Wang, Z.; Yuan, Z.Y. Ultrasmall Co confined in the silanols of dealuminated beta zeolite: A highly active and selective catalyst for direct dehydrogenation of propane to propylene. J. Catal. 2020, 383, 77–87. [Google Scholar] [CrossRef]
- Chen, C.; Sun, M.L.; Hu, Z.P.; Ren, J.T.; Zhang, S.M.; Yuan, Z.Y. New insight into the enhanced catalytic performance of ZnPt/HZSM-5 catalysts for direct dehydrogenation of propane to propylene. Catal. Sci. Technol. 2019, 9, 1979–1988. [Google Scholar] [CrossRef]
- Hill, J.M.; Cortright, R.D.; Dumesic, J.A. Silica- and L-zeolite-supported Pt, Pt/Sn and Pt/Sn/K catalysts for isobutane dehydrogenation. Appl. Catal. A 1998, 168, 9–21. [Google Scholar] [CrossRef]
- Ohta, M.; Ikeda, Y.; Igarashi, A. Additive effect on properties of Pt/ZnO catalyst for dehydrogenation of isobutane at low temperatures. J. Jpn. Petrol. Inst. 2002, 45, 150–155. [Google Scholar] [CrossRef][Green Version]
- Miura, H.; Itoh, T. Selective deposition of Sn on the Pt surface of Pt/ZnAl2O4 catalyst and addition effect on isobutane dehydrogenation. React. Kinet. Catal. Lett. 1999, 66, 189–194. [Google Scholar] [CrossRef]
- Rashidi, M.; Nikazar, M.; Rahmani, M.; Mohamadghasemi, Z. Kinetic modeling of simultaneous dehydrogenation of propane and isobutane on Pt-Sn-K/Al2O3 catalyst. Chem. Eng. Res. Des. 2015, 95, 239–247. [Google Scholar] [CrossRef]
- Zangeneh, F.T.; Sahebdelfar, S.; Bahmani, M. Propane Dehydrogenation over a Commercial Pt-Sn/Al2O3 Catalyst for Isobutane Dehydrogenation: Optimization of Reaction Conditions. Chin. J. Chem. Eng. 2013, 21, 730–735. [Google Scholar] [CrossRef]
- Tolek, W.; Suriye, K.; Praserthdam, P.; Panpranot, J. Enhanced stability and propene yield in propane dehydrogenation on PtIn/Mg(Al)O catalysts with various In loadings. Top. Catal. 2018, 61, 1624–1632. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Song, Z.; Liu, S.; Wang, J.; Zhang, L. Hierarchical PtIn/Mg(Al)O derived from reconstructed PtIn-hydrotalcite-like compounds for highly efficient propane dehydrogenation. Catalysts 2019, 9, 767. [Google Scholar] [CrossRef]
- Xia, K.; Lang, W.; Li, P.; Long, L.; Yan, X.; Guo, Y. The influences of Mg/Al molar ratio on the properties of PtIn/Mg(Al)O-x catalysts for propane dehydrogenation reaction. Chem. Eng. J. 2016, 284, 1068–1079. [Google Scholar] [CrossRef]
- Xia, K.; Lang, W.; Li, P.; Yan, X.; Guo, Y. The properties and catalytic performance of PtIn/Mg(Al)O catalysts for the propane dehydrogenation reaction: Effects of pH value in preparing Mg(Al)O supports by the co-precipitation method. J. Catal. 2016, 338, 104–114. [Google Scholar] [CrossRef]
- Ma, Z.; Wu, Z.; Miller, J.T. Effect of Cu content on the bimetallic Pt-Cu catalysts for propane dehydrogenation. Catal. Struct. React. 2017, 3, 43–53. [Google Scholar] [CrossRef]
- Liu, Y.; Xia, C.; Wang, Q.; Zhang, L.; Huang, A.; Ke, M.; Song, Z. Direct dehydrogenation of isobutane to isobutene over Zn-doped ZrO2 metal oxide heterogeneous catalysts. Catal. Sci. Technol. 2018, 8, 4916–4924. [Google Scholar] [CrossRef]
- Silvestre-Albero, J.; Sanchez-Castillo, M.A.; He, R.; Sepulveda-Escribano, A.; Rodriguez-Reinoso, F.; Dumesic, J.A. Microcalorimetric, reaction kinetics and DFT studies of Pt-Zn/X-zeolite for isobutane dehydrogenation. Catal. Lett. 2001, 74, 17–25. [Google Scholar] [CrossRef]
- Siddiqi, G.; Sun, P.; Galvita, V.; Bell, A.T. Catalyst performance of novel Pt/Mg(Ga)(Al)O catalysts for alkane dehydrogenation. J. Catal. 2010, 274, 200–206. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, F.; Liu, G.; Zeng, L.; Zhao, Z.J.; Gong, J.L. Effects of Ga doping on Pt/CeO2-Al2O3 catalysts for propane dehydrogenation. AIChE J. 2016, 62, 4365–4376. [Google Scholar] [CrossRef]
- Yang, K.; Yin, Y.; Lai, S.; Zhu, L.; Zhang, J.; Lai, W.; Lian, Y.; Fang, W. Aromatization of n-Butane and i-Butane over PtSnK/ZSM-5 Catalysts: Influence of SiO2/Al2O3 Ratio. Catal. Lett. 2018, 148, 3570–3582. [Google Scholar] [CrossRef]
- Li, Q.; Yang, G.B.; Wang, K.; Wang, X.T. Preparation of carbon-doped alumina beads and their application as the supports of Pt-Sn-K catalysts for the dehydrogenation of propane. React. Kinet. Mech. Catal. 2020, 129, 805–817. [Google Scholar] [CrossRef]
- Ballarini, A.D.; de Miguel, S.R.; Castro, A.A.; Scelza, O.A. n-Decane dehydrogenation on Pt, PtSn and PtGe supported on spinels prepared by different methods of synthesis. Appl. Catal. A 2013, 467, 235–245. [Google Scholar] [CrossRef]
- Rimaz, S.; Chen, L.W.; Kawi, S.; Borgna, A. Promoting effect of Ge on Pt-based catalysts for dehydrogenation of propane to propylene. Appl. Catal. A 2019, 588, 117266. [Google Scholar] [CrossRef]
- Ji, Z.H.; Miao, D.Y.; Gao, L.J.; Pan, X.L.; Bao, X.H. Effect of pH on the catalytic performance of PtSn/B-ZrO2 in propane dehydrogenation. Chin. J. Catal. 2020, 41, 719–729. [Google Scholar] [CrossRef]
- Wu, G.; Wang, X.; Wei, W.; Sun, Y. Fluorine-modified Mg-Al mixed oxides: A solid base with variable basic sites and tunable basicity. Appl. Catal. A 2010, 377, 107–113. [Google Scholar] [CrossRef]
- Wu, G.; Wang, X.; Chen, B.; Li, J.; Zhao, N.; Wei, W.; Sun, Y. Fluorine-modified mesoporous Mg-Al mixed oxides: Mild and stable base catalysts for O-methylation of phenol with dimethyl carbonate. Appl. Catal. A 2007, 329, 106–111. [Google Scholar] [CrossRef]
- Liu, J.; Liu, C.; Ma, A.; Rong, J.; Da, Z.; Zheng, A.; Qin, L. Effects of Al2O3 phase and Cl component on dehydrogenation of propane. Appl. Surf. Sci. 2016, 368, 233–240. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, H.; Zhang, S.; Wang, G.; Zhu, X.; Li, C. Dehydrogenation of isobutane over a Ni-P/SiO2 ceatalyst: Effect of P addition. Ind. Eng. Chem. Res. 2019, 58, 7834–7843. [Google Scholar] [CrossRef]
- Matveyeva, A.N.; Wärnå, J.; Pakhomov, N.A.; Murzin, D.Y. Kinetic modeling of isobutane dehydrogenation over Ga2O3/Al2O3 catalyst. Chem. Eng. J. 2020, 381, 122741. [Google Scholar] [CrossRef]
- Dong, A.H.; Wang, K.; Zhu, S.Z.; Yang, G.B.; Wang, X.T. Facile preparation of PtSn-La/Al2O3 catalyst with large pore size and its improved catalytic performance for isobutane dehydrogenation. Fuel Process. Technol. 2017, 158, 218–225. [Google Scholar] [CrossRef]
- Sun, G.; Huang, Q.; Li, H.; Liu, H.; Zhang, Z.; Wang, X.; Wang, Q.; Wang, J. Different supports-supported Cr-based catalysts for oxidative dehydrogenation of isobutane with CO2. Chin. J. Catal. 2014, 32, 1424–1429. [Google Scholar] [CrossRef]
- Deng, L.D.; Zhou, Z.J.; Shishido, T. Behavior of active species on Pt-Sn/SiO2 catalyst during the dehydrogenation of propane and regeneration. Appl. Catal. A 2020, 606, 117826. [Google Scholar] [CrossRef]
- Otroshchenko, T.P.; Kondratenko, V.A.; Rodemerck, U.; Linke, D.; Kondratenko, E.V. Non-oxidative dehydrogenation of propane, n-butane, and isobutane over bulk ZrO2-based catalysts: Effect of dopant on the active site and pathways of product formation. Catal. Sci. Technol. 2017, 7, 4499–4510. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, W.; Jiang, D.; Wu, W.; Miao, C.; Wang, Y.; Ma, X. Isobutane dehydrogenation over InPtSn/ZnAl2O4 catalysts: Effect of indium promoter. Ind. Eng. Chem. Res. 2018, 57, 11265–11270. [Google Scholar] [CrossRef]
- Vu, B.K.; Song, M.B.; Ahn, I.Y.; Suh, Y.W.; Suh, D.J.; Kim, W.I.; Koh, H.L.; Choi, Y.G.; Shin, E.W. Pt-Sn alloy phases and coke mobility over Pt-Sn/Al2O3 and Pt-Sn/ZnAl2O4 catalysts for propane dehydrogenation. Appl. Catal. A 2011, 400, 25–33. [Google Scholar] [CrossRef]
- Zhang, M.; Song, Z.; Guo, M.Q.; Li, X.X.; Lin, Y.J.; Zhang, L.H. Effect of reduction atmosphere on structure and catalytic performance of PtIn/Mg(Al)O/ZnO for propane dehydrogenation. Catalysts 2020, 10, 485. [Google Scholar] [CrossRef]
- Wu, P.; Xia, L.; Liu, Y.; Wu, J.S.; Chen, Q.Y.; Song, S.X. Simultaneous sorption of arsenate and fluoride on calcined Mg-Fe-La hydrotalcite-like compound from water. ACS Sustain. Chem. Eng. 2018, 6, 16287–16297. [Google Scholar] [CrossRef]
- Mimura, N.; Takahara, I.; Saito, M.; Sasaki, Y.; Murata, K. Dehydrogenation of ethylbenzene to styrene in the presence of CO2 over calcined hydrotalcite-like compounds as catalysts. Catal. Lett. 2002, 78, 125–128. [Google Scholar] [CrossRef]
- Sun, P.; Siddiqi, G.; Vining, W.C.; Chi, M.; Bell, A.T. Novel Pt/Mg(In)(Al)O catalysts for ethane and propane dehydrogenation. J. Catal. 2011, 282, 165–174. [Google Scholar] [CrossRef]
- Yang, X.; Liu, G.; Li, Y.; Zhang, L.; Wang, X.; Liu, Y. Novel Pt-Ni bimetallic batalysts Pt(Ni)-LaFeO3/SiO2 via lattice atomic-confined reduction for highly efficient isobutane dehydrogenation. Trans. Tianjin Univ. 2018, 25, 245–257. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Xu, J.; Ouyang, X.; Hou, X.; Chen, D.; Wang, R.; Shen, G. Flexible coaxial-type fiber supercapacitor based on NiCo2O4 nanosheets electrodes. Nano Energy 2014, 8, 44–51. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Ge, M.; Chen, X.; Guo, M.; Zhang, L. Perovskite-derived Pt-Ni/Zn(Ni)TiO3/SiO2 catalyst for propane dehydrogenation to propene. Catal. Lett. 2019, 149, 2552–2562. [Google Scholar] [CrossRef]
- Passos, F.B.; Aranda, D.A.G.; Schmal, M. Characterization and catalytic activity of bimetallic Pt-In/Al2O3 and Pt-Sn/Al2O3 catalysts. J. Catal. 1998, 178, 478–488. [Google Scholar] [CrossRef]
- Marchesini, F.A.; Gutierrez, L.B.; Querini, C.A.; Miro, E.E. Pt, In and Pd, In catalysts for the hydrogenation of nitrates and nitrites in water. FTIR characterization and reaction studies. Chem. Eng. J. 2010, 159, 203–211. [Google Scholar] [CrossRef]
- Im, J.; Choi, M. Physicochemical stabilization of Pt against sintering for a dehydrogenation catalyst with high activity, selectivity, and durability. ACS Catal. 2016, 6, 2819–2826. [Google Scholar] [CrossRef]
- Xia, K.; Lang, W.; Li, P.; Yan, X.; Guo, Y. Analysis of the catalytic activity induction and deactivation of PtIn/Mg(Al)O catalysts for propane dehydrogenation reaction. RSC Adv. 2015, 5, 64689–64695. [Google Scholar] [CrossRef]
- Chen, H.; Jie, Y.; Chang, L.; Chen, X. Reaction behavior of MgF2 powder in hexafluoropropylene/air atmospheres at high temperatures. Solid State Ion. 2019, 340, 115016. [Google Scholar] [CrossRef]
- Han, W.; Zhang, C.; Wang, H.; Zhou, S.; Tang, H.; Yang, L.; Wang, Z. Sub-nano MgF2 embedded in carbon nanofibers and electrospun MgF2 nanofibers by one-step electrospinning as highly efficient catalysts for 1,1,1-trifluoroethane dehydrofluorination. Catal. Sci. Technol. 2017, 7, 6000–6012. [Google Scholar] [CrossRef]
- Chen, X.; Ge, M.; Li, Y.; Liu, Y.; Wang, J.; Zhang, L. Fabrication of highly dispersed Pt-based catalysts on γ-Al2O3 supported perovskite Nano islands: High durability and tolerance to coke deposition in propane dehydrogenation. Appl. Surf. Sci. 2019, 490, 611–621. [Google Scholar] [CrossRef]
- Zhao, S.Y.; Xu, B.L.; Yu, L.; Fan, Y.N. Catalytic dehydrogenation of propane to propylene over highly active PtSnNa/gamma-Al2O3 catalyst. Chin. Chem. Lett. 2018, 29, 475–478. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Wan, L.; Xue, M.; Duan, Y.; Liu, X. Effect of magnesium addition on catalytic performance of PtSnK/γ-Al2O3 catalyst for isobutane dehydrogenation. Fuel Process. Technol. 2011, 92, 1632–1638. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Shi, J.; Sheng, X.; Duan, Y.; Zhou, S.; Zhang, Z. Effect of zinc addition on catalytic properties of PtSnK/γ-Al2O3 catalyst for isobutane dehydrogenation. Fuel Process. Technol. 2012, 96, 220–227. [Google Scholar] [CrossRef]
- Vaezifar, S.; Faghihian, H.; Kamali, M. Dehydrogenation of isobutane over Sn/Pt/Na-ZSM-5 catalysts: The effect of SiO2/Al2O3 ratio, amount and distribution of Pt nanoparticles on the catalytic behavior. Korean J. Chem. Eng. 2010, 28, 370–377. [Google Scholar] [CrossRef]
- Li, B.; Xu, Z.; Jing, F.; Luo, S.; Chu, W. Facile one-pot synthesized ordered mesoporous Mg-SBA-15 supported PtSn catalysts for propane dehydrogenation. Appl. Catal. A 2017, 533, 17–27. [Google Scholar] [CrossRef]




| Samples | Binding Energy (eV) | In3+/In0 a | |||
|---|---|---|---|---|---|
| In 3d5/2 | In 3d3/2 | ||||
| In0 | In3+ | In0 | In3+ | ||
| PtInHTR | 444.5 | 445.2 | 452.2 | 452.7 | 3.5 |
| PtInHTR-MgF2 | 444.5 | 445.2 | 452.2 | 452.7 | 4.2 |
| Catalysts | Pt Contents (wt%) | WHSV (h−1) | Isobutane Conversion (%) b | Isobutene Selectivity (%) b | References |
|---|---|---|---|---|---|
| PtInHTR-MgF2 | 0.5 | 3 | 50–58 | 96–95 | Present work |
| InPtSn/ZnAl2O4 | 0.4 | 4 | 54–38 | 94–96 | [33] |
| PtNi/LaFeO3/SiO2 | 0.3 | 3 | 39–39 | 84–91 | [39] |
| PtSnKMg/Al2O3 | 0.5 | 2 | 34–29 | 80–95 | [50] |
| PtSnKZn/Al2O3 | 0.5 | 2 | 36–32 | 96–96 | [51] |
| PtSnNa/ZSM-5 | 0.5 | 2.5 | 52–52 | 84–84 | [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Z.; Wang, J.; Liu, F.; Zhang, X.; Matusse, É.; Zhang, L. MgF2-Modified Hydrotalcite-Derived Composites Supported Pt-In Catalysts for Isobutane Direct Dehydrogenation. Catalysts 2021, 11, 478. https://doi.org/10.3390/catal11040478
Song Z, Wang J, Liu F, Zhang X, Matusse É, Zhang L. MgF2-Modified Hydrotalcite-Derived Composites Supported Pt-In Catalysts for Isobutane Direct Dehydrogenation. Catalysts. 2021; 11(4):478. https://doi.org/10.3390/catal11040478
Chicago/Turabian StyleSong, Zhen, Jiameng Wang, Fanji Liu, Xiqing Zhang, Énio Matusse, and Lihong Zhang. 2021. "MgF2-Modified Hydrotalcite-Derived Composites Supported Pt-In Catalysts for Isobutane Direct Dehydrogenation" Catalysts 11, no. 4: 478. https://doi.org/10.3390/catal11040478
APA StyleSong, Z., Wang, J., Liu, F., Zhang, X., Matusse, É., & Zhang, L. (2021). MgF2-Modified Hydrotalcite-Derived Composites Supported Pt-In Catalysts for Isobutane Direct Dehydrogenation. Catalysts, 11(4), 478. https://doi.org/10.3390/catal11040478