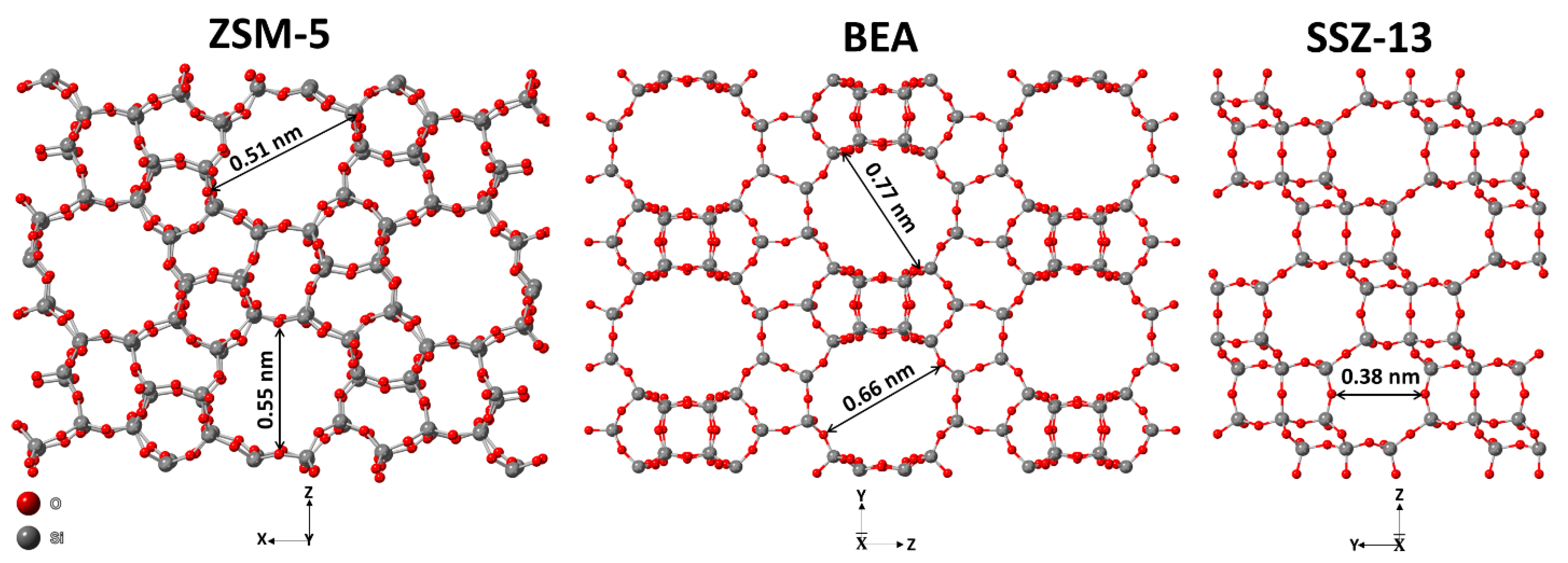
Analysis of Ion-Exchanged ZSM-5, BEA, and SSZ-13 Zeolite Trapping Materials under Realistic Exhaust Conditions
Abstract
1. Introduction
2. Results and Discussion
2.1. Hydrocarbon Trapping
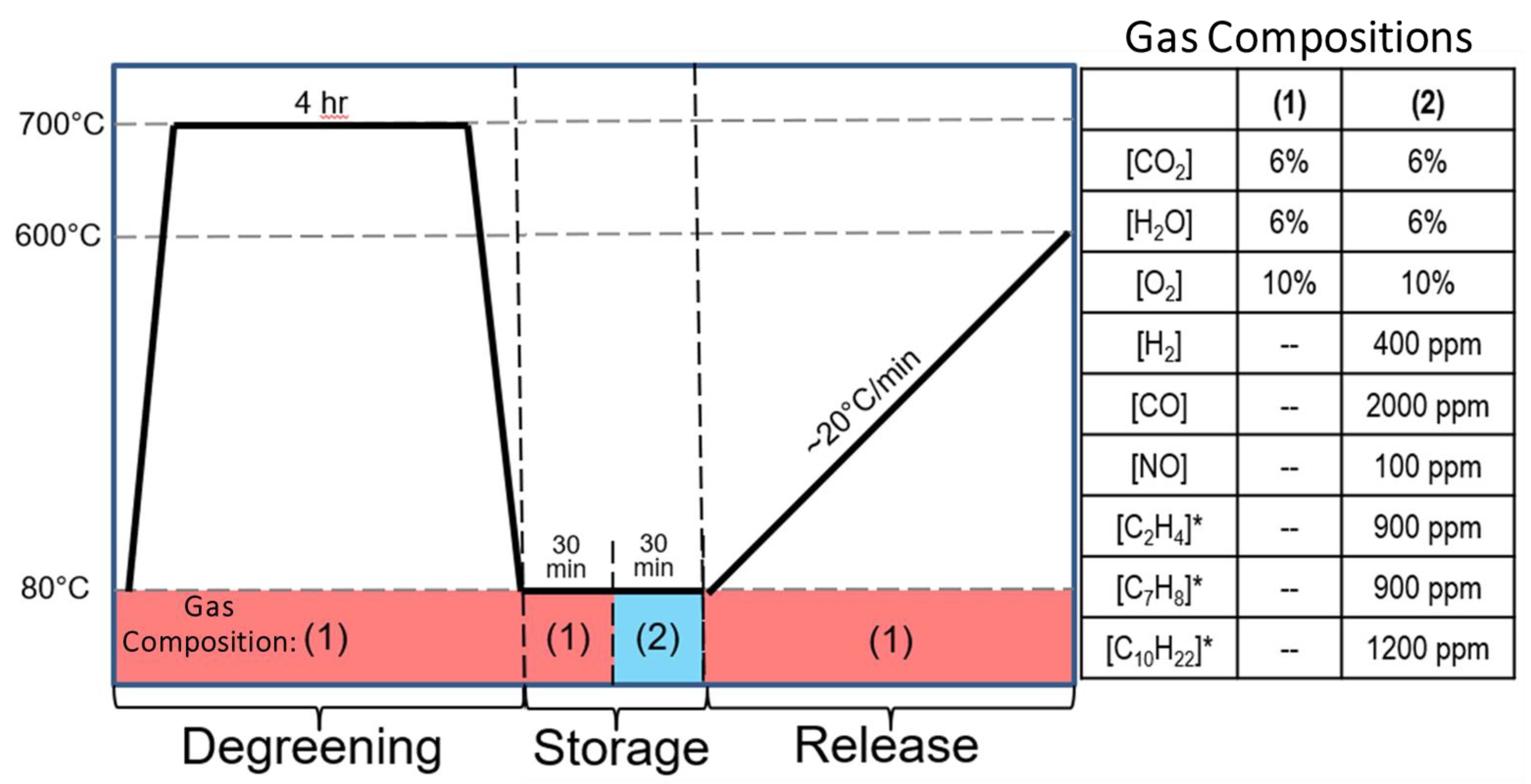
 ) in Figure 2a and is quantified in Figure 2b. The second portion up to 30 min is related to total storage capacity (white with blue dots,
) in Figure 2a and is quantified in Figure 2b. The second portion up to 30 min is related to total storage capacity (white with blue dots,  , in Figure 2a,b), but as can be seen here, the sample was not fully saturated as the bypass concentration was not reached. After the 30-min storage, the reactant gases are turned off, and the sample is quickly heated to 600 °C. During this portion of the experiment the HCs desorb from the sample, and some of them react to form CO and CO2. As suggested by the protocol [38,39], the release profile is described by three characteristic temperatures. The first is the temperature of 10% release (T10), i.e., the temperature where 10% of the observed desorbed HCs were released. This is also shown in Figure 2a with a dark blue shading (■) beginning at 30 min (0% release) and continuing to ~33 min (10% release); the temperature is also indicated with a dark blue arrow. Figure 2c indicates this value with the dark blue (■) portion of the bar. The next segment indicates the temperature of 50% release (T50). In Figure 2a this is represented with the medium blue shading (■) that reaches from 10% to 50% and the temperature is indicated with medium blue arrow and by the second section of the bar in Figure 2c (■). The next segment of interest is for 90% release of the HCs, which is represented by a light blue shading (■) for the 50–90% release in Figure 2a. The T90 is represented by a light blue arrow in Figure 2a and the top segment of the bar in Figure 2c (■).
, in Figure 2a,b), but as can be seen here, the sample was not fully saturated as the bypass concentration was not reached. After the 30-min storage, the reactant gases are turned off, and the sample is quickly heated to 600 °C. During this portion of the experiment the HCs desorb from the sample, and some of them react to form CO and CO2. As suggested by the protocol [38,39], the release profile is described by three characteristic temperatures. The first is the temperature of 10% release (T10), i.e., the temperature where 10% of the observed desorbed HCs were released. This is also shown in Figure 2a with a dark blue shading (■) beginning at 30 min (0% release) and continuing to ~33 min (10% release); the temperature is also indicated with a dark blue arrow. Figure 2c indicates this value with the dark blue (■) portion of the bar. The next segment indicates the temperature of 50% release (T50). In Figure 2a this is represented with the medium blue shading (■) that reaches from 10% to 50% and the temperature is indicated with medium blue arrow and by the second section of the bar in Figure 2c (■). The next segment of interest is for 90% release of the HCs, which is represented by a light blue shading (■) for the 50–90% release in Figure 2a. The T90 is represented by a light blue arrow in Figure 2a and the top segment of the bar in Figure 2c (■).2.2. NO Storage
 ) and 30 (
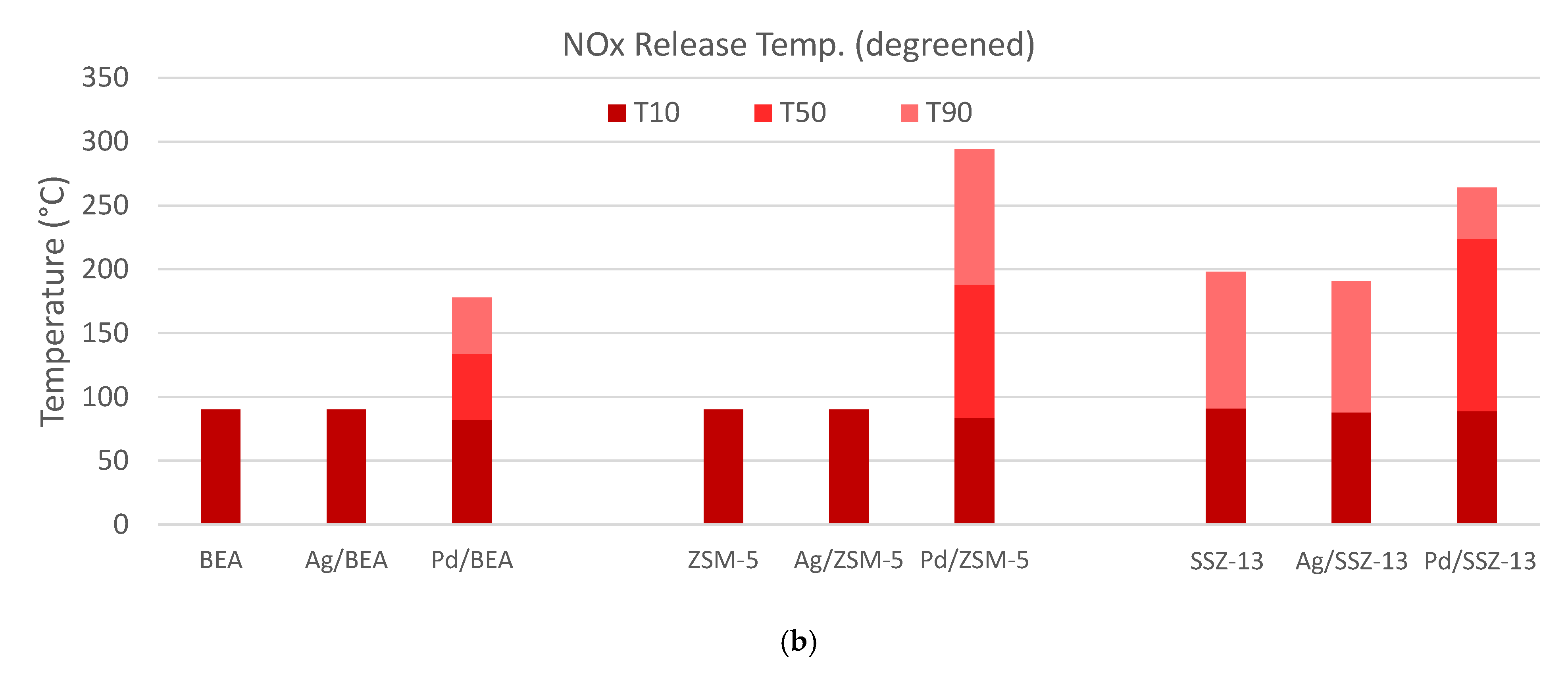
) and 30 ( ) minutes (Figure 4b), and the temperatures of 10%, 50% and 90% NOx release are determined (Figure 4c). The data for all the samples are shown in Figure 5 with the specific values listed in Table 2. From these data two important observations are apparent. First, the Pd-zeolites are the only samples to have significant uptake, and second, only Pd/ZSM-5 and Pd/SSZ-13 maintains 50% of the NOx above 150 °C; in fact, over 50% of the NOx is still on Pd Pd/SSZ-13 at 224 °C. This finding is consistent with other reports of Pd/SSZ-13 being one of the best PNAs [56,57] and that NO storage only occurs on ion-exchanged Pd sites [41]. Neither catalyst closed the NOx balance during release, suggesting there is some direct reduction in the stored NOx from HCs. This typically yields some N2O formation, but this reactor was not equipped with an analyzer that would allow N2O to be measured accurately.
) minutes (Figure 4b), and the temperatures of 10%, 50% and 90% NOx release are determined (Figure 4c). The data for all the samples are shown in Figure 5 with the specific values listed in Table 2. From these data two important observations are apparent. First, the Pd-zeolites are the only samples to have significant uptake, and second, only Pd/ZSM-5 and Pd/SSZ-13 maintains 50% of the NOx above 150 °C; in fact, over 50% of the NOx is still on Pd Pd/SSZ-13 at 224 °C. This finding is consistent with other reports of Pd/SSZ-13 being one of the best PNAs [56,57] and that NO storage only occurs on ion-exchanged Pd sites [41]. Neither catalyst closed the NOx balance during release, suggesting there is some direct reduction in the stored NOx from HCs. This typically yields some N2O formation, but this reactor was not equipped with an analyzer that would allow N2O to be measured accurately.2.3. Hydrothermal Aging
3. Experimental
3.1. Synthesis of Ion-Exchanged Zeolites
3.2. Trapping Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dürnholz, M.; Eifler, G.; Endres, H. Exhaust-Gas Recirculation—A Measure to Reduce Exhaust Emissions of DI Diesel Engines; No. 920725; SAE Technical Paper; SAE International: Warrendale, PA, USA, 1992. [Google Scholar] [CrossRef]
- Johnson, T.V. Review of Diesel Emissions and Control. Int. J. Engine Res. 2009, 10, 275–285. [Google Scholar] [CrossRef]
- Kyriakidou, E.A.; Toops, T.J.; Choi, J.-S.; Lance, M.J.; Parks, J.E., II. Exhaust Treatment Catalysts with Enhanced Hydrothermal Stability and Low-Temperature Activity. U.S. Patent 10,427,137 B2, 1 October 2019. [Google Scholar]
- Wong, A.P.; Kyriakidou, E.A.; Toops, T.J.; Regalbuto, J.R. The Catalytic Behavior of Precisely Synthesized Pt-Pd Bimetallic Catalysts for Use as Diesel Oxidation Catalysts. Catal. Today 2016, 267, 145–156. [Google Scholar] [CrossRef]
- Kim, M.-Y.; Kyriakidou, E.A.; Choi, J.-S.; Toops, T.J.; Binder, A.J.; Thomas, C.; Parks, J.E., II; Schwartz, V.; Chen, J.; Hensley, D.K. Enhancing Low-Temperature Activity and Durability of Pd-based Diesel Oxidation Catalysts Using ZrO2 Supports. Appl. Catal. B Environ. 2016, 187, 181–194. [Google Scholar] [CrossRef]
- Hoang, S.; Guo, Y.; Binder, A.; Tang, W.; Wang, S.; Liu, J.; Huan, T.; Lu, X.; Wang, Y.; Ding, Y.; et al. Activating Low-Temperature Diesel Oxidation by Single-Atom Pt on TiO2 Nanowire Array. Nat. Commun. 2020, 11, 1–10. [Google Scholar]
- Wang, A.; Olsson, L. The Impact of Automotive Catalysis on the United Nations Sustainable Development Goals. Nat. Catal. 2019, 2, 566–570. [Google Scholar] [CrossRef]
- Johnson, T.V. Diesel Emissions in Review. SAE Int. J. Engines 2011, 4, 143–157. [Google Scholar] [CrossRef]
- Zelinsky, R.; Epling, W. Effects of Multicomponent Hydrocarbon Feed on Hydrocarbon Adsorption—Desorption and Oxidation Light-Off Behavior on a Pd/BEA Hydrocarbon Trap. Catal. Lett. 2019, 149, 3194–3202. [Google Scholar] [CrossRef]
- Beale, A.M.; Gao, F.; Lezcano-Gonzalez, I.; Peden, C.H.F.; Szanyi, J. Recent Advances in Automotive Catalysis for NOx Emission Control by Small-Pore Microporous Materials. Chem. Soc. Rev. 2015, 44, 7371–7405. [Google Scholar] [CrossRef]
- Naseri, M.; Aydin, C.; Mulla, S.; Conway, R.; Chatterjee, S. Development of Emission Control Systems to Enable High NOx Conversion on Heavy Duty Diesel Engines. SAE Int. J. Engines 2015, 8, 1144–1151. [Google Scholar] [CrossRef]
- Squaiella, L.L.F.; Martins, C.A.; Pedro, T.L. Strategies for Emission Control in Diesel Engine to Meet Euro VI. Fuel 2013, 104, 183–193. [Google Scholar] [CrossRef]
- Lao, C.T.; Akroyd, J.; Eaves, N.; Smith, A.; Morgan, N.; Nurkowski, D.; Bhave, A.; Kraft, M. Investigation of the Impact of the Configuration of Exhaust After-Treatment System for Diesel Engines. Appl. Energy 2020, 267, 114844. [Google Scholar] [CrossRef]
- Lyu, M.; Bao, X.; Zhu, R.; Matthews, R. State-of-the-Art Outlook for Light-Duty Vehicle Emission Control Standards and Technologies in China. Clean Technol. Environ. Policy 2020, 22, 757–771. [Google Scholar] [CrossRef]
- Weilenmann, M.; Favez, J.-Y.Y.; Alvarez, R. Cold-Start Emissions of Modern Passenger Cars at Different Low Ambient Temperatures and Their Evolution over Vehicle Legislation Categories. Atmos. Environ. 2009, 43, 2419–2429. [Google Scholar] [CrossRef]
- Iliyas, A.; Zahedi-Niaki, M.H.; Eić, M.; Kaliaguine, S. Control of Hydrocarbon Cold-Start Emissions: A Search for Potential Adsorbents. Microporous Mesoporous Mater. 2007, 102, 171–177. [Google Scholar] [CrossRef]
- Lee, J.; Theis, J.R.; Kyriakidou, E.A. Vehicle Emissions Trapping Materials: Successes, Challenges, and the Path Forward. Appl. Catal. B Environ. 2019, 243, 397–414. [Google Scholar] [CrossRef]
- Curran, S.; Prikhodko, V.; Cho, K.; Sluder, C.S.; Parks, J.; Wagner, R.; Kokjohn, S.; Reitz, R.D. In-Cylinder Fuel Blending of Gasoline/Diesel for Improved Efficiency and Lowest Possible Emissions on a Multi-Cylinder Light-Duty Diesel Engine; No. 2010-01-2206; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2010. [Google Scholar]
- Zheng, M.; Asad, U.; Graham, T.; Reader, Y.T.; Wang, M. Energy Efficiency Improvement Strategies for a Diesel Engine in Low-Temperature Combustion. Int. J. Energy Res. 2009, 33, 8–28. [Google Scholar] [CrossRef]
- Zammit, M.; DiMaggio, C.; Kim, C.; Lambert, C.; Muntean, G.; Peden, C.; Parks, J.; Howden, K. Future Automotive Aftertreatment Solutions: The 150 °C Challenge Workshop Report. Available online: https://cleers.org/wp-content/uploads/2012_The_150C_Challenge_Workshop_Report.pdf (accessed on 29 March 2021).
- Environmental Protection Agency, U.S. EPA-420-F-13-016a; U. S. Environmental Protection Agency: Washington, DC, USA, 2013; pp. 1–4.
- Environmental Protection Agency, U.S.; Department of Transportation, National Highway Traffic Safety Administration. 2017 and Later Model Year Light-Duty Vehicle Greenhouse Gas Emissions and Corporate Average Fuel Economy Standards. Fed. Regist. 2012, 77, 62623–63200. [Google Scholar]
- Sarshar, Z.; Zahedi-Niaki, M.H.; Huang, Q.; Eić, M.; Kaliaguine, S. MTW Zeolites for Reducing Cold-Start Emissions of Automotive Exhaust. Appl. Catal. B Environ. 2009, 87, 37–45. [Google Scholar] [CrossRef]
- Burke, N.R.; Trimm, D.L.; Howe, R.F. The Effect of Silica:Alumina Ratio and Hydrothermal Ageing on the Adsorption Characteristics of BEA Zeolites for Cold Start Emission Control. Appl. Catal. B Environ. 2003, 46, 97–104. [Google Scholar] [CrossRef]
- Huuhtanen, M.; Rahkamaa-Tolonen, K.; Maunula, T.; Keiski, R.L. Pt-Loaded Zeolites for Reducing Exhaust Gas Emissions at Low Temperatures and in Lean Conditions. Catal. Today 2005, 100, 321–325. [Google Scholar] [CrossRef]
- López, J.M.; Navarro, M.V.; García, T.; Murillo, R.; Mastral, A.M.; Varela-Gandía, F.J.; Lozano-Castelló, D.; Bueno-López, A.; Cazorla-Amorós, D. Screening of Different Zeolites and Silicoaluminophosphates for the Retention of Propene Under Cold Start Conditions. Microporous Mesoporous Mater. 2010, 130, 239–247. [Google Scholar] [CrossRef]
- Kim, D.J.; Kim, J.W.; Yie, J.E.; Moon, H. Temperature-Programmed Adsorption and Characteristics of Honeycomb Hydrocarbon Adsorbers. Ind. Eng. Chem. Res. 2002, 41, 6589–6592. [Google Scholar] [CrossRef]
- Kim, H.; Jang, E.; Jeong, Y.; Kim, J.; Kang, C.Y.; Kim, C.H.; Baik, H.; Lee, K.-Y.; Choi, J. On the Synthesis of a Hierarchically-Structured ZSM-5 Zeolite and the Effect of its Physicochemical Properties with Cu Impregnation on Cold-Start Hydrocarbon Trap Performance. Catal. Today 2018, 314, 78–93. [Google Scholar] [CrossRef]
- Westermann, A.; Azambre, B.; Chebbi, M.; Koch, A. Modification of Y Faujasite Zeolites for the Trapping and Elimination of a Propene-Toluene-Decane Mixture in the Context of Cold-Start. Microporous Mesoporous Mater. 2016, 230, 76–88. [Google Scholar] [CrossRef]
- Azambre, B.; Westermann, A.; Finqueneisel, G.; Can, F.; Comparot, J.D. Adsorption and Desorption of a Model Hydrocarbon Mixture Over HY Zeolite under Dry and Wet Conditions. J. Phys. Chem. C 2015, 119, 315–331. [Google Scholar] [CrossRef]
- Zheng, Y.; Kovarik, L.; Mark, H.; Engelhard, Y.W.; Wang, Y.; Gao, F.; Szanyi, J. Low-Temperature Pd/Zeolite Passive NOx Adsorbers: Structure, Performance, and Adsorption Chemistry. J. Phys. Chem. C 2017, 121, 15793–15803. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, X.; Bhat, A.; Tang, X.; Yi, H.; Lin, X.; Schwank, J.W. Activation of Passive NOx Adsorbers by Pretreatment with Reaction Gas Mixture. Chem. Eng. J. 2020, 399, 125727. [Google Scholar] [CrossRef]
- Ryou, Y.; Lee, J.; Lee, H.; Kim, C.H.; Kim, D.H. Effect of Various Activation Conditions on the Low Temperature NO Adsorption Performance of Pd/SSZ-13 Passive NOx Adsorber. Catal. Today 2019, 320, 175–180. [Google Scholar] [CrossRef]
- Malamis, S.A.; Harold, M.P.; Epling, W.S. Coupled NO and C3H6 Trapping, Release and Conversion on Pd/BEA: Evaluation of the Lean Hydrocarbon NOx Trap. Ind. Eng. Chem. Res. 2019, 58, 22912–22923. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Mulla, S.; Weigert, E.; Camm, K.; Ballinger, T.; Cox, J.; Blakeman, P. Old Start Concept (CSCTM): A Novel Catalyst for Cold Start Emission Control. SAE Int. J. Fuels Lubr. 2013, 6, 372–381. [Google Scholar] [CrossRef]
- Lupescu, J.; Chanko, T.; Richert, J.; DeVries, J. Treatment of Vehicle Emissions from the Combustion of E85 and Gasoline with Catalyzed Hydrocarbon Traps. SAE Int. J. Fuels Lubr. 2009, 2, 485–496. [Google Scholar] [CrossRef]
- Nunan, J.; Lupescu, J.; Denison, G.; Ball, D.; Moser, D. HC Traps for Gasoline and Ethanol Applications. SAE Int. J. Fuels Lubr. 2013, 6, 430–449. [Google Scholar] [CrossRef]
- Aftertreatment Protocols for Catalyst Characterization and Performance Evaluation: Low-Temperature Storage Catalyst Test Protocol. Available online: https://cleers.org/wp-content/uploads/2018/03/2018_LTAT_Low-Temperature-Storage-Protocol.pdf (accessed on 29 March 2021).
- Kenneth, G.; Rappé, C.D.; Josh, A.; Pihl, J.R.; Theis, S.; Oh, H.; Galen, B.; Fisher, J.P.; Vencon, G.; Easterling, M.Y.; et al. Aftertreatment Protocols for Catalyst Characterization and Performance Evaluation: Low-Temperature Oxidation, Storage, Three-Way, and NH3-SCR Catalyst Test Protocols. Emiss. Control Sci. Technol. 2019, 5, 183–214. [Google Scholar] [CrossRef]
- Kyriakidou, E.A.; Lee, J.; Choi, J.-S.; Lance, M.; Toops, T.J. A Comparative Study of Silver- and Palladium-Exchanged Zeolites in Propylene and Nitrogen Oxide Adsorption and Desorption for Cold-Start Applications. Catal. Today 2020. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Collier, J.E.; Liu, D.; Mantarosie, L.; Durán-Martín, D.; Novák, V.; Rajaram, R.R.; Tompsett, D. Low Temperature NO Storage of Zeolite Supported Pd for Low Temperature Diesel Engine Emission Control. Catal. Lett. 2016, 146, 1706–1711. [Google Scholar] [CrossRef]
- Westermann, A.; Azambre, B.; Finqueneisel, G.; Da Costa, P.; Can, F. Evolution of Unburnt Hydrocarbons Under ‘Cold-Start’ Conditions from Adsorption/Desorption to Conversion: On the Screening of Zeolitic Materials. Appl. Catal. B 2014, 158–159, 48–59. [Google Scholar] [CrossRef]
- Kang, S.B.; Kalamaras, C.; Balakotaiah, V.; Epling, W. Hydrocarbon Trapping over Ag-Beta Zeolite for Cold-Start Emission Control. Catal. Lett. 2017, 147, 1355–1362. [Google Scholar] [CrossRef]
- Khivantsev, K.; Jaegers, N.R.; Kovarik, L.; Hanson, J.C.; Tao, F.; Tang, Y.; Zhang, X.; Koleva, I.Z.; Aleksandrov, H.A.; Vayssilov, G.N.; et al. Achieving Atomic Dispersion of Highly Loaded Transition Metals in Small-Pore Zeolite SSZ-13: High-Capacity and High Efficiency Low Temperature CO and Passive NOx Adsorbers. Angew. Chem. 2018, 57, 16672–16677. [Google Scholar] [CrossRef]
- Ryou, Y.; Lee, J.; Cho, S.J.; Lee, H.; Kim, C.H.; Kim, D.H. Activation of Pd/SSZ-13 Catalyst by Hydrothermal Aging Treatment in Passive NO Adsorption Performance at Low Temperature for Cold Start Application. Appl. Catal. B 2017, 212, 140–149. [Google Scholar] [CrossRef]
- Lee, J.; Ryou, Y.S.; Hwang, S.; Kim, Y.; Cho, S.J.; Lee, H.; Kim, C.H.; Kim, D.H. Comparative Study of the Mobility of Pd Species in SSZ-13 and ZSM-5, and its Implication for their Activity as Passive NOx Adsorber (PNAs) after Hydro-Thermal Aging. Catal. Sci. Technol. 2019, 9, 163–173. [Google Scholar] [CrossRef]
- Ball, D.; Zammit, M.; Wuttke, J.; Buitrago, C. Investigation of LEV-III Aftertreatment Designs. SAE Int. J. Fuels Lubr. 2011, 4, 1–8. [Google Scholar] [CrossRef]
- Gao, Z.; Kim, M.-Y.; Choi, J.-S.; Daw, C.S.; Parks, J.E., II; Smith, D.E. Cold-Start Emissions Control in Hybrid Vehicles Equipped with a Passive Adsorber for Hydrocarbons and Nitrogen Oxides. J. Automob. Eng. 2012, 226, 1396–1407. [Google Scholar] [CrossRef]
- McLeary, E.E.; Jansen, J.C.; Kapteijn, F. Zeolite Based Films, Membranes and Membrane Reactors: Progress and Prospects. Microporous Mesoporous Mater. 2006, 90, 198–220. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Yu, J. Applications of Zeolites in Sustainable Chemistry. Chem 2017, 3, 928–949. [Google Scholar] [CrossRef]
- Jonsson, R.; Woo, J.; Skoglundh, M.; Olsson, L. Zeolite Beta Doped with La, Fe, and Pd as a Hydrocarbon Trap. Catalysts 2020, 10, 173. [Google Scholar] [CrossRef]
- Park, J.H.; Park, S.J.; Ahn, H.A.; Nam, I.S.; Yeo, G.K.; Kil, J.K.; Youn, Y.K. Promising Zeolite-Type Hydrocarbon Trap Catalyst by a Knowledge-Based Combinatorial Approach. Microporous Mesoporous Mater. 2009, 117, 178–184. [Google Scholar] [CrossRef]
- Schaber, P.M.; Colson, J.; Higgins, S.; Thielen, D.; Anspach, B.; Brauer, J. Thermal Decomposition (Pyrolysis) of Urea in an Open Reaction Vessel. Thermochim. Acta 2004, 424, 131–142. [Google Scholar] [CrossRef]
- Xu, M.; Wang, J.; Yu, T.; Wang, J.; Shen, M. New Insight into Cu/SAPO-34 Preparation Procedure: Impact of NH4-SAPO-34 on the Structure and Cu Distribution in Cu-SAPO-34 NH3-SCR Catalysts. Appl. Catal. B Environ. 2018, 220, 161–170. [Google Scholar] [CrossRef]
- Rahkamaa-Tolonen, K.; Maunula, T.; Lomma, M.; Huuhtanen, M.; Keiski, R.L. The Effect of NO2 on the Activity of Fresh and Aged Zeolite Catalysts in the NH3-SCR Reaction. Catal. Today 2005, 100, 217–222. [Google Scholar] [CrossRef]
- Konstantin, K.; Feng, G.; Libor, K.; Yong, W.; János, S. Molecular Level Understanding of How Oxygen and Carbon Monoxide Improve NOx Storage in Palladium/SSZ-13 Passive NOx Adsorbers: The Role of NO+ and Pd(II)(CO)(NO) Species. J. Phys. Chem. C 2018, 122, 10820–10827. [Google Scholar] [CrossRef]
- Yongwoo, K.; Sungha, H.; Jaeha, L.; YoungSeok, R.; Hyokyoung, L.; Chang, H.K.; Do Heui, K. Comparison of NOx Adsorption/Desorption Behaviors over Pd/CeO2 and Pd/SSZ-13 as Passive NOx Adsorbers for Cold Start Application. Emiss. Control Sci. Technol. 2019, 5, 172–182. [Google Scholar] [CrossRef]
 ) and 30 (
) and 30 ( ) minutes of adsorption during uptake and the zones/temperatures associated with 10% (T10, ■), 50% (T50, ■), or 90% (T90, ■) of THC released during desorption, which are also indicated with arrows. These characteristics are then represented by bar charts for both (b) adsorption and (c) desorption.
) minutes of adsorption during uptake and the zones/temperatures associated with 10% (T10, ■), 50% (T50, ■), or 90% (T90, ■) of THC released during desorption, which are also indicated with arrows. These characteristics are then represented by bar charts for both (b) adsorption and (c) desorption.
 ) and 30 (
) and 30 ( ) minutes of adsorption during uptake and the zones/temperatures associated with 10% (T10, ■), 50% (T50, ■), or 90% (T90, ■) of THC released during desorption, which are also indicated with arrows. These characteristics are then represented by bar charts for both (b) adsorption and (c) desorption.
) minutes of adsorption during uptake and the zones/temperatures associated with 10% (T10, ■), 50% (T50, ■), or 90% (T90, ■) of THC released during desorption, which are also indicated with arrows. These characteristics are then represented by bar charts for both (b) adsorption and (c) desorption.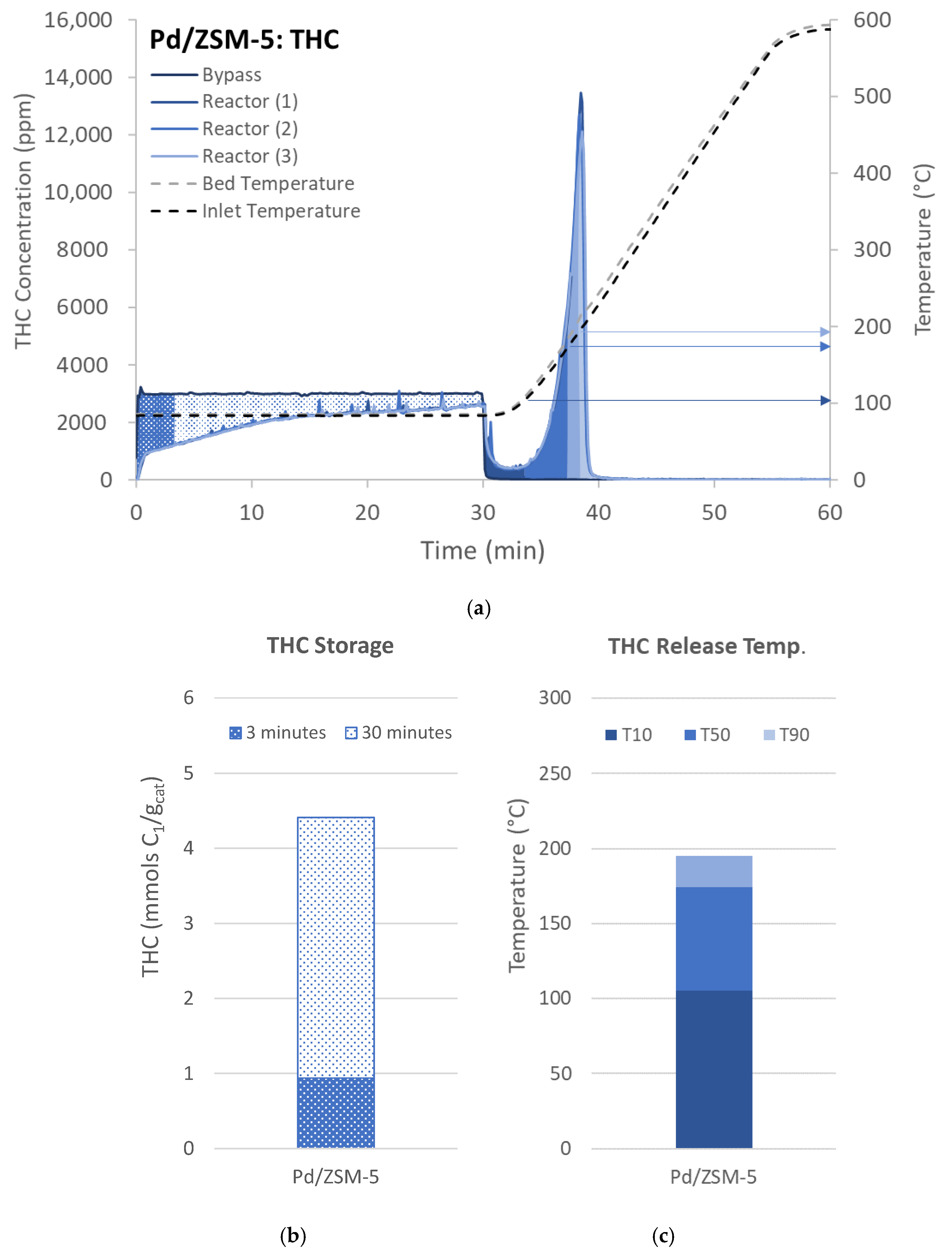
 ) and 30 (
) and 30 ( ) minutes for each of the materials studied. (b) The temperature where 10% (T10, ■), 50% (T50, ■), or 90% (T90, ■) of THC is released.
) minutes for each of the materials studied. (b) The temperature where 10% (T10, ■), 50% (T50, ■), or 90% (T90, ■) of THC is released.
 ) and 30 (
) and 30 ( ) minutes for each of the materials studied. (b) The temperature where 10% (T10, ■), 50% (T50, ■), or 90% (T90, ■) of THC is released.
) minutes for each of the materials studied. (b) The temperature where 10% (T10, ■), 50% (T50, ■), or 90% (T90, ■) of THC is released.
 ) and 30 (
) and 30 ( ) minutes for Pd ZSM-5, and (c) the temperature where 10% (T10, ■), 50% (T50, ■), or 90% (T90, ■) of the NOx is released for PD/ZSM-5.
) minutes for Pd ZSM-5, and (c) the temperature where 10% (T10, ■), 50% (T50, ■), or 90% (T90, ■) of the NOx is released for PD/ZSM-5.
 ) and 30 (
) and 30 ( ) minutes for Pd ZSM-5, and (c) the temperature where 10% (T10, ■), 50% (T50, ■), or 90% (T90, ■) of the NOx is released for PD/ZSM-5.
) minutes for Pd ZSM-5, and (c) the temperature where 10% (T10, ■), 50% (T50, ■), or 90% (T90, ■) of the NOx is released for PD/ZSM-5.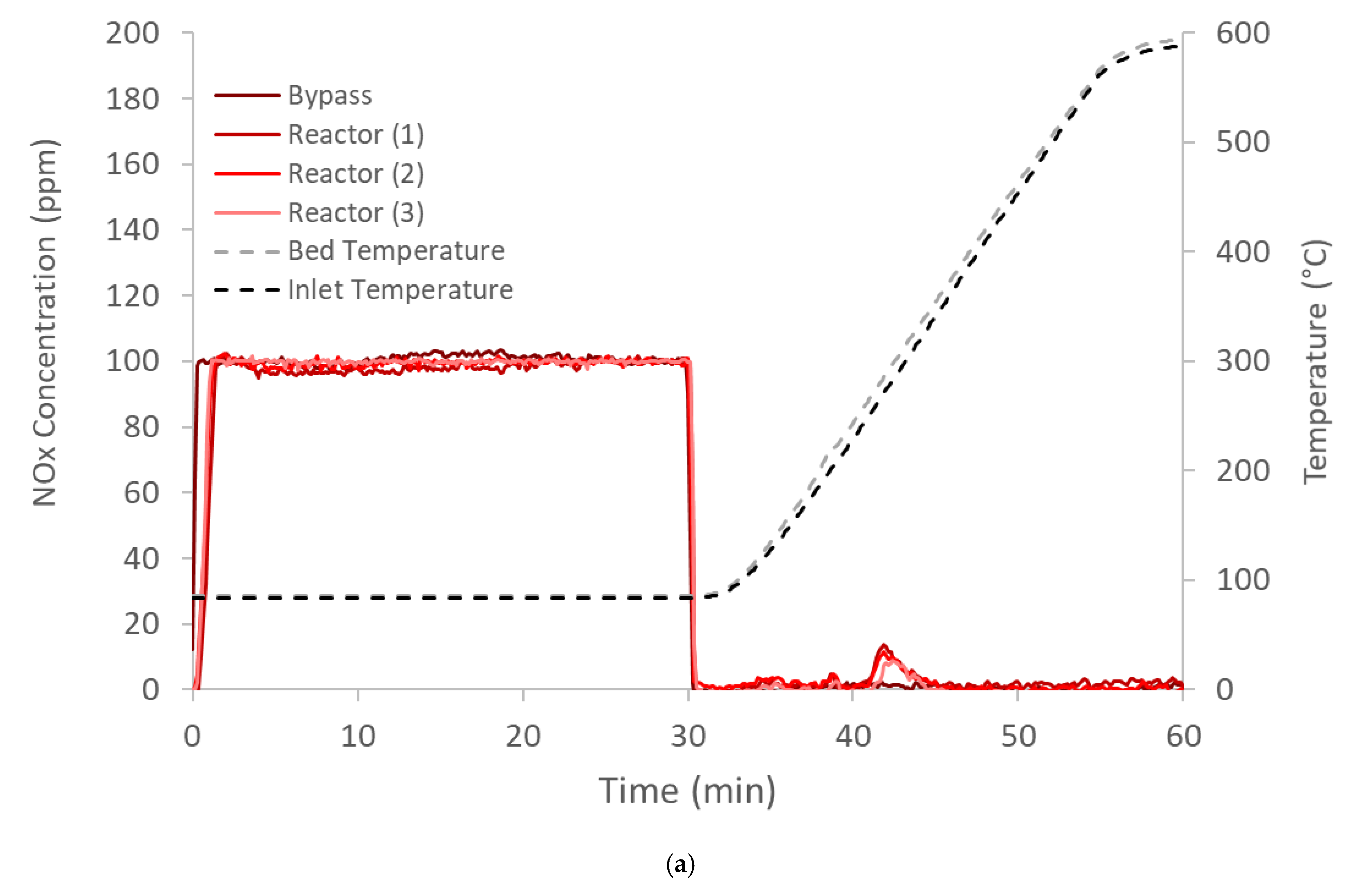
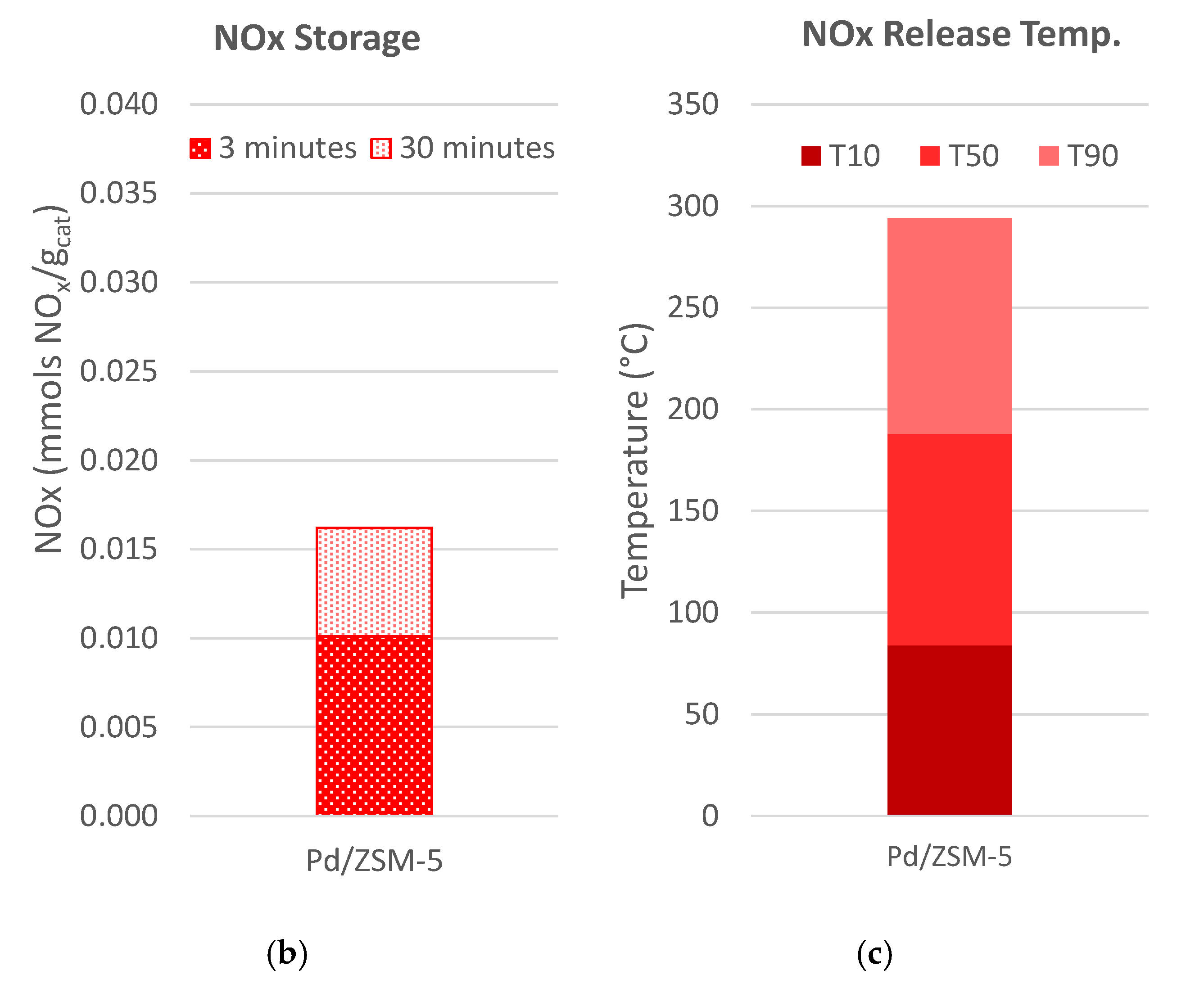
 ) and 30 (
) and 30 ( ) minutes for each of the materials studied. (b) The temperature where 10% (T10, ■), 50% (T50, ■), or 90% (T90, ■) of the NOx is released.
) minutes for each of the materials studied. (b) The temperature where 10% (T10, ■), 50% (T50, ■), or 90% (T90, ■) of the NOx is released.
 ) and 30 (
) and 30 ( ) minutes for each of the materials studied. (b) The temperature where 10% (T10, ■), 50% (T50, ■), or 90% (T90, ■) of the NOx is released.
) minutes for each of the materials studied. (b) The temperature where 10% (T10, ■), 50% (T50, ■), or 90% (T90, ■) of the NOx is released.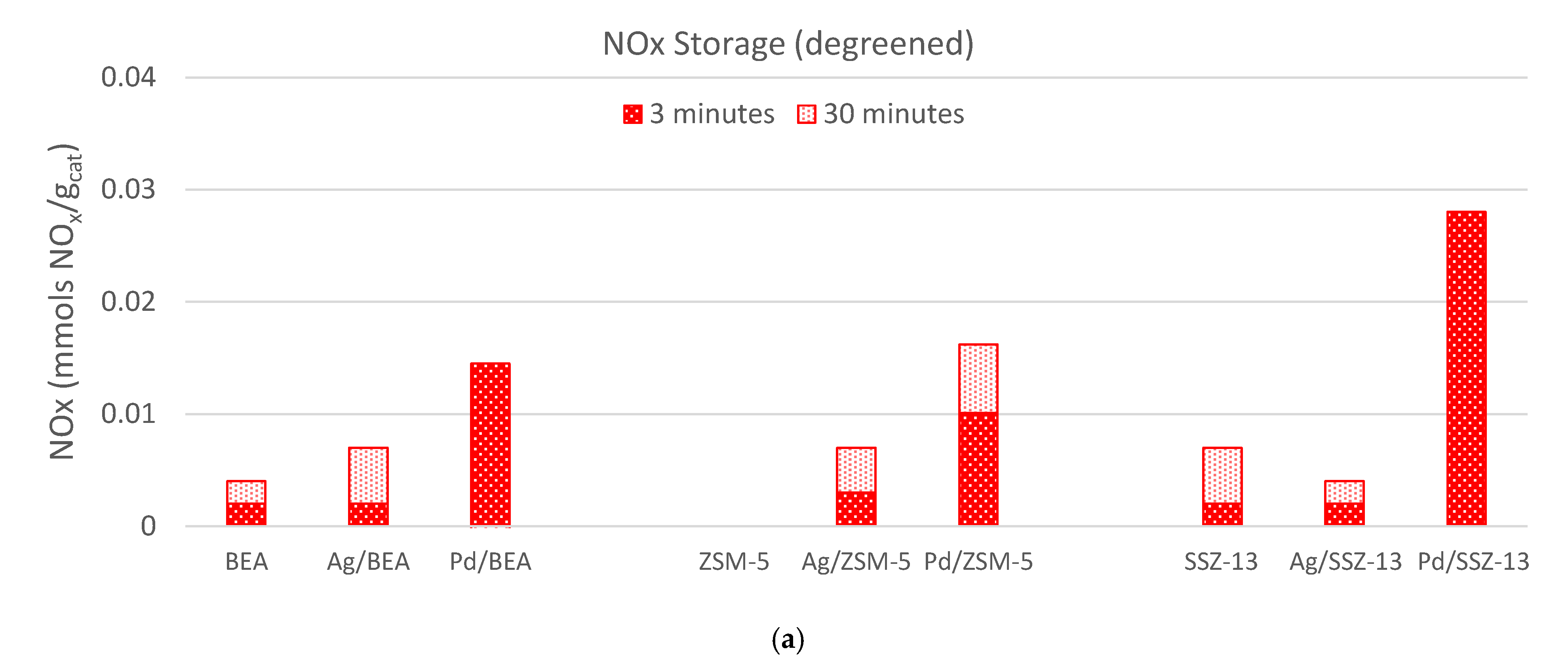
 ) and 30 (
) and 30 ( ) minutes for each of the materials studied, and (b) the temperature where 10% (T10, ■), 50% (T50, ■), or 90% (T90, ■) of THC is released.
) minutes for each of the materials studied, and (b) the temperature where 10% (T10, ■), 50% (T50, ■), or 90% (T90, ■) of THC is released.
 ) and 30 (
) and 30 ( ) minutes for each of the materials studied, and (b) the temperature where 10% (T10, ■), 50% (T50, ■), or 90% (T90, ■) of THC is released.
) minutes for each of the materials studied, and (b) the temperature where 10% (T10, ■), 50% (T50, ■), or 90% (T90, ■) of THC is released.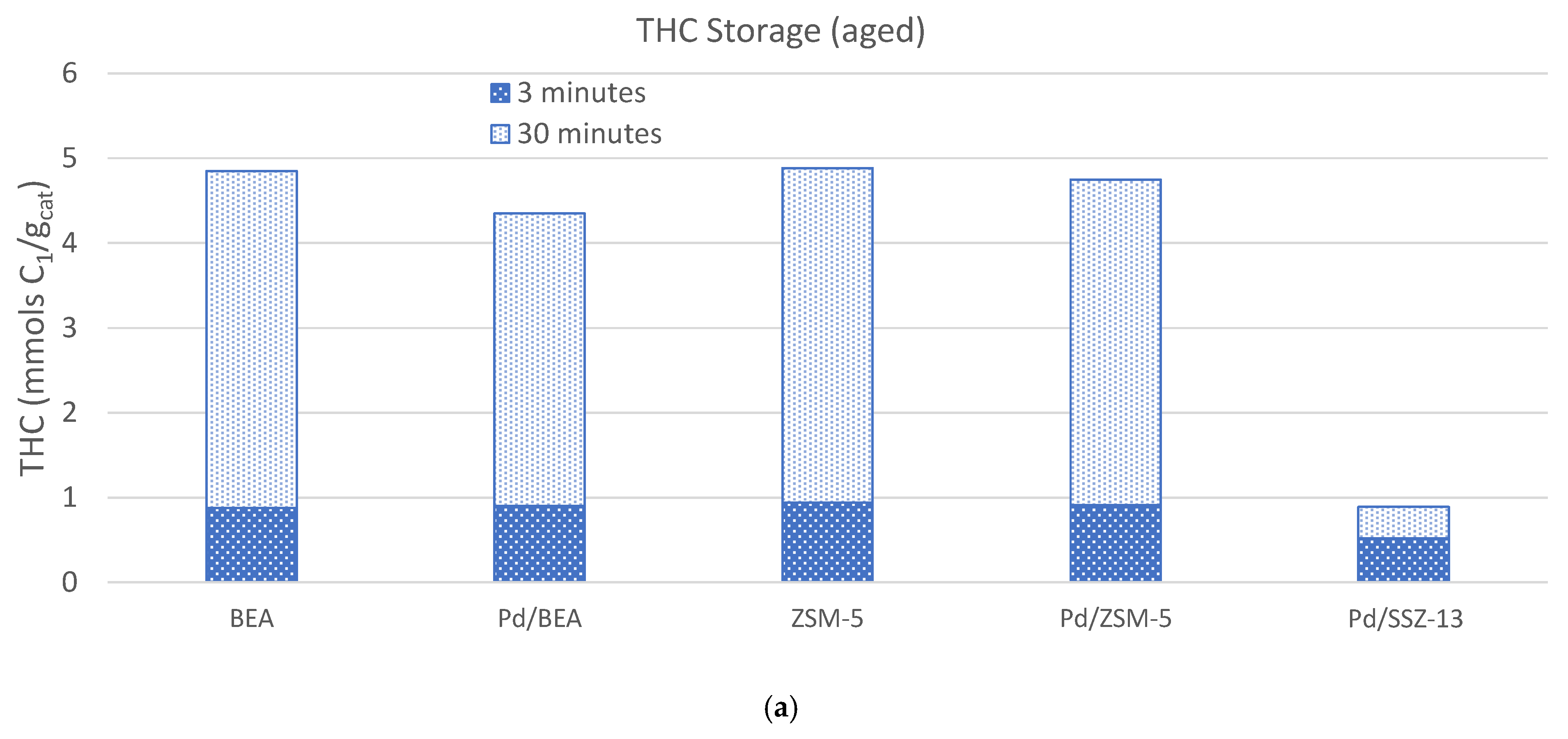
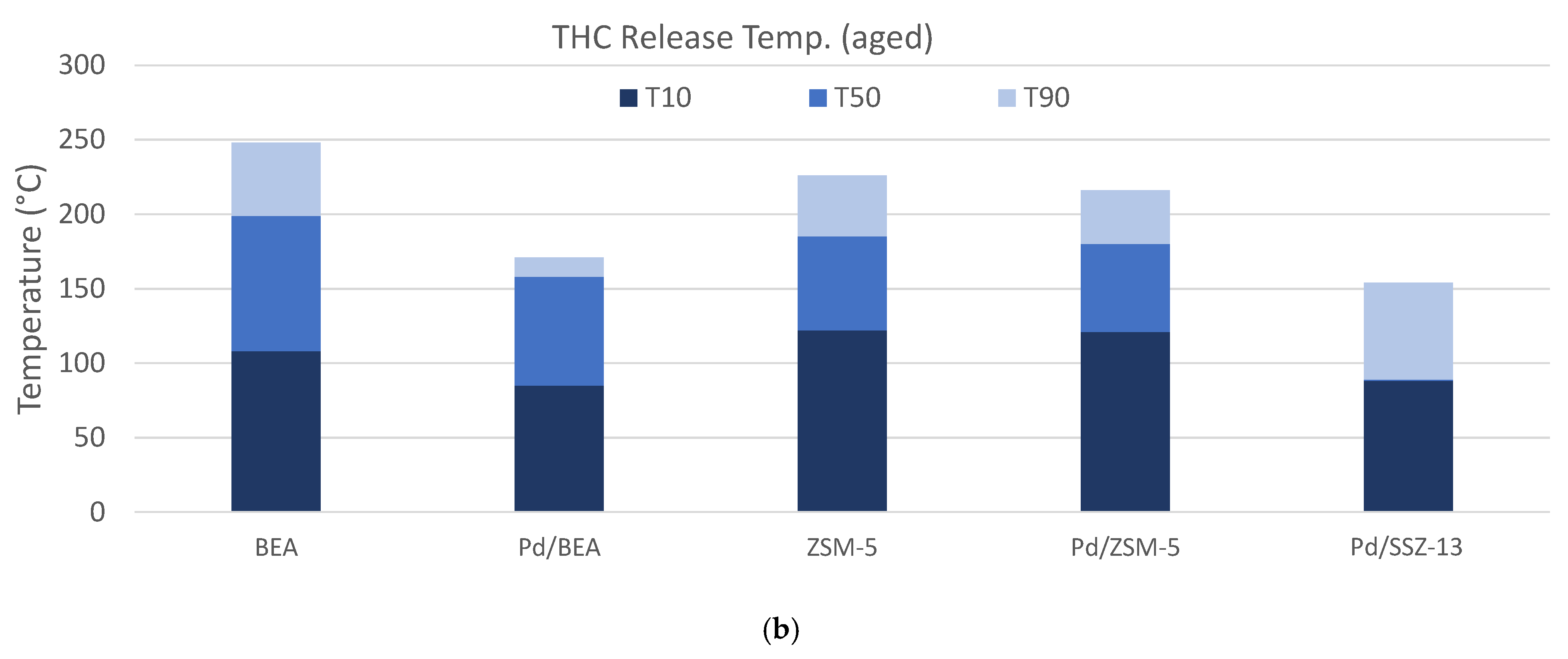
 ) and 30 (
) and 30 ( ) minutes for each of the materials studied, and (b) the temperature where 10% (T10, ■), 50% (T50, ■), or 90% (T90, ■) of the NOx is released.
) minutes for each of the materials studied, and (b) the temperature where 10% (T10, ■), 50% (T50, ■), or 90% (T90, ■) of the NOx is released.
 ) and 30 (
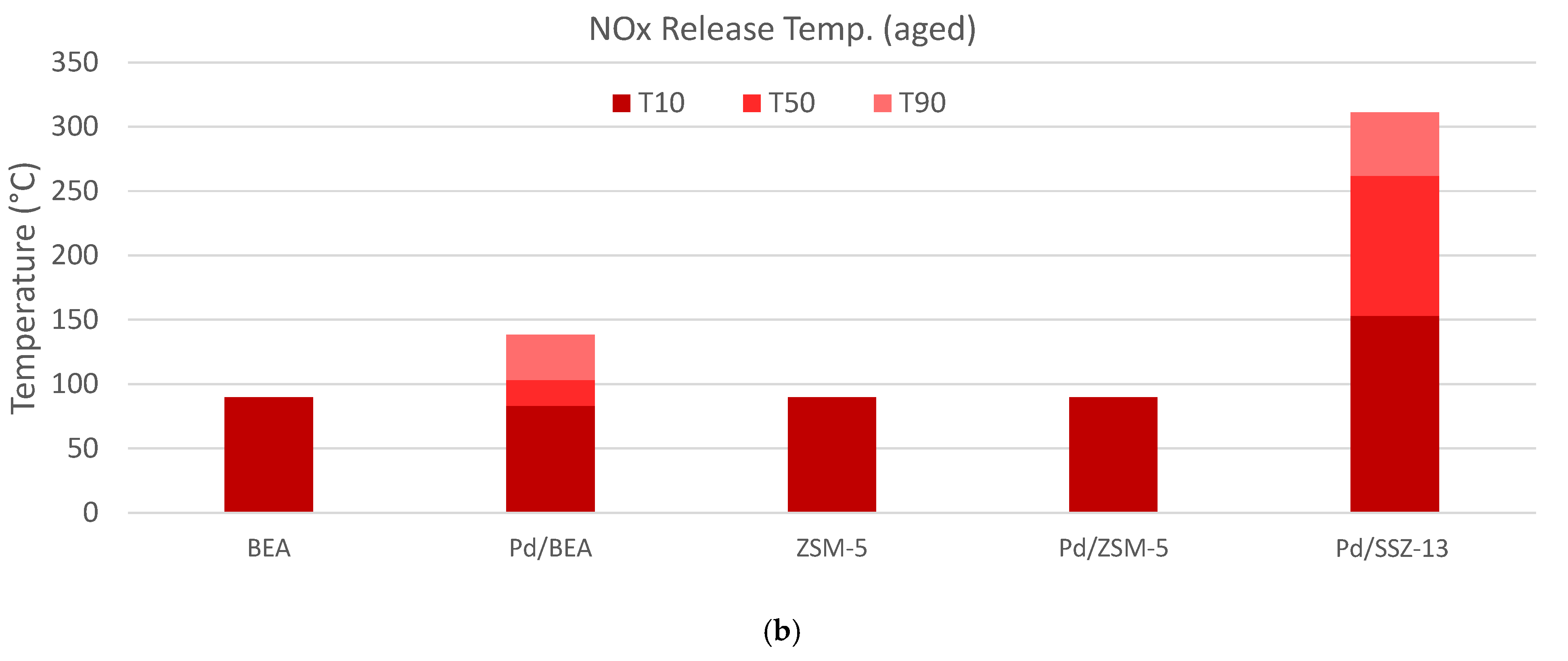
) and 30 ( ) minutes for each of the materials studied, and (b) the temperature where 10% (T10, ■), 50% (T50, ■), or 90% (T90, ■) of the NOx is released.
) minutes for each of the materials studied, and (b) the temperature where 10% (T10, ■), 50% (T50, ■), or 90% (T90, ■) of the NOx is released.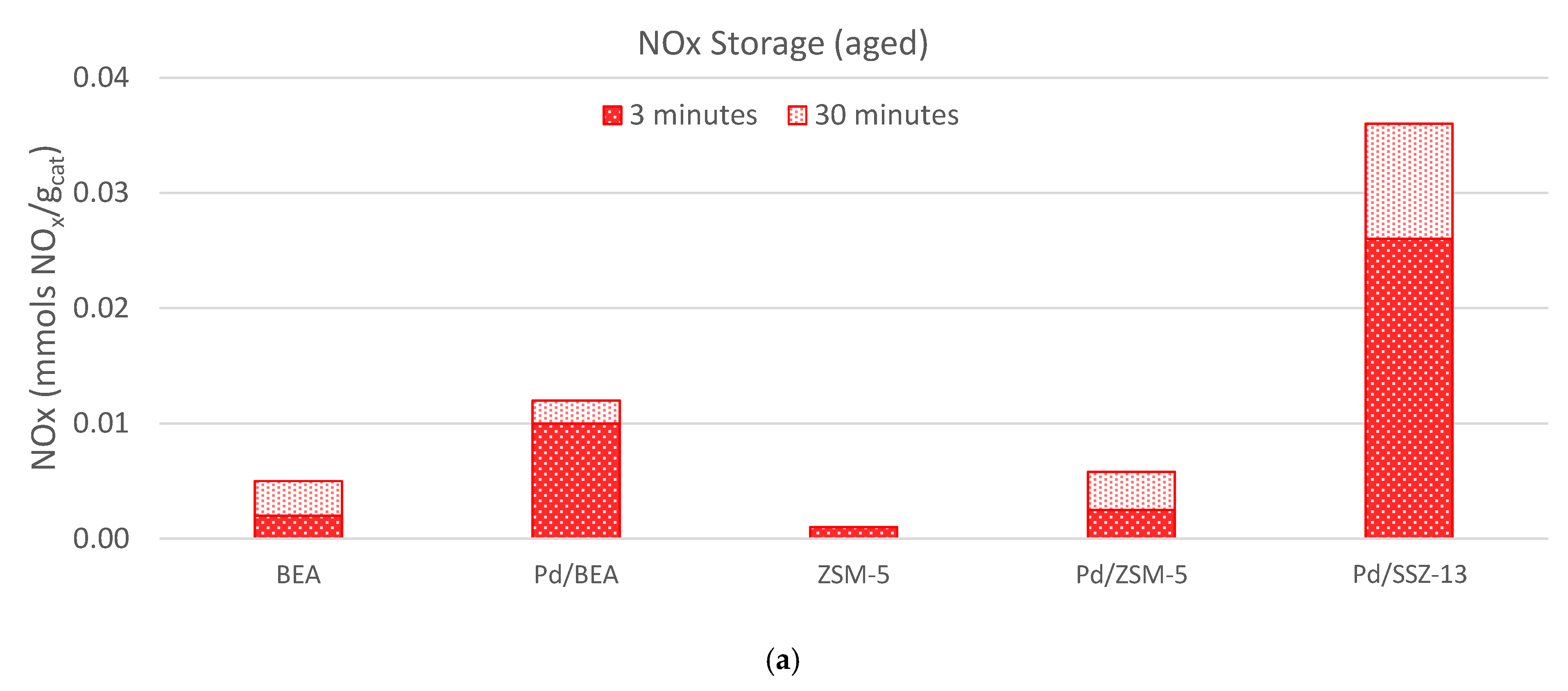
| LTC-D (Storage) | THC Storage | THC Release | Release Temperature | |||
|---|---|---|---|---|---|---|
| Degreened Zeolite | (mmols C1/gcat) | (mmols C1/gcat) | T10 | T50 | T90 | |
| 3 min | 30 min | 15 min | (°C) | (°C) | (°C) | |
| BEA | 0.9 | 5.1 | 5.2 | 109 | 205 | 251 |
| Ag/BEA | 1.0 | 5.1 | 4.5 | 101 | 203 | 262 |
| Pd/BEA | 1.0 | 4.8 | 2.0 | 82 | 160 | 170 |
| ZSM-5 | 1.0 | 4.3 | 4.7 | 123 | 190 | 231 |
| Ag/ZSM-5 | 1.0 | 5.0 | 5.0 | 116 | 185 | 224 |
| Pd/ZSM-5 | 0.9 | 4.4 | 3.8 | 105 | 174 | 195 |
| SSZ-13 | 0.6 | 0.9 | 1.0 | 91 | 102 | 168 |
| Ag/SSZ-13 | 0.7 | 1.0 | 1.0 | 88 | 97 | 164 |
| Pd/SSZ-13 | 0.6 | 0.9 | 0.8 | 89 | 100 | 144 |
| LTC-D (Storage) | NOx Storage | NOx Release | Release Temperature | |||
|---|---|---|---|---|---|---|
| Degreened Zeolite | (mmols NOx/gcat) | (mmols NOx/gcat) | T10 | T50 | T90 | |
| 3 min | 30 min | 15 min | (°C) | (°C) | (°C) | |
| BEA | 0.002 | 0.004 | 0.001 | 90 | 90 | 90 |
| Ag/BEA | 0.002 | 0.007 | 0.001 | 90 | 90 | 90 |
| Pd/BEA | 0.015 | 0.012 | 0.007 | 82 | 134 | 178 |
| ZSM-5 | 0.000 | 0.000 | 0.000 | 90 | 90 | 90 |
| Ag/ZSM-5 | 0.003 | 0.007 | 0.000 | 90 | 90 | 90 |
| Pd/ZSM-5 | 0.010 | 0.016 | 0.007 | 84 | 188 | 294 |
| SSZ-13 | 0.002 | 0.007 | 0.001 | 91 | 91 | 198 |
| Ag/SSZ-13 | 0.002 | 0.004 | 0.002 | 88 | 88 | 191 |
| Pd/SSZ-13 | 0.028 | 0.028 | 0.008 | 89 | 224 | 264 |
| LTC-D (Storage) | NOx Storage | NOx Release | Release Temperature | |||
|---|---|---|---|---|---|---|
| Aged Zeolite | (mmols C1/gcat) | (mmols C1/gcat) | T10 | T50 | T90 | |
| 3 min | 30 min | 15 min | (°C) | (°C) | (°C) | |
| BEA | 0.9 | 4.8 | 4.9 | 108 | 199 | 248 |
| Pd/BEA | 0.9 | 4.3 | 2.1 | 85 | 158 | 171 |
| ZSM-5 | 0.9 | 4.9 | 4.8 | 122 | 185 | 226 |
| Pd/ZSM-5 | 0.9 | 4.7 | 4.5 | 121 | 180 | 216 |
| Pd/SSZ-13 | 0.5 | 0.9 | 0.5 | 88 | 89 | 154 |
| LTC-D (Storage) | Storage | Release | Release Temperature | |||
|---|---|---|---|---|---|---|
| Aged Zeolite | (mmols NOx/gcat) | (mmols NOx/gcat) | T10 | T50 | T90 | |
| 3 min | 30 min | 15 min | (°C) | (°C) | (°C) | |
| BEA | 0.002 | 0.005 | 0.002 | 90 | 90 | 90 |
| Pd/BEA | 0.010 | 0.012 | 0.004 | 83 | 103 | 138 |
| ZSM-5 | 0.001 | 0.001 | 0.000 | 90 | 90 | 90 |
| Pd/ZSM-5 | 0.003 | 0.006 | 0.000 | 90 | 90 | 90 |
| Pd/SSZ-13 | 0.026 | 0.036 | 0.021 | 153 | 262 | 311 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toops, T.J.; Binder, A.J.; Kunal, P.; Kyriakidou, E.A.; Choi, J.-S. Analysis of Ion-Exchanged ZSM-5, BEA, and SSZ-13 Zeolite Trapping Materials under Realistic Exhaust Conditions. Catalysts 2021, 11, 449. https://doi.org/10.3390/catal11040449
Toops TJ, Binder AJ, Kunal P, Kyriakidou EA, Choi J-S. Analysis of Ion-Exchanged ZSM-5, BEA, and SSZ-13 Zeolite Trapping Materials under Realistic Exhaust Conditions. Catalysts. 2021; 11(4):449. https://doi.org/10.3390/catal11040449
Chicago/Turabian StyleToops, Todd J., Andrew J. Binder, Pranaw Kunal, Eleni A. Kyriakidou, and Jae-Soon Choi. 2021. "Analysis of Ion-Exchanged ZSM-5, BEA, and SSZ-13 Zeolite Trapping Materials under Realistic Exhaust Conditions" Catalysts 11, no. 4: 449. https://doi.org/10.3390/catal11040449
APA StyleToops, T. J., Binder, A. J., Kunal, P., Kyriakidou, E. A., & Choi, J.-S. (2021). Analysis of Ion-Exchanged ZSM-5, BEA, and SSZ-13 Zeolite Trapping Materials under Realistic Exhaust Conditions. Catalysts, 11(4), 449. https://doi.org/10.3390/catal11040449








