The β-Fructofuranosidase from Rhodotorula dairenensis: Molecular Cloning, Heterologous Expression, and Evaluation of Its Transferase Activity
Abstract
1. Introduction
2. Results
2.1. Molecular Characterization of the Gene RdINV and Analysis of the Amino Acid Sequence Encoded
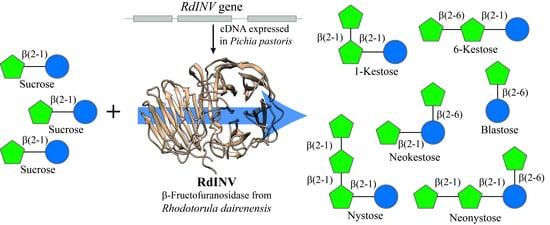
2.2. Functionality of the Gene RdINV and Heterologous Protein Size Analyses
2.3. Analysis of the β-Fructofuranosidase Activity Expressed in P. pastoris
3. Discussion
4. Materials and Methods
4.1. Organisms, Growth and Expression Media
4.2. Protein Purification, Quantification and Spectrometric Analysis
4.3. Hydrolase Activity and Kinetic Analysis
4.4. Transferase Activity, Fructooligosaccharides Production, and HPLC Analysis
4.5. DNA Techniques and Cloning of the R. dairenensis β-Fructofuranosidase
4.6. Protein Sequence Analysis
4.7. Nucleotide Sequence Accession Number
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Swennen, K.; Courtin, C.M.; Delcour, J.A. Non-digestible oligosaccharides with prebiotic properties. Crit. Rev. Food Sci. Nutr. 2006, 46, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Battson, M.L.; Lee, D.M.; Weir, T.L.; Gentile, C.L. The gut microbiota as a novel regulator of cardiovascular function and disease. J. Nutr. Biochem. 2018, 56, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Whisner, C.M.; Castillo, L.F. Prebiotics, bone and mineral metabolism. Calcif. Tissue Int. 2018, 102, 443–479. [Google Scholar] [CrossRef]
- Schachter, J.; Martel, J.; Lin, C.S.; Chang, C.J.; Wu, T.R.; Lu, C.C.; Ko, Y.F.; Lai, H.C.; Ojcius, D.M.; Young, J.D. Effects of obesity on depression: A role for inflammation and the gut microbiota. Brain Behav. Immun. 2018, 69, 1–8. [Google Scholar] [CrossRef]
- Chen, D.; Yang, X.; Yang, J.; Lai, G.; Yong, T.; Tang, X.; Shuai, O.; Zhou, G.; Xie, Y.; Wu, Q. Prebiotic effect of Fructooligosaccharides from Morinda officinalis on Alzheimer’s disease in rodent models by targeting the microbiota-gut-brain axis. Front. Aging Neurosci. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Man, S.; Liu, T.; Yao, Y.; Lu, Y.; Ma, L.; Lu, F. Friend or foe? The roles of inulin-type fructans. Carbohydr. Polym. 2021, 252, 117155. [Google Scholar] [CrossRef] [PubMed]
- Sabater-Molina, M.; Larqué, E.; Torrella, F.; Zamora, S. Dietary fructooligosaccharides and potential benefits on health. J. Physiol. Biochem. 2009, 65, 315–328. [Google Scholar] [CrossRef]
- Kilian, S.; Kritzinger, S.; Rycroft, C.; Gibson, G.; du Preez, J. The effects of the novel bifidogenic trisaccharide, neokestose, on the human colonic microbiota. World J. Microbiol. Biotechnol. 2002, 18, 637–644. [Google Scholar] [CrossRef]
- Wu, J.S.; Chang, J.Y.; Chen, C.W.; Lin, M.T.; Sheu, D.C.; Lee, S.M. Neokestose suppresses the growth of human melanoma A2058 cells via inhibition of the nuclear factor-κB signaling pathway. Mol. Med. Rep. 2017, 16, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Chang, J.Y.; Wu, J.S.; Sheu, D.C. Antineoplastic effect of a novel chemopreventive agent, neokestose, on the Caco-2 cell line via inhibition of expression of nuclear factor-κB and cyclooxygenase-2. Mol. Med. Rep. 2015, 12, 1114–1118. [Google Scholar] [CrossRef]
- Ni, D.; Xu, W.; Zhu, Y.; Pang, X.; Lv, J.; Mu, W. Insight into the effects and biotechnological production of kestoses, the smallest fructooligosaccharides. Crit. Rev. Biotechnol. 2021, 41, 34–46. [Google Scholar] [CrossRef]
- Singh, S.P.; Jadaun, J.S.; Narnoliya, L.K.; Pandey, A. Prebiotic oligosaccharides: Special focus on fructooligosaccharides, its biosynthesis and bioactivity. Appl. Biochem. Biotechnol. 2017, 183, 613–635. [Google Scholar] [CrossRef]
- Flores-Maltos, D.A.; Mussatto, S.I.; Contreras-Esquivel, J.C.; Rodríguez-Herrera, R.; Teixeira, J.A.; Aguilar, C.N. Biotechnological production and application of fructooligosaccharides. Crit. Rev. Biotechnol. 2016, 36, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Marín-Navarro, J.; Talens-Perales, D.; Polaina, J. One-pot production of fructooligosaccharides by a Saccharomyces cerevisiae strain expressing an engineered invertase. Appl. Microbiol. Biotechnol. 2015, 99, 2549–2555. [Google Scholar] [CrossRef] [PubMed]
- Álvaro-Benito, M.; de Abreu, M.; Fernández-Arrojo, L.; Plou, F.J.; Jiménez-Barbero, J.; Ballesteros, A.; Polaina, J.; Fernández-Lobato, M. Characterization of a β-fructofuranosidase from Schwanniomyces occidentalis with transfructosylating activity yielding the prebiotic 6-kestose. J. Biotechnol. 2007, 132, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Linde, D.; Rodríguez-Colinas, B.; Estévez, M.; Poveda, A.; Plou, F.J.; Fernández Lobato, M. Analysis of neofructooligosaccharides production mediated by the extracellular β-fructofuranosidase from Xanthophyllomyces dendrorhous. Bioresour. Technol. 2012, 109, 123–130. [Google Scholar] [CrossRef]
- Ramírez-Escudero, M.; Gimeno-Pérez, M.; González, B.; Linde, D.; Merdzo, Z.; Fernández-Lobato, M.; Sanz-Aparicio, J. Structural analysis of β-fructofuranosidase from Xanthophyllomyces dendrorhous reveals unique features and the crucial role of N-glycosylation in oligomerization and activity. J. Biol. Chem. 2016, 291, 6843–6857. [Google Scholar] [CrossRef] [PubMed]
- Sainz-Polo, M.A.; Ramírez-Escudero, M.; Lafraya, A.; González, B.; Marín-Navarro, J.; Polaina, J.; Sanz-Aparicio, J. Three-dimensional structure of Saccharomyces invertase: Role of a non-catalytic domain in oligomerization and substrate specificity. J. Biol. Chem. 2013, 288, 9755–9766. [Google Scholar] [CrossRef]
- Álvaro-Benito, M.; Polo, A.; González, B.; Fernández-Lobato, M.; Sanz-Aparicio, J. Structural and kinetic analysis of Schwanniomyces occidentalis invertase reveals a new oligomerization pattern and the role of its supplementary domain in substrate binding. J. Biol. Chem. 2010, 285, 13930–13941. [Google Scholar] [CrossRef]
- Álvaro-Benito, M.; Sainz-Polo, M.A.; González-Pérez, D.; González, B.; Plou, F.J.; Fernández-Lobato, M.; Sanz-Aparicio, J. Structural and kinetic insights reveal that the amino acid pair Gln-228/Asn-254 modulates the transfructosylating specificity of Schwanniomyces occidentalis β-fructofuranosidase, an enzyme that produces prebiotics. J. Biol. Chem. 2012, 287, 19674–19686. [Google Scholar] [CrossRef]
- Rubio, M.C.; Runco, R.; Navarro, A.R. Invertase from a strain of Rhodotorula glutinis. Phytochemistry 2002, 61, 605–609. [Google Scholar] [CrossRef]
- Hernalsteens, S.; Maugeri, F. Purification and characterisation of a fructosyltransferase from Rhodotorula sp. Appl. Microbiol. Biotechnol. 2008, 79, 589–596. [Google Scholar] [CrossRef]
- Canli Tasar, O. Enhanced β-fructofuranosidase biosynthesis by Rhodotorula glutinis using Taguchi robust design method. Biocatal. Biotransform. 2017, 35, 191–196. [Google Scholar] [CrossRef]
- Barbosa, P.M.G.; de Morais, T.P.; de Andrade Silva, C.A.; da Silva Santos, F.R.; Garcia, N.F.L.; Fonseca, G.G.; Leite, R.S.R.; da Paz, M.F. Biochemical characterization and evaluation of invertases produced from Saccharomyces cerevisiae CAT-1 and Rhodotorula mucilaginosa for the production of fructooligosaccharides. Prep. Biochem. Biotechnol. 2018, 48, 506–513. [Google Scholar] [CrossRef]
- Gutiérrez-Alonso, P.; Fernández-Arrojo, L.; Plou, F.J.; Fernández-Lobato, M. Biochemical characterization of a β-fructofuranosidase from Rhodotorula dairenensis with transfructosylating activity. FEMS Yeast Res. 2009, 9, 768–773. [Google Scholar] [CrossRef][Green Version]
- Lammens, W.; Le Roy, K.; Schroeven, L.; Van Laere, A.; Rabijns, A.; Van Den Ende, W. Structural insights into glycoside hydrolase family 32 and 68 enzymes: Functional implications. J. Exp. Bot. 2009, 60, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Pons, T.; Naumoff, D.G.; Martínez-Fleites, C.; Hernández, L. Three acidic residues are at the active site of a β-propeller architecture in Glycoside Hydrolase families 32, 43, 62, and 68. Proteins Struct. Funct. Genet. 2004, 54, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Trollope, K.M.; van Wyk, N.; Kotjomela, M.A.; Volschenk, H. Sequence and structure-based prediction of fructosyltransferase activity for functional sub-classification of fungal GH32 enzymes. FEBS J. 2015, 282, 1–15. [Google Scholar] [CrossRef]
- Goordial, J.; Raymond-Bouchard, I.; Riley, R.; Ronholm, J.; Shapiro, N.; Woyke, T.; LaButti, K.M.; Tice, H.; Amirebrahimi, M.; Grigoriev, I.V.; et al. Improved high-quality draft genome sequence of the eurypsychrophile Rhodotorula sp. JG1b, isolated from permafrost in the hyperarid upper-elevation McMurdo Dry Valleys, Antarctica. Genome Announc. 2016, 4, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Magbanua, Z.; Arick, M.; French, T.; Bridges, S.M.; Burgess, S.C.; Lawrence, M.L. Genome Sequence of the oleaginous yeast Rhodotorula glutinis ATCC 204091. Genome Announc. 2014, 2, 46–60. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, S.; Liu, H.; Shen, H.; Lin, X.; Yang, F.; Zhou, Y.J.; Jin, G.; Ye, M.; Zou, H.; et al. A multi-omic map of the lipid-producing yeast Rhodosporidium toruloides. Nat. Commun. 2012, 3, 1–12. [Google Scholar] [CrossRef]
- Nagem, R.A.P.; Rojas, A.L.; Golubev, A.M.; Korneeva, O.S.; Eneyskaya, E.V.; Kulminskaya, A.A.; Neustroev, K.N.; Polikarpov, I. Crystal structure of exo-inulinase from Aspergillus awamori: The enzyme fold and structural determinants of substrate recognition. J. Mol. Biol. 2004, 344, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Gimeno-Pérez, M.; Linde, D.; Fernández-Arrojo, L.; Plou, F.J.; Fernández-Lobato, M. Heterologous overproduction of β-fructofuranosidase from yeast Xanthophyllomyces dendrorhous, an enzyme producing prebiotic sugars. Appl. Microbiol. Biotechnol. 2015, 99, 3459–3467. [Google Scholar] [CrossRef]
- Linde, D.; Macias, I.; Fernández-Arrojo, L.; Plou, F.J.; Jiménez, A.; Fernández-Lobato, M. Molecular and biochemical characterization of a β-fructofuranosidase from Xanthophyllomyces dendrorhous. Appl. Environ. Microbiol. 2009, 75, 1065–1073. [Google Scholar] [CrossRef]
- Nagaya, M.; Kimura, M.; Gozu, Y.; Sato, S.; Hirano, K.; Tochio, T.; Nishikawa, A.; Tonozuka, T. Crystal structure of a β-fructofuranosidase with high transfructosylation activity from Aspergillus kawachii. Biosci. Biotechnol. Biochem. 2017, 81, 1786–1795. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, H.; Denman, S.E.; Campuzano, I.D.G.; Ademark, P.; Master, E.R.; Teeri, T.T.; Brumer, H. N-linked glycosylation of native and recombinant cauliflower xyloglucan endotransglycosylase 16A. Biochem. J. 2003, 375, 61–73. [Google Scholar] [CrossRef]
- Han, M.; Wang, X.; Ding, H.; Jin, M.; Yu, L.; Wang, J.; Yu, X. The role of N-glycosylation sites in the activity, stability, and expression of the recombinant elastase expressed by Pichia pastoris. Enzyme Microb. Technol. 2014, 54, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, J.; Li, Y.; Wang, X.; Li, Q. Heterologous expression of Hordeum vulgare protein Z4 in Pichia pastoris shows increased structural stability. Process Biochem. 2016, 51, 828–837. [Google Scholar] [CrossRef]
- Zambelli, P.; Fernandez-Arrojo, L.; Romano, D.; Santos-Moriano, P.; Gimeno-Perez, M.; Poveda, A.; Gandolfi, R.; Fernández-Lobato, M.; Molinari, F.; Plou, F.J. Production of fructooligosaccharides by mycelium-bound transfructosylation activity present in Cladosporium cladosporioides and Penicilium sizovae. Process Biochem. 2014, 49, 2174–2180. [Google Scholar] [CrossRef][Green Version]
- Santos-Moriano, P.; Fernandez-Arrojo, L.; Poveda, A.; Jimenez-Barbero, J.; Ballesteros, A.O.; Plou, F.J. Levan versus fructooligosaccharide synthesis using the levansucrase from Zymomonas mobilis: Effect of reaction conditions. J. Mol. Catal. B Enzym. 2015, 119, 18–25. [Google Scholar] [CrossRef]
- Rodrigo-Frutos, D.; Piedrabuena, D.; Sanz-Aparicio, J.; Fernández-Lobato, M. Yeast cultures expressing the Ffase from Schwanniomyces occidentalis, a simple system to produce the potential prebiotic sugar 6-kestose. Appl. Microbiol. Biotechnol. 2019, 103, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, F.V.; Neifar, S.; Merdzo, Z.; Viña-Gonzalez, J.; Fernandez-Arrojo, L.; Ballesteros, A.O.; Fernandez-Lobato, M.; Bejar, S.; Plou, F.J. A three-step process for the bioconversion of whey permeate into a glucose D-free tagatose syrup. Catalysts 2020, 10, 647. [Google Scholar] [CrossRef]
- Gimeno-Pérez, M.; Santos-Moriano, P.; Fernandez-Arrojo, L.; Poveda, A.; Jimenez-Barbero, J.; Ballesteros, A.O.; Fernandez-Lobato, M.; Plou, F.J. Regioselective synthesis of neo-erlose by the β-fructofuranosidase from Xanthophyllomyces dendrorhous. Process Biochem. 2014, 49, 423–429. [Google Scholar] [CrossRef]
- Piedrabuena, D.; Míguez, N.; Poveda, A.; Plou, F.J.; Fernández-Lobato, M. Exploring the transferase activity of Ffase from Schwanniomyces occidentalis, a β-fructofuranosidase showing high fructosyl-acceptor promiscuity. Appl. Microbiol. Biotechnol. 2016, 100, 8769–8778. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ojeda, F.J.; Plaza-Díaz, J.; Sáez-Lara, M.J.; Gil, A. Effects of sweeteners on the gut microbiota: A review of experimental studies and clinical trials. Adv. Nutr. 2019, 10, S31–S48. [Google Scholar] [CrossRef]
- Lenhart, A.; Chey, W.D. A systematic review of the effects of polyols on gastrointestinal health and irritable bowel syndrome. Adv. Nutr. 2017, 8, 587–596. [Google Scholar] [CrossRef]
- Zhang, J.; Xin, L.; Shan, B.; Chen, W.; Xie, M.; Yuen, D.; Zhang, W.; Zhang, Z.; Lajoie, G.A.; Ma, B. PEAKS DB: De novo sequencing assisted database search for sensitive and accurate peptide identification. Mol. Cell. Proteom. 2012, 11. [Google Scholar] [CrossRef]
- Wang, K.; Ussery, D.W.; Brunak, S. Analysis and prediction of gene splice sites in four Aspergillus genomes. Fungal Genet. Biol. 2009, 46 (Suppl. 1), S14–S18. [Google Scholar] [CrossRef]
- Van Den Ent, F.; Löwe, J. RF cloning: A restriction-free method for inserting target genes into plasmids. J. Biochem. Biophys. Methods 2006, 67, 67–74. [Google Scholar] [CrossRef]
- Kelley, L.A.; Sternberg, M.J.E. Protein structure prediction on the Web: A case study using the Phyre server. Nat. Protoc. 2009, 4, 363–371. [Google Scholar] [CrossRef] [PubMed]




| β-Fructofuranosidase (U/mL) | ||
|---|---|---|
| Substrate | P. pastoris | R. dairenensis |
| Sucrose | 20.5 ± 1.4 | 12.4 ± 1.1 |
| 1-Kestose | 4.1 ± 0.03 | 1.8 ± 0.02 |
| Nystose | 0.4 ± 0.04 | 0.4 ± 0.02 |
| Raffinose | 16.7 ± 0.2 | 11.0 ± 0.3 |
| Inulin | 3.2 ± 0.05 | 2.8 ± 0.03 |
| Substrate | Km (mM) | kcat (1/s) | kcat/Km (1/s mM) | |
|---|---|---|---|---|
| P. pastoris | Sucrose | 6.2 ± 1.0 | 2236 ± 356 | 360 ± 57 |
| 1-Kestose | 56 ± 5 | 613 ± 49 | 11 ± 1 | |
| R. dairenensis | Sucrose | 6.4 ± 0.9 | 4458 ± 628 | 698 ± 98 |
| 1-Kestose | 57 ± 4 | 1753 ± 136 | 30 ± 2 |
| Monosaccharides | Disaccharides | Polyols | |||
|---|---|---|---|---|---|
| Acceptor | New Product a | Acceptor | New Product a | Acceptor | New Product a |
| Fructose | + | Trehalose | + | Erythritol | + |
| Galactose | - | Palatinose | + | Galactitol | + |
| Glucose | + | Lactose | + | Sorbitol | + |
| Xylose | - | Lactulose | + | Mannitol | + |
| Arabinose | - | Leucrose | + | Ribitol | - |
| Mannose | - | Maltose | + | Xylitol | - |
| Melibiose | - | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gimeno-Pérez, M.; Merdzo, Z.; Castillo-Rosa, E.; Hijas, C.M.d.; Fernández-Lobato, M. The β-Fructofuranosidase from Rhodotorula dairenensis: Molecular Cloning, Heterologous Expression, and Evaluation of Its Transferase Activity. Catalysts 2021, 11, 476. https://doi.org/10.3390/catal11040476
Gimeno-Pérez M, Merdzo Z, Castillo-Rosa E, Hijas CMd, Fernández-Lobato M. The β-Fructofuranosidase from Rhodotorula dairenensis: Molecular Cloning, Heterologous Expression, and Evaluation of Its Transferase Activity. Catalysts. 2021; 11(4):476. https://doi.org/10.3390/catal11040476
Chicago/Turabian StyleGimeno-Pérez, María, Zoran Merdzo, Eva Castillo-Rosa, Carlos Martín de Hijas, and María Fernández-Lobato. 2021. "The β-Fructofuranosidase from Rhodotorula dairenensis: Molecular Cloning, Heterologous Expression, and Evaluation of Its Transferase Activity" Catalysts 11, no. 4: 476. https://doi.org/10.3390/catal11040476
APA StyleGimeno-Pérez, M., Merdzo, Z., Castillo-Rosa, E., Hijas, C. M. d., & Fernández-Lobato, M. (2021). The β-Fructofuranosidase from Rhodotorula dairenensis: Molecular Cloning, Heterologous Expression, and Evaluation of Its Transferase Activity. Catalysts, 11(4), 476. https://doi.org/10.3390/catal11040476