Effects of Surface Oxygen-Containing Groups of the Flowerlike Carbon Nanosheets on Palladium Dispersion, Catalytic Activity and Stability in Hydrogenolytic Debenzylation of Tetraacetyldibenzylhexaazaisowurtzitane
Abstract
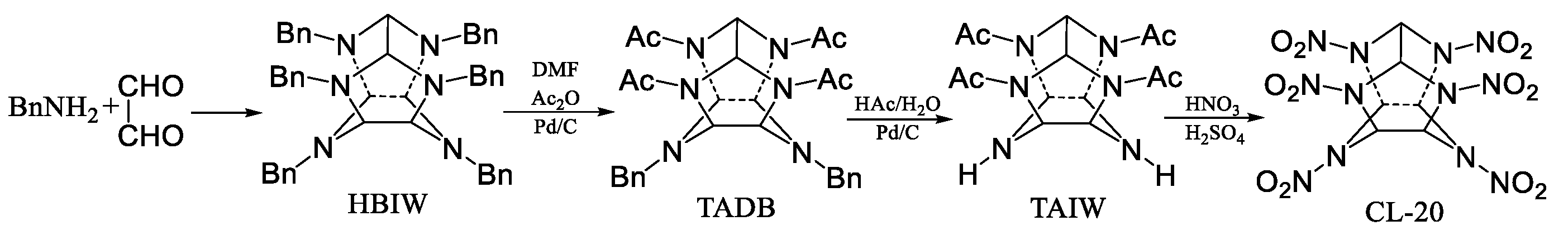
1. Introduction
2. Results and Discussion
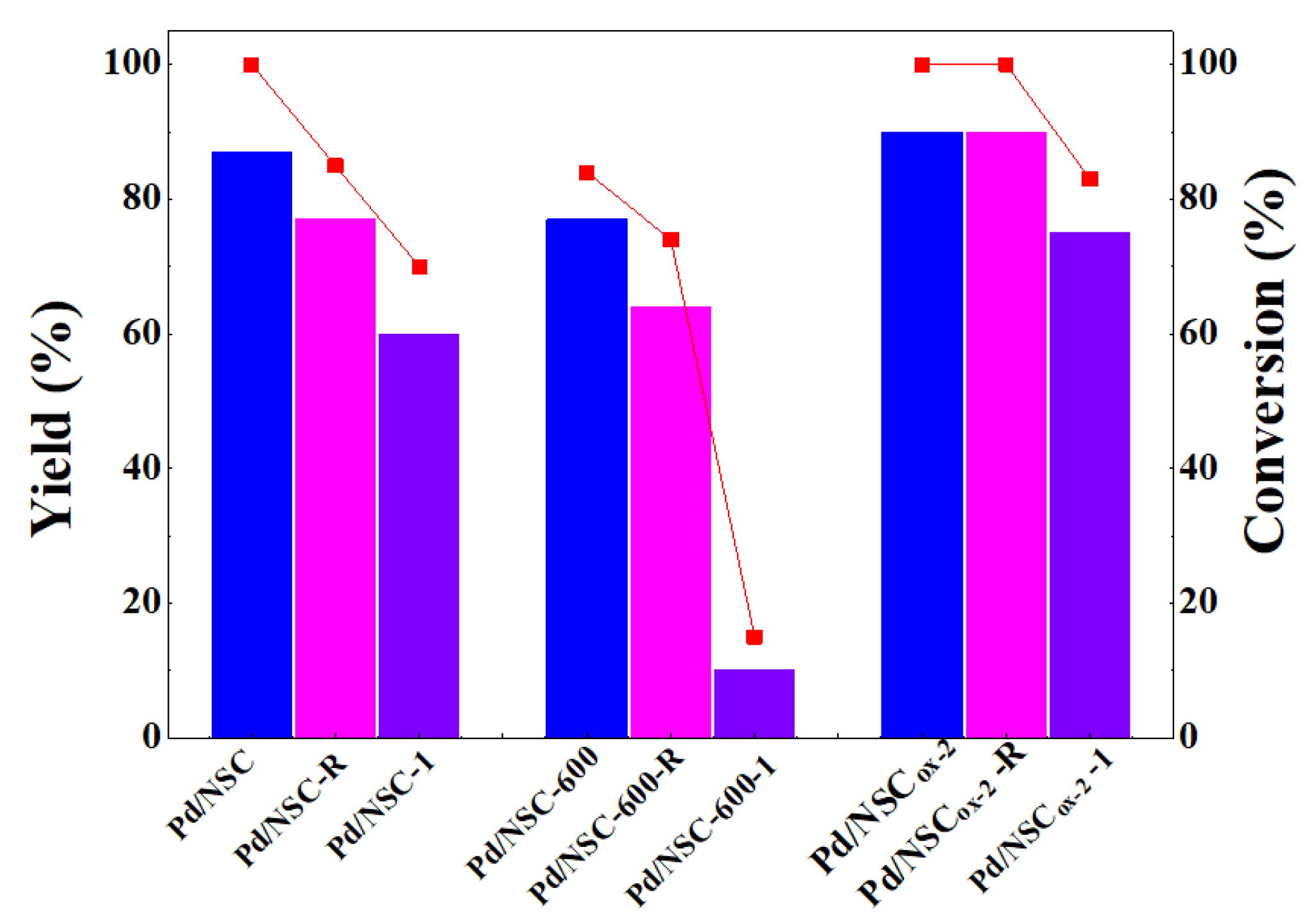
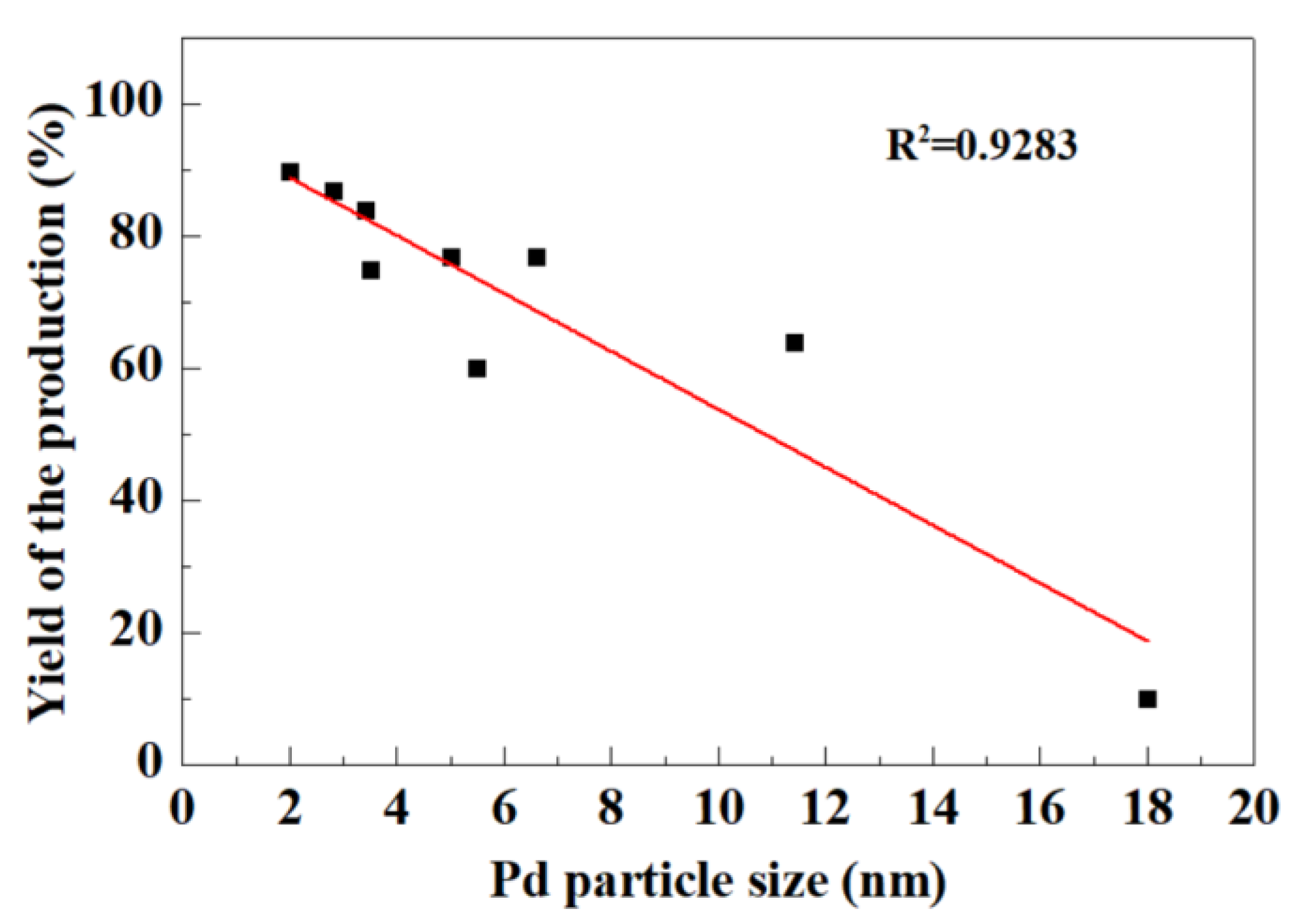
2.1. Activities and Stabilities of the Catalysts in Hydrogenolytic Debenzylation of TADB
2.2. Characterization of the Carbon Supports
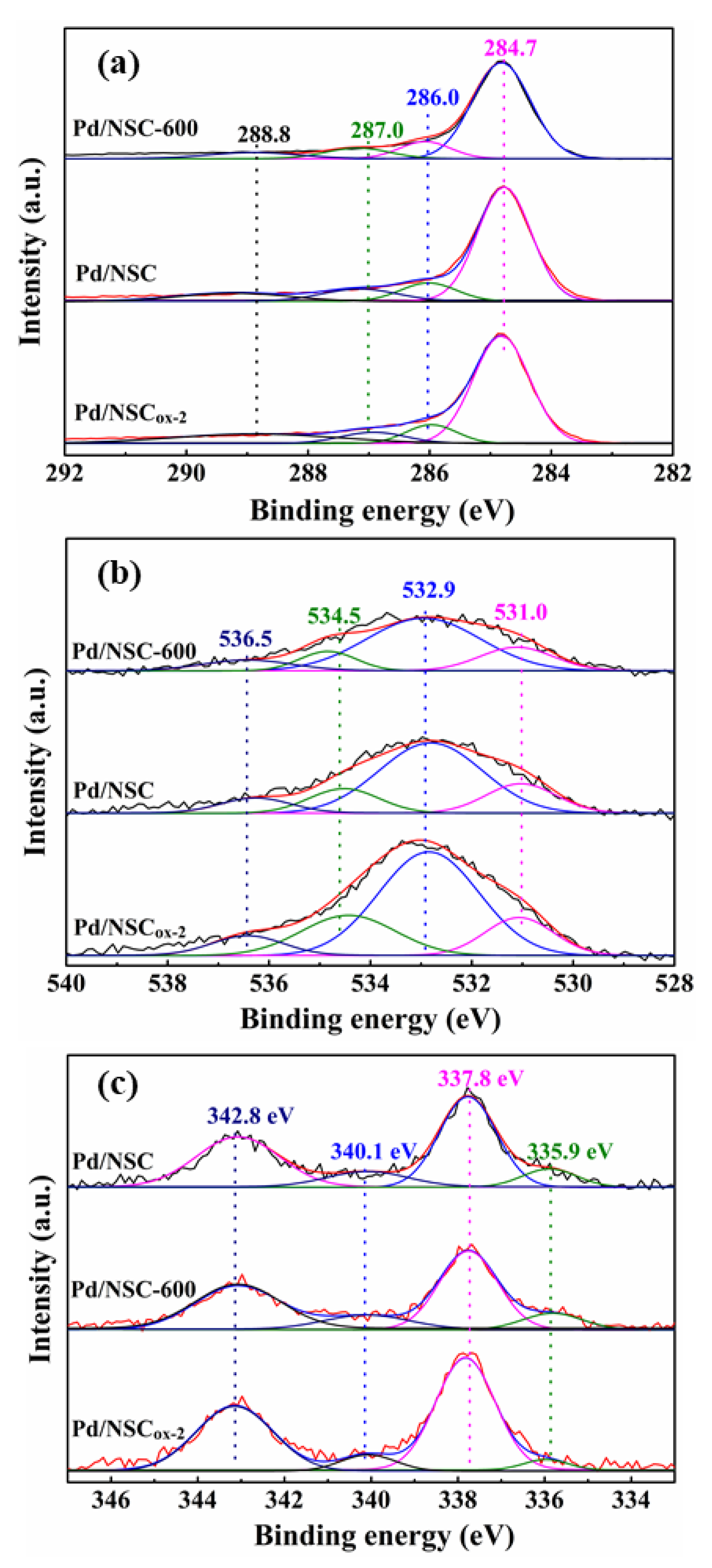
2.3. Characterization of the Catalysts
3. Experimental
3.1. Chemicals
3.2. Preparation of the Carbon Support and the Reaction Substrate
3.3. Treatment of the Carbon Supports
3.4. Catalyst Preparation
3.5. Catalytic Activity Tests
3.6. Carbon Supports and Catalysts Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Morishita, T. Nitrogen-doped carbon materials. Carbon 2018, 132, 104–140. [Google Scholar] [CrossRef]
- Toebes, M.L.; Dillen, J.A.V.; Jong, K.P.D. Synthesis of supported palladium catalysts. J. Mol. Catal. A Chem. 2001, 173, 75–98. [Google Scholar] [CrossRef]
- Semikolenov, V.A. Modern approaches to the preparation of palladium on charcoal catalysts. Russ. Chem. Rev. 1992, 61, 168–174. [Google Scholar] [CrossRef]
- Liu, X.; Astruc, D. Development of the applications of palladium on charcoal in organic synthesis. Adv. Synth. Catal. 2018, 360, 3426–3459. [Google Scholar] [CrossRef]
- Simagina, V.I.; Netskina, O.V.; Tayban, E.S.; Komova, O.V.; Grayfer, E.D.; Ischenko, A.V.; Pazhetnov, E.M. The effect of support properties on the activity of Pd/C catalysts in the liquid-phase hydrodechlorination of chlorobenzene. Appl. Catal. A Gen. 2010, 379, 87–94. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, X.Y.; Wu, H.; Lei, Y.; Li, J. Highly efficient reduction of nitro compounds: Recyclable Pd/C-catalyzed transfer hydrogenation with ammonium formate or hydrazine hydrate as hydrogen source. Synth. Commun. 2018, 48, 2475–2484. [Google Scholar] [CrossRef]
- Cabiac, A.; Cacciaguerra, T.; Trens, P.; Durand, R.; Delahay, G.; Medevielle, A.; Plee, D.; Coq, B. Influence of textural properties of activated carbons on Pd/carbon catalysts synthesis for cinnamaldehyde hydrogenation. Appl. Catal. A Gen. 2008, 340, 229–235. [Google Scholar] [CrossRef]
- Kang, M.; Song, M.W.; Kim, K.L. Palladium catalysts supported on activated carbon with different textural and surface chemical properties. React. Kinet. Catal. Lett. 2002, 76, 207–212. [Google Scholar] [CrossRef]
- Contreras, R.C.; Guicheret, B.; Machado, B.F.; Rivera-Carcamo, C.; Curiel Alvarez, M.A.; Valdez Salas, B.; Ruttert, M.; Placke, T.; Favre Reguillon, A.; Vanoye, L.; et al. Effect of mesoporous carbon support nature and pretreatments on palladium loading, dispersion and apparent catalytic activity in hydrogenation of myrcene. J. Catal. 2019, 372, 226–244. [Google Scholar] [CrossRef]
- Cabiac, A.; Delahay, G.; Durand, R.; Trens, P.; Coq, B.; Plee, D. Controlled preparation of Pd/AC catalysts for hydrogenation reactions. Carbon 2007, 45, 3–10. [Google Scholar] [CrossRef]
- Okhlopkova, L.B.; Lisitsyn, A.S.; Likholobov, V.A.; Gurrath, M.; Boehm, H.P. Properties of Pt/C and Pd/C catalysts prepared by reduction with hydrogen of adsorbed metal chlorides influence of pore structure of the support. Appl. Catal. A Gen. 2000, 204, 229–240. [Google Scholar] [CrossRef]
- Heal, G.R.; Mkayula, L.L. The preparation of palladium metal catalysts supported on carbon part II: Deposition of palladium and metal area measurement. Carbon 1988, 26, 815–823. [Google Scholar] [CrossRef]
- Simonov, P.A.; Romanenko, A.V.; Prosvirin, I.P.; Moroz, E.M.; Boronin, A.I.; Chuvilin, A.L.; Likholobov, V.A. On the nature of the interaction of H2PdC14 with the surface of graphite-like carbon materials. Carbon 1997, 35, 73–82. [Google Scholar] [CrossRef]
- Ma, J.; Ji, Y.; Sun, H.; Chen, Y.; Tang, Y.; Lu, T.; Zheng, J. Synthesis of carbon supported palladium nanoparticles catalyst using a facile homogeneous precipitation-reduction reaction method for formic acid electrooxidation. Appl. Surf. Sci. 2011, 257, 10483–10488. [Google Scholar] [CrossRef]
- Harada, T.; Ikeda, S.; Miyazaki, M.; Sakata, T.; Mori, H.; Matsumura, M. A simple method for preparing highly active palladium catalysts loaded on various carbon supports for liquid-phase oxidation and hydrogenation reactions. J. Mol. Catal. A Chem. 2007, 268, 59–64. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, Y.; Cha, C.; Carroll, W.M. A new method for the preparation of highly dispersed metal/carbon-Pd/C catalyst and its properties. Electrochimi. Acta 1993, 38, 2333–2341. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, Y.; Qin, R.; Mo, S.; Chen, G.X.; Gu, L.; Chevrier, D.; Zhang, P.; Zheng, N. Photochemical route for synthesizing atomically dispersed palladium catalysts. Science 2016, 352, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.; El-Sayed, M.A. Effect of catalysis on the stability of metallic nanoparticles: Suzuki reaction catalyzed by PVP-palladium nanoparticles. J. Am. Chem. Soc. 2003, 125, 8340–8347. [Google Scholar] [CrossRef]
- Gugliotti, L.A.; Feldheim, D.L.; Eaton, B.E. RNA-mediated metal-metal bond formation in the synthesis of hexagonal palladium nanoparticles. Science 2004, 304, 850–852. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Chen, J.; Wiley, B.; Xia, Y.; Yin, Y. Understanding the role of oxidative etching in the polyol synthesis of Pd nanoparticles with uniform shape and size. J. Am. Chem. Soc. 2005, 127, 7332–7333. [Google Scholar] [CrossRef]
- Cabiac, A.; Delahay, G.; Durand, R.; Trens, P.; Plee, D.; Medevielle, A.; Coq, B. The influence of textural and structural properties of Pd/carbon on the hydrogenation of cis, trans, trans-1,5,9-cyclododecatriene. Appl. Catal. A Gen. 2007, 318, 17–21. [Google Scholar] [CrossRef]
- Tamai, H.; Nobuaki, U.; Yasuda, H. Preparation of Pd supported mesoporous activated carbons and their catalytic activity. Mater. Chem. Phys. 2009, 114, 10–13. [Google Scholar] [CrossRef]
- Qiu, W.; Liu, H.; Dong, K.; Sun, C.; Pang, S.; Bai, G.; Zi, X.; Zhang, G.; He, H. Preparation of Pd(OH)2/C catalyst for hydrogenolytic debenzylation of hexabenzylhexaazaisowurtzitane. Chin. J. Energetic Mater. 2014, 22, 441–446. [Google Scholar]
- Zhang, M.; Liu, S.; Li, L.; Li, X.; Huang, H.; Yin, J.; Shao, X.; Yang, J. Effect of carbon supports on Pd catalyst for hydrogenation debenzylation of hexabenzylhexaazaisowurtzitane (HBIW). J. Energetic Mater. 2017, 35, 251–264. [Google Scholar] [CrossRef]
- Tang, M.; Mao, S.; Li, M.; Wei, Z.; Xu, F.; Li, H.; Wang, Y. RuPd alloy nanoparticles supported on N-doped carbon as an efficient and stable catalyst for benzoic acid hydrogenation. ACS Catal. 2015, 5, 3100–3107. [Google Scholar] [CrossRef]
- Xu, X.; Tang, M.; Li, M.; Li, H.; Wang, Y. Hydrogenation of benzoic acid and derivatives over Pd nanoparticles supported on N-doped carbon derived from glucosamine hydrochloride. ACS Catal. 2014, 4, 3132–3135. [Google Scholar] [CrossRef]
- Pang, S.; Zhang, Y.; Huang, Y.; Yuan, H.; Shi, F. N/O-doped carbon as a ‘‘solid ligand” for nano-Pd catalyzed biphenyl- and triphenylamine syntheses. Catal. Sci. Technol. 2017, 7, 2170–2182. [Google Scholar] [CrossRef]
- Zhu, M.; Gao, X.; Luo, G.; Dai, B. A novel method for synthesis of phosphomolybdic acid-modified Pd/C catalysts for oxygen reduction reaction. J. Power Sources 2013, 225, 27–33. [Google Scholar] [CrossRef]
- Li, J.; Ma, L.; Li, X.; Lu, C.; Liu, H. Effect of nitric acid pretreatment on the properties of activated carbon and supported palladium catalysts. Ind. Eng. Chem. Res. 2005, 44, 5478–5482. [Google Scholar] [CrossRef]
- Saleh, T.A. The influence of treatment temperature on the acidity of MWCNT oxidized by HNO3 or a mixture of HNO3/H2SO4. Appl. Surf. Sci. 2011, 257, 7746–7751. [Google Scholar] [CrossRef]
- Lu, A.H.; Li, W.; Muratova, N.; Spliethoff, B.; Schuth, F. Evidence for C-C bond cleavage by H2O2 in a mesoporous CMK-5 type carbon at room temperature. Chem. Commun. 2005, 41, 5184–5186. [Google Scholar] [CrossRef]
- Han, W.; Huang, X.; Lu, G.; Tang, Z. Carefully designed oxygen-containing functional groups and defects of porous carbon spheres with UV-O3 treatment and their enhanced catalytic performance. Appl. Surf. Sci. 2017, 436, 747–755. [Google Scholar] [CrossRef]
- Lu, C.; Chiu, H. Chemical modification of multiwalled carbon nanotubes for sorption of Zn2+ from aqueous solution. Chem. Eng. J. 2008, 139, 462–468. [Google Scholar] [CrossRef]
- Gu, Y.; Li, Y.; Zhang, J.; Zhang, H.; Guo, Z. Effects of pretreated carbon supports in Pd/C catalysts on rosin disproportionation catalytic performance. Chem. Eng. Sci. 2020, 216, 115588. [Google Scholar] [CrossRef]
- Martin, S.; Hans-Ulrich, B. Influence of catalyst type, solvent, acid and base on the selectivity and rate in the catalytic debenzylation of 4-chloro-N, N-dibenzylaniline with Pd/C and H2. J. Mol. Catal. A Chem. 1996, 112, 437–445. [Google Scholar]
- Kosydar, R.; Szewczyk, I.; Natkanski, P.; Duraczynska, D.; Gurgul, J.; Kustrowski, P.; Drelinkiewicz, A. New insight into the effect of surface oxidized groups of nanostructured carbon supported Pd catalysts on the furfural hydrogenation. Surf. Interfaces 2019, 17, 100379. [Google Scholar] [CrossRef]
- Sandee, A.J.; Chintada, T.J.; Groen, C.; Donkervoort, G.J.; Terorde, R. Optimized palladium on activated carbon formulation for N-hydrogenolysis reactions. Chim. Oggi 2013, 31, 20–23. [Google Scholar]
- Nielsen, A.T.; Nissan, R.A.; Vanderah, D.J.; Coon, C.L.; Gilardi, R.D.; George, C.F.; Flippen-Anderson, J. Polyazapolycyclics by condensation of aldehydes with amines. 2. Formation of 2,4,6,8,10,12-hexabenzyl-2,4,6,8,10,12-hexaazatetracyclo[5.5.0.05.9.03,11]dodecanes from glyoxal and benzylamines. Cheminform 1990, 55, 1459–1466. [Google Scholar] [CrossRef]
- Koskin, A.P.; Simakova, I.L.; Parmon, V.N. Reductive debenzylation of hexabenzylhexaazaisowurtzitane-the key step of the synthesis of polycyclic nitramine hexanitrohexaazaisowurtzitane. Russ. Chem. Bull. 2007, 56, 2370–2375. [Google Scholar] [CrossRef]
- Lou, D.Y.; Wang, H.S.; Liu, S.; Li, L.; Zhao, W.; Chen, X.H.; Wang, J.L.; Li, X.Q.; Wu, P.F.; Yang, J. PdFe bimetallic catalysts for debenzylation of hexabenzylhexaazaisowurtzitane (HBIW) and tetraacetyldibenzylhexaazaisowurtzitane (TADBIW). Catal. Commun. 2018, 109, 28–32. [Google Scholar] [CrossRef]
- Chen, Y.; Qiu, W.; Sun, J.; Li, S.; Bai, G.; Li, S.; Sun, C.; Pang, S. Synthesis of flowerlike carbon nanosheets from hydrothermally carbonized glucose: An in situ self-generating template strategy. RSC Adv. 2019, 9, 37355–37364. [Google Scholar] [CrossRef]
- Agostini, G.; Lamberti, C.; Pellegrini, R.; Leofanti, G.; Giannici, F.; Longo, A.; Groppo, E. Effect of pre-reduction on the properties and the catalytic activity of Pd/carbon catalysts: A comparison with Pd/Al2O3. ACS Catal. 2014, 4, 187–194. [Google Scholar] [CrossRef]
- Lazzarini, A.; Piovano, A.; Pellegrini, R.; Leofanti, G.; Agostini, G.; Rudic, S.; Chierotti, M.R.; Gobetto, R.; Battiato, A.; Spoto, G.; et al. A comprehensive approach to investigate the structural and surface properties of activated carbons and related Pd-based catalysts. Catal. Sci. Technol. 2016, 6, 4910–4922. [Google Scholar] [CrossRef]
- Aksoylu, A.E.; Madalena, M.; Freitas, A.; Fernando, M.; Pereira, R.; Figueiredo, J.L. The effects of different activated carbon supports and support modifications on the properties of Pt/AC catalysts. Carbon 2001, 39, 175–185. [Google Scholar] [CrossRef]
- Ehrburger, P.; Mahajan, O.P.; Walker, P.L. Carbon as a support for catalysts: 1. Effect of surface heterogeneity of carbon on dispersion of platinum. J. Catal. 1976, 43, 61–67. [Google Scholar] [CrossRef]
- Ehrburger, P.; Walker, P.L. Carbon as a support for catalysts: Ⅱ. Size distribution of platinum particles on carbons of different heterogeneity before and after Sintering. J. Catal. 1978, 55, 63–70. [Google Scholar] [CrossRef]
- Derbyshire, F.J.; Debeer, V.H.J.; Abotsi, G.M.K.; Scaroni, A.W.; Solar, J.M.; Skrovanek, D.J. The influence of surface functionality on the activity of carbon-supported catalysts. Appl. Catal. 1986, 27, 117–131. [Google Scholar] [CrossRef]
- Cheng, N.; Lv, H.; Wang, W.; Mu, S.; Pan, M.; Marken, F. An ambient aqueous synthesis for highly dispersed and active Pd/C catalyst for formic acid electrooxidation. J. Power Sources 2010, 195, 7246–7249. [Google Scholar] [CrossRef]
- Jurkiewicz, K.; Kamiński, M.; Glajcar, W.; Woznica, N.; Julienne, F.; Bartczak, P.; Polanski, J.; Lelatko, J.; Zubko, M.; Burian, A. Paracrystalline structure of gold, silver, palladium and platinum nanoparticles. J. Appl. Crystallogr. 2018, 51, 411–419. [Google Scholar] [CrossRef]
- Burton, A.W.; Ong, K.; Rea, T.; Chan, I.Y. On the estimation of average crystallite size of zeolites from the Scherrer equation: A critical evaluation of its application to zeolites with one-dimensional pore systems. Microporous Mesoporous Mater. 2009, 117, 75–90. [Google Scholar] [CrossRef]
- Korzec, M.; Bartczak, P.; Niemczyk, A.; Szade, J.; Kapkowski, M.; Zenderowska, P.; Balin, K.; Lelatko, J.; Polanski, J. Bimetallic nano-Pd/PdO/Cu system as a highly effective catalyst for the Sonogashira reaction. J. Catal. 2014, 313, 1–8. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, A.; Suarez-Garcia, F.; Martinez-Alonso, A.; Tascon, J.M.D. Surface modification of nanocast ordered mesoporous carbons through a wet oxidation method. Carbon 2013, 62, 193–203. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef]
- Janus, P.; Janus, R.; Kustrowski, P.; Jarczewski, S.; Wach, A.; Silvestre-Albero, A.M.; Rodríguez-Reinoso, F. Chemically activated poly (furfuryl alcohol)-derived CMK-3 carbon catalysts for the oxidative dehydrogenation of ethylbenzene. Catal. Today 2014, 235, 201–209. [Google Scholar] [CrossRef]
- An, N.; Dai, Y.; Tang, C.; Yuan, X.; Dong, J.; Shen, Y.; Zhou, W. Design and preparation of a simple and effective palladium catalyst and the hydrogenation performance toward dibenzylbiotinmethylester. J. Colloid Interf. Sci. 2016, 470, 56–61. [Google Scholar] [CrossRef] [PubMed]






| Sample | SBET /m2 g−1 | Smic /m2 g−1 | Vmic/Vtot | Vtot /cm3 g−1 |
|---|---|---|---|---|
| NSC | 595 | 280 | 0.19 | 0.90 |
| NSC-600 | 600 | 364 | 0.30 | 0.68 |
| NSCox-2 | 510 | 227 | 0.13 | 0.81 |
| Pd/NSC | 560 | 262 | 0.12 | 0.72 |
| Pd/NSC-600 | 472 | 180 | 0.12 | 0.54 |
| Pd/NSCox-2 | 485 | 167 | 0.10 | 0.68 |
| Samples | Carboxylic-1 | Carboxylic-2 | Anhydride | Lactone | ||||
|---|---|---|---|---|---|---|---|---|
| TM (°C) | A (μmol/g) | TM (°C) | A (μmol/g) | TM (°C) | A (μmol/g) | TM (°C) | A (μmol/g) | |
| NSC-600 | - | - | - | - | - | - | 733 892 | 234 34 |
| NSC | 210 | 356 | 315 | 614 | 525 | 627 | - | - |
| NSCox-2 | 188 | 680 | 272 | 942 | 427 | 1293 | - | - |
| Samples | Anhydride | Phenol | Carbonyl-Quinone | |||
|---|---|---|---|---|---|---|
| TM (°C) | A (μmol/g) | TM (°C) | A (μmol/g) | TM (°C) | A (μmol/g) | |
| NSC-600 | - | - | - | - | 771 920 | 318 160 |
| NSC | 509 | 322 | 613 | 562 | 762 | 386 |
| NSCox-2 | 432 | 514 | 559 | 968 | 730 | 572 |
| Sample | Atomic Concentration (mol%) | |||||||
|---|---|---|---|---|---|---|---|---|
| C 1s | O 1s | |||||||
| C=C sp2 C-C sp3 | C-OH | C=O | COOH | C=O | OH, COOH N-O | COOH | H2O | |
| 284.7 | 286.0 | 287.0 | 288.8 | 531.0 | 532.9 | 534.5 | 536.5 | |
| Pd/NSC-600 | 70.2 | 12.1 | 9.7 | 8.0 | 16.9 | 67.8 | 11.2 | 4.1 |
| Pd/NSC | 67.9 | 11.4 | 10.1 | 10.6 | 15.4 | 66.2 | 16.2 | 1.7 |
| Pd/NSCox-2 | 65.1 | 10.1 | 7.6 | 17.2 | 17.2 | 55.8 | 22.8 | 4.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Ding, X.; Qiu, W.; Song, J.; Nan, J.; Bai, G.; Pang, S. Effects of Surface Oxygen-Containing Groups of the Flowerlike Carbon Nanosheets on Palladium Dispersion, Catalytic Activity and Stability in Hydrogenolytic Debenzylation of Tetraacetyldibenzylhexaazaisowurtzitane. Catalysts 2021, 11, 441. https://doi.org/10.3390/catal11040441
Chen Y, Ding X, Qiu W, Song J, Nan J, Bai G, Pang S. Effects of Surface Oxygen-Containing Groups of the Flowerlike Carbon Nanosheets on Palladium Dispersion, Catalytic Activity and Stability in Hydrogenolytic Debenzylation of Tetraacetyldibenzylhexaazaisowurtzitane. Catalysts. 2021; 11(4):441. https://doi.org/10.3390/catal11040441
Chicago/Turabian StyleChen, Yun, Xinlei Ding, Wenge Qiu, Jianwei Song, Junping Nan, Guangmei Bai, and Siping Pang. 2021. "Effects of Surface Oxygen-Containing Groups of the Flowerlike Carbon Nanosheets on Palladium Dispersion, Catalytic Activity and Stability in Hydrogenolytic Debenzylation of Tetraacetyldibenzylhexaazaisowurtzitane" Catalysts 11, no. 4: 441. https://doi.org/10.3390/catal11040441
APA StyleChen, Y., Ding, X., Qiu, W., Song, J., Nan, J., Bai, G., & Pang, S. (2021). Effects of Surface Oxygen-Containing Groups of the Flowerlike Carbon Nanosheets on Palladium Dispersion, Catalytic Activity and Stability in Hydrogenolytic Debenzylation of Tetraacetyldibenzylhexaazaisowurtzitane. Catalysts, 11(4), 441. https://doi.org/10.3390/catal11040441







